Abstract
This is a review of the published contributions made by Brazilian researchers between 2010 and 2020 on the natural history of human toxocariasis and the effects of human toxocariasis on nonhuman paratenic hosts.
Keywords: Toxocara, Toxocara canis, toxocariasis, visceral larva migrans syndrome, Brazilian contribution
Introduction
Human toxocariasis, first described in 1952 by Beaver et al,1 is currently considered an important zoonosis and the main cause of visceral larva migrans (VLM) and other syndromes, such as ocular larva migrans (OLM), covert toxocariasis (CT), and neurological toxocariasis.2–5 Signs and symptoms of the main clinical types of human toxocariasis are6–8
Visceral Larva Migrans – fever, pallor, malaise, irritability, weight loss, cutaneous rash, hepatomegaly, respiratory and nervous disturbs, myocarditis, hypergammaglobulinemia, leukocytosis and eosinophilia, elevated anti-A and anti-B isohemagglutinins; Ocular Larva Migrans – visual loss, strabismus, retinal granuloma and detachment, endophthalmitis, chorioretinitis, uveitis; Covert Toxocariasis – coughing, abdominal pain, headache, sleep and behavioral disturbances.
An analysis of the published toxocariasis research from 1932 to 2015 showed that researchers from the United States of America and Japan were responsible for 18.5% of a total of 2765 papers identified in the Scopus database, followed by researchers from Brazil and the United Kingdom, each responsible for 6.5% of the published papers.9
In 2009, Chieffi et al10 published a paper reviewing the contributions of Brazilian researchers to the main aspects of the natural history of human toxocariasis. The aim of this current paper is to update the Brazilian contributions on the natural history from 2010 to 2020. The MEDLINE and LILACS databases were used and representative articles consisting of papers published by Brazilian researchers were selected.
Toxocara canis Infection in Dogs and Soil Contamination by Toxocara Eggs
Dogs and other canids are the natural hosts of Toxocara canis; they can be infected via several routes; however, the transplacental and transmammary migration of third-stage larva are the principal mechanisms leading to the maintenance of high levels of infection in young dogs.11 Under certain conditions, T. canis can infect cats and develop into adult worms. Additionally, T. cati can infect dogs.12 In humans, with few exceptions, Toxocara infections lead to the presence and migration of larvae, which remain in the third developmental stage.
Stray dogs are usually controlled in the urban areas of Brazil; however, control does not necessarily extend to many suburban or rural localities, where stray dogs are not dewormed, which thus negatively impacts public health.
The most important route for human infection is the accidental ingestion of the embryonated eggs of Toxocara. The prevalence of T. canis infections in dogs, therefore, as reflected by soil contaminated by ascarid eggs, should be considered a good index of the risk of human infection by Toxocara larvae. Chieffi et al10 previously referred to parasitological soil surveys conducted in several Brazilian locations, which showed huge variations in the rates of T. canis infections in dogs, ranging from 5.5% to 39%.
Some publications from 2010 to 2020 by Brazilian researchers reported on the rates of T. canis infection in dogs and confirmed the rates that had already been established.13–15 However, a serological investigation in Salvador (Bahia State) revealed that 82.7% of 301 dogs were positive for anti-Toxocara IgG antibodies.16 On the other hand, Merigueti et al17 examined 165 dogs at a veterinary hospital and found T. canis eggs in 6.7% of samples of dog hair that were mainly collected in or near the dog tail.
Several Brazilian researchers investigated the soils of public spaces for Toxocara eggs between 2010 and 2020 (Table 1) and almost always found high positivity rates.18–25 Santarém et al19 found higher numbers of Toxocara eggs in the soil of public parks with mean monthly temperatures and monthly amounts of rainfall. Queiroz et al26 had also noticed increased soil contamination at a similar time.
Table 1.
Soil Contamination by Toxocara Eggs in Public Places in Brazilian Locations, from 2010 to 2020
| Authors | Local | n | Method | Positivity (%) |
|---|---|---|---|---|
| Manini et al (2012)18 | Maringá (Paraná State) | 15 | Water-sedmentation | 100* |
| Santarém et al (2012)19 | Presidente Prudente (São Paulo State) | 25 | Centrifuge-flotation | 96.0 |
| Marques et al (2012)20 | Guarulhos (São Paulo State) | 47 | Spontaneous-flotation | 74.5 |
| Ribeiro et al (2013)21 | Belo Horizonte (Minas Gerais State) | 42 | Spontaneous-flotation | 42.4 |
| Sprenger et al (2014)22 | Curitiba (Paraná State) | 69 | Centrifuge-flotation | 9.6 |
| Capella et al (2018)23 | Pelotas (Rio Grande do Sul State) | 100 | Spontaneous-flotation | 3.0 |
| Mello et al (2019)24 | Pelotas (Rio Grande do Sul State) | 79 | Spontaneoussedimentation | 11.9 |
| Leon et al (2020)25 | Laguna dos Patos (Rio Grande do Sul State) | 6 | Centrifuge-flotation | 8.3 |
Note: (*) 100% of public squares, 18.9% of peridomicile, 23.1% in schools.
Frequency and Characteristics of Human Infections Due to Toxocara
Researchers aiming to assess the frequency of human Toxocara infections in Brazil began to emerge in the late 1980s. The main serological surveys carrying out this aim up to 2009 have been summarized elsewhere.10 Tables 2 and 3 summarize the rates of infection in adults and in children. The tables reveal broad variations in infection rates, but highlight the importance of the morbidity of Toxocara infections in humans, especially children, and mainly is those aged from 1 to 8 years.27
Table 2.
Percentage of Human Seropositivity by Toxocara in Adults in Some Brazilian Regions
| Authors, Year | Location | N | Frequency (%) |
|---|---|---|---|
| Colli et al (2010)27 | Maringá (Paraná State) | 376 | 51.6 |
| Dattoli et al (2011)28 | Salvador (Bahia State) | 306 | 46.3* |
| Souza et al (2011)29 | Salvador (Bahia State) | 338 | 52.0 |
| Prestes-Carneiro et al (2013)30 | Pontal do Paranapanema (São Paulo State) | 194 | 14.4 |
| Negri et al (2013)31 | Presidente Prudente (São Paulo State) | 253 | 8.7 |
| Santos et al (2015)32 | Rio Grande (Rio Grande do Sul State) | 280 | 6.4+ |
| Pereira et al (2016)33 | Brasília (DF) | 311 | 7.4+ |
| Araújo et al (2018)34 | Rural population of Rio Grande do Sul State | 344 | 71.8 |
Note: (*) Blood donors (+) pregnant women.
Table 3.
Percentage of Human Seropositivity by Toxocara in Children in Some Brazilian Regions, Tested by ELISA
| Authors, Year | Local | N | Frequency (%) |
|---|---|---|---|
| Correa and Bismarck (2010)35 | Campinas (São Paulo State) | 100 | 28.0 |
| Santarém et al (2011)36 | Presidente Prudente (São Paulo State) | 252 | 11.1 |
| Fragoso et al (2011)37 | Vitória (Espírito Santo State) | 391 | 51.6 |
| Marchioro et al (2011)38 | Maringá (Paraná State) | 1199 | 32.2 |
| Mattia et al (2012)39 | Maringá (Paraná State) | 353 | 36.8 |
| Manini et al (2012)18 | Maringá (Paraná State) | 90 | 17.8 |
| Mendonça et al (2013)40 | Salvador (Bahia State) | 1309 | 48.4 |
| Guilherme et al (2013)41 | Maringá (Paraná State) | 167 | 4.2 |
| Schoenardie et al (2013)42 | Rio Grande (Rio Grande do Sul State) | 427 | 50.6 |
| Oliart-Guzmán et al (2014)43 | Assis Brasil (Acre State) | 421 | 12.3 |
| Cassenote et al (2014)44 | Rio Preto (São Paulo State) | 252 | 15.5 |
| Marchioro et al (2015)45 | Maringá (Paraná State) | 544 | 25.0 |
| Silva et al (2017)46 | São Francisco do Conde (Bahia State) | 791 | 63.6 |
| Araújo et al (2020)47 | Rio Grande (Rio Grande do Sul State) | 41 | 43.9 |
It is important to note, however, the scarcity of surveys based on samples that are statistically representative of the population being investigated, which perhaps accounts for the varying rates that have been sometimes found in the same regions or from areas nearby.
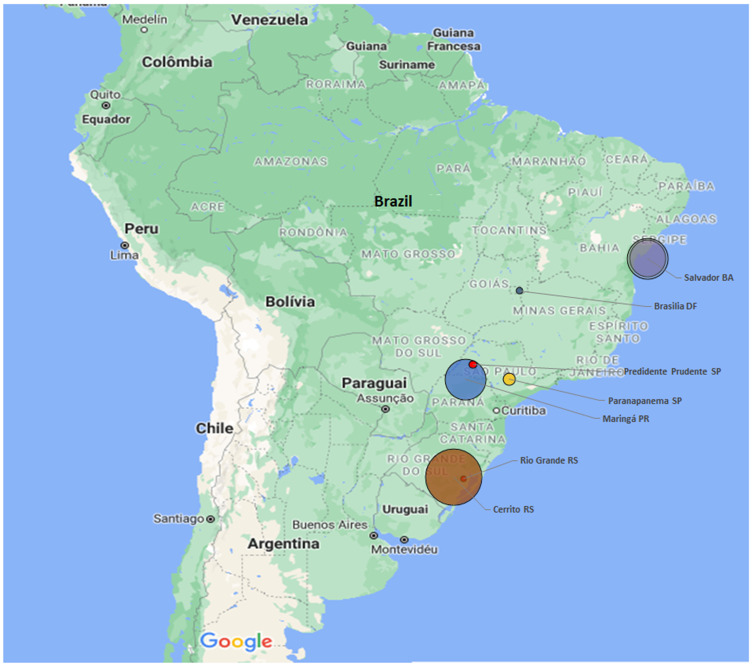
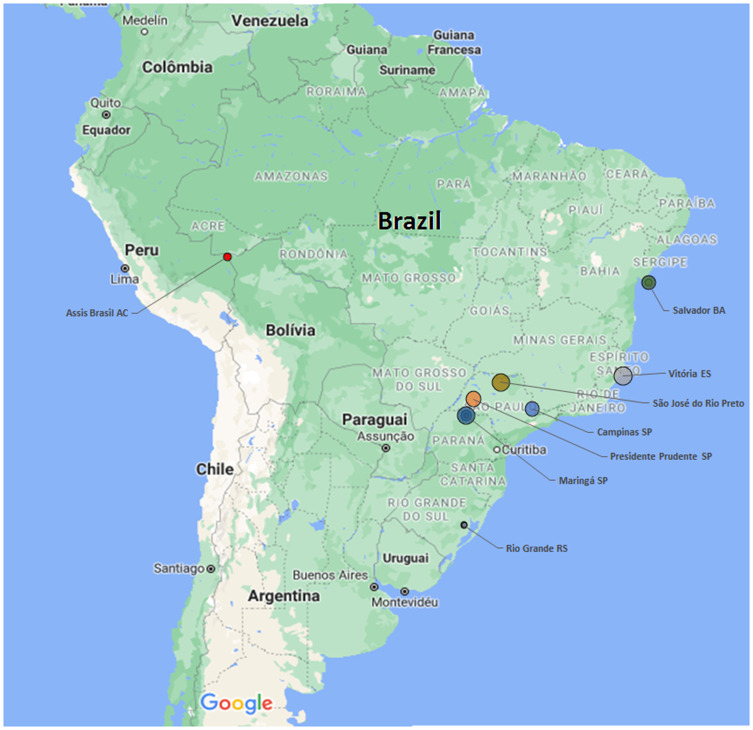
Figures 1 and 2 show the geographical location in Brazil of positive tests for antiToxocara antibodies in adults and children, examined by ELISA.
Figure 1.
Geographical distribution in Brazil of anti-Toxocara antibodies in adults examined by ELISA from 2010 to 2020.
Figure 2.
Geographical distribution in Brazil of anti-Toxocara antibodies in children examined by ELISA from 2010 to 2020.
In Rio Grande in Southern Brazil, 8% of 200 pregnant women were found to be coinfected with Toxocara canis and Toxoplasma gondii. This coinfection was associated with neonates with low birth weights.48,49
Frequent contact with soil or with dogs and cats has been identified as a risk for human Toxocara infection, especially in children.18,29,31,33,36,40 According to Colli et al,27 contact with the soils of recreational areas of schools is a higher risk of Toxocara infection in children than frequent contact with the soils of public squares.
Mattos et al50 performed an interesting study that determined the prevalence of infection and risk factors of infection in researchers who worked frequently with Toxocara in the laboratory. The control group consisted of researchers without contact with Toxocara in their laboratory activities. They found that the group working with Toxocara showed a lower frequency of infection compared to the controls (13.8% vs 15.6%, respectively), indicating that the Toxocara researchers, possibly because they were taking special precautions, were less exposed to the risk of Toxocara infection than the controls.
Changes in liver and pulmonary parameters are frequent in human toxocariasis, mainly in children. Although less frequent, ocular and brain lesions and allergic manifestations can occur.51 Carvalho et al52 used abdominal ultrasound to reveal hepatic changes such as hypoechoic liver lesions and/or enlarged lymph nodes in 29.7% of 37 children with toxocariasis.
The relevance of allergic manifestations in patients with toxocariasis is controversial among Brazilian researchers. Zaia et al53 observed acute allergic inflammation of the airways of mice infected by T. canis. Silva et al54 reported a patient with Loeffler syndrome who had been infected by T. canis. The patient only showed marked improvement after treatment with thiabendazole. On the other hand, Grama et al55 and Cadore et al56 did not find an association between atopy and an increased risk of asthmatic manifestations in children with serological tests positive for anti-Toxocara antibodies. In 2012, Mendonça et al51 did not find an association between positivity for aeroallergens and Toxocara infections in children living in poor areas of Salvador (Bahia State) and postulated that increased polyclonal IgE levels and the induction of a modified Th2 immune response to Toxocara infection might prevent the development of skin hypersensitivity to aeroallergens. On the other hand, Fialho and Corrêa57 found an association between asthma and increased body mass index in children and adolescents living in Campinas (São Paulo State), and Fialho et al58,59 confirmed a positive association between urticaria and infection with Toxocara in children.
Recuero et al60 described a case of eosinophilic panniculitis as a rare skin manifestation associated with toxocariasis in a 5-year-old girl with an ELISA positive for Toxocara. Albendazole treatment (10 mg/kg/day for 10 days) provided complete resolution of her condition. Another uncommon complication of toxocariasis in a child was reported by Salvador et al,61 who described the concomitant involvement of the cerebrum, cerebellum, and the peripheral nervous system of a 5-year-old boy from Porto Alegre (Rio Grande do Sul State).
Acute joint manifestations are common in toxocariasis; however, chronic polyarthritis as an isolated manifestation is very uncommon. Viola et al62 reported the case of a 3-year-old girl with chronic severe, painful polyarthritis and 30 min of morning stiffness that lasted longer than 10 weeks. The patient was positive for anti-Toxocara antibodies on ELISA. Treatment with paracetamol and thiabendazole for 10 days led to reduction in all the clinical manifestations of the infection.
Laboratory Diagnosis of Human Toxocariasis and Toxocariasis in Other Paratenic Hosts
Parasitologists usually prefer to diagnose parasitic infections by demonstrating the presence of the parasite in the host organism. The diagnosis of human toxocariasis is only possible through a biopsy, which is both extremely invasive and is sometimes neither safe nor accurate. The use of immunohistochemical techniques, in turn, allows identification of Toxocara antigen expression in histological sections.63 However, because biopsies are invasive, they are not common, and indirect diagnostic techniques are therefore more common.
Traditional indirect laboratory methods employed for the diagnosis of human toxocariasis rely usually on enzyme-linked immunosorbent assays (ELISAs) and Western blots. However, the production of T. canis antigens for use in those evaluations is arduous and time-consuming, taking at least 60 days,64 and does not eliminate possible cross-reactions with other similar antigens of other intestinal parasites.65 Several Brazilian researchers tried to resolve the shortcomings of the methods used in the diagnosis of human toxocariasis during the period from 2010 to 2020.
In an attempt to improve the sensitivity and specificity of immunological tests for the diagnosis of human toxocariasis, several researchers sought to identify possible markers of infections and the immunodominant antigens of T. canis.64,66–70
Roldán et al71 showed that IgM antibodies used for the serodiagnosis of human toxocariasis showed low specificity, and Santos et al72 found that Toxocara recombinant antigens developed with the use of the yeast Pichia pastoris instead of Escherichia coli showed low sensitivity for the immunodiagnosis of Toxocara infections. On the other hand, when Santos et al73 previously used recombinant antigens of low molecular weight (30 kDa), they obtained better results, especially in surveys that included children.
Some researchers have dedicated themselves to the study of the somatic and surface proteins of T. canis. They have obtained results that, in addition to contributing to the improvement of immunological tests, have provided information that allows the development of potential vaccines.74,75
The laboratory diagnosis of human ocular toxocariasis represents a greater challenge than the diagnosis of the visceral form because the levels of serum antibodies in the host are usually low and sometimes undetectable.76,77 Imaging modalities such as ultrasonography or optical coherence tomography should be useful additions to the clinical examination of patients suspected to have ocular toxocariasis.78,79 However, if ocular toxocariasis is suspected, despite the risks, an assessment of specimens of the aqueous or vitreous humor is recommended.
Aspects of the immune response of nonhuman paratenic hosts, with possible benefits for surveys aimed at determining the frequency of infections by Toxocara larvae, have been studied in vivo, in mouse or rabbit models.80–90 Serological surveys carried out on synanthropic animals that are paratenic hosts of T. canis revealed significant natural frequencies of infection, indicating that there is a risk involved in the human consumption of raw or undercooked meat from these animals.91–94 Recombinant proteins used in ELISAs and Western blot assays for the diagnosis of Toxocara infections of cattle, horses, and sheep have obtained good results.95
Treatment and Prophylaxis of Human Toxocariasis
Although the anthelminthic treatment of dogs with patent intestinal infections of T. canis shows good results; the anthelminthic treatment of Toxocara larvae encysted in canine tissues has been ineffective.6 In human toxocariasis, ascarid larvae migrate or encyst in tissues; thus, the use of various anthelmintics such as diethylcarbamazine and benznidazole derivatives has not led to the complete elimination of encysted larvae but has usually reduced or controlled the symptoms of those infections.96
Some Brazilian researchers also have tested anthelmintics in rodents infected by T. canis. Lescano et al97 compared the effect of nitazoxanide, a drug recently used for the treatment of several human protozoal and helminthic infections, with mebendazole, a benznidazole derivative known to reduce the Toxocara larval load in experimental rodent infections. They found a greater reduction in the number of larvae in the rodents treated with mebendazole than in those treated with nitazoxanide.
Another group of researchers tested the effects of quinone derivatives against Toxocara larvae in vitro, as well as their anthelmintic action in experimental rodent infections.98,99 They found good in vitro larvicidal activity and an anthelmintic activity similar to that found with benznidazole derivatives. Sinott et al,100 in turn, reported that the essential oil extracted from Brazilian red propolis showed good Toxocara larvicidal activity. On the other hand, Avila et al101 showed an increase in the larval burden of Toxocara in mice treated with cyclophosphamide or dexamethasone, drugs commonly used in immunosuppressive treatments.
Probiotics have recently been studied by some researchers, including researchers in Brazil, as possible alternatives for the control or even treatment of human toxocariasis.64 Avila et al102 found a significant reduction in the burden of Toxocara larvae in mice that were treated with Saccharomyces boulardii for 15 days. However, in a subsequent in vitro experiment they noticed a lack of action by S. boulardii on T. canis larvae in vitro, indicating that an interaction with the intestinal mucosa is mandatory for the protective effect of S. boulardii.103 The same researchers postulated that this type of protection could be provided through the modulation of cytokine expression, especially IL-12104,105 Walcher et al106 found that the probiotic Lactobacillus rhamnosus showed protective results similar to those provided by S. boulardii.
Another line of investigation, which is focused on the prophylaxis of toxocariasis, is the possible deleterious effects of some fungi on Toxocara eggs. Thus, the in vitro studies of the fungus Pochonia chlamydosporia showed its deleterious effects on T. canis and T. vitulorum eggs.107–109 Other species of fungi in the Trichoderma and Fusarium genera also showed deleterious in vitro activity against Toxocara eggs.110,111
Verocai et al112 showed that Toxocara eggs were resistant to solutions of sodium hypochlorite, benzalkonium chloride, and formaldehyde-based disinfectants. However, 70% ethanol led to the complete degeneration of Toxocara eggs in a few days.
Some researchers have focused on the use of synanthropic animals such as chickens as sentinels for soil contaminated by Toxocara eggs.113,114
Experimental Studies in Nonhuman Paratenic Hosts
Several researchers have focused on the roles and importance of the vertical and transmammary transmission of T. canis. Schoenardie et al41 verified the vertical transmission of T. canis in mice either with chronic infections or infections during the acute phase. The importance of the transmammary route in the transmission of T. canis in mice was emphasized by a group of researchers from the Federal University of Rio Grande, in the state of Rio Grande do Sul.115,116
The migration patterns of T. canis were studied in experimental infections of mice and gerbils. In Rattus norvegicus, a significant difference was found between the migration patterns according to the sex of the animals. Female rats showed a greater number of larvae in the liver than males.117 Resende et al82 studied the immunopathology of T. canis infections in mice and found similar larval migration patterns, with innate induction of TH17/TH2 responses during early infection. The migration patterns of T. canis larvae in the gerbil Meriones unguiculatus were similar to the patterns already observed in mice and rats; however, in reinfected animals, more larvae were apparently retained within hepatic granulomas, without evident signs of destruction.118
There are numerous references in the literature on behavioral changes in rodents experimentally infected with T. canis, which are associated with larvae located in the central nervous system.119,120 Before reaching the nervous system, the larvae pass through muscle tissues, being able to determine alterations.7 Santos et al121 observed a decrease in the strength of the muscles of rats (R. norvegicus) infected with T. canis or T. cati. The reduction in muscle strength was greater in rats infected with T. cati than in rats infected with T. canis. Researchers from the same group26,122 studied the patterns of behavioral changes in mice and rats concomitantly infected with T. canis and Toxoplasma gondii and observed that the obvious changes that occurred in animals infected with T. canis only or T. gondii only were not manifested in mice and rats with concomitant infections of both parasites.
The hamster (Mesocricetus auratus) is another species of rodent that is frequently used in the laboratory. It has been characterized by histopathological, immunohistochemical, and immunoelectron microscopic findings as a good animal model for research on toxocariasis.123
Conclusions
Evidence on the occurrence of human toxocariasis in Brazil indicates that additional well designed and comprehensive epidemiological studies are needed. The studies should be applied to the entire nation in order to understand the spatial distribution of this zoonosis and provide reliable information for implementing a national program to control it.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Beaver PC, Snyder H, Carrera G, Dent J, Lafferty I. Chronic eosinophilia due to visceral larva migrans. Pediatrics. 1952;9:7–19. [PubMed] [Google Scholar]
- 2.Taylor MRH, Keane CT, O’Connor P, Girdwood RW, Smith H. Clinical features of covert toxocariasis. Scand J Infect Dis. 1987;19:696–699. doi: 10.3109/00365548709117206 [DOI] [PubMed] [Google Scholar]
- 3.Rubinsky-Elefant G, Hirata CE, Yamamoto JH, Ferreira MU. Human toxocariasis: diagnosis, worldwide seroprevalences and clinical expression of the systemic and ocular forms. Ann Trop Med Parasitol. 2010;104:3–23. doi: 10.1179/136485910x12607012373957 [DOI] [PubMed] [Google Scholar]
- 4.Torgerson PR, Macpherson CNL. The socioeconomic burden of parasitic zoonoses: global trends. Vet Parasitol. 2011;182:79–95. doi: 10.1016/j.vetpar.2011.07.017 [DOI] [PubMed] [Google Scholar]
- 5.Fialho PM, Corrêa CR. A systematic review of toxocariasis: a neglected but high-prevalence disease in Brazil. Am J Trop Med Hyg. 2016;94:1193–1199. doi: 10.4269/ajtmh.15-0733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith H, Holland C, Taylor M, Magnaval JF, Schantz P, Maizels R. How common is human toxocariasis? Towards standardizing our knowledge. Trends Parasitol. 2009;25:182–188. doi: 10.1016/j.pt.2009.01.006 [DOI] [PubMed] [Google Scholar]
- 7.Raissi V, Sohrabi Z, Getso M, et al. Risk factors and prevalence of toxocariasis in pregnant women and diabetic patients compared to healthy adults in Ilam Province, Western Iran. EXCLI J. 2018;17:983–988. doi: 10.17179/excli2018-1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raissi V, Masoumi M, Ibrahim A, et al. Spatial analysis of Toxocara spp. eggs in soil as a potential for serious human infection. Comp Immunol Microbiol Inf Dis. 2021;75:101619. doi: 10.1016/j.cimid.2021.101619 [DOI] [PubMed] [Google Scholar]
- 9.Zyoud SH. Global toxocariasis research trends from 1932 to 2015: a bibliometric analysis. Health Res Policy Syst. 2017;15:14. doi: 10.1186/s12961-017-0178-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chieffi PP, Santos SV, Queiroz ML, Lescano SA. Human toxocariasis: contribution by Brazilian researchers. Rev Inst Med Trop Sao Paulo. 2009;51:301–308. doi: 10.1590/s0036-46652009000600001 [DOI] [PubMed] [Google Scholar]
- 11.Overgaauw PA. Aspects of Toxocara epidemiology: toxocarosis in dogs and cats. Crit Rev Microbiol. 1997;23:233–251. doi: 10.3109/10408419709115138 [DOI] [PubMed] [Google Scholar]
- 12.Fava NMN, Cury MC, Santos HA, et al. Phylogenetic relationships among Toxocara spp. and Toxascaris sp. from different regions of the world. Vet Parasitol. 2020;282:109133. doi: 10.1016/j.vetpar.2020.109133 [DOI] [PubMed] [Google Scholar]
- 13.Dantas-Torres F, Otranto D. Dogs, cats, parasites, and humans in Brazil: opening the black box. Parasit Vectors. 2014;7:22. doi: 10.1186/1756-3305-7-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramos DGS, Zocco BKA, Torres MM, Braga IA, Pacheco RC, Sinkoc AL. Helminths parasites of stray dogs (Canis lupus familiaris) from Cuiabá, Midwestern of Brazil. Semin Cienc Agrar. 2015;36:889–894. doi: 10.5433/1679-0359.2015v36n2p889 [DOI] [Google Scholar]
- 15.Lima VF, Cringoli G, Rinaldi L, et al. A comparison of mini-FLOTAC and FLOTAC with classic methods to diagnosing intestinal parasites of dogs from Brazil. Parasitol Res. 2015;114:3529–3533. doi: 10.1007/s00436-015-4605-x [DOI] [PubMed] [Google Scholar]
- 16.Regis SC, Mendonça LR, Silva Ndos S, Dattoli VC, Alcântara-Neves NM, Barrouin-Melo SM. Seroprevalence and risk factors for canine toxocariasis by detection of specific IgG as a marker of infection in dogs from Salvador, Brazil. Acta Trop. 2011;120:46–51. doi: 10.1016/j.actatropica.2011.05.011 [DOI] [PubMed] [Google Scholar]
- 17.Merigueti Y, Santarém VA, Ramires LM, et al. Protective and risk factors associated with the presence of Toxocara spp. eggs in dog hair. Vet Parasitol. 2017;244:39–43. doi: 10.1016/j.vetpar.2017.07.020 [DOI] [PubMed] [Google Scholar]
- 18.Manini MP, Marchioro AA, Colli CM, Nishi L, Falavigna-Guilherme AL. Association between contamination of public squares and seropositivity for Toxocara spp. in children. Vet Parasitol. 2012;188:48–52. doi: 10.1016/j.vetpar.201203.011 [DOI] [PubMed] [Google Scholar]
- 19.Santarém VA, Pereira VC, Alegre BC. Contamination of public parks in Presidente Prudente (São Paulo, Brazil) by Toxocara spp. eggs. Rev Bras Parasitol Vet. 2012;3:323–325. doi: 10.1590/S1984-29612012000300029 [DOI] [PubMed] [Google Scholar]
- 20.Marques JP, Guimarães CR, Boas AV, Carnaúba PU, Moraes J. Contamination of public parks and squares from Guarulhos (São Paulo State, Brazil) by Toxocara spp. and Ancylostoma spp. Rev Inst Med Trop Sao Paulo. 2012;54:267–271. doi: 10.1590/S0036-466.520120005500006 [DOI] [PubMed] [Google Scholar]
- 21.Ribeiro LM, Dracz RM, Mozzer LR, Lima WDS. Soil contamination in public squares in Belo Horizonte, Minas Gerais, by canine parasites in different developmental stages. Rev Inst Med Trop Sao Paulo. 2013;55:229–231. doi: 10.1590/S0036-46652013000400002 [DOI] [Google Scholar]
- 22.Sprenger LK, Green KT, Molento MB. Geohelminth contamination of public areas and epidemiological risk factors in Curitiba, Brazil. Rev Bras Parasitol Vet. 2014;23:69–73. doi: 10.1590/S1984-29612014009 [DOI] [PubMed] [Google Scholar]
- 23.Capella GDA, Pinto NB, Perera SC, et al. Environmental contamination by parasitic forms in a socially vulnerable community in southern Rio Grande do Sul state: a serious public health problem. Braz J Vet Res Anim Sci. 2018;55:1–8e132007. doi: 10.11606/issn.1678-4456.bjvras.2018.132007 [DOI] [Google Scholar]
- 24.Mello CCS, Nizoli LQ, Ferraz A, Chagas BC, Azario WJD, Villela MM. Helminth eggs with zoonotic potential in the vicinity of public schools in southern Brazil. Rev Bras Parasitol Vet. 2020;29(1):e016419. doi: 10.1590/S1984-29612019102 [DOI] [PubMed] [Google Scholar]
- 25.Leon IF, Strothmann AL, Islabão CL, Jeske S, Villela MM. Geohelminths in the soil of the Laguna dos Patos in Rio Grande do Sul state, Brazil. Braz J Biol. 2020;80:839–843. doi: 10.1590/15196984.222590 [DOI] [PubMed] [Google Scholar]
- 26.Queiroz ML, Viel TA, Papa CH, Lescano SA, Chieffi PP. Behavioral changes in Rattus norvegicus coinfected by Toxocara canis and Toxoplasma gondii. Rev Inst Med Trop Sao Paulo. 2013;55:51–53. doi: 10.1590/s0036-46652013000100009 [DOI] [PubMed] [Google Scholar]
- 27.Colli CM, Rubinsky-Elefant G, Paludo ML, et al. Serological, clinical and epidemiological evaluation of toxocariasis in urban areas of south Brazil. Rev Inst Med Trop Sao Paulo. 2010;52:69–74. doi: 10.1590/S0036-46652010000200002 [DOI] [PubMed] [Google Scholar]
- 28.Dattoli VC, Freire SM, Mendonça LR, Santos PC, Meyer R, Alcantara-Neves NM. Toxocara canis infection is associated with eosinophilia and total IgE in blood donors from a large Brazilian centre. Trop Med Int Health. 2011;16:514–517. doi: 10.1111/j.1365-3156.2010.02719.x [DOI] [PubMed] [Google Scholar]
- 29.Souza RF, Dattoli VC, Mendonça LR, et al. [Prevalence and risk factors of human infection by Toxocara canis in Salvador, State of Bahia, Brazil]. Rev Soc Bras Med Trop. 2011;44::516–519. doi: 10.1590/S0037-86822011000400024 [DOI] [PubMed] [Google Scholar]
- 30.Prestes-Carneiro LE, Rubinsky-Elefant G, Ferreira AW, et al. Seroprevalence of toxoplasmosis, toxocariasis and cysticercosis in a rural settlement, São Paulo State, Brazil. Pathog Glob Health. 2013;107:88–95. doi: 10.1179/2047773213Y.0000000079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Negri EC, Santarém VA, Rubinsky-Elefant G, Giuffrida R. AntiToxocara spp. antibodies in an adult healthy population: serosurvey and risk factors in Southeast Brazil. Asian Pac J Trop Biomed. 2013;3:211–216. doi: 10.1016/S2221-1691(13)60052-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santos PC, Lehmann LM, Lorenzi C, et al. The seropositivity of Toxocara spp. antibodies in pregnant women attended at the university hospital in southern Brazil and the factors associated with infection. PLoS One. 2015;10:e0131058. doi: 10.1371/journal.pone.0131058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pereira LC, Elefant GR, Nóbrega YM, et al. Toxocara spp. seroprevalence in pregnant women in Brasília, Brazil. Rev Soc Bras Med Trop. 2016;49:641–643. doi: 10.1590/0037-8682-0106-2016 [DOI] [PubMed] [Google Scholar]
- 34.Araújo AC, Villela MM, Sena-Lopes A, et al. Seroprevalence of Toxoplasma gondii and Toxocara canis in a human rural population of Southern Rio Grande do Sul. Rev Inst Med Trop Sao Paulo. 2018;60:e28. doi: 10.1590/s1678-9946201860028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Correa CR, Bismarck CM. Toxocariasis: incidence, prevalence and the time serum remains positive in school children from Campinas, SP, Brazil. J Trop Pediatr. 2010;56:215–216. doi: 10.1093/tropej/fmp095 [DOI] [PubMed] [Google Scholar]
- 36.Santarém VA, Leli FN, Rubinsky-Elefant G, Giuffrida R. Protective and risk factors for toxocariasis in children from two different social classes of Brazil. Rev Inst Med Trop Sao Paulo. 2011;53:67–72. doi: 10.1590/s0036-46652011000200002 [DOI] [PubMed] [Google Scholar]
- 37.Fragoso RP, Monteiro MB, Lemos EM, Pereira FE. Anti-Toxocara antibodies detected in children attending elementary school in Vitoria, State of Espírito Santo, Brazil: prevalence and associated factors. Rev Soc Bras Med Trop. 2011;44:461–466. doi: 10.1590/s003786822011000400012 [DOI] [PubMed] [Google Scholar]
- 38.Marchioro AA, Colli CM, Mattia S, et al. Avaliação eosinofílica e soropositividade para anticorpos IgG anti-Toxocara em crianças atendidas pelo Sistema Único de Saúde [Eosinophilic count and seropositivity for IgG antibodies to Toxocara spp. in chidren assisted at the public health service. 52 - Clinical and ultrasound liver impairment in children with toxocariasis]. Rev Paul Pediatr. 2011;29:80–84. doi: 10.1590/S0103-05822011000100013 [DOI] [Google Scholar]
- 39.Mattia S, Colli CM, Adami CM, et al. Seroprevalence of Toxocara infection in children and environmental contamination of urban areas in Paraná State, Brazil. J Helminthol. 2012;86:440–445. doi: 10.1017/S0022149X11000666 [DOI] [PubMed] [Google Scholar]
- 40.Mendonça LR, Figueiredo CA, Esquivel R, et al. Seroprevalence and risk factors for Toxocara infection in children from an urban large setting in Northeast Brazil. Acta Trop. 2013;128:90–95. doi: 10.1016/j.actatropica.2013.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guilherme EV, Marchioro AA, Araujo SM, et al. Toxocariasis in children attending a public health service pneumology unit in Paraná State, Brazil. Rev Inst Med Trop Sao Paulo. 2013;55:189–192. doi: 10.1590/s0036-46652013000300009 [DOI] [PubMed] [Google Scholar]
- 42.Schoenardie ER, Scaini CJ, Brod CS, et al. Seroprevalence of Toxocara infection in children from southern Brazil. J Parasitol. 2013;99:537–539. doi: 10.1645/ge-3182 [DOI] [PubMed] [Google Scholar]
- 43.Oliart-Guzmán H, Delfino BM, Martins AC, et al. Epidemiology and control of child toxocariasis in the western Brazilian Amazon - a population-based study. Am J Trop Med Hyg. 2014;90:670–681. doi: 10.4269/ajtmh.13-0506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cassenote AJ, Lima AR, Pinto Neto JM, Rubinsky-Elefant G. Seroprevalence and modifiable risk factors for Toxocara spp. in Brazilian school children. PLoS Negl Trop Dis. 2014;8:e2830. doi: 10.1371/journal.pntd.0002830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marchioro AA, Colli CM, Ferreira EC, Viol BM, Araújo SM, Falavigna-Guilherme AL. Risk factors associated with toxoplasmosis and toxocariasis in populations of children from nine cities in southern Brazil. J Helminthol. 2015;89:428–432. doi: 10.1017/s0022149x14000212 [DOI] [PubMed] [Google Scholar]
- 46.Silva MB, Amor ALM, Santos LN, et al. Risk factors for Toxocara spp. seroprevalence and its association with atopy and asthma phenotypes in school-age children in a small town and semi-rural areas of Northeast Brazil. Acta Trop. 2017;174:158–164. doi: 10.1016/j.actatropica.2016.04.005 [DOI] [PubMed] [Google Scholar]
- 47.Araújo GMS, Walcher DL, Previtali IF, et al. Frequency of enteroparasitic infections and serum positivity for Toxocara spp. in children from a public day care center in southern Brazil. Braz J Biol. 2020;80:305–310. doi: 10.1590/1519-6984.200952 [DOI] [PubMed] [Google Scholar]
- 48.Santos PC, Telmo PL, Lehmann LM, et al. Risk and other factors associated with toxoplasmosis and toxocariasis in pregnant women from southern Brazil. J Helminthol. 2017a;91:534–538. doi: 10.1017/s0022149x16000481 [DOI] [PubMed] [Google Scholar]
- 49.Santos PC, Telmo PL, Lehmann LM, et al. Frequency of Toxocara spp. antibodies in umbilical cords of newborns attended at the University Hospital in Southern Brazil and factors associated with infection. Acta Trop. 2017b;170:43–47. doi: 10.1016/j.actatropica.2017.02.003 [DOI] [PubMed] [Google Scholar]
- 50.Mattos GT, Santos PC, Telmo PL, Berne ME, Scaini CJ. Human toxocariasis: prevalence and factors associated with biosafety in research laboratories. Am J Trop Med Hyg. 2016;95:1428–1431. doi: 10.4269/ajtmh.16-0196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mendonça LR, Veiga RV, Dattoli VC, et al. Toxocara seropositivity, atopy and wheezing in children living in poor neighbourhoods in urban Latin American. PLoS Negl Trop Dis. 2012;6:e1886. doi: 10.1371/journal.pntd.0001886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carvalho EAA, Lunardi Rocha R, Pinto da silva RA. Comprometimento hepático clínico e ultrassonográfico em crianças com toxocaríase. Rev Med Minas Gerais. 2015;25:523–528. doi: 10.5935/2238-3182.20150117 [DOI] [Google Scholar]
- 53.Zaia MG, Oliveira SR, Castro CA, et al. Toxocara canis and the allergic process. Mem Inst Oswaldo Cruz. 2015;110:726–731. doi: 10.1590/0074-02760150051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silva DCCE, Medeiros YRC, Kametani EI, et al. Loeffler syndrome in the differential diagnosis of severe asthma. Pediatr Pulmonol. 2016;51(S42):S59–S60. doi: 10.1002/ppul.23409 [DOI] [Google Scholar]
- 55.Grama DF, Lescano SZ, Pereira Mota KC, et al. Seroprevalence of Toxocara spp. in children with atopy. Trans R Soc Trop Med Hyg. 2014;108:797–803. doi: 10.1093/trstmh/tru165 [DOI] [PubMed] [Google Scholar]
- 56.Cadore PS, Zhang L, Lemos Lde L, et al. Toxocariasis and childhood asthma: a case-control study. J Asthma. 2016;53:601–606. doi: 10.3109/02770903.2015.1064951 [DOI] [PubMed] [Google Scholar]
- 57.Fialho PM, Corrêa CRS. Toxocaríase, asma e índice de massa corporal em crianças e adolescentes em Campinas-SP, 1996 a 1998. Epidemiol Serv Saúde[Toxocariasis, asthma and body mass index in children and adolescent in Campinas, São Paulo State, Brazil, 1996-1998. 79 - Ultrasonographic findings in ocular toxocariasis]. 2014;23:361–368. doi: 10.5123/S1679-49742014000200018 [DOI] [Google Scholar]
- 58.Fialho PM, Correa CRS, Lescano SZ. Asthma and seroconversion from Toxocara spp. infection: which comes first? Biomed Res Int. 2018;2018:4280792. doi: 10.1155/2018/4280792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fialho PM, Correa CRS, Lescano SZ. Seroprevalence of toxocariasis in children with urticaria: a population-based study. J Trop Pediatr. 2017;63:352–357. doi: 10.1093/tropej/fmw094 [DOI] [PubMed] [Google Scholar]
- 60.Recuero JK, Binda G, Kiszewski AE. Eosinophilic panniculitis associated with toxocariasis in a child. An Bras Dermatol. 2019;94:243–254. doi: 10.1590/abd1806-4841.20198172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Salvador S, Ribeiro R, Winckler MI, Ohlweiler L, Riesgo R. Pediatric neurotoxocariasis with concomitant cerebral, cerebellar, and peripheral nervous system involvement: case report and review of the literature. J Pediatr (Rio J). 2010;86:531–534. doi: 10.2223/jped.2037 [DOI] [PubMed] [Google Scholar]
- 62.Viola GR, Giacomin MF, França CM, Sallum AM, Jacob CM, Silva CA. Chronic polyarthritis as isolated manifestation of toxocariasis. Rev Bras Reumatol. 2016;56:185–187. doi: 10.1016/j.rbre.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 63.Brito T, Chieffi PP, Peres BA, et al. Immunohistochemical detection of toxocaral antigens in human liver biopsies. Int J Surg Pathol. 1994;2:117–124. doi: 10.1177/106689699400200206 [DOI] [Google Scholar]
- 64.Moreira GM, Telmo Pde L, Mendonça M, et al. Human toxocariasis: current advances in diagnostics, treatment, and interventions. Trends Parasitol. 2014;30:456–464. doi: 10.1016/j.pt.2014.07.003 [DOI] [PubMed] [Google Scholar]
- 65.Felicetti CPD, Sinnott F, Monte LG, et al. Diagnostic potential of AntiRTE30 polyclonal antibodies In a blocking Elisa for Toxocara canis detection. J Parasitol. 2019;105:64–68. doi: 10.1645/17-59 [DOI] [PubMed] [Google Scholar]
- 66.Rubinsky-Elefant G, Hoshino-Shimizu S, Jacob CM, Sanchez MC, Ferreira AW. Potential immunological markers for diagnosis and therapeutic assessment of toxocariasis. Rev Inst Med Trop Sao Paulo. 2011;53:61–65. doi: 10.1590/s0036-46652011000200001 [DOI] [PubMed] [Google Scholar]
- 67.Peixoto PL, Nascimento E, Cançado GG, et al. Identification of candidate antigens from adult stages of Toxocara canis for the serodiagnosis of human toxocariasis. Mem Inst Oswaldo Cruz. 2011;106:200–206. doi: 10.1590/s0074-02762011000200014 [DOI] [PubMed] [Google Scholar]
- 68.Carvalho EA, Rocha RL. Visceral larva migrans syndromes associated with toxocariasis: epidemiology, clinical and laboratory aspects of human toxocariasis. Curr Trop Med Rep. 2014;1:74–79. doi: 10.1007/s40475-013-0011-6 [DOI] [Google Scholar]
- 69.Zhan B, Ajmera R, Geiger SM, et al. Identification of immunodominant antigens for the laboratory diagnosis of toxocariasis. Trop Med Int Health. 2015;20:1787–1796. doi: 10.1111/tmi.12607 [DOI] [PubMed] [Google Scholar]
- 70.Roldán WH, Elefant GR, Ferreira AW. Deglycosylation of Toxocara excretory-secretory antigens improves the specificity of the serodiagnosis for human toxocariasis. Parasite Immunol. 2015;37:557–567. doi: 10.1111/pim.12248 [DOI] [PubMed] [Google Scholar]
- 71.Roldán WH, Elefant GR, Ferreira AW. Immunoglobulin M antibodies are not specific for serodiagnosis of human toxocariasis. Parasite Immunol. 2017;39:e12447. doi: 10.1111/pim.12447 [DOI] [PubMed] [Google Scholar]
- 72.Santos LMD, Cerqueira MP, Gaboardi GC, et al. Evaluation of Toxocara canis glycosylated TES produced in Pichia pastoris for immunodiagnosis of human toxocariasis. Braz Arch Biol Technol. 2020;63:e20190148. doi: 10.1590/1678-4324-2020190148 [DOI] [Google Scholar]
- 73.Santos LMD, Magalhães CG, Telmo PL, et al. Sensitivity and specificity of recombinant proteins in Toxocara spp. for serodiagnosis in humans: differences in adult and child populations. PLoS One. 2018;13:e0208991. doi: 10.1371/journal.pone.0208991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sperotto RL, Kremer FS, Aires berne ME, et al. Proteomic analysis of Toxocara canis excretory and secretory (TES) proteins. Mol Biochem Parasitol. 2017;211:39–47. doi: 10.1016/j.molbiopara.2016.09.002 [DOI] [PubMed] [Google Scholar]
- 75.Silva MB, Urrego AJ, Oviedo Y, et al. The somatic proteins of Toxocara canis larvae and excretory-secretory products revealed by proteomics. Vet Parasitol. 2018;259:25–34. doi: 10.1016/j.vetpar.2018.06.015 [DOI] [PubMed] [Google Scholar]
- 76.Rubinsky-Elefant G, Yamamoto JH, Hirata CE, Prestes-Carneiro LE. Toxocariasis: critical analysis of serology in patients attending a public referral center for ophthalmology in Brazil. Jpn J Ophthalmol. 2018;62:77–83. doi: 10.1007/s10384-017-0543-8 [DOI] [PubMed] [Google Scholar]
- 77.Souto FMS, Giampietro BV, Takiuti JT, Campos LMA, Hirata CE, Yamamoto JH. Clinical features of paediatric uveitis at a tertiary referral centre in São Paulo, SP, Brazil. Br J Ophthalmol. 2019;103:636–640. doi: 10.1136/bjophthalmol-2018-312313 [DOI] [PubMed] [Google Scholar]
- 78.Lago A, Andrade R, Muccioli C, Belfort R Jr. Optical coherence tomography in presumed subretinal Toxocara granuloma: case report. Arq Bras Oftalmol. 2006;69:403–405. doi: 10.1590/s0004-27492006000300022 [DOI] [PubMed] [Google Scholar]
- 79.Morais FB, Maciel AL, Arantes TE, Muccioli C, Allemann N. Achados ultrassonográficos em toxocaríase ocular. Arq Bras Oftalmol. 2012;75:43–47. doi: 10.1590/s0004-27492012000100009 [DOI] [PubMed] [Google Scholar]
- 80.Lescano SA, Nakhle MC, Ribeiro MC, Chieffi PP. IgG antibody responses in mice coinfected with Toxocara canis and other helminths or protozoan parasites. Rev Inst Med Trop Sao Paulo. 2012;54:145–152. doi: 10.1590/s0036-46652012000300006 [DOI] [PubMed] [Google Scholar]
- 81.Schoenardie ER, Scaini CJ, Avila LF, et al. Determination of IgG avidity in BALB/c mice experimentally infected with Toxocara canis. Rev Bras Parasitol Vet. 2014;23:403–406. doi: 10.1590/s1984-29612014060 [DOI] [PubMed] [Google Scholar]
- 82.Resende NM, Gazzinelli-Guimarães PH, Barbosa FS, et al. New insights into the immunopathology of early Toxocara canis infection in mice. Parasit Vectors. 2015;8:354. doi: 10.1186/s13071-015-0962-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Raposo RS, Santarém VA, Merigueti YF, et al. Kinetic and avidity of IgY anti-Toxocara antibodies in experimentally infected chickens. Exp Parasitol. 2016;171:33–41. doi: 10.1016/j.exppara.2016.09.009 [DOI] [PubMed] [Google Scholar]
- 84.Bin LLC, Santarém VA, Laposy CB, Rubinsky-Elefant G, Roldán WH, Giuffrida R. Kinetics and avidity of anti-Toxocara antibodies (IgG) in rabbits experimentally infected with Toxocara canis. Bras J Vet Parasitol. 2016;25:99–104. doi: 10.1590/S1984-29612015067 [DOI] [PubMed] [Google Scholar]
- 85.Fonseca GRE, Santos SVD, Chieffi PP, Paula FM, Gryschek RCB, Lescano SAZ. Experimental toxocariasis in BALB/c mice: relationship between parasite inoculum and the IgG immune response. Mem Inst Oswaldo Cruz. 2017;112:382–386. doi: 10.1590/0074-02760160341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rodolpho JMA, Camillo L, Araújo MSS, et al. Robust phenotypic activation of eosinophils during experimental Toxocara canis infection. Front Immunol. 2018;9:64. doi: 10.3389/fimmu.2018.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Santos LMD, de Moura MQ, Azevedo ML, et al. Reactivity of recombinant Toxocara canis TES-30/120 in experimentally infected mice. Parasite Immunol. 2018;40:e12568. doi: 10.1111/pim.12568 [DOI] [PubMed] [Google Scholar]
- 88.Moura MQ, Macedo MRP, Terto W, et al. Detection of Toxocara canis DNA in tissues of experimentally infected mice. Acta Trop. 2018;187:51–56. doi: 10.1016/j.actatropica.2018.07.017 [DOI] [PubMed] [Google Scholar]
- 89.Moura MQ, Terto W, Avila L, et al. Quantification of Toxocara canis DNA by qPCR in mice inoculated with different infective doses. Parasitol Int. 2020;78:102134. doi: 10.1016/j.parint.2020.102134 [DOI] [PubMed] [Google Scholar]
- 90.Garcés LFS, Santiago LF, Santos SPO, et al. Immunogenicity and protection induced by recombinant Toxocara canis proteins in a murine model of toxocariasis. Vaccine. 2020;38:4762–4772. doi: 10.1016/j.vaccine.2020.04.072 [DOI] [PubMed] [Google Scholar]
- 91.Rassier GL, Borsuk S, Pappen F, et al. Toxocara spp. seroprevalence in sheep from southern Brazil. Parasitol Res. 2013;112:3181–3186. doi: 10.1007/s00436-013-3499-8 [DOI] [PubMed] [Google Scholar]
- 92.Dutra GF, Pinto NS, De avila LF, et al. Risk of infection by the consumption of liver of chickens inoculated with low doses of Toxocara canis eggs. Vet Parasitol. 2014;203:87–90. doi: 10.1016/j.vetpar.2014.03.025 [DOI] [PubMed] [Google Scholar]
- 93.Campos-da-silva DR, da Paz JS, Fortunato VR, Beltrame MA, Valli LC, Pereira FE. Natural infection of free-range chickens with the ascarid nematode Toxocara sp. Parasitol Res. 2015;114:4289–4293. doi: 10.1007/s00436-015-4669-7 [DOI] [PubMed] [Google Scholar]
- 94.Oliveira AC, Rubinsky-Elefant G, Merigueti Y, Batista ADS, Santarém VA. Frequency of anti-Toxocara antibodies in broiler chickens in southern Brazil. Rev Bras Parasitol Vet. 2018;27:141–145. doi: 10.1590/s1984-296120180025 [DOI] [PubMed] [Google Scholar]
- 95.Santos LMD, Donassolo RA, Berne ME, et al. The serodiagnostic potential of recombinant proteins TES-30 and TES-120 in an indirect ELISA in the diagnosis of toxocariasis in cattle, horses, and sheep. PLoS One. 2019;14:e0213830. doi: 10.1371/journal.pone.0213830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Magnaval JF, Gligman LT, Dorchies P, Morassin B. Highlights of human toxocariasis. Korean J Parasitol. 2001;39:1–11. doi: 10.3347/kjp.2001.39.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lescano SA, Santos SV, Assis JM, Chieffi PP. Efficacy of nitazoxanide against Toxocara canis: larval recovery and humoral immune response in experimentally infected mice. Rev Inst Med Trop Sao Paulo. 2015;57:337–341. doi: 10.1590/s0036-46652015000400011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mata-Santos T, Pinto NF, Mata-Santos HA, et al. Anthelmintic activity of lapachol, β-lapachone and its derivatives against Toxocara canis larvae. Rev Inst Med Trop Sao Paulo. 2015;57:197–204. doi: 10.1590/s0036-46652015000300003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mata-Santos T, Mata-Santos HA, Carneiro PF, et al. Toxocara canis: anthelmintic activity of quinone derivatives in murine toxocarosis. Parasitology. 2016;143:507–517. doi: 10.1017/s0031182016000068 [DOI] [PubMed] [Google Scholar]
- 100.Sinott FA, Sena-Lopes A, Leal KS, et al. Essential oil from Brazilian Red Propolis exhibits anthelmintic activity against larvae of Toxocara cati. Exp Parasitol. 2019;200:37–41. doi: 10.1016/j.exppara.2019.03.014xz [DOI] [PubMed] [Google Scholar]
- 101.Avila LFC, Da Fonseca JS, Dutra GF, et al. Evaluation of the immunosuppressive effect of cyclophosphamide and dexamethasone in mice with visceral toxocariasis. Parasitol Res. 2012;110:443–447. doi: 10.1007/s00436-011-2510-5 [DOI] [PubMed] [Google Scholar]
- 102.Avila LFC, Conceição FR, Telmo PL, et al. Saccharomyces boulardii reduces infection intensity of mice with toxocariasis. Vet Parasitol. 2012;187:337–340. doi: 10.1016/j.vetpar.2012.01.002 [DOI] [PubMed] [Google Scholar]
- 103.Avila LF, Telmo Pde L, Martins LH, et al. Protective effect of the probiotic Saccharomyces boulardii in Toxocara canis infection is not due to direct action on the larvae. Rev Inst Med Trop Sao Paulo. 2013;55:363–365. doi: 10.1590/s0036-46652013000500012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Avila LFC, De leon PM, De moura MQ, Berne ME, Scaini CJ, Leivas Leite FP. Modulation of IL-12 and IFNγ by probiotic supplementation promotes protection against Toxocara canis infection in mice. Parasite Immunol. 2016;38:326–330. doi: 10.1111/pim.12314 [DOI] [PubMed] [Google Scholar]
- 105.Moura MQ, Da silva terto WD, Jeske ST, et al. Evaluation of the transcription of interleukin-12 in the intestinal mucosa of mice subjected to experimental toxocariasis and supplemented with Saccharomyces boulardii. Vet Parasitol. 2017;242:59–62. doi: 10.1016/j.vetpar.2017.05.012 [DOI] [PubMed] [Google Scholar]
- 106.Walcher DL, Cruz LAX, De lima Telmo P, et al. Lactobacillus rhamnosus reduces parasite load on Toxocara canis experimental infection in mice, but has no effect on the parasite in vitro. Parasitol Res. 2018;117:597–602. doi: 10.1007/s00436-017-5712-7 [DOI] [PubMed] [Google Scholar]
- 107.Braga FR, Ferreira SR, Araújo JV, et al. Predatory activity of Pochonia chlamydosporia fungus on Toxocara (syn. Neoascaris) vitulorum eggs. Trop Anim Health Prod. 2010;42:309–314. doi: 10.1007/s11250-009-9422-8 [DOI] [PubMed] [Google Scholar]
- 108.Carvalho RO, Araújo JV, Braga FR, Araujo JM, Alves CD. Ovicidal activity of Pochonia chlamydosporia and Paecilomyces lilacinus on Toxocara canis eggs. Vet Parasitol. 2010;169:123–127. doi: 10.1016/j.vetpar.2009.12.037 [DOI] [PubMed] [Google Scholar]
- 109.Hiura E, Del Carmen Garcia Lopes A, da Paz JS, et al. Fungi predatory activity on embryonated Toxocara canis eggs inoculated in domestic chickens (Gallus gallus domesticus) and destruction of second stage larvae. Parasitol Res. 2015;114:3301–3308. doi: 10.1007/s00436-015-4553-5 [DOI] [PubMed] [Google Scholar]
- 110.Maia Filho FS, Fonseca AOS, Persici BM, et al. Trichoderma virens as a biocontrol of Toxocara canis: in vivo evaluation. Rev Iberoam Micol. 2017;34:32–35. doi: 10.1016/j.riam.2016.06.004 [DOI] [PubMed] [Google Scholar]
- 111.Maia Filho FS, Nunes Vieira J, Aires Berne ME, et al. Fungal ovicidal activity on Toxocara canis eggs. Rev Iberoam Micol. 2013;30:226–230. doi: 10.1016/j.riam.2012.12.009 [DOI] [PubMed] [Google Scholar]
- 112.Verocai GG, Tavares PV, De A. ribeiro F, Correia TR, Scott FB. Effects of disinfectants on Toxocara canis embryogenesis and larval establishment in mice tissues. Zoonoses Public Health. 2010;57:e213–6. doi: 10.1111/j.1863-2378.2010.01330.x [DOI] [PubMed] [Google Scholar]
- 113.Merigueti Y, da Silva Raposo R, Zampieri BP, de Lima Cerazo LM, Pereira L, Santarém VA. Dispersion and infectivity of Toxocara canis eggs after passage through chicken intestine. Parasitol Res. 2018;117:3481–3486. doi: 10.1007/s00436-018-6045-x [DOI] [PubMed] [Google Scholar]
- 114.von Sohsten AL, Vieira da Silva A, Rubinsky-Elefant G, et al. Chickens bred extensively as sentinels from soil contamination by Toxocara. Exp Parasitol. 2020;211:107852. doi: 10.1016/j.exppara.2020.107852 [DOI] [PubMed] [Google Scholar]
- 115.Aguiar PS, Furtado RD, de Avila LF, et al. Transmammary infection in BALB/c mice with chronic toxocariasis. Parasitol Int. 2015;64:145–147. doi: 10.1016/j.parint.2014.04.010 [DOI] [PubMed] [Google Scholar]
- 116.Telmo PL, Avila LF, Santos CA, et al. Elevated trans-mammary transmission of Toxocara canis larvae in BALB/c mice. Rev Inst Med Trop Sao Paulo. 2015;57:85–87. doi: 10.1590/s0036-46652015000100013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Santos SV, Yazawa santos FH, Zevallos lescano SA, et al. Migration pattern of Toxocara canis larvae in experimentally infected male and female Rattus norvegicus. Rev Soc Bras Med Trop. 2017;50:698–700. doi: 10.1590/0037-8682-0076-2017 [DOI] [PubMed] [Google Scholar]
- 118.Flecher MC, Musso C, Martins IV, Pereira FE. Larval migration of the ascarid nematode Toxocara canis following infection and re-infection in the gerbil Meriones unguiculatus. J Helminthol. 2016;90:569–576. doi: 10.1017/s0022149x15000760 [DOI] [PubMed] [Google Scholar]
- 119.Cox DM, Holland CV. The influence of mouse strain, infective dose and larval burden in the brain on activity in Toxocara-infected mice. J Helminthol. 2001a;75:23–32. doi: 10.1079/JOH200027 [DOI] [PubMed] [Google Scholar]
- 120.Cox DM, Holland CV. Relationship between three intensity levels of Toxocara canis larvae in the brain effects on exploration, anxiety, learning and memory in the murine host. J Helminthol. 2001b;75:33–41. doi: 10.1079/JOH200028 [DOI] [PubMed] [Google Scholar]
- 121.Santos SV, Moura JV, Lescano SA, Castro JM, Ribeiro MC, Chieffi PP. Behavioural changes and muscle strength in Rattus norvegicus experimentally infected with Toxocara cati and T. canis. J Helminthol. 2015;89:465–470. doi: 10.1017/s0022149x14000303 [DOI] [PubMed] [Google Scholar]
- 122.Corrêa FM, Chieffi PP, Lescano SA, Santos SV. Behavioral and memory changes in Mus musculus coinfected by Toxocara canis and Toxoplasma gondii. Rev Inst Med Trop Sao Paulo. 2014;56:353–356. doi: 10.1590/s0036-46652014000400014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Silva AM, Chieffi PP, Da silva WL, et al. The hamster (Mesocricetus auratus) as an experimental model of toxocariasis: histopathological, immunohistochemical, and immunoelectron microscopic findings. Parasitol Res. 2015;114:809–821. doi: 10.1007/s00436-014-4246-5 [DOI] [PubMed] [Google Scholar]