Abstract
Patient: Female, 27-year-old
Final Diagnosis: Brain death
Symptoms: Loss of consciousness
Medication:—
Clinical Procedure: —
Specialty: Critical Care Medicine • Obstetrics and Gynecology
Objective:
Rare disease
Background:
The care and management of brain-dead pregnant women is surrounded by legal and ethical controversies. Gestational age is directly proportional to newborn survival. We report a case of a brain-dead pregnant woman at the 16th week of gestation and the successful delivery of a healthy child after 117 days of maternal somatic support.
Case Report:
A 27-year-old pregnant woman at 16 weeks’ gestation with large intracerebral hematoma after rupture of an arteriovenous malformation was admitted to our intensive care unit. Signs of brain death developed early, and the woman was confirmed to be brain dead after day 6 of hospitalization. The decision-making process regarding course of medical treatment was complex and accompanied by uncertainties arising from the absence of a legal, ethical, and professional framework. A complex multidisciplinary approach was followed. The main aim was to maintain the brain-dead woman’s homeostasis to allow for proper development of the fetus. Monitoring of fetal growth was considered the best endpoint, and satisfactory fetus development was achieved. A healthy child was delivered with a birth weight of 2140 g. Her Apgar score was 10/10/10 at 1, 5, and 10 minutes, respectively, and favorable outcomes were observed at a 1-year follow-up.
Conclusions:
Brain death during pregnancy is an extremely rare but increasingly common condition. Guidelines for care management are lacking, and reporting these cases may help establish medical treatment in future cases. We show that somatic support of the body of a brain-dead pregnant woman for an extended period of time can lead to successful delivery of a healthy child.
Keywords: Brain Death; Case Reports; Critical Care; Delivery, Obstetric; Life Support Care; Pregnancy
Background
Several medical emergencies directly or indirectly affecting the brain may lead to the development of brain death (BD). Continuation of somatic support after BD is diagnosed is cornerstone and common praxis in organ-donation programs. Development of BD during pregnancy is rare, and only 30 cases have been described in the medical literature since 1982, involving 19 infants who were born and survived the neonatal period [1,2]. Homeostasis and overall organ function must be maintained in brain-dead pregnant women to ensure proper development of the fetus. The gestational age is directly proportional to the survival of the baby, and thus attempts to prolong the somatic support of brain-dead pregnant women should be made even in early phases of pregnancy, because doing so can lead to the successful delivery of a healthy child [1,3].
We report a case of a brain-dead pregnant woman at the 16th week of gestation and the successful delivery of a healthy child after 117 days of maternal somatic support, with overall good 1-year outcomes for the child. This case report was prepared according to CARE guidelines [4]. The patient’s family provided written informed consent for the publication of this report.
Case Report
A 27-year-old woman, secundigravida, in the 16th week of pregnancy, with a prior uncomplicated course of pregnancy in regular obstetric care, was found unconscious for an unknown period of time at home. She was referred to the emergency department.
She was a nonsmoker in good physical condition. She was not taking any medicines that increased the risk of bleeding. Her first pregnancy in 2016 was complicated during delivery by paroxysms and generalized seizures, and an arteriovenous malformation (AVM) of the left frontal-parietal brain lobe was diagnosed. She was administered levetiracetam and underwent gamma knife surgery during the same year. In 2018, she experienced another episode of seizures, and persistent AVM was diagnosed through magnetic resonance imaging. Regarding the localization and anatomy of the AVM, no therapeutic option was offered to the patient. Although she knew of the high risk of AVM rupture, she became pregnant in 2019 and she did not provide an advance life directive in the event that she would not be able to speak for herself. The pregnancy was complicated in early stages by AVM rupture, as described above. In the initial physical examination, she was in coma with Glasgow Coma Scale score of 3 points, her pupils were fixed and dilated at 5 mm, photoreaction was absent bilaterally, her blood pressure was 110/75 mmHg, and her heart rate was 70 beats/min.
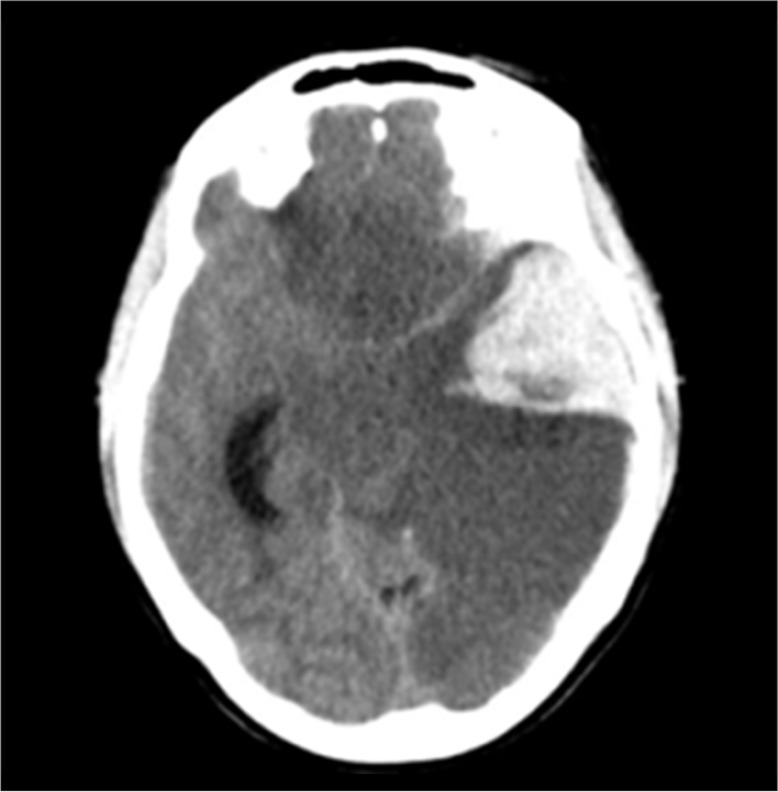
A computed tomography (CT) scan showed a large intracerebral hematoma and ischemia of the left cerebral hemisphere, part of the right frontal lobe and the brainstem, with signs of expansion and cerebral edema (Figure 1). CT angiography was performed to identify the source of bleeding. The source could not be identified, owing to extensive edema and the hemorrhage itself.
Figure 1.
CT scan of head on admission showing an intracerebral hematoma and ischemia of the left cerebral hemisphere, part of the right frontal lobe, and the brainstem, with signs of expansion and cerebral edema.
Regarding the patient’s history and results of CT showing a large hematoma localized to the region of the AVM, a diagnosis of AVM rupture was determined with a high level of certainty. Other causes of sudden unconsciousness, such as cerebral venous sinus thrombosis, were also considered but were deemed unlikely. According to the clinical status of the patient and the CT findings, the prognosis of the patient was assessed as poor. However, the obstetric ultrasound examination showed a viable fetus. Because of the medical, legal, and ethical uncertainty associated with whether somatic support should be continued or stopped, the patient was admitted to the intensive care unit (ICU). A discussion with family members began after admission to the ICU. The agency in the decision-making process was given to the father of the child, but other family members’ opinions were also considered. With respect for the family members’ wishes, a decision to proceed with the somatic support was made by the ICU team leader, in consensus with all ICU team members. A multidisciplinary approach was used to determine the best management of care in this case. With respect for the wishes of the family members, the multidisciplinary team developed a special medical management plan in an attempt to save the child. Clinical signs of BD were diagnosed on hospitalization day (HD) 1 and, according to the laws of the Czech Republic, the BD was assessed on the basis of an absence of defined brainstem reflexes on clinical examination (an apnea test is not mandatory and was not performed because of the possible negative effects of maternal hypoxia on the fetus). This clinical estimation was confirmed on HD 6, on the basis of brainstem auditory evoked potentials. The confirmation was mandatory for diagnosis of BD, and one of the instrumental methods that must be used by law.
Standard monitoring of vital signs and laboratory testing was performed during the entire ICU stay. Endocrinology laboratory tests were performed regularly once per week (TSH, fT4, and serum cortisol), and vitamin D levels were verified once per week. Fetal monitoring was performed daily for a period of 15 minutes with cardiotocography and ultrasound examination of the fetal heart rate.
Somatic support of the brain-dead woman was continued with the aim of reaching at least 32 weeks of pregnancy and delivering a viable fetus. The main goal of the somatic support was to provide a sufficient oxygen supply to all maternal organs, including the placenta. During the entire course of hospitalization, the lung protective strategy of artificial ventilation was applied with peak inspiratory pressures below 20 cmH2O. Vasopressors were administered to maintain the mean arterial pressure. Intravenous norepinephrine was replaced with dopa-mine on HD 53. Circulatory support was not needed after HD 99.
Hypothalamic-pituitary dysfunction was managed with hormone supplemental therapy driven by plasma serum concentrations of hormones, as discussed with an endocrinologist. The serum levels of cortisol and thyroxine were examined once per week, and substitutional therapy was used to maintain serum levels in normal ranges.
Levothyroxine was administered via a nasogastric tube, and the dose was increased throughout the ICU stay from 75 mcg on HD 17–24 to 100 mcg on HD 25–29, 125 mcg on HD 30–78, and 125 mcg 5 days per week +137 mcg 2 days per week on HD 79–117. A dose of 100 mg of hydrocortisone was given intravenously twice per day and was decreased gradually to 50 mg daily on HD 30–50. Subsequently, the dose was switched to 30 mg daily, divided into 2 doses (20 mg in the morning and 10 mg in the evening). In the case of infection, the dose was increased to 200 mg daily and was administered intravenously. Diabetes insipidus developed on HD 1, and thus daily administration of desmopressin was necessary. We started with 60 mcg of desmopressin per day, and the dose was then titrated according to its effects. From HD 67, the dose was stabilized at 90 mcg of desmopressin per day and administered for the remainder of the ICU stay.
Infections were treated with antibiotics. Pneumonia developed twice. The first episode was caused by Staphylococcus aureus plus Streptococcus pneumoniae and was treated with intravenous administration of ampicillin/sulbactam. The second episode was caused by Klebsiella pneumoniae and was treated with intravenous piperacillin/tazobactam. Urinary tract infections developed 3 times. The first episode was caused by Klebsiella oxytoca and Escherichia coli with extended-spectrum beta lactamase (ESBL), and was treated with meropenem. The second episode was caused by E. coli ESBL and was treated with meropenem. The etiology of the third urinary tract infection was mixed and was caused by E. coli ESBL and Enterococcus sp. This infection was treated with meropenem and ampicillin. Vaginal infection was treated once and was caused by E. coli ESBL. Local application of nifuratel was used for the treatment. The choice of antibiotics was made after considering culture results, local antibiotic policy, and the safety of each antibiotic during pregnancy. All infections were treated successfully, and amniotic fluid bacteriological cultures after delivery were negative.
Low molecular weight heparin (LMWH) was administered subcutaneously for deep-vein thrombosis and pulmonary embolism prevention. The dose of LMWH was set according to the manufacturer’s recommendation and based on body weight. LMWH was applied subcutaneously once daily after HD 14. Neither hemorrhagic nor thrombotic complications were diagnosed during hospitalization.
To estimate the daily dose of calories and proteins to meet the nutritional demands of both the brain-dead mother and the developing fetus, the standard formula for critically ill patients was used. The actual body weight (ABW) in kilograms was multiplied by 25 calories and by 1.5 g to determine the daily caloric and protein demand, respectively. From HD 24, the targets increased to 30 calories per kilogram ABW and 1.6 g protein, respectively. These amounts were further increased to 35 calories per kg ABW and 1.8 g protein from HD 40. Finally, the targets were set at 45 calories and 2 g protein per kilogram ABW from HD 51. To meet these nutritional targets, a high-protein high-energy formula for enteral nutrition was continuously administered from HD 3, and from HD 42, the administration of nutrition switched to boluses. Special attention was paid to vitamin D replacement. The dose of oral preparation of cholecalciferol was set according to serum levels, and daily administration of 1500 IU was necessary to maintain the serum levels in normal ranges.
The body of the brain-dead woman was positioned in a semi-recumbent position to prevent the possible negative hemodynamic effects of an enlarged uterus compressing the inferior vena cava. From gestation week 27, she was positioned only laterally. Physiotherapy was performed by a physiotherapist 2 times per day for 60 minutes. To simulate the mother’s walking, we used a MOTOmed letto2 leg/arm device (MOTOmed®, RECK-Technik GmbH & Co. KG Medizintechnik, Reckstraße 1–5, 88422 Betzenweiler, Germany).
The organ system monitoring and support that was given is summarized in Table 1. To accelerate fetal lung maturity, we administered betamethasone on HD 53 (gestation week 25) and on HD 86 (gestation week 30). The development of the fetus was monitored regularly by an obstetrician. Ultrasound examination with biometry of the fetus was performed weekly to assess the growth of the fetus and rule out any organ development malformation. The progress of fetal growth is outlined in Table 2. A clinical geneticist was consulted to assess the risk of a genetic disorder and chromosomal aberration on HD 11, and the results were negative.
Table 1.
Summary of organ support and monitoring.
| Organ system | Parameter | Assessment | Intervention | |
|---|---|---|---|---|
| The woman | Cardiovascular | Blood pressure, heart rate | Continuous | Fluids if considered hypovolemic, inotropes otherwise, preferentially dopamine |
| Respiratory | Blood gas analysis | 3 times a day | Lung protective mechanical ventilation, set to keep blood gases in normal range | |
| Gastrointestinal/nutrition | Growth of the fetus Total serum protein, albumin and prealbumin Serum vitamin D levels |
Once a week Once a week Once a week |
Gradual increase of the dose of nutrition Gradual increase of the dose of nutrition Substitution to keep serum level in normal range |
|
| Renal | Serum ions/urine specific gravity | 3 times a day/in case of polyuria | Substitution, desmopressin in case of polyuria | |
| Endocrine | Serum hormone levels (thyroxine, cortisol) | Once a week | Substitution to keep serum levels in normal range | |
| The fetus | Cardiovascular | Cardiotocography | Twice a day | – |
| Fetal growth | Ultrasound biometry | Once a week | – | |
| Organ development malformation | Ultrasound | Once a week | – |
Table 2.
Fetal growth.
| Hospitalization day | Gestational age | Estimated weight |
|---|---|---|
| HD 44 | 23+2 | 580 g |
| HD 65 | 26+2 | 980 g |
| HD 79 | 29+2 | 1350 g |
| HD 86 | 30+0 | 1500 g |
| HD 93 | 31+5 | 1700 g |
| HD 107 | 32+5 | 2100 g |
| HD 117 | 34+0 | 2200 g |
Basal stimulation of the fetus was also performed. Each manipulation of the brain-dead woman’s body was preceded by caressing of her abdomen and calling the fetus by name. The end of the manipulation was marked by a sound made by a toy. The family members were involved in basal stimulation, part of which involved gentle tactile stimulation of the brain-dead woman’s abdomen, playing music, and reading fairy tales. The family members were supported by psychologists throughout the hospitalization.
On HD 117 (gestation week 35), a female infant was delivered via post-mortem cesarean section, with a birth weight of 2140 g and an Apgar score of 10/10/10 at 1, 5, and 10 minutes, respectively. After the delivery, the somatic support of the mother’s body was terminated.
The newborn was admitted to the neonatal department and discharged after 11 days of uncomplicated hospitalization. Her weight at discharge was 2220 g. The baby was examined by a neurologist at 4 weeks, and 4, 9, and 12 months. Her neurologic status and psychomotor development were found to be in normal ranges. Key points of the case are summarized in the timeline and are shown in Figure 2.
Figure 2.
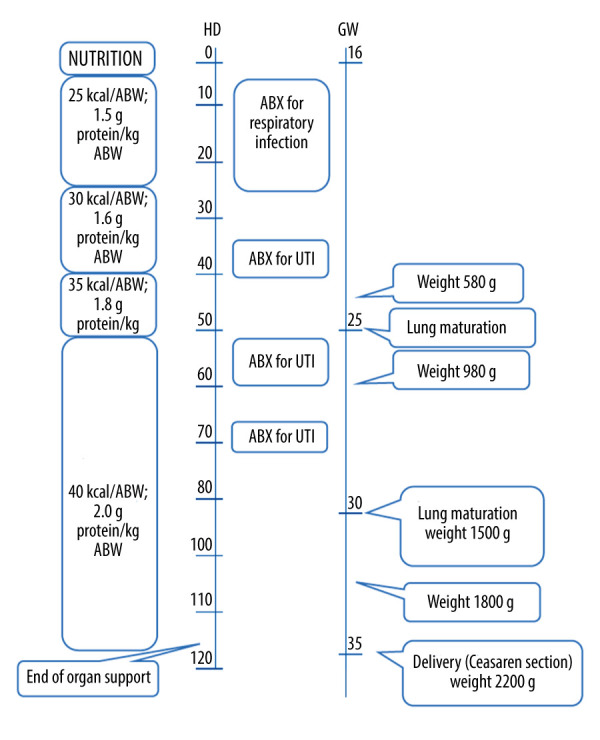
Key actions and milestones during the course of treatment. ABW – actual body weight; ABX – antibiotics; UTI – urinary tract infection.
Discussion
Progress in intensive care, technology and patient care in recent decades has brought about a unique situation in which somatic support of brain-dead pregnant women is possible to prolong the pregnancy until a viable fetus can be delivered. This situation is quite uncommon, and to date, only 25 cases have been described in the medical literature [1]. To our knowledge, descriptions of only 3 cases in which the somatic support exceeded 100 days have been published [5–7].
The decision to continue somatic support of the body of a pregnant woman after a diagnosis of BD is a controversial ethical and legal issue. There are no specific legal regulations associated with somatic support after BD is diagnosed during pregnancy in the Czech Republic, and a wide range of options are present in different countries. The International Federation of Gynecology and Obstetrics has published guidelines for the management of the social and ethical challenges presented by BD during pregnancy [8]. The guidelines outline 6 key recommendations for the management of brain-dead pregnant women. Each recommendation was discussed, and attempts to follow the guidelines were made. The recommendations, and how they were addressed by the medical team, are summarized as follows: 1. Women have the right to die in dignity. This right should be respected in the treatment of every single patient. 2. Questions regarding maintaining pregnancy must be answered in consultation with the remaining family members. In the present case, the family members were involved in the decision-making process form the very beginning. The mother did not provide an advance life directive before BD occurred, and her wishes about whether to continue life-sustaining therapy in the event of BD were unknown. However, the father of the child acted as the woman’s agent and declared that in his and her family members’ opinion, the woman would have wished that every effort be made to save her child. 3. Decisions on delivery in the event of BD should be made according to fetal viability. Surveillance of fetal well-being should be implemented. The fetus was not viable when BD was diagnosed. The woman was in stable condition, and no organ dysfunction was apparent at that time. Fetal well-being was assessed by an obstetrician, and no signs of fetal damage were found. 4. There is no low gestational age limit for the onset of fetal rescue after maternal BD. The gestational age of the fetus, and therefore the viability, was low, but this aspect was not considered. 5. The interest of the fetus must be considered. Efforts should be made to promote the birth of a mature, brain-intact infant. In our opinion, the best way to ensure this was to maximize efforts to secure the stability of the brain-dead pregnant mother of the fetus. All organ systems were carefully monitored and supported adequately as necessary. 6. The fetus should be allowed to die in utero if maternal or fetus distress calls for immediate delivery with a high probability of an unfavorable outcome. To address this issue, the medical team assessed the level of organ support of the brain-dead mother on a daily basis, taking precautions not to provide organ support beyond the level usually provided for brain-dead organ donors; eg, the use of excessive vasopressor dosing to maintain adequate blood pressure or renal replacement therapy if acute renal failure develops. According to these guidelines, we focused primarily on the interest of the fetus, and the agency in the decision-making process was given to the father of the child.
Independent legal and professional advice was sought, but because of the lack of legal regulations regarding this specific situation, opinions varied substantially, ranging from immediately stopping supportive care to full support as long as possible. After discussions with all members of the medical team and the family members, a decision to continue with somatic support was made, and the support was sustained until the fetus reached maturity. The decision-making process was influenced by the information that the woman and the father had known about the risks of a pregnancy associated with the woman’s AVM and the potentially fatal complications for both the woman and the child in the event of AVM rupture.
If somatic support of a brain-dead mother is initiated in an early stage of pregnancy, when the fetus is not yet viable, a rational effort should be made to maintain the pregnancy as long as possible and thus to allow for fetal development until viability is achieved. Neonatal disability is proportional to gestational age. The chance of survival of a baby born in gestational week 22 is 1% and rises to 44% in gestational week 25. The probability of survival without a disability at 30 months shows a similar course and is approximately 0.7% in gestational week 22 and 23% in gestational week 25. From gestational week 32, the survival rate rises to 98%, with a risk of neurological complications lower than 2%; therefore, making efforts to maintain the pregnancy until that time seems rational [9].
Because only individual cases of prolonged somatic support of the bodies of brain-dead pregnant women have been reported, there are no guidelines for the medical management of these women. Our management was based on our experience with non-pregnant brain-dead patients, and we considered the review by Mallampalli et al, who have discussed the physiologic changes and provided recommendations regarding organ support for brain-dead pregnant women [10].
Long-term management of a brain-dead patient may be associated with several complications.
The most severe complications are those leading to hemodynamic instability of the brain-dead pregnant woman, because this threatens the oxygen supply to the fetus. The pathophysiological changes associated with BD lead to vasoplegia and hypotension. The usual management consists of the administration of intravenous fluids and vasoactive agents.
In our case, to maintain a mean arterial pressure above 65 mmHg, we administered vasoactive agents. Norepinephrine was used for the first 53 HDs. Because of the detection of placental infarctions in an ultrasound examination, norepinephrine was replaced with dopamine on HD 53, because of the potentially negative effects of norepinephrine on placental perfusion [11].
After the onset of BD, the hypothalamic-pituitary axis is disrupted, thus leading to various clinical manifestations of panhypopituitarism. Steroids, vasopressors, levothyroxine, and desmopressin were administered to maintain maternal endocrine homeostasis. However, data supporting a choice of hormonal therapy, dosing, and effect on neonatal outcomes in brain-dead pregnant women are lacking. In our case, we adjusted the dosage of hormone replacement therapy in cooperation with an endocrinologist, according to the measured serum levels.
Infections are frequent and serious complications of prolonged intensive care [12]. Hemodynamic instability associated with sepsis may negatively affect the development of the fetus and, if not successfully treated, can lead to fetal death. In our case, infections were successfully treated with antibiotics 6 times. The duration of antibiotic treatment was 56 out of 117 HDs in total. The choice of antimicrobial agents was made after discussion with a microbiologist, considering the safety of the individual antibiotics during pregnancy. Intravenous ampicillin/sulbactam, meropenem, and piperacillin/tazobactam were used to treat respiratory and urinary tract infections.
Special attention was paid to ensure adequate nutrition, because fetal weight is a crucial factor for adequate fetal physiological development and a successful outcome of pregnancy. The increase in fetal weight throughout the pregnancy corresponded to the normal growth curve, and the birth weight was in the 75th percentile.
Efforts were made to positively support the neurobehavioral development of the fetus. Music, gentle tactile stimulation, and talking have been shown to have positive effects on the fetus. Prenatal music activities positively affect early brain maturation and neurodevelopment, and may also promote good infant temperament [13,14].
Conclusions
BD during pregnancy is an extremely rare but increasingly common condition, and its management is associated with medical, ethical, and legal uncertainties and controversies. Guidelines for care management are lacking, and reporting these cases may help establish medical treatment in future cases. We showed that somatic support of the body of a brain-dead pregnant woman for an extended period of time can lead to the successful delivery of a healthy child. This possibility should be considered during decision-making processes regarding whether to commence somatic support after BD is diagnosed in pregnant women.
Footnotes
Conflicts of Interests
None.
References:
- 1.Boran ÖF, Yazar FM, Bakacak M, et al. Assessment of somatic support process for pregnant brain death patients occurring in a transition country between Asia and Europe from medical, ethical, legal and religious aspects. J Relig Health. 2020;59(6):2935–50. doi: 10.1007/s10943-019-00952-1. [DOI] [PubMed] [Google Scholar]
- 2.Pikto-Pietkiewicz I, Okniński A, Wójtowicz R, et al. The management of a thirteen weeks pregnant woman rendered brain-dead following a ruptured aneurysm. J Crit Care Med (Targu Mures) 2019;5(3):111–14. doi: 10.2478/jccm-2019-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esmaeilzadeh M, Dictus C, Kayvanpour E, et al. One life ends, another begins: Management of a brain-dead pregnant mother – a systematic review. BMC Med. 2010;8:74. doi: 10.1186/1741-7015-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: Explanation and elaboration document. J Clin Epidemiol. 2017;89:218–35. doi: 10.1016/j.jclinepi.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein IM, Watson M, Simmons GM, et al. Maternal brain death and prolonged fetal survival. Obstet Gynecol. 1989;74:434–37. [PubMed] [Google Scholar]
- 6.Spike J. Brain death, pregnancy, and posthumous motherhood. J Clin Ethics. 1999;10:57–65. [PubMed] [Google Scholar]
- 7.Said A, Amer AJ, Masood UR, et al. A brain-dead pregnant woman with prolonged somatic support and successful neonatal outcome: A grand rounds case with a detailed review of literature and ethical considerations. Int J Crit Illn Inj Sci. 2013;3:220. doi: 10.4103/2229-5151.119205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Čartolovni A, Habek D. Guidelines for the management of the social and ethical challenges in brain death during pregnancy. Int J Gynecol Obstet. 2019;146:149–56. doi: 10.1002/ijgo.12871. [DOI] [PubMed] [Google Scholar]
- 9.Serenius F, Källén K, Blennow M, et al. Neurodevelopmental outcome in extremely preterm infants at 2.5 years after active perinatal care in Sweden. JAMA. 2013;309(17):1810–20. doi: 10.1001/jama.2013.3786. [DOI] [PubMed] [Google Scholar]
- 10.Mallampalli A, Guy E. Cardiac arrest in pregnancy and somatic support after brain death. Crit Care Med. 2005;33:S325–31. doi: 10.1097/01.ccm.0000182788.31961.88. [DOI] [PubMed] [Google Scholar]
- 11.Souza JP, Oliveira-Neto A, Surita FG, et al. The prolongation of somatic support in a pregnant woman with brain-death: A case report. Reprod Health. 2006;3:3. doi: 10.1186/1742-4755-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richards MJ, Edwards JR, Culver DH, et al. Nosocomial infections in medical intensive care units in the United States. National Nosocomial Infections Surveillance System. Crit Care Med. 1999;27(5):887–92. doi: 10.1097/00003246-199905000-00020. [DOI] [PubMed] [Google Scholar]
- 13.Mastnak W. Perinatal music therapy and antenatal music classes: Principles, mechanisms, and benefits. J Perinat Educ. 2016;25:184–92. doi: 10.1891/1058-1243.25.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang ZW, Hua J, Xu YH. The relationship between gentle tactile stimulation on the fetus and its temperament 3 months after birth. Behav Neurol. 2015;2015:71906. doi: 10.1155/2015/371906. [DOI] [PMC free article] [PubMed] [Google Scholar]