Abstract
The use of chitosan as an alternative for fungicides has received more attention worldwide. Hence, this study aimed to evaluate in vitro and in vivo antifungal activity of chitosan against Fusarium solani causing root rot in fenugreek. Chitosan treatments ranged from 0.1 to 2gL−1 were tested against F. solani on to potato dextrose agar and in potato dextrose broth. The results revealed that increase in concentrations of chitosan significantly reduced growth, dried biomass, sporulation and spore germination of F. solani. The hyphal swellings and distortion of F. solani mycelia were induced by chitosan. Fenugreek seeds treated with chitosan at 2 gL−1 and 0.5 gL−1 showed reduced F. solani infection and increased seed germination, respectively. In pot and field studies, fenugreek seeds treated with chitosan at 2.0 gL−1 greatly reduced root rot disease severity and also enhanced yield parameters. The activity of defence enzymes, such as chitinase, β-1, 3-glucanase and total phenol were increased in chitosan treated in fenugreek plants. This increased activity offered protection to fenugreek plants against F. solani to a greater extent. The results showed that chitosan could be used as inducer of defense response and has the potential of controlling fenugreek root rot disease.
Keywords: Trigonella foenum-graecum, Chitosan, Antifungal, Fusarium solani, Defense enzymes, Disease control
Introduction
Fenugreek (Trigonella foenum-graecum L.), a leafy vegetable and seed spice with medicinal properties and, has economic importance in national and international market. Fusarium solani is a soil-borne fungal pathogen and incitant of root rot disease in fenugreek in India (Ramteke et al., 2019). Fusarium solani can also infect different economically important crops like tomato, potato and pepper causing huge crop loss. To manage the root rot disease of fenugreek, growers are using different formulations of many synthetic fungicides. The sustainable use of synthetic fungicides is hazardous and toxic to both the people and domestic animals and that leads to environmental pollution (Ragab et al. 2012). Hence, there is a need of the hour to explore possible eco-friendly alternatives for synthetic fungicides that can reduce disease severity and crop loss in plants. The use of chitosan is one such environment friendly approach in managing root rot diseases in different crops. Chitosan is a deacetylated N-glucosamine natural polycationic linear biopolymer obtained from chitin. Chitin is a polymer N-acetyl glucosamine present in fungal cell walls and insect exoskeletons. Chitosan has a great demand in the market due to its nontoxicity, biodegradability, biocompatibility and fungicidal properties (Kumar 2000; Rinaudo 2006). The antifungal efficacy of chitosan is based on its solubility, degree of deacetylation, molecular weight and fungal species (Park et al. 2002; Goy et al. 2009). Chitosan controls pathogenic microorganisms by different mechanism viz., preventing growth, sporulation, spore viability, germination and disrupting cell and induction of different defense responses in host plant during the plant pathogen interactions (Hassan and Chang 2017). Several studies have showed that the chitosan has antifungal activity against a wide range of plant pathogens (Hernandez-Lauzardo et al. 2011; Bhattacharya 2013; Jabnoun-Khiareddine et al. 2015; Zivkovic et al. 2018; Mohammed et al. 2019). Currently, interest has been developed among the growers for the use of chitosan due to its antifungal properties in plant protection. Therefore, innate ability of chitosan to resist fungal pathogens using seed treatment has been enhanced. The several studies have demonstrated that the use of seed coating of chitosan controlled the different fungal pathogens. Bhaskara Reddy et al. (1999) reported that chitosan treatment of wheat seeds reduces seed borne F. graminearum infection and Lizarraga-Paulin et al. (2011) reported that seed coating on maize to control F. moniliforme. Recently, Abd-El-Kareem et al. (2018) showed that bean root rot disease caused by F. solani, Sclerotium rolfsii and Rhizoctonia solani was reduced by the application of chitosan under greenhouse conditions. Moreover, the use of chitosan increases photosynthesis, enhances plant growth, stimulates nutrient uptake, increases seed germination and plant vigour (Gavhane et al. 2013). Survey of literature showed potential of chitosan to manage F. solani causing root rot on fenugreek is unexplored. Therefore, the present investigation has been undertaken to evaluate the antifungal activities of chitosan in terms of reduction in mycelial growth, dry biomass, sporulation, spore germination of F. solani and to study its impact on root rot disease development under glasshouse and field conditions.
Materials and methods
Pathogen
Fusarium solani FS5 (NFCCI-4501) isolate was obtained from National Fungal Culture Collection of India, Agharkar Research Institute, Pune, India. Earlier studied by Ramteke et al. (2019) investigated the F. solani (FS5) causes root rot disease of fenugreek in India. Fusarium solani was maintained on potato dextrose agar (HiMedia, India), in the dark at 25 °C and stored at 4 °C for further studies.
Fenugreek seeds
The seeds of fenugreek cv. Jaora were purchased from local market in Pune, India.
Fungicide
The commercial grade Carbendazim (Dhanustin 50% WP) was provided by Dhanuka Agritech Ltd., India.
Chitosan
The extra pure chitosan powder with a degree of deacetylation 76.4% was procured from ISF Chitin and Marine Products, LLP (Ernaculum, Kerala, India). Chitosan was dissolved in 0.25 N HCl followed by centrifugation at 1000 rpm for 15 min to remove undissolved particles. To precipitate the chitosan, the viscous solution was neutralized with 2.5 N NaOH solution. The precipitated chitosan was filtrated through two layered muslin cloth and thoroughly washed with distilled water to remove salts and impurities. Further, chitosan was dried at 60 °C for 24 h and used for different studies. A 20 gL−1 stock solution of chitosan was prepared by dissolving it in 0.1 M glacial acetic acid and heated to 55 °C with constant agitation for 24 h. The final volume of chitosan solution was taken to 200 mL adjusted with distilled water and maintained pH of 5.6 by adding 1 N NaOH. Chitosan stock solution was autoclaved for 20 min at 121 °C.
Effect of chitosan on radial growth of F. solani
An antifungal activity of different concentrations of chitosan was determined against F. solani by a radial hyphal growth bioassay on PDA medium (Al-Hetar et al. 2011). Conical flasks containing PDA medium amended with each concentration of chitosan viz. 0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.75, 1, 1.5 and 2 gL−1 and poured in sterilized Petri plates of 90 mm diameter. A 5 mm culture plug of F. solani from seven days old colony was placed upside down in the center of the Petri plates. Four replications were maintained for each concentration of chitosan and the experiment was repeated thrice in a completely randomized design. The plates were sealed with parafilm and incubated in dark at 27 ± 1 °C. The plates without chitosan were treated as control. The radial growth of the test pathogen F. solani was measured on every day for up to 8 days of post-inoculation (dpi) along with the control. The untreated control Petri plates showed complete growth of 90 mm at 8 dpi. The percentage of inhibition zone of radial growth of F. solani was calculated using the formula (Tiru et al. 2013) as follows: IC % = (C1 − C2)/C1 × 100, where: IC % is the percentage of inhibition, C1 is the mean diameter of growth in the control and C2 is the mean diameter of growth in chitosan treatment. The mycelia from the margin of 6 days old colonies of F. solani grown in chitosan amended and control plates were observed for morphological changes under compound microscope model LB 460 (Labline, India).
Effect of chitosan on growth of F. solani in potato dextrose broth
The chitosan solutions and potato dextrose broth (PDB) medium were autoclaved separately at 121 °C for 20 min and combined after autoclaving to obtain various concentrations viz. 0, 0.1, 0.5, 0.75, 1 and 2 gL−1. The in vitro assay was performed in 24-well plate with four replications for each treatment. In each well poured 2 mL of PDB medium amended with different concentrations of chitosan as mentioned above. The well poured only with PDB medium was treated as a control. The 100 µL of conidial suspension of F. solani was added in each well and incubated at 25 °C. After 10 days of incubation the dry biomass of the fungus was determined by drying at 60 °C for 24 h. The percentage inhibition of mycelial growth based on dry weight of biomass was calculated according to the method described by Al-Hetar et al. (2011). This experiment was repeated thrice and average values were presented in final dataset.
Effect of chitosan on sporulation of F. solani
The effect of chitosan on sporulation of F. solani was assessed using PDB medium amended as described above. Mycelial growth of F. solani was permitted to extend to the edge of the control plates and after 14 days of incubation at room temperature, four plates for each treatment were flooded with 10 mL sterile-distilled water, which was then placed on an orbital shaker running at 140 rpm for 20 min to liberate spores from the mycelia, 100 µl of suspension was pipette out and counted the number of spores per mL using a Neubauer-hemocytometer under the compound microscope (40×). Four replications were maintained for each concentration and the experiment was repeated thrice. The percentage inhibition of sporulation (PS) was calculated using the formula: PS = sporulation in control − sporulation in chitosan treatment/sporulation in control × 100.
Effect of chitosan on spore germination of F. solani
To assess the effect of chitosan on spore germination of F. solani, a conidial suspension was prepared from 14 days old culture and 100 μL aliquot of a conidial suspension of 1 × 105 spores mL−1 were plated on 0.5% agar plate containing different concentration of chitosan viz, 0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.75, 1, 1.5 and 2 gL−1 separately and plates were incubated at 27 °C. After 8 h of incubation, the spore germination was measured for approximately 200 spores per treatment under compound microscope (100×). The spores were scored as germinated when germ tube length was equal or more than the diameter of spore. The experiment was repeated three times with three replications of each concentration. The percent inhibition of spore germination (% SG) was calculated using the formula (Plascencia et al. 2003). The percent inhibition of spore germination (% SG) = percent inhibition of spore germination in control − percent inhibition of spore germination in chitosan treatment/percent inhibition of spore germination in control × 100.
Effect of seed treatment on seed infection by F. solani
The fenugreek seeds were treated with different concentration of chitosan viz., 0.5, 1 and 2 gL−1. The sterile distilled water used as a negative control and carbendazim 50% WP fungicide @ 0.5 gL−1 was used as a positive control. After 24 h of treatment, 25 seeds were kept in 90 mm Petri dish and 1 × 105 mL−1 conidial suspension was sprayed on seeds using hand atomizer and incubated at 25 °C under 12 h alternate light and darkness. Four replications were maintained for each concentration and the experiment was repeated twice. After 5 days of incubation, F. solani infection was observed under stereomicroscope (5×) and percentage of infected seeds were calculated using formula: percent seed infection = number of seed infected/total number seed observed × 100.
Effect of seed treatment with chitosan on disease incidence in pot experiments
The plastic pots having diameter of 15 cm were sterilized with 2% formalin and left for 2 h to dry and filled with autoclaved sandy loam soils. About 100 fenugreek seeds were treated with 0.5, 1 and 2 gL−1 of chitosan. The untreated seeds served as negative control and carbendazim 50% WP at 0.5 gL−1 treated seeds as a fungicide control. Each pot was filled with 5 kg of sterile soil and nine replications of each treatment were maintained. Each pot was infested with 100 mL of F. solani spore suspensions (1 × 105 conidia mL−1). After 24 h, 50 seeds of respective treatments were sown in each pot and sterile water irrigated at an interval of 24 h. After 7 days, seed germination was recorded and radicle length was measured using camel ruler (30 cm). After 30 days, 25 seedlings were randomly selected and root rot disease was recorded using the rating scale as follows: 0 = no root rot; 1 = 1 to 25% of roots with visible lesions or root rot; 2 = approximately 26 to 50% of the roots rot or damaged; 3 = 51 to 75% of the root rot and 4 = 76 to 100% root rot or completely damaged, four categories were used previously by Abo-Elyousr et al. 2014; Ali et al. 2018 and per cent disease index (PDI) was estimated according to Ali et al. (2018).
Field experiments for assessment of chitosan against root rot disease
In order to assess the effect of chitosan on fenugreek root rot disease under field conditions, two experiments were conducted during November–December, 2019 and July–August, 2020 at Green Cultivate Agro Farm, Manjari, Pune, Maharashtra, India. (Longitude 18.511974 °N and Latitude 73.982261 ºE). About 1 kg of fenugreek seeds were treated with chitosan at the concentration of 0.5, 1 and 2 gL−1 and carbendazim 50% WP (0.5 gL−1) as fungicide control. For seed sowing, 10 × 6 sq. feet area of soil bed was prepared. The fenugreek seeds were sowed in the main field after 15-min soaking in respective treatment solution. The experiment was arranged in a randomized block design (RBD) and each treatment was replicated by four times. After 30 days of sowing, the observation of root rot disease incidence was recorded and PDI was estimated according to Ali et al. (2018).
Plant growth parameters in fenugreek plants
For studying plant growth parameters, 25 seeds were sown in plastic pots containing sandy loam soil. The seedlings were grown in a pot and field conditions. Application of chitosan with different concentrations was carried out by soaking fenugreek seeds in chitosan solution for 15 min before sowing. At 30 days old, the plants were harvested and plant height was recorded using camel ruler (30 cm). The number of leaves per plant was also measured. Fresh weight and dry weight of total plant were also measured along with the control. For dry weight, plant tissues were oven-dried at 80 °C for 24 h. Four replications were maintained for each concentration and the experiment was repeated twice.
Total phenol content in fenugreek plants
The 30-day-old fenugreek plants were dried at 40 °C in hot air oven and whole fenugreek plant material (shoot + root) of (10 g) from each sample was used for preparation of extract. Samples were subjected for extraction with 75 mL (80% v/v) ethanol at 40 °C for 10 min; the extraction process was repeated thrice. The total phenolic content in plant extracts was determined using the Folin–Ciocalteu reagent method described by Ainsworth and Gillespie (2007). The 0.2 mL extract was mixed with 0.6 mL of water and 0.2 mL of Folin–Ciocalteu’s reagent (1:1). After 5 min, 1 mL of (8% w/v Na2CO3 in water) sodium carbonate was added in the mixture and the volume was made up to 3 mL using distilled water. The reaction was kept in the dark for 30 min and the absorbance was measured at 765 nm against the reagent blank using a spectrophotometer (UV–Vis Spectrophotometer). The phenolic content was calculated as gallic acid equivalents GAE/g of dry of fenugreek plant on the basis of a standard curve of gallic acid (5–500 mg L−1). This test was carried out in triplicate and repeated twice.
Chitinase and β-1,3-glucanase enzymes assay
Fenugreek seedlings from pot and field studies were harvested after 30 days of seed treatment for evaluating defense-related enzyme activities and harvested seedlings were immediately kept in liquid nitrogen, lyophilized and then crushed to a fine powder using mortar and pestle. 1 g of crushed powder was added in 10 mL of 0.05 M sodium phosphate buffer (pH 6.5) and dissolved by vortex for 2 min and then centrifuged at 20,000 rpm for 15 min at 4 °C. After centrifugation, the supernatant was considered as the crude extract and used for its protein estimation and enzyme activity. The total protein content in crude extract was determined by comparison with bovine serum albumin (BSA) standard (Bradford 1976).
The chitinase activity was performed by the dinitrosalicylic acid method (Dai et al. 2011). For assay, mixture containing 20 µg mL−1 colloidal chitin as the substrate and 1 mL aliquot of enzyme crude extract was incubated at 40 °C for 24 h, the reaction was stopped by the addition of 0.8 ml of 0.03 M dinitrosalicylic acid and boiled for 10 min. After cooling, the reducing sugar released during reaction by chitinase activity was measured at 575 nm (Nelson 1944). The activity of chitinase enzyme was expressed as µg of glucosamine (GlcN) per mg−1 of protein per min−1 of incubation period. The β-1, 3-glucanase activity was performed according to the method described by Chandrasekaran et al. (2017) with slight modifications. Briefly, the extract from both, the control and inoculated plants were used for the assay. The reaction mixture (1 mL) consisted of 200 mL extract sample, 600 µL buffer (0.05 M sodium phosphate buffer (pH 6.5) and 200 µL of 2% laminarin as a substrate. This reaction mixture incubated at 40 °C for 1 h. After incubation, the released glucose was further assayed using the method of dinitrosalicylic acid (DNSA). The β-1, 3-glucanase activity was calculated using the standard curve procedure.
Statistical analysis
The data obtained during in vitro and in vivo (pot and field experiments) were analyzed using analysis of variance (ANOVA) through SAS version 9.3; SAS Institute Inc., Cary, NC, USA. The mean value of treatments was compared using Tukey’s studentized range test using SAS system.
Results
Effect of chitosan on in vitro growth, dry weight, sporulation, spore germination and morphology of F. solani
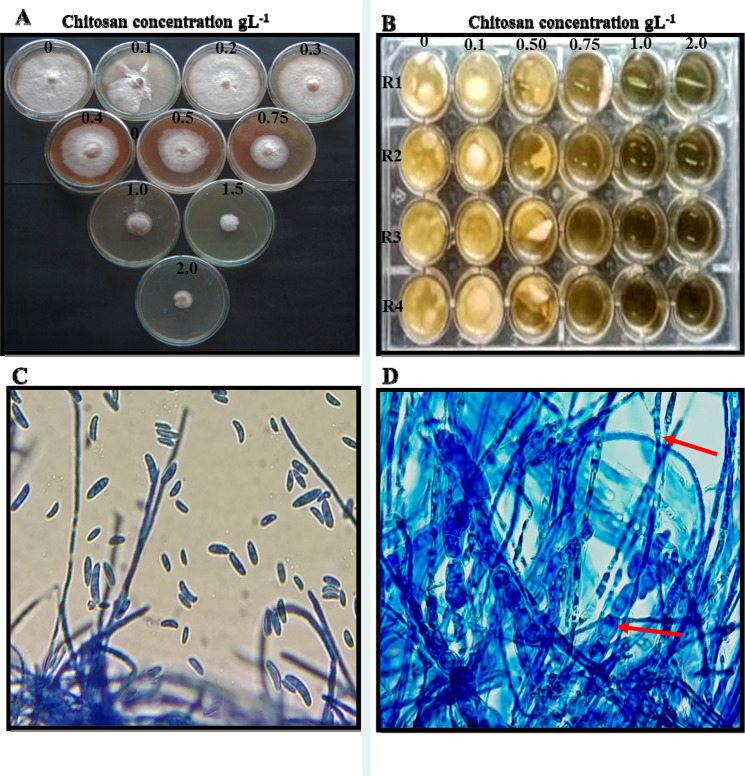
An antifungal activity of different concentrations of chitosan was determined against F. solani by a radial hyphal growth bioassay on to PDA medium. The chitosan amended PDA medium inhibited radial mycelial growth of F. solani. The inhibitory effect of chitosan on F. solani was increased with the increase in concentration up to 2 gL−1. The inhibition obtained was alone due to chitosan concentration, with a maximum radial growth inhibition of 98.04% recorded at 2 gL−1 (Table1, Fig. 1A). After 7 days of incubation, dry biomass of F. solani was determined and presented in Table 1 and Fig. 1B. The dry biomass was significantly decreased with an increase of chitosan concentrations (0.1, 0.5, 0.75, 1 and 2 gL−1) when compared with control. The maximum inhibition (96.71%) of dry biomass was recorded at chitosan (2 gL−1). Also, microscopic observation showed that the chitosan-induced morphological changes, such as hyphal swelling, distortion mycelium of F. solani (Fig. 1C). Moreover, chitosan significantly reduced the sporulation of F. solani with increased tested concentrations i.e., 86.37% at 2 gL−1 (Table 1). The results obtained for spore germination were presented in Table 1. After 18 h of incubation, chitosan at all tested concentrations (0.1–2 gL−1) were showed reduced spore germination of F. solani ranged from 30.67 to 99.7% as compared to spore germination (10.73%) in untreated control. Microscopic observation of F. solani grown on PDA (without chitosan) did not show any morphological anomaly (Fig. 1C). The morphological changes, such as hyphal swelling and distortion in F. solani were induced on PDA amended with chitosan (Fig. 1D).
Table 1.
Effect of chitosan on radial growth, dry biomass, sporulation and spore sporulation of F. solani FS 5
| Chitosan concentration (gL−1) | Inhibition (%) | |||
|---|---|---|---|---|
| Radial growth | Dry biomass | Sporulation | Spore germination | |
| 0 | 0.00h | 0.0e | 00.00f | 10.73h |
| 0.1 | 9.02g | 11.10d | 18.18e | 30.67g |
| 0.2 | 10.98g | ND | 25.49de | 52.13f |
| 0.3 | 14.90f | ND | 27.24de | 62.43e |
| 0.4 | 19.99e | ND | 31.85cd | 69.97d |
| 0.5 | 47.71d | 80.04c | 37.62c | 74.93c |
| 0.75 | 60.59c | 89.34b | 47.20b | 79.3b |
| 1.0 | 94.90b | 94.06ab | 78.64a | 95.27ab |
| 1.5 | 96.86ab | ND | 82.71a | 98.47a |
| 2.0 | 98.04a | 96.71a | 86.37a | 99.7a |
| CD | 2.71 | 6.31 | 9.30 | 4.13 |
The bold numbers indicate the CD value obtained by statistical analysis of the data, values within columns followed by the same letters are not significant different P = 0.05 according to Tukey’s Studentized Range (HSD) Test
ND not determined
Fig. 1.
A Inhibition of radial growth of F. solani FS5 by different concentrations of chitosan amended in potato dextrose agar. B Growth inhibition of F. solani FS5 at concentrations of chitosan amended in potato dextrose broth in 24 well plate. Microscopic observation (400×) mycelial of F. solani FS5, C grown on PDA; D grown on PDA amended with 0.5 gL−1 chitosan. Red arrow color showing distortion of F. solani mycelium
In vitro fenugreek seed infection assay and germination efficacy
Six days after inoculation, the infection of F. solani on fenugreek seed was recorded 89.60% in untreated control (T1) (Table 2, Fig. 2A). Although, on chitosan treated seeds at 0.5–2.0 gL−1, the infection was reduced from 44.00 to 11.20%. Moreover, in carbendazim (0.5 gL−1) treated seeds, the infection was 8.80%. The carbendazim was found to inhibit the highest infection (8.80%) of F. solani on fenugreek seeds followed by chitosan dose @ 2.0, 1.0 and 0.5 gL−1. The seed germination was found to be significantly higher (98.40%) at chitosan (0.5 gL−1) concentration as compared to control (96.80%) and carbendazim treated seeds (97.60). The radicle length of fenugreek seedlings due to chitosan (0.5 gL−1) was also observed significantly higher (3.76 cm) over control (2.26 cm) and carbendazim (3.34 cm) (Table 2, Fig. 2A).
Table 2.
Effect of chitosan on fenugreek root rot disease incidence and growth parameters
| Treatment | In vitro seed treatment | ||
|---|---|---|---|
| F. solani infection (%) | Seed germination efficacy (%) | Radicle length (cm) | |
| Control | 89.60d | 96.80a | 2.26c |
| Chitosan 0.5 gL−1 | 44.00c | 98.40a | 3.76a |
| Chitosan 1 gL−1 | 14.40b | 96.80a | 3.32ab |
| Chitosan 2 gL−1 | 11.20b | 96.00a | 2.80bc |
| Carbendazim 0.5 gL−1 | 8.80a | 97.60a | 3.34ab |
| CD | 1.80 | 4.01 | 0.57 |
A number indicated in bold are the CD values obtained by statistical analysis of the data, values within columns followed by the same letters indicates no significant difference at P = 0.05 according to Tukey’s Studentized Range (HSD) Test
Fig. 2.
Effect of chitosan seed treament (2 gL−1) on fenugreek seed germination, radicle growth, infection of F. solani. In vitro study: A control, B treated; pot study: C control, D treated; field study: E control, F treated
Effect of chitosan on root rot disease incidence in fenugreek plants in pot and field experiments
The percent disease index (PDI) of root rot disease in pot and field experiments was presented in Table 3. The root rot disease incidence was recorded since initiation of the disease, i.e., at 16 DAT (days after treatment) and to the last i.e., terminal observation which was recorded at 30 DAT. In pot experiment, untreated control PDI was 68.25%. Although, the lowest mean of PDI 9.75 was recorded in carbendazim treated seedlings. However, PDI 13.75 followed by 16.5 and 20.5 were recorded at 2, 1 and 0.5 gL−1 chitosan concentrations, respectively. Moreover, in field experiment conducted during November–December2019; chitosan (2 gL−1) treatment showed PDI 22.00 as compared to untreated control (PDI 66.70), but slightly more than fungicide control (PDS 18.50). The efficacy of chitosan increased with increase in concentrations in pot and field experiments. However, in field experiment conducted during July–August2020, the lowest root rot incidence was recorded at chitosan (2 gL−1) treatment (PDI 21.50) as compared to untreated control (PDI 72.20), but statistically at par with fungicide control (PDI 21.20).
Table 3.
Effect of chitosan on root rot disease incidence and growth parameters in fenugreek
| Treatment | Pot experiment | Field experiment | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nov–Dec 2019 | Jul–Aug 2020 | ||||||||||||||
| PDI | Number of leaves | Plant Height (cm) | Fresh weight (g) | Dry weight (g) | PDI | No. of leaves | Plant height (cm) | Fresh weight (g) | Dry weight (g) | PDI | Number of leaves | Plant height (cm) | Fresh weight (g) | Dry weight (g) | |
| Control | 68.25d | 18.50a | 16.60c | 0.93c | 0.19c | 66.70e | 16.90 c | 15.30 c | 0.76c | 0.15c | 72.20d | 17.70d | 15.60c | 0.78c | 0.12d |
| Chitosan 0.5 gL−1 | 20.5c | 24.60b | 22.10b | 1.23b | 0.25b | 38.40d | 27.70 b | 21.90 b | 1.09b | 0.22b | 38.11c | 24.40c | 24.20b | 1.21b | 0.15b |
| Chitosan 1 gL−1 | 16.5cd | 27.60a | 25.20ab | 1.38a | 0.27a | 27.60c | 28.50 ab | 25.10ab | 1.26ab | 0.25ab | 27.00b | 27.80c | 27.50a | 1.38a | 0.28b |
| Chitosan 2 gL−1 | 13.75bc | 28.20a | 27.40a | 1.41a | 0.28a | 22.00b | 30.40a | 27.60a | 1.38a | 0.28a | 21.50a | 29.90a | 28.20a | 1.41a | 0.33c |
| Carbendazim 0.5 gL−1 | 9.75a | 24.70b | 21.50b | 1.24b | 0.25b | 18.50a | 26.52 b | 22.60b | 1.13b | 0.23b | 21.20a | 28.70ab | 27.90a | 1.39a | 0.25b |
| CD | 5.80 | 1.80 | 3.88 | 0.09 | 0.01 | 2.65 | 2.11 | 3.74 | 0.18 | 0.037 | 2.62 | 1.94 | 2.14 | 0.11 | 0.018 |
A number indicated in bold are the CD values obtained by statistical analysis of the data, values within columns followed by the same letters indicates no significant difference at P = 0.05 according to Tukey’s Studentized Range (HSD) Test
Effect of chitosan on growth parameters in fenugreek plants
The number of leaves, plant height, fresh weight and dry weight of fenugreek plants after 30 days chitosan seed treatment were evaluated and presented in Table 3. In pot experiment, the number of leaf and height of plant (28.20, 25.20 cm) were recorded the highest at 2gL−1 chitosan when compared with untreated control (18.50, 16.60 cm) and fungicide control (24.70, 21.50 cm). The fresh and dry weights contents of fenugreek plants also significantly higher (1.41 g, 0.28 g) at 2gL−1 chitosan over untreated control (0.93 g, 0.19 g) and fungicide control (1.24 g, 0.25 g). Moreover, in 1st and 2nd field trials, significance difference was observed in number of plants, plant height, fresh and dry weights among chitosan treated plants, untreated control and fungicide control plants. In 1st field trial, the highest increase in no. of leaves (30.40), plant height (27.60 cm), fresh weight (1.38 g) and dry weight (0.28 g) were recorded at 2 gL−1 chitosan as compared to untreated control and fungicide control. More or less similar values for growth parameters were recorded in 2nd field trial. Overall, chitosan (2 gL−1) was found to be superior among pot and field trials (Fig. 2B and C).
Total phenolic content
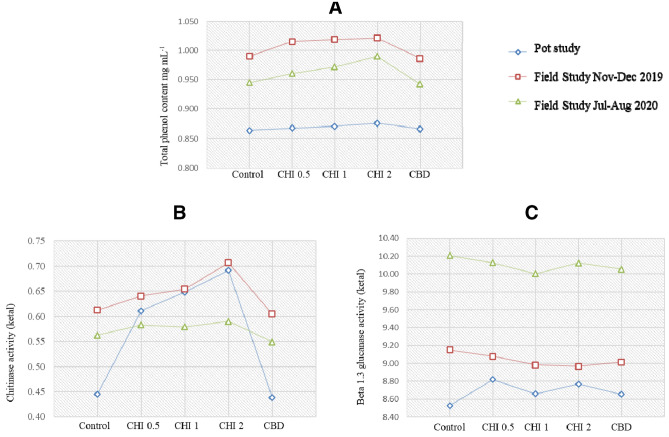
Phenolic activity was increased with increasing chitosan concentrations (0.5–2 gL−1). At higher chitosan (2 gL−1) concentration, total phenolic content was recorded the highest activity in 1st field trial (Nov–Dec, 2019) and found superior over untreated control and fungicide control (Fig. 3A).
Fig. 3.
Effect of chitosan on total phenol content (A), chitinase (B) and beta 1, 3 glucanase activity (C) in fenugreek., Control: untreated fenugreek plant, CHI 0.5, 1, 2: Chitosan 0.5 gL−1, 1 gL−1and 2 gL−1, respectively, CBD: carbendazim (50%WP) 0.5 gL−1
Induction of chitinase and β-1,3-glucanase enzymes
The results presented in Fig. 3B and C showed higher enzyme activity of chitinase and β-1, 3-glucanase in chitosan treated fenugreek plants as compared to fungicide control after 30 days. In field trial (Nov–Dec 2019), chitinase activity was increased followed by pot experiment and field trial at higher chitosan at 2 gL−1). The β -1, 3-glucanase activity was observed slightly higher in untreated control followed by chitosan at 0.5 gL−1) and chitosan at1 gL−1). However, β-1, 3-glucanase activity suddenly increased at chitosan (2 gL−1) and observed superior over fungicide control in field trial (July–August, 2020). Similar trend was observed in pot experiment and 1st field trial.
Discussion
Fusarium root rot disease caused by F. solani is the most common disease in fenugreek in India (Ramteke et al. 2019). For, management of this disease, farmers are dependent on the application of several number chemical fungicides. Although the sustained use of chemicals is hazardous and adversely affecting human health and environment. Hence, chitosan was evaluated for antifungal activity and induced resistance in fenugreek plants against F. solani. In the present study, the antifungal activities of chitosan against F. solani, a causa agent of root rot disease of fenugreek under in vitro and in vivo conditions were investigated. Chitosan showed significant effect on reduction in the radial growth of F. solani in PDA medium with increase in concentrations as compared to the control. The maximum mycelial growth reduction was observed using chitosan at 2 gL−1. Moreover, the dry biomass of F. solani was significantly decreased with increase in chitosan concentrations (i.e., 0.1, 0.5, 0.75, 1 and 2 gL−1) over control. Similarly, the results were recorded by many researchers with different crops against different pathogens. Mycelial growth of F. solani f. sp. phaseoli and F. solani f. sp. pisi was inhibited at the minimum concentrations of 12 and 18 mgmL−1, respectively (Kendra and Hadwiger 1984). The degree of inhibition of fungi is mostly correlated with chitosan concentration representing the application of appropriate dose of chitosan related to its performance (Baustista Banos et al. 2006). The mycelial growth of F. oxysporum the causative agent of wilt disease of pepper (Capsicum annum L.) was completely inhibited at 4.5 gL−1 chitosan (Ragab et al. 2012). Similarly, Bhattacharya (2013) reported that maximum inhibition of mycelial growth and sporulation of F. solani causing root rot of Coleus forskohlii by chitosan was achieved at 0.20% concentration. In addition to this, Jabnoun-Khiareddine et al. (2015) reported that chitosan applied at concentrations varying from 0.5 to 4 gL−1 is effective in inhibiting the radial growth of ten tomato pathogenic fungi, i.e., F. oxysporum f. sp. lycopersici, F. oxysporum f. sp. radicis-lycopersici, F. solani, Verticillium dahlia, Colletotrichum coccodes, Rhizoctonia solani, Pythium aphanidermatum, Sclerotinia sclerotiorum, Botrytis cineria and Alternaria solani. Complete inhibition of linear growth of Rhizoctonia solani and Sclerotium rolfsii causing root rot disease in bean (Phaseolus vulgaris L.) plants were obtained with chitosan at 8 gL−1 concentration (Abd-El-Kareem et al. 2018). Mohammed et al. (2019) showed that chitosan completely inhibited mycelial growth of Rhizoctonia solani causing black scurf disease in potato at 1% concentration. Al-Hetar et al. (2011) investigated the high sensitivity of F. oxysporum f. sp. cubense in PDB where mycelial growth completely inhibited at tested concentrations of chitosan. Similar results were also obtained on broth cultures in the present study. In general, sporulation of fungi treated with chitosan is reported to be lower than in untreated fungi. Moreover, in some reports, no spore formation was observed after chitosan treatment. However, recently, Ramteke (2019) studied the complete inhibition of spore formation of F. solani at 10 gL−1 chitosan treatment. The antifungal activity of chitosan is related to its ability to interfere with the plasma membrane function (Leuba and Stossel 1986) and the interaction of chitosan with fungal DNA (Hadwiger and Loschk 1981). Chitosan’s antifungal activities are due to its polycationic nature, so that cationic chitosan interacts negatively with charged fungal cell membrane components such as proteins, phospholipids, thus interfering with the normal growth and metabolism of the fungal cells (Bautista-Banos et al. 2006). The present results showed that chitosan works at threshold concentration for inhibiting mycelial growth, sporulation and spore germination of F. solani. Microscopic observation of F. solani treated with chitosan showed that the morphological changes, such as mycelial swelling and hyphal distortion in mycelium of F. solani. The present finding was in accordance with those of Al-Hetar et al. (2011) who reported that chitosan at concentrations of more than 1.6 mgmL−1 was found to induce morphological changes in F. oxysporum f. sp. cubense and in Alternaria alternata, Botrytis cineria, Penicillium expansum and Rhizopus stolonifer four phytopathogenic fungi Oliveira-Jr. et al. (2012). Moreover, in vitro assays showed that chitosan treated fenugreek seeds significantly reduced F. solani infection and improved seed germination and seedling radicle growth. Photchanachai et al. (2006) also reported that chitosan seed treatment reduced Colletotrichum sp. infection and improved seedling performance.
The PDI of root rot disease in fenugreek plants in pot and field studies was also significantly reduced after 30 days of chitosan seed treatment. Chitosan at higher concentration at 2 gL−1 showed great reduction in root rot disease severity caused by F. solani and found superior over untreated control and at par with standard fungicide. Chitosan applied to fenugreek seeds provided protection to fenugreek plants better or similar to that of carbendazim from root rot incidence. The protective in vivo effect of chitosan against pathogens has also been reported by numerous researchers. The use of chitosan for maize seed coating inhibited the growth of F. moniliforme (Lizarraga-Paulin et al. 2011). Jabnoun-Khiareddine et al. (2015) reported that chitosan applied as soil drench at 4 gL−1 concentration, showed varied degree of protection against Verticillium wilt, Fusarium wilt and Fusarium crown and root diseases of tomato in Tunisia. Abd-El-Kareem et al. (2018) showed that chitosan at 8 gL−1 was found effective in reducing bean root rot disease caused by F. solani, Sclerotium rolfsii and Rhizoctonia solani under greenhouse conditions.
Chitosan has been used as a natural seed treatment and plant growth enhancer in agriculture as an eco-friendly biopesticide to boost the innate ability of plants to defend themselves against infection of fungal pathogens (El-Sawy et al. 2010). In the present study, chitosan treatment enhanced yield contributing parameters in fenugreek such as number of leaves, height, fresh and dry weight of plants in pot and field experiments over untreated control and fungicide treated control. El-Tantawy (2009) also found that spraying the tomato plants with chitosan increased all vegetative growth parameters viz., plant height, number of branches, number of leaves, fresh and dry weight of plants. Similarly, Mondal et al. (2012) reported that application of chitosan increased similar growth parameters along with some biochemical and physiological parameters such as nitrate reductase and photosynthesis which enhanced the plant growth and development in okra (Hibiscus esculentus L.). In the current study, total phenolic contents were found to be accumulated more in chitosan treated fenugreek plants as compared to the untreated control and fungicide treated control in pot and field experiments. Similar findings were obtained in chitosan treated potato tubers against Fusarium species (Mejdoub-Trabelsi et al. 2020). It was interesting to note that the contents of chitinase, β-1,3-glucanase enzymes were found to be increased in chitosan treated plants over untreated control and fungicide treated plants in pot and field experiments. The reduction of root rot disease severity in fenugreek plants treated with chitosan treatments might be due to enhanced activity of defence enzymes produced against F. solani. Avdiushko et al. (1993) reported that the oxidative enzymes such as polyphenol oxidase, peroxidase and other enzymes, such as chitinase and β-1,3-glucanase enzymes are associated with defence reactions against plant pathogens. Ryals et al. (1996) stated that the development of systemic acquired resistance (SAR) is often found associated with various cellular defense enzyme responses such as synthesis of pathogenesis related (PR) proteins, phytoalexins and accumulation of reactive-oxygen species, rapid alterations in cell wall and enhanced activity of various defense related enzymes. Chitinase and β-1,3- glucanase degrades the fungal cell wall and cause lysis of fungal cells. These enzymes are associated with the host–pathogen interactions for protection of plants against fungal infections. The results from the present study showed chitosan offered a protection to fenugreek plants in pot and field experiments against F. solani in the form of SAR. Benhamou et al. (1998) observed that chitosan seed treatment induces resistance in susceptible tomato plants against F. oxysporum f. sp. radicis-lycopersici by restricting fungal growth to the outer rot tissues and eliciting defense reactions including structural barriers. The results revealed that chitosan has a dual effect. It acts as antimicrobial agent as well as activates plant defense response during host–pathogen interactions. These findings were in line with the recent studies on potato plants wherein chitosan increased the expression of defence-related genes and synthesis of lytic enzymes against pathogen example Rhizoctonia solani (Mohammed et al. 2019) and Fusarium species (Fitza et al. 2013; Mejdoub-Trabelsi et al.2020). Chitosan reduced root rot incidence and provided protection to fenugreek plants at a great extent. This may be due to induction of plant defense reactions responsible for creation of a toxic environment which adversely affecting the pathogen by causing fungal growth inhibition (Sarwar et al. 2005). The dual effects of chitosan led to reduced disease severity due to callose deposition, lignification, synthesis of abscisic acid, phytoalexins and PR proteins (Bautista-Banos et al. 2006; Cavalcanti et al. 2007; Jabnoun-Khiareddine et al. 2015). The current findings showed that chitosan is very effective against F. solani in vitro and in vivo field experiments and reduced root rot disease severity by induction of resistance with increased growth parameters of fenugreek plants. Further work is needed on detailed understanding on the exact mode of action of chitosan at molecular level.
In conclusion, present study showed inhibitory activity and plant defense response of chitosan against F. solani causing root rot disease of fenugreek. Moreover, growth parameters of fenugreek plant were increased by chitosan treatment. Based on the findings, from the present study, chitosan could be used as an alternative to fungicides to control root rot disease and enhance the growth of fenugreek. Due to nontoxic, elicitor as well as antifungal activities, chitosan has found to be the potential plant protectant against Fusarium root rot disease.
Acknowledgements
Authors are thanks to director of ICAR-National Research Centre for Grapes, Vasumitra Life Energies Pvt Ltd and principal of Raja Shripatrao Bhagawantrao Mahavidyalaya for permission of this work. Authors are also thanks to ISF chitin and Marine products LLP for suppling chitosan powder and Owner of Green Cultivate Agro Farm for providing land for field trial.
Author contributions
MRG project in charge, research plan and monitoring all the experiment, manuscript writing. PKR in vitro and pot study activity of chitosan against Fusarium solani. SDR total phenol estimation. PSK enzyme activity estimation. AL field observation. SKH in vitro antagonistic activity, microscopic observation and Manuscript writing. HJ manuscript writing.
Funding
NA.
Availability of data and material
Data will available on request.
Declarations
Conflict of interest
There is no conflict interest of authors.
Ethics approval
All the authors mentioned in the manuscript have agreed for authorship, read and approved the manuscript, and given consent for submission and subsequent publication of the manuscript.
Consent to participate
All the authors mentioned in the manuscript have agreed for authorship, read and approved the manuscript, and given consent for submission of the manuscript to 3 Biotech Journal.
Consent for publication
All the authors mentioned in the manuscript have agreed for authorship, read and approved the manuscript, and given consent for publication of the manuscript in 3 Biotech Journal.
References
- Abd-El-Kareem F, Saied NM, El-Mohamedy RSR. Seed treatment with chitosan and ethanol-extracted Propolis for suppression bean root rot disease under greenhouse conditions. J Mater Environ Sci. 2018;9:2356–2361. [Google Scholar]
- Abo-Elyousr KAM, Zein E-A, Hassan MHA, El-Sheakh MM. Enhance suppressive effect of compost on soybean Rhizoctonia root rot by soil treatment with Trichodermaharzianum. J Plant Physiol Pathol. 2014;2:2. doi: 10.4172/2329-955X.1000122. [DOI] [Google Scholar]
- Ainsworth EA, Gillespie KM. Estimation of total phenolic and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat Protoc. 2007;2:875–877. doi: 10.1038/nprot.2007.102. [DOI] [PubMed] [Google Scholar]
- Al-Hetar MY, ZainalAbidin MA, Sariah M, Wong MY. Antifungal activity of chitosan against Fusariumoxysporum f. sp. cubense. J Appl Polym Sci. 2011;120:2434–2439. [Google Scholar]
- Ali MZH, El-Sheikh Aly MM, Ghaly HMA, Abd-Elaziz MA. Biological control of damping-off and root rot of fenugreek. J Phytopathol Pest Manag. 2018;5:59–75. [Google Scholar]
- Avdiushko SA, Ye XS, Kuc J. Detection of several enzymatic activities in leaf prints cucumber plants. Physiol Mol Plant Pathol. 1993;42:441–454. [Google Scholar]
- Bautista-Banos S, Hernandez-Louzardo AN, Velazquez-del Valle MG, Hernandez-Lopez M, Ait BE, Bosquez-Molina E, Wilson CL. Chitosan as a potential natural compound to control pre and postharvest diseases of horticultural commodities. Crop Prot. 2006;25:108–118. [Google Scholar]
- Benhamou N, Kloepper JW, Tuzunm S. Induction of resistance against Fusarium wilt of tomato by combination of chitosan with an endophytic bacteria strain: ultrastructure and cytochemistry of the host response. Planta. 1998;204:153–168. [Google Scholar]
- Bhaskara Reddy MV, Arul J, Angers P, Couture L. Chitosan treatment of wheat seeds induces resistance to Fusariumgraminearum and improves seed quality. J Agric Food Chem. 1999;47:1208–1216. doi: 10.1021/jf981225k. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A. Fungicidal potential of chitosan against phytopathogenic Fusariumsolani. J Exp Biol Agric Sci. 2013;1:259–263. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cavalcanti FR, Resende MLV, Carvalho CPS, Silveira JAG, Oliveira JTA. An aqueous suspension of Crinipellisperniciosa mycelium activates tomato defense responses against Xanthomonasvesicatoria. Crop Prot. 2007;26:729–738. [Google Scholar]
- Chandrasekaran M, Belachew ST, Yoon E, Chun SC. Expression of β-1,3-glucanase (GLU) and phenylalanine ammonia-lyase (PAL) genes and their enzymes in tomato plants induced after treatment with Bacillussubtilis CBR05 against Xanthomonascampestris pv. vesicatoria. J Gen Plant Pathol. 2017;83:7–13. [Google Scholar]
- Dai DH, Hu WL, Huang GR, Li W. Purification and characterization of a novel extracellular chitinase from thermophilic Bacillus sp. Hu1. Afr J Biotech. 2011;10:2476–2485. [Google Scholar]
- El-Sawy NM, Hassan El-Rehim HAA, Elbarbary AM, Hegazy EA. Radiation-induced degradation of chitosan for possible use as a growth promoter in agricultural purposes. Carbohyd Polym. 2010;79:555–562. [Google Scholar]
- El-Tantawy EM. Behaviour of tomato plants as affected by spraying with chitosan and aminofort as natural stimulator substances under application of soil organic amendments. Pak J Biol Sci. 2009;12:1164–1173. doi: 10.3923/pjbs.2009.1164.1173. [DOI] [PubMed] [Google Scholar]
- Fitza KN, Payn KG, Steenkamp ET, Myburg AA, Naidoo S. Chitosan application improves resistance to Fusariumcircinatum in Pinuspatula. S Afr J Bot. 2013;85:70–78. [Google Scholar]
- Gavhane YN, Gurav AS, Yadav AV. Chitosan and its applications: a review of literature. Int J Res Pharm Biomed Sci. 2013;4:312–331. [Google Scholar]
- Goy RC, Britto DD, Assis OB. A review of the antimicrobial activity of chitosan. Polimeros. 2009;19:241–247. [Google Scholar]
- Hadwiger LA, Loschk DC. Molecular communication in host-parasite interactions: hexosamine polymers (chitosan) as regulator compounds in race-specific and other interactions. Phytopathology. 1981;71:756–762. [Google Scholar]
- Hassan O, Chang T. Chitosan for eco-friendly control of plant disease. Asian J Plant Pathol. 2017;11:53–70. [Google Scholar]
- Hernandez-Lauzardo AN, Velazquez-del Valle MG, Guerra-Sanchez MG. Current status of action mode and effect of chitosan against phytopathogens fungi. Afr J Microbiol Res. 2011;5:4243–4247. [Google Scholar]
- Jabnoun-Khiareddine H, El-Mohamedy RSR, Abdel-Kareem F, Aydi Ben Abdallah R, Gueddes-Chahed M, Daami-Remadi M. Variation in chitosan and salicylic acid efficacy towards soil-borne and air borne fungi and their suppressive effect of tomato wilt severity. J Plant Pathol Microbiol. 2015;6:325–334. [Google Scholar]
- Kendra DF, Hadwiger LA. Characterization of the smallest chitosan oligomer that is maximally antifungal to Fusariumsolani and elicits pisatin formation by Pisumsativum. Exp Mycol. 1984;8:276–281. [Google Scholar]
- Kumar MNR. A review of chitin and chitosan applications. React Funct Polym. 2000;46:1–27. [Google Scholar]
- Leuba JL, Stossel P. Chitosan and other polyamines: antifungal activity and interaction with biological membranes. In: Muzzarelli R, Jeuniaux C, Graham GW, editors. Chitin in nature and technology. New York: Plenum Press; 1986. pp. 215–222. [Google Scholar]
- Lizarraga-Paulin EG, Torres-Pacheco I, Moreno-Martínez E, Miranda-Castro SP. Chitosan application in maize (Zeamays) to counteract the effects of abiotic stress at seedling level. Afr J Biotech. 2011;10:6439–6446. [Google Scholar]
- Mejdoub-Trabelsi B, Touihri S, Ammar N, Riahi A, Daami-Remadi M. Effect of chitosan for the control of potato diseases caused by Fusarium species. J Phytopathol. 2020;168:18–27. [Google Scholar]
- Mohammed SR, Zeitar EM, Eskov ID. Inhibition of mycelia growth of Rhizoctoniasolani by chitosan in vitro and in vivo. Open Agric J. 2019;13:156–161. [Google Scholar]
- Mondal MMA, Malek MA, Puteh AB, Ismail MR, Ashrafuzzaman M, Naher L. Effect of foliar application of chitosan on growth and yield in okra. Aust J Crop Sci. 2012;6:918–921. [Google Scholar]
- Nelson N. A photometric adaption of the Somogyi method for the determination of glucose. J Biol Chem. 1944;153:375–380. [Google Scholar]
- Oliveira-Jr EN, El Gueddari NE, Moerschbacher BM, Franco TT. Growth rate inhibition of phytopathogenic fungi by characterized chitosans. Braz J Microbiol. 2012;43:800–809. doi: 10.1590/S1517-83822012000200046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Marsh KS, Rhim JW. Characteristics of different molecular weight chitosan film affected by the type of organic solvents. J Food Sci. 2002;67:194–197. [Google Scholar]
- Photchanachai S, Singkaew J, Thamthong J. Effects of chitosan seed treatment on Colletotrichum sp. and seedling growth of chilli cv. ‘Jinda’. Acta Hortic. 2006;712:585–590. [Google Scholar]
- Plascencia MG, Viniegra G, Olayo R, Castillo M, Shirai K. Effect of chitosan and temperature on spore germination of Aspergillusniger. Macromol Biosci. 2003;3:582–586. [Google Scholar]
- Ragab MM, Ashour AMA, Abdel-Kader MM, El-Mohamady R, Abdel-Aziz A. In vitro evaluation of some fungicide’s alternatives against Fusariumoxysporum the causal of wilt disease of pepper (Capsicumannum L.) Int J Agric for. 2012;2:70–77. [Google Scholar]
- Ramteke PK. Effect of resistance inducers on in vitro inhibition of mycelial growth and sporulation of Fusariumsolani causing root rot of fenugreek. Plant Pathol Quar. 2019;9:198–209. [Google Scholar]
- Ramteke PK, Ghule MR, Ramteke SD. First report of Fusariumsolani causing root rot on fenugreek (Trigonellafoenum-graecum L) in India. Plant Dis. 2019;104:992. [Google Scholar]
- Rinaudo M. Chitin and chitosan: properties and applications. Prog Polym Sci. 2006;31:603–632. [Google Scholar]
- Ryals JA, Neuenschwander MG, Willits A, Molina A, Steiner HY, Hunt MD. Systemic acquired resistance. Plant Cell. 1996;8:1809–1819. doi: 10.1105/tpc.8.10.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwar N, Zahid CMH, Ikramul HAQ, Jamil FF. Induction of systemic resistance in chickpea against Fusarium wilt by seed treatment with salicylic acid and bion. Pak J Bot. 2005;37:989–995. [Google Scholar]
- Tiru M, Muleta D, Bercha G, Adugna G. Antagonistic effects of rhizobacteria against coffee wilt disease caused by Gibberellaxylarioides. Asian J Plant Pathol. 2013;7:109–122. [Google Scholar]
- Zivkovic S, Stevanovic M, Durovic S, Ristic D, Stosic S. Antifungal activity of chitosan against Alternariaalternata and Colletotrichumgloeosporioides. Pestic Phytomed. 2018;33:197–204. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will available on request.