Abstract
Neurofibromatosis type 1 (NF1) is an incurable genetic condition that frequently includes the development of plexiform neurofibromas (PNs) in patients. A systematic literature review was conducted to identify data on the natural history, disease burden, and treatment patterns among patients diagnosed with NF1 and PN, as well as to identify evidence gaps in these areas. MEDLINE and MEDLINE In-Process, Embase, and Cochrane Library Searches were searched using predefined terms. Potential references underwent two phases of screening by two independent researchers. A total of 39 references focusing on populations of patients with both NF1 and PN were included in this review. The wide range of PN-related complications creates a substantial quality-of-life (QOL) burden for patients, including pain, social functioning, physical function impact, stigma, and emotional distress. The severe burden of NF1 with PN on the QOL of patients demonstrates the high unmet need for an effective treatment option that can reduce tumor burden and improve QOL. The heterogeneity of measurement tools used to evaluate QOL and the gap in data evaluating the health economic burden of PN should be the focus of future research.
Keywords: neurofibromatosis type 1, plexiform neurofibromas, quality of life, natural history, review
Introduction
Neurofibromatosis type 1 (NF1) is an incurable genetic condition that affects 1 in 3000 newborns worldwide.1 Neurofibromatosis type 1 is caused by a germline NF1 tumor suppressor pathogenic or likely pathogenic variant.2–4 The severity of signs and symptoms associated with NF1 can be highly variable and may include widespread manifestations across different body systems.1 Diagnostic criteria include the presence of at least six café-au-lait macules, optic pathway gliomas, or bony dysplasia.5,6 Patients with NF1 develop malignant gliomas and neurofibromas in addition to behavioral, cognitive, motor, and pigmentary abnormalities.4
Plexiform neurofibromas (PNs) are benign tumors of peripheral nerves that are distinguished by a plexiform growth pattern.7 Based on the National Neurofibromatosis Foundation International Database that collects information on NF1, approximately 20% of children aged 0–19 develop PNs.8 Plexiform neurofibromas cause significant morbidity because they are diffuse, grow alongside nerves, and may involve multiple nerve branches and plexi. The growth rate of PNs is unpredictable and there may be periods of rapid growth, followed by periods of relative inactivity.6 Common complications of NF1 with PN include pain, difficulty with motor functioning, and motor deficit or weakness.9,10 Rare PN-related comorbidities include vision reduction, bowel and bladder dysfunction, and obstructive sleep apnea.11 Potentially life-threatening complications associated with PNs include their transformation to malignant peripheral nerve sheath tumors (MPNSTs)12 and airway or spinal cord compression.13 Patients with NF1 have an 8% to 13% cumulative lifetime risk of developing MPNSTs. Malignant peripheral nerve sheath tumors are aggressive, deadly tumors that have a high rate of metastases with a survival typically less than five years. More than 80% of patients presenting with an MPNST had a coexisting or preexisting benign neurofibroma, which suggests that benign PNs can transform into MPNSTs.4 Some patients undergo surgical resection in an attempt to remove or reduce tumor volume. In many patients, complete resection may be impossible without causing significant damage because of encasement of vital structures, invasiveness, or extensiveness of tumor growth.14 In addition, the outcomes from surgery are questionable, as tumors may regrow following resection.15 Effective drug therapies are not widely available, although the mitogen-activated protein kinase (MEK) 1/2 inhibitor treatment selumetinib recently has been approved by the Food and Drug Administration (FDA) for the treatment of pediatric patients 2 years of age and older with NF1 who have symptomatic, inoperable PN.16
A systematic literature review (SLR) was conducted to identify data on the natural history, disease burden, and treatment patterns among patients diagnosed with NF1 with PN, as well as to identify evidence gaps in these areas.
Materials and Methods
This SLR was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.17 MEDLINE and MEDLINE In-Process (using the PubMed platform), Embase (using the Dialog Platform), and the Cochrane Library were searched using predefined terms (Tables S1-S5, Supplementary Material) for publications in English. Articles related to health care resource use and costs, practice patterns and guidelines, and treatments were restricted to publication dates ranging from January 1, 2009, to May 31, 2019. Articles related to identification of disease overview information were restricted to publication dates ranging from January 1, 2014, to May 31, 2019, to identify the most recent and relevant information on this topic. A search was conducted for key patient registries and associations. Conference proceedings from the American Society of Clinical Oncology and the European Society for Medical Oncology were included in the database searches and followed the same restrictions. Reference lists of relevant SLRs also were searched for further studies of interest with no time frame restriction on publication dates. Criteria for study inclusion are presented in Table 1. An assessment of study quality was not conducted due to the types of studies that were included.
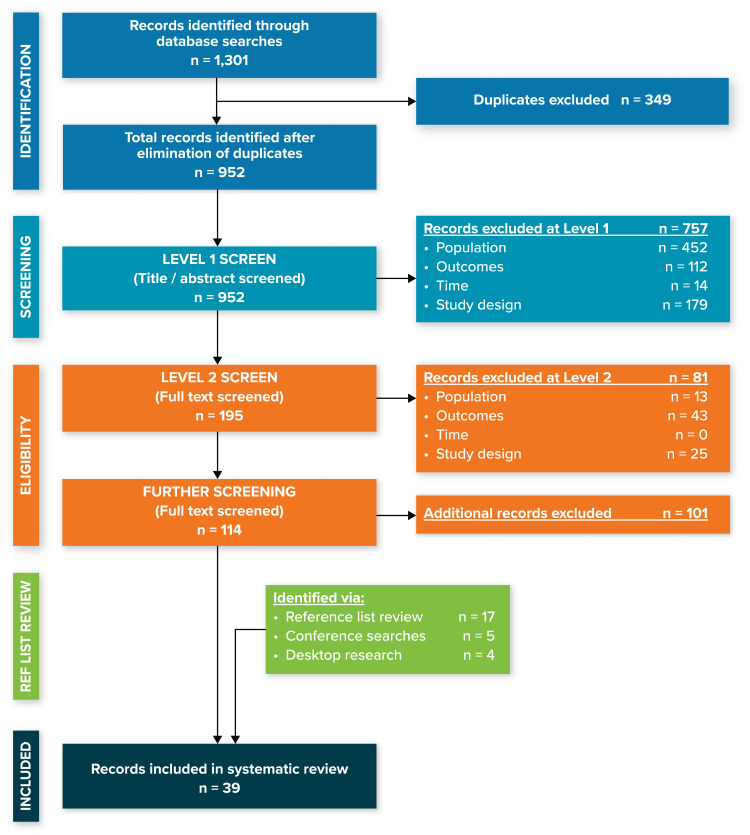
Figure 1.
PRISMA diagram.
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Results
The search strategy identified 952 unique articles and conference abstracts. These references underwent two phases of screening by two independent researchers (Figure 1). A total of 13 of these 952 references were selected for inclusion, and an additional 26 references were identified via a review of the reference lists of SLRs (17 references), conference searches (5 references), and desktop research (4 references). A total of 39 references, focusing on populations of patients with both NF1 and PN, were included in this review.
Prevalence and Incidence
The birth incidence of NF1 in Europe is 1 in 2500–3000, while the prevalence is 1 in 3000–5000.18–20 An older study estimated that 20% of patients (n = 1728) in an international NF1 disease registry with NF1 had PN;8 however, this is likely an underestimate because of the lack of advanced imaging tools that might be necessary to detect PNs that are internal, benign, or asymptomatic.21,22 Mautner et al21 and Nguyen et al22 conducted studies in Germany, using whole-body magnetic resonance imaging (WBMRI) to determine the frequency of PNs in patient with NF1. Mautner et al21 observed 56% (22/39) of patients (median age: 30.5 years) with NF1 had PNs, while Nguyen et al22 found 57% (37/65) of children (median age: 11.5 years) with NF1 had PNs. This apparent increase could be due to improved technology for detection of internal PNs.
Natural History
Characterization of Plexiform Neurofibromas in Neurofibromatosis Type 1
A medical record review of 520 patients with NF1 and PN in the United States (US) found that 58% of patients with NF1 and PN were male and 80.5% of these patients were white.12 The study did not provide further data explaining the demographic composition. The median age of patients in this study who received a diagnosis of NF1 with PN was 5.5 years (range: 0–18 years). The age at symptomatic PN identification had a bimodal distribution, with most identifications occurring during early childhood (birth-3 years) and adolescence (11–18 years). Symptomatic PNs were most often found in the head and neck, followed by the extremities and the trunk. The majority of symptomatic PNs found in the head and extremities were recognized before patients were 6 years of age. Symptomatic mediastinal PNs were rare and only recognized during infancy. Patients in this study with more than four tumors were more likely to receive a diagnosis before age 3 years.
Plexiform neurofibromas can grow invasively out from and alongside the nerve with resulting complications of disfiguring lesions, erosion of bone, and displacement to organs.4 Additionally, there may be an inverse relationship between tumor volume and height (ie, greater tumor volume is associated with shorter height).23 A prospective study conducted in Germany between 2003 and 2009 in 65 children with PNs found that the ratio of symptomatic to asymptomatic PNs varied across body regions and that symptomatic PNs were significantly larger than asymptomatic ones.22 However, in this study, symptomatic or asymptomatic PNs appeared more closely aligned with specific regions of the body than with the size of the PN. For example, all PNs on the arms were symptomatic and all PNs on the abdomen were asymptomatic. Most PNs in the thorax and paravertebral regions and the legs were asymptomatic, and most PNs on the head/neck were symptomatic.
Plexiform Neurofibromas Complications or Manifestations
A retrospective review of 150 patients with 159 PNs treated in a United Kingdom neurofibromatosis clinic in 2017–2018 found a broad range of complications.24 Baseline complications of patients included pain (35%), disfigurement (30%), growth (13%), neurological deficit (13%), and hemorrhage (6%). Of the patients with symptomatic PNs, 26% were referred for debulking surgery and 9% had biopsy only. Four new MPNSTs were observed in the patient population.
Kim et al13 evaluated the pre-enrollment characteristics of pediatric patients with NF1 and PN who enrolled in early phase investigational drug treatment trials at the National Institutes of Health Clinical Center between August 1996 and July 2007, to characterize PN disease burden and associated complications in these patients. The 59 enrolled patients had PNs that were large, centrally located, and unresectable. The patients had a number of manifestations of NF1 with PN, including pain (53%), cognitive deficits (51%), skeletal complications (39%), history of other tumors (18%), and hypertension (8%). Some patients had a PN associated with a potentially life-threatening complication, such as airway compression (4/59 or 7%) or spinal cord compression (14/59 or 24%), major physical deformity (43/59 or 73%), or loss of function (27/59 or 46%). Caution should be used in generalizing these manifestations or complications to all children with NF1 and PN, as they represented a clinical trial population.
Airway compression and spontaneous bleeding are lethal complications of NF1 with PN.12 A medical record review conducted at the Neurofibromatosis Center at Cincinnati Children’s Hospital between 1997 and 2007 in 520 patients with NF1 and symptomatic PN assessed clinical characteristics and the disease impact on mortality.12 Malignant peripheral nerve sheath tumors were associated with a known symptomatic PN in all cases. Patients with NF1 and symptomatic PN had a higher mortality rate (5/154 or 3.2%) than patients with NF1 without PN or with asymptomatic PN (2/366 or 0.5%; P = 0.024). In the study, MPNSTs were the cause of death in 3 of 154 patients with symptomatic PNs. Reasons for mortality in patients with asymptomatic PNs included airway compression in one pediatric patient (age 3 years) and hypovolemic shock from a large hemothorax in an 18 year old.
Plexiform Neurofibromas Growth
Dombi et al25 conducted a longitudinal study in the US to analyze changes in PN volume in relation to age and body growth in children and young adults with NF1 and inoperable, symptomatic, or progressive PNs, using a sensitive, automated method of volumetric magnetic resonance imaging (MRI) analysis (Table 1,Table 2). The 49 included patients were aged 25 years and younger (median age: 8.3 years) and had entered into a natural history study or were in treatment trials and had volumetric MRI over ≥16 months. A total of 69% of patients experienced an increase of more than 20% of PN volume during the median 34-month observation period (range: 18–70 months). The PN volume increased more rapidly than body weight over time (P = 0.026). There was no relationship noted between the PN growth rate and the site of the PN or the volume of the PN at baseline.
Table 1.
Criteria for Study Inclusion
| Inclusion Criteria | |
|---|---|
| Populations | Patients with NF1 with PN |
| Patients with NF1 without PN | |
| Outcomes | Disease overview |
| Demographics | |
| Clinical characteristics | |
| Disease progression/natural history | |
| Disease complications | |
| Impact on patient functioning and health-related quality of life | |
| Mortality/survival | |
| Treatment | |
| Practice patterns/treatment patterns | |
| Medications | |
| Surgery | |
| Resource-use estimates | |
| Drug utilization | |
| Outpatient and emergency department visits | |
| Imaging costs (eg, MRI, CT, PET) | |
| Number of hospitalizations and length of stay | |
| Management of drug-related adverse events | |
| Direct costs | |
| Medication costs | |
| Outpatient visit costs | |
| Hospitalization costs | |
| Emergency department visits | |
| Laboratory costs | |
| Diagnostic costs | |
| Physician costs | |
| Costs of managing drug-related adverse events | |
| Indirect or other costs of interest, including the following | |
| Productivity loss of patient (wages lost from absences) | |
| Out-of-pocket expenses | |
| Travel costs for patient | |
| Caregiver burden | |
| Time | The database searches will be performed for the past 10 years, except for disease overview, which will be for the past 5 years |
| Study design | Prospective (including clinical trials), retrospective, cross-sectional, or other studies |
| Systematic literature reviews | |
| Other | Electronic database searches will be limited to articles published in the English language |
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging; NF1, neurofibromatosis type 1; PET, positron emission tomography; PN, plexiform neurofibroma.
Table 2.
Summaries of Plexiform Neurofibromas Growth
| Reference | Study Details | Population | Follow-Up Period | Key Findings |
|---|---|---|---|---|
| Dombi et al25 | US longitudinal study to analyze changes in PN volume in relation to age and body growth in children and young adults with NF1 and inoperable, symptomatic, or progressive PNs, using a sensitive, automated method of volumetric MRI analysis | N = 49 Median age: 8.3 years |
Median: 34 months |
|
| Nguyen et al26 | German retrospective study to assess internal PNs using volumetric WBMRI | N = 171 Median age: 28.6 years |
Median 2.2 years |
|
| Gross et al11 | Retrospective review of patients enrolled in the National Cancer Institute Natural History Study of Patients with Neurofibromatosis Type 1 (NCT00924196) using volumetric MRI analysis | N = 41 Median age: 8 years |
At least yearly until age 18, then every 1–3 years |
|
| Tucker et al27 | German study to characterize the growth of PNs in patients with NF1 between 1990 and 2006 using serial MRIs | N = 34 Mean age: 10 years |
Median: 6.0 years |
|
| Dagalakis et al28 | Retrospective study using data from the National Cancer Institute NF1 Natural History Study to evaluate the relationship between pubertal progression and change in PN burden over time in pediatric and young adult patients with NF1 and PN | N = 41 Actively progressing through puberty (n = 16); age range: 7–20 years Peripubertal (n = 25); age range: 6–11 years and 17–33 years |
Mean: 20 months |
|
Abbreviations: MRI, magnetic resonance imaging; NF1, neurofibromatosis type 1; PN, plexiform neurofibroma; WBMRI, whole-body magnetic resonance imaging.
Nguyen et al26 conducted a retrospective study in Germany to assess internal PNs by using volumetric WBMRI in 171 unselected patients of various ages with NF1 who were followed for tumor growth over a median of 2.2 years. A total of 71 of the 171 patients (41.5%) had internal PNs. The median growth rate in whole-body tumor burden attributable to internal PNs, expressed as a percentage of the total volume of tumors measured in the patient on first examination, was 3.7% per year, with a range of –13.4% to 111.1% per year. This growth rate of 3.7% per year correlated with larger whole-body tumor volume (P < 0.001) and younger age (P = 0.004). A total of 27 of the 200 individual tumors (13.5%) that were followed longitudinally by MRI increased in size by more than 20% per year, on average. The majority of these tumors (19/27) were found in pediatric patients (<18 years old) and represented 29% (19/66) of the tumors found in the pediatric age group.
A retrospective review11 of patients enrolled in the National Cancer Institute Natural History Study of Patients with Neurofibromatosis Type 1 (NCT00924196) used volumetric MRI analysis to evaluate 57 PNs in 41 patients with a median age of 8 years (range: 3–25 years). Of the 57 PNs, 49 (86%) had a more than a 20% increase in tumor volume from baseline to maximum assessment. There was a median 108.9% change in PN volume between baseline and maximum assessment and an observed 15.9% per year median growth rate. Young patients (3–5 years) had a median PN growth rate of 35.1% per year compared with 13.1% per year for patients aged 11–25 years. Gross et al11 also found that rapid growth appeared to be associated with increased pain. Patients with NF1 and PN who reported increased use of pain medication experienced 21% growth in PNs per year, while those with NF1 and PN who did not report increased use of pain medication experienced only 13% growth in PNs per year.
Tucker et al27 characterized the growth of PNs using serial MRIs for 44 tumors in 34 patients (19 males and 15 females) with NF1 between 1990 and 2006 in Hamburg, Germany. The mean age at first examination was 17.3 years (standard deviation: 12.2; range: 1–45) for males and 13.5 years (standard deviation: 13.0; range: 1–47) for females. Follow-up was a median 6.0 years with a mean 3.0-year interval between scans. Of the 14 symptomatic PNs, four were superficial and displacing, three were superficial and invasive, two were deep and displacing, and five were deep and invasive. Superficial tumors grew significantly more quickly than deep tumors (P = 0.034). The growth rate of tumors in patients younger than 10 years at initial examination (0.7 cm2/year) was significantly greater than that of tumors in patients older than 10 years at initial examination (0.03 cm2/year; P = 0.014). Only 7 (30%) of the 23 PNs identified in the 18 patients aged younger than 10 years were symptomatic, which included 4 of 9 (44%) rapidly progressing tumors. This observation suggests that each tumor should be followed separately and that one tumor cannot be used to represent the growth rate of all tumors in an individual.
Dagalakis et al28 evaluated the relationship between pubertal progression and change in PN burden over time in pediatric and young adult patients with NF1 and PN in a retrospective study using data from the National Cancer Institute NF1 Natural History Study. Patients were divided into two groups, based on whether they were actively progressing through puberty (n = 16) or were peripubertal (n = 25) and followed for an average of 20 months. There were no statistically significant differences in tumor burden change over time between the groups (P = 0.31) or in peripubertal and pubertal PN growth rates (P = 0.90). These findings indicate that PN growth proceeds at a similar pace before, during, and after puberty.
Disease Burden
Quality-of-Life Burden
Four studies reviewed the quality of life (QOL) of persons with NF1 with PN in the US using the Patient-Reported Outcomes Measurement Information System (PROMIS) module, the eight-item Quality of Life in Neurological Disorders (Neuro-QOL), the Impact of Pediatric Illness Scale, the Numeric Rating Scale, the Brief Pain Inventory Pain Interference Scale, or the NF1 Pediatric Quality of Life Inventory (PedsQL) Scale (Table 3).29–32 Lai et al29 developed a model to conceptualize the experience of patients with NF1 and PN using information from interviews conducted with 16 adults (≥18 years old) with NF1 and PN, 15 children and adolescents with NF1 and PN, and 17 parents of the children and adolescents with NF1 and PN. The conceptual framework identified five domains that represented the most important symptoms or concerns for patients with NF1 with PN: Pain, Social functioning, Physical function impact, Stigma, and Emotional distress. The most frequently reported concerns for patients across all age groups included pain, appearance/disfigurement, social activity/role participation, stigma, and anxiety. The parents of patients were primarily concerned with physical functioning, followed by pain, social activity/role participation, appearance/disfigurement, and social relationships. Although the types of identified concerns were similar across age groups, there was variability in the level of importance placed on different concerns. The knowledge and responsibility of disease management in adult patients was associated with an increase in anxiety. Stigma related to body image concerns also affected adolescent and adult patients more than pediatric patients.
Table 3.
Summary of Quality of Life Burden Data
| Reference | Study Design | Population | PRO Instrument(s) | Key Findings |
|---|---|---|---|---|
| Lai et al29 | Concept elicitation interviews to develop a conceptual model of NF1 with PN experience | Adults with NF1 with PN (n=16) Children and adolescents with NF1 with PN (n=15) Parents of children with NF1 with PN (n=17) |
None |
|
| Lai et al30 | Qualitative study | Children with NF1 with PN n =140 |
PROMIS; Neuro-QOL |
|
| Rosser32 | Prospective study of patients who enrolled in NF Clinical Trials Consortium PN treatment trials | Adolescents with NF1 with PN n=38 |
Numeric Rating Scale; Brief Pain Inventory; Pain Interference Scale; NF1 PedsQL Scale |
|
| Wolters et al31 | Prospective natural history study | Children and adolescents with NF1 with PN n=41 |
Impact of Pediatric Illness Scale |
|
Abbreviations: Neuro-QOL, Quality of Life in Neurological Disorders; NF1, neurofibromatosis type 1; PedsQL, Pediatric Quality of Life Inventory; PN, plexiform neurofibroma; PRO, patient-reported outcome; PROMIS, Patient-Reported Outcomes Measurement Information System; QOL, quality of life.
A qualitative study used the pediatric PROMIS module and the eight-item Neuro-QOL to evaluate QOL in 140 children with NF1 with PN.30 Children with NF1 and PN reported significantly worse scores than the population norms on all domains except fatigue and pain interference. Children with NF1 with PN who had at least one family member with a diagnosis of NF1 and those with pain reported significantly worse symptoms and functioning on all QOL domains. Male children also reported experiencing significantly worse symptoms (ie, pain interference, stigma, meaning and purpose, mobility function, and upper extremity function) than female children. The most important concerns reported by parents regarding their children were sadness (29.5% always or often), physical health (23.9% poor or fair), and trouble sleeping when the child had pain (23.5% often or almost always).
Rosser32 collected patient-reported outcomes prior to treatment in patients with NF1 and PN enrolled in clinical trials. Data on the impact of PN on daily functioning and QOL were collected from 38 adolescents and young adults using the Numeric Rating Scale, the Brief Pain Inventory Pain Interference Scale, and the NF1 PedsQL Scale. Patients reported experiencing considerable pain, with 42% of patients taking pain medication regularly and 23% of patients receiving prescription pain medication. Patients who were not regularly taking pain medication reported significantly worse tumor pain, pain interference, total functioning, worry, pain/hurt, and paresthesia than patients who were regularly taking pain medication. The burden of PN also affected employment; only 32% of patients with NF1 and PN were employed, despite the fact that 68% had completed high school or some college.
A prospective natural history study assessed the impact of pain interference and its relationship to disease factors, social-emotional functioning, and QOL in 41 children and adolescents aged 10 to 18 years with NF1 and PN, as well as 59 caregivers of children and adolescents aged 6 to 18 years with NF1 and PN, using the Impact of Pediatric Illness Scale.31 Severity of NF1 symptoms were classified as mild, moderate, or severe by caregivers using the NF1 Symptom Severity Scale, tumor characteristics, impact on motor function, and learning difficulties. Pain was found to interfere with daily functioning in most youths, even when they used pain medication. Caregivers of children aged 6 to 18 years indicated that pain was interfering with their child’s daily activities to some degree. Caregiver ratings of their child’s pain interference were similar to patient self-reported ratings. When disease severity groups were compared by proxy and by self-report, pain interference was significantly higher in youths who had moderate to severe NF1 compared with youths who had mild disease. Parents reported that 33% of all participants, including 27% of children and 36% of adolescents, were taking pain medication regularly. Despite taking pain medication regularly, 93% of these adolescents and 100% of their caregivers rated pain as interfering with functioning at least to some degree. Based on caregiver ratings, more anxiety symptoms and larger tumor volumes predicted greater pain interference; caregiver ratings also indicated that greater pain interference, worse depressive symptoms, and more disease complications predicted poorer QOL. Based on adolescent ratings, more anxiety symptoms predicted greater pain interference; adolescent ratings also indicated that greater pain interference and social stress predicted poorer QOL. Pain interference in this sample of youths with NF1 and PN was not significantly affected by age, sex, socioeconomic status, or familial versus sporadic NF1. The impact of pain interference, disease severity, and social-emotional problems on QOL highlights the interaction between physical and psychological states in patients with NF1 and PN.
Health Economic Burden
Limited information was found regarding health care resource use and costs for patients with NF1 and PN, which indicates a large data gap in the literature. The only identified study assessed the medical needs of French patients with NF1 (n = 201; age range: 7–84 years) and the financial cost of the resources used relative to disease severity.33 The most commonly reported reasons for hospitalization for these patients were excision of multiple PNs (n = 51), MPNSTs (n = 21), reconstructive surgery of PN (n = 9), and spinal compression (n = 6).
Treatment Patterns
Medical Management
Because NF1 with PN has many diverse manifestations, there is no standard of care. Once the diagnosis is considered, referral is made to any clinician skilled in NF1 for diagnostic confirmation; further disease management is based on the complications that develop in an individual patient. This review identified no studies that outlined treatment patterns and disease management, indicating another gap in the literature. Guidance provided by Ferner et al6 noted that it is essential to obtain expert advice from an experienced soft-tissue tumor or plastic surgeon before removing a PN. This guidance noted removal of a benign PN can be complex and has the potential for life-threatening hemorrhage, particularly with facial PN.
Phase 1 and 2 studies have been conducted to determine the extent to which several products (imatinib, sirolimus, tipifarnib, pirfenidone, peginterferon, trametinib, selumetinib, and cabozantinib) reduce tumor volume in patients with NF1 with PN; of these products, selumetinib appears to have the most promising efficacy and patient-reported outcomes data.9,34–41 Wolters42 observed that treatment with selumetinib for reduction of tumor volume was associated with clinically significant improvements in PN-related pain, as well as significant improvements in parent total QOL, physical domain, emotional domain, and social domain scores (each P < 0.01) and child physical domain scores (P < 0.05). Gross et al9 noted a mean 28% reduction in PN volume from baseline among 50 patients, aged 3.5 to 17.4 years, with inoperable PNs. These findings indicate clear clinical benefits in the setting of PN volume reduction in children with NF1 and PN. For MEK inhibitors, phase 1 and 2 studies showed a partial response rate of 71% for selumetinib (17/24 patients),40 46% for trametinib (12/26 patients),39 and 42% for cabozantinib (8/19 patients).41
Surgical Treatment
Five retrospective studies reported types of surgery and surgical outcomes for patients with NF1 and PN.12,14,15,43,44 Patients aged younger than 10 years who had lesions of the head, neck, face, and trunk were unlikely to have long-term benefits after surgery because the resection is often incomplete.15 For these patients, therapeutic options are needed that could either induce regression to render the surgically inoperable lesion completely resectable or arrest tumor growth to delay progression for the youngest patients until an age at which tumor recurrence may be less likely.15
In patients with NF1 and PN, many lesions are very vascular and significant bleeding can complicate surgical procedures. To minimize blood loss during tumor excision, vascular tourniquets are used in lesions located in the distal part of extremities.14 Lesions located in the proximal part of the extremities and the trunk, where tourniquet application to prevent bleeding is not possible, represent a challenge for surgery, as many can be large, very vascular, and have a tendency to infiltrate the proximal musculature, even involving bone and filling the pelvic cavity.14 Postoperative progression of PN may not be significantly different from the natural growth behavior of these tumors, suggesting that postoperative tumor growth could be unrelated to surgery.43 However, a retrospective review of 121 pediatric patients found that the extent of surgical resection, as well as tumor age and location, are all statistically significant predictors of recurrence.15 More specifically, a greater extent of surgical resection predicted both a lower risk of tumor progression and a longer interval to progression (P < 0.0001). In addition, children aged 10 years and younger had a shorter interval of tumor control following surgical resection than children aged older than 10 years (P = 0.0004). Tumors of the head, neck, and face were most likely to progress following resection, while tumors of the extremities were significantly less likely to progress (P = 0.0003). In multivariate analysis, older age and tumor location in the extremities were predictors of a longer interval to progression.
Discussion
This SLR includes a total of 39 studies that met eligibility and described the impact of PN on patients with NF1 during the patient journey from natural history to QOL burden and limited treatment options. The heterogeneous nature of PN creates a challenge for presenting a unified narrative of patients’ experience. Instead, the impact of this disease on patients should be considered individually. Symptomatic PN can generally be detected before the patient is 6 years old,12 but asymptomatic PN can go unnoticed without advanced imaging technology.21,22 Patients with PN experience a wide range of complications,24 including the potential for PN to transform into malignant tumors (eg, MPNSTs).4 These complications cause a substantial burden on QOL for patients, including interference with daily functioning, reduction in social activity, increased anxiety, and decreased mobility.29–31 There are limited options for managing NF1 with PN. Surgical removal of PN is difficult, with the potential for life-threatening hemorrhage.6 In addition, children with PN located on the trunk or face are unlikely to have complete resections, thus minimizing the benefit from surgery.15 The detrimental impact of PN on QOL combined with the current lack of effective surgical options highlight the need for effective drug therapies that can reduce the size of tumors. However, the approval of selumetinib by the FDA in April 2020 for the treatment of pediatric patients 2 years of age and older with NF1 who have symptomatic, inoperable PN suggests that the treatment landscape may be changing.16
This SLR identified a substantial gap in the assessment of the economic burden posed by NF1 with PN. Only one study evaluated the cost of PN,33 and that study was performed as part of a broader assessment of resource use among patients with NF1. One factor contributing to the gap in economic assessments may be the lack of tools for specifically assessing the financial burdens of PN. Future research could develop conceptual models or frameworks to detail the impact on patients and health care systems, which then could inform the choice of tools used to quantify the extent of the burden.
Conversely, there were a variety of different tools used to assess QOL burden without a recommended standard, including the PROMIS module, the eight-item Neuro-QOL, the Impact of Pediatric Illness Scale, the Numeric Rating Scale, the Brief Pain Inventory Pain Interference Scale, and the NF1 PedsQL Scale.29–32 The large number of measures used in the literature to assess the highly variable QOL burden of PN reflect the heterogeneity of this disease and the unmet need for treatment.
There were limitations associated with this study. We did not assess study quality for included articles due to the nature of the study designs. Additionally, the synthesis of information in a review is limited by the available data in published journal articles. Several studies did not specify the population of patients with NF1 and PNs, so these patients could have been overlooked for the purposes of this SLR.
Conclusions
This SLR provides data on the natural history, disease burden, and treatment patterns among patients diagnosed with NF1 with PN. The severe burden of this disease on the QOL of patients demonstrates the high unmet need for an effective treatment option that can reduce tumor burden and improve QOL. The heterogeneity of measurement tools used to evaluate QOL and the gap in data evaluating the health economic burden of PN should be the focus of future research.
Acknowledgments
The authors acknowledge Brian Samsell of RTI Health Solutions for medical writing assistance.
Funding Statement
The financial support for the study was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and AstraZeneca. RTI Health Solutions received funding under a research contract with Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, to conduct this study and provide editorial support in the form of manuscript writing, styling, and submission.
Disclosure
CCM and MJ are full-time employees of RTI Health Solutions, an independent nonprofit research organization, which received funding pursuant to a contract from Merck to conduct the study, which is the subject of this manuscript. XY is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and holds shares in Merck & Co., Inc., Kenilworth, NJ, USA stock. SSS was an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, at the time the study was conducted. SA and HKY are employees of AstraZeneca and hold shares in AstraZeneca stock.
References
- 1.Genetics Home Reference. Neurofibromatosis type 1. Available from: https://ghr.nlm.nih.gov/condition/neurofibromatosis-type-1#statistics.. Accessed May 3, 2020.
- 2.Menon AG, Gusella JF, Seizinger BR. Progress towards the isolation and characterization of the genes causing neurofibromatosis. Cancer Surv. 1990;9(4):689–702. [PubMed] [Google Scholar]
- 3.Menon AG, Gusella JF, Seizinger BR. Progress toward the isolation and characterization of the genes causing neurofibromatosis. Brain Pathol. 1990;1(1):33–40. doi: 10.1111/j.1750-3639.1990.tb00636.x [DOI] [PubMed] [Google Scholar]
- 4.Monroe CL, Dahiya S, Gutmann DH. Dissecting clinical heterogeneity in neurofibromatosis type 1. Annu Rev Pathol. 2017;12:53–74. doi: 10.1146/annurev-pathol-052016-100228 [DOI] [PubMed] [Google Scholar]
- 5.National Institutes of Health Consensus Development Conference. Neurofibromatosis. Conference statement. Arch Neurol. 1988;45(5):575–578. doi: 10.1001/archneur.1988.00520290115023 [DOI] [PubMed] [Google Scholar]
- 6.Ferner RE, Huson SM, Thomas N, et al. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J Med Genet. 2007;44(2):81–88. doi: 10.1136/jmg.2006.045906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carroll SL, Ratner N. How does the Schwann cell lineage form tumors in NF1? Glia. 2008;56(14):1590–1605. doi: 10.1002/glia.20776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedman JM, Birch PH. Type 1 neurofibromatosis: a descriptive analysis of the disorder in 1728 patients. Am J Med Genet. 1997;70(2):138–143. doi: [DOI] [PubMed] [Google Scholar]
- 9.Gross AM, Wolters P, Baldwin A, et al. SPRINT: Phase II study of the MEK 1/2 inhibitor selumetinib (AZD6244, ARRY-142886) in children with neurofibromatosis type 1 (NF1) and inoperable plexiform neurofibromas (PN). J Clin Oncol. 2018;36(15 suppl):10503. doi: 10.1200/JCO.2018.36.15_suppl.10503 [DOI] [Google Scholar]
- 10.Korf BR. Plexiform neurofibromas. Am J Med Genet. 1999;89(1):31–37. doi: [DOI] [PubMed] [Google Scholar]
- 11.Gross AM, Singh G, Akshintala S, et al. Association of plexiform neurofibroma volume changes and development of clinical morbidities in neurofibromatosis 1. Neuro Oncol. 2018;20(12):1643–1651. doi: 10.1093/neuonc/noy067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prada CE, Rangwala FA, Martin LJ, et al. Pediatric plexiform neurofibromas: impact on morbidity and mortality in neurofibromatosis type 1. J Pediatr. 2012;160(3):461–467. doi: 10.1016/j.jpeds.2011.08.051 [DOI] [PubMed] [Google Scholar]
- 13.Kim A, Gillespie A, Dombi E, et al. Characteristics of children enrolled in treatment trials for NF1-related plexiform neurofibromas. Neurology. 2009;73(16):1273–1279. doi: 10.1212/WNL.0b013e3181bd1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canavese F, Krajbich JI. Resection of plexiform neurofibromas in children with neurofibromatosis type 1. J Pediatr Orthop. 2011;31(3):303–311. doi: 10.1097/BPO.0b013e31820cad77 [DOI] [PubMed] [Google Scholar]
- 15.Needle MN, Cnaan A, Dattilo J, et al. Prognostic signs in the surgical management of plexiform neurofibroma: the Children’s Hospital of Philadelphia experience, 1974-1994. J Pediatr. 1997;131(5):678–682. doi: 10.1016/S0022-3476(97)70092-1 [DOI] [PubMed] [Google Scholar]
- 16.Food and Drug Administration. FDA approves selumetinib for neurofibromatosis type 1 with symptomatic, inoperable plexiform neurofibromas. 2020. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-selumetinib-neurofibromatosis-type-1-symptomatic-inoperable-plexiform-neurofibromas. Accessed June2, 2020.
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans DG, Howard E, Giblin C, et al. Birth incidence and prevalence of tumor-prone syndromes: estimates from a UK family genetic register service. Am J Med Genet A. 2010;152A(2):327–332. doi: 10.1002/ajmg.a.33139 [DOI] [PubMed] [Google Scholar]
- 19.Huson SM, Compston DA, Clark P, Harper PS. A genetic study of von Recklinghausen neurofibromatosis in south east Wales. I. Prevalence, fitness, mutation rate, and effect of parental transmission on severity. J Med Genet. 1989;26(11):704–711. doi: 10.1136/jmg.26.11.704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lammert M, Friedman JM, Kluwe L, Mautner VF. Prevalence of neurofibromatosis 1 in German children at elementary school enrollment. Arch Dermatol. 2005;141(1):71–74. doi: 10.1001/archderm.141.1.71 [DOI] [PubMed] [Google Scholar]
- 21.Mautner VF, Asuagbor FA, Dombi E, et al. Assessment of benign tumor burden by whole-body MRI in patients with neurofibromatosis 1. Neuro Oncol. 2008;10(4):593–598. doi: 10.1215/15228517-2008-011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen R, Kluwe L, Fuensterer C, Kentsch M, Friedrich RE, Mautner VF. Plexiform neurofibromas in children with neurofibromatosis type 1: frequency and associated clinical deficits. J Pediatr. 2011;159(4):652–655. (). doi: 10.1016/j.jpeds.2011.04.008 [DOI] [PubMed] [Google Scholar]
- 23.Lemberg K Lower height percentiles in neurofibromatosis type 1 (NF1) patients on the NCI NF-1 natural history study inversely correlate with plexiform neurofibroma (PN) tumor volume. Joint Global Neurofibromatosis Conference. Paris, France; 2018. [Google Scholar]
- 24.Williams V Plexiform neurofibromas in 150 complex NF1 patients 2017-2018. Joint Global Neurofibromatosis Conference. Paris, France; 2018. [Google Scholar]
- 25.Dombi E, Solomon J, Gillespie AJ, et al. NF1 plexiform neurofibroma growth rate by volumetric MRI: relationship to age and body weight. Neurology. 2007;68(9):643–647. doi: 10.1212/01.wnl.0000250332.89420.e6 [DOI] [PubMed] [Google Scholar]
- 26.Nguyen R, Dombi E, Widemann BC, et al. Growth dynamics of plexiform neurofibromas: a retrospective cohort study of 201 patients with neurofibromatosis 1. Orphanet J Rare Dis. 2012;7:75. doi: 10.1186/1750-1172-7-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tucker T, Friedman JM, Friedrich RE, Wenzel R, Funsterer C, Mautner VF. Longitudinal study of neurofibromatosis 1 associated plexiform neurofibromas. J Med Genet. 2009;46(2):81–85. doi: 10.1136/jmg.2008.061051 [DOI] [PubMed] [Google Scholar]
- 28.Dagalakis U, Lodish M, Dombi E, et al. Puberty and plexiform neurofibroma tumor growth in patients with neurofibromatosis type I. J Pediatr. 2014;164(3):620–624. doi: 10.1016/j.jpeds.2013.10.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lai JS, Jensen SE, Patel ZS, Listernick R, Charrow J. Using a qualitative approach to conceptualize concerns of patients with neurofibromatosis type 1 associated plexiform neurofibromas (pNF) across the lifespan. Am J Med Genet A. 2017;173(1):79–87. doi: 10.1002/ajmg.a.37987 [DOI] [PubMed] [Google Scholar]
- 30.Lai JS, Jensen SE, Charrow J, Listernick R. Patient reported outcomes measurement information system and quality of life in neurological disorders measurement system to evaluate quality of life for children and adolescents with neurofibromatosis type 1 associated plexiform neurofibroma. J Pediatr. 2019;206:190–196. doi: 10.1016/j.jpeds.2018.10.019 [DOI] [PubMed] [Google Scholar]
- 31.Wolters PL, Burns KM, Martin S, et al. Pain interference in youth with neurofibromatosis type 1 and plexiform neurofibromas and relation to disease severity, social-emotional functioning, and quality of life. Am J Med Genet A. 2015;167A(9):2103–2113. doi: 10.1002/ajmg.a.37123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosser T Substantial pain and reduced quality of life (QoL) in adolescents and young adults (AYA) with neurofibromatosis type 1 (NF1) and plexiform neurofibromas (PNs) enrolled in NF Consortium PN clinical trials. Joint Global Neurofibromatosis Conference. Paris, France; 2018. [Google Scholar]
- 33.Wolkenstein P, Durand-Zaleski I, Moreno JC, Zeller J, Hemery F, Revuz J. Cost evaluation of the medical management of neurofibromatosis 1: a prospective study on 201 patients. Br J Dermatol. 2000;142(6):1166–1170. doi: 10.1046/j.1365-2133.2000.03543.x [DOI] [PubMed] [Google Scholar]
- 34.Robertson KA, Nalepa G, Yang FC, et al. Imatinib mesylate for plexiform neurofibromas in patients with neurofibromatosis type 1: a Phase 2 trial. Lancet Oncol. 2012;13(12):1218–1224. doi: 10.1016/S1470-2045(12)70414-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiss B. Corrigendum: sirolimus for progressive neurofibromatosis type 1-associated plexiform neurofibromas: a neurofibromatosis clinical trials consortium phase II study. Neuro Oncol. 2015;17(6):905. doi: 10.1093/neuonc/nou235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Widemann BC, Dombi E, Gillespie A, et al. Phase 2 randomized, flexible crossover, double-blinded, placebo-controlled trial of the farnesyltransferase inhibitor tipifarnib in children and young adults with neurofibromatosis type 1 and progressive plexiform neurofibromas. Neuro Oncol. 2014;16(5):707–718. doi: 10.1093/neuonc/nou004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Widemann BC, Babovic-Vuksanovic D, Dombi E, et al. Phase II trial of pirfenidone in children and young adults with neurofibromatosis type 1 and progressive plexiform neurofibromas. Pediatr Blood Cancer. 2014;61(9):1598–1602. doi: 10.1002/pbc.25041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jakacki RI, Dombi E, Steinberg SM, et al. Phase II trial of pegylated interferon alfa-2b in young patients with neurofibromatosis type 1 and unresectable plexiform neurofibromas. Neuro Oncol. 2017;19(2):289–297. doi: 10.1093/neuonc/now158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moertel C Trametinib in pediatric patients with neurofibromatosis type 1 (NF-1)-associated plexiform neurofibroma: a phase I/IIa study. Joint Global Neurofibromatosis Conference. Paris, France; 2018. [Google Scholar]
- 40.Dombi E, Baldwin A, Marcus LJ, et al. Activity of selumetinib in neurofibromatosis type 1-related plexiform neurofibromas. N Engl J Med. 2016;375(26):2550–2560. doi: 10.1056/NEJMoa1605943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fisher M Neurofibromatosis Clinical Trials Consortium (NFCTC) phase II study of cabozantinib (XL184) for neurofibromatosis type 1 (NF1) associated plexiform neurofibromas. Joint Global Neurofibromatosis Conference. Paris, France; 2018. [Google Scholar]
- 42.Wolters P Prospective patient-reported outcomes (PROs) document clinical benefit in children with neurofibromatosis type 1 (NF1) and inoperable plexiform neurofibromas (PNs) on SPRINT: a phase II trial of the MEK 1/2 inhibitor selumetinib (AZD6244, ARRY-142886). Joint Global Neurofibromatosis Conference. Paris, France; 2018. [Google Scholar]
- 43.Nguyen R, Ibrahim C, Friedrich RE, Westphal M, Schuhmann M, Mautner VF. Growth behavior of plexiform neurofibromas after surgery. Genet Med. 2013;15(9):691–697. doi: 10.1038/gim.2013.30 [DOI] [PubMed] [Google Scholar]
- 44.Chan SH, Chew W, Ishak NDB, et al. Clinical relevance of screening checklists for detecting cancer predisposition syndromes in Asian childhood tumours. NPJ Genom Med. 2018;3(1):30. doi: 10.1038/s41525-018-0070-7 [DOI] [PMC free article] [PubMed] [Google Scholar]