Introduction
Herpetic geometric glossitis (HGG) represents an atypical manifestation of herpes simplex virus (HSV) infection that usually affects immunocompromised patients. It was first described in 1993 by Grossman et al.1 Clinical presentation may include longitudinal fissures with perpendicular ramifications, papules, erythematous nodules and ulcers over the tongue, all of which are associated with extreme pain and good response to antiviral therapy.2,3
Reports of this entity in the literature are scarce, even in its classic clinical manifestation. We report a case of HGG exhibiting an atypical pattern of vegetative plaques.
Case report
A 32-year-old man presented to the emergency room because of progressive shortness of breath for the last 2 months, unintentional weight loss of 40 pounds, and a recent diagnosis of AIDS due to HIV infection. His chest radiograph and bacteriological studies confirmed Pneumocystis jiroveci pneumonia.
On the seventh day of his hospital stay, the patient developed stomatitis with involvement of the upper lip, vermilion border of the lower lip and the middle and anterior third of the dorsal surface of the tongue. Physical examination revealed numerous painful papules around 3 mm in diameter that tended to cluster and form vegetative plaques measuring 0.6-1.2 cm with defined and scalloped borders, with an ulcerated surface with blood that rested on a constitutional fissured tongue (Fig 1). The remainder of the cutaneous examination was unremarkable. We requested a venereal disease research laboratory test, a fluorescent treponemal antibody absorption, and a Tzanck smear. Serological tests were negative, whereas the Tzanck smear revealed multinucleated giant cells with basophilic and pale cytoplasm (Fig 2).
Fig 1.
Herpetic geometric glossitis; visual appearance.
Fig 2.
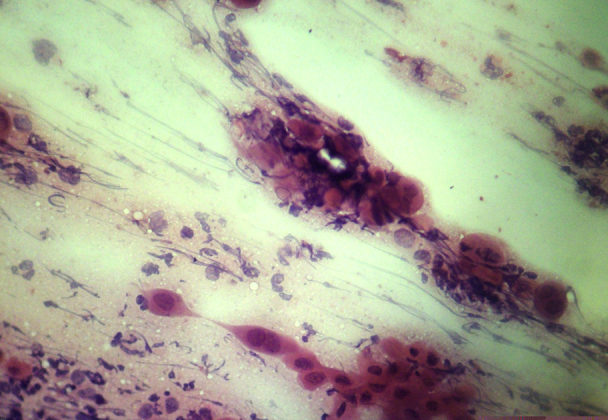
Tzanck smear with giant multinucleated epithelial cells.
Based on the presence of multinucleated giant epithelial cells, the morphology of the lesions, and a history of cold sores in the medical interview, we diagnosed HGG. The patient was treated with acyclovir 400 mg every 8 hours for 10 days. He experienced symptomatic relief over the first 48 hours and exhibited complete remission on the sixth day of treatment (Fig 3).
Fig 3.
Clinical remission after 10 days of treatment.
Discussion
The seroprevalence of infection with HSV types 1 and 2 among persons aged 14-49 years living in the United States was 53.9% and 15.7% from 1999 to 2010, respectively,4 and has shown stable declines in both sexes up to 2016.5
Clinically, HSV reactivations affect the vermilion border and adjacent keratinized oral mucosa. Nevertheless, immunocompromising conditions are associated with more chronic, severe, and atypical reactivations with involvement of multiple sites and an increased risk of viral spread, which persists up to 3 weeks after the clinical resolution of the lesions.6 Of these atypical reactivations, HGG shows a characteristic branching pattern that resembles the dendritic corneal ulcers produced by HSV. Other clinical manifestations of HGG include papules, erythematous nodules, and ulcers over the tongue, all of which are associated with severe pain.1, 2, 3,7
During the evaluation of patients with chronic HIV infection and sudden development of oral lesions, it is important to rule out other infectious conditions, such as secondary syphilis. Oral manifestations of Treponema pallidum can mimic oral herpes infection, candidiasis, and oral hairy leukoplakia, and may also be observed without the classical rash with macules and papules that involves palms and soles; for this reason, venereal disease research laboratory and fluorescent treponemal antibody absorption tests in HIV patients are justified.8 Also, it is important to keep in mind that atypical hypertrophic variants of HSV that resemble squamous cell carcinoma of the anogenital region have been described in patients with HIV, which highlights the need to biopsy lesions with rapid growth before any therapeutic procedure is offered.9
The diagnosis of HSV is based on the onset of classic symptoms and morphology and distribution of lesions, but in immunocompromised patients it is often necessary to confirm the diagnosis with laboratory tests. The Tzanck smear is an economical, accessible, and fast tool available in most clinics; nevertheless, it does not have the specificity to distinguish between different types of herpes viruses. Since HIV-positive patients have an elevated cancer risk, use of cytology as a diagnostic test is not recommended in chronic cases of tumor-like, vegetative, or ulcerated HSV lesions because it has only moderate reliability for tumoral diseases; thus, malignancies could be overlooked. Viral culture allows the precise identification of HSV 1 or 2 and also identifies acyclovir-resistant HSV. Polymerase chain reaction for HSV has 95% sensitivity and specificity and is considered the gold standard for the diagnosis of herpetic meningitis and encephalitis. Histological analysis with immunostaining also confirms the diagnosis, and it can also differentiate between HSV 1 and 2.6,9,10
Treatment of uncomplicated mucocutaneous lesions can be achieved with oral acyclovir 400 mg every 8 hours, famciclovir 500 mg every 12 hours, or valacyclovir 1 g every 12 hours for 5-10 days. For severe mucocutaneous HSV infections, intravenous acyclovir treatment is recommended initially at a dose of 5 mg/kg every 8 hours; when lesions begin to improve, therapy can be switched to the oral route, and antiviral therapy must be continued until healing of lesions is complete. Patient with HIV who have severe or frequent recurrences are candidates for chronic suppressive therapy, which should be evaluated annually by an infectious disease specialist.10
This case report describes the rare clinical presentation of HGG as vegetative plaques that coexisted on a constitutional fissured tongue and highlights the quick favorable response to acyclovir treatment. Also, this case underscores the importance of considering atypical HSV clinical manifestations in the differential diagnosis of oral or anogenital dermatoses that present in patients with HIV.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
References
- 1.Grossman M.E., Stevens A.W., Cohen P.R. Brief report: herpetic geometric glossitis. N Engl J Med. 1993;329(25):1859–1860. doi: 10.1056/NEJM199312163292506. [DOI] [PubMed] [Google Scholar]
- 2.Theriault A., Cohen P.R. Herpetic geometric glossitis in a pediatric patient with acute myelogenous leukemia. Am J Clin Oncol. 1997;20(6):567–568. doi: 10.1097/00000421-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Pereira C.M., Souza C.A., Correa M.E.P. Herpetic geometric glossitis: acyclovir resistant case in a patient with acute myelogenous leukemia. Indian J Pathol Microbiol. 2010;53(1):133–134. doi: 10.4103/0377-4929.59205. [DOI] [PubMed] [Google Scholar]
- 4.Bradley H., Markowitz L.E., Gibson T., McQuillan G.M. Seroprevalence of herpes simplex virus types 1 and 2–United States, 1999-2010. J Infect Dis. 2014;209(3):325–333. doi: 10.1093/infdis/jit458. [DOI] [PubMed] [Google Scholar]
- 5.Chemaitelly H., Nagelkerke N., Omori R., Abu-Raddad L.J. Characterizing herpes simplex virus type 1 and type 2 seroprevalence declines and epidemiological association in the United States. PLoS One. 2019;14(6):e0214151. doi: 10.1371/journal.pone.0214151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fatahzadeh M., Schwartz R.A. Human herpes simplex virus infections: epidemiology, pathogenesis, symptomatology, diagnosis, and management. J Am Acad Dermatol. 2007;57(5):737–763. doi: 10.1016/j.jaad.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 7.Mirowski G.W., Goddard A. Herpetic geometric glossitis in an immunocompetent patient with pneumonia. J Am Acad Dermatol. 2009;61(1):139–142. doi: 10.1016/j.jaad.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Ramírez-Amador V., Anaya-Saavedra G., Crabtree-Ramírez B., Esquivel-Pedraza L., Saeb-Lima M., Sierra-Madero J. Clinical spectrum of oral secondary syphilis in HIV-infected patients. J Sex Transm Dis. 2013;2013:892427. doi: 10.1155/2013/892427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ranu H., Lee J., Chio M., Sen P. Tumour-like presentations of anogenital herpes simplex in HIV-positive patients. Int J STD AIDS. 2011;22(4):181–186. doi: 10.1258/ijsa.2010.010204. [DOI] [PubMed] [Google Scholar]
- 10.Panel on Opportunistic Infections in Adults and Adolescents with HIV Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/Adult_OI.pdf




