Abstract
Introduction
India has the largest burden of cases and deaths related to tuberculosis (TB). Undernutrition is the leading risk factor accounting for TB incidence, while severe undernutrition is a common risk factor for mortality in patients with TB in India. The impact of nutritional supplementation on TB incidence is unknown, while few underpowered studies have assessed its impact on TB mortality. We designed an open-label, field-based cluster randomised trial to assess the impact of nutritional supplementation (with food rations) on TB incidence in a group at higher risk of TB infection and disease, viz household contacts (HHC) of patients with microbiologically confirmed pulmonary TB (PTB) in Jharkhand, a state with a high prevalence of undernutrition.
Methods and analysis
We shall enrol 2800 adult patients with PTB of the national TB programme, across 28 treatment units in 4 districts, and their approximately 11 200 eligible contacts. The sample size has 80% power to detect the primary outcome of 50% reduction in incidence of active TB in HHC over 2 years of follow-up. Patients and HHC in both the arms will undergo nutritional assessment and counselling. Patients will receive monthly food rations (supplying 1200 kcal and 52 g proteins/day) and multivitamins along with antitubercular treatment. The HHC in the intervention arm will receive food rations (supplying 750 kcal and 23 g proteins/day) and multivitamins while HHC in control arm will be on usual diet. The secondary outcomes in HHC will include effects on nutritional status, non-TB infections. Secondary outcomes in patients are effects on TB mortality, adherence, adverse effects, nutritional and performance status. Substudies will examine micronutrient status and effects on dietary intake, body composition, muscle strength and immune function.
Ethics and dissemination
The institutional ethics committee of ICMR-NIRT, Chennai, approved the study (289/NIRT-IEC/2018). The results will be disseminated in publications and presentations.
Trial registration number
Clinical Trial Registry of India: CTRI/2019/08/020490.
Keywords: nutrition & dietetics, tuberculosis, preventive medicine, public health
Strengths and limitations of the study.
The Reducing Activation of Tuberculosis by Improvement of Nutritional Status study is the first trial addressing undernutrition to reduce tuberculosis (TB) incidence in communities with high prevalence of poverty, undernutrition and low prevalence of HIV infection.
It is the largest trial to evaluate the impact of nutritional support on TB mortality in a programme-based cohort of patients with pulmonary TB and a high prevalence of undernutrition.
The follow-up period of 2 years will ensure detection of most cases of incident TB in household contacts and recurrence of TB in index cases.
The quantum of food rations per participant is standardised, and in the absence of individual needs assessment, the extent to which the intervention meets individual needs is unknown.
Food sharing in the families in the control arm and extra food consumption from other sources cannot be ruled out.
Introduction
Tuberculosis (TB) is a global public health problem leading to significant morbidity and mortality, especially in low and middle income countries. An estimated 10 million people developed TB and 1.2 million (HIV-negative) people succumbed to it in 2019.1 India was the major contributor to the global TB burden with an estimated 2.6 million new cases (27% of global) and 0. 4 million (35% of global) TB deaths in HIV-negative people in 2019.1
The United Nations Sustainable Development Goals-3 (SDG-3) has a target for ending the TB epidemic by 2030 and aims to reduce TB incidence and TB deaths by 80% and 90% of the 2015 levels, respectively.2 As per the National Strategic Plan (2017–2025), the Revised National Tuberculosis Control Programme (renamed as National Tuberculosis Elimination Programme, NTEP) in India has set an ambitious target of achieving the 2030 SDG milestone by 2025, 5 years ahead of the global target.3
The end TB strategy will require a mix of biomedical, public health and social interventions to achieve these goals.2 The strategy requires acceleration of the current decline of 1.5% to 10%–17% per year.4 The present biomedical approach to TB prevention based on vaccination and TB preventive treatment (TPT) has its limitations. The efficacy of BCG vaccine is limited to prevention of severe forms of childhood TB.5 The TPT with isoniazid in countries like India currently covers only select groups of contacts like children under 6 years of age and people living with HIV.6 WHO recently made a conditional recommendation of offering TPT to all household contacts (HHC).7 However, there are considerable logistical and technical challenges in countries with a high burden of latent TB infection (LTBI).8
Rationale for the trial
The end TB strategy recognises the need for new tools, interventions and strategies to address the problem of TB incidence and adverse outcomes.2 Globally, an estimated 1.7 billion people or 23% of the population have LTBI, which remains latent in 90% in the presence of innate and cell-mediated immunity.9 Risk factors like HIV, undernutrition, uncontrolled diabetes, smoking and alcohol impair immunity, lead to active TB, and act as drivers of the TB epidemic. Undernutrition results from deficient intake or assimilation of energy and nutrients, often in association with disease-associated inflammation, and is a part of the broader spectrum of malnutrition, which includes both undernutrition, overweight and obesity and deficiencies of micronutrients. Undernutrition in children is commonly defined by the well-accepted WHO indicators of low birth weight in newborns, underweight (low weight for age), stunting (low height for age) and wasting (low weight for height) in preschool children and by age and gender-specific cut-offs for body mass index (BMI) in those aged 6–18 years. In adults, undernutrition is based on a low BMI, which reflects low body energy stores or chronic energy deficiency. The BMI cut-off for underweight proposed by WHO of <18.5 kg/m2 for populations10 has also been accepted as a criterion for clinical diagnosis of malnutrition/undernutrition in a recent consensus statement.11 In addition, there have been proposals for diagnosis of undernutrition based on altered body composition, and for higher BMI cut-offs in patients undergoing significant involuntary weight loss, which require further validation.11 12
Undernutrition is the leading cause of impaired immunity globally,13 with a consistent inverse exponential relationship between nutritional status measured by BMI and TB incidence.14 According to the global TB report 2020, undernutrition is a leading risk factor accounting for 2.2 million cases (19%), more than HIV and diabetes (accounting for 0.76 and 0.35 million cases, respectively).1 Undernutrition is also a consistent risk factor for TB mortality, regardless of HIV infection, and drug susceptibility.15 Its prevalence was as high as 23% in women and 19% in men (BMI: <18.5 kg/m2) in the most recent National Family Health Survey (NFHS-4) in India.16 It is higher in the poor, rural residents and those belonging to the scheduled castes and tribes, who also suffer a high burden of TB disease.16 17 The WHO has estimated that 0.6 million cases of TB in India are attributable to undernutrition,1 while other studies indicate that this estimate may be higher.18 A majority of Indian patients with active TB have severe levels of undernutrition (macronutrient and micronutrient), which are associated with twofold–fourfold higher risk of mortality.19
A single unit increase in BMI could reduce TB incidence by 14%,14 and a modelling study has shown that TB incidence and mortality could decline by 40%–71% with nutritional interventions.20 There is no randomised controlled trial on the effect of nutritional supplementation on TB incidence. The studies on the impact of nutritional supplementation on TB mortality have been limited, small and underpowered.21
The Reducing Activation of Tuberculosis by Improvement of Nutritional Status (RATIONS) study is a cluster randomised trial to assess the impact of nutritional supplementation on TB incidence among HHC of patients with microbiologically confirmed pulmonary TB (PTB), living in a community with a high prevalence of undernutrition. They are a group at higher risk of TB infection and disease,22 with a prevalence of 10-fold–60-fold higher than in the general population.23 TB incidence was 4.8% in the HHC and 21.4% in child contacts in a previous study from Peru.24 Food insecurity and undernutrition are strong and modifiable risk factors of TB in the HHC.25 26 The trial is being conducted in Jharkhand (meaning ‘Land of Forests’) a state in eastern India which has a high prevalence of undernutrition in children and adults. According to the National Family Health Survey-4 (2015–6), the state has the highest levels of underweight (47.8%), wasting (29.0%), and the second highest level of stunting(45.3%) in children under 6 years of age in India.16 27 Similarly, more than two of out of every five (41%) of adult rural women in Jharkhand had a BMI of <18.5 kg/m2, and had the highest prevalence of anaemia in adult women in India,(65.9%), which is largely related to nutritional deficiencies of iron and folic acid.16 27
Objectives
The objectives and the outcome variables have been tabulated in table 1.
Table 1.
Objectives of the RATIONS trial and the outcome variables
| Objective | Outcome variables | Index case | HHC |
| Primary Objective | |||
| Effect of household nutritional supplementation in reducing TB incidence among HHC of patients with microbiologically confirmed PTB | Difference in number of incident cases of active TB (all forms) in the two arms detected by active case finding over a follow-up period of 2 years after diagnosis of index case | ✓ | |
| Secondary objectives | |||
| Effect of nutritional supplementation on anthropometric indicators over 6 months | Anthropometric indicators such as weight and BMI | ✓ | ✓ |
| Non-TB infectious morbidity and mortality in HHC in both the arms | Malaria, diarrhoea, lower respiratory tract infection, hospitalisation with fever of any cause or death with fever of any cause <15 days in duration | ✓ | |
| Adherence to anti-TB therapy | Proportion completing the therapy successfully | ✓ | |
| Mortality during treatment | Proportion of index cases who died during treatment | ✓ | |
| Adverse effects | Severe adverse effects with TB drugs | ✓ | |
| Recurrence of TB within 2 years after cure | Relapse rate of microbiologically confirmed TB | ✓ | |
| Performance status | Change in ECOG scale at 1 month, 2 months and 6 months compared with baseline | ✓ | |
| Dietary substudy | |||
| Evaluate the difference in dietary intake of calories and proteins | Calorie and protein intake at baseline, and end of treatment in intervention and control arms | ✓ | ✓ |
| Micronutrient substudy | |||
| Assess vitamins A and D (25-hydroxyvitamin D) levels | Level of vitamins A and D at baseline | ✓ | ✓ |
| Body composition substudy | |||
| Evaluation of body composition | Estimate fat-free mass, fat mass and other bioimpedance analysis parameters at baseline, and 6 months after treatment | ✓ | ✓ |
| Substudy on grip strength | |||
| Evaluate muscle strength using hand grip dynamometer | Grip strength at baseline and 6 months | ✓ | |
| Substudy of immune function | |||
| Evaluate cellular immunity in patients and HHC | Lymphocyte subsets (CD4, CD8, natural killer cells and B lymphocytes), fourth generation IGRA at baseline and end of treatment | ✓ | ✓ |
BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; HHC, household contacts; IGRA, Interferon Gamma Release Assay; PTB, pulmonary tuberculosis; RATIONS, Reducing Activation of Tuberculosis by Improvement of Nutritional Status.
Primary objective
The primary objective is to evaluate the effect of household nutritional supplementation in reducing TB incidence among HHC of patients with microbiologically confirmed PTB.
Secondary objectives in HHC
The secondary objective is to evaluate the effect of nutritional supplementation on anthropometric indicators, and non-TB infectious morbidity and mortality.
Secondary outcomes in index cases
To evaluate the effect of nutritional supplementation on adherence to treatment, mortality, frequency of adverse effects due to treatment, performance status of patients as measured by the Eastern Cooperative Oncology Group (ECOG),28 and relapse of microbiologically confirmed TB on follow-up.
Substudies: six substudies have been planned in a subset of index cases and HHC
Dietary intakes: to evaluate the difference in dietary intake of calories, proteins at baseline, and at the end of treatment in a subsample of the patients in both the arms.
Micronutrients: vitamin A (serum retinol) and 25-hydroxyvitamin D levels in a subsample of index patient and HHC at baseline.
Body composition: to evaluate the difference in body composition between patients in the two arms at baseline and 6 months by a multifrequency bioelectric impedance analyzer (Bodystat Quadscan 4000).
Muscle function (grip strength) in a subsample of index cases at baseline, and end of treatment using a digital handheld dynamometer.
Immune function: to evaluate select aspects of immunity in index patient and their HHC before and after treatment using the lymphocyte subsets (CD4, CD8, natural killer cells and B lymphocytes) and kinetics of interferon γ responses (by CD4 and CD8 cells) in a fourth generation Interferon Gamma Release Assay (QuantiFERON-TB Gold Plus: QFT-Plus).
Qualitative study in a subset of stakeholders: a qualitative study will also be conducted in a subset of the stakeholders (patients, contacts and field staff) at the end of the intervention period to assess the perceptions and experiences of nutrition intervention.
Methods and analysis
Study design and oversight
This is a cluster randomised open-label parallel-arm, superiority trial of nutritional supplementation in households with microbiologically confirmed patients with PTB in the state of Jharkhand, Eastern India. The study will randomise 28 TB units (TUs) in four districts (Ranchi, East-Singhbhum, West-Singhbhum and Seraikela-Kharsawan) into control and intervention arms, each with 1400 adult PTB patients. It is supported by the India TB Research Consortium of the Indian Council of Medical Research (ICMR) and implemented by the Yenepoya (deemed to be University), in association with the National Institute for Research in Tuberculosis (ICMR-NIRT) and National Institute of Nutrition. The enrolment began on the 16 August 2019.
Study setting: under the NTEP, each district has one district TB centre and there are subdistrict administrative units called TUs. The population is predominantly rural (75%) and indigenous communities classified as ‘scheduled tribes’ who comprise 28% of the population (national-8%) and are historically disadvantaged groups with regard to social, economic and health indicators. According to NFHS-4, the prevalence of undernutrition in Jharkhand was 23.8% and 31.6% in adult men and women, respectively, significantly higher than the national average.16 A total of 44 000 TB cases were notified in the year 2017 when this trial was proposed.29
Eligibility criteria
The inclusion and exclusion criteria are mentioned in table 2.
Table 2.
Eligibility criteria for RATIONS trial participants
| Index cases | HHC |
| Inclusion criteria | |
| Patients ≥18 years of age with microbiologically confirmed PTB | Persons living in the same house, eating from same kitchen as index case for ≥one night or for frequent or extended periods during the day during the 3 months before diagnosis in index case |
| Exclusion criteria | |
| Non eligible HHC | Current smear or GeneXpert or LPA or culture confirmed TB |
| Time interval between initiation of treatment and enrolment is >14 days | Clinically diagnosed PTB or extra-PTB and currently on treatment |
HHC, household contacts; LPA, line probe assay; PTB, pulmonary tuberculosis; RATIONS, Reducing Activation of Tuberculosis by Improvement of Nutritional Status.
Inclusion criteria: adult patients (≥18 years) with microbiologically confirmed PTB (irrespective of drug sensitivity) will constitute the index cases and will be eligible to enrol in the study. The HHC will be persons who have lived in the same house (and eating from the same kitchen), for one or more nights or for frequent or extended periods during the day with the index case during the preceding 3 months.
Exclusion criteria: an index case with no eligible HHC and any HHC currently on treatment for TB will be excluded.
Study interventions
Nature and quantity
The study intervention includes macronutrients and micronutrient supplementation along with nutritional counselling as per national guidelines.30 The index patients (in both arms) and the HHC (in the intervention arm) will receive a food basket and a recommended daily allowance of vitamins and micronutrients every month, as described in table 3. This will be either delivered by the study staff or may be picked up from a depot as per the participant preference.
Table 3.
Nutritional supplementation in the RATIONS trial
| Intervention arm | Control arm | |
| Index case*, quantity per person per month | Nutritional counselling 5 kg of rice 3 kg roasted Bengal gram powder (locally called as sattu) 1.5 kg of milk powder 500 mL vegetable oil One RDA of micronutrient |
Nutritional counselling 5 kg of rice 3 kg roasted Bengal gram powder (locally called as sattu) 1.5 kg of milk powder 500 mL vegetable oil One RDA of micronutrient |
| Household contact†, quantity per person per month | Nutritional counselling 5 kg rice 1.5 kg pulses (split pigeon peas) One RDA of micronutrient per adult/adolescent HHC Half of this amount for children less than 10 years |
Nutritional counselling Usual food assistance available to eligible households through public distribution system |
*Approximately 1200 kcal of energy and 52 g proteins/day.
†Approximately 750 kcal of energy and 23 g of proteins/day.
HHC, household contacts; RATIONS, Reducing Activation of Tuberculosis by Improvement of Nutritional Status; RDA, recommended dietary allowance.
Frequency and duration
The food basket will be provided for 6 months for new patients and 12 months for patients with multidrug resistant TB (MDR-TB) (and their HHC in intervention arm). Extension of the intervention period to 12 months, for a patient with non-MDR-TB will be considered if there is evidence of undernutrition (BMI: <18.5 kg/m2) in the index case even at the end of 6 months. Extension of rations to an HHC will be considered if an adult contact has a BMI of <16 kg/m2; children (<10 years) have weight-for-age z-score <−2SD and adolescents (10–18 years) have BMI-for-age z-scores <−2SD.
Nutritional counselling and assessing adherence
The patients and the HHC will be counselled about the importance of a balanced diet for the nutritional recovery of the patient and the protection of the health status of the family. The families will be instructed about the optimal utilisation of the food rations in locally acceptable food recipes. The field staff will undertake follow-up visits to monitor weight gain (a proxy indicator for adherence) and check the empty packets of the milk powder as an indicator of consumption by the patient.
Co-interventions permitted during the trial
The patients as well as the HHC will continue to access public distribution system, supplementary feeding programmes (Integrated Child Development Services Scheme, mid-day meals) as usual and additional INR 500/month as direct benefit transfer availed by patients with TB in India. The eligible children under 6 years of age and those with HIV infection who have been advised by the NTEP staff to take chemoprophylaxis with isoniazid after an evaluation will continue to do so.
Risk assessment and referral
The patients will be evaluated for nutritional status, oxygen saturation, blood pressure and presence of complications at baseline and at follow-up. Patients with severe undernutrition with oedema, extremely severe undernutrition (BMI: <14 kg/m2), breathlessness or low oxygen saturation (SpO2: <94) will be referred for inpatient care as per national guidelines.30
Randomisation and intervention allocation
This is an open label trial; the participants and field staff are not blinded after assignment. All the TUs from the selected districts were line-listed (list of TUs is available in online supplemental file 1) and randomised equally to both the arms by computer-generated random numbers using restricted randomisation by the statistician at ICMR-NIRT, Chennai. The cluster allocation was kept confidential until the end of training of the field staff and the TUs were ready for implementation.
bmjopen-2020-047210supp001.pdf (40.1KB, pdf)
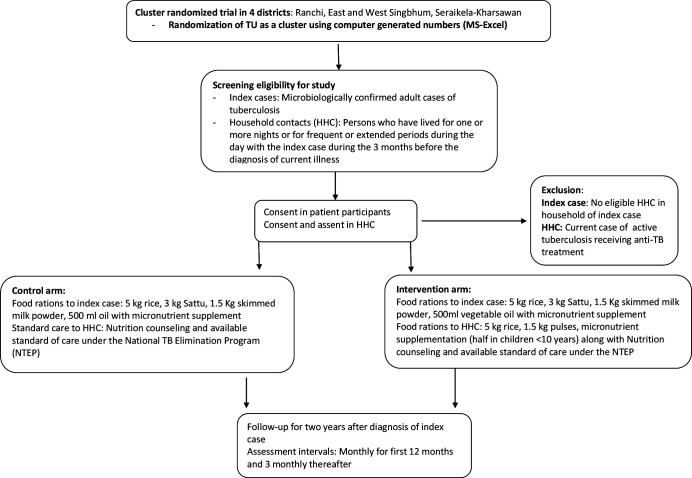
Enrolment of index cases and HHC
Figure 1 describes the study flow. Consecutive patients diagnosed with microbiologically confirmed PTB in selected TUs will be enrolled after due consent process, during a 6–12-month period. Information about the study will be given to the HHC during a home visit by the trial staff and enrolled after elicitation of voluntary written informed consent. The need for adherence to treatment and food rations, cooperation with study procedures, the stability of residence and the willingness to permit home visits will be discussed with the index cases and HHC during enrolment.
Figure 1.
Study flow for the Reducing Activation of Tuberculosis by Improvement of Nutritional Status trial. TU, tuberculosis unit.
Baseline evaluation of index cases and HHC
The study procedures at baseline and follow-up are denoted in table 4. Demographic characteristics, including gender, occupation, caste, marital status, education, socioeconomic assessment with an asset score and education, will be noted. The presence of self-reported risk factors such as diabetes, alcohol consumption and tobacco use, and family/history of TB will also be recorded.
Table 4.
Schedule of enrolment, assessments at baseline and follow-up in the RATIONS trial
| Investigations | Baseline | M1 | M2 | M3 | M4 | M5 | M6 | M7 | M8 | M9 | M10 | M11 | M12 | M15 | M18 | M21 | M24 | |
| In index cases | ||||||||||||||||||
| 1 | Informed consent | X | ||||||||||||||||
| 2 | Demography, socio-economic status, comorbidities, household characteristics and access to PDS | X | ||||||||||||||||
| 3 | Clinical evaluation | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| 4 | Anthropometry (height, weight and MUAC) and SpO2 pedal oedema | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| 5 | *Laboratory evaluation RBS, HIV and Hb | X | ||||||||||||||||
| 6 | Compliance to nutritional supplement in new cases (12 months in MDR-TB) | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| 7 | Performance status (modified ECOG Scale) | X | X | X | X | X | X | X | X | X | X | |||||||
| 8 | *CB-NAAT | X | (in case of symptoms of recurrent disease) | |||||||||||||||
| 9 | *CXR | X | X | |||||||||||||||
| In subsample of index cases | ||||||||||||||||||
| 1 | Dietary recall of calories and protein intake | X | X | |||||||||||||||
| 2 | Body composition (by BIA) | X | X | |||||||||||||||
| 3 | Micronutrient estimation (vitamins A and D) | X | ||||||||||||||||
| 4 | Hand grip strength | X | X | |||||||||||||||
| 5 | Immunological tests (fourth-generation IGRA, lymphocyte subsets) | X | X | |||||||||||||||
| In household contacts | ||||||||||||||||||
| 1 | Informed consent | X | ||||||||||||||||
| 2 | Baseline demography, socioeconomic status, comorbid conditions, household characteristics and access to PDS | X | ||||||||||||||||
| 3 | Symptom screen | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| 4. | Clinical evaluation if symptomatic | X | In case of symptoms of active TB | |||||||||||||||
| 5 | Anthropometry (height, weight and MUAC) | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| 6 | Compliance to nutritional supplement (intervention arm) (12 months in MDR-TB) | X | X | X | X | X | X | X | ||||||||||
| 7. | Review of non-TB infectious illnesses† | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| 8 | Sputum smear, CB-NAAT | In case of symptoms of active TB | ||||||||||||||||
| 9 | CXR‡ | X | In case of symptoms of active TB | |||||||||||||||
| In subsample of household contacts | ||||||||||||||||||
| 1. | Dietary recall of calories, protein intake | X | X | |||||||||||||||
| 2 | Body composition (by BIA) | X | X | |||||||||||||||
| 3 | Micronutrient estimation (vitamins A and D) | X | ||||||||||||||||
| 4 | Immunological tests (fourth-generation IGRA and lymphocyte subsets) | X | X | |||||||||||||||
*Through public health system.
†Respiratory infections, diarrhoea, hospitalisation with fever of any cause and death due to fever <15 days duration.
‡For children under 5 years of age, symptomatic contacts.
BIA, Bioimpedance analysis; CB-NAAT, cartridge-based nucleic acid amplification test; CXR, chest X-ray; ECOG, Eastern Cooperative Oncology Group; Hb, haemoglobin; HIV, HIV immunodeficiency virus; IGRA, Interferon Gamma Release Assay; MDR-TB, multidrug resistant tuberculosis; MUAC, mid upper arm circumference; MUAC, mid-upper arm circumference; PDS, public distribution system; RATIONS, Reducing Activation of Tuberculosis by Improvement of Nutritional Status; RBS, random blood sugar; SpO2, oxygen saturation; TB, tuberculosis.
Clinical examination of index cases
Weight will be measured with a digital weighing scale (SECA 803) with accuracy of 100 g, and height using a portable stadiometer (SECA 213) with accuracy of 0.1 cm using standard procedures. Mean of two measurements of weight will be taken for calculation of BMI. Undernutrition is defined as a BMI of <18.5 kg/m2 according to the underweight definition approved by the WHO.10 Patients will further be categorised into mild underweight in case the BMI is 17.0–18.49 kg/m2, moderate underweight if the BMI is 16.0–16.99 kg/m2 and severely underweight if the BMI is <16 kg/m2 as suggested by WHO.31 An additional category of extremely severe underweight is used to classify those with a BMI of ≤14 kg/m2.32 Mid-upper arm circumference (MUAC) will be measured to the nearest of 0.1 cm on the non-dominant arm, if the patient is unable to stand. Presence of oedema, blood pressure using a digital instrument (OMRON) and oxygen saturation using pulse oximeter will be noted. Assessment of performance status will be done using ECOG Scale as described in table 5.28
Table 5.
Eastern Cooperative Oncology Group (ECOG) Scale for functional assessment24
| ECOG categories | Additional description | Score |
| Able to carry out normal activity without restriction | No physical restriction | 0 |
| Unable to do physically strenuous activity, but ambulatory and able to carry out light work | Able to walk around the neighbourhood, but unable to do any income-generating work | 1 |
| Ambulatory and capable of all self-care, but unable to carry out any work; up and about <50% of waking hours | Able to walk around the house and backyard | 2 |
| Capable of only limited self-care; confined to bed or chair >50% of waking hours | Able to go to the bathroom | 3 |
| Completely disabled; cannot carry out any self-care and totally confined to bed or chair | Unable to go to the bathroom | 4 |
Clinical examination in contacts
This will consist of anthropometric measurements like weight, height, MUAC (if unable to stand and in children under 5 years of age), the presence of oedema and BCG scar.
Laboratory evaluation of index cases
The results of the sputum smear microscopy, cartridge-based nucleic acid amplification test (CB-NAAT), Gene Xpert MTB/RIF test, blood glucose and HIV tests (if available) will be retrieved from the NTEP records. Haemoglobin will be measured during the home visit using HemoCue Hb 201+ using standard procedures and precautions. Chest X-ray (CXR) of patients at baseline will be done wherever feasible.
Laboratory evaluation of HHC
Symptom screening for TB will be done in all HHC and CXR will be done wherever feasible as per the NTEP guidelines.6 In case of symptoms of presumptive TB or an abnormal CXR, the HHC will be referred for sputum examination. Children with symptoms/abnormal CXR will be referred for further evaluation by sputum smear and CB-NAAT (if cough is productive) and induced sputum/gastric aspirate, if unable to produce sputum at a referral hospital.
Follow-up of index cases and HHC
The enrolled index cases and their HHC will be followed-up for 2 years after the diagnosis of the index case. Jharkhand is a state with potential seasonal labour migration from rural areas. All attempts (including telephonic contact) will be made to retain follow-up in case of temporary migration with an in-person visit on their return. Participants will be termed as lost to follow-up if in-person or telephonic contact is not made for ≥2 months in the intervention period or for ≥6 months in the follow-up period. All participants lost to follow-up will be approached for an end of study evaluation to ascertain information on the primary and relevant secondary outcomes.
The schedule of follow-up and assessments is described in table 4. Evaluation will be done for current symptoms, any adverse effect related to treatment, adherence to treatment and rations. All HHC will be evaluated for symptoms of active TB on each visit, consumption of rations (in intervention arm) and review of non-TB infectious morbidity and mortality based on symptoms, hospitalisation or death.
Patients will undergo repeat sputum examination on follow-up as per NTEP guidelines. Contacts that develop any symptoms of active TB (pulmonary or extrapulmonary) will undergo appropriate investigations.
The cases of active TB in HHC will be classified as co-prevalent or incident according to the time of diagnosis of the index case. A co-prevalent case is HHC diagnosed with active TB (microbiologically confirmed active TB or as clinically diagnosed TB) at the baseline, or within 2 months of the baseline screening and evaluation of the HHC. An incident case is a new case of TB in an HHC (microbiologically confirmed active TB or as clinically diagnosed) that was diagnosed more than 2 months following the initial negative screening and evaluation. The definition of microbiologically confirmed case and clinically diagnosed cases is as per table 6.
Table 6.
Case definitions for outcomes used in RATIONS trial6
| Outcome | Case definition |
| Active TB | Any patient with microbiologically confirmed TB or clinically diagnosed TB |
| Microbiologically confirmed TB in adults or children | A patient who has a positive sputum smear for Mycobacterium tuberculosis and/or
|
| Clinically diagnosed PTB | A patient who has symptoms suggestive of TB, is smear negative and/or negative on CB-NAAT, and/or who has CXR is suggestive of TB, and where there is no alternative clinical diagnosis |
| Clinically diagnosed extra-PTB | A patient who is either negative on microbiological testing and/or CB-NAAT, or where an appropriate specimen is not available, and the findings (clinical/ biochemical/cytological/histopathological/radiological or direct visualisation procedures) are suggestive of TB, and where alternative diagnosis has been ruled out |
| Clinically diagnosed PTB in children | A patient who has symptoms suggestive of active PTB (fever, cough, weight loss or the absence of weight gain), and/or a CXR is suggestive of TB, and there is absence of alternative diagnosis, who is negative on CB-NAAT on gastric aspirate or induced sputum, or when bacteriological confirmation has not been possible |
CB-NAAT, Cartridge-based nucleic acid amplification test; CXR, chest X-ray; PTB, pulmonary tuberculosis; RATIONS, Reducing Activation of Tuberculosis by Improvement of Nutritional Status.
Qualitative study about the nutritional intervention
We will use a phenomenological approach to generate qualitative data through the in-depth interview of TB patients and their HHC. The participants will be purposively selected till conceptual saturation and triangulation is reached, and will be interviewed using topic guides prepared in line with the study objectives. Interviews will be tape-recorded, transcribed verbatim and translated to English. Open Code software will be used to facilitate analysis. This substudy will be conducted at the end of the intervention period.
Discontinuation of study intervention and withdrawal of study participants
Study participants will be asked about consumption of rations and micronutrients at every visit. Rarely, they may choose to discontinue consumption of the study intervention during the intervention period, due to an unrelated illness or perceived adverse effects. The reasons for their discontinuation of study intervention will be recorded, but these participants will remain in the study and undergo protocol-specified follow-up procedures. However, if the participant also explicitly withdraws consent for follow-up and collection of additional information in addition to discontinuation of consumption of study intervention, the withdrawal of consent will be recorded, and only the data collected prior to withdrawal of consent will be used in the study. Study participants will be free to withdraw at any time during the trial. The reasons for the withdrawal will be documented, which may include refusal of follow-up, lost to follow-up, participant request, death or if the study sponsors decide to stop or cancel the study. Unless the participants withdraw consent for further follow-up, attempts will be made to ascertain outcomes as mentioned earlier.
Study outcomes
The primary outcome in HHC is the difference in number of incident cases of active TB (all forms) in the two arms by active case finding over a follow-up period of 2 years. The secondary outcomes are improvement in the nutritional indicators over 6 months, frequency of malaria, diarrhoea, lower respiratory tract infection, hospitalisation with fever of any cause or death with fever of any cause less than 15 days in duration in both the trial arms.
The secondary outcomes in the index cases are successful treatment completion, TB-related deaths, improvement in performance status, adverse effects and recurrence of TB during 2-year follow-up. The ascertainment of primary outcomes of incident TB in contacts is by NTEP staff (not part of the trial team).
Participant timeline
The trial has a preparatory phase of 3 months for site selection, staff recruitment and training, and preparation of manual of procedures. The intervention phase will be 6 months for drug-susceptible cases and 12 months in the MDR-TB. The follow-up phase will continue for 2 years from the initiation of treatment.
Sample size estimation
The estimated incidence rate of PTB in the general population in India is 217/100 000 population (0.208 %).4 The incidence rate ratio of TB in HHC is 15.9 (IQR: 2.6–21.4) compared with the general population, translating into 4% incidence in the HHC.22 Assuming a higher burden of TB and undernutrition in India, and recent emerging evidence of significantly higher risk of TB disease following infection in close contacts,33 we considered TB incidence in HHC to be 5% over the study period. We assume 50% reduction in TB incidence at household level with intervention.34
Our sample size considers design effect at three levels; the TU level, the families of index cases and finally their HHC.35 We assumed approximately 100 index cases (80–120) and their families in a cluster per annum, a correlation coefficient of 0.2 for the outcome in HHC and 0.01 between members of the same cluster,22 and thus a design effect of 6.75. Thus, a sample size of 28 clusters with 2800 patients and approximately 11 200 contacts equally distributed in both the arms would have 80% power to detect 50% reduction of TB incidence in intervention arm with a type-1 error of 5%.
The substudy sample sizes were estimated based on the assumptions related to the objective of the substudy. The sample size of 250 cases (125/arm) and 250 contacts (125/arm) was based on the prevalence of multiple vitamin deficiencies in patients with PTB,36 and the prevalence of vitamin D deficiency in apparently healthy individuals in India.37
A sample size of 352 contacts (176/arm) was assumed to be needed to detect a 10% difference in mean CD4 counts in the contacts of the two arms after 6 months of intervention, with 90% power. This proportion is about 3% of the HHC and we will enrol a similar 3% of the index cases (50/arm) to assess determine the immune function at baseline and after intervention in them.
The sample size for the dietary intake substudy assumes an SD of 525 kcal, over a wide range of caloric intakes.38 Assuming a mean difference in caloric intake between both the arms as 400 kcal and 20% loss to follow-up, we will enrol 45 contacts and 45 patients in each arm.
Data collection and management
The data will be collected by field investigators working in close collaboration with the NTEP staff. Study data will be collected and managed using Research Electronic Data Capture, an electronic data capture tools39 hosted at ICMR-NIRT. The data capture will be done real time using a handheld device, will be subjected to range and logic checks and will be monitored by the project technical team. A periodic quality check will be performed for accuracy and completeness by the data management team at ICMR-NIRT, which will minimise missing data. Appropriate imputation methods will be used for missing values in the analysis if required. The final dataset will be accessible to the investigators based in Yenepoya (deemed to be University) and ICMR-NIRT and will be deposited in electronic format with the trial sponsor, ICMR, at the end of the study.
All essential trial documents and consent forms will be stored under lock and key at the recruitment site under the supervision of investigators. Electronic data will be password protected and the records will be retained for a period of 5 years after completion of the study.
We will constitute a data safety management board (DSMB) comprising of subject experts in clinical trials, TB and nutrition along with independent biostatistician and ethicist.
Apart from the regular monitoring by the project team, there is periodic reporting to the ICMR, to the institutional ethics committee (IEC) of ICMR-NIRT, the trial advisory committee and the DSMB.
Data analysis
The primary outcome is TB incidence among contacts, expressed as events per 100 000 person-months of follow-up. Follow-up is defined as time from date of randomisation until the earliest endpoint, that is, documented TB disease or censoring (death, loss to follow-up or end of the study).
Cox proportional hazards model, accounting for varying follow-up times and clustering effect, will be used to compare the rate of progression of TB infection to disease among contacts between the arms and to assess its association with risk factors. Unadjusted and adjusted HRs along with their 95% CIs will be reported. The crude effect of the intervention will be calculated using Kaplan-Meier survival plots and compared using the log rank test.
The primary analysis will be intention to treat. Per-protocol analysis will also be done. The models will be adjusted for relevant risk factors (age, smoking, diabetes and duration of exposure) during the sensitivity analysis.
The secondary outcomes of change in weight and z-scores in patients and HHC, and the performance status in patients, will be compared using unpaired and paired t-tests and Bonferroni correction for multiple comparisons. Linear mixed regression models adjusted for age, gender, TU, caste, asset score, family size and baseline weight will be done.
The secondary outcomes of frequency of non-TB morbidities and deaths due to infections in the HHC, and the frequency of deaths, adverse effects, defaults and relapse in the index cases in the two arms, will be compared using the χ2 test and Cox proportional hazards regression for time to first event.
The patients enrolled in the substudies will be compared in their baseline characteristics as these have been drawn by non-random sampling of patients from the main trial. The changes in dietary intake of calories and proteins, body composition parameters and lymphocyte will be assessed among index and contacts. Interactions between treatment and change in nutritional and body composition indicators will be tested using likelihood ratio tests.
The effect of subgroups will be analysed based on age, gender, caste, residence, BMI, asset score and possession of below poverty line card, alcohol use and family history of TB. A p<0.05 (two-tailed) will be considered statistically significant. All data will be analysed using STATA V.16.1 (StataCorp, Texas, USA).
Interim analysis will be performed on attaining 50% of outcomes in the control arm (approximately 230 cases) with p<0.0054 as statistically significant. The final analysis will be done at the end of attainment of planned sample size and completion of follow-up, considering p<0.0492 as statistically significant.
Harm
The intervention involves locally consumed food items, which are part of the daily diet and hence no specific adverse events are expected. Patients who have lactose intolerance will be offered alternatives. The possibility of ‘re-feeding syndrome’ in severely undernourished patients will be prevented by training the field staff to offer a graded increase in food intake in such patients.
Ethics, participant information and consent
Ethics clearance has been obtained from IEC of ICMR-NIRT, Chennai (NIRT-IEC number: 2018020), to which all the amendments of the protocol will be communicated. Patients and their HHC who are enrolled in the study will receive a detailed ‘Participant Information Sheet’ in local language before administering the informed consent. A separate consent form will be used for the adult participants enrolled in the substudy on micronutrient status and immune function. No blood specimen will be stored for any future use. A unique numerical code will be allocated to each participant for purpose of their identification and for maintaining confidentiality. Personal identifiers will be deleted in the final research database for analysis. All forms with personal identifiers will be under lock and key with the trial team.
Responsibility for ancillary care during the trial
Any index case or HHC found to have an acute illness other than TB during the follow-up visits will be facilitated by the field staff to reach the nearest government health set-up.
Patient and public involvement statement
Patients were not directly involved in the development of the research question. The components of food basket were discussed with community health workers during the preparatory phase of the trial. The training of the field staff involved interaction with TB survivors and two of the field staff in the trial are TB survivors.
Dissemination plan
The impact of nutritional support on TB incidence and outcomes in this trial will be of relevance to NTEP, India. The results will be disseminated through publications, conference presentations and briefs for the programme managers, Jharkhand’s department of health, policy-makers and other stakeholders. We intend to share the published results in simple language with the participants and community leaders.
Trial status
The trial was started on 16 August 2019 after trial registration and an intensive 2 weeks training of 56 field staff, two project consultants and one project director by state NTEP staff, nutritionist and specialists in TB, ethicist, TB champions, communication experts and social scientists. We have enrolled 2488 index cases and 9125 HHC in the trial as of 31 October 2020.
Supplementary Material
Acknowledgments
We wish to gratefully acknowledge the critical inputs of Professor Dick Menzies, Director McGill International TB Centre and Professor Andrea Benedetti, of McGill University's Departments of Epidemiology, Biostatistics and Occupational Health and Medicine, on early drafts of the protocol. We also wish to thank Dr Soumya Swaminathan, former Director-General, Indian Council of Medical Research, and Chief Scientist (WHO) for her support in exploring nutritional interventions to address the tuberculosis and undernutrition syndemic in India.
Footnotes
MB and BV contributed equally.
Contributors: AB conceived the research question. AB, BV, MB, KT and BK designed the study protocol and drafted the manuscript. BW, MS, RD, AM, RRP, KR and KSS reviewed the study protocol and provided critical intellectual content. All authors carefully read and approved the final version of the manuscript.
Funding: The Reducing Activation of Tuberculosis by Improvement of Nutritional Status study is supported by the India Tuberculosis Research Consortium, Indian Council of Medical Research, New Delhi, India, vide grant number 5/8/5/57/TB Consortium/Call India Project/2017/ECD-1. The funder has no role in the study design and writing of the protocol, and will not have any role in collection, management, analysis and interpretation of data; the writing and the decision to submit any future reports for publication.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.World Health Organization . Global tuberculosis report 2020. Geneva: World Health Organization, 2020. [Google Scholar]
- 2.World Health Organization . Implementing the end TB strategy: the essentials. WHO/HTM/TB/2015.31. Geneva: WHO, 2015. [Google Scholar]
- 3.Central TB Division, Ministry of Health and Family Welfare, Government of India . National strategic plan for TB elimination (2017-2025) 2017. http://tbcindia.gov.in/WriteReadData/NSP%20Draft%2020.02.2017%201.pdf
- 4.World Health Organization . Global tuberculosis report 2016. Geneva: World Health Organization, 2016. [Google Scholar]
- 5.Mangtani P, Abubakar I, Ariti C, et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis 2014;58:470–80. 10.1093/cid/cit790 [DOI] [PubMed] [Google Scholar]
- 6.Revised National TB Control Programme . Technical and operational guidelines for tuberculosis control in India. New Delhi: Central TB Division, Ministry of Health and Family Welfare, 2016. [Google Scholar]
- 7.WHO consolidated guidelines on tuberculosis . Module 1: prevention – tuberculosis preventive treatment. Geneva: World Health Organization, 2020. [PubMed] [Google Scholar]
- 8.Paton NI, Borand L, Benedicto J, et al. Diagnosis and management of latent tuberculosis infection in Asia: review of current status and challenges. Int J Infect Dis 2019;87:21–9. 10.1016/j.ijid.2019.07.004 [DOI] [PubMed] [Google Scholar]
- 9.Houben RMGJ, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med 2016;13:e1002152. 10.1371/journal.pmed.1002152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization . Physical status: the use and interpretation of anthropometry: report of a WHO expert Committee. Geneva: World Health Organization, 1995. [PubMed] [Google Scholar]
- 11.Cederholm T, Bosaeus I, Barazzoni R, et al. Diagnostic criteria for malnutrition - An ESPEN Consensus Statement. Clin Nutr 2015;34:335–40. 10.1016/j.clnu.2015.03.001 [DOI] [PubMed] [Google Scholar]
- 12.Cederholm T, Jensen GL, Correia MITD, et al. GLIM criteria for the diagnosis of malnutrition - A consensus report from the global clinical nutrition community. Clin Nutr 2019;38:1–9. 10.1016/j.clnu.2018.08.002 [DOI] [PubMed] [Google Scholar]
- 13.Bhargava A. Undernutrition, nutritionally acquired immunodeficiency, and tuberculosis control. BMJ 2016;355:i5407. 10.1136/bmj.i5407 [DOI] [PubMed] [Google Scholar]
- 14.Lönnroth K, Williams BG, Cegielski P, et al. A consistent log-linear relationship between tuberculosis incidence and body mass index. Int J Epidemiol 2010;39:149–55. 10.1093/ije/dyp308 [DOI] [PubMed] [Google Scholar]
- 15.Waitt CJ, Squire SB. A systematic review of risk factors for death in adults during and after tuberculosis treatment. Int J Tuberc Lung Dis 2011;15:871–85. 10.5588/ijtld.10.0352 [DOI] [PubMed] [Google Scholar]
- 16.International Institute for Population Sciences, ICF . National family health survey (NFHS-4), 2015–16: India. Mumbai: IIPS Mumbai, 2017. [Google Scholar]
- 17.Mazumdar S, Satyanarayana S, Pai M. Self-Reported tuberculosis in India: evidence from NFHS-4. BMJ Glob Health 2019;4:e001371. 10.1136/bmjgh-2018-001371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhargava A, Benedetti A, Oxlade O, et al. Undernutrition and the incidence of tuberculosis in India: national and subnational estimates of the population-attributable fraction related to undernutrition. Natl Med J India 2014;27:128–33. [PubMed] [Google Scholar]
- 19.Bhargava A, Chatterjee M, Jain Y, et al. Nutritional status of adult patients with pulmonary tuberculosis in rural central India and its association with mortality. PLoS One 2013;8:e77979. 10.1371/journal.pone.0077979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oxlade O, Huang C-C, Murray M. Estimating the impact of reducing under-nutrition on the tuberculosis epidemic in the central eastern states of India: a dynamic modeling study. PLoS One 2015;10:e0128187. 10.1371/journal.pone.0128187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grobler L, Nagpal S, Sudarsanam TD. Nutritional supplements for people being treated for active tuberculosis. Cochrane Database Syst Rev 2016;6:CD006086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox GJ, Barry SE, Britton WJ, et al. Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Respir J 2013;41:140–56. 10.1183/09031936.00070812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Enarson DA, Fanning EA, Allen EA. Case-finding in the elimination phase of tuberculosis: high risk groups in epidemiology and clinical practice. Bull Int Union Tuberc Lung Dis 1990;65:73–4. [PubMed] [Google Scholar]
- 24.Grandjean L, Gilman RH, Martin L, et al. Transmission of multidrug-resistant and drug-susceptible tuberculosis within households: a prospective cohort study. PLoS Med 2015;12:e1001843. 10.1371/journal.pmed.1001843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jubulis J, Kinikar A, Ithape M, et al. Modifiable risk factors associated with tuberculosis disease in children in Pune, India. Int J Tuberc Lung Dis 2014;18:198–204. 10.5588/ijtld.13.0314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morán-Mendoza O, Marion SA, Elwood K, et al. Risk factors for developing tuberculosis: a 12-year follow-up of contacts of tuberculosis cases. Int J Tuberc Lung Dis 2010;14:1112–9. [PubMed] [Google Scholar]
- 27.Food and Nutrition Security Analysis, India, 2019 . Ministry of statistics and programme implementation, the world food programme 2019.
- 28.de Vallière S, Barker RD. Poor performance status is associated with early death in patients with pulmonary tuberculosis. Trans R Soc Trop Med Hyg 2006;100:681–6. 10.1016/j.trstmh.2005.09.007 [DOI] [PubMed] [Google Scholar]
- 29.Central TB Division, Ministry of Health and Family Welfare, Government of India . India TB report 2018. New Delhi: Central TB Division, Ministry of Health and Family Welfare, Government of India, 2018. [Google Scholar]
- 30.Central TB Division, Ministry of Health and Family Welfare, Government of India . Guidance document on nutritional care and support for patients with tuberculosis in India. New Delhi: Central TB Division, Ministry of Health and Family Welfare, Government of India, 2017. [Google Scholar]
- 31.WHO Expert Consultation . Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–63. 10.1016/S0140-6736(03)15268-3 [DOI] [PubMed] [Google Scholar]
- 32.Alpers DH. Manual of nutritional therapeutics. 5th ed. New York: Lippincott Williams & Wilkins, 2008. [Google Scholar]
- 33.Trauer JM, Moyo N, Tay E-L, et al. Risk of active Tuberculosis in the five years following infection … 15%? Chest 2016;149:516–25. 10.1016/j.chest.2015.11.017 [DOI] [PubMed] [Google Scholar]
- 34.Bhargava A, Pai M, Bhargava M, et al. Can social interventions prevent tuberculosis?: the Papworth experiment (1918-1943) revisited. Am J Respir Crit Care Med 2012;186:442–9. 10.1164/rccm.201201-0023OC [DOI] [PubMed] [Google Scholar]
- 35.Teerenstra S, Lu B, Preisser JS, et al. Sample size considerations for Gee analyses of three-level cluster randomized trials. Biometrics 2010;66:1230–7. 10.1111/j.1541-0420.2009.01374.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oh J, Choi R, Park H-D, et al. Evaluation of vitamin status in patients with pulmonary tuberculosis. J Infect 2017;74:272–80. 10.1016/j.jinf.2016.10.009 [DOI] [PubMed] [Google Scholar]
- 37.Beloyartseva M, Mithal A, Kaur P, et al. Widespread vitamin D deficiency among Indian health care professionals. Arch Osteoporos 2012;7:187–92. 10.1007/s11657-012-0096-x [DOI] [PubMed] [Google Scholar]
- 38.Hall JC. A method for the rapid assessment of sample size in dietary studies. Am J Clin Nutr 1983;37:473–7. 10.1093/ajcn/37.3.473 [DOI] [PubMed] [Google Scholar]
- 39.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-047210supp001.pdf (40.1KB, pdf)