Abstract
Background
The prothrombotic phenotype and diffuse intravascular coagulation observed in COVID-19 reflect endothelial dysfunction, which is linked to blood flow delivery deficiencies and cardiovascular risk. Assessments of detect vascular deficiencies among newly diagnosed and hospitalized patients due to COVID-19 have yet to be determined.
Objective
To assess endothelial function characteristics in relation to length of hospitalization and mortality in patients diagnosed with COVID-19 and compare to patients without COVID-19.
Methods
A prospective observational study involving 180 patients with confirmed COVID-19 (COVID-19 group) or suspected and ruled out COVID-19 (Non-COVID-19 group). Clinical evaluation and flow mediated vasodilation (FMD) were performed between the first 24–48 h of hospitalization. Patients were followed until death or discharge.
Results
We evaluated 98 patients (COVID-19 group) and 82 (Non-COVID-19 group), COVID-19 group remained hospitalized longer and more deaths occurred compared to the Non-COVID-19 group (p = 0.01; and p < 0.01). Patients in COVID-19 group also had a significantly greater reduction in both FMDmm and FMD% (p < 0.01 in both). We found that absolute FMD≤0.26 mm and relative FMD≤3.43% were the ideal cutoff point to predict mortality and longer hospital stay. In Kaplan Meyer's analysis patients had a high probability of death within a period of up to 10 days of hospitalization.
Conclusion
Patients hospitalized for COVID-19 present endothelial vascular dysfunction early, remained hospitalized longer and had a higher number of deaths, when compared with patients without COVID-19.
Keywords: COVID-19, Flow-mediated dilation (FMD), Endothelium, Hospitalization
Abbreviations: SARS, Severe acute respiratory syndrome; ACE2, type 2 angiotensin converting enzyme; ARDS, Acute Respiratory Discomfort Syndrome; FMD, Flow-mediated dilation; FMD (mm), Absolut Flow-mediated dilation (in millimeters); FMD (%), Relative/percentage Flow-mediated dilation; ICU, intensive care unit; RT-PCR, Real-time reverse-transcriptase polymerase chain reaction; PPE, personal protective equipment
1. Introduction
Coronavirus disease 2019 (COVID-19), caused by SARS-CoV-2, was first reported in December 2019 in Wuhan city, China, was first reported in December 2019 in the city of Wuhan, China [1]. The route of entry is the respiratory tract where the type 2 angiotensin converting enzyme (ACE2) is a functional receptor sequestered by SARS-CoV-2 for entry into the host cell [2]. ACE2 is a protein expressed in the lungs, heart, kidneys, vascular endothelium and intestine, suggesting broad clinical consequences of SARS-CoV-2 infection that make COVID-19 a multiorgan disease [3]. The disease progresses to a severe form in 10–30% of patients infected, requiring hospitalization and potential intensive care unit (ICU) treatment [4].
In this sense, endothelial cells have recently been implicated as a primary source for the initiation and spread of Acute Respiratory Discomfort Syndrome (ARDS) caused by SARS-CoV-2, resulting in severe endothelial injury and generalized thrombosis [5]. It has been proposed that the loss of endothelial ACE2 function is related to lung injury, where negative regulation and the fall of this enzyme can lead to dysfunction of the renin-angiotensin system and potentially impair vascular function among individuals with COVID-19 infection [6].
The prothrombotic phenotype and diffuse intravascular coagulation observed in COVID-19 reflect endothelial dysfunction, which stimulates thrombosis, leading to the exposure of pro-thrombotic subendothelial material, platelet aggregation, regulation of coagulation cascades, activation of thrombin and fibrin production, as well as changes in vascular muscle tone and blood flow [5,7]. In addition, the association between a positive diagnosis for SARS-CoV-2 and risk of stroke [8], as well as myocardial infarction [9] suggests a link between blood flow delivery deficiencies and cardiovascular risk during the acute stages of COVID-19 infection.
Although recent studies have suggested that endothelial biomarker and vascular function tests should be performed [10], we have not yet identified any early assessments of vascular deficiencies among newly diagnosed and hospitalized patients with SARS-CoV-2. In this sense, the flow-mediated dilation (FMD) technique of the brachial artery is a non-invasive method used to assess systemic vascular function [11], which strongly correlates with coronary vascular function [12] and is predictive of future cardiovascular events [13].
Therefore, the aim of the current study was to assess the relationship between FMD measures of endothelial function on hospitalization days and mortality in patients diagnosed with COVID-19, as well as compare FMD measurements and outcomes to patients without COVID-19. The hypothesis of our study is that patients with COVID-19 may present with early endothelial dysfunction compared to patients without COVID-19 and this finding will be related to differences in days of hospitalization and mortality risk.
2. Methods
2.1. Design and ethical approval
This is an observational, cohort prospective study, performed in hospitals of São Carlos - SP. This study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) [14]. The study was approved by the local ethics committees (protocol number: 33265220.9.0000.5504) of Federal University of Sao Carlos (UFSCAR) and adhered to Resolution 466/2012 of the National Health Council and followed the ethical guidelines of the Declaration of Helsinki (1975) [15].
2.2. Subjects
All patients included in this study were over 18 years of age and were of both sexes, hospitalized (i.e., ward or ICU) at the University Hospital of São Carlos and Santa Casa of Misericordia, from July to December 2020. Measurements included in this study occurred between 24 and 48 h after hospitalization. Patients with positive diagnosis for COVID-19 by real-time reverse-transcriptase polymerase chain reaction (RT-PCR) from the nasopharyngeal swab [16] were included in the COVID-19 group. Patients without a negative diagnosis for COVID-19 RT-PCR, but with hospitalized with similar signs and respiratory symptoms (e.g., cough, fever, runny nose, pain in the body and throat) comprised the Non-COVID-19 group.
It should be noted that in hospitals where patients were evaluated, specific wards were created for patients with respiratory symptoms. When these patients arrived at the hospital and were assessed urgently for suspected COVID-19, they were admitted to the special ward or ICU. A positive or negative COVID-19 diagnosis was confirmed within 48 h of hospitalization.
Exclusion criteria of the present study consisted of: 1) Patients who did not agree to participate in the study and/or did not sign the Informed Consent Form; 2) severe forms of COVID-19 in the initial phase of hospitalization that culminated with sedation and intubation with invasive mechanical ventilation; 3) patients in palliative care; and 4) cases of readmission.
2.3. Experimental procedures
Patients were screened and recruited by members of the research team who visited the hospital on a daily basis. A brief evaluation of the patient's medical record was carried out to confirm the potential for eligibility. In the first face-to-face meeting, the patients or responsible family members received a description of the study and informed consent for them to read, have any questions answered and, if they agreed to participate, sign the Informed Consent. Patients who consented were then familiarized with the procedures and data collection was initiated. Members of the research team were properly equipped with personal protective equipment (PPE), including a waterproof apron, goggles, latex gloves, mask n95, disposable mask, disposable cap and face shield, in accordance with World Health Organization recommendations (WHO) [17].
2.4. Measurements
All assessments were carried out at the same time of the day (afternoon), avoiding different physiological responses due to the influence of the circadian cycle. The evaluations took place at the bedside as soon as possible following hospital admission, always within the first 24–48 h of admission. It is also noteworthy that all assessments were carried out with a minimum time of 1 h after patient feeding or any intervention by the staff, avoiding any confounding factor in the endothelial function. The following evaluations were carried out:
2.4.1. Clinical and epidemiological evaluation
Clinical data, including age, sex, weight, height, race, comorbidities, medication use and oxygen supplementation were collected from the medical records to characterize the sample.
2.4.2. Blood count, arterial blood gas analysis and vital signs
Blood count, arterial blood gas analysis and vital signs were analyzed from medical records. Hemoglobin (g/dL), Leukocytes (N/mm3), Platelets (μL), Lymphocytes (N/mm3), Neutrophils (N/mm3), Eosinophil (N/mm3), C-reactive protein (mg/L), Troponin-1 (ng/L), Total creatine kinase (CK) (U/L), Creatinine (mg/L), Hydrogen potential (pH), PaO2 (mmHg), PaCO2 (mmHg), HCO3 (mmol/L), heart rate (HR) (bpm), Systolic blood pressure (SBP) (mmHg), Diastolic blood pressure (DBP) (mmHg), Peripheral oxygen saturation (SpO2) (%), Respiratory rate (bpm) and dyspnea (MRC scale) were all collected.
2.4.3. Flow-mediated dilation (FMD)
Endothelium-dependent brachial vascular function was evaluated using ultrasonography (Sonosite turbo M, Fujifilme, Bothell, WA, USA). Briefly, patients were asked to rest for 20 min in the supine position. An inflatable cuff was placed 1–3 cm distal to the antecubital fossa on the forearm. Baseline artery diameter was measured along with pulsed Doppler signals for flow velocity analysis. The brachial artery was imaged in the longitudinal plane, 5–11 cm proximal to the medial epicondyle using a high frequency (10–5 MHz) linear–array probe [18].
The ultrasound measured the arterial diameter (B-mode) continuously in 60 s segments and Doppler (blood flow velocity) at each time point. After baseline recordings, reactive hyperemia was induced by inflation of a blood pressure cuff to a pressure of 220 mmHg for 5 min [19]. Arterial diameter was subsequently measured immediately following blood pressure cuff release for 3 min. The analyses were performed with Brachial Analyzer software (Medical Imaging Applications LLC, Iowa, USA). Before and after starting each evaluation, the equipment was cleaned with 3% hydrogen peroxide, according to the manufacturer's guidelines, in order to avoid cross-contamination of the virus.
The variables analyzed at baseline and immediately post-hyperemia were Absolute FMD (mm) (diameter before cuff – blood vessel diameter after cuff), percentage (%) FMD [(peak diameter − baseline diameter)/baseline diameter) × 100] [18].
2.4.4. Follow-up
After the evaluations, the research team followed patient's outcomes through medical record review, verifying days of hospitalization, outcome of hospital discharge, and deaths.
2.5. Statistical analysis
The results are presented as mean and standard deviation (SD) for continuous variables and percentages for categorical variables. The Kolmogorov-Smirnov test was used to verify the distribution of the data. The Student's t-test and chi-square test were used for comparison between COVID-19 and Non-COVID-19 groups.
Analysis of the receiver's operating characteristic curve (ROC) was used to identify the ideal threshold values of the FMD on hospitalization days and mortality in patients diagnosed with COVID-19. Both FMD mm and FMD% optimal predictive cut points were determined in patients with COVID-19. The 95% confidence interval (95% CI) were also calculated with the lower limit being greater than 0.50. Subsequently, the cutoff points of the variables that obtained significant areas under the ROC curve were identified, with the respective values of sensitivity and specificity. Survival was calculated using the Kaplan-Meyer's analysis and the log-rank test, using as exposures FMDmm, deaths number and hospitalization days. Cox proportional univariate and multivariate regression models (adjusted for FMD mm, age, sex, comorbidities and BMI) were performed, with associations expressed as risk ratios (HRs) and 95% CIs. All tests were made in Graphpad Prism 8.0 (GraphPad Software, California, USA) with statistical significance set at p ≤ 0.05.
3. Results
235 patients were initially recruited with 55 excluded. Reasons for exclusion were: 1) 2 were excluded due to refusing to participate and not signing the Informed Consent Form; 2) 25 patients were sedated during the first 24–48 h of hospitalization; 3) 19 patients were severe cases and required palliative care; and 4) 9 patients were readmitted to the hospital for other diseases. Of the remaining 180 patients, 98 patients were positive on the RT-PCR test for COVID-19 and were allocated to the COVID-19 group and 82 were negative on the RT-PCR test for COVID-19 and were allocate to the Non-COVID-19 group (Fig. 1 ).
Fig. 1.
Flow chart of study.
3.1. General characteristics
Clinical data, comorbidities, number of deaths, hospitalization days, medication use, blood count and FMD results are listed in Table 1 .
Table 1.
Clinical data, comorbidity, number of deaths, hospitalization days, medications, blood count and FMD in patients with Non-COVID-19 and COVID-19 confirmed.
| Variables | Non-COVID-19 (n = 82) | COVID-19 (n = 98) | P value |
|---|---|---|---|
| Age (years) | 63 ± 17 | 61 ± 16 | 0.45 |
| Sex, n (%) | 0.28 | ||
| Male | 40 (49) | 55 (56) | |
| Woman | 42 (51) | 43 (44) | |
| Weight, (kg) | 72 ± 25 | 81 ± 18 | 0.01 |
| Height (m) | 1.66 ± 0.10 | 1.67 ± 0.09 | 0.31 |
| Body Mass Index (BMI) (kg/m2) | 25 ± 7 | 28 ± 5 | 0.01 |
| Race, n (%) | 0.28 | ||
| White | 53 (65) | 64 (65) | |
| Brown | 24 (29) | 24 (25) | |
| Black | 5 (6) | 10 (10) | |
| Evaluation location, n (%) | <0.01 | ||
| Nursery | 73 (89) | 80 (82) | |
| Intensive care unit (ICU) | 7 (9) | 18 (18) | |
| Emergency | 2 (2) | – | |
| Comorbidity, n (%) | |||
| Hypertension | 43 (54) | 52 (63) | <0.01 |
| COPD | 18 (22) | 11 (11) | <0.01 |
| Diabetes | 21 (26) | 29 (30) | <0.01 |
| Hospitalization days | 5.7 ± 4.8 | 8.3 ± 7.6 | 0.01 |
| Symptom days | 5 ± 5 | 7 ± 4 | <0.01 |
| Death, n (%) | 4 (5) | 11 (12) | <0.01 |
| Oxygen supplementation,n (%) | 39 (48) | 60 (61) | <0.01 |
| Liters | 2.2 ± 1.0 | 2.8 ± 2.3 | 0.07 |
| Interfaces | |||
| Nasal catheter | 39 (48) | 52 (51) | <0.01 |
| Venturi Mask | 0 (0) | 8 (9) | <0.01 |
| Medications during hospitalization, n (%) | |||
| Inhibits platelet aggregation | 9 (11) | 23 (24) | <0.01 |
| Antibiotics | 51 (62) | 69 (71) | <0.01 |
| Antivirals | 1 (1) | 8 (9) | <0.01 |
| Blood count | |||
| Hemoglobin (g/dL) | 12 ± 3 | 12 ± 3 | 0.52 |
| Leukocytes (N/mm3) | 11669 ± 6005 | 8560 ± 4325 | 0.01 |
| Platelets (μL) | 275681 ± 110098 | 230761 ± 95071 | 0.03 |
| Lymphocytes (N/mm3) | 1830 ± 1960 | 1479 ± 1759 | 0.77 |
| Neutrophils (N/mm3) | 8571 ± 5406 | 6527 ± 4081 | 0.04 |
| Eosinophil (μL) | 161 ± 135 | 100 ± 93 | <0.01 |
| C-reactive protein (mg/L) | 9 ± 10 | 11 ± 10 | 0.29 |
| Troponin-1 (ng/L) | 1.2 ± 3.6 | 1.2 ± 3.7 | 0.97 |
| Total CK (U/L) | 91 ± 126 | 99 ± 146 | 0.72 |
| Creatinine (mg/dL) | 1.1 ± 1.60 | 1.3 ± 2 | 0.48 |
| D-Dimer (mcg/mL) | 1.8 ± 1.5 | 2.2 ± 3.1 | 0.54 |
| Arterial blood gas analysis | |||
| Ph | 7.40 ± 0.1 | 7.43 ± 0.1 | 0.04 |
| PaO2 (mmHg) | 67 ± 18 | 69 ± 25 | 0.58 |
| PaCO2 (mmHg) | 40 ± 13 | 38 ± 13 | 0.19 |
| HCO3 (mmHg) | 25 ± 6 | 23 ± 5 | 0.10 |
Abbreviations: COPD: Chronic obstructive pulmonary disease; FMD: Flow-mediated dilation. Test t Student and chi square test.
Patients in the COVID-19 and Non-COVID-19 groups presented with similar values regarding age, sex, height and race. However, weight and body mass index (BMI) were significantly higher in the COVID-19 group (p = 0.01). The COVID-19 group had higher admissions to the ICU (p = <0.01). The most common comorbidities found in hospitalized patients were hypertension, chronic obstructive pulmonary disease (COPD) and diabetes, in which the COVID-19 group had more hypertension (p = <0.01) and diabetes (p = <0.01) compared to the Non-COVID-19 group.
The COVID-19 group remained in hospital longer, required more oxygen supplementation and had a higher mortality rate compared to the Non-COVID-19 group (p ≤ 0.01). Patients with COVID-19 were evaluated with 7 days of symptoms, while patients in the non-COVID19 group with 5 days (p = <0.01). In the COVID-19 group, in which there are 98 patients, there were 11 deaths representing an 11.2% mortality rate.
In relation to the interfaces used for oxygen supplementation, the COVID-19 group used more nasal catheters and venturi masks, indicating a more severe clinical status (p < 0.01). In addition, both groups used medications in their hospitalization, with concomitant medications, but when compared, the COVID-19 group made greater use of platelet aggregation inhibitors, antibiotics and antivirals (p = <0.01) than Non-COVID-19 group.
With respect to blood analysis, the number of leukocytes (p = 0.01), platelets (p = 0.03), neutrophils (p = 0.04) and eosinophils (p = <0.01) were lower in the COVID-19 group when compared to the Non-COVID-19 group.
As for vital signs, HR (Non-COVID-10 group: 95 ± 23bpm and COVID-19 group: 88 ± 17bpm, p = 0.01), SBP (Non-COVID-10 group: 139 ± 28 mmHg and COVID-19 group: 129 ± 29 mmHg, p = 0.01) and DBP (Non-COVID-10 group: 85 ± 17 mmHg and COVID-19 group: 80 ± 12 mmHg, p = 0.02) were higher in patients in the Non-COVID-19 group in relation to patients with COVID-19. While the SpO2 (Non-COVID-10 group: 92 ± 7% and COVID-19 group: 91 ± 4%, p = 0.63), respiratory rate (Non-COVID-10 group: 23 ± 6bpm and COVID-19 group: 23 ± 9bpm, p = 0.87) and dyspnea (MRC scale) (Non-COVID-10 group: 1.97 ± 1.49 and COVID-19 group: 1.86 ± 1.46, p = 0.62) had no difference.
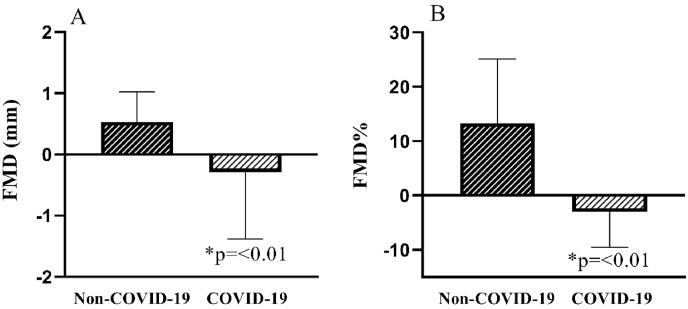
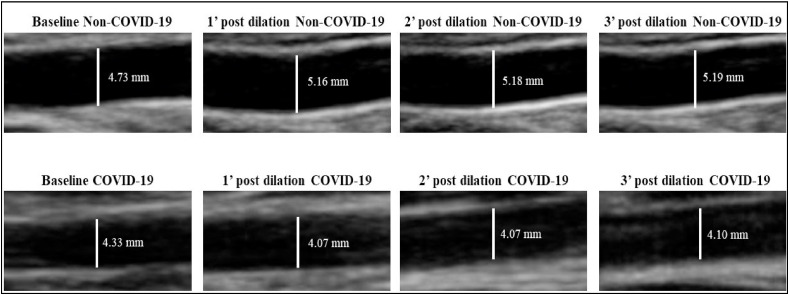
In relation to FMD measurements, patients in the COVID-19 group had a reduction in both FMD% and FMDmm (p < 0.01) ( Fig. 2 ). Fig. 3 illustrates the baseline brachial artery diameter at rest of a representative subject of each group and after 3-min of ischemic stimulation in Non-COVID-19 group compared with COVID-19 group.
Fig. 2.
Comparison of FMD% and FMDmm between COVID-19 and Non-COVID-19 groups.
Fig. 3.
Illustration of the brachial artery diameter of Non-COVID-19 group and COVID-19 group on Baseline and post 3 min of ischemic stimulation. Legend: ' = minute.
The ROC analysis showed that an absolute FMD≤0.26 mm and relative FMD≤3.43% were the ideal cutoff point to predict mortality and longer hospital stay for patients with COVID-19, showing a sensitivity of 75% and 85% and specificity of 73% and 84%, respectively (Table 2 and Fig. 4 ). We emphasize that we performed the prediction cutoff points for mortality and longer hospital stay also for Non-COVID-19 patients, however, due to the low number of deaths [4], there was no difference.
Table 2.
Cutoff values, AUC, sensitivity and specificity of FMD% and FMD (mm) in COVID-19.
| Variables | COVID-19 (N = 98) |
|||||
|---|---|---|---|---|---|---|
| Cutoff | Sensitivity | Specificity | AUC [CI 95%] | Positive likelihood | Negative likelihood | |
| FMD (%) | ≤3.43 | 85 | 84 | 0.922 [0.872 to 0.957] | 5.63 | 0.17 |
| FMD (mm) | ≤0.26 | 75 | 73 | 0.806 [0.741 to 0,861] | 2.81 | 0.34 |
Abbreviations: mm: millimeter; FMD: Flow-mediated dilation.
Fig. 4.
ROC curves of patients with COVID-19.
When dividing patients in the COVID-19 group according to the FMD threshold values for the mortality-based ROC curve analyses, there was no difference in age, sex, weight, height and BMI when comparing patients with FMD≤3.43% and FMD>3.43% or FMD≤0.26 mm and FMD>0.26 mm.
The groups with the worst FMD outcomes (≤3.43% and ≤0.26 mm) remained in the hospital longer (p = 0.04 and p = 0.01), required more oxygen supplementation (p = 0.03 and p = 0.02) and had a higher mortality rate (p = <0.01 in both) compared with the groups with more favorable FMD measures (>3.43% and>0.26).
As for the blood count, there was an increase in the number of troponin-1 and total CK in patients with COVID-19 who had an FMD≤0.26 mm when compared to patients with an FMD>0.26 mm (p = <0.01 in both). Likewise, CK showed higher values in those with an FMD ≤3.43% compared to those with an FMD>3.43% (p = 0.01) ( Table 3 ).
Table 3.
Clinical data, hospitalization days, number of deaths, blood count and FMD in patients with COVID-19, divided at the FMD cutoff points.
| Variables | FMD ≤3.43% (n = 84) | FMD >3.43% (n = 14) |
P value | FMD ≤0.26 mm (n = 74) |
FMD >0.26 mm (n = 24) |
P value |
|---|---|---|---|---|---|---|
| Age (years) | 61 ± 16 | 61 ± 14 | 0.89 | 62 ± 15 | 58 ± 17 | 0.32 |
| Sex, n (%) | 0.38 | 0.43 | ||||
| Male | 49 (58) | 6 (43) | 45 (61) | 13 (54) | ||
| Woman | 35 (42) | 8 (57) | 29 (39) | 11 (46) | ||
| Weight, (kg) | 79 ± 18 | 87 ± 17 | 0.19 | 79 ± 16 | 85 ± 22 | 0.20 |
| Height (m) | 1.67 ± 0.09 | 1.66 ± 0.05 | 0.72 | 1.67 ± 0.09 | 1.67 ± 0.08 | 0.95 |
| Body Mass Index (kg/m2) | 28 ± 5.6 | 31 ± 6.5 | 0.08 | 28 ± 5.4 | 30 ± 6.7 | 0.17 |
| Hospitalization days | 10 ± 7.8 | 5.4 ± 2.0 | 0.04 | 10 ± 8.4 | 5.3 ± 2.3 | 0.01 |
| Death, n (%) | 9 (82) | 2 [18] | <0.01 | 11 (100) | 0 (0) | <0.01 |
| Oxygen supplementation,n (%) | 54 (65) | 6 (43) | 0.03 | 48 (65) | 9 [35] | 0.02 |
| Liters | 2.5 ± 2.0 | 4.3 ± 3.7 | 0.33 | 2.9 ± 2.3 | 2.1 ± 1.9 | 0.25 |
| Interfacer | ||||||
| Nasal catheter | 48 (58) | 4 [29] | 0.15 | 41 (56) | 8 [32] | 0.25 |
| Venturi mask | 6 [7] | 2 [14] | 0.07 | 7 [9] | 1 [3] | 0.11 |
| Blood countr | ||||||
| Troponin-1 (ng/L) | 1.8 ± 4.4 | 0.7 ± 1.99 | 0.18 | 3.23 ± 15.14 | 1.87 ± 4.77 | <0.01 |
| Total CK (U/L) | 145 ± 339 | 123 ± 124 | <0.01 | 164 ± 358 | 70 ± 94 | <0.01 |
| Arterial blood gas analysis | ||||||
| Ph | 7.43 ± 0.05 | 7.42 ± 0.06 | 0.69 | 7.43 ± 0.06 | 7.42 ± 0.05 | 0.62 |
| PaO2 (mmHg) | 69 ± 23 | 77 ± 35 | 0.51 | 67 ± 22 | 77 ± 33 | 0.27 |
| PaCO2 (mmHg) | 38 ± 14 | 39 ± 7 | 0.78 | 38 ± 13 | 37 ± 9 | 0.78 |
| HCO3 (mmHg) | 23 ± 6 | 25 ± 3 | 0.14 | 23 ± 5 | 23 ± 7 | 0.89 |
| Flow-mediated dilationr | ||||||
| FMD (mm) | −0.38 ± 1.0 | 0.27 ± 0.48 | <0.01 | −0.74 ± 1.1 | 0.76 ± 0.43 | <0.01 |
| FMD (%) | −5.25 ± 4.8 | 7.9 ± 4.3 | <0.01 | −4.4 ± 5.2 | 1.27 ± 8.0 | <0.01 |
Abbreviations: chronic obstructive pulmonary disease (COPD); CK: Creatine kinase. Test t Student and chi square test.
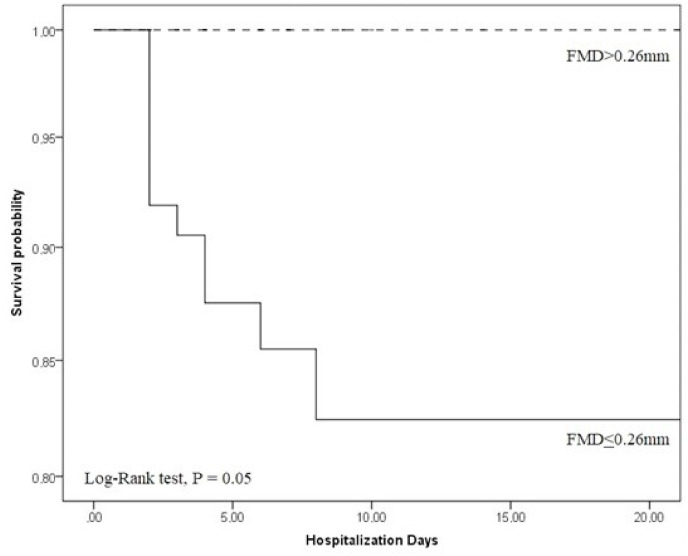
In Kaplan Meyer's analysis when we analyzed FMD≤0.26 mm (n = 74), patients had a high probability of death within a period of up to 10 days of hospitalization, with a significant Log-rank test (p = 0.05) ( Fig. 5 ). In relation to FMD%, there was no significant difference in the Kaplan Meyer's analysis (p = 0.25). This fact was possibly due to the fact that we had a low number of deaths in our study and the difference in the distribution of patients with COVID-19 in the cutoff points stipulated by the ROC curve (84 patients with FMD≤3.43% and 14 with FMD>3.43%).
Fig. 5.
Kaplan-Meyer's analysis for FMDmm in COVID-19 patients.
Modeling FMD≤0.26 mm as a principal variable in the Cox regression model, we found a significant difference for the risk of mortality (95% CI, 0.41 to 0.60; p=<0.01) and body mass index (BMI) (95% CI, 1.12 to 7.04; p = 0.02). For sex, age, hypertension and diabetes our model was not significant (Table 4 ). Therefore, the Cox regression model showed that there was no influence of comorbidities on endothelial dysfunction, which was a consequence of the COVID-19 infection.
Table 4.
Cox regression for risk factors according to cutoff point FMD≤0.26 mm.
| Covariates | Coefficient | Standard Error | Hazard Ratio | P value |
|---|---|---|---|---|
| Deaths (0: No, 1: Yes) | −1.84 | 0.68 | 0.15 | <0.01 |
| Age (0: <60 years, 1: >60 years) | 0.52 | 0.46 | 1.68 | 0.26 |
| Sex (0: M, 1: F) | −0.70 | 0.43 | 0.49 | 0.10 |
| BMI (0: <25 kg/m2, 1: >25 kg/m2) | 1.03 | 0.46 | 2.81 | 0.02 |
| Hypertension (0: No, 1: Yes) | 0.36 | 0.41 | 1.44 | 0.37 |
| Diabetes (0: No, 1: Yes) | −0.54 | 0.50 | 0.57 | 0.27 |
Abbreviations: Flow-mediated dilation (FMD); Body Mass Index (BMI); Male (M) and Female (F).
4. Discussion
To our knowledge, this is the first study that evaluated early FMD in hospitalized patients with suspected (Non-COVID-19) and confirmed COVID-19. We found that there is early endothelial vascular dysfunction in patients hospitalized for confirmed COVID-19 when comparing them to hospitalized patients with respiratory symptoms, but without COVID-19 (Non-COVID-19). In addition, patients with COVID-19 spent more days in hospital and had a higher mortality rate. These findings confirmed our hypothesis. Another new important result of our study was the clinically relevant cutoff points for FMD% and FMDmm in patients with COVID-19. Specifically, we demonstrated FMDmm was significant predictor of mortality in patients with COVID-19, indicating patients with endothelial dysfunction are more likely to die in 10 days of hospitalization. As such, FMD measurement may be valuable in patients hospitalized with COVID-19 to determine those at particularly high risk for death.
The mortality rate in patients with COVID-19 was 11.2% after a median of 10 days of hospitalization. Young Hu et al., demonstrated the risks of high COVID-19 severity and mortality ranged from 12.6 to 23.5% [20]. Grasseli et al. a 26% mortality rate in critically ill older patients with COVID-19 admitted to ICUs in Italy [21] and Xie et al. reported a 25.7% mortality rate in patients with COVID-19 hospitalized in China after median 14-day follow-up [22]. From these studies, we can say that the mortality rate in our cohort was lower, and may thus impact the prognostic ability of FMD in other cohorts hospitalized with COVID-19 with differing mortality rates.
To this point, a study by Ratchford et al. is the only other analysis using FMD assessment in patients with COVID-19. However, this study only included 20 young adults without chronic diseases and relatively healthy, assessed up to 4 weeks following infection (i.e., after the acute process of infection) [23]. Even so, the results indicated that COVID-19 may have detrimental effect on the systemic vasculature in young adults. Thus, we emphasize the novelty of our work, as we know that the highest prevalence of poor outcomes in COVID-19 is in elderly patients and in those with chronic diseases. Therefore, identifying non-invasive methods that can assist in the treatment of these patients is of great importance [23].
In relation to blood counts, patients with poorer FMD outcomes had higher values for troponin-1 and total CK. As in our study, Lombardi et al. elevated troponin levels were an independent predictor of hospital mortality and increased risk of cardiovascular and non-cardiovascular complications in a multicenter study including 614 patients hospitalized for COVID-19 [24]. As for total CK, Ponti et al. and Guzik et al. also found hospitalized patients with COVID-19 had elevated levels of this marker. CK parameters correlate with severe prognosis and poor outcomes in patients with COVID-19 and can be used as a predictive biomarker, helping to stratify patients positive for COVID-19 into risk categories, which would be extremely important in the clinical setting and in therapeutic management [25,26].
Coronavirus is known to invade the human cell directly after the glycoprotein binds to the ectodomain of ACE2, with ACE2 being the main input mechanism for COVID-19 [27,28]. The endocytosis of ACE2 linked to the virus particle reduces the number of ACE2 enzymes on the cell surface and therefore weakens tissue protection, thereby impacting cardiac and vascular changes and dysfunction [28]. Thus, when SARS-CoV-2 is bound to the ACE2 receptor, the expression of the receptor is unregulated, which in turn induces vascular endothelial dysfunction, which activates the prothrombotic cascade and ends up leading to vascular thrombosis and risks of heart disease, already observed in patients with COVID-19 [29], supporting our findings. Therefore, it is extremely important to identify these changes early, which can be done through the cutoff points outlined in our FMD study (≤3.43% and ≤0.26 mm). In this context, the previous pro-inflammatory state associated with SARS-CoV-2 infection can directly cause inactivation of the ACE2 receptor, which is associated with higher death rates and days of hospitalization (Table 1). A previous study by our group showed that endothelial dysfunction is present in patients with sepsis [30], which is related to higher death rates. In this line of evidence, noninvasive measurements of endothelial function may be important tool to detect systemic endothelial dysfunction in the early stages of infection by SARS-CoV-2.
In addition, the clinical utility of the FMD measurement, with cutoff points determined even on the first day of hospitalization of patients with COVID-19, is potentially viable simple and rapid measure to determine severity and risks, as well as assess the effects of pharmacological interventions and non-pharmacological and direct therapies. Thus, noninvasive measures of endothelial dysfunction can help health professionals introduce interventions aimed at improving endothelial function through interventions such as noninvasive ventilation, respiratory care [31] and aerobic exercise training [32]. These interventions can provide a decrease in arterial resistance [33] and to increased nitric oxide synthesis and bioavailability [34], thus improving the local muscle response and favoring the activity of the endothelium in smooth muscle cells; in addition to positively impacting hemodynamic responses [32].
Finally, our results showed differences in some aspects in the groups (Non-COVID-19 vs COVID-19), such as the highest number of comorbidities, in BMI and oxygen supplementation in patients with COVID-19, being factors that can influence the endothelial function. Because of this, we performed the analysis of subgroups with the cutoff points stipulated by the ROC curve in patients with COVID-19 to determine the severity, risk of death and days of hospitalization of these patients, thus verifying whether the endothelial dysfunction in these patients occurred due to infection by the SARS-CoV-2 and not by other factors.
4.1. Limitation of the study
The study has some limitations that must be considered. First, it must be recognized that the hospital environment, in which evaluations were carried out, is not the best environment for a controlled research study, as factors such as noise, light and stress of patients during hospitalization were not controlled.
In second place, the lack of baseline FMD results before COVID-19 infection makes it difficult to compare with the results during COVID-19 infection, as these patients have comorbidities and advanced age. As it is the first study to assess endothelial function in this population, there is still no data to compare the results, but hypotheses based on reviews. It is also noteworthy that we obtained a low mortality rate among to the number of cases of patients evaluated. However, fortunately, number of deaths confirmed in the São Carlos hospitals was lower compared to other cities of the country (131 deaths for 9.639 confirmed cases of the COVID-19 until February of 2021).
Third, in relation to blood count, arterial blood gas analysis and vital signs, we can see that some variables did not have differences or did not demonstrate the values expected according to some epidemiological studies [21,35]. This may be due to the fact that our sample is smaller in relation to these studies and because they are not severely ill patients, but rather mild to moderate cases at the beginning of hospitalization. In addition, these variables did not influence our main outcomes (FMDmm and FMD%).
Our fourth limitation refers to the lack of tests that could confirm our results of endothelial dysfunction, such as platelet function and NO levels. Finally, the most severe patients, who arrived at the hospital already in need of invasive ventilatory support and sedation in 24–48 h, were excluded. As our study has the potential to use FMD as an early event predictor, sedation and the use of invasive ventilatory support may be a potential confounding factor in our findings. Thus, the results of the present study are limited to patients with mild to moderate degree of respiratory dysfunction in the first 48 h and who required hospitalization for clinical stability.
4.2. Clinical perspectives
This is the first study to emphasize the importance of evaluating FMD in patients with COVID-19, we demonstrated through our cutoff point that this population may have early endothelial dysfunction. Therefore, strategies to minimize this dysfunction are of paramount importance, prioritizing the performance of aerobic and resistance exercises both for prevention and for the immediate admission of the patient to the hospital, associated with oxygen supplementation strategies and medications, thus being able to reduce the days hospitalization and mortality in this population. In this context, it is highly recommendable early endothelial function assessment in order that the staff initiates, when necessary, antithrombotic measures from the initial moment of hospitalization, in order to prevent thromboembolic events.
5. Conclusion
We conclude that patients hospitalized for COVID-19 present endothelial vascular dysfunction early, remained hospitalized longer and had a higher number of deaths, when compared with patients without COVID-19. In addition, it was the first study to establish cutoff points for FMD (FMD≤3.43% and FMD≤0.26 mm) for patients infected with COVID-19. In addition, we identified that FMD≤0.26 mm is a strong predictor of mortality risk in a 10-day hospitalization period due to COVID-19.
Our data suggest that these patients are more likely to have endothelial dysfunction and consequently risks of cardiovascular diseases, but that, from the cutoff points established, the FMD assessed early can help in the recognition of these patients and in clinical treatment.
Sources of funding
This study is supported by a research grant from Fundação de Amparo à Pesquisa do Estado de São Paulo, São Paulo, Brazil (FAPESP) Process N◦ 2015/26501–1 and N◦ 2018/03233-0, by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior- Brasil (CAPES - 001) (88887.507811/2020-00) and by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq): 141803/2019 -3. Audrey Borghi-Silva is an established Investigator (level IB) of the CNPq, Brazil.
Declaration of competing interest
No potential conflict of interest was reported by the authors.
References
- 1.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan , China: a retrospective cohort study. Lancet [Internet] 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet [Internet] 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017;39(5):529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res [Internet] 2020;191(April):145–147. doi: 10.1016/j.thromres.2020.04.013. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clerkin K.J., Fried J.A., Raikhelkar J., Sayer G., Griffin J.M., Masoumi A., et al. COVID-19 and cardiovascular disease. Circulation. 2020;2019:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 7.Wang M., Hao H., Leeper N.J., Zhu L. Thrombotic regulation from the endothelial cell perspectives. Arterioscler. Thromb. Vasc. Biol. 2018;38(6):e90–e95. doi: 10.1161/ATVBAHA.118.310367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oxley T.J., Mocco J., Majidi S., Kellner C.P., Shoirah H., Singh I.P., et al. Large-vessel stroke as a presenting feature of covid-19 in the young. N. Engl. J. Med. 2020 May 14;382(20):e60. doi: 10.1056/NEJMc2009787. http://www.nejm.org/doi/10.1056/NEJMc2009787 [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahmud E., Dauerman H.L., FGP Welt, Messenger J.C., Rao S.V., Grines C., et al. Management of acute myocardial infarction during the COVID ‐19 pandemic. Cathet. Cardiovasc. Interv. 2020 Aug 13;96(2):336–345. doi: 10.1002/ccd.28946. https://onlinelibrary.wiley.com/doi/10.1002/ccd.28946 [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 10.Evans P.C., Rainger G.E., Mason J.C., Guzik T.J., Osto E., Stamataki Z., et al. Endothelial dysfunction in COVID-19: a position paper of the ESC working group for atherosclerosis and vascular biology, and the ESC Council of basic cardiovascular science. Cardiovasc. Res. 2020;116(14):2177–2184. doi: 10.1093/cvr/cvaa230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Celermajer D., Sorensen K., Gooch V., Spiegelhalter D., Miller O., Sullivan I., et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet (London, England) 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 12.Broxterman R.M., Witman M.A., Trinity J.D., Groot H.J., Rossman M.J., Park S.-Y., et al. Strong relationship between vascular function in the coronary and brachial arteries. Hypertension. 2019 Jul;74(1):208–215. doi: 10.1161/HYPERTENSIONAHA.119.12881. https://www.ahajournals.org/doi/10.1161/HYPERTENSIONAHA.119.12881 [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeboah J., Folsom A.R., Burke G.L., Johnson C., Polak J.F., Post W., et al. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009;120(6):502–509. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malta M., Cardoso L.O., Bastos F.I., Magnanini M.M.F., da Silva C.M.F.P. Iniciativa STROBE: subsídios para a comunicação de estudos observacionais. Rev. Saude Publica. 2010;44(3):559–565. doi: 10.1590/s0034-89102010000300021. [DOI] [PubMed] [Google Scholar]
- 15.Shephard D.A. The 1975 declaration of Helsinki and consent. Can. Med. Assoc. J. 1976;115(12):1191–1192. [PMC free article] [PubMed] [Google Scholar]
- 16.Bwire G.M., Majigo M.V., Njiro B.J., Mawazo A. Detection profile of SARS-CoV-2 using RT-PCR in different types of clinical specimens: a systematic review and meta-analysis. J. Med. Virol. 2021;93(2):719–725. doi: 10.1002/jmv.26349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization Rational use of personal protective equipment for COVID-19 and considerations during severe shortages: interim guidance. 23 December 2020. https://www.who.int/publications/i/item/rational-use-of-personal-protective-equipment-for-coronavirus-disease-(covid-19)-and-considerations-during-severe-shortages Available from: 2020, December, 1, 32.
- 18.Thijssen D.H.J., Bruno R.M., Van Mil A.C.C.M., Holder S.M., Faita F., Greyling A., et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur. Heart J. 2019;40(30):2534–2547. doi: 10.1093/eurheartj/ehz350. [DOI] [PubMed] [Google Scholar]
- 19.Corretti M.C., Anderson T.J., Benjamin E.J., Ms C., Celermajer D., Charbonneau F., et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery A report of the international brachial artery reactivity task force. J Am Coll Cardiol [Internet] 2002;39(2):257–265. doi: 10.1016/S0735-1097(01)01746-6. Available from: [DOI] [PubMed] [Google Scholar]
- 20.Hu Y., Sun J., Dai Z., Deng H., Li X., Huang Q., et al. Prevalence and severity of corona virus disease 2019 (COVID-19): a systematic review and meta-analysis. J Clin Virol [Internet. 2020;127(April):104371. doi: 10.1016/j.jcv.2020.104371. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the lombardy region. Italy. JAMA - J Am Med Assoc. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie J., Covassin N., Fan Z., Singh P., Gao W., Li G., et al. Association between hypoxemia and mortality in patients with COVID-19. Mayo Clin. Proc. 2020 Jun;95(6):1138–1147. doi: 10.1016/j.mayocp.2020.04.006. https://linkinghub.elsevier.com/retrieve/pii/S0025619620303670 [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ratchford S.M., Stickford J.L., Province V.M., Stute N., Augenreich M.A., Koontz L.K., et al. Vascular alterations among young adults with SARS-CoV-2. Am. J. Physiol. Cell Physiol. 2020 doi: 10.1152/ajpheart.00897.2020. November 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lombardi C.M., Carubelli V., Iorio A., Inciardi R.M., Bellasi A., Canale C., et al. Association of troponin levels with mortality in Italian patients hospitalized with coronavirus disease 2019: results of a multicenter study. JAMA Cardiol. 2020;5(11):1274–1280. doi: 10.1001/jamacardio.2020.3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ponti G., Maccaferri M., Ruini C., Tomasi A., Ozben T. Biomarkers associated with COVID-19 disease progression. Crit Rev Clin Lab Sci [Internet] 2020;57(6):1–11. doi: 10.1080/10408363.2020.1770685. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guzik T.J., Mohiddin S.A., Dimarco A., Patel V., Savvatis K., Marelli-Berg F.M., et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc. Res. 2020;116(10):1666–1687. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vrints C.J.M., Krychtiuk K.A., Van Craenenbroeck E.M., Segers V.F., Price S., Heidbuchel H. Endothelialitis plays a central role in the pathophysiology of severe COVID-19 and its cardiovascular complications. Acta Cardiol. 2020:1–16. doi: 10.1080/00015385.2020.1846921. [Internet] Available from: 0(0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar A., Narayan R.K., Kumari C., Faiq M.A., Kulandhasamy M., Kant K., et al. SARS-CoV-2 cell entry receptor ACE2 mediated endothelial dysfunction leads to vascular thrombosis in COVID-19 patients. Med. Hypotheses. 2020;145(September):110320. doi: 10.1016/j.mehy.2020.110320. [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonjorno J.C., Caruso F.R., Mendes R.G., Da Silva T.R., de Campos Biazon T.M.P., Rangel F., et al. Noninvasive measurements of hemodynamic, autonomic and endothelial function as predictors of mortality in sepsis: a prospective cohort study. PloS One. 2019;14(3):1–16. doi: 10.1371/journal.pone.0213239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gautam A.P., Arena R., Dixit S., Borghi-Silva A. Pulmonary rehabilitation in COVID-19 pandemic era: the need for a revised approach. Respirology. 2020;25(12):1320–1322. doi: 10.1111/resp.13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.da Luz Goulart C., Caruso F.R., Garcia de Araújo A.S., Tinoco Arêas G.P., Garcia de Moura S.C., Catai A.M., et al. Non-invasive ventilation improves exercise tolerance and peripheral vascular function after high-intensity exercise in COPD-HF patients. Respir. Med. 2020;173(July) doi: 10.1016/j.rmed.2020.106173. [DOI] [PubMed] [Google Scholar]
- 33.Ashor A.W., Lara J., Siervo M., Celis-Morales C., Mathers J.C. Effects of exercise modalities on arterial stiffness and wave reflection: a systematic review and meta-analysis of randomized controlled trials. PloS One. 2014;9(10) doi: 10.1371/journal.pone.0110034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiu S., Cai X., Yin H., Sun Z., Zügel M., Steinacker J.M., et al. Exercise training and endothelial function in patients with type 2 diabetes: a meta-analysis. Cardiovasc. Diabetol. 2018;17(1):1–12. doi: 10.1186/s12933-018-0711-2. [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terpos E., Ntanasis-Stathopoulos I., Elalamy I., Kastritis E., Sergentanis T.N., Politou M., et al. Hematological findings and complications of COVID-19. Am. J. Hematol. 2020;95(7):834–847. doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]