Abstract
Background
COVID-19 has resulted in the largest pandemic experienced since 1918, accounting for over 2 million deaths globally. Frail and older people are at the highest risk of mortality. The main objective of the present research was to quantify the impact of clinical frailty scale (CFS) by increasing severity of frailty and to identify other personal prognostic factors associated with increased mortality from COVID-19.
Methods
This study offers a contemporary systematic review and meta-analysis to analyse the stratified mortality risk by increasing CFS sub-categories (1–3, 4–5 and 6–9). Databases searched included EMBASE, MEDLINE, CAB Abstracts, PsychInfo, and Web of Science with end-search restriction the 18th December 2020. Publications identified via MedRevix were followed up on the 23rd March 2021 in peer-reviewed database search, and citations were updated as published. Prospective and retrospective cohort studies which reported the association between CFS and COVID-19 mortality were included. Thirty-four studies were eligible for systematic review and seventeen for meta-analysis, with 81–87% (I2) heterogeneity.
Findings
All studies [N: 34] included patients from a hospital setting, comprising a total of 18,042 patients with mean age 72.8 (Min: 56; Max: 86). The CFS 4–5 patient group had significantly increased mortality when compared to patients with CFS 1–3 [(RE) OR 1.95 (1.32 (95% CI), 2.87 (95% CI)); I2 81%; p = 0.0008]. Furthermore, CFS 6–9 patient group displayed an even more noticeable mortality increase when compared to patients with CFS 1–3 [(RE) OR 3.09 (2.03, 4.71); I2 87%; p<0.0001]. Generic inverse variance analysis of adjusted hazard ratio among included studies highlighted that CFS (p = 0.0001), male gender (p = 0.0009), National Early Warning Score (p = 0.0001), Ischaemic Heart Disease (IHD) (p = 0.07), Hypertension (HT) (p<0.0001), and Chronic Kidney Disease (CKD) (p = 0.0009) were associated with increased COVID-19 mortality.
Interpretation
Our findings suggest a differential stratification of CFS scores in the context of COVID-19 infection, in which CFS 1–3 patients may be considered at lower risk, CFS 4–5 at moderate risk, and CFS 6–9 at high risk of mortality regardless of age. Overall, our study not only aims to alert clinicians of the value of CFS scores, but also highlight the multiple dimensions to consider such as age, gender and co-morbidities, even among moderately frail patients in relation to COVID-19 mortality.
Funding
None.
Research in Context.
Evidence before this study
COVID-19 has resulted in the largest pandemic experienced since 1918, with detrimental effects upon most vulnerable and frail populations. The association between frailty and consequently CFS with COVID-19 mortality has been shown in multiple prospective and retrospective studies, a finding which has promoted the incorporation of CFS in the management of COVID-19 patients as suggested by the NICE guidelines.
Added value of this study
This study offers a contemporary systematic review and meta-analysis to analysis the stratified mortality risk by increasing CFS sub-categories (1–3, 4–5 and 6–9).
Implication of all the available evidence
Differential stratification of the CFS scores, in the context of COVID-19 infection, is proposed in which CFS 1–3 patients may be considered at lower risk, CFS 4–5 at moderate and CFS 6–9 at high risk of mortality. Overall, our study not only aims to alert clinicians of the value of CFS scores, but also the multiple dimensions to consider such as age, gender and co-morbidities, even among moderately frail patients in relation to COVID-19 mortality.
Alt-text: Unlabelled box
1. Introduction
COVID-19, a novel coronavirus identified in late 2019, has rapidly spread across the globe resulting in the largest pandemic experienced since 1918 [1]. Among the infected patients, up to 20% develop severe disease which requires hospitalisation [2,3]. The association between frailty and consequently Clinical Frailty Scale (CFS) with COVID-19 mortality has been shown in multiple prospective and retrospective studies, a finding which has promoted the incorporation of CFS in the management of COVID-19 patients as suggested by the National Institute for Heath and Care Excellence (NICE) guidelines, despite caution as advised by certain studies against CFS overestimation in COVID-19 prognosis [4], [5], [6], [7]. Frailty prevalence is varied and affects approximately 40% of middle-aged to older patients with age commonly, but not always, correlating with higher scores in the CFS [8]. To date, a limited number of systematic reviews and even fewer meta-analyses have confirmed the association between CFS and patient mortality with inherent limitations originating from the incorporated studies [7,9]. Nonetheless, until present and to the best of our knowledge, no study has attempted to correlate increasing level of CFS scores, in the context of modifiable and non-modifiable patient factors, with mortality outcomes.
Furthermore, multiple confounding factors have been shown to worsen COVID-19 prognosis. Common complications include acute kidney injury (AKI), cardiac injury and sudden cardiac death, whilst hyperactive delirium complicates the management of two-thirds of critically ill patients [10,11]. How these variables interplay with the CFS patient categorisation for poorer or improved COVID-19 outcomes remains elusive and present a ground for clarification in the present study.
Overall, our study aims to quantify the impact of CFS by increasing severity of frailty and to identify other personal prognostic factors associated with increased mortality from COVID-19 infection.
2. Methods
2.1. Search strategy and selection criteria
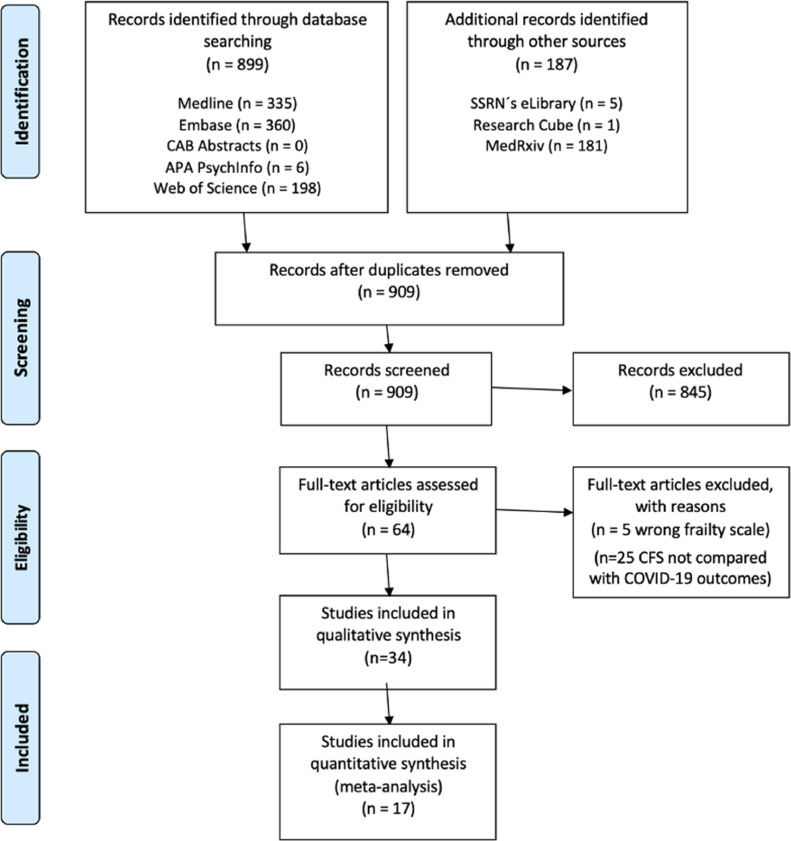
A systematic literature review was conducted according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Fig. 1) [12]. A study protocol was not registered for the present study.
Fig. 1.
Prisma 2009 Flow Diagram. Search strategy included and excluded studies.
Independent literature search for relevant studies was performed up to 18th December 2020 on five databases: EMBASE, MEDLINE, CAB Abstracts, PsychInfo, and Web of Science. Additional records were identified through other sources, including SSRN´s eLibrary, Research Square and MedRxiv. The MedRxiv search was simplified according to database search functionality. Publications identified via MedRevix were followed up on the 23rd March 2021 in peer-reviewed database search, and citations were updated as published. The references of the included studies were scrutinized for additional relevant studies. Search limitations included human participants and English language articles. The following search term was used in OVID: (clinical frailty scale OR clinical frailty score OR frail*).mh,tw,ab,hw,kw. AND (2019 Novel Coronavirus Disease OR 2019 Novel Coronavirus Infection OR 2019-nCoV Disease OR 2019-nCoV Infection OR COVID-19 Pandemic OR COVID-19 Pandemics OR COVID-19 Virus Disease OR COVID-19 Virus Infection OR COVID19 OR Coronavirus Disease 2019 OR Coronavirus Disease-19 OR SARS Coronavirus 2 Infection OR SARS-CoV-2 Infection).mp. limit to (English language and humans). The same search strategy was adapted for the remaining databases.
Prospective and retrospective cohort studies were included examining COVID-19 mortality in the context of CFS scale (Table S1). Restrictions included English language and human. Restrictions were not applied to participant age, gender or ethnicity. Full-text exclusion criteria were: Different frailty measurement tool other than the CFS [Hospital Frailty Risk Score (HFRS); Fried Frailty Scale (FRIED)]; including only deceased patients; not reporting specific mortality numbers; and CFS and caregiver association rather than mortality. Excluded studies and justifications are recorded in Table S3.
After removing duplicates, citations were screened by title and abstract, then full texts were appraised to determine their eligibility by three authors (GK, SK, SP) (Fig. 1). Three authors (GK, SK, SP) independently conducted the abstract and full text screening. Disagreements were resolved by a consensus meeting. Peer-reviewed full-text papers that reported mortality outcome were selected.
Data from each article was extracted by two authors (GK, SP) and validated independently by a third researcher (SK): (1) Total number of participants, type of study, setting of study (hospital), length of hospital stay, discharge, Clinical Frailty Scale score, patient characteristics: age, gender, method of COVID-19 diagnosis, co-morbidities (respiratory, cardiovascular (CVS), chronic kidney disease (CKD), cancer, diabetes mellitus, delirium development %, National Early Warning Score (NEWS). More specifically, the term “cardiovascular co-morbidities” included hypertension, heart failure (HF) dyslipidaemia, previous cerebrovascular accident, ischaemic heart disease (IHD), myocardial infarction. The term “respiratory co-morbidities’ included chronic obstructive pulmonary disease (COPD), asthma and interstitial lung fibrosis. Type 1 and type 2 diabetes mellitus were analysed together and mentioned with the umbrella term “Diabetes”. CKD and AKI represented two different entities and analysed separately. The CKD term did not include AKI patients as this parameter could not confidently be identified as an independent factor to the active COVID-19 infection. Patients diagnosed with solid tumours or haematological malignancies were grouped together under the umbrella term “Cancer”.
Quality of the included studies were assessed by three independent reviewers (GK, SK, SP) using the Newcastle-Ottawa Scale (NOS) for observational studies [13]. Bias analysis was conducted via the Cochrane recommended tool (RevMan V. 5.4). Studies were considered to be high quality if they had a NOS score ≥6. Adequate follow-up was considered to be ≥ 30 days (Table S2).
2.2. Data analysis
Clinicalstudy, context and design, were compared and those in which, populations were considered suitably homogeneous, were pooled [14]. The meta-analysis was conducted by computing the odds ratio (OR), random effects (RE) from the original data using the DerSimonian-Laird method with Review Manager (RevMan) v5.4 software using a random-effect model. Statistical heterogeneity was quantified using I2 statistics and Cochrane Q tests.
The primary outcome of this study was to identify COVID-19 associated mortality among CFS groups. Confounding factors of increased mortality were assessed using generic inverse variance model regression (IVR), adjusted with covariates consistent with the primary outcome. Variables assessed included age (continuous variable), gender (categorical variable), smoking status (categorical), BMI (continuous), NEWS (categorical), active cancer diagnosis (categorical), respiratory conditions (categorical), diabetes (categorical), hypertension (categorical), IHD (categorical), CKD (categorical), AKI (categorical), and delirium (categorical). Both crude hazard ratio (HR) and adjusted HR were presented with associated 95% Confidence Intervals (CI). HR (95% CI) per Model, where Model 1 (Adjusted for Age, Gender), Model 2 (adjusted for Age, Gender, Co-morbidities), and Model 3 (Adjusted for Age; Gender; Co-morbidities; Biochemical findings) are depicted in the associated tables to explore heterogeneity. Collective adjusted HR were used for forest plots, unstratified for adjusted parameters.
Asymmetry was assessed by funnel plot, and asymmetry was assessed formally by rank correlation test (Begg's test; RevMan V. 5.4) [15]. Sensitivity analyses were conducted to assess the impact of individual potential confounding variables. Publication bias was assessed visually by funnel plot, and asymmetry was assessed formally by rank correlation test (Begg's test) [15].
2.3. Role of the funding source
There was no funding source for this study. All authors confirm that they had full access to the dataset of the study and accept responsibility to submit for publication.
3. Results
Following the PRISMA guidelines on systematic review search, we identified 64 studies eligible for full-text screening. Full-text screening excluded 30 studies (Table S3, companion file). A total of 34 studies remained [4,5,[16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47]], all of which were included in the systematic review while 17 were included in the meta-analysis [4,5,[33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47]] (Fig. 1; Table S1). Given that all available studies examining the CFS under the prism of COVID-19 mortality were observational, we employed the NOS for quality assessment [13]. Twenty-two studies were graded as good, two as fair and ten studies as poor according to independent grading as per NOS selection, comparability and outcome parameters (Table S2).
All studies [N:34] included patients from a hospital setting [4,5,[16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47]], comprising a total of 18,042 patients with mean age 72.8 (Min: 56; Max: 86). Over half (57.2%) [10,320/18,042] [4,5,[16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47]] of the COVID-19-positive patients were male (Fig. S1C), 30.6% [4691/15,430] [4,5,[16], [17], [18], [19], [20], [21], [22], [23], [24],27,[29], [30], [31], [32], [33],[35], [36],38,40,42,[45], [46], [47]] of the patients suffered from cardiovascular co-morbidities, 12.19% [1556/12,761] [4,[16], [17], [18], [19],21,[23], [24],27,[29], [30], [31],33,[35], [36],38,40,42, [45], [46], [47]] had a pre-existing respiratory co-morbidity, 22.6% [3487/15,430] [4,5,[16], [17], [18], [19], [20],24,27,[29], [30], [31], [32], [33],[35], [36],38,40,42,[45], [46], [47]] were diabetic, cancer patients comprised a total of 7.8% [895/11,469] [4,16,18,23,24,27,[29], [30], [31],33,35,38,46,47] of the analysed patient population, whilst 12.4% [1329/10,716] [5,16,[18], [19],[23], [24],27, [30], [31],33,35,38,40,46] of patients were suffering from a CKD (Fig. S1C). The overall mortality% across studies was 31.1% (SD: 14.1) [5611/18,042] [4,5,[16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47]] (Fig. S1C). A mean of 32.7% (SD: 35.5) [1546/4727] [4,[16], [17], [18],20,21,23,30,31,33,35,38,46] required intubation and mean overall admission to Intensive Care Unit (ICU) was 11.0% (SD: 6.3) [586/5330] [16,17,22,25,29,33,38,39,42,44,46] (Fig. S1C). Length of hospital stay mean was 12.2 days (SD: 13.3) [18,19,21,[24], [25], [26],30,34,38,39,41,43,44] (Fig. S1C). A 60.3% (SD: 16.8) [8807/14,606] [4,5,17,[19], [20], [21], [22], [23],25,27,28,32,33,35,36,[38], [39], [40],42,46,47] of patients were discharged within the timeline of the included studies (Fig. S1C). Interestingly, almost a quarter of patients, 24.1% (SD: 14.5) [1742/7226] [22,24,25,27,28,36,38,41,47] either presented or developed delirium during their hospital admission (Fig. S1C). Observed I2 was significantly decreased with sensitivity analysis as per NOS scale grading (Fig. S4).
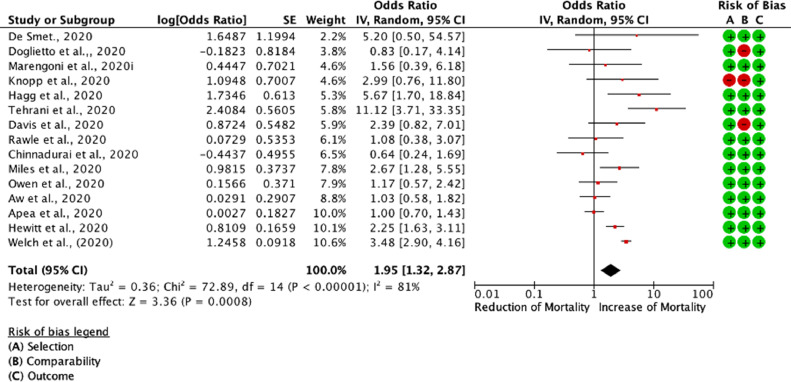
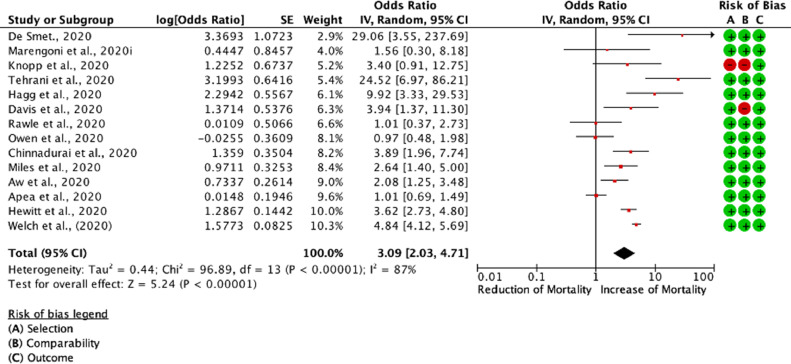
Of the thirty-four studies, seventeen [4,5,[33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47]] were included in the meta-analysis, whilst a total of seventeen studies were excluded because mortality was either not reported in CFS increments or crude patient numbers were unavailable (Table S4). Patient groups were stratified according to their CFS hierarchical scale upon hospital admission. CFS numerically ranks frailty taking into account patient co-morbidities and functional status spanning from 1 to 9. Patients scoring 1 are considered to be very fit, 2 - well, 3 - managing well, 4 - vulnerable, 5 - mildly frail, 6 - moderately frail, 7 - severely frail, 8 - very severely frail, and 9 - terminally ill [48]. For the purposes of this meta-analysis, we stratified patients with CFS 1–3,CFS 4–5, and CFS 6–9, and assessed their respective OR of mortality between CFS sub-groups (Fig. 2, Fig. 3, Fig. S3). Control group was set as the CFS 1–3. We found that the CFS 4–5 patient group had significantly increased mortality when compared to patients with CFS 1–3 [(RE) OR 1.95 (1.32,2.87); I2 81%; p = 0.0008]. Furthermore, mortality in the CFS 6–9 patient group was noticeably increased when compared to patients with CFS 1–3 [(RE) OR 3.09 (2.03, 4.71); I2 87%; p<0.0001]. Comparison between the CFS 4–5 vs. CFS 6–9 also returned significant results [(RE) OR 1.51 (1.23, 1.84)] (Fig. S2). These results remained significant even when included studies were restricted to only those with standard error (SE) [log (OR)] <0.6 and mapped to NOS scale (Fig. S4) [49].
Fig. 2.
Odds associated with increased frailty (CFS 4–5 vs. CFS 1–3). DerSimonian and Laird statistical method with odds ratio as output only for included observational studies. Forrest plot of included observational studies of the meta-analysis (patients with CFS 4–5 COVID-19 mortality vs. patients with CFS 1–3 COVID-19 mortality) representing respective reduction or increase in mortality in the frailest population, is shown in Fig. S3, random effects odds ratio 1.95 (1.32,2.87; I2 81%). Forrest and associated funnel plots (Fig. S3) were generated with Review Manager V. 5.4 Cochrane Tool for meta-analysis.
Fig. 3.
Odds associated with increased frailty (CFS 6–9 vs. CFS 1–3). DerSimonian and Laird statistical method with odds ratio as output only for included observational studies. Forrest plot of included observational studies of the meta-analysis (patients with CFS 6–9 COVID-19 mortality vs. patients with CFS 1–3 COVID-19 mortality), representing respective reduction or increase in mortality in the frailest population, random effects odds ratio 3.09 (2.03, 4.71); I2 87%. Forrest and associated funnel plots (Fig. S3) were generated with Review Manager V. 5.4 Cochrane Tool for meta-analysis.
Lastly, we sought to identify confounding factors that may correlate with COVID-19 mortality across all included studies (Fig. S5, Fig. S6). Overall, CFS, male gender, NEWS, Diabetes, CVS co-morbidities (including Hypertension and IHD) and CKD were associated with increased COVID-19 mortality among CFS groups in unadjusted HR (Fig. S5) and adjusted HR analysis as reported in each study (Fig. S6). CFS, male gender and increased NEWS were statistically significant, predictors of poor prognosis in the adjusted for age, gender, co-morbidities and biochemical findings HR, IVR analysis for COVID-19 mortality across studies (Model 3; Fig. S6). These findings highlight the multitude of dimensions at interplay dictating COVID-19 mortality along with the CFS scale.
4. Discussion
Globally, over 100 million confirmed cases of COVID-19 have been reported with more than 2 million deaths. As we strive to improve patient management, achieve population vaccination and maintain distancing measures, the elderly population continues to be subjected to the highest mortality among all infected patient groups, with up to 8.1 times higher risk [50]. Whilst previous meta-analyses [9] have been conducted with smaller numbers of included studies, given the rapidly evolving literature on the subject we have attempted to correlate not only the entirety of the CFS scale with mortality outcomes but also CFS-specific stratified patient groups, by incorporating a total of 34 studies, 17 of which were included in the meta-analysis.
As expected, the difference among the CFS 1–3 AND CFS 6–9 patient groups were very noticeable with favoured survival in the CFS 1–3 group [(RE) OR 3.09 (2.03, 4.71); p<0.0001]. Equally, significant differences were also observed between fit (CFS 1–3) and vulnerable patients (CFS 4–5), with [(RE) OR 1.95 (1.32, 2.87); p <0.0008]. This analysis indicated an almost stepwise increase in mortality as we moved up the scale of frailty. Given this finding, we can hypothesise that even subtle changes in the CFS scale can significantly worsen patient outcomes. Previous work has suggested a 12% incremental increase of mortality per 1-point in the CFS scale in a linear fashion, a finding which was not consistent with our findings. We identified variable increase of mortality between single CFS scores (Fig. S7), whilst linear increase was identified between CFS groups (1–3 vs. 4–5; vs. 6–9) rather than single CFS groups. Furthermore, we have identified modifiable and non-modifiable variables that associate with poorer outcomes for COVID-19 mortality across all CFS groups. Whilst age, male gender and cardiovascular co-morbidities have been previously associated with worse prognosis [51], [52], [53], this work has also identified CKD as another poor prognosticator.
It is now established that COVID-19 disproportionately affects patients with pre-existing comorbidities [54]. The National Kidney Foundation has already advised caution for patients of CKD stage 3 and above or those receiving dialysis. The molecular pathway through which COVID-19 infection worsens CKD pathophysiology remains elusive, nonetheless. Molecular work has indicated that whilst the mechanism may be multifactorial, the angiotensin‐converting enzyme 2 (ACE2) pathway may be under increased scrutiny [55]. Further factors that may worsen outcomes of CKD patients may be the extensive albuminuria and proteinuria as observed in COVID‐19 hospitalized patients, which on top of pre-existing CKD may induce an acute-on-chronic kidney injury [56]. Previous work has also suggested that patients with pre-existing cardiovascular disease and overall decreasing CVS integrity are more prone to COVID-19 complications and poorer survival outcomes [52]. Targeted cardiac assessment is currently recommended in patients with new-onset HF (including left HF and acute cor pulmonale), unexplained cardiac arrhythmias, or ECG changes (more specifically ST elevation) as well as pre-existing CVS co-morbidities [51]. The pathophysiology of acute on chronic cardiovascular injury upon chronic infection is also, as expected, multifactorial, mainly disseminating via direct viral invasion of the myocardium through ACE2 receptor and Transmembrane protease serine 2 (TMPRSS2) protease and Angiotensin II–mediated inflammatory response [57,58].
Other positively associated factors with COVID-19 mortality include delirium and active cancer - albeit a lack of statistical significance. Delirium has been shown to be noticeably prevalent in elderly and potentially more frail adults, although it is not exclusive to increased age, and high delirium rates have been observed amongst all critically ill patients [11]. Whilst delirium cannot be considered an independent confounder of COVID-19 induced mortality, its synergistic association is evident despite being elusive mechanistically. More research is required in order to comprehend why patients with hyperactive delirium are more prone to increased COVID-19 mortality. Furthermore, our data concur with previous literature associating male gender and NEWS with poorer COVID-19 outcomes in the elderly [50], [51], [52], [53], [54], [55], [56], [57], [58], [59]. Of note previous pioneering work in the acute setting, prior to the COVID-19 pandemic, had already indicated that higher NEWS and CFS correlate, predicting illness severity, mortality and readmission rate, in the frailer population [59].
While the molecular background rendering male patients more susceptible to severe COVID-19 complications and death remains obscure, studies have suggested that higher ACE-2 expression, sex-immunological differences (e.g. interleukin (IL) IL-4, IL-10, and the IL-12 expression levels), lifestyle factors, as well as overall attitudes towards the COVID-19 pandemic, play pivotal roles in the observed outcomes [60], [61], [62], [63], [64]. Lastly, we identified increased intubation % to be associated with mortality, a finding that should be taken into context; only patients most in need of mechanical ventilation due to COVID-19 infection receive this. Mortality among patients requiring invasive ventilation, regardless of age group, has been shown to be as high as 42.7% [65]. Considering that older age groups, which comparatively suffer from a higher number of pre-existing co-morbidities [54], it is not surprising that intubation requirement % was found to be positively associated with increased mortality among the included studies. Overall, our study, in alignment with previous research, not only highlights the importance of CFS scale in correlation with COVID-19 mortality prediction, but also emphasises the multiple demographic and physiological variables at interplay that may impact on COVID-19 mortality prediction. Consequently, as in the context of other medical conditions, especially in the geriatric population, a comprehensive geriatric assessment is not only desired but necessary to identify patient medical, psychosocial, and functional limitations that impact patient outcomes in the context of COVID-19 infection.
Our study suffers from the inherent limitations of the contemporarily published observational studies and the evident lack of randomised control trials (RCT). Whilst RCT studies are extremely difficult to be formulated amidst the COVID-19 pandemic, their outcomes would be extremely valuable in on-going patient care. Additionally, small patient numbers and variable CFS and co-morbidities reporting across studies increased overall reporting and selection bias amidst included studies. Whilst taking into account the inherent limitations of a meta-analysis without included randomised controlled trials, our systematic review and meta-analysis has been conducted according to the Cochrane standards to provide rigorous results under statistician guidance. We have identified and addressed sources of heterogeneity by NOS subgrouping, sensitivity and IVR analysis. By preserving the integrity of frailty assessment across studies, by selecting only those reporting on CFS system, we were able to make comparisons across studies [7]. Whilst previous work has highlighted the linearity of CFS and COVID-19 mortality in dose-response fashion [7], [8], [9], the present work is the first to provide scaled CFS score association with mortality risk in the context of patient modifiable and non-modifiable confounding factors. These include gender, cardiovascular, renal and endocrine co-morbidities as well as acute deterioration indicators such as the NEWS and delirium development.
In terms of clinical intuition, it is expected that CFS 7–9 corresponds with an increased risk of mortality. Whilst this holds true, this work underlines that even patients with lower CFS scores (e.g., 4–5) display significant mortality risks in comparison to the CFS 1-3 groups. This finding should uniformly change the way we view CFS amidst the COVID pandemic and encourage clinicians to use more comprehensive patient assessment and management - even when patients are considered to be vulnerable to frailty but are not yet frail. Our findings suggest a differential stratification of the CFS scores, in the context of COVID-19 infection, in which CFS 1–3 patients may be considered at lower risk, CFS 4–5 at moderate risk and CFS 6–9 at high risk of mortality. Patients’ CFS score should be assessed within the context of a comprehensive clinical assessment, taking into account demographic and physiological variables, to efficiently predict COVID-19 mortality and act proactively towards improving patient outcomes.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
Author Contributions
Search and data extraction: GK, SLK, SP
Data meta-analysis and statistical analysis: SLK, BC
Writing - Original draft preparation: SLK, GK
Conceptualisation: SLK, PKM
Expert opinion: PKM, JH
Final manuscript and approval: All
Funding
None.
Data sharing statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.100896.
Appendix. Supplementary materials
References
- 1.WHO Director-General's remarks at the media briefing on 2019-nCoV on 11 February 2020. Who.int. http://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020. Accessed January 25, 2021.
- 2.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Labenz C., Kremer W.M., Schattenberg J.M. Clinical frailty scale for risk stratification in patients with SARS-CoV-2 infection. J Investig Med. 2020;68(6):1199–1202. doi: 10.1136/jim-2020-001410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hewitt J., Carter B., Vilches-Moraga A. The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. Lancet Public Health. 2020;5(8):e444–e451. doi: 10.1016/S2468-2667(20)30146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.NICE guideline. COVID-19 rapid guideline: critical care in adults (NG 159). https://www.nice.org.uk/guidance/ng159. Accessed December 20, 2021. [PubMed]
- 7.Cosco T.D., Best J., Bryden D. What is the relationship between validated frailty scores and mortality for adults with COVID-19 in acute hospital care? a systematic review. Age Ageing. 2020:afab008. doi: 10.1093/ageing/afab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanlon P., Nicholl B.I., Jani B.D., Lee D., McQueenie R., Mair F.S. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: a prospective analysis of 493 737 UK Biobank participants. Lancet Public Health. 2018;3(7):e323–e332. doi: 10.1016/S2468-2667(18)30091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pranata R., Henrina J., Lim M.A. Clinical frailty scale and mortality in COVID-19: a systematic review and dose-response meta-analysis. Arch Gerontol Geriatr. 2021;93 doi: 10.1016/j.archger.2020.104324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arentz M., Yim E., Klaff L. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helms J., Kremer S., Merdji H. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;4(23):2268–2270. doi: 10.1056/NEJMc2008597. 382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D., Liberati A., Tetzlaff J., Altman D.G., Prisma Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peterson J., Welch V., Losos M., Tugwell P.J. Ottawa Hospital Research Institute; Ottawa: 2011. The Newcastle-Oottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [Google Scholar]
- 14.Training.cochrane.org; 2021. Chapter 24: including non-randomized studies on intervention effects.https://training.cochrane.org/handbook/current/chapter-24 Accessed January 28. [Google Scholar]
- 15.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 16.Baker K., Hanrath A., Schim van der Loeff I. COVID-19 management in a UK NHS foundation trust with a high consequence infectious diseases centre: a retrospective analysis. Med Sci. 2021;9(1):6. doi: 10.3390/medsci9010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brill S., Jarvis H., Ozcan E. COVID-19: a retrospective cohort study with focus on the over-80s and hospital-onset disease. BMC Med. 2020;18(1) doi: 10.1186/s12916-020-01665-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cobos-Siles M., Cubero-Morais P., Arroyo-Jiménez I. Cause-specific death in hospitalized individuals infected with SARS-CoV-2: more than just acute respiratory failure or thromboembolic events. Intern Emerg Med. 2020;15(8):1533–1544. doi: 10.1007/s11739-020-02485-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conway J., Gould A., Westley R. Clinical characteristics and progression of COVID-19 confirmed cases admitted to a single British clinical centre—A brief case series report. Int J Clin Pract. 2020;31:e13807. doi: 10.1111/ijcp.13807. [DOI] [PubMed] [Google Scholar]
- 20.Hoek R.A., Manintveld O.C., Betjes M.G. Covid-19 in solid organ transplant recipients: a single-center experience. Transpl Int. 2020;33(9):1099–1105. doi: 10.1111/tri.13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Izzi-Engbeaya C., Distaso W., Amin A. Adverse outcomes in COVID-19 and diabetes: a retrospective cohort study from three London teaching hospitals. BMJ Open Diabetes Res Care. 2021;9(1) doi: 10.1136/bmjdrc-2020-001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knights H., Mayor N., Millar K. Characteristics and outcomes of patients with COVID-19 at a district general hospital in Surrey, UK. Clin Med (Lond) 2020;20(5):e148–e153. doi: 10.7861/clinmed.2020-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kokoszka-Bargieł I., Cyprys P., Rutkowska K., Madowicz J., Knapik P. Intensive care unit admissions during the first 3 months of the COVID-19 pandemic in poland: a single-center, cross-sectional study. Med Sci Monit. 2020;26 doi: 10.12659/MSM.926974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maguire D., Woods M., Richards C. Prognostic factors in patients admitted to an urban teaching hospital with COVID-19 infection. J Transl Med. 2020;18(1):354. doi: 10.1186/s12967-020-02524-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marengoni A., Zucchelli A., Vetrano D.L. Beyond chronological age: frailty and multimorbidity predict in-hospital mortality in patients with coronavirus disease 2019. J Gerontol A Biol Sci Med Sci. 2020;76(3):e38–e45. doi: 10.1093/gerona/glaa291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mcloughlin B., Miles A., Webb T. Functional and cognitive outcomes after COVID-19 delirium. Eur Geriatr Med. 2020;11(5):857–862. doi: 10.1007/s41999-020-00353-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mendes A., Serratrice C., Herrmann F.R. Predictors of in-hospital mortality in older patients with COVID-19: the COVID age study. J Am Med Dir Assoc. 2020;21(11):1546–1554. doi: 10.1016/j.jamda.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osuafor C.N., Davidson C., Mackett A.J. Research Square; 2020. Clinical features and inpatient trajectories of older inpatients with COVID-19: a retrospective observational study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poco P.C., Aliberti M.J., Dias M.B. Divergent: age, frailty, and atypical presentations of COVID-19 in hospitalized patients. J Gerontol A Biol Sci Med Sci. 2020;76(3):e41–e46. doi: 10.1093/gerona/glaa280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson J.V., Meghani N.J., Powell B.M. Patient characteristics and predictors of mortality in 470 adults admitted to a district general hospital in England with Covid-19. Epidemiol Infect. 2020;148:e285. doi: 10.1017/S0950268820002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vallecillo G., Anguera M., Martin N., Robles M.J. Effectiveness of an Acute Care for Elders unit at a long-term care facility for frail older patients with COVID-19. Geriatr Nurs. 2020 doi: 10.1016/j.gerinurse.2020.10.004. S0197-4572(20)30304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vilches-Moraga A., Price A., Braude P. Increased care at discharge from COVID-19: the association between pre-admission frailty and increased care needs after hospital discharge; a multicentre European observational cohort study. BMC Med. 2020;18(1):1–19. doi: 10.1186/s12916-020-01856-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Apea V., Wan Y., Dhairyawan R. Ethnicity and outcomes in patients hospitalised with COVID-19 infection in East London: an observational cohort study. BMJ Open. 2021;11(1) doi: 10.1136/bmjopen-2020-042140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aw D., Woodrow L., Ogliari G., Harwood R. Association of frailty with mortality in older inpatients with Covid-19: a cohort study. Age Ageing. 2020;49(6):915–922. doi: 10.1093/ageing/afaa184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chinnadurai R., Ogedengbe O., Agarwal P. Older age and frailty are the chief predictors of mortality in COVID-19 patients admitted to an acute medical unit in a secondary care setting-a cohort study. BMC Geriatr. 2020;20(1):1–11. doi: 10.1186/s12877-020-01803-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis P., Gibson R., Wright E. Atypical presentations in the hospitalised older adult testing positive for SARS-CoV-2: a retrospective observational study in Glasgow, Scotland. Scott Med J. 2020;0(0):1–9. doi: 10.1177/0036933020962891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Smet R., Mellaerts B., Vandewinckele H. Frailty and mortality in hospitalized older adults with COVID-19: retrospective observational study. J Am Med Dir Assoc. 2020;21(7):928–932. doi: 10.1016/j.jamda.2020.06.008. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doglietto F., Vezzoli M., Gheza F. Factors associated with surgical mortality and complications among patients with and without coronavirus disease 2019 (COVID-19) in Italy. JAMA Surg. 2020;155(8):691–702. doi: 10.1001/jamasurg.2020.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bradley P., Frost F., Tharmaratnam K., Wootton D. Utility of established prognostic scores in COVID-19 hospital admissions: multicentre prospective evaluation of CURB-65, NEWS2 and qSOFA. BMJ Open Respir Res. 2020;7(1) doi: 10.1136/bmjresp-2020-000729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hägg S., Jylhävä J., Wang Y. Age, frailty, and comorbidity as prognostic factors for short-term outcomes in patients with coronavirus disease 2019 in geriatric care. J Am Med Dir Assoc. 2020;21(11):1555–1559. doi: 10.1016/j.jamda.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knopp P., Miles A., Webb T. Presenting features of COVID-19 in older people: relationships with frailty, inflammation and mortality. Eur Geriatr Med. 2020;11(6):1089–1094. doi: 10.1007/s41999-020-00373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marengoni A., Zucchelli A., Grande G., Fratiglioni L., Rizzuto D. The impact of delirium on outcomes for older adults hospitalised with COVID-19. Age Ageing. 2020;49(6):923–926. doi: 10.1093/ageing/afaa189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miles A., Webb T., Mcloughlin B. Outcomes from COVID-19 across the range of frailty: excess mortality in fitter older people. Eur Geriatr Med. 2020;11(5):851–855. doi: 10.1007/s41999-020-00354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Owen R.K., Conroy S.P., Taub N. Comparing associations between frailty and mortality in hospitalised older adults with or without COVID-19 infection: a retrospective observational study using electronic health records. Age Ageing. 2020;50(2):307–3716. doi: 10.1093/ageing/afaa167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rawle M.J., Bertfield D.L., Brill S.E. Atypical presentations of COVID-19 in care home residents presenting to secondary care: a UK single centre study. Aging Med. 2020;3(4):237–244. doi: 10.1002/agm2.12126. (Milton) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tehrani S., Killander A., Åstrand P., Jakobsson J., Gille-Johnson P. Risk factors for death in adult COVID-19 patients: frailty predicts fatal outcome in older patients. Int J Infect Dis. 2020;102:415–421. doi: 10.1016/j.ijid.2020.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Welch C., Alsahab M., Beishon L. Age and frailty are independently associated with increased COVID-19 mortality and increased care needs in survivors: results of an international multi-centre study. Age Ageing. 2021:1–14. doi: 10.1093/ageing/afab026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rockwood K., Song X., MacKnight C. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Donnelly K., Brocchi R., Hewitt J., Routledge P.A., Carter B. Benzodiazepines, Z-drugs and the risk of hip fracture: a systematic review and meta-analysis. PLoS ONE. 2017;12(4) doi: 10.1371/journal.pone.0174730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yanez N.D., Weiss N.S., Romand J.A., Treggiari M.M. COVID-19 mortality risk for older men and women. BMC Public Health. 2020;20(1):1–7. doi: 10.1186/s12889-020-09826-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Driggin E., Madhavan M.V., Bikdeli B. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75(18):2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ssentongo P., Ssentongo A.E., Heilbrunn E.S., Ba D.M., Chinchilli V.M. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: a systematic review and meta-analysis. PLoS ONE. 2020;15(8) doi: 10.1371/journal.pone.0238215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arentz M., Yim E., Klaff L. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu C., Chen X., Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Malha L., Mueller F.B., Pecker M.S., Mann S.J., August P., Feig P.U. COVID-19 and the renin-angiotensin system. Kidney Int Rep. 2020;5(5):563–565. doi: 10.1016/j.ekir.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diao B., Feng Z., Wang C. MedRxiv; 2020. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu P.P., Blet A., Smyth D., Li H. The science underlying COVID-19: implications for the cardiovascular system. Circulation. 2020;142(1):68–78. doi: 10.1161/CIRCULATIONAHA.120.047549. [DOI] [PubMed] [Google Scholar]
- 58.Gheblawi M., Wang K., Viveiros A. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020;126(10):1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Subbe C.P., Burford C., Le Jeune I., Masterton-Smith C., Ward D. Relationship between input and output in acute medicine–secondary analysis of the society for acute medicine's benchmarking audit 2013 (SAMBA ‘13) Clin Med. 2015;15(1):15. doi: 10.7861/clinmedicine.15-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li W., Sui J., Huang I.C. The S proteins of human coronavirus NL63 and severe acute respiratory syndrome coronavirus bind overlapping regions of ACE2. Virology. 2007;367(2):367–374. doi: 10.1016/j.virol.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao Y., Zhao Z., Wang Y., Zhou Y., Ma Y., Zuo W. BioRxiv; 2020. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 63.Shim E., Tariq A., Choi W., Lee Y., Chowell G. Transmission potential and severity of COVID-19 in South Korea. Int J Infect Dis. 2020;93:339–344. doi: 10.1016/j.ijid.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De L.a., Vega R., Barquín R.R., Boros S., Szabo A. Could Attitudes toward COVID-19 in Spain render men more vulnerable than women? Glob Public Health. 2020;15(9):1278–1291. doi: 10.1080/17441692.2020.1791212. [DOI] [PubMed] [Google Scholar]
- 65.King C.S., Sahjwani D., Brown A.W. Outcomes of mechanically ventilated patients with COVID-19 associated respiratory failure. PLoS ONE. 2020;15(11) doi: 10.1371/journal.pone.0242651. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.