Abstract
Background
A significant proportion of individuals experience lingering and debilitating symptoms following acute COVID-19 infection. The National Institute for Health and Care Excellence (NICE) have coined the persistent cluster of symptoms as post-COVID syndrome. This has been further sub-categorised into acute post-COVID syndrome for symptoms persisting three weeks beyond initial infection and chronic post-COVID syndrome for symptoms persisting beyond twelve weeks. The aim of this review was to detail the prevalence of clinical features and identify potential predictors for acute and chronic post-COVID syndrome.
Methods
A systematic literature search, with no language restrictions, was performed to identify studies detailing characteristics and outcomes related to survivorship of post-COVID syndrome. The last search was performed on 6 March 2021 and all pre-dating published articles included. A means of proportion meta-analysis was performed to quantify characteristics of acute and chronic post-COVID syndrome. Study quality was assessed with a specific risk of bias tool. PROSPERO Registration: CRD42020222855
Findings
A total of 43 studies met the eligibility criteria; of which, 38 allowed for meta-analysis. Fatigue and dyspnoea were the most prevalent symptoms in acute post-COVID (0·37 and 0·35) and fatigue and sleep disturbance in chronic post-COVID syndrome (0·48 and 0·44), respectively. The available evidence is generally of poor quality, with considerable risk of bias, and are of observational design.
Interpretation
In conclusion, this review highlights that flaws in data capture and interpretation, noted in the uncertainty within our meta-analysis, affect the applicability of current knowledge. Policy makers and researchers must focus on understanding the impact of this condition on individuals and society with appropriate funding initiatives and global collaborative research.
Keywords: Post covid syndrome, Long covid, SARS-CoV 2, COVID-19, Systematic review, Coronavirus
Panel: Research in context.
Evidence before this study
The emergence of post-COVID syndrome, in which recovering SARS-CoV-2 patients suffer from persistent symptoms extending several months beyond their initial diagnosis is gaining increasing recognition. However, there is a need for a greater understanding for diagnosis and management strategies. We searched Ovid in Medline, EMBASE, health management information consortium (HMIC), and PsycINFO databases without language restriction. The search was conducted in March 2021 using a list of terms relating to COVID-19 and persistent symptoms. Studies were included if they focussed on describing ‘long-COVID’ or ‘post-COVID syndrome’, the incidence of reported symptoms or predictors. Studies detailing a follow-up period shorter than 21 days; case series and articles focussing on other non-COVID-19 related conditions were excluded.
Added value of this study
To the best of our knowledge, this is the first systematic review and meta-analysis describing symptom prevalence and predictors for acute and chronic subtypes of post-COVID syndrome. However, during the process, several limitations of current literature surfaced. The significant heterogeneity in the field limits clinical applicability and high-quality evidence are urgently needed.
Implications of all available evidence
This review highlights that flaws in data capture and interpretation, noted in the uncertainty within our meta-analysis, affect the applicability of current knowledge. Moreover, the majority of studies displayed significant risk of bias, were typically of observational design, based within a limited number of countries and of inconsistent methodologies. There is an urgent need for global collaboration and recruitment into COVID-19 trials to tackle this.
Alt-text: Unlabelled box
1. Introduction
As of 4th February 2021, SARS-CoV-2 has infected over 70 million individuals globally and has directly attributed to over 1.6 million deaths [1]. While hospitals continue to grapple with the challenges of acute COVID-19, there is evidence to suggest the emergence of an associated secondary syndrome, labelled as either post-COVID or long-COVID syndrome, in which recovering SARS-CoV-2 patients suffer from persistent and, often, debilitating symptoms extending several months past their initial diagnosis [2], [3], [4].
In contrast to the scientific community's rapidly developing understanding of acute SARS-CoV-2 infection, characterisation of post-COVID syndrome remains sparse. It is suggested that upwards of 20% of SARS-CoV-2 positive individuals go on to develop post-COVID syndrome [5]. Its inception stems from a collective created through patients sharing a more complex course of recovery from their acute illness on social media platforms [6]. This was given further traction with healthcare professionals recovering and sharing similar experiences; it has enveloped to incorporate broader patient perspectives of recovery, extending beyond a negative test result for COVID-19, encompassing a cohort of individuals who did not require hospitalisation but suffer morbidity [7,8]. As such, there is an urgent medical, financial and societal need to understand the survivorship burden associated with this phenomenon [9], [10], [11].
Of note, there is a particular lack of understanding as to whether post-COVID syndrome constitutes a singular disease process. It has been suggested that the post-COVID syndrome may be characterised into either an acute or chronic subtype, depending on whether symptoms extend beyond 12 weeks following initial diagnosis [2,12]. However, it is not currently understood as to whether chronic post-COVID is either an extension of acute post-COVID or is a separate disease subtype that carries a distinct risk profile. Clearly delineating the clinical features between post-COVID subtypes could prove to be a crucial step in (i) empowering clinicians to accurately diagnose post-COVID in the patients that they manage in both primary and secondary care settings, (ii) counselling patients on how to manage their particular syndrome subtypes as well as (iii) ensuring appropriate resource allocation in order to cater for the specific health and social care needs associated with each subtype cohort. Moreover, these goals could be further supplemented by the prospective identification of patients who are at highest risk of developing post-COVID syndrome of any description, who may benefit from enhanced surveillance programmes upon discharge from hospital.
As such, the primary aim for this study aims to characterise the clinical features between acute and chronic post-COVID syndrome. The secondary aim is to identify predictors for post-COVID syndrome, irrespective of subtype, in order to understand the risk factors and the acute clinical course that is associated with syndrome development.
2. Methods
2.1. Design
This systematic review was conducted in accordance to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [13]. The review was registered at the International Prospective Register of Systematic Reviews (PROSPERO ID: CRD42020222855). .
2.2. Research questions
This review sought to answer the following questions:
-
(1)
What are the clinical features associated with acute and chronic post-COVID syndrome?
-
(2)
Which features predict the development of post-COVID syndrome?
2.3. Search Strategy and databases
A systematic search, with expert librarian support, was performed using electronic databases through Ovid in Medline, EMBASE, health management information consortium (HMIC), and PsycINFO databases without language restriction. The search was conducted using a list of terms relating to COVID-19 and persistent symptoms; the complete search strategy is available in Appendix 1. Further studies not captured by the search were identified through bibliometric cross-referencing. Grey literature was additionally searched.
All identified studies were uploaded to Covidence (Melbourne, Australia), a Cochrane supported systematic review package tool [14]. Initial screening was conducted by two investigators (FI and KL) to determine if the eligibility criteria were met. Discrepancies resolved by discussion. Studies meeting the inclusion criteria underwent full-text screening; supplemental references were scrutinised for additional relevant articles.
2.4. Study selection criteria and outcome measures
The inclusion criteria for study selection were focussed on studies describing ‘long-COVID’ or ‘post-COVID syndrome’, the incidence of reported symptoms and predictors. The last search was performed in March 2021. No language restrictions were placed.
Given the rapidly expanding literature surrounding COVID-19, a wide range of publications were included, (e.g., feature articles). Studies detailing a follow-up period shorter than 21 days; case series and articles focussing on other non-COVID-19 related conditions were excluded.
2.5. Data extraction
Outcome measures were the prevalence of symptoms indicative of acute and chronic post-COVID syndrome.
All included study characteristics and outcome measures were independently extracted by two investigators (FI and KL) with consensus achieved. All full text reports of studies identified as potentially eligible after title and abstract review were obtained for further review.
2.6. Quality assessment (risk of bias)
Risk of bias was assessed using a validated quality assessment checklist for prevalence studies [15]. This consists of ten domains for assessing internal (e.g., methods for data collection, clear case definition, reliability, duration of follow-up) and external validity (e.g., representation of sample population, selection of population, response rate); and an additional cumulative risk of bias for the assessed study. Quality assessment was assessed by one reviewer (FI) and validated by a second (KL).
2.7. Data analysis
We characterised studies describing symptom clusters with a follow-up period of 12 weeks or more into chronic post-COVID syndrome and studies detailing a follow-up period shorter than 12 weeks as acute post-COVID, in keeping with the definitions by the National Institute for Health and Care Excellence (NICE) [12].
A meta-analysis of proportions was performed in RStudio version 3.6.3 (R Studio, Boston, MA, USA using the metaphor package and metaprop command (Appendix 2) [16]. Forest plots were generated for all included studies. Heterogeneity was assessed with the I2 statistic. We considered a value less than 30% as low heterogeneity, between 30-60% moderate, and over 60% as high.
Chest pain and chest tightness were grouped into one variable, given their close clinical relationship [17]. Halpin et al [18]. represented an intensive care and non-intensive care cohort. Carvalho-Schneider et al [19]. reported repeated outcomes at days 30 and 60. Therefore, separate cohorts within these papers have been displayed.
2.8. Funding
No funding was received for this study; all authors had access to the data and decided to submit for publication.
3. Results
3.1. Study characteristics
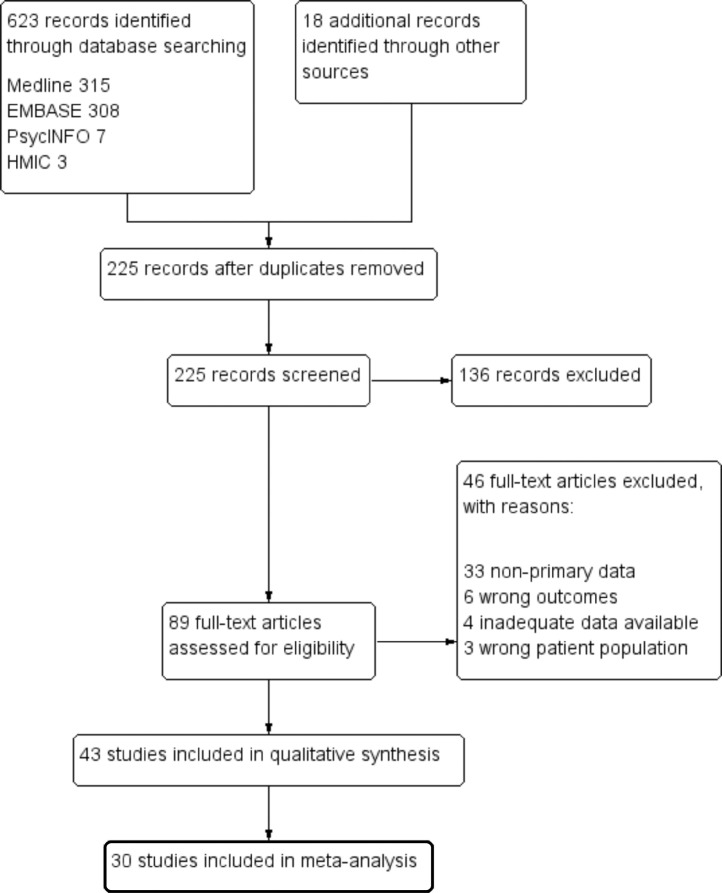
A total of 623 citations were retrieved through literature searches. An additional 18 articles were found from bibliography cross-referencing. Full text review was performed for 89 articles with 43 meeting the inclusion criteria for analysis, of which, 30 allowed for meta-analysis. Studies were conducted in 18 countries most of which are deemed as high-income. Included studies were observational in design with a mixture of previously hospitalised and non-hospitalised individuals recruited into the trials; the characteristics are shown in Tables 1 and 2. A PRISMA flow diagram can be seen in Figure 1.
Table 1.
Characteristics of included studies.
| Study | Country | Study type | COVID-19 status | Sampling | Risk of Bias |
|---|---|---|---|---|---|
| Arnold et al. [45] | UK | Cohort | RT-PCR confirmed cases or clinic-radiological diagnosis. | Previously hospitalised |  |
| Bongiovanni et al. [21] | Italy | Cohort | RT-PCR confirmed cases | Previously hospitalised |  |
| Carfi et al. [46] | Italy | Cohort | RT-PCR confirmed cases | Previously hospitalised |  |
| Carvalho-Schneider et al. [26] | France | Cohort | RT-PCR confirmed cases | Previously hospitalised |  |
| Daynes et al. [40] | UK | Cohort | RT-PCR confirmed cases or suspected ventilated cases | Previously hospitalised |  |
| D'Cruz et al. [31] | UK | Cohort | RT-PCR confirmed cases | Previously hospitalised |  |
| Garrigues et al. [39] | France | Cohort | RT-PCR confirmed cases or CT findings. | Previously hospitalised |  |
| Halpin et al. [18] | UK | Cross sectional | RT-PCR confirmed cases | Previously hospitalised |  |
| Jacobs et al. [24] | USA | Cohort | RT-PCR confirmed cases | Previously hospitalised |  |
| Liu et al. [25] | China | Cross sectional | RT-PCR confirmed cases | Previously hospitalised |  |
| Liu, Zhang et al. [47] | China | Cohort | RT-PCR confirmed cases | Previously hospitalised | - |
| Mandal et al. [23] | UK | Cross sectional | RT-PCR confirmed cases | Previously hospitalised |  |
| Pellaud et al. [48] | Switzerland | Cohort | RT-PCR confirmed cases | Previously hospitalised |  |
| Rahmani et al. [22] | Iran | Cohort | RT-PCR confirmed cases or CT findings | Previously hospitalised |  |
| Rosales-Castillo et al. [49] | Spain | Cohort | RT-PCR confirmed cases | Previously hospitalised |  |
| Sonnweber et al. [50] | Austria | Cohort | RT-PCR confirmed cases | Previously hospitalised |  |
| Taboada et al. [51] | Spain | Cohort | RT-PCR confirmed cases in intensive care | Previously hospitalised |  |
| Tomasoni et al. [52] | Italy | Cohort | RT-PCR confirmed cases or CT findings | Previously hospitalised |  |
| Venturelli et al | Italy | Cohort | RT-PCR or serologically confirmed and suspected cases | Previously hospitalised |  |
| Wang et al. [53] | China | Cohort | RT-PCR confirmed cases | Previously hospitalised |  |
| Kingstone et al. [43] | UK | Qualitative | RT-PCR confirmed cases and persistent symptoms in suspected | NS | - |
| Sollini et al. [20] | Italy | Cohort | Persisting symptoms for >30 days in recovered cases | NS |  |
| Blair et al. [54] | USA | Cohort | RT-PCR confirmed cases | Non-hospitalised |  |
| Boscolo-Rizzo et al. [55] | Italy | Cross sectional | RT-PCR confirmed cases | Non-hospitalised |  |
| Brandao Neto et al. [56] | Brazil | Cohort | RT-PCR confirmed cases | Non-hospitalised |  |
| Chiesa-Estomba et al. [57] | Belgium, France, Spain | Cohort | RT-PCR confirmed cases | Non-hospitalised |  |
| Fjaeldstad et al. [58] | Denmark | Cross sectional | RT-PCR confirmed cases or suspected cases | Non-hospitalised |  |
| Lovato et al. [59] | Italy | Cross sectional | RT-PCR confirmed cases | Non-hospitalised |  |
| Petersen et al. [41] | Faroe Islands | Cross sectional | RT-PCR confirmed cases | Non-hospitalised |  |
| Stavem et al. [30] | Norway | Cross sectional | RT-PCR confirmed cases | Non-hospitalised |  |
| Vaes et al. [60] | The Netherlands & Belgium | Cross sectional | RT-PCR confirmed and suspected cases | Non-hospitalised |  |
| Villarreal et al. [61] | Spain | Cohort | RT-PCR confirmed cases | Non-hospitalised |  |
| Darley et al. [62] | Australia | Cohort | RT-PCR confirmed cases | Mixed |  |
| Goertz et al. [28] | The Netherlands & Belgium | Cross sectional | RT-PCR confirmed cases or suspected cases | Mixed |  |
| Hopkins et al. [63] | UK | Cross sectional | RT-PCR or serologically confirmed cases and suspected cases | Mixed |  |
| Islam et al. [64] | Bangladesh | Cross sectional | NS | Mixed |  |
| Jacobson et al. [29] | USA | Cross sectional | RT-PCR confirmed cases | Mixed |  |
| Lampl et al. [65] | Germany | Cohort | RT-PCR confirmed cases | Mixed |  |
| Mazza et al. [66] | Italy | Cohort | RT-PCR confirmed cases | Mixed |  |
| Poncet-Megemont et al. [67] | France | Cohort | RT-PCR confirmed cases or CT findings | Mixed |  |
| Puntmann et al. [33] | Germany | Cohort | RT-PCR confirmed cases | Mixed |  |
| Townsend et al. [68] | UK | Cross sectional | RT-PCR confirmed cases | Mixed |  |
| Townsend et al. [69] | UK | Cross sectional | RT-PCR confirmed cases | Mixed |  |
| Vaira et al. [70] | Italy | Cohort | RT-PCR confirmed cases | Mixed |  |
| van den Borst et al. [42] | The Netherlands | Cohort | RT-PCR confirmed cases and community suspected cases | Mixed |  |
 low;
low;  medium;
medium;  high
high
Table 2.
Summary of study data.
| Study | N | Mean Age, SD (y) | Female (%) | BAME (%) | Mean BMI, SD (kg/m2) | Common comorbidities | Follow-up timepoint | Data collected |
|---|---|---|---|---|---|---|---|---|
| Arnold et al.[45] | 110 | 60 (46-73)* | 44 | 20·9 | 32·1 | Chronic lung disease, hypertension, DM, CHD | 84 days from admission | Symptom reporting at follow-up clinic, SF-36, WEMWBS |
| Blair et al.[54] | 118 | 56 (50-63)* | 52·5 | 57·6 | 30 (26-30)* | Hypertension, asthma, DM, COPD | 28-60 days | Self-reported symptom questionnaires |
| Bongiovanni et al.[21] | 125 | 65·7 | NS | NS | NS | NS | 19·9 days from discharge | IES-R; PCL-5; ZSDS; BDI-13; STAI; MOS; WHIIRS; OCI scales |
| Boscolo-Rizzo et al.[55] | 187 | 56 (20-89)* | 55·1 | NS | NS | NS | 28 days from diagnosis | Self-reported symptom questionnaires: ARTIQ, SNOT-22 |
| Brandao Neto et al.[56] | 143 | 37·7 | 64·7 | NS | NS | Hypertension, DM, asthma | 76 (66-88)* days | Self-reported symptom questionnaires |
| Carfi et al.[46] | 143 | 56·5 (14·6) | 37·1 | NS | 26·3 (4·4) | Hypertension, Thyroid disorder, Immune disorders, COPD | 60·3 (13·6) days¥ since symptom onset 36·1 (12·9)¥ days since discharge |
Demographics, Covid characteristics, symptom, EuroQoL collected at outpatient visits. |
| Carvalho-Schneider et al.[26] | 130 | 49 (15) | 55·8 | NS | NS | obesity, COPD, CKD, CHD, DM, immune disorder | 30 & 60 days | EHR/phone call collected demographic & symptom data |
| Chiesa-Estomba et al.[57] | 121 | 41 | 63·5 | NS | NS | NS | 47 (30-71)* days from diagnosis | sQOD-NS; self reporting symptoms |
| Darley et al.[62] | 78 | 47 (16) | 34·6 | NS | NS | Hypertension, asthma | 69 (64-83)* days from diagnosis | Self-reported symptom questionnaires |
| Daynes et al.[40] | 131 | 60 (14) | 41·2 | NS | NS | Asthma, COPD | 32 (18) days¥ | Phone call for demographics, CAT, HADS anxiety & depression, FACIT, symptom questionnaires |
| D'Cruz et al.[31] | 119 | 58·7 (14·4)¥ | 38 | 70 | 30·0 (25·9–35·2)* | CHD, COPD, CKD | 61 (51–67) days from discharge | Self-reported symptom questionnaires |
| Fjaeldstad et al.[58] | 109 | 39·4 | 79 | NS | NS | NS | 30 days from symptom onset | Self-reported symptom questionnaires |
| Garrigues et al.[39] | 120 | 63·2 (15·7) | 37·5 | NS | 29·2% normal/underweight 47·5% ≥ overweight |
DM, hypertension | 110·9 (11·1) days¥ following admission | Phone call collected mMRC and EuroQoL questionnaires |
| Goertz et al.[28] | 2113 | 47 (39-54)* | 85 | NS | 25 (23-29)* | NS | 79 (17) days¥ since symptom onset | Demographics, online symptom questionnaires from two long-COVID Facebook groups |
| Halpin et al.[18] | 100 | 70·5 (20-93)*† 58·5 (34-84)*‡ |
48·5 † 40·6‡ |
10·3† 34·4‡ |
36·8% overweight† 17·6% obese† 33·3% overweight‡ 40% Obese‡ |
asthma, COPD, CKD, DM | 48 (10·3) days¥ | Phone call collected symptom questionnaires, EuroQoL, demographics. |
| Hopkins et al.[63] | 434 | NS | 74·9 | NS | NS | NS | 6 months | Self-reported online questionnaires |
| Islam et al.[64] | 1002 | 34·7 (13·9( | 42·1 | NS | 47·3% obese | DM, hypertension, CHD, malignancy, asthma | NS | Self-reported questionnaires |
| Jacobs et al.[24] | 183 | 57 (48-68)* | 38·5 | 45·9 | 30 (27·3-33·5)* | DM, hypertension, CHD, asthma, hyperlipidaemia | 35 (± 5) days from hospital discharge | Email or telephone collected symptom questionnaires |
| Jacobson et al.[29] | 118 | 43·4 (14·4) | 46·6 | 63·6 | 30·4 (6·3) | NS | 119·3 (33)¥ days from diagnosis | Symptom reporting at follow-up clinic |
| Kingstone et al.[43] | 24 | 43·2 | 79·1 | 0 | NS | Asthma, IBD | 3-4 months - not explicitly stated. | Semi-structured interviews |
| Lampl et al.[65] | 419 | 44 (30-57)* | 56·6 | NS | 16·7% obese | NS | 42 days after symptom onset | Phone call collected symptom questionnaires |
| Liu et al.[25] | 675 | 55 (41-66)* | 53 | NS | NS | NS | 37 days from discharge | GAD-7; PHQ-9; PCL-5; self-reported symptom questionnaires |
| Liu, Zhang et al.[47] | 149 | 43 (36-56)* | 55 | NS | NS | Hypertension | 21 days from discharge | CT-imaging |
| Lovato et al.[59] | 121 | 46·7 | 59·5 | NS | NS | NS | 38 (3)¥ days from diagnosis | Phone call collected symptom questionnaires |
| Mandal et al.[23] | 384 | 59·9 | 38 | 43 | NS | Hypertension, DM, Asthma, COPD, CKD, CHD | 54 (57-59) days* | Demographics, biochemistry, imaging; in person or telephone collected follow-up data (symptom, PHQ-2 questionnaire) |
| Mazza et al.[66] | 402 | 57·8 (13·3) | 34·3 | NS | NS | NS | 31 (16)¥ days from discharge | IES-R; PCL-5; ZSDS; BDI-13; STAI; MOS; WHIIRS; OCI scales |
| Pellaud et al.[48] | 196 | 70 (60-80)* | 39 | 20·9% obese | NS | DM, OSA, COPD, CHD, hypertension, cancer | 30 days from symptom onset | Telephone call/EHR collected data. |
| Petersen et al.[41] | 180 | 39·9 (19·4) | 54·5 | NS | NS | Asthma, DM, Hypertension, COPD | 125 (18) days¥ | Telephones & interview collected demographics, baseline & follow-up symptoms, mMRC scale |
| Poncet-Megemont et al.[67] | 139 | 48·5 (15·3) | 62·6 | NS | NS | NS | 79 (17)¥ days from symptom onset | Self-reported symptom questionnaires/semi-structured interviews. |
| Puntmann et al.[33] | 100 | 49 (14) | 47 | NS | 25 (23-28)* | Hypertension, DM, COPD, asthma, CHD | 71 (64-92) days from diagnosis* | Demographics, Cardiac MRI data, hs-CRP, hs-TnT, NT-proBNP |
| Rahmani et al.[22] | 176 | 60 (14) | 46·9 | NS | 26 ± 4* moderate disease 27 ± 4* severe disease |
Hypertension, CHD, DM | 56 days from discharge | Phone call collected symptom questionnaires. |
| Rosales-Castillo et al.[49] | 118 | 60·2 (15·1) | 44·1 | NS | 29·7 (15·1) | Hypercholesterolaemia, DM, COPD, CHD, hypertension | 50·8 (6.02)¥ days from discharge | Specialist discussion at follow-up |
| Stavem et al.[30] | 458 | 4.6 | 56 | NS | 26·9 (5·2) | DM, asthma, arthrosis, COPD, CHD | 117·5 (41-200)* days from diagnosis | Self-reported symptom questionnaires. |
| Sollini et al.[20] | 10 | 58 | 30 | NS | NS | NS | NS | PET/CT results, demographics. |
| Sonnweber et al.[50] | 135 | 57 (14) | 43 | NS | 26 (5) | CHD, hypertension, COPD, asthma, DM | 100 days from diagnosis | Self-reported symptom questionnaires, mMRC scores, clinical review at follow up visits. |
| Taboada et al.[51] | 91 | 65·5 (10·4) | 35·2 | NS | NS | Hypertension, hypercholesterolaemia, DM, asthma | 6 months | Interview collected data |
| Tomasoni et al.[52] | 105 | 55 (43-65)* | 27 | NS | NS | NS | 46 (43-48) days* from discharge | Self-reported symptom questionnaire. |
| Townsend et al.[68] | 128 | 49·5 | 53·9 | NS | 28·7 | NS | 72 (62-87) days* | Outpatient appointment, demographics, biochemistry, covid characteristics, symptom questionnaires (CFQ-11) |
| Townsend et al.[69] | 153 | 50·4 (12.8) | 42·5 | 24·8 | NS | NS | 75 (62-117)* days from diagnosis | Self-reported symptom questionnaires |
| Vaes et al.[60] | 1837 | 47 (38-54)* | 86·1 | NS | 25·1 | NS | 79 (17)¥ days from symptom onset | Self-reported symptom diaries and questionnaires. |
| Vaira et al.[70] | 138 | 50·7 | 51·2 (8·8) | 29% obese | NS | Cardiovascular, pulmonary disorder, DM | 60 days from symptom onset | self-reported symptoms; CCCRC test |
| van den Borst et al.[42] | 124 | 59 (14) | 40 | NS | NS | asthma, COPD, CHD, hypertension | 10·0 (1·7) weeks¥ since discharge | Demographics, imaging, laboratory results, mMRC scale, CFS, SF-36, TICCS, PTSS, IES-R, CFQ, HADS questionnaires |
| Venturelli et al | 767 | 63 (13·6) | 32·9 | 22·4% obese | NS | Hypertension, CHD< DM, COPD | 81 (66-106)* days from discharge | Self-reported symptom questionnaires |
| Villarreal et al.[61] | 230 | 43 (18-62)* | 85 | NS | NS | NS | 28 days from symptom onset | VAS symptom scales |
| Wang et al.[53] | 131 | 49 (36-62)* | 55 | NS | NS | hypertension | 28 days from discharge | Self-reported symptom questionnaires |
BAME: Black Asian Minority Ethnic; BMI: body mass index
median (range); ¥ mean (SD)
for ward patients
for intensive care patients; NS: not specified; COPD: chronic obstructive pulmonary disease; DM: diabetes mellitus; CKD: chronic kidney disease; CHD: coronary heart disease; IBD: inflammatory bowel disease; hs-CRP: highly sensitive c-reactive protein; hs-TnT: highly sensitive troponin T; NT-proBNP: of N-terminal pro-brain natriuretic peptide; mMRC: modified medical research council dyspnoea scale; CFQ-11: Chalder Fatigue Score; EHR: electronic health records; HADS: Hospital anxiety and Depression Scale; FACIT: Functional assessment of chronic illness therapy; CAT: COPD assessment test; IES-R: Impact of Event Scale-Revised; TICS: Telephone Interview of cognitive status; CFS: Cognitive Failure Questionnaire; PTSS: Post traumatic stress syndrome; NCSI: Nijmegen Clinical Screening Instrument; CCCRC: Connecticut Chemosensory Clinical Research Center orthonasal olfaction test; ARTIQ: acute respiratory tract infection questionnaire; WEMWBS: Warwick-Edinburgh Mental Wellbeing Scales
Fig. 1.
Study selection.
3.2. Clinical features
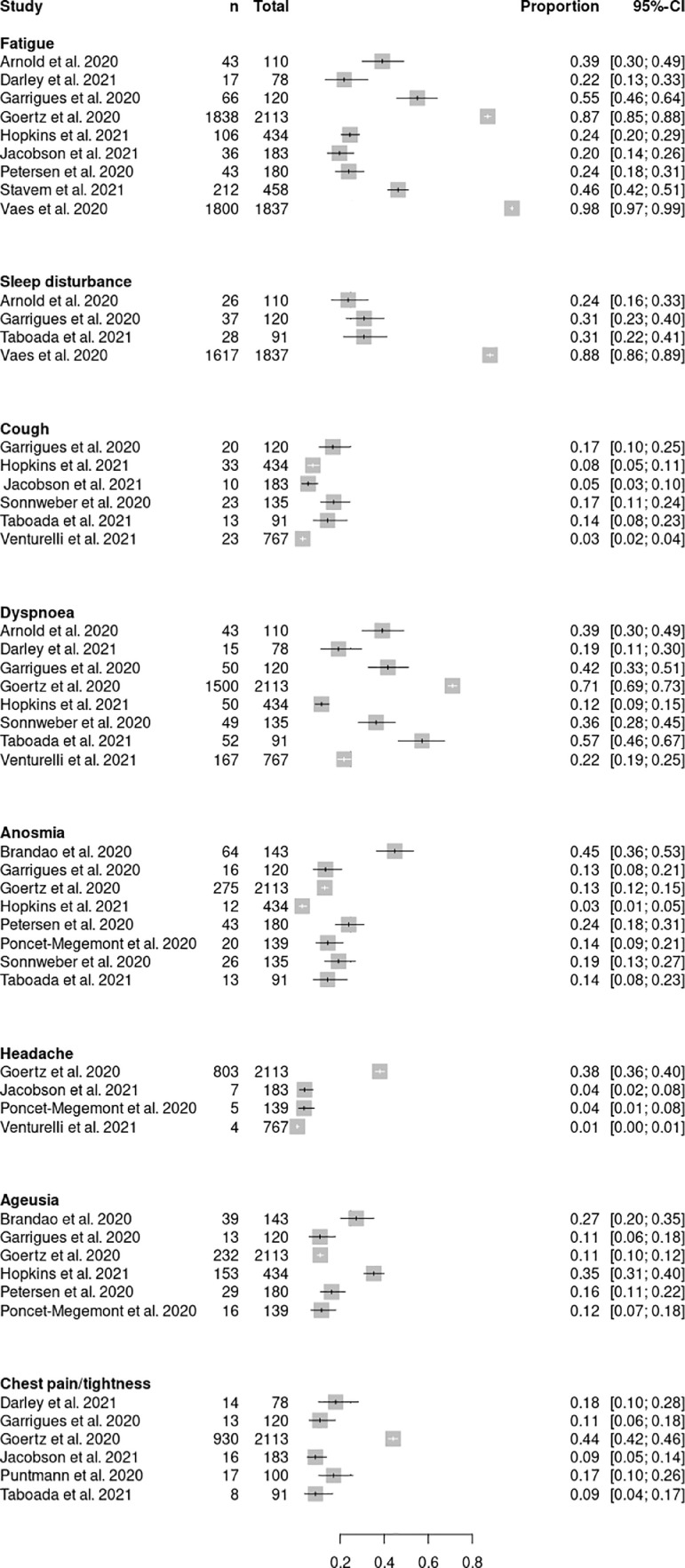
All studies reporting the prevalence of clinical features for acute and chronic post-COVID syndrome are shown in Fig. 2, Fig. 3, respectively.
Fig. 2.

Forest plot of studies describing clinical features in acute post-COVID syndrome.
Fig. 3.
Forest plot of studies describing clinical features in chronic post-COVID syndrome.
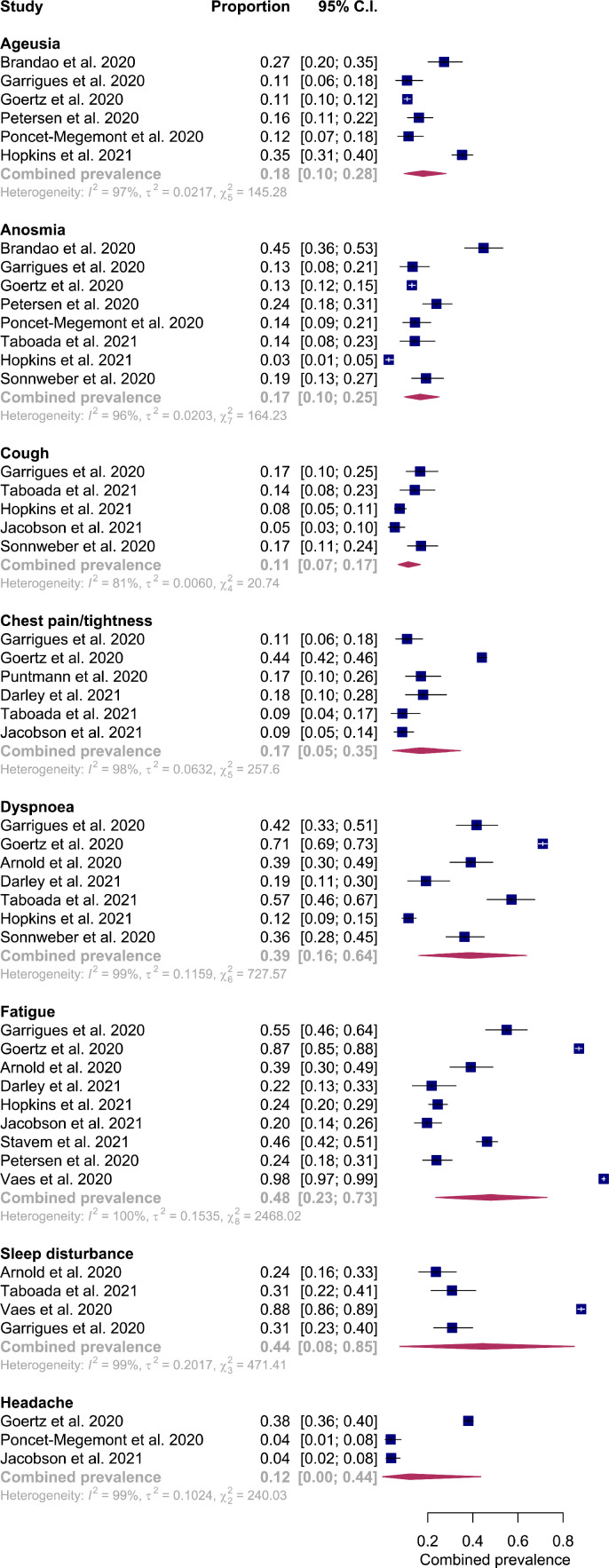
To meta-analyse the acute post-COVID cohort, seven studies were removed. Two studies study failed to include demographics or characteristics of the included individuals appropriately and was excluded from the sub-group analysis [20,21]. Additionally, Bongiovanni et al. describe potential for inaccurate PCR testing, as such, this study was also excluded from our sub-group analysis [21]. Another study inadequately described follow-up protocols and ascertainment of results [22]. The use of unvalidated questionnaires for retrospective recall in pre-infective functional status and against ‘maximum symptoms’ risks a significant inherent recall bias [23,24]. In addition, strong risks for sample bias from two studies precluded pooled comparisons, including exclusion of hospitalised individuals admitted to intensive care which may underestimate symptom burden [25,26]. The pooled prevalence of clinical features for acute post-COVID syndrome is shown in Fig. 4.
Fig. 4.
Forest plot of pooled prevalence of clinical features reported in acute post-COVID syndrome.
In the acute post-COVID phase, studies reported 13 predominant symptoms allowing for pooled analysis (Fig. 3). The most frequently reported symptoms were fatigue (0·37; 95% CI 0·20-0·56, I2 = 98%), dyspnoea (0·35; 95% CI 0·16-0·562, I2 = 97%) and anxiety (0·29; 95% CI 0·19-0·40, I2 = 88%).
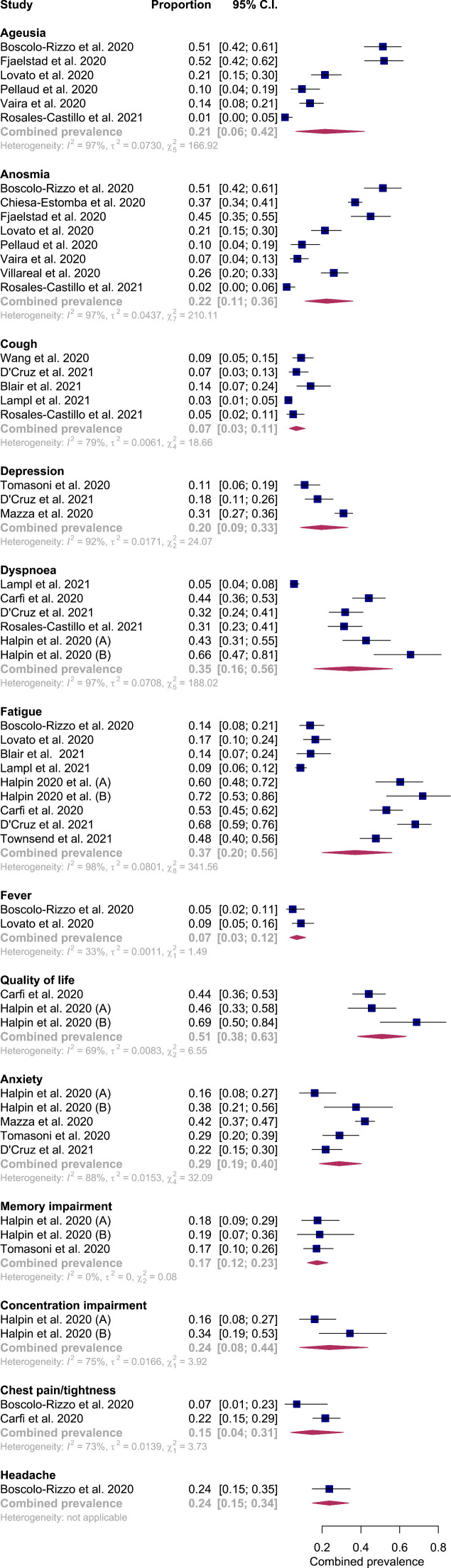
The lack of standardisation between enrolment and assessments into the trial precluded one study for the chronic post-COVID syndrome sub-group analysis [27]. Studies reported 8 predominant symptoms allowing for pooled analysis (Fig. 5). Fatigue (0·48; 95% CI 0·23–0·73, I2 = 100%), sleep disturbance (0·44; 95%CI 0·08–0·85, I2 = 99%), and dyspnoea (0·39; 95% CI 0·16–0·64, I2 = 99%) were reported as most prevalent symptoms.
Fig. 5.
Forest plot of pooled prevalence of clinical features reported in chronic post-COVID syndrome.
3.3. Predictors of post-COVID syndrome
Studies detailing predictors of post-COVID syndrome were limited to five studies. Carvalho-Schneider et al. reported that hospitalisation during the acute infection (odds ratio [OR] 2·9, 95% CI 1·3–6·9) and an age between 40-49 years (OR 15·3, 95% CI 2·8–83·9) were deemed the most significant predictors of developing post-COVID syndrome. The presence of initial symptoms (chest pain, dyspnoea, fever, anosmia, ageusia), gender or number of comorbidities did not predict post-COVID syndrome [19]. However, Goertz et al. contrasts these findings by suggesting that the number of symptoms present during initial infection was most responsible for predicting the number of symptoms at three months [28]. Furthermore, a multivariable analysis adjusting for gender, ethnicity, age, BMI, and hospitalisation status reported that only the presence of fatigue accounted for long-term activity impairment (OR 6·0, 95% CI 1·0–34·9) [29]. Similarly, those with a higher symptom load during the initial infection had greater odds of persistent fatigue [30].
Moreover, the severity of initial infection (i.e., need for critical care admission or invasive ventilation) was associated with patient-reported impairment, although no relationships between age or pre-existing comorbidities and the persistence of post-COVID symptoms were observed [31].
3.4. Risk of bias assessment
Twenty-two included studies were deemed to be at high risk of bias; 17 studies were deemed as moderate, and the remaining were considered low risk (Table 1). Frequently, risk of biases surfaced due to lack of control arms, potential effects from confounding variables (e.g., severity of symptoms during acute COVID-19 infection) or a result of strong recall biases given the varied data collection methodology. In addition, limited descriptions of participant recruitment and response rates across studies were noted.
3.5. Heterogeneity
Overall, the pooled analyses display significant heterogeneity urging for cautious interpretation of our results. The finding of heterogeneity is partly expected given the pragmatic choice of studies from a range of settings with different study populations (e.g., hospitalised, non-hospitalised, and mixed) with differing co-morbidity demographics (Table 2); differing follow-up timepoints; the varied use of validated and unvalidated questionnaires with significant diverse data collection protocols, such as telephone and face-to-face, are likely to contribute to the observed effects.
In order to explore the sources of substantial heterogeneity, stratification by sampling methodology (previously hospitalised, community, and mixed) was conducted for symptoms displaying an I-squared of over 95% (Appendix 3). Overall, the heterogeneity was lower although, values remained high, particularly in the mixed cohorts (Appendix 3). Further stratification for other factors which we hypothesised to be contributory to the heterogeneity could not be further explored given the insufficient number of papers available for sub-group analysis.
4. Discussion
This study suggests that there is a broad range of symptoms that persist beyond the acute phase of COVID-19 in patients with post-COVID syndrome. Fatigue and sleep disturbance were reported to be most common symptoms in acute post-COVID syndrome and fatigue, anxiety and dyspnoea were the most common in chronic post-COVID syndrome. The sizeable prevalence of extra-respiratory (e.g., anosmia) and functional (e.g., fatigue) symptoms illustrates the multi-system burden that post-COVID syndrome imposes upon individuals. Moreover, we also noted that the number of symptoms associated with chronic post-COVID is lower in comparison to the acute post-COVID experience. Lastly, although studies do comment upon specific predictors of post-COVID development, they report varied results, thus hindering clinical application of this knowledge. It seems, however, that severity of initial infection or symptom load during the acute phase of illness is associated with a greater likelihood of continued post-COVID symptomatology.
Given the prevalence of respiratory symptoms in acute COVID infection, the persistence of respiratory symptoms in post-COVID syndrome can be expected. Furthermore, persistent respiratory symptoms are in keeping with previous outbreaks of SARS-CoV which have demonstrated a restrictive pattern of lung function metrics consistent with the resultant muscle weakness six to eight weeks following hospital discharge [32]. Cardiac symptoms were also noted across both acute and chronic post-COVID syndromes. Cardiovascular involvement on cardiac MRI, with myocardial inflammation being the most prevalent abnormality, was observed in 78% of individuals having recovered from acute COVID-19 infection regardless of pre-existing conditions, severity and course of the initial presentation, or presence of cardiac symptoms [33]. The persistence of functional symptoms (e.g., fatigue) could be exacerbated in the context of social distancing and isolation. The pathophysiology of post-COVID syndrome is poorly understood, theories relating to hyperinflammatory state, oxidative stress, cytokine storm, and DNA damage have been hypothesised but on-going research is required for targeting potential treatments [34].
To combat post-COVID syndrome effectively, a multi-faceted approach will be required [2,35]. Current practice consists of following up individuals through self-reported symptoms and remote outpatient clinics. However, the investigations of choice for various symptoms, subsequent monitoring, and need for referral to specialist care has not yet been standardised [2]. The COVID-19 pandemic has seen a marked adoption in health technology. Innovation in technologies have allowed for remote monitoring to take precedent with several trials and evaluations underway [36,37]. One area of future research could see the utilisation of wearable sensors to monitor recovery from COVID-19.
We highlight the limited literature predicting post-COVID syndrome, indicating the need for enhanced surveillance programmes to be employed. Comparisons can be drawn from cancer survivorship in which the development of evidence-based frameworks (e.g., the National Cancer Survivorship Initiative) are deemed essential for the provision of personalised care [11]. Individuals with post-COVID syndrome may experience long lasting effects requiring long lasting support. It has been reported that 15% of individuals were absent from work due to illness at the time of follow-up [18]. It is imperative that this cohort is not forgotten about and broad education is provided to the public to enable better acceptability and understanding.
Policymakers should aim to educate the public and clinicians concerning post-COVID syndrome, thus recognising it as a legitimate health condition [11]. There is demand for a tailored approach towards recovery to pre-empt issues in advance. To achieve this, and given the scarcity of current data, there is an urgent need to drive recruitment into COVID-19 trials to improve our understanding and better identify predictors of symptom clusters [38].
Despite the importance of our work, a series of limitations are to be mentioned. Whilst the broad inclusion of studies, including those deemed at high risk of bias, in our analysis resulted in significant heterogeneity (with high I-squared values), it allowed for a broad insight into the prevalence and predictors of post-COVID syndrome based on the current literature in a condition with a limited but growing evidence base. Studies reported a mixture of cohort sampling (previously hospitalised, community, and mixed), follow-up timepoints, and data collection protocols which likely contributed to the existing heterogeneity. Further sub-group analyses assisted in providing insight into this heterogeneity, with mixed cohorts displaying large I-squared values (Appendix 3). However, given the pragmatic nature of study inclusion, this was expected. Calls to incorporate post-COVID sufferers’ perceptions within its evolving definition suggests the grouping of these cohorts within our analysis may assist in depicting the overall disease burden. Furthermore, the majority of published literature excludes low-income countries, an important omission in the midst of a global pandemic given available resources distinctly tailor potential available strategies for surveillance and treatment. Moreover, included cross-sectional studies consisted of small sample sizes; were single-centre; and involved questionnaires requiring retrospective recall of symptoms resulting in potential for recall bias and subjective assessment [18,19,[39], [40], [41], [42]]. Indeed, this methodology fails to capture the evolution of symptoms over time. In one study, attempts to overcome this through multiple phone calls at various time points was made; however, only a small proportion of participants responded to repeated calling [41]. A further study identified participants through long-COVID groups on Facebook, and eligible individuals were invited to join a registry and then respond to questionnaires [28]. However, this relied on technological literacy, risking selection and ascertainment bias. Qualitative experiences were measured on individuals that did not require hospitalisation for either the acute COVID-19 infection nor for post-COVID symptoms; participants were predominantly female and under-representative of BAME communities, reducing overall generalisability [43]. Lastly, the literature on predictors for post-COVID syndrome remains limited with one study excluding severe (intensive care) COVID-19 cases and including participants younger in age, many of whom were healthcare professionals, limiting generalisability to the public [19].
Varied terminology (e.g., ‘long COVID’, and ‘post-COVID syndrome’) have contributed to heterogenous research; given the adoption of the latter by NICE guidelines, widespread adoption of the term ‘post-COVID syndrome’ is required to aid homogenisation of future symptom data, allowing predictors to be accurately described [12]. The introduction of clinical codes for chronic post-COVID syndrome may aid identification of cases from administrative clinical datasets [44]. Moreover, prospectively designed trials with appropriate control arms are required (including low- and middle-income countries) to establish relationships between post-COVID syndrome and i) age, particularly as several studies excluded elderly populations which are most at risk of severe symptoms; ii) ethnicity status; and iii) characteristics and severity of initial acute infection (e.g., requirement of intensive care, need for supplemental oxygen).
In conclusion, the applicability of current knowledge on post-COVID syndrome is limited by the quality of available data, a result of the flaws in data capture and interpretation, as demonstrated in the uncertainty of our meta-analysis and there is need for global collaboration to further understand the prevalence, clinical characteristics, and prognosis of this novel disease. Clinicians, policy makers, and researchers must focus on understanding the impact of this condition on individuals and society.
Declaration of Competing Interest
None.
Acknowledgments
Acknowledgments
Infrastructure support for this research was provided by the NIHR Imperial Biomedical Research Centre (BRC) and the NIHR Imperial Patient Safety Translational Research Centre (PSTRC). JC acknowledges support from The Wellcome Trust grant 215938/Z/19/Z which supports the Sir Henry Wellcome Postdoctoral fellowship.
Data sharing statement: All data presented in this study were extracted from published original data. The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Author Contributions
FMI conceptualised the idea and drafted the manuscript. FMI and KL independently screened and reviewed all included articles and graded the quality of included studies. HA, JC, FMI, and KL performed the statistical analysis. KL, VS, HA, and AD all contributed to significant amendments to the final manuscript.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.100899.
Appendix. Supplementary materials
References
- 1.World Health Organisation. WHO Coronavirus Disease (COVID-19) Dashboard. 2020. https://covid19.who.int/?gclid=CjwKCAjw2Jb7BRBHEiwAXTR4jTyiJ83RwLwaLTHzkxD3tqRLxDjbB8GqAxU09OFw-RB3F3Q0P4URcRoC46sQAvD_BwE (accessed Sept 19, 2020).
- 2.Greenhalgh T, Knight M, A'Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. 2020;370 doi: 10.1136/bmj.m3026. [DOI] [PubMed] [Google Scholar]
- 3.Nabavi N. Long covid: How to define it and how to manage it. BMJ. 2020;370:m3489. doi: 10.1136/bmj.m3489. [DOI] [PubMed] [Google Scholar]
- 4.Office for National Statistics (ONS). Coronavirus (COVID-19) Infection Survey. 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/coronaviruscovid19infectionsurveypilot/4december2020 (accessed Dec 23, 2020).
- 5.Updated estimates of the prevalence of long COVID symptoms - Office for National Statistics. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/healthandlifeexpectancies/adhocs/12788updatedestimatesoftheprevalenceoflongcovidsymptoms (accessed March 9, 2021).
- 6.Callard F, Perego E. How and why patients made Long Covid. Soc Sci Med. 2021;268 doi: 10.1016/j.socscimed.2020.113426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anonymous. Long COVID: let patients help define long-lasting COVID symptoms. Nature. 2020;586:170. doi: 10.1038/d41586-020-02796-2. [DOI] [PubMed] [Google Scholar]
- 8.Alwan NA. Track COVID-19 sickness, not just positive tests and deaths. Nature. 2020;584:170. doi: 10.1038/d41586-020-02335-z. [DOI] [PubMed] [Google Scholar]
- 9.How long does COVID-19 last? https://covid.joinzoe.com/post/covid-long-term?fbclid=IwAR1RxIcmmdL-EFjh_aI- (accessed Dec 12, 2020).
- 10.Alwan NA. Surveillance is underestimating the burden of the COVID-19 pandemic. Lancet. 2020;396:e24. doi: 10.1016/S0140-6736(20)31823-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iqbal FM, Lam K, Sounderajah V, Elkin S, Ashrafian H, Darzi A. Understanding the survivorship burden of long COVID. EClinicalMedicine. 2021:33. doi: 10.1016/j.eclinm.2021.100767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Institute for Health and Care Excellence. COVID-19 rapid guideline: managing the long-term effects of COVID-19. NICE guideline (NG188). 2020; published online Dec 18. https://www.nice.org.uk/guidance/ng188 (accessed Jan 29, 2021).
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available at https://www.covidence.org.
- 15.Hoy D, Brooks P, Woolf A. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65:934–939. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 16.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. [Google Scholar]
- 17.Arora G, Bittner V. Chest pain characteristics and gender in the early diagnosis of acute myocardial infarction. Curr Cardiol Rep. 2015;17:5. doi: 10.1007/s11886-014-0557-5. [DOI] [PubMed] [Google Scholar]
- 18.Halpin SJ, McIvor C, Whyatt G. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross-sectional evaluation. J Med Virol. 2020 doi: 10.1002/jmv.26368. [DOI] [PubMed] [Google Scholar]
- 19.Carvalho-Schneider C, Laurent E, Lemaignen A. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sollini M, Ciccarelli M, Cecconi M. Vasculitis changes in COVID-19 survivors with persistent symptoms: an [18F]FDG-PET/CT study. Eur J Nucl Med Mol Imaging. 2020 doi: 10.1007/s00259-020-05084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bongiovanni M, Vignati M, Giuliani G. The dilemma of COVID-19 recurrence after clinical recovery. J. Infect. 2020 doi: 10.1016/j.jinf.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahmani H, Davoudi-Monfared E, Nourian A. Comparing outcomes of hospitalized patients with moderate and severe COVID-19 following treatment with hydroxychloroquine plus atazanavir/ritonavir. DARU, J Pharm Sci. 2020 doi: 10.1007/s40199-020-00369-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mandal S, Barnett J, Brill SE. Long-COVID’: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. 2020 doi: 10.1136/thoraxjnl-2020-215818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobs LG, Gourna Paleoudis E, Lesky-Di Bari D. Persistence of symptoms and quality of life at 35 days after hospitalization for COVID-19 infection. PLoS One. 2020;15 doi: 10.1371/journal.pone.0243882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu D, Baumeister RF, Veilleux JC. Risk factors associated with mental illness in hospital discharged patients infected with COVID-19 in Wuhan, China. Psychiatry Res. 2020 doi: 10.1016/j.psychres.2020.113297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.C. C-S, E. L, A. L Follow-up of adults with non-critical COVID-19 two months after symptoms’ onset. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venturelli S, Benatti SV., Casati M. Surviving COVID-19 in Bergamo Province: a post-acute outpatient re-evaluation. Epidemiol Infect. 2021;149 doi: 10.1017/S0950268821000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goërtz Y, Van Herck M, Delbressine J. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res. 2020;6:1–10. doi: 10.1183/23120541.00542-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobson KB, Rao M, Bonilla H. Patients with uncomplicated COVID-19 have long-term persistent symptoms and functional impairment similar to patients with severe COVID-19: a cautionary tale during a global pandemic. Clin Infect Dis. 2021;ciab103 doi: 10.1093/cid/ciab103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stavem K, Ghanima W, Olsen MK, Gilboe HM, Einvik G. Prevalence and determinants of fatigue after COVID-19 in non-hospitalized subjects: a population-based study. 2021. doi:10.3390/ijerph18042030. [DOI] [PMC free article] [PubMed]
- 31.D'Cruz RF, Waller MD, Perrin F. Chest radiography is a poor predictor of respiratory symptoms and functional impairment in survivors of severe COVID-19 pneumonia. ERJ Open Res. 2021;7:00655–02020. doi: 10.1183/23120541.00655-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan KS, Zheng JP, Mok YW. SARS: prognosis, outcome and sequelae. Respirology. 2003;8:S36–S40. doi: 10.1046/j.1440-1843.2003.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puntmann VO, Carerj ML, Wieters I. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bektas A, Schurman SH, Franceschi C, Ferrucci L. A public health perspective of aging: do hyper-inflammatory syndromes such as COVID-19, SARS, ARDS, cytokine storm syndrome, and post-ICU syndrome accelerate short- and long-term inflammaging? Immun Ageing. 2020;17:23. doi: 10.1186/s12979-020-00196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lutchmansingh DD, Knauert MP, DE Antin-Ozerkis. A clinic blueprint for post-COVID-19 RECOVERY: learning from the past, looking to the future. Chest. 2020 doi: 10.1016/j.chest.2020.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iqbal FM, Joshi M, Davies G, Khan S, Ashrafian H, Darzi A. Design of the pilot, proof of concept REMOTE-COVID trial: remote monitoring use in suspected cases of COVID-19 (SARS-CoV-2) Pilot Feasibility Stud. 2021;7:62. doi: 10.1186/s40814-021-00804-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vindrola-Padros C, Singh KE, Sidhu MS. Remote home monitoring (virtual wards) during the COVID-19 pandemic: a living systematic review. medRxiv. 2020 doi: 10.1101/2020.10.07.20208587. [DOI] [Google Scholar]
- 38.Darzi A, Goddard A, Henderson K. Increasing recruitment into covid-19 trials. BMJ. 2021;372:n235. doi: 10.1136/bmj.n235. [DOI] [PubMed] [Google Scholar]
- 39.Garrigues E, Janvier P, Kherabi Y. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020 doi: 10.1016/j.jinf.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daynes E, Gerlis C, Briggs-Price S, Jones P, Singh SJ. COPD assessment test for the evaluation of COVID-19 symptoms. Thorax. 2020 doi: 10.1136/thoraxjnl-2020-215916. [DOI] [PubMed] [Google Scholar]
- 41.Petersen MS, Kristiansen MF, Hanusson KD. Long COVID in the Faroe Islands - a longitudinal study among non-hospitalized patients. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van den Borst B, Peters JB, Brink M. Comprehensive health assessment three months after recovery from acute COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kingstone T, Taylor AK, O'Donnell CA, Atherton H, Blane DN, Chew-Graham CA. Finding the ‘right’ GP: a qualitative study of the experiences of people with long-COVID. BJGP open. 2020 doi: 10.3399/bjgpopen20X101143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.SNOMED CT COVID-19 related content - announcements - SNOMED Confluence. https://confluence.ihtsdotools.org/display/snomed/SNOMED+CT+COVID-19+Related+Content (accessed March 12, 2021).
- 45.Arnold DT, Hamilton FW, Milne A. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax. 2020 doi: 10.1136/thoraxjnl-2020-216086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carfi A, Bernabei R, Landi F. Group GAC-19 P-ACS. persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu D, Zhang W, Pan F. The pulmonary sequalae in discharged patients with COVID-19: a short-term observational study. Respir Res. 2020 doi: 10.1186/s12931-020-01385-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pellaud C, Grandmaison G, Pham Huu Thien HP. Characteristics, comorbidities, 30-day outcome and in-hospital mortality of patients hospitalised with COVID-19 in a Swiss area - a retrospective cohort study. Swiss Med Wkly. 2020;150:w20314. doi: 10.4414/smw.2020.20314. [DOI] [PubMed] [Google Scholar]
- 49.Rosales-Castillo A, García de los Ríos C, Mediavilla García JD. Persistent symptoms after acute COVID-19 infection: importance of follow-up. Med Clin. 2021;156:35–36. doi: 10.1016/j.medcle.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sonnweber T, Sahanic S, Pizzini A. Cardiopulmonary recovery after COVID-19 – an observational prospective multi-center trial. Eur Respir J. 2020 doi: 10.1183/13993003.03481-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taboada M, Moreno E, Cariñena A. Quality of life, functional status, and persistent symptoms after intensive care of COVID-19 patients. Br. J. Anaesth. 2021;126:e110–e113. doi: 10.1016/j.bja.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tomasoni D, Bai F, Castoldi R. Anxiety and depression symptoms after virological clearance of COVID-19: a cross-sectional study in Milan, Italy. J Med Virol. 2020 doi: 10.1002/jmv.26459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang X, Xu H, Jiang H. Clinical features and outcomes of discharged coronavirus disease 2019 patients: a prospective cohort study. QJM. 2020 doi: 10.1093/qjmed/hcaa178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blair PW, Brown DM, Jang M. The clinical course of COVID-19 in the outpatient setting: a prospective cohort study. Open Forum Infect Dis. 2021;8 doi: 10.1093/ofid/ofab007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boscolo-Rizzo P, Borsetto D, Fabbris C. Evolution of altered sense of smell or taste in patients with mildly symptomatic COVID-19. JAMA Otolaryngol Head Neck Surg. 2020 doi: 10.1001/jamaoto.2020.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brandão Neto D, Fornazieri MA, Dib C. Chemosensory Dysfunction in COVID-19: prevalences, recovery rates, and clinical associations on a large Brazilian Sample. Otolaryngol - Head Neck Surg. 2020 doi: 10.1177/0194599820954825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chiesa-Estomba CM, Lechien JR, Radulesco T. Patterns of smell recovery in 751 patients affected by the COVID-19 outbreak. Eur J Neurol. 2020 doi: 10.1111/ene.14440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fjaeldstad AW. Prolonged complaints of chemosensory loss after COVID-19. Dan Med J. 2020;67(8) [PubMed] [Google Scholar]
- 59.Lovato A, Galletti C, Galletti B, de Filippis C. Clinical characteristics associated with persistent olfactory and taste alterations in COVID-19: a preliminary report on 121 patients. Am. J. Otolaryngol. - Head Neck Med. Surg. 2020 doi: 10.1016/j.amjoto.2020.102548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vaes AW, Machado FVC, Meys R. Care dependency in non-hospitalized patients with COVID-19. J Clin Med. 2020;9 doi: 10.3390/jcm9092946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Villarreal IM, Morato M, Martínez-RuizCoello M. Olfactory and taste disorders in healthcare workers with COVID-19 infection. Eur Arch Oto-Rhino-Laryngology. 2020 doi: 10.1007/s00405-020-06237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Darley DR, Dore GJ, Cysique L. Persistent symptoms up to four months after community and hospital-managed SARS-CoV-2 infection. Med J Aust. 2021 doi: 10.5694/mja2.50963. mja2.50963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hopkins C, Surda P, Vaira LA. Six month follow-up of self-reported loss of smell during the COVID-19 pandemic. Rhinology. 2020;59(1):26–31. doi: 10.4193/Rhin20.544. [DOI] [PubMed] [Google Scholar]
- 64.Islam MS, Ferdous MZ, Islam US, Mosaddek ASM, Potenza MN, Pardhan S. Treatment, persistent symptoms, and depression in people infected with COVID-19 in Bangladesh. Int J Environ Res Public Health. 2021;18:1453. doi: 10.3390/ijerph18041453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lampl BMJ, Buczovsky M, Martin G, Schmied H, Leitzmann M, Salzberger B. Clinical and epidemiological data of COVID-19 from Regensburg, Germany: a retrospective analysis of 1084 consecutive cases. Infection. 2021;1:3. doi: 10.1007/s15010-021-01580-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mazza MG, De Lorenzo R, Conte C. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. 2020 doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Poncet-Megemont L, Paris P, Tronchere A. High prevalence of headaches during Covid-19 infection: a retrospective cohort study. Headache. 2020 doi: 10.1111/head.13923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Townsend L, Dyer AH, Jones K. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One. 2020;15 doi: 10.1371/journal.pone.0240784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Townsend L, Dowds J, O'Brien K. Persistent poor health post-COVID-19 is not associated with respiratory complications or initial disease severity. Ann Am Thorac Soc. 2021 doi: 10.1513/annalsats.202009-1175oc. published online Jan 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vaira LA, Hopkins C, Petrocelli M. Smell and taste recovery in coronavirus disease 2019 patients: A 60-day objective and prospective study. J Laryngol Otol. 2020 doi: 10.1017/S0022215120001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.