Abstract
Excessive pain during medical procedures is a pervasive health challenge. This study tested the (additive) analgesic efficacy of combining hypnotic analgesia and virtual reality (VR) pain distraction. A single blind, randomized, and controlled trial was used to study 205 undergraduate volunteers aged 18 to 20. The individual and combined effects of hypnotic analgesia (H) and VR distraction on experimentally induced acute thermal pain were examined using a 2 X 2, between-groups parallel design (4 groups total). Participants in groups that received hypnosis remained hypnotized during the test phase pain stimulus. The main outcome measure was “worst pain” ratings. Hypnosis reduced acute pain even for people who scored low on hypnotizability. As predicted, H+VR was significantly more effective than VR distraction alone. However, H+VR was not significantly more effective than hypnotic analgesia alone. Being hypnotized during thermal pain enhanced VR distraction analgesia.
Keywords: virtual reality, analgesia, distraction, hypnotizability, hypnosis, pain control
Zusammenfassung:
Exzessiver Schmerz während medizinischer Maßnahmen stellt eine allgegenwärtige Herausforderung im Gesundheitswesen dar. In dieser Studie wurde die (zusätzliche) analgetische Effizienz in der Kombination von hypnotischer Analgesie und Virtueller Realität (VR) zur Ablenkung von Schmerz untersucht. 205 Freiwillige, Studierende zwischen 18 und 20 Jahren, nahmen an der kontrollierten, randomisierten, einfach verblindeten Studie teil. Die individuellen und kombinierten Effekte hypnotischer Analgesie (H) und VR Ablenkung von experimentell erzeugtem akutem Hitzeschmerz wurden in einem 2 × 2 Zwischengruppen Parallel-Design (insgesamt 4 Gruppen) untersucht. Diejenigen, welche Hypnose erhielten, blieben während des Schmerzreizes der Testphase in Hypnose. Das hauptsächliche Maß für das Ergebnis war die Einstufung „schlimmster Schmerz“. Selbst bei Testpersonen mit als niedrig eingestufter Hypnotisierbarkeit reduzierte Hypnose den akuten Schmerz. Wie vorausgesagt war H + VR signifikant effektiver als VR Ablenkung allein. Allerdings war H + VR nicht signifikant effektiver als hypnotische Analgesie allein. Während des Hitzeschmerzes hypnotisiert zu sein förderte die Analgesie durch VR Ablenkung.
Alida Iost-Peter
Dipl.-Psych.
Résumé:
La douleur excessive pendant les procédures médicales est un défi de santé omniprésent. Cette étude a testé l’efficacité analgésique (additive) associant l’analgésie hypnotique et la distraction de la douleur en réalité virtuelle (RV). Un essai en simple aveugle, randomisé et contrôlé a été utilisé pour étudier 205 volontaires de premier cycle âgés de 18 à 20 ans.
Les effets individuels et combinés de l’analgésie par hypnose (H) et par la distraction VR sur la douleur thermique aiguë induite expérimentalement ont été examinés en utilisant une approche 2 X 2 entre les groupes parallèles (4 groupes au total).
Les participants des groupes qui ont reçu de l’hypnose sont restés hypnotisés pendant la phase de test de stimulation de la douleur. Le principal critère de jugement était l’évaluation de la «pire douleur». L’hypnose a réduit la douleur aiguë, même pour les personnes dont l’hypnotisabilité était faible. Comme prévu, H + VR était significativement plus efficace que la distraction VR seule. Cependant, H + VR n’était pas significativement plus efficace que l’analgésie hypnotique seule. Être hypnotisé pendant la douleur thermique a amélioré l’analgésie par distraction VR.
Gerard Fitoussi, M.D.
Président of the European Society of Hypnosis
Resumen:
El dolor excesivo durante procedimientos médicos es un reto generalizado de salud. Este estudio evaluó la eficacia analgésica (aditiva) al combinar analgesia hipnótica y distracción del dolor mediante realidad virtual (RV). Se utilizó un ensayo clínico, simple-ciego, aleatorio para evaluar 205 voluntarios de licenciatura entre 18 y 20 años. Se evaluaron los efectos individuales y combinados de la analgesia hipnótica (H) y la distracción mediante RV sobre dolor térmico agudo inducido experimentalmente usando un diseño paralelo entre grupos 2 X 2 (con un total de 4 grupos). Los participantes en grupos que recibieron hipnosis se mantuvieron hipnotizados durante la fase de prueba del estímulo de dolor. La principal medida objetivo fue una clasificación del “peor dolor.” La hipnosis redujo el dolor aún en personas con puntuaciones bajas en hipnotizabilidad. Como se esperaba, H + RV resultó significativamente más eficaz que la únicamente la distracción con RV. Sin embargo, H + RV no fue significativamente más eficaz que la analgesia hipnótica por sí misma. Estar hipnotizado durante el dolor térmico mejoró la analgesia por distracción mediante RV.
Omar Sánchez-Armáss Cappello
Autonomous University of San Luis Potosi, Mexico
Controlling acute pain associated with medical procedures is a pervasive health challenge. Although analgesic medications can help reduce pain during painful medical procedures, the side effects of pharmacologic interventions limit dose levels and frequency of use. In light of the current opioid addiction/overdose death crisis, (Chen et al., 2019), developing effective adjunctive nonpharmacologic analgesic techniques for acute pain management is a national priority (Keefe et al., 2018; Van Denburg et al., 2018).
Hypnosis is a psychological pain control technique that can help reduce acute procedural pain (Kendrick et al., 2016; Lang et al., 2000,Montgomery et al., 2000; Patterson, 2010; Patterson & Jensen, 2003; see also Kihlstrom, 1998; Lynn & Rhue, 1991; Rainville et al., 1997; Tataryn & Kihlstrom, 2017). Immersive virtual reality (VR) is another nonpharmacologic analgesic for which there is growing empirical support (Hoffman et al., 2000, 2011; Soltani et al., 2018). In the case of VR analgesia, people look into a pair of VR goggles in order to “go into” a virtual world that is designed to help distract them from pain. The current study explores whether these two interventions, hypnosis and VR, which are each known to be individually effective, can be combined or integrated to reduce induced acute pain in the laboratory.
To our knowledge, a study published in 2006 was the first to examine systematically the combination of hypnotic analgesia and an immersive VR distraction intervention (Patterson et al., 2006). In that study, healthy volunteer college students were randomly assigned to one of four experimental conditions: (1) no VR/no hypnosis, (2) yes VR/no hypnosis, (3) no VR/yes hypnosis, and (4) yes VR/yes hypnosis. Using a parallel design in their study, participants were randomly assigned to one of four treatment groups. Groups 1, 2, 3, and 4 all received the same introductory instructions prior to the baseline pain measurement. During baseline, all participants received several brief thermal stimuli, and they identified a temperature they found “painful but tolerable for 30 seconds” that they were willing to receive for one more thermal stimulus later in the study. In Patterson et al. (2006), no participants received VR or pain stimuli during the Phase 1 intermission. During Phase 1, half of the participants listened to sounds from nature with no hypnotic suggestions, and the other half listened to post hypnotic suggestions via a prerecorded audio. Those who received post hypnotic suggestions were re-alerted before the Phase 2 test thermal stimulus. During Phase 2 of Patterson et al. (2006), the test phase, the participants received one brief test phase thermal stimulus. Some of their participants were looking into a pair of VR goggles and were interacting with a computer-generated world during the test phase thermal stimulus, and other participants received no VR during the test phase stimulus.
It is important to note that in Patterson et al. (2006), participants who received hypnosis listened to an audio recording that provided a hypnotic induction followed by posthypnotic suggestions for analgesia; that is, participants received suggestions that they would experience less pain later, after they were no longer hypnotized. The participants in that study were then invited to return to a state of full alertness (i.e., no longer hypnotized) before receiving a brief pain stimulus and rating their pain (the test phase). These previous researchers in Patterson et al., 2006, found mixed results regarding the beneficial effects of posthypnotic suggestion on pain; posthypnotic suggestions for analgesia significantly reduced laboratory pain but follow-up analyses revealed that posthypnotic suggestions only reduced pain for the subset of people who scored high on the Stanford Hypnotic Clinical Scale (Morgan & Hilgard, 1978). In that previous study (Patterson et al., 2006), the posthypnotic suggestions did not reduce pain for participants who scored low or medium on the Stanford Hypnotic Clinical Scale. Their finding that posthypnotic suggestions were modulated/limited by hypnotizability is consistent with previous research by Miller and Bowers (1986, 1993). Furthermore, in Patterson et al. (2006), VR distraction was effective regardless of hynotizability, whereas hypnosis was only effective for people who scored high on the Stanford. Consistent with the relatively low benefits from posthypnotic suggestions, in Patterson et al. (2006), hypnosis combined with VR was not significantly more effective than VR distraction alone.
It is also mentioned in Patterson et al. (2006) that their use of “posthypnotic” suggestions may have been a limitation. Regarding future directions for how to increase hypnotic analgesia, the investigators Patterson et al. (2006) noted that, “during hypnosis” interventions (remaining hypnotized during the pain stimulus) may be more powerful than posthypnotic suggestions for reducing perceived pain. Towards our overall goal of developing more effective nonpharmacological approaches for pain management, in the current study we sought to evaluate the effects of inviting participants to remain in a hypnotic state during the pain stimulus, rather than relying on posthypnotic suggestions. Here, in the current study, participants were invited to remain hypnotized during the pain stimulus and asked to return to a state of full awareness only after they had already finished receiving the pain stimulus and already reported their pain ratings. Further, unlike Patterson et al. (2006), in the current study participants in the group that received both hypnosis + VR treatments were asked to remain hypnotized when they received VR distraction (“during hypnosis” + VR distraction during the test pain stimulus). We hypothesized that not only would audio hypnosis alone have a significant direct effect on pain but that participants in the hypnosis condition would allow themselves to “go into” a 3D computer-generated world to a greater extent than those who experienced VR without hypnosis. As a result, in the current study we hypothesized that participants in the hypnosis + VR condition would experience more analgesia than those in the VR-alone condition. Moreover, given previous evidence that VR distraction is effective for reducing acute pain, we predicted that participants in the VR + hypnosis condition would report more pain reduction than those in the hypnosis condition alone. In summary, the current study tested the (additive) analgesic efficacy of combining hypnotic analgesia and VR pain distraction in a manner designed to maximize their individual and combined analgesic effectiveness.
Method
Participants
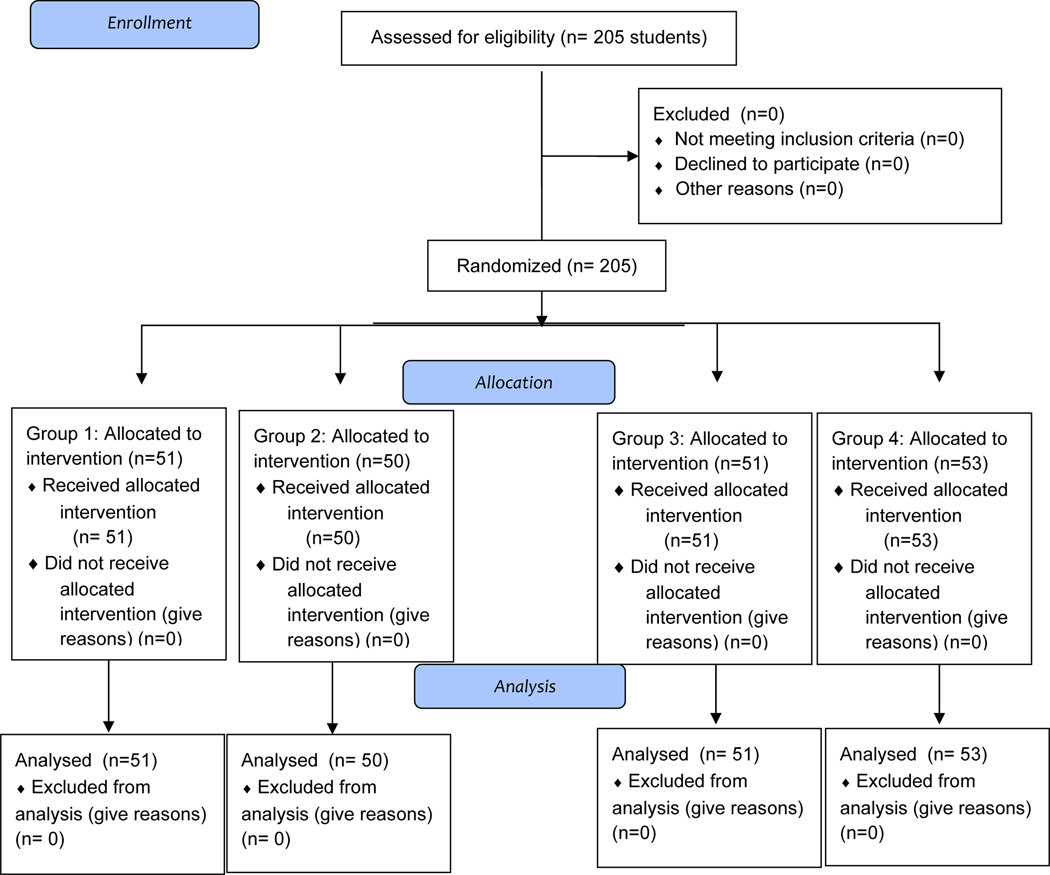
Two hundred and five students from a large university, aged 18 to 20 years old, volunteered to participate. Participants were fully informed of what the study would entail before consenting; each person verbally agreed to participate and signed an informed consent form approved by the University’s Human Subjects IRB. Students received course credit in their psychology classes for participating.
Measures
Participants’ subjective rating of worst pain intensity during the thermal stimulus was the primary study outcome. Pain unpleasantness and time spent thinking about pain during the stimulus served as secondary outcomes. Graphic Rating Scales (GRS; Jensen & Karoly, 2001) were used to assess each of these outcome domains.
Primary outcome variable: Worst pain intensity.
Participants received the following instructions. “Please rate your WORST pain intensity during the most recent stimulus on the following scale.” The GRS used provided respondents with a 10cm line with small hatch marks and numerical labels from 0 to 10. It also included verbal descriptors for different numbers and ranges of number (0 for no pain, 1–4 for mild pain, 5–6 for moderate pain, 7–9 for severe pain, and 10 for excruciating pain). The verbal descriptors were selected on the basis of research that suggests that they represent reasonable cutoffs for mild, moderate, and severe pain ratings (Jensen et al., 2001; Mendoza et al., 2004; Serlin et al., 1995). Strong associations with other measures of pain intensity, and their ability to detect treatment effects have been used to validate these measures (Jensen & Karoly, 2001). Respondents could either make a mark on the line (if they wanted to select a specific point between any two integers), or select a specific number, e.g.:
Rate your WORST PAIN during the most recent pain stimulus (pain intensity).
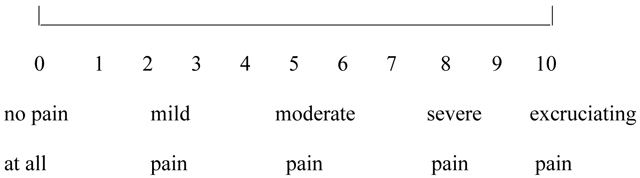
As shown in the example above, the pain rating scales used in the current study consisted of a numerical pain rating scale, with verbal labels/identifiers. According to Cook et al. (2013, p. s50) “pain intensity….is a narrow construct often measured using a 1 item scale.” Although a review by Cook et al., 2013, identified verbal rating scales as the most common subjective pain rating for adults, the NIH toolbox recommends a 0-to-10 numeric rating scale for measuring pain intensity (Cook et al., 2013). Similarly, Karcioglu et al. (2018) analyzed and compared the Visual Analogue Scale, the Verbal Scale, and the Numerical Rating Scale and concluded that …” all three scales are valid, reliable, and appropriate for use in clinical practice” (Karcioglu et al., 2018, p. 707).
The secondary outcome variables were pain unpleasantness, time spent thinking about pain during the painful stimulus, and the amount of fun experienced during the pain stimulus.
Participants were instructed to “Please indicate your answer to the following questions by making a mark on the line or circling one of the following numbers: you can use fractions if you want (e.g., your answers do not have to be whole numbers).” The verbal descriptors associated with the pain unpleasantness rating were not unpleasant at all (0), mildly unpleasant (1–4), moderately unpleasant (5–6), severely unpleasant (7–9), and excruciatingly unpleasant (10). Participants rated how much time they spent thinking about pain as a measure of a cognitive component of pain (Jensen, 2003), none of the time (0), some of the time (1–4), half of the time (5), most of the time (6–9), and all of the time (10). As a surrogate measure of positive affect, participants rated how much fun they had during the painful stimulus (Hoffman, Patterson et al., 2004; Sharar et al., 2016). The verbal descriptors associated with the fun rating were no fun at all (0), mildly fun (1–4), moderately fun (5–6), pretty fun (7–9), and extremely fun (10). Previous studies indicate that these secondary measures are sensitive to manipulations of the VR system immersiveness (Hoffman, Sharar, et al., 2004; Hoffman, Patterson, et al., 2004; Sharar et al., 2016; Wender et al., 2009).
Hypnotizability.
The trait-like tendency to respond to hypnotic suggestions (Jensen & Patterson, 2014) was assessed using the Stanford Hypnotic Clinical Scale (SHCS; Hilgard & Hilgard, 1975; Morgan & Hilgard, 1978). Trained research assistants blind to experimental treatment group gave participants a hypnotic induction followed by five suggestions designed to elicit five responses from the participants: (1) hand movement, (2) age regression, (3) having a dream, (4) a posthypnotic suggestion (coughing or clearing their throat), and (5) posthypnotic amnesia. For each suggestion, the hypnotist gave participants a 0 if they did not respond or a 1 if they met the criteria for responding to the suggestion. Total scores ranged from 0 (no hypnotizability) to 5 (highest hypnotizability). The SHCS has demonstrated validity through its strong correlation with other measures of hypnotizability (Hilgard & Hilgard, 1975). A reliability estimate for the clinical scale was obtained from the product-moment correlation (.72) between the total scores on the SHCS and the Stanford Form C (Morgan & Hilgard, 1978; Weitzenhoffer & Hilgard, 1962).
Pain catastrophizing.
Participants’ pain catastrophizing was assessed using the validated Pain Catastrophizing Scale (Sullivan et al.,1995). The Pain Catastrophizing Scale (PCS) has 13 items and assesses the degree of pain catastrophizing in three components: helplessness (H), magnification (M), and rumination (R). The total score indicates the degree of pain catastrophizing. Each individual item is assessed on a scale ranging from 0 (not at all) to 4 (always), and hence, the total score range is from 0 to 52. A higher score of pain catastrophizing indicates a state of higher occurrence of cognitive and affective thought processes that makes the person emotionally vulnerable and relates strongly to exaggerated negative orientation towards noxious stimuli, e.g., negative appraisal about pain and its consequences (Sullivan et al., 1995).
Experimental Thermal Pain Model
A commercially available Quantitative Sensory Testing system (Medoc Advanced Medical Systems U.S., Durham, NC; http://www.medoc-web.com) involving a Peltier thermode was used to provide noxious heat at a painful but tolerable and safe temperature (Roldan & Abdi, 2015).
Baseline. Identification of a painful but tolerable temperature.
In the current study, the psychophysical method of ascending levels was used. A thermode was connected to the top of the participants’ right foot. Each participant selected a temperature they found painful but tolerable. The highest temperature permitted was 48 °C (only 4 participants elected to go this high). Each participant began by receiving the lowest 30-second heat stimulus (always 44 °C for the first stimulus, which all participants found tolerable). After each stimulus, the participant was asked to rate the stimulus using the 0-to-10 GRS for current pain. They were then asked if they wanted to use that temperature or go up 1 °C (e.g., to 45 °C), and this sequence was continued until the participant identified a stimulus that was “painful but tolerable” and that they were willing to receive again for their second pain stimulus at that temperature (for 30 seconds), later in the study. To avoid excessive pain, stimulus increments of less than 1 °C were sometimes administered at the researcher’s discretion as participants approached a “painful but tolerable” temperature for that individual. In general, an attempt was made to achieve either a pain unpleasantness or worst pain intensity rating of 7 out of 10, but a number of participants chose to stop before achieving a pain intensity rating of 7. Individualized stimulus temperatures that were rated as painful but tolerable ranged from 44 °C to 48 °C (M = 45.82 °C, SD = 1.01) and were associated with baseline ratings of worst pain ranging from 2 to 9 out of 10 (M = 6.23; 81% of participants rated baseline pain between 5 and 7).
Phase 1 “intermission” treatment conditions: No Hypnosis.
The participants assigned to the no hypnosis condition received the following instructions:
You will now get a brief intermission during which you will listen to a relaxation audio tape called Relaxing Sounds from Nature. Please close your eyes, make yourself comfortable, and listen to the tape until it stops in about 10 minutes. There will be no pain stimuli during the intermission. After the intermission, you will receive one additional 30-second thermal pain stimulus and will then rate how painful you found that stimulus.
Participants then listened to the control audiotape, “Relaxing Sounds from Nature.”
Phase 1 “intermission” Yes Hypnosis audio.
Participants who were randomly assigned to receive “during hypnosis” analgesia were asked to close their eyes and listen to a digital audio file.
You will now get a brief intermission during which you will listen to a relaxation audiotape. Please close your eyes, make yourself comfortable, and listen to the tape until it stops in about 10 minutes. You will imagine yourself going down ten stairs and you’ll count each stair from one to ten as you pass them on the way down. At the bottom, you will hear suggestions that you feel relaxed and comfortable. There will be no pain stimuli during the intermission. After the intermission, you will receive one additional 30-second thermal pain stimulus and will then rate how painful you found that stimulus.
The hypnotic induction largely consisted of suggestions for the participant to become increasingly comfortable and relaxed as they imagined themselves walking down a staircase, one step at a time, from 1 to 10. At the bottom of the staircase, upon reaching 10, all participants who received hypnosis received the same hypnotic suggestions for absorption and complete distraction in SnowWorld during the subsequent test phase immediately after the intermission/hypnosis phase (see Hypnosis suggestions script, below). The hypnotic suggestions all hypnosis participants received were as follows:
Hypnosis suggestions script:
Deeply, deeply relaxed. And as you focus on my voice, in a while, you are going to find yourself entering a very special place. We call that place, SnowWorld. It may be that you see SnowWorld in your imagination, maybe you’ll see it with your own eyes, or maybe it will be some of both. It really doesn’t matter. What is only important at this point, is how completely you will be absorbed in this world. You’ll find yourself flying around SnowWorld, and looking all around you, there will be tall canyon walls with blue icicles on them. At the bottom there will be a river flowing. Above you will see the blue sky. When you fly around SnowWorld, you will not have a care in the world. You will be completely, and utterly, at ease. In fact, you will feel so drawn to this special world, that you will not be able to feel anything else. The only thing that you will feel will be comfort, and relaxation, and also the excitement of being in the world around you. So you’ll be comfortable and relaxed, but you will also, interestingly, be excited, and absorbed in your world. Everything else will fall into the background. You will be so completely wrapped in the world, that you will be attentive only to what is happening here and now. You will forget about everything else. I’m sure you’ve had the experience in your life of being so completely, pleasantly absorbed in an experience that everything else just seems to disappear. Maybe you can remember an experience in your past like that now. A moment where you were so completely captivated, in which nothing else seemed to matter. That is what it will be like when you enter SnowWorld. Whether you remember an experience like this or not, isn’t really important. What is important is the experience of being lost in something that fascinates you. Time ceases to have any meaning. In addition, even things that might have been annoying or irritating to you seem to disappear from your consciousness—disappear from your attention altogether. Just complete absorption, in the world you are about to enter. Whether you are imaging yourself in a world in your mind, or whether it is right there, in front of you, soon you will find yourself going into SnowWorld. While you are in SnowWorld, we will be turning on the heat machine to your right foot again. Only this time, you will likely find yourself so absorbed in your present experience, that you may not even notice any changes in sensation, at all.
Although they were no longer listening to audiotapes during Phase 2, participants assigned to receive hypnosis during the Phase 1 intermission remained hypnotized throughout Phase 2 (i.e., during the Phase 2 test phase thermal pain stimulus).
Phase 2. Test phase (thermal stimulus). VR analgesia condition.
Approximately 50% of the study participants were assigned to receive VR distraction during their test phase pain stimulus. Participants in groups assigned to receive VR distraction received their test phase pain stimulus while they were looking into a pair of VR goggles and interacting with a 3D computer generated VR world.
For VR distraction, an immersive, three-dimensional, interactive, computer-simulated environment was computer generated using a Dell 530 personal computer workstation with dual 2GHz central processing units, 2GB of RAM, and a GeForce 6800 Ultra video card (Santa Clara, CA; http://www.nvidia.com) running the VR software on a Microsoft Windows operating system paired with a ProView SR 80 (Kaiser Electro-Optics, Carlsbad CA) VR helmet with mouse tracking, and no head tracking (because we used the articulated arm goggle holder our team typically uses when treating severe burn patients). The software used in this study is known as SnowWorld, http://www.vrpain.com, (see Figure 1), and was developed as an empirical and clinical intervention for pain associated with burn care procedures (Hoffman et al., 2001).
Figure 1.
SnowWorld, image by Ari Hollander and Howard Rose, copyright Hunter Hoffman, www.vrpain.com
In the VR condition, the participant assigned to receive VR during the test phase followed a predetermined path, “gliding” through an icy 3-D virtual canyon. This computer-generated VR distraction condition included mouse tracking (e.g., participants saw the sky when they moved the mouse to look up, a canyon wall when they pushed the computer mouse to the left, and a river when they pushed the computer mouse forward to look down); sound effects (e.g., a splash when a snowball hit the river); and animated green, blue, or white colored explosions. Participants were able to aim with their computer mouse and pushed (left clicked) a mouse button to shoot virtual snowballs at virtual snowmen, igloos, flying fish, and penguins (see Figure 1). Although the original version of SnowWorld (Hoffman et al., 2001) was designed and created by earlier teams of programmers/worldbuilders (University of Washington, Seattle and MultiGen-Paradigm Inc.), the more recent version of SnowWorld used in the current study was built by Firsthand.com and is owned by the University of Washington, Seattle (www.vrpain.com).
Study Design and Procedures
To help reduce bias in the results, participants were blinded to treatment condition (Karanicolas et al., 2010). In other words, in the current study, group allocation was concealed from participants. Group allocation was also concealed from the subset of researchers who helped enroll and consent the participants and who also later administered theSHCS.
Randomization to treatment condition.
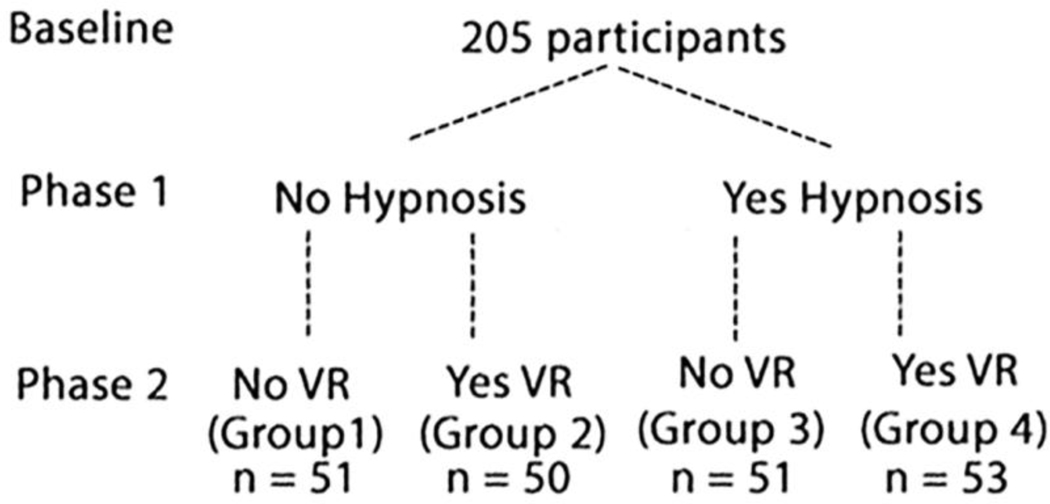
To help minimize differences between treatment groups in the current study, participants were randomly assigned to one of four treatment groups using computer generated random numbers from random.org. Blocked randomization was not used in the current study (but is recommended for future research). Using a parallel design, the study participants were randomly assigned to one of the four mutually exclusive experimental conditions (see Table 1, Study Design Figure 2 and Consort Diagram, Figure 3). (Group 1 = No VR/No H), (Group 2 = Yes VR/No H), (Group 3 = No VR/Yes H), (Group 4 =Yes VR/Yes H). Hypnosis vs. No Hypnosis was manipulated during the Phase 1 intermission immediately before the test phase pain stimulus. VR versus no VR distraction was manipulated during the Phase 2 test pain stimulus.
Table 1.
Mutually Exclusive Experimental Conditions
| Baseline calibration of heat tolerance (pain stimuli with no treatments) | Preparation (no thermal stimulation) Phase 1. | Thermal Pain Test Phase 2 | Additional treatments, if any | |
|---|---|---|---|---|
| Group 1 (n = 51), Age 18–20, 44% males, 56% female | Several 30 second thermal stim with no pain treatments | No hypnosis – listen to relaxing soundtrack via digital audio recordings | 30 sec thermal pain stimulation with No VR distraction during the pain stimulus | NA |
| Group 2 (n = 50) Aged 18–20 43% males, 57% female | Several 30 second thermal stim with no pain treatments | No hypnosis – listen to relaxing soundtrack via digitized audio recordings | 30 sec thermal pain stimulation WITH VR distraction during the pain stimulus | NA |
| Group 3 (n = 51) Aged 18–20 55% male, 45% female | Several 30 second thermal stim with no pain treatments | Hypnotic induction (going down staircase) followed by “during-hypnosis” suggestions for analgesia and presence, via digitized audio recordings | 30 sec thermal pain stimulation while still hypnotized, with No VR distraction during the pain stimulus | Digitized audio recording returns patients to a state of full alertness, awake and refreshed, after the test pain stimulus and pain ratings. |
| Group 4 (n = 53) Aged 18–20 38% male, 62% female | Several 30 second thermal stim with no pain treatments | Hypnotic induction (going down staircase) followed by “during-hypnosis” suggestions for analgesia and presence via digitized audio recordings | 30 sec thermal pain stimulation while still hypnotized and WITH VR distraction during the pain stimulus. | Digitized audio recording returns patients to a state of full alertness, awake and refreshed, after the test pain stimulus and pain ratings. |
Figure 2.
Study Design Diagram
Figure 3.
Consort Flow Diagram
Groups 1, 2, 3, and 4 all received the same introductory instructions prior to baseline. During baseline, all participants received several brief thermal stimuli, and they identified a temperature they found “painful but tolerable for 30 seconds” that they were willing to receive for one more thermal stimulus later in the study.
No participants received VR during Phase 1 (during the 15-minute intermission that separated the baseline pain stimulus from the second test phase pain stimulus). During Phase 1 intermission, participants were randomly assigned to one of two possible audio treatment groups. Half of the participants listened to sounds from nature with no hypnotic suggestions, and the other half listened to hypnotic suggestions via a prerecorded audio. Those who received hypnotic suggestions remained hypnotized during the Phase 2 test stimulus. All intermission audios were completed before participants began the test phase pain stimulus. In other words, participants were not listening to hypnosis audios or sounds from nature during the test (Phase 2) pain stimulus.
During Phase 2, (the test phase), participants received one brief test phase thermal stimulus. Some participants (Group 2 and Group 4) were looking into a pair of VR goggles and were interacting with a computer generated world during the test phase thermal stimulus, and other participants (Group 1 and Group 4), received no VR during the test phase stimulus. (See Table 1 and Figure 2).
Group 1: No VR/No H.
During the 15-minute intermission, Group 1 listened to sounds from nature (no talking). After the sounds from nature audio ended, the sounds from nature audio was turned off, participants received the test phase pain stimulus and rated their pain.
Group 2: Yes VR/No H.
During the 15-minute intermission, Group 2 listened to sounds from nature (no talking). After the sounds from nature audio ended (was turned off), participants looked into a pair of VR goggles and interacted with the 3D computer generated VR world they could see in the VR goggles, during the test phase pain stimulus, and rated their pain.
Group 3: No VR/Yes H.
During the 15-minute intermission, Group 3 listened to the hypnosis audio. After the hypnosis audio ended, but while still hypnotized, with their eyes closed, based on audio hypnotic suggestions they had received during the intermission, they imagined going into the self-generated world during the second pain stimulus (i.e., the test phase) and then rated their pain. After the pain ratings, participants were re-alerted via digital audio suggestions.
Group 4: Yes VR/Yes H.
During the 15-minute intermission, Group 4 listened to the hypnosis audio. After the hypnosis audio ended, but while still hypnotized, they looked into the VR goggles and interacted with the 3D computer-generated world (used a computer mouse to throw snowballs at objects in VR), during the test phase pain stimulus, and rated their pain after the 30 second test phase pain stimulus. After the pain ratings, participants were re-alerted via digital audio suggestions.
Thermal pain test phase (Phase 2).
During the test phase, all participants received 30 seconds of painful stimulation shortly after they finished listening to the Phase 1 (intermission) hypnosis audio (Groups 3 and 4) or shortly after they listened to the Phase I (intermission) nature sounds audio recording (Groups 1 and 2). During Phase 2, they received the pain stimuli either while interacting with the computer-generated VR world (Groups 2 and 4) or while not looking into the VR goggles (Groups 1 and 3). Participants assigned to be engaged in the computer-generated VR world during Phase 2 looked into the VR goggles and experienced the VR technology for a total of 2.5 minutes. This included a 1.5-minute acclimatization period in VR, after which participants received their second 30-second pain stimulus while still in VR. They stopped looking into the VR goggles and answered the pain ratings shortly after the thermal pain stimulus ended.
Participants assigned to receive no VR (during Phase 2) did not look into any VR goggles. After the thermal stimulus, all participants rated worst pain intensity, pain unpleasantness, fun, and time spent thinking about pain using the series of 10-point GRSs described above. After all graphic rating pain measures were completed, the participants who had been hypnotized received an audio recording instructing them to imagine themselves returning up the stairs and to become increasingly alert, awake, and refreshed as the audiotaped voice counted from 10 to 1.
Poststimulation Measures
After the 30-second of painful stimulation and after the GRS pain ratings were completed by participants, hypnotized participants were re-alerted, the Pain Catastrophizing Scale was administered to all participants, and afterwards participants’ trait hypnotizability was assessed in another room by a second (different) trained research assistant who was blind to the treatment condition. Each participant’s trait hypnotizability was measured using the SHCS (described in the Measures section).
Sample Size.
The following a priori power analysis was conducting to determine if there would be enough power to detect a significant interaction effect of hypnosis on VR analgesia. Variance was estimated to be 1.6 SE. Type I error rate is 5% using a 2-tailed test. In order to estimate the number of subjects needed, we assumed that in the presence of VR distraction, hypnosis should provide an approximately 20% increase in the efficacy. This translates to an effect size (d) of .141, which represents a “small” effect. Hence, we estimated we would require a fairly large sample size. With at least 50 subjects per group (200 total), we would be able to detect the hypothesized hypnotic enhancement of VR Distraction with 90% power.
Data Analysis
Following computation of difference (absolute change) scores in the outcome variables (baseline pain ratings during the first [baseline] 30-second stimulus minus participants pain ratings obtained just after the test phase 30-second stimulus), a series of four univariate analyses of variance (ANOVAs) were computed to test the effects of hypnosis and VR on the outcome variables. In these analyses, Hypnosis condition (Yes, No), and VR condition (Yes, No), were the independent variables, see Figure 2.
Results
Primary Outcome: Worst Pain Intensity
An ANOVA with absolute change in worst pain intensity as the dependent variable showed significant main effects for computer-generated VR, F(1, 201) = 10.47, p < .005, MSE = 26.79, partial eta squared = .05, and hypnosis, F(1, 201) = 53.13, p < .001, partial eta squared = .21, MSE = 135.94. In addition, a significant interaction between the hypnosis and VR conditions emerged, F(1, 201) = 5.13, p < .05, MSE = 13.13, partial eta squared = .025. Given the significant interaction, between-groups pairwise comparisons between each of the four possible groups were then performed, using independent t-tests (see Table 2).
Table 2.
Worst Pain
Mean difference scores: Difference between participants GRS ratings during baseline pain stimulus minus GRS ratings during the test phase pain stimulus.
| Group 1: | Group 2: |
| No VR/No Hypnosis | VR only |
| Mean = .03 (SD = 1.10) | Mean difference = 1.22, (SD = 1.66) |
| Group 3: | Group 4: |
| Hypnosis only | VR/Hypnosis combined |
| Mean difference = 2.06 (SD = 1.80) | Mean difference = 2.32, (SD = 1.75) |
Between Groups independent t-tests: Worst pain.
Group 1 (Mean = −.03, SD = 1.10) vs. Group 2 (Mean = 1.22, SD = 1.66), t(98) = 4.46, p < .001, Mean Difference = −1.26, Standard Error of the Difference = .28, CI = −1.81 to .69.
Group 1 (Mean = −.03, SD = 1.10) vs. Group 3 (Mean = 2.06, SD = 1.80), t(101) = 7.11, p < .001, Mean Difference = −2.09, Standard Error of the Difference = .29, CI = 2.68 to −1.51.
Group 1 (Mean = −.03, SD = 1.10) vs. Group 4 (Mean = 2.32, SD = 1.75), t(102) = 8.17, p < .001, Mean Diffference = −2.38, SE dif = .29, CI = −2.92 to −1.78.
Group 2 (Mean = 1.22, SD = 1.66) vs. Group 3 (Mean = 2.06, SD = 1.80), t(99) = 2.44, p = .017, Mean Difference = −.84, SE dif = .35, CI = −1.53 to – .16
Group 2 (mean = 1.22, SD = 1.66) vs. Group 4 (Mean = 2.32, SD = 1.75), t(100) = −3.25, p < .005, Mean difference = −1.10, SD dif = .34, CI = −1.77 to −.43.
Group 3 (Mean = 2.11, SD = 1.79) vs. Group 4 (Mean = 2.32, SD = 1.75), t(102) = −.63, NS, Mean Difference = −.22, CI = −.91 to .47.
Secondary Outcome: Pain Unpleasantness
An ANOVA with absolute change in pain unpleasantness as the dependent variable showed significant main effects for No VR versus Yes computer-generated VR, F(1, 201) = 7.44, p = .007, partial eta squared = .036, MSE = 23.47, and a significant main effect for No Hypnosis versus Yes Hypnosis, F(1, 201) = 42.42, p = .000, partial eta squared = .174, MSE = 133.76. In addition, a significant interaction between the hypnosis and VR conditions emerged, F(1, 201) = 6.25, p = .013, MSE = 19.72, partial eta squared = .030. Given the significant interaction, between-groups pairwise comparisons between each of four possible groups were then performed, using independent t-tests (see Table 3).
Table 3.
Pain Unpleasantness
Mean difference scores: Difference between participants GRS ratings during baseline pain stimulus minus GRS ratings during the test phase pain stimulus.
| Group 1: | Group 2: |
| No VR/No Hypnosis | VR only |
| Mean = .32, (SD = 1.39) | Mean difference = 1.63, (SD = 2.02) |
| Group 3: | Group 4: |
| Hypnosis only | VR/Hypnosis combined |
| Mean difference = 2.52 (SD = 1.83) | Mean difference = 2.61, (SD = 1.82) |
Between Groups independent t-tests: Pain Unpleasantness
Group 1 (Mean = .32, SD = 1.39) vs. Group 2 (Mean = 1.63, SD = 2.02), t(98) = 3.78, p < .001 Mean difference = −1.31, Standard Error Difference = .35, CI = −2.00 to −.62.
Group 1 (Mean = .32, SD = 1.39) vs. Group 3 (Mean = 2.52, SD = 1.83), t(101) = 6.89, p < .001 Mean difference = −2.20, Standard Error Difference = .32, CI = −2.84 to −1.57.
Group 1 (Mean = .32, SD = 1.39) vs. Group 4 (Mean = 2.61, SD = 2.61), t(102) = −7.20, p < .001 Mean difference = −2.29, Standard Error Difference = .32, CI = −2.92 to −1.66.
Group 2 (Mean = 1.63, SD = 2.02) vs. Group 3 (Mean = 2.52, SD = 1.83), t(99) = 2.34, p < .05, Mean difference = −.90, Standard Error Difference = .38, CI = −1.66 to −.14.
Group 2 (Mean = 1.63, SD = 2.02) to Group 4 (Mean = 2.61, SD = 1.82), t(100) = 2.58, p < .05 Mean difference = −.98, Standard Error of the Difference = .38, CI = −1.74 to −.23.
Group 3 = (Mean = 2.52, SD = 1.83) to Group 4 (Mean = 2.61, SD = 1.82), t(103) < 1, ns.
Secondary Outcome: Time Spent Thinking About Pain
An ANOVA with absolute change in time spent thinking about pain as the dependent variable showed significant main effects for No VR versus Yes computer-generated VR, F(1, 201) = 26.88, p < .001, partial eta squared = .118, MSE = 104.57, and a significant main effect for No Hypnosis versus Yes Hypnosis, F(1, 201) = 47.08, p < .001, partial eta squared = .19, MSE = 183.17. In addition, a significant interaction between the hypnosis and VR conditions emerged, F(1, 201) = 16.60, p < .001, MSE = 64.58, partial eta squared = .076. Given the significant interaction, between-groups pairwise comparisons between each of four possible groups were then performed, using independent t-tests (see Table 4).
Table 4.
Time Spent Thinking About Pain
Mean difference scores: Difference between participants GRS ratings during baseline pain stimulus minus GRS ratings during the test phase pain stimulus.
| Group 1: | Group 2: |
| No VR/No Hypnosis | VR only |
| Mean = .78 (SD = 1.88) | Mean difference = 3.38, (SD = 1.84) |
| Group 3: | Group 4: |
| Hypnosis only | VR/Hypnosis combined |
| Mean difference = 3.74 (SD = 2.16) | Mean difference = 4.10, (SD = 2.00) |
Between Groups independent t-tests: Time Spent Thinking about Pain.
Group 1 (mean = .78, SD = 1.88) vs Group 2 (mean = 3.38, SD = 1.84), t(98) = 7.00, p < .001, Mean difference = −2.60, Standard Error Difference = .37, CI = −3.34 to −1.86.
Group 1 (mean = .78, SD = 1.88) vs Group 3 (mean = 3.74, SD = 2.16), t(101) = 7.41, p < .001, Mean difference = −2.96, Standard Error Difference = .40, CI = −3.75 to −2.17.
Group 1 (mean = .78, SD = 1.88) vs Group 4 (mean = 4.10, SD = 2.00), t(102) = 8.73, p < .001, Mean difference = −3.32, Standard Error Difference = .38, CI = −4.07 to −2.57.
Group 2 (mean = 3.38, SD = 1.84) vs. Group 3 (mean = 3.74, SD = 2.16), t(99) < 1, ns.
Group 2 (mean = 3.38, SD = 1.84) vs. Group 4 (mean 4.10, SD = 2.00), t(100) = 1.89, p = .06 NS, Mean difference = −.72, Standard Error Difference = .38, CI = −1.48 to .034.
Group 3 (mean = 3.74, SD = 2.16) vs. Group 4 (Mean = 4.10, SD = 2.00), t(103) < 1, ns.
Secondary Outcome: Fun
An ANOVA with absolute change in fun as the dependent variable showed significant main effects for No VR versus Yes computer-generated VR, F(1, 200) = 69.81, p = .000, partial eta squared = .259, MSE = 199.01, and a significant main effect for No Hypnosis versus Yes Hypnosis, F(1, 200) = 20.56, p = .000, partial eta squared = .093, MSE = 58.61. In addition, a significant interaction between the hypnosis and VR conditions emerged, F(1, 200) = 15.97, p = .000, partial eta squared = .074, MSE = 45.52. Given the significant interaction, between-groups pairwise comparisons between each of four possible groups were then performed, using independent t-tests (see Table 5).
Table 5.
Fun During Pain Stimulus
Mean difference scores: Difference between participants GRS ratings during baseline pain stimulus minus GRS ratings during the test phase pain stimulus.
| Group 1: | Group 2: |
| No VR/No Hypnosis | VR only |
| Mean = −.01 (SD = 1.08) | Mean difference = −2.95, (SD = 2.16) |
| Group 3: | Group 4: |
| Hypnosis only | VR/Hypnosis combined |
| Mean difference = −2.03 (SD = 1.55) | Mean difference = −3.06, (SD = 1.78) |
Group 1 (Mean = −.01 (SD = 1.08) vs. Group 2 (Mean = − 2.95) (2.16)
t(97) = 8.57, p < .001, Mean dif = 2.94, SE dif = .34, CI = 2.26 to 3.62.
Group 1 (Mean = −.01) (SD = 1.08) vs. Group 3 (Mean = −2.03 (1.55)
t(100) = 7.58, p < .001, mean dif = 2.02, SE dif = .26, CI = 1.49 to 2.54.
Group 1 (mean = −.01) (SD = 1.08) vs. Group 4 (Mean = −3.06 (1.78)
t(101) = 10.44, p < .001, mean dif = 3.04, SE dif = .29, CI = 2.47 to 3.63
Group 2 (Mean = 2.95) SD = 2.16) vs Group 3 (Mean = −2.03 (1.55)
t(99) = −2.48, p < .05, mean dif = −.92, SE dif = .37, CI = −1.66 to −.183
Group 3 (Mean = −2.03) vs Group 4 (Mean = −3.06, SE = 1.78)
t(103) = 3.17, p < .005, mean dif = 1.03, SE dif = .33, CI = .39 to 1.68.
Correlations
As shown in Table 6, no significant correlations were found between scores on the Pain Catastrophizing Scale, scores on the SHCS, and Worst pain ratings (during No VR) on a graphic ratings scale.
Table 6.
No significant correlations were found between scores on the Pain Catastrophizing Scale, the Stanford Hypnotic Clinical Scale, and worst pain graphic ratings (pain intensity).
| Correlations | ||||
|---|---|---|---|---|
| Catast | Stanford | Worst pain | ||
| Catast | Pearson Correlation | 1 | −.009 | .057 |
| Sig. (2-tailed) | .912 | .462 | ||
| N | 168 | 168 | 168 | |
| Stanford | Pearson Correlation | −.009 | 1 | .125 |
| Sig. (2-tailed) | .912 | .077 | ||
| N | 168 | 200 | 200 | |
| Worst pain | Pearson Correlation | .057 | .125 | 1 |
| Sig. (2-tailed) | .462 | .077 | ||
| N | 168 | 200 | 205 | |
Discussion
The main goal of the present study was to test the hypothesis that hypnotic suggestions would potentiate pain reduction from VR distraction, and vice versa. Specifically, we investigated whether combining hypnosis with immersive VR would have a stronger analgesic effect on laboratory induced pain than either intervention used alone. We designed the hypnotic intervention so that it would provide suggestions for analgesia as well as suggestions designed to increase the participants’ sense of presence in VR (presence in the VR literature refers to the illusion of going into the computer generated virtual world, as if it is a place the participant is visiting). It is important to note that, in this study, hypnotic analgesia was based on participants being invited to remain in a hypnotic state during pain stimuli, rather than relying on posthypnotic suggestions.
The results indicate that, as hypothesized, both hypnosis alone and VR alone each significantly reduced pain. Furthermore, as also hypothesized, the combination of hypnosis and VR significantly decreased pain ratings, relative to VR distraction used alone. This is consistent with the possibility that hypnosis can be used to increase the impact of VR analgesia. However, in a separate comparison, and contrary to our predictions, the combined effect of VR distraction and hypnosis was not significantly superior to hypnosis alone.
In our previous study Patterson et al. (2006), hypnotic analgesia was given as a posthypnotic suggestion; that is, a suggestion to experience analgesia after participants had been alerted. In that study, participants listened to suggestions that they were becoming increasingly alert, awake and refreshed BEFORE they received their test pain stimulus. In the present study, participants were asked to remain in a hypnotic state during the test phase thermal stimuli. In the current study, only AFTER they completed the test pain stimulus and pain ratings, participants received suggestions to become alert, awake, and refreshed. When provided in this way, hypnosis treatment had a strong analgesic effect on pain ratings. This is consistent with a large body of research indicating the efficacy of hypnosis, particularly for acute pain (Montgomery et al., 2000, Patterson & Jensen, 2003; Patterson, 2010). However, in the current study, asking the participants to remain in a relaxed, hypnotic-like state during the pain stimulus may have had such a large effect that this created a ceiling effect, limiting the additional benefits that might have been obtained with participation in the computer-generated world. This might explain why the combination of VR and hypnosis was superior to VR alone, but the combined treatment was not superior to hypnosis used alone. However, the current study did not offer a direct comparison of these two hypnotic approaches (actual hypnotic states vs. posthypnotic suggestions), and we are unaware of any studies that have done so.
In our previous study, posthypnotic analgesia was moderated by hypnotizability and VR was not (Patterson et al., 2006; see also Montgomery et al., 2000; Patterson & Jensen, 2003). In the present study, the effects of the during hypnosis condition on pain relief were not moderated/limited by hypnotizability. One explanation for this may be that the participants in the current study were asked to stay in a hypnotic state during the test phase thermal pain stimulus, rather than relying on posthypnotic suggestions as in the previous study. Using the during hypnosis technique, in the current study even low hypnotizable participants reported reductions in pain from hypnosis. The during hypnosis technique may potentially benefit a greater number of people overall. It is possible that greater hypnotizability may be required to respond to posthypnotic suggestions for pain relief, whereas more individuals may be able to respond to hypnotic analgesia during the hypnotic procedures (i.e., receiving the painful stimulus and rating their pain before the subject is asked to become alert). Another possibility, and one limitation of the current study, is the measure used to assess hypnotizability in this study. We used the 5-item Stanford Hypnotic Clinical Scale (Hilgard & Hilgard, 1975) rather than the longer scales (Form A or Form C; Hilgard & Hilgard, 1975; Weitzenhoffer & Hilgard, 1959, 1962) that would have given a larger response range (12 items) in the assessment of hypnotizability. Most (but not all) studies that have examined the role of hypnotizability as a moderator of the effects of hypnosis on pain in individuals have used the 12-item Stanford A or C scales (see Thompson et al., 2019, for a review). It is possible that the longer Stanford A or C scales may be more reliable or psychometrically stronger than the Stanford Hypnotic Clinical Scale and that a greater moderating role for hypnotizability may have emerged had we used another scale (see also the Elkins Hypnotizability Scale, Kekecs et al., 2016).
Consistent with numerous clinical and experimental studies, in the current study, VR alone reduced ratings of pain effectively. VR distraction remains a promising nonpharmacologic approach to reducing acute pain (Hoffman et al., 2011; Soltani et al., 2018), and more exploration of methods to increase its efficacy are needed (Lynn et al., 2017). Hypnosis has long been known to enhance the impact of psychotherapy (Kirsch et al, 1995). For example, hypnosis has recently been demonstrated to enhance the effectiveness of pain education for chronic pain (Rizzo et al., 2018), and hypnosis has also been shown to enhance the effectiveness of cognitive behavioral therapy for chronic pain (Lee et al., 2019; De la Vega et al., 2019). In the current study, hypnosis was combined with VR in a way designed to enhance the effects of computer-generated distraction. The hypnotic suggestions in this study were designed to enhance a sense of presence in the VR world. VR is thought to provide analgesia by absorbing attention and leaving less cognitive resources available for processing pain (Hoffman et al., 2000). Our results suggest that hypnotic suggestions designed to enhance presence in VR had analgesic effects beyond the use of computer-generated distraction alone.
Part of the methodology used in this study deserves particular attention. The suggestions used in the hypnotic suggestions closely mirrored what the participants experienced in the computer-generated VR world. In the VR condition, some participants went (virtually) into the virtually generated world, a computer-generated environment designed to reduce burn pain. Other participants imagined/self-generated the analgesic environment. Future research should explore whether the analgesic effects of an imagined world have the same impact on pain as a world that participants experience with the sights and sounds that come with a live experience of a computer-generated VR world.
There were a number of limitations to our study. The VR goggles used in the current study for VR distraction were relatively high quality, but we used a robot-like arm goggle holder, customized to be used with severe burn patients who cannot wear VR helmets (no head tracking was used in the current study). Our use of a VR system designed for clinical use may increase the generalizability of our VR analgesia results to clinical patients, but more immersive VR systems that have recently become commercially available (e.g., Oculus Rift, HTC VIVE Pro, or XTAL by VRgineers.com, with head tracking) may prove considerably more distracting than the immobilized VR system used in the present study.
Future research is needed on how to maximize responsiveness to hypnotic suggestions (Lynn et al., 2017). Our study design asked participants to remain hypnotized during the pain stimulus rather than relying on posthypnotic suggestions. Although the current results suggest that staying hypnotized during the pain stimulus is more effective, the current study did not compare during hypnosis analgesia to analgesia from posthypnotic suggestions. In a future study, it would be of interest to compare, directly, the relative effectiveness of a hypnotic intervention that is provided during painful stimuli versus posthypnotic suggestion.
Acknowledgments
The work reported here was supported by National Institutes of Health awards R01 GM042725 and R01 AR054115 (principal investigator D. R. Patterson). The extensive efforts of Maryam Soltani and Sydney Drever in preparing this manuscript are gratefully acknowledged.
References
- Chen Q, Larochelle MR, Weaver DT, Lietz AP, Mueller PP, Mercaldo S, Wakeman SE, Freedberg KA, Raphel TJ, Knudsen AB, Pandharipande PV, & Chhatwal J. (2019). Prevention of prescription opioid misuse and projected overdose deaths in the United States. JAMA Network Open, 2(2), e187621. 10.1001/jamanetworkopen.2018.7621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook KF, Dunn W, Griffith JW, Morrison MT, Tanquary J, Sabata D, Victorson D, Carey LM, Macdermid JC, Dudgeon BJ, Gershon RC (2013). Pain assessment using the NIH toolbox. Neurology, 80(Suppl 3): S49–53. 10.1212/WNL.0b013e3182872e80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Vega R, Mendoza ME, Chan JF, & Jensen MP (2019). Case study: Cognitive restructuring hypnosis for chronic pain in a quadriplegic patient. American Journal of Clinical Hypnosis, 61(4), 394–408. 10.1080/00029157.2018.1537973 [DOI] [PubMed] [Google Scholar]
- Hilgard ER, & Hilgard JR (1975). Hypnosis in the Relief of Pain. William Kaufmann. [Google Scholar]
- Hoffman HG, Chambers GT, Meyer WJ 3rd., Arceneaux LL, Russell WJ, Seibel EJ, Richards TL, Sharar SR, & Patterson DR (2011). Virtual reality as an adjunctive non-pharmacologic analgesic for acute burn pain during medical procedures. Annals of Behavioral Medicine, 41(2), 183–191. 10.1007/s12160-010-9248-9247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman HG, Doctor JN, Patterson DR, Carrougher GJ, & Furness TA 3rd. (2000). Use of virtual reality as an adjunctive treatment of adolescent burn pain during wound care: A case report. Pain, 85(1–2), 305–309. [DOI] [PubMed] [Google Scholar]
- Hoffman HG, Patterson DR, Carrougher GJ, & Sharar SR (2001). Effectiveness of virtual reality-based pain control with multiple treatments. Clinical Journal of Pain, 17(3), 229–235. [DOI] [PubMed] [Google Scholar]
- Hoffman HG, Patterson DR, Magula J, Carrougher GJ, Zeltzer K, Dagadakis S, & Sharar SR (2004). Water-friendly virtual reality pain control during wound care. Journal of Clinical Psychology, 60(2), 189–195. [DOI] [PubMed] [Google Scholar]
- Hoffman HG, Sharar SR, Coda B, Everett JJ, Ciol M, Richards T, & Patterson DR (2004). Manipulating presence influences the magnitude of virtual reality analgesia. Pain, 111(1–2), 162–168. [DOI] [PubMed] [Google Scholar]
- Jensen MP (2003). The validity and reliability of pain measures in adults with cancer. Journal of Pain, 4(1), 2–21. [DOI] [PubMed] [Google Scholar]
- Jensen MP, & Karoly P. (2001). Self-report scales and procedures for assessing pain in adults. In Turk DC & Melzack R (Eds.), Handbook of pain assessment (2nd ed., pp. 15–34). Guilford Publications. [Google Scholar]
- Jensen MP, & Patterson DR (2014). Hypnotic approaches for chronic pain management: Clinical implications of recent research findings. American Psychologist, 69(2), 167–177. 10.1037/a0035644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MP, Smith DG, Ehde DM, & Robinson LR (2001). Pain site and the effects of amputation pain: Further clarification of the meaning of mild, moderate, and severe pain. Pain, 91(3), 317–322. [DOI] [PubMed] [Google Scholar]
- Karanicolas PJ, Farrokhyar F, Bhandari M. (2010). Practical tips for surgical research: blinding: who, what, when, why, how? Canadian Journal of Surgery, 53(5), 345–348. [PMC free article] [PubMed] [Google Scholar]
- Karcioglu O, Topacoglu H, Dikme O, & Dikme O. (2018). A systematic review of the pain scales in adults: Which to use? American Journal of Emergency Medicine, 36(4), 707–714. [DOI] [PubMed] [Google Scholar]
- Keefe FJ, Main CJ, & George SZ (2018). Advancing psychologically informed practice for patients with persistent musculoskeletal pain: Promise, pitfalls, and solutions. Physical Therapy, 98(5), 398–407. 10.1037/a0035644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekecs Z, Bowers J,Johnson A, Kendrick C, Elkins G. (2016). The Elkins Hypnotizability Scale: Assessment of reliability and validity. International Journal of Clinical and Experimental Hypnosis, 64(3), 285–304. 10.1080/00207144.2016.1171089 [DOI] [PubMed] [Google Scholar]
- Kendrick C, Sliwinski J, Yu Y, Johnson A, Fisher W, Kekecs Z, Elkins G. (2016). Hypnosis for acute procedural pain: A critical review. International Journal of Clinical and Experimental Hypnosis, 64(1), 75–115. 10.1080/00207144.2015.1099405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihlstrom JF (1998). Attributions, awareness, and dissociation: In memoriam Kenneth S. Bowers, 1937–1996. American Journal of Clinical Hypnosis, 40(3), 194–205. [DOI] [PubMed] [Google Scholar]
- Kirsch I, Montgomery G, & Sapirstein G. (1995). Hypnosis as an adjunct to cognitive-behavioral psychotherapy: A meta-analysis. Journal of Consulting and Clinical Psychology, 63(2), 214–220. [DOI] [PubMed] [Google Scholar]
- Lang EV, Benotsch EG, Fick LJ, Lutgendorf S, Berbaum ML, Berbaum KS, Logan H & Spiegel D. (2000). Adjunctive non-pharmacological analgesia for invasive medical procedures: A randomized trial. Lancet, 355(9214), 1486–1490. [DOI] [PubMed] [Google Scholar]
- Lee JK, Zubaidah JO, Fadhilah ISI, Normala I, & Jensen MP (2019). Prerecorded hypnotic peri-surgical intervention to alleviate risk of chronic postsurgical pain in total knee replacement: A randomized controlled pilot study. International Journal of Clinical and Experimental Hypnosis, 67(2), 217–245. 10.1080/00207144.2019.1580975 [DOI] [PubMed] [Google Scholar]
- Lynn SJ, Maxwell R, Green JP (2017). The hypnotic induction in the broad scheme of hypnosis: A sociocognitive perspective. American Journal of Clinical Hypnosis, 59(4), 363–384. 10.1080/00029157.2016.1233093 [DOI] [PubMed] [Google Scholar]
- Lynn SJ, & Rhue JW (1991). Theories of hypnosis: Current models and perspectives. Guilford Press. [Google Scholar]
- Mendoza TR, Chen C, Brugger A, Hubbard R, Snabes M, Palmer SN, Zhang Q & Cleeland CS (2004). Lessons learned from a multiple-dose post-operative analgesic trial. Pain, 109(1–2), 103–109. [DOI] [PubMed] [Google Scholar]
- Miller ME, & Bowers KS (1986). Hypnotic analgesia and stress inoculation in the reduction of pain. Journal of Abnormal Psychology, 95(1), 6–14. [DOI] [PubMed] [Google Scholar]
- Miller ME, & Bowers KS (1993). Hypnotic analgesia: Dissociated experience or dissociated control? Journal of Abnormal Psychology, 102(1), 29–38. [DOI] [PubMed] [Google Scholar]
- Montgomery GH, DuHamel KN, & Redd WH (2000). A meta-analysis of hypnotically induced analgesia: How effective is hypnosis? International Journal of Clinical and Experimental Hypnosis, 48(2), 138–153. [DOI] [PubMed] [Google Scholar]
- Morgan AH, & Hilgard JR (1978). The Stanford Hypnotic Clinical Scale for adults. American Journal of Clinical Hypnosis, 21(2–3), 134–147. [DOI] [PubMed] [Google Scholar]
- Patterson DR (2010). Clinical hypnosis for pain control. American Psychological Association. [Google Scholar]
- Patterson DR, Hoffman HG, Palacios AG, & Jensen MJ (2006). Analgesic effects of posthypnotic suggestions and virtual reality distraction on thermal pain. Journal of Abnormal Psychology, 115(4), 834–841. [DOI] [PubMed] [Google Scholar]
- Patterson DR, & Jensen MP (2003). Hypnosis and clinical pain. Psychological Bulletin, 129(4), 495–521. [DOI] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, & Bushnell MC (1997). Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science, 277(5328), 968–971. [DOI] [PubMed] [Google Scholar]
- Rizzo RRN, Medeiros FC, Pires LG, Pimenta RM, McAuley JH, Jensen MP, & Costa LOP (2018). Hypnosis enhances the effects of pain education in patients with chronic nonspecific low back pain: A randomized controlled trial. The Journal of Pain, 19(10), 1103.e1–1103.e9. 10.1016/j.jpain.2018.03.013 [DOI] [PubMed] [Google Scholar]
- Roldan CJ, & Abdi S. (2015). Quantitative sensory testing in pain management. Pain Management, 5(6), 483–491. 10.2217/pmt.15.37 [DOI] [PubMed] [Google Scholar]
- Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, & Cleeland CS (1995). When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain, 61(2), 277–284. [DOI] [PubMed] [Google Scholar]
- Sharar SR, Alamdari A, Hoffer C, Hoffman HG, Jensen MP, & Patterson DR (2016). Circumplex model of affect: A measure of pleasure and arousal during virtual reality distraction analgesia. Games for Health Journal, 5(3), 197–202. 10.1089/g4h.2015.0046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltani M, Drever SA, Hoffman HG, Sharar SR, Wiechman SA, Jensen MP, & Patterson DR (2018). Virtual reality analgesia for burn joint flexibility: A randomized controlled trial. Rehabilitation Psychology, 63(4), 487–494. 10.1037/rep0000239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MJL, Bishop SR, & Pivik J. (1995). The pain catastrophizing scale: Development and validation. Psychological Assessment, 7(4), 524–532. [Google Scholar]
- Tataryn DJ, & Kihlstrom JF (2017). Hypnotic tactile anesthesia: Psychophysical and signal-detection analyses. International Journal of Clinical and Experimental Hypnosis, 65(2), 133–161. 10.1080/00207144.2017.1276358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson T, Terhune DB, Oram C, Sharangparni J, Rouf R, Solmi M, Veronese N, & Stubbs B. (2019). The effectiveness of hypnosis for pain relief: A systematic review and meta-analysis of 85 controlled experimental trials. Neuroscience & Biobehavioral Reviews, 99, 298–310. 10.1016/j.neubiorev.2019.02.013 [DOI] [PubMed] [Google Scholar]
- Van Denburg AN, Vilardaga JP, Shelby RA, & Keefe FJ (2018). Opioid therapy and persistent pain: Can cognitive behavioral therapy help? Pain, 159(3), 411–415. 10.1097/j.pain.0000000000001091 [DOI] [PubMed] [Google Scholar]
- Weitzenhoffer AM, & Hilgard ER (1959). Stanford Hypnotic Susceptibility Scale, Forms A and B. Consulting Psychologists Press. [Google Scholar]
- Weitzenhoffer AM, & Hilgard ER (1962). Stanford Hypnotic Susceptibility Scale, Form C. Consulting Psychologists Press. [Google Scholar]
- Wender R, Hoffman HG, Hunner HH, Seibel EJ, Patterson DR, & Sharar SR (2009). Interactivity influences the magnitude of virtual reality analgesia. Journal of Cybertherapy and Rehabilitation, 2(1), 27–33. [PMC free article] [PubMed] [Google Scholar]