Abstract
Purpose
Patients with fibromyalgia (FM) may demonstrate low cortisol concentrations during diagnostic evaluation. However, it remains unclear whether low cortisol reflects underlying pituitary dysfunction. We aimed to determine if a subset of patients with FM have concomitant secondary adrenal insufficiency (SAI) and growth hormone deficiency (GH).
Patients and Methods
This is a retrospective study of all patients with FM diagnosed with SAI based on abnormal insulin tolerance test (ITT) between June 2002 and August 2019. Patients were excluded if they had other reasons for SAI. Measurements include cortisol and GH during ITT in all patients, and peak cortisol during cosyntropin stimulation test in a subset of patients.
Results
We identified 22 patients (median age of 38 years (range 19–65), 18 (82%) women) diagnosed with secondary AI based on abnormal ITT (peak median cortisol level of 11 mcg/dL (range 5.4–17)). Concomitant GH deficiency was diagnosed in 19 (86%) patients. Cosyntropin stimulation test was performed in 14 (64%) patients and was normal in 11 (79%) (peak cortisol ≥18 mcg/dL). MRI pituitary imaging was performed in 20 patients and showed no significant pituitary pathology. All patients were started on physiologic glucocorticoid replacement, and 5 patients were started on GH replacement. Of the 13 patients with follow-up, 8 (62%) reported symptom improvement after starting treatment.
Conclusion
Patients with FM can have concurrent SAI and GH deficiency. Cosyntropin stimulation test should not be used to exclude SAI in patients with FM. Appropriate glucocorticoid and/or GH replacement may improve symptoms in some patients.
Keywords: hypothalamus-pituitary dysfunction, cosyntropin stimulation test, insulin tolerance test
Introduction
Fibromyalgia (FM) is a common disorder characterized by widespread pain, fatigue, unrefreshing sleep, and multiple other symptoms.1 The exact pathophysiology is unknown, but FM is associated with central sensitization whereby dysfunction of neural pathways causes increased response to mechanical stimuli and amplification of signaling in the central nervous system.2 Alterations in neuroendocrine hormones can also occur, leading to impaired function of the hypothalamus-pituitary axis.3 As such, abnormalities in both cortisol and growth hormone (GH) levels have been observed in patients with FM, and these changes are more pronounced when FM is severe.4,5
Patients with FM have lower plasma and urinary cortisol and lower plasma GH concentrations compared to healthy controls, although these levels are still in the normal range.4–8 They can also have an abnormal hormonal response to physiologic stress or pharmacologic stimulation, including a less robust ACTH response to hypoglycemia and an impaired cortisol and GH surge with acute exercise.6,9 Despite these observations, studies have not been designed to diagnose or treat secondary adrenal insufficiency (SAI) or GH deficiency in patients with FM. Therefore, our objective was threefold: 1) to determine if a subset of patients with FM have SAI and/or GH deficiency using the gold-standard insulin tolerance test (ITT), 2) to see if treating these deficiencies was beneficial, and 3) to assess the response to Cosyntropin stimulation test (CST) in patients with confirmed SAI on ITT.
Patients and Methods
We performed a retrospective review of the electronic medical records of all patients with ITT performed at our institution between July 1997 and August 2019 (n=482). Patients were included in our study if they had a diagnosis of fibromyalgia and demonstrated an abnormal response to insulin during ITT, defined as a peak cortisol < 18 mcg/dL. Patients were excluded if they had other reasons for SAI, including steroid exposure in the last 6 months, daily opioid use ≥ 60 morphine equivalents, or pituitary/hypothalamic pathology. No patients were on oral estrogen. Fibromyalgia was confirmed through the use of clinical notes. Symptoms and exam findings were used to support the diagnosis, but individual widespread pain index or tender point exam scores are not available as this was a retrospective study.
ITT is the gold standard test for the diagnosis of SAI and uses an established protocol to ensure consistency of administering medications, monitoring vital signs, and drawing blood for laboratory testing. This test allows for a concomitant assessment of GH deficiency. In brief, ITT is performed at the Endocrine Testing Center at Mayo Clinic, Rochester, Minnesota by administering a weight-based dose of intravenous insulin after baseline labs are collected. Capillary glucose is checked every 2–5 minutes according to a protocol. The next set of labs is obtained when capillary glucose is < 40 mg/dL and patient has symptoms of hypoglycemia (around 30 minutes). Once blood is collected, the patient is given oral glucose, and a 10% dextrose infusion is started and continued until the glucose is > 70 mg/dL at two time points at least 5 minutes apart. Blood samples are collected at baseline and 30, 60, 90, and 120 minutes after hypoglycemia occurs to measure glucose, cortisol, and GH. Peak cortisol of 18 mcg/dl or more and GH of 4 ng/mL or more are considered normal. We chose a threshold of < 4 ng/dL to diagnose GH deficiency on ITT as guidelines recommended using a cutoff of 3.0 to 5.1 ng/dL.10–12 Cosyntropin testing is performed in the morning (8–10 am) at the Endocrine Testing Center. In brief, 1 or 250 µg Cosyntropin (Mylan Institutional, Amsterdam, Netherlands) is reconstituted with 2 mL 0.9% sodium chloride and administered over 30 seconds. Samples at baseline and 30 and 60 minutes following injection are obtained and analyzed for total cortisol measurements. Peak cortisol of 18 mcg/dl or more are considered normal.
Serum cortisol (normal range 7–22 mcg/dL) and GH (ng/dL) were measured using a competitive binding immunoenzymatic assay (Beckman Coulter, Brea, CA). Corticotropin (ACTH) (normal range 7.2–63 pg/mL) was measured using Roche Elecsys ACTH assay (Los Angeles, CA). Dehydroepiandrosterone sulfate (DHEAS) (µg/dL, normal range varies) was measured by automated chemiluminescent competitive immunoassay on the IMMULITE® 2000 platform (Siemens Diagnostics, Tarrytown, NY). IGF-1 was measured by Q-EXACTIVE HRAM spectrometer (Thermo Scientific, Waltham, MA) with a z-score < −2.0 being abnormal. We summarized continuous data as median values and ranges, and categorical data were summarized as number (%).
Results
Patients
We identified 22 patients (18 women, 82%) with FM who were diagnosed with SAI by abnormal ITT at a median age of 38 years (range 19–65). Fibromyalgia symptoms included fatigue (22, 100%), pain (22, 100%), sleep disturbance (15, 68%), bowel changes (13, 59%), and headache (12, 55%). Symptoms such as dizziness, difficulty concentrating/brain fog, paresthesias, dyspareunia, and urinary symptoms occurred less frequently. All patients had at least 2 symptoms of FM in addition to pain. Most patients had psychiatric disease including depression (4, 18%), anxiety (4, 18%), or both depression and anxiety (6, 27%). Eight patients (36%) underwent formal evaluation in our FM clinic, while others were diagnosed by internal medicine, family medicine, or rheumatology providers.
Baseline Measurements
Baseline laboratory studies showed a median morning cortisol concentration of 8.6 mcg/dL (range 1.1–11), ACTH concentration of 15.5 pg/mL (range 7.7–54), and IGF-1 z-score of −0.94 (range −1.96 to 1.70), Table 1. DHEA-S levels were obtained in 8 patients with a median concentration of 56.9 mcg/dL (range 32.6–206). Morning cortisol concentrations were below the normal range (<7 mcg/dL) in 9 patients (41%) and 10 patients (45%) had an IGF-1 z-score between −1.0 and −2.0, Table 1. Magnetic resonance imaging was performed in 20 patients (91%) and confirmed no significant pituitary pathology.
Table 1.
Summary of Laboratory Data and Dynamic Testing for 22 Patients with Fibromyalgia and Secondary Adrenal Insufficiency
| Demographic Data | Baseline Measurements | CST | Insulin Tolerance Test | Imaging | Therapy and Outcomes | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Age (Years) | Cortisol (mcg/dL) | ACTH (pg/mL) | IGF-1 (Z-score) | Peak Cortisol (mcg/dl) | Peak Cortisol (mcg/dl) | Peak GH (ng/mL) | Negative Pituitary MRI | Treatment | Symptom Improvement |
| F | 25 | 1.1 | 29 | −1.65 | 16 | <1 | 0.62 | Yes | HC | Unknown |
| F | 48 | 4.2 | 7.7 | 1.22 | 22 | 5.4 | 0.05 | Yes | HC | Yes |
| F | 37 | 9.7 | 12 | - | 15 | 5.4 | 0.73 | Yes | HC | No |
| F | 25 | 6.3 | 23 | −0.84 | 19 | 5.7 | 1.64 | No | HC | Yes |
| M | 56 | 6.9 | 26 | 0.67 | 7.2 | 3.83 | Yes | HC | Unknown | |
| F | 22 | 5.7 | 12 | −1.04 | 18 | 7.5 | 12 | Yes | HC | Unknown |
| F | 43 | 9.4 | 54 | 0.13 | 8.1 | 0.22 | Yes | HC, GH | Unknown | |
| F | 61 | 9.1 | 13 | −1.37 | 20 | 8.1 | 0.15 | Yes | HC | Yes |
| F | 39 | 6.5 | 14 | −1.13 | 19 | 8.4 | 0.27 | Yes | HC | Unknown |
| F | 39 | 8.5 | 36 | 1.70 | 8.5 | 3.7 | Yes | HC | Yes | |
| F | 34 | 11 | 19 | −1.96 | 10 | 0.5 | Yes | HC, GH | Unknown | |
| F | 53 | 7.3 | 20 | 0.84 | 20 | 11 | 0.06 | Yes | HC, GH | Yes to HC, no to GH |
| F | 47 | 9.3 | 8 | – | 22 | 11 | 4.9 | Yes | Pred | No |
| F | 21 | 11 | 17 | – | 11 | 0.26 | Yes | HC | Unknown | |
| M | 32 | 11 | 10 | 1.08 | 11 | 0.07 | Yes | HC | Unknown | |
| F | 41 | 4 | 8.6 | −0.17 | 12 | 0.36 | Yes | HC | No | |
| M | 32 | 5.7 | 34 | −0.84 | 26 | 13 | 0.03 | Yes | HC, GH | Unknown |
| F | 65 | 6.8 | 9.6 | −1.82 | 26 | 14 | 0.69 | Yes | HC | Yes |
| F | 39 | 11 | 13 | −1.71 | 14 | 14 | – | Yes | HC | No |
| F | 19 | 9.1 | 9.2 | −1.04 | 23 | 17 | 1.37 | Yes | HC | yes |
| M | 26 | 8.7 | 19 | −1.82 | 29 | 17 | 0.06 | Yes | HC, GH | No to HC, yes to GH |
| F | 25 | 11 | 21 | – | 17 | 3.68 | No | HC | Yes | |
Abbreviations: CST, cosyntropin stimulation test; HC, hydrocortisone; GH, growth hormone; Pred, prednisone; MRI, magnetic resonance imaging.
Cosyntropin Stimulation Test
Cosyntropin stimulation test was performed in 14 (64%) patients, including a low dose 1 mcg test in 2 patients and a standard dose 250 mcg test in 12 patients. The median peak cortisol was 20 mcg/dL (range 14–29). Only three patients (21%) did not reach a peak cortisol of 18 mcg/dL or greater, Table 1.
Insulin Tolerance Test
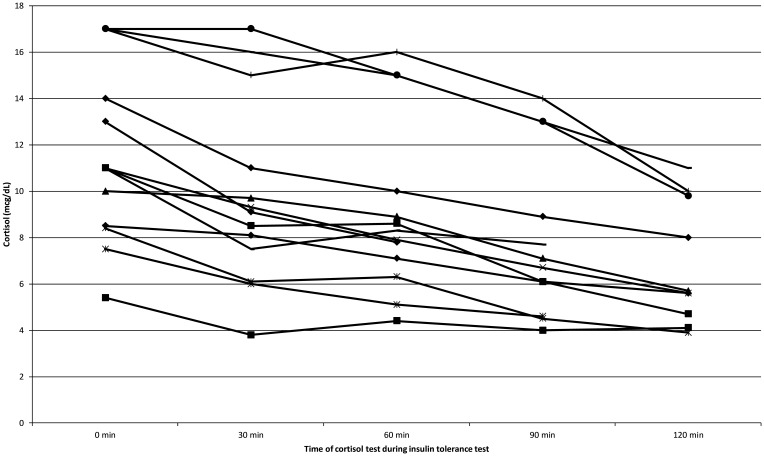
All patients achieved hypoglycemia with a plasma glucose ≤40 mg/dL during ITT. The peak median cortisol level was 11 mcg/dL (range 5.4–17). More than half of the patients (13, 59%) demonstrated a paradoxical response to hypoglycemia where cortisol concentrations decreased with hypoglycemia, and the peak value was observed at the start of the ITT, Figure 1.
Figure 1.
Thirteen patients with paradoxical cortisol response to hypoglycemia during Insulin Tolerance Test.
Nineteen patients (86%) had concurrent GH deficiency, demonstrating a peak median GH concentration of 0.36 ng/mL (range 0.03–3.83). Three patients (14%) had a paradoxical GH response to hypoglycemia (GH was lower during hypoglycemia). Six (27%) patients experienced sustained hypoglycemia despite eating and intravenous dextrose, requiring hydrocortisone therapy during the test. Otherwise, no complications occurred during ITT.
Management
Following the diagnosis of SAI, all patients were started on glucocorticoid replacement (21 patients on hydrocortisone and 1 patient on prednisone). In addition, 5 of 19 (26%) patients with GH deficiency were initiated on GH replacement. Laboratory and clinical follow-up data is available for 13 patients with a median length of follow-up of 1.5 years (range 0.3 to 7.0). Symptoms improved in 8 patients (36%), 5 patients (23%) did not note improvement, and it is unknown how 9 patients (41%) responded to glucocorticoid therapy.
Discussion
In this study, we demonstrate that a subset of patients with FM may be present with concomitant SAI and GH deficiency. The diagnosis of SAI in patients with FM can be challenging. Notably, the majority of these patients had a normal baseline cortisol concentration above 7 mcg/dL, and CST was falsely normal in half of the patients who had this testing. Clinicians need a high index of suspicion for SAI since baseline labs are still in the normal range and may be falsely reassuring. The majority of patients also had concomitant GH deficiency. For patients where SAI is confirmed, we recommend evaluation of growth hormone deficiency with an IGF-1 level and dynamic testing based on clinical suspicion.
We do not propose that all patients with FM should be treated with hydrocortisone and GH replacement therapy. Rather, we suggest a step-wise approach to evaluating patients with FM for SAI, starting with baseline laboratory testing followed by dynamic testing if indicated. When the screening cortisol is low or low-normal (<10 mcg/dL), this should be followed by a repeat morning cortisol and ACTH. A repeat morning cortisol < 10 mcg/dL raises suspicion for SAI and referral to an endocrinologist should be considered.13,14 A cortisol value of < 3 mcg/dL is consistent with SAI and additional testing is often unnecessary.13 For baseline cortisol levels above 3 mcg/dL, additional testing can help determine if a patient has SAI. A cosyntropin stimulation test is helpful if the test is abnormal, but a normal cortisol response does not rule out SAI. A systematic review and meta-analysis of the accuracy of cosyntropin stimulation test to diagnose SAI reported a high specificity but low sensitivity of 64–83%.15 Reported sensitivity of cosyntropin stimulation test in patients with FM is even lower, potentially misdiagnosing up to 50% of cases.16–18 The sensitivity of cosyntropin stimulation test in this study was also quite low at 21.4%. Therefore, CST may be a helpful test to diagnose SAI in patients with FM but cannot reliably rule out disease.
Alternative testing such as an ITT or potentially a metyrapone stimulation test may be needed.16 Both SAI and GH deficiency can be assessed simultaneously with an ITT, but the test is cumbersome and contraindicated in patients with cardiac disease or epilepsy. A metyrapone stimulation test is a good option for patients with FM when there is concern for SAI as it assesses hypothalamic-pituitary function but has fewer contraindications to administration compared to ITT.19 The test is performed by administering a weight-based dose of metyrapone at 11:00 pm followed by a blood draw at 8:00 am the next day to test cortisol and 11-deoxycortisol levels. Metyrapone is generally well tolerated but can cause dizziness, sedation or rarely bone marrow suppression. It can also give patients symptoms of adrenal insufficiency, so administration should be avoided in patients with baseline cortisol levels of less than 4 mcg/dL. Hydrocortisone can be administered immediately after the blood draws if adrenal insufficiency symptoms are present.
Our study has potential clinical implications. First, this study demonstrates that at least some patients with FM may have concomitant hypothalamic-pituitary dysfunction that is difficult to diagnose and may remain untreated. As symptoms of FM overlap with those of SAI, clinical history and physical exam may not be as revealing as in other causes of SAI. Secondly, a subset of patients with FM and hypothalamic-pituitary dysfunction in our study reported improvement in their symptoms with treatment. Several trials and studies have tested the use of hydrocortisone or GH in FM, but these often included patients who did not have a documented endocrine deficiency or represent clinical trials without published results.20–22 Prospective studies are needed to fully understand the objective change in symptoms and functionality of patients with FM and treat endocrine hormone deficiencies. Finally, several studies demonstrated a greater degree of endocrine dysfunction is present in severe FM and improvement in endocrine dysfunction and resiliency of the hypothalamic-pituitary axis can occur with intensive treatment of FM.5,23 The exact cause and effect relationship between endocrine dysfunction and FM remains unclear. However, intense treatment of FM with cognitive behavioral therapy and medications may lead to recovery from endocrine dysfunction and should be further assessed in prospective studies.
The strengths of this study included diagnosing SAI and GH deficiency based on ITT, which is considered the gold standard test, uniform assessment under a single protocol in the Endocrine Testing Center, and availability of detailed medical records in regard to symptoms. Limitations include those inherent to the retrospective design with possible selection, information, and lack of follow-up bias. Details about the diagnosis of fibromyalgia are not available, and these patients were diagnosed by a variety of providers with various levels of training. Objective assessment of symptom change with starting endocrine hormone replacement was not possible and relied on documentation in the medical record. Not all patients had cosyntropin stimulation test and several baseline measures, and not all had followed up to assess for symptom improvement. Our institution is also likely to see more severe cases of FM, so these patients may not be representative of all patients with FM. While we demonstrated that patients with FM can develop endocrine hormonal deficiencies, our study was not designed to determine the cause of these deficiencies. Alterations in the function of both the hypothalamus and pituitary have been implicated, but the exact mechanism is poorly understood.4,7,9,24
In conclusion, patients with FM can have co-existing SAI and GH deficiency as defined by insulin-induced hypoglycemia. These patients can have normal cortisol and IGF-1 z-scores, so a high level of suspicion is needed to make this diagnosis. An abnormal peak cortisol during cosyntropin stimulation test can be used to diagnose SAI in patients with FM, but a normal result does not rule out SAI. Replacing the underlying deficiency improved symptoms in some patients demonstrating certain FM symptoms may overlap with symptoms of SAI and GH deficiency. Future studies are needed to understand the relationship between endocrine hormone deficiencies, severity of FM symptoms, and changes in these symptoms with endocrine hormone replacement.
Ethics Statement
The Institutional Review Board at Mayo Clinic, Rochester, MN approved this study. As this was a retrospective study, direct consent was not obtained for participants in this study. However, any patient who signed a form denying use of their protected health information for research was not included in the study as dictated by Minnesota state law. Guidelines outlined by the Declaration of Helsinki were followed.
Acknowledgments
This research was supported partially by the James A. Ruppe Career Development Award in Endocrinology, Catalyst Award for Advancing in Academics from Mayo Clinic, and the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health USA under the award K23 DK121888. The views expressed are those of the authors(s) and not necessarily those of the National Institutes of Health USA. The abstract of this paper was presented at the Endocrine Society Conference 2020 as a poster presentation with interim findings. The poster’s abstract was published in the Journal of the Endocrine Society, Volume 4, Issue Supplement_1, April-May 2020, SUN-307, https://doi.org/10.1210/jendso/bvaa046.922.
Disclosure
Irina Bancos reports advisory board participation/consulting with Corcept Therapeutics, Sparrow Pharmaceutics, Strongbridge, Adrenas Pharmaceutics, and HRA Pharma outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Vincent A, Lahr BD, Wolfe F, et al. Prevalence of fibromyalgia: a population-based study in Olmsted County, Minnesota, utilizing the Rochester Epidemiology Project. Arthritis Care Res (Hoboken). 2013;65(5):786–792. doi: 10.1002/acr.21896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chinn S, Caldwell W, Gritsenko K. Fibromyalgia pathogenesis and treatment options update. Curr Pain Headache Rep. 2016;20(4):25. doi: 10.1007/s11916-016-0556-x [DOI] [PubMed] [Google Scholar]
- 3.Crofford LJ, Engleberg NC, Demitrack MA. Neurohormonal perturbations in fibromyalgia. Baillieres Clin Rheumatol. 1996;10(2):365–378. doi: 10.1016/S0950-3579(96)80022-7 [DOI] [PubMed] [Google Scholar]
- 4.Crofford LJ, Pillemer SR, Kalogeras KT, et al. Hypothalamic–pituitary–adrenal axis perturbations in patients with fibromyalgia. Arthritis Rheum. 1994;37(11):1583–1592. doi: 10.1002/art.1780371105 [DOI] [PubMed] [Google Scholar]
- 5.Gur A, Cevik R, Sarac AJ, Colpan L, Em S. Hypothalamic-pituitary-gonadal axis and cortisol in young women with primary fibromyalgia: the potential roles of depression, fatigue, and sleep disturbance in the occurrence of hypocortisolism. Ann Rheum Dis. 2004;63(11):1504–1506. doi: 10.1136/ard.2003.014969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paiva ES, Deodhar A, Jones KD, Bennett R. Impaired growth hormone secretion in fibromyalgia patients: evidence for augmented hypothalamic somatostatin tone. Arthritis Rheum. 2002;46(5):1344–1350. doi: 10.1002/art.10209 [DOI] [PubMed] [Google Scholar]
- 7.Leal-Cerro A, Povedano J, Astorga R, et al. The growth hormone (GH)-releasing hormone-GH-insulin-like growth factor-1 axis in patients with fibromyalgia syndrome. J Clin Endocrinol Metab. 1999;84(9):3378–3381. doi: 10.1210/jcem.84.9.5982 [DOI] [PubMed] [Google Scholar]
- 8.Buchwald D, Umali J, Stene M. Insulin-like growth factor-I (somatomedin C) levels in chronic fatigue syndrome and fibromyalgia. J Rheumatol. 1996;23(4):739–742. [PubMed] [Google Scholar]
- 9.Adler GK, Kinsley BT, Hurwitz S, Mossey CJ, Goldenberg DL. Reduced hypothalamic-pituitary and sympathoadrenal responses to hypoglycemia in women with fibromyalgia syndrome. Am J Med. 1999;106(5):534–543. doi: 10.1016/S0002-9343(99)00074-1 [DOI] [PubMed] [Google Scholar]
- 10.Ho KKY. Consensus guidelines for the diagnosis and treatment of adults with GH deficiency II: a statement of the GH Research Society in association with the European Society for Pediatric Endocrinology, Lawson Wilkins Society, European Society of Endocrinology, Japan Endocrine Society, and Endocrine Society of Australia.. Eur J Endocrinol. 2007;157(6):695–700. doi: 10.1530/EJE-07-0631 [DOI] [PubMed] [Google Scholar]
- 11.Yuen KCJ, Biller BMK, Radovick S, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of growth hormone deficiency in adults and patients transitioning from pediatric to adult care. Endocr Pract. 2019;25(11):1191–1232. [DOI] [PubMed] [Google Scholar]
- 12.Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Vance ML, Endocrine S. Evaluation and treatment of adult growth hormone deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(6):1587–1609. doi: 10.1210/jc.2011-0179 [DOI] [PubMed] [Google Scholar]
- 13.Hagg E, Asplund K, Lithner F. Value of basal plasma cortisol assays in the assessment of pituitary-adrenal insufficiency. Clin Endocrinol (Oxf). 1987;26(2):221–226. doi: 10.1111/j.1365-2265.1987.tb00780.x [DOI] [PubMed] [Google Scholar]
- 14.Deutschbein T, Unger N, Mann K, Petersenn S. Diagnosis of secondary adrenal insufficiency in patients with hypothalamic-pituitary disease: comparison between serum and salivary cortisol during the high-dose short synacthen test. Eur J Endocrinol. 2009;160(1):9–16. doi: 10.1530/EJE-08-0600 [DOI] [PubMed] [Google Scholar]
- 15.Ospina NS, Al Nofal A, Bancos I, et al. ACTH stimulation tests for the diagnosis of adrenal insufficiency: systematic review and meta-analysis. J Clin Endocrinol Metab. 2016;101(2):427–434. doi: 10.1210/jc.2015-1700 [DOI] [PubMed] [Google Scholar]
- 16.Calis M, Gokce C, Ates F, et al. Investigation of the hypothalamo-pituitary-adrenal axis (HPA) by 1 microg ACTH test and metyrapone test in patients with primary fibromyalgia syndrome. J Endocrinol Invest. 2004;27(1):42–46. doi: 10.1007/BF03350909 [DOI] [PubMed] [Google Scholar]
- 17.Kirnap M, Colak R, Eser C, Ozsoy O, Tutus A, Kelestimur F. A comparison between low-dose (1 microg), standard-dose (250 microg) ACTH stimulation tests and insulin tolerance test in the evaluation of hypothalamo-pituitary-adrenal axis in primary fibromyalgia syndrome. Clin Endocrinol (Oxf). 2001;55(4):455–459. doi: 10.1046/j.1365-2265.2001.01373.x [DOI] [PubMed] [Google Scholar]
- 18.Holtorf K. Diagnosis and treatment of Hypothalamic-Pituitary-Adrenal (HPA) axis dysfunction in patients with Chronic Fatigue Syndrome (CFS) and Fibromyalgia (FM). J Chronic Fatigue Syndr. 2008;14(3). [Google Scholar]
- 19.Grossman AB. Clinical Review#: the diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. 2010;95(11):4855–4863. doi: 10.1210/jc.2010-0982 [DOI] [PubMed] [Google Scholar]
- 20.Cuatrecasas G, Riudavets C, Guell MA, Nadal A. Growth hormone as concomitant treatment in severe fibromyalgia associated with low IGF-1 serum levels. A pilot study. BMC Musculoskelet Disord. 2007;8(1):119. doi: 10.1186/1471-2474-8-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maximilians L. Efficacy study of low dose hydrocortisone treatment for fibromyalgia; 2005. Accessed 1/19/2020.
- 22.KGaA M. Growth hormone in neuroendocrine dysfunction with severe fibromyalgia syndrome; 2009. Accessed 1/19/2020.
- 23.Bonifazi M, Suman AL, Cambiaggi C, et al. Changes in salivary cortisol and corticosteroid receptor-alpha mRNA expression following a 3-week multidisciplinary treatment program in patients with fibromyalgia. Psychoneuroendocrinology. 2006;31(9):1076–1086. doi: 10.1016/j.psyneuen.2006.06.008 [DOI] [PubMed] [Google Scholar]
- 24.Riedel W, Schlapp U, Leck S, Netter P, Neeck G. Blunted ACTH and cortisol responses to systemic injection of corticotropin-releasing hormone (CRH) in fibromyalgia: role of somatostatin and CRH-binding protein. Ann N Y Acad Sci. 2002;966(1):483–490. doi: 10.1111/j.1749-6632.2002.tb04251.x [DOI] [PubMed] [Google Scholar]