Abstract
Background
Krüppel like factor 10 (KLF10), which is also known as TGF-β Inducible Early Gene-1 (TIEG1), plays a crucial role in regulating cell proliferation, cell apoptosis and inflammatory reaction in human carcinoma cells. Moreover, KLF10 knockout in mice leads to severe defects associated with muscle, skeleton and heart etc. However, the function of KLF10 in intervertebral disc degeneration (IVDD) has not been reported yet.
Methods
The relationship between KLF10 and IVDD were investigated in nucleus pulposus (NP) tissues from human and rats. The role of KLF10 in NP cells was explored via loss or gain of function experiments. IVDD rat models were constructed through needle puncture and the effects of KLF10 in IVDD model of rats were investigated via intradiscal injection of KLF10.
Results
We first found that KLF10 was lowly expressed in degenerative NP tissues and the level of KLF10 showed negative correlation with the disc grades of IVDD patients. Loss or gain of function experiments demonstrated that KLF10 could inhibit apoptosis and enhance migration and proliferation of IL-1β induced NP cells. And KLF10 overexpression reduced extracellular matrix (ECM) degeneration and enhanced ECM synthesis, whereas knockdown of KLF10 resulted in adverse effects. These positive effects of KLF10 could be reversed by the inhibition of TGF-β signaling pathway. In vivo, KLF10 overexpression alleviated IVDD.
Conclusions
This is the first study to reveal that KLF10 was dysregulated in IVDD and overexpressed KLF10 could alleviate IVDD by regulating TGF-β signaling pathway both in vitro and in vivo, which were involved in prohibiting apoptosis, promoting proliferation and migration of NP cells.
The translational potential of this article: Overexpression of KLF10 might be an effective therapeutic strategy in the treatment of IVDD.
Keywords: KLF10, Intervertebral disc degeneration, TGF-β pathway, Nucleus pulposus, Therapeutic strategy
1. Introduction
Intervertebral disc degeneration (IVDD) is regarded as the main cause of low back pain and other spine-related diseases [1]. This condition has already led to a major economic and social burden worldwide, affecting the quality of life of millions of individuals [2,3]. IVDD is characterized by highly expressed proinflammatory cytokines, which break the balances between catabolism and anabolism of degenerative nucleus pulposus (NP) tissues [4,5]. This process is involved in excessive production of extracellular matrix (ECM) enzymes and increased apoptosis of NP cells [6]. However, the pathogenesis of IVDD progression still remains unclear. Subsequently, it is necessary for us to further explore the molecular mechanism of IVDD and discover new therapeutic strategies for treating IVDD.
Krüppel like factor 10 (KLF10), which is also known as TGF-β Inducible Early Gene-1 (TIEG1), was first discovered in human osteoblasts after TGF-β treatment. KLF10 contained three zinc finger domains for binding DNA sequences to mediate gene transcription [7]. KLF10 was found to be involved in many diseases since its discovery. Previous studies have suggested that KLF10 plays a crucial role in regulating cell proliferation and apoptosis in multiple tumor cells [8,9], except that KLF10 knockout in mice leads to severe defects associated with muscle, skeleton and heart etc [[10], [11], [12]]. Researchers in recent years have reported that KLF10 exerts its function mainly through mimicking TGF-β function. KLF10 also could enhance Smad2 phosphorylation by repressing Smad7 to further activate TGF-β/Smad signaling pathway [[13], [14], [15]]. Prior researches have demonstrated that TGF-β could protect ECM against degradation and promote ECM synthesis [16,17]. Moreover, treatment of TGF-β1 in rat NP cells also promotes cell proliferation, reduces cell death and ameliorates inflammatory response [17,18]. Therefore, whether KLF10 could activate TGF-β pathway to prevent IVDD requires an answer.
Hence, in this study, we first discovered that KLF10 was downregulated in degenerative NP tissues from human and rat models and the expression level of KLF10 showed negative correlation with the disc grades of IVDD patients. These data indicated that KLF10 might play a key role in IVDD development. The loss or gain of function experiments demonstrated that KLF10 could improve viability and function of IVDD cells and regulated the expression anabolic/catabolic makers of IVDD. Mechanically, KLF10 exerts its protective role in IVDD by activating TGF-β signaling pathway. In vivo, KLF10 local injection was shown to alleviate IVDD. These results suggest that KLF10 could be an effective therapeutic target against IVDD.
2. Materials and methods
2.1. Human NP tissue specimens
Eighty-six degenerative NP tissues were collected from lumber disc degeneration patients who accepted spinal surgery at the Shanghai East Hospital, Tongji University School of Medicine, Shanghai, China from February 2019 to June 2020. All NP samples were stored in liquid nitrogen to prevent RNA from degradation. The intervertebral disc samples of the patients were divided into four categories (II, III, IV and V) according to the Pfirrmann grades of T2-weighted MRI images [19]. The clinical information of patients was summarized in Table 1. Informed consent was obtained from patients before collecting the NP samples. Our research was approved by the Ethics Committee of Shanghai East Hospital.
Table 1.
Clinical information of human degenerative disc samples from 86 patients.
| Variables | Number |
|---|---|
| Sex | |
| Male | 49 |
| Female | 37 |
| Age | |
| <50 | 22 |
| ≥50 | 64 |
| Diagnosis | |
| Lumbar disc herniation | 21 |
| Lumbar spinal stenosis | 65 |
| Level | |
| L1/2 | 2 |
| L2/3 | 8 |
| L3/4 | 18 |
| L4/5 | 27 |
| L5/S1 | 31 |
| Degenerative grades | |
| II | 24 |
| III | 30 |
| IV | 19 |
| V | 13 |
2.2. NP cells lentivirus transfection
Human NP cells were purchased from ScienCell, Carlsbad, CA, and cultured in human NP cell medium (ScienCell) at 37 °C in 5% CO2 as previously described [20]. Lentivirus-mediated stably overexpressed or knockdown of KLF10 in NP cells were constructed according to the manufacturer's instructions (GenePharma, Shanghai, China). The target sequences of KLF10-shRNAs were as follows: KLF10 shRNA1 GCAAGAAAGAACATACCATGT, KLF10 shRNA2 GGAGTGACCATTTGACCAAGC. The efficiency of overexpressed and knocked down cells was certified by qRT-PCR and immunoblotting. After transfection, NP cells were treated with or without 10 ng/ml of interleukin-1β (IL-1β, Sigma) and 300 nmol/L of LY364947(a TGF-β Receptor I Kinase Inhibitor, APExBIO) in the culture medium for 12 h.
2.3. RNA extraction and quantitative reverse transcription-PCR (qRT-PCR)
Before RNA extraction, NP tissue was placed in a mortar containing liquid nitrogen and ground with a pestle. Total RNA of NP tissues and NP cells were extracted by Trizol reagent (Takara Biotechnology) according to the manufacturer's instructions. The RNA concentration was determined using spectrophotometry. cDNAs were reverse-transcribed with TaqMan RT Reagents Kit (Applied Biosystems). Quantitative PCR was carried out using a QuantiTect SYBR Green PCR Kit (Takara) in an Applied Biosystems 7500 System. GAPDH gene expression was used as an endogenous control. The 2−ΔΔCT method was used to analyze the relative fold changes of target genes. The sequences of primers were shown in Supplementary Table 1.
2.2. Cell immunofluorescence
The NP cells were fixed in 4% paraformaldehyde for 15 min, incubated with 0.1% Triton X-100 for 25 min, and then blocked with 5% BSA containing 0.1% Triton X-100 for 30 min at room temperature. After that, the cells were incubated with primary antibodies against KLF10 (#130408, diluted 1:250, Santa), collagen II (#34712 diluted 1:1000, Abcam) and MMP-3 (#53015, diluted 1:500, Abcam) for overnight at 4 °C. Subsequently, the cells were incubated in relevant secondary antibodies in the dark for 1 h and counterstained in DAPI for another 5 min. The fluorescence was obtained by using fluorescence microscope (Leica DMI3000B, Leica Microsystems, Inc).
2.5. Western blot analysis
Protein lysate was obtained by using RIPA buffer supplemented with protease and phosphatase inhibitors and protein concentrations were measured by BCA Protein Assay Kit (Thermo, Waltham, MA, USA). Western blot was performed as described previously [21]. The primary antibodies are as follows: anti-KLF10 (#130408, diluted 1:1000) was supplied by Santa Cruz Biotechnology (Santa Cruz, CA, USA); anti-caspase-3 (#9664, diluted 1:1000), anti-caspase-7 (#9491, diluted 1:1000), anti-caspase-9 (#9505, diluted 1:1000), anti-TGF-β (#3709, diluted 1:1000), anti-Phospho-Smad2/Smad3 (#8828, diluted 1:1000) and anti-Smad2/3 (#8685, diluted 1:1000) were purchased from Cell Signaling Technology (CST, Beverly, MA, USA). The Smad7 antibody (#MAB2029, diluted 0.5 mg/mL) was obtained from R&D Systems (Minneapolis, MN, USA). Anti-aggrecan (#36861, diluted 1:1000), anti-collagen II (#34712, diluted 1:5000), anti-MMP-3 (#53015, diluted 1:1000), anti-MMP-13 (#39012, diluted 1:3000), anti-ADAMTS4 (#185722, diluted 1:1000), anti-ADAMTS5 (#41037, diluted 1:250) and anti-GAPDH (#9485, diluted 1:2500) were purchased from Abcam (Cambridge, MA, USA). Secondary antibodies including HRP-linked anti-rabbit IgG or HRP-linked anti-mouse IgG (#7074, #7076, diluted 1:1000) were purchased from CST. Protein bands were detected using an ECL chemiluminescence kit (Thermo Scientific CA, USA) according to the manufacturer's specifications and the results were quantified using ImageJ software (US National Institutes of Health).
2.6. Flow cytometry analysis
NP cells at a density of 1 × 105 cells per well were seeded into six-well plates. An Annexin V/PI apoptosis detection kit (BD Biosciences, Franklin Lakes, NJ, USA) was used to determine the apoptotic rate of cells. NP cells were washed twice with cold PBS, suspended in 400 ul Annexin V binding buffer (1X) and stained with 5 μl Annexin V-FITC and 10 μl PI for 15 min in the dark. The stained cells were analyzed using the FACScan flow cytometry system (Beckman Coulter, CA, USA)
2.7. Cell migration
Cells migration assays were measured using transwell filter with 8.0 μm pores (Costar, USA). Cells at a density of 50,000 cells were seeded in the upper chamber including 200 ul serum-free DMEM/F12 medium, while 600 ul complete medium was added into the lower chamber. After culturing for 2 days, the cells were fixed with 4% paraformaldehyde and stained via 5% crystal violet solution. The migrated cells were photographed with a light microscope and analyzed using ImageJ software.
2.8. Cell counting Kit-8 (CCK8)
CCK-8 assay was performed to detect cellular proliferation. NP cells were seeded in 96-well plates at a density of 3000 cells per well with 100 ul medium per well for 24 h. The cell nutrient medium was then substituted by 100ul fresh cell medium including 10% CCK8 (Dojindo, Japan) at 0, 1, 3, and 5 days. The cells were incubated for another 3 h and then the absorbance was read at 450 nm using a microplate reader.
2.9. Construction of IVDD rat model and intradiscal injection of lentivirus
A total number of 24 adult male Sprague–Dawley (SD, SPF and 250–300g), aged 3 months, were supplied by Shanghai Slac Laboratory Animal Co. Ltd. The SD rats were randomly divided into four groups (Control, IVDD, IVDD+KLF10, and IVDD+NC). The IVDD rat model was constructed as described previously [22,23]. Briefly, the rats were anaesthetized by intraperitoneal injection of 5% chloral hydrate (6 mL/kg) and Co7/8 of rat discs were punctured using 21-gauge needle. The punctured depth was 5 mm from the AF to the NP in the center of the disc and the needle was rotated 180° and then held for 30s. After that, a total of 10 μl construct of Lenti-KLF10 and Lenti-NC (approximately 5 × 108 TU/mL) were slowly injected into the punctured discs. No further treatment was carried out in control and IVDD groups. After injection, the rats were placed in their respective cages. Our animal protocol was authorized by the Ethics Committee of Shanghai East Hospital.
2.10. X-ray and MRI examination
All animals in the groups underwent X-ray examination at 0, 4 and 8 weeks after IVDD puncture. As mentioned previously, the disc height index (DHI) was used to assess the height of the disc [24]. Changes in DHI of disc were expressed as % DHI (%DHI = post-punctured DHI/pre-punctured DHI × 100%) [25,26]. After last X-ray examination, rats in each group underwent MRI examination on 3.0T MRI system (Philips Eclipse, Germany). The specific parameters of MRI scanning in T2-weighted sagittal and transverse plane were referred to the previous study [27]. The disc degeneration grades of all rats were evaluated based on the Pfirrmann classification [19].
2.11. Histological and immunohistochemical staining
All disc samples were fixed in 4% paraformaldehyde, embedded in paraffin and cut into sections (5 μm). The degenerative disc samples of patients underwent hematoxylin–eosin (HE) staining. All rat discs were used for HE and safranin-O/fast green staining. The histological photograph of the disc was acquired by a light microscope. Histological scores of rat discs obtained were based on a modified histologic grading system [28,29]. Immunohistochemical staining was performed to detect the expression of KLF10, MMP3, collagen II and aggrecan in human degenerative NP tissues and the expression of KLF10, TGF-β1, caspase-3, MMP-3 and collagen II in the discs of rats of different groups. The slices then underwent antigen retrieval, blocked and incubated with primary antibodies and relevant secondary antibodies. Finally, immunohistochemical photographs were obtained under a light microscope and analyzed by ImageJ software. Reagents used in histological and immunohistochemical staining were supplied by the Shanghai Record Biological Technology Co. Ltd.
2.12. Statistical analysis
Statistical analyses were performed with GraphPad Prism 7 Software (San Diego, CA, USA) and all data were represented as means ± standard deviations. The normal distribution of data was examined by Shapiro–Wilk test and Kolmogorov–Smirnov test. The homogeneity of variances was examined by Levene test. Student's t-test and ANOVA was used to assess the statistically significant differences and the Spearman's correlation coefficient was used to assess the correlation between KLF10 expression and Pfirrmann grade of human degenerative intervertebral disc (∗, p<0.05; ∗∗, p<0.01; ∗∗∗, p<0.001).
3. Results
3.1. Discovery of KLF10 related to IVDD from patient samples
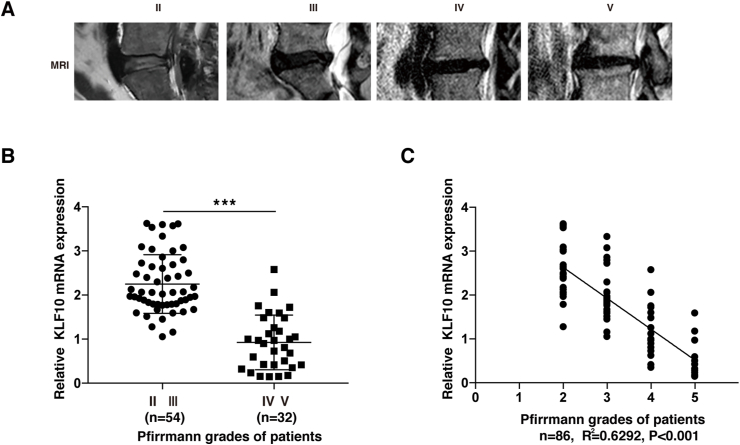
To detect the level of KLF10 in different degenerative disc samples, a total of 86 degenerative NP tissues were collected from IVDD patients, and divided them into 4 groups (grades II, III, IV, and V) according to the Pfirrmann grades of MRI images. Fig. 1A showed the representative T2-weighted MRI images. The basic information of the patients was shown in Table 1. Next, qRT-PCR was performed to detect the mRNA levels of KLF10 in NP tissues. The result showed that KLF10 mRNA expression was significantly downregulated in IVDD patients with Pfirrmann grades Ⅳ and Ⅴ when compared with Pfirrmann grades Ⅱ and Ⅲ patients (P<0.001) (Fig. 1B) and KLF10 expression was negatively correlated with the Pfirrmann grades of IVDD patients (n = 86; R2 = 0.6292, P<0.001) (Fig. 1C).
Figure 1.
Discovery of KLF10 related to IVDD from patient samples. (A) The representative T2-weighted MRI images of different Pfirrmann grades. (B) KLF10 mRNA level was lower in grade Ⅳ and Ⅴ of NP tissues (n = 32) than in grade Ⅱ and Ⅲ (n = 54) (P<0.001). (C) The level of KLF10 in NP tissues from IVDD patients was negatively correlated with the disc degeneration grade (n = 86, R2 = 0.6292 and P<0.001). Data is presented as the mean ± SD.
3.2. KLF10 is downregulated in degenerative NP tissues of both humans and rats
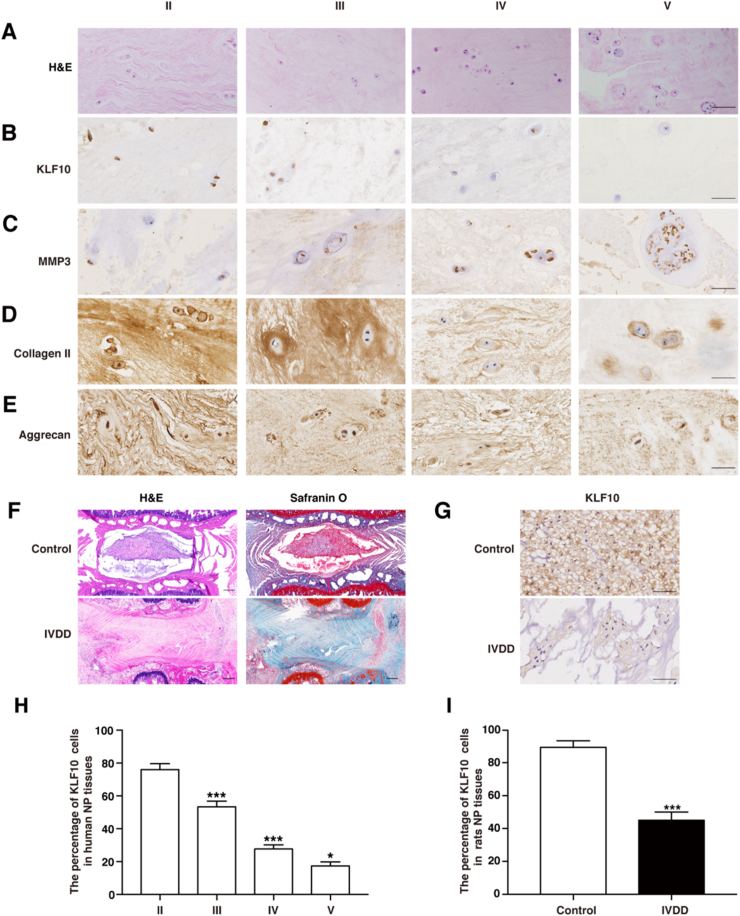
Fig. 2A showed HE staining pictures of different degenerative grades of patient's discs. IHC staining and quantitative analysis indicated that the expression levels of KLF10 were decreased with increasing grade of human disc degeneration (P<0.05) (Fig. 2B and H). Obviously, KLF10 expression level detected by IHC staining was shown to be similar to that of the important component of ECM such as collagen II and aggrecan but was different from MMP3 expression (Fig. 2C-E). To further explore the effects of KLF10 on IVDD, IVDD rat model was successfully established by needle puncturing, which was confirmed by HE and Safranin O-fast green staining (Fig. 2F). IHC quantitative analysis then revealed that the levels of KLF10 were downregulated greatly in the degenerative discs of IVDD rat models when compared with normal rat discs, which was consistent with that in the patient samples (P<0.001) (Fig. 2G and I). These findings indicated that KLF10 might have a great effect on IVDD.
Figure 2.
KLF10 is downregulated in degenerative NP tissues of both humans and rats. (A) Representative hematoxylin–eosin (HE) staining pictures of each Pfirrmann grade. n = 5. Scale bars:50 μm. (B–E) Immunohistochemical staining of KLF10, MMP3, collagen II and aggrecan in different degenerative disc. n = 5. Scale bars:50 μm. (F) HE and safranin-O staining of normal and IVDD rat discs. n = 5. Scale bars: 250 μm. (G) Immunohistochemical staining of KLF10 in normal and IVDD rat discs. n = 5. Scale bars:50 μm. (H) Quantitative analysis of the expression of KLF10 in human degenerative disc. (I) Quantitative analysis of the expression of KLF10 in normal and IVDD rat discs. Data are presented as the mean ± SD. ∗, P<0.05; ∗∗, P<0.01; ∗∗∗, P<0.001.
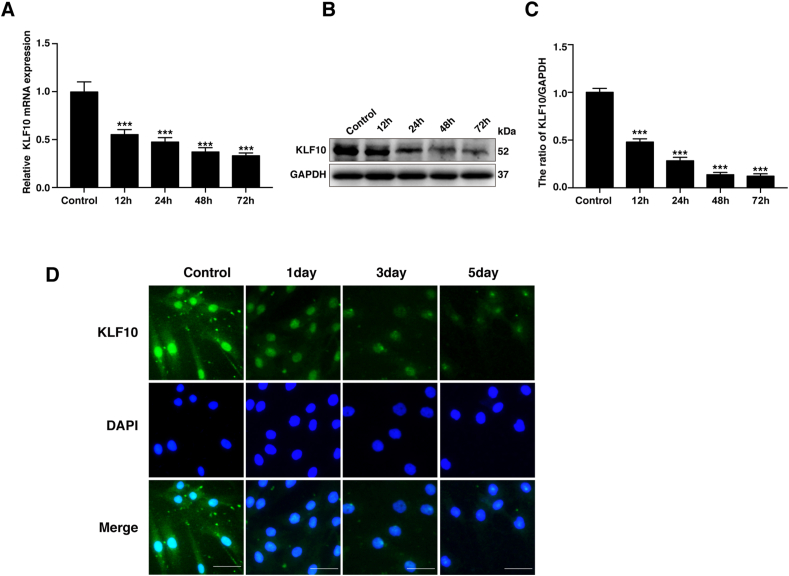
3.3. IL-1β treatment can decrease the KLF10 levels in NP cells in vitro
The aggregation of inflammatory cytokines such as IL-1β, TNF and IL-1α are shown to be a great characteristic during IVDD [4]. To further investigate the levels of KLF10 during IVDD in vitro, NP cells were stimulated with IL-1β (10 ng/mL). The results revealed that the mRNA and protein levels of KLF10 were simultaneously decreased after IL-1β treatment for 12, 24, 48, or 72h (P<0.05) (Fig. 3A–C). Consistently, immunofluorescence results also confirmed the declination in the levels of KLF10 in NP cells after IL-1β treatment for 1, 3 or 5 days. Immunofluorescence results further indicated that KLF10 basically localized in the nucleus and IL-1β treatment can decrease the expression levels of KLF10 (Fig. 3D).
Figure 3.
KLF10 level was decreased in the NP cells with IL-1β treatment. (A–C) NP cells were treated with IL-1β (10 ng/mL) for different times (12, 24, 48, or 72 h). The mRNA and protein level of KLF10 detected by qRT-PCR and western blotting, respectively. (D) Immunofluorescence of KLF10 in NP cells induced with IL-1β (10 ng/mL) for 1, 3 or 5 days. Scale bars: 50 μm. All experiments are repeated at least three times, and GAPDH is used as an internal control. Data are presented as the mean ± SD. ∗, P<0.05; ∗∗, P<0.01; ∗∗∗, P<0.001.
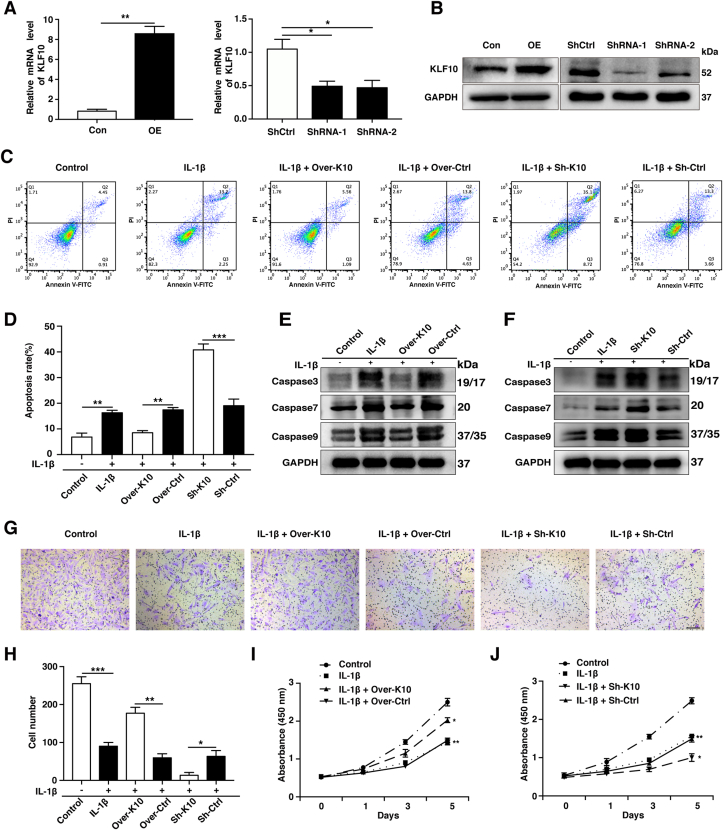
3.4. The effects of KLF10 overexpression or silencing on NP cells viability and function
To better explore the role of KLF10 in the pathogenesis of IVDD, overexpressed and knocked down KLF10 NP cells were constructed by lentiviral transfection. The increased or decreased expression levels of KLF10 were confirmed by qRT-PCR and western blotting (P<0.05) (Fig. 4A and B). Based on the knockdown efficiency, KLF10 shRNA1 was chose for subsequent experiments. Our results revealed that KLF10 overexpression significantly reduced NP cells apoptosis induced by IL-1β(P<0.01). However, KLF10 knockdown significantly enhanced the apoptotic rate of NP cells after IL-1β stimulation (P<0.001) (Fig. 4C and D). Furthermore, western blotting assay indicated that IL-1β treatment increased the elevated caspase protein levels such as caspase 3, caspase 7 and caspase 9, which could be suppressed by KLF10 overexpression and be raised by KLF10 knockdown in NP cells (Fig. 4E and F). With respect to NP cells migration, the number of migrated cells can be reduced remarkably in IL-1β treated group, which was increased after KLF10 overexpression and further decreased by KLF10 knockdown when compared with relevant control group (P<0.05) (Fig. 4G and H). NP cell proliferation of different groups was detected by CCK-8 assay. Our results demonstrated that KLF10 overexpression has remarkably increased the proliferation rate, while its knockdown dominantly decreased the proliferation rate in IL-1β induced NP cells when compared with its corresponding control group at 3 and 5 days (P<0.05) (Fig. 4I and J). These data suggest that the upregulation of KLF10 inhibited cells apoptosis and promoted migration and proliferation of IL-1β induced NP cells.
Figure 4.
The effects of KLF10 overexpression or silencing on NP cells viability and function. (A, B) QRT-PCR and western blotting was used to evaluated KLF10 expression after transfection. (C, D) The NP cells apoptosis rate of each group as treated above was detected by flow cytometry. (E, F) Western blot analyzes protein expression of apoptotic effector caspases (caspase-3, caspase-7 and caspase-9) of each group as treated above. (G, H) The NP cells migratory ability of each group as treated above was determined by transwell assay. Scale bar: 200 μm. (I, J) NP cells proliferation of each group as treated above was determined by CCK8 assay. All experiments were repeated three times, and GAPDH is used as an internal control. Data are presented as the mean ± SD. ∗, P<0.05; ∗∗, P<0.01; ∗∗∗, P<0.001.
3.5. KLF10 regulated IL-1β induced human NP cells degenerative phenotype
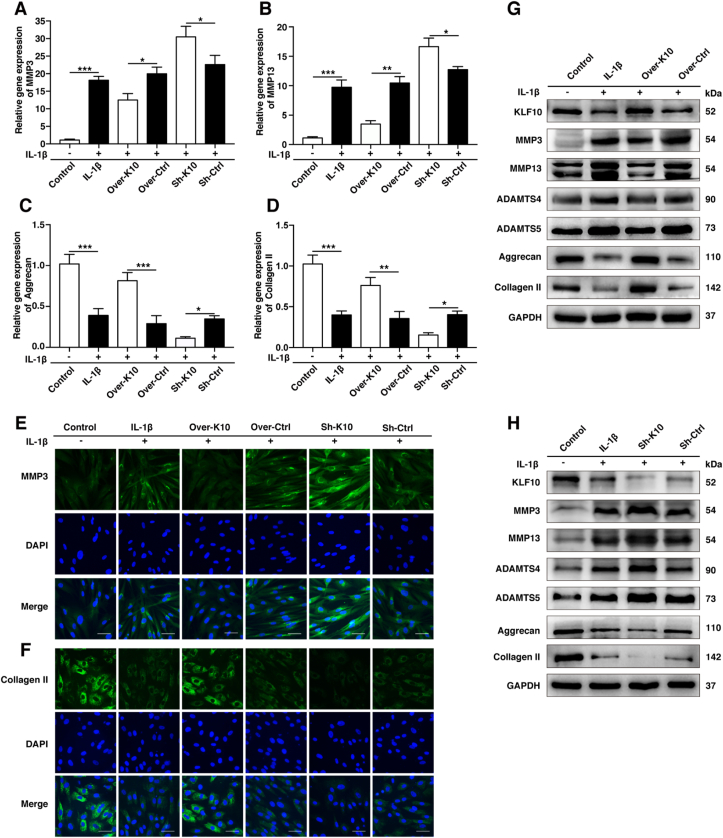
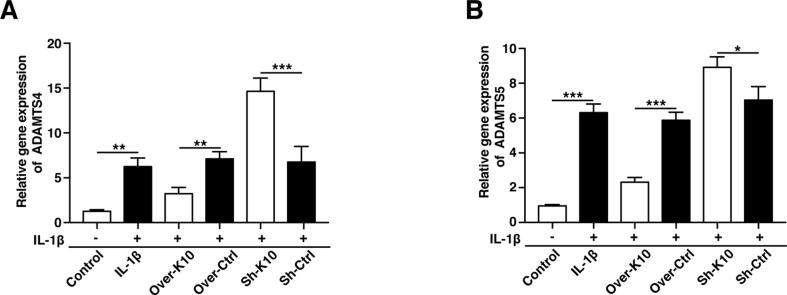
To further investigation on whether KLF10 regulates the expression of anabolic/catabolic markers of IVDD, gain-of-function and loss-of-function experiment of KLF10 were included. QRT-PCR, immunofluorescence and western blotting were performed to detect the levels of anabolic factors (collagen II and aggrecan) and catabolic factors (MMP3, MMP13, ADAMTS4, and ADAMTS5) in NP cells. Our experiments showed inflammatory factors strikingly cut down the mRNA levels of collagen II and aggrecan, but raised the mRNA levels of MMP3, MMP13, ADAMTS4, and ADAMTS5 (P<0.01). Interestingly, overexpression of KLF10 has increased the mRNA levels of anabolic markers and decreased the mRNA levels of catabolic markers, while its knockdown showed adverse effects (P<0.05) (Fig. 5A–D and Fig. S1). These results were consistent with those of immunofluorescence and western blotting results (Fig. 5E-H).
Figure 5.
KLF10 regulated IL-1β induced human NP cells degenerative phenotype. (A–D) The mRNA levels of MMP-3, MMP-13, aggrecan and collagen II in NP cells of each group as treated above was detected by qRT-PCR assay. (E, F) The representative MMP3 and collagen II were detected by the immunofluorescence. Scale bars: 50 μm. (G, H) The protein levels of KLF10, MMP3, MMP13, ADAMTS4, ADAMTS5, aggrecan and collagen II were detected by western blot. All experiments were repeated three times, and GAPDH is used as an internal control. Data are presented as the mean ± SD. ∗, P<0.05; ∗∗, P<0.01; ∗∗∗, P<0.001.
3.6. KLF10 regulates IVDD by modulating TGF-β signaling pathway
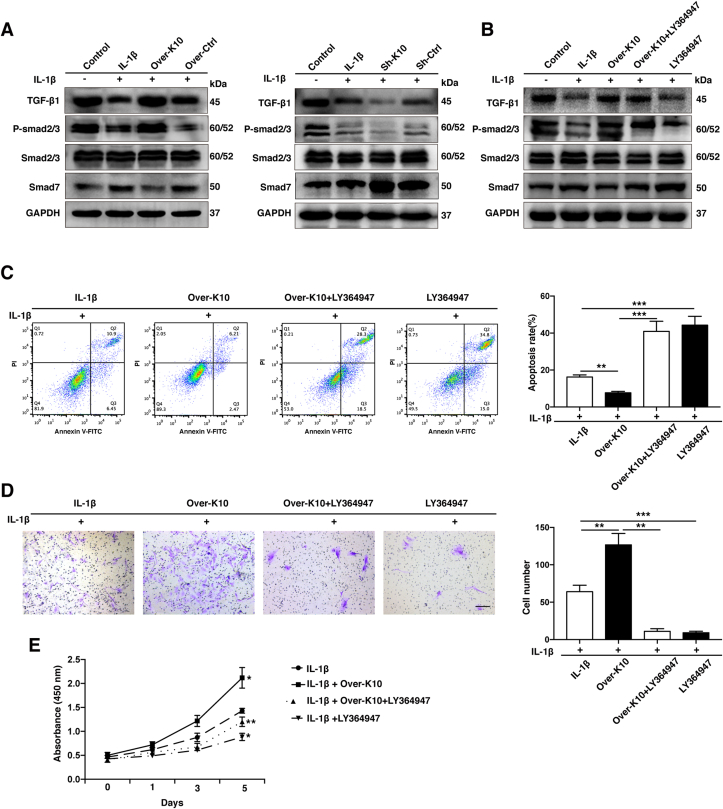
Previous studies showed that KLF10 could exert its function by TGF-β signaling pathway [13,14]. In order to explore the molecular mechanism of KLF10 in IVDD, western blotting assay was performed to detect the protein levels of TGF-β1, P-smad2/3, Smad2/3, and Smad7 in each group. These results demonstrated that the treatment of IL-1β effectively suppressed the protein levels of TGF-β1, P-smad2/3 but increased the levels of Smad7 when compared to its control group, indicating the inhibition of TGF-β signaling pathway(P<0.001). However, in contrast to their negative control group, KLF10 overexpression could increase the protein level of TGF-β1, P-smad2/3 but decrease Smad7 expression, which indicated that TGF-β signaling pathway was re-activated, whereas KLF10 knockdown further inactivated TGF-β signaling pathway after IL-1β treatment (P<0.001) (Fig. 6A). Treatment NP cells with an inhibitor of TGF-β signaling pathway, LY364947(300 nmol/L), significantly decreased the protein level of P-Smad3 whereas the P-Smad2 expression was not remarkably reduced (Fig. 6B). By flow cytometry, transwell, and CCK8 assays, we found that the inhibition of TGF-β signaling pathway can remarkably reverse the positive effects of KLF10 overexpression in IL-1β treated NP cells. Moreover, we found that IL-1β combined with LY364947 further enhanced apoptosis, inhibited proliferation and migration of NP cells compared with IL-1β induced alone (P<0.05) (Fig. 6C-E). The above results implied that TGF-β signaling pathway may play a positive effect on rescuing NP cells treated by inflammatory cytokines and KLF10 exerts its protective role on IVDD via activating TGF-β signaling pathway.
Figure 6.
KLF10 regulate IVDD through modulate TGF-β signaling pathway. (A, B) The protein levels of TGF-β1, P-smad2/3, Smad2/3 and Smad7 in NP cells of each group as treated above were determined by western blotting. (C) The NP cells apoptosis rate of each group was detected by flow cytometry. (D) The NP cells migratory ability of each group was determined by transwell assay. Scale bar: 200 μm. (E) The NP cells proliferation of each group was determined by CCK8 assay. All experiments were repeated three times, and GAPDH is used as an internal control. Data are presented as the mean ± SD. ∗, P<0.05; ∗∗, P<0.01; ∗∗∗, P<0.001.
3.7. KLF10 overexpression alleviates IVDD in a rat model
In vitro experimental results have prompted us to investigate whether KLF10 could alleviate IVDD in vivo.
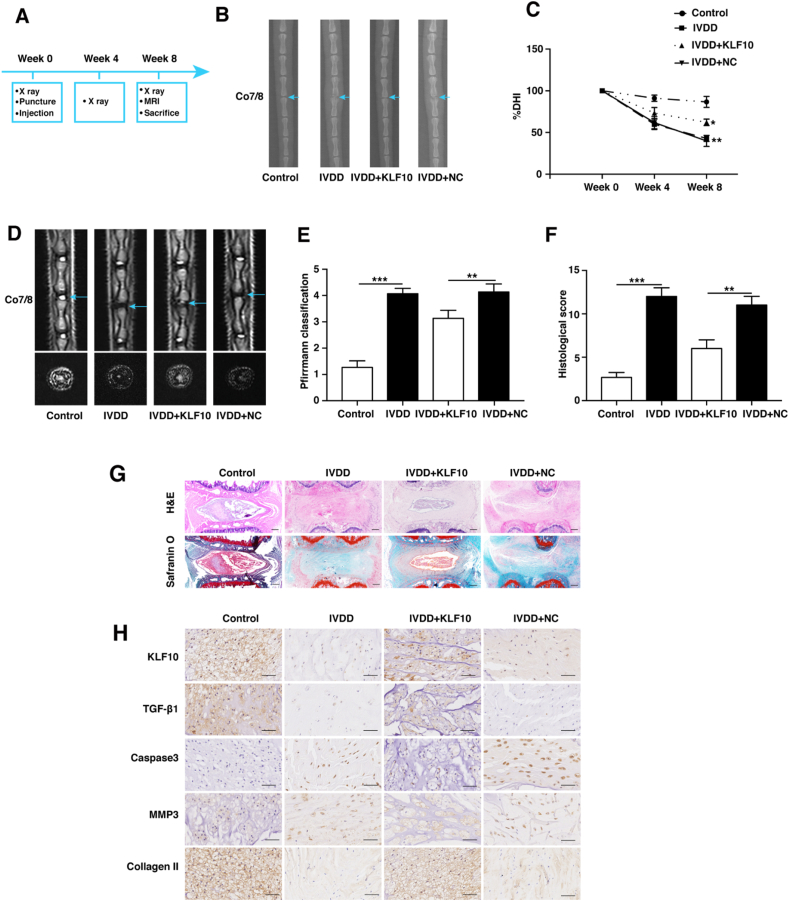
A total number of 24 adult male SD, aged 3 months, were randomly divided into four groups (Control, IVDD, IVDD+KLF10, and IVDD+NC). The IVDD rat model was successfully established by (Annulus Fibrosus) AF needle puncturing. Briefly, the rats were anaesthetized by intraperitoneal injection of 5% chloral hydrate (6 mL/kg) and Co7/8 of rat discs were punctured using 21-gauge needle. After that, a total of 10 μl construct of Lenti-KLF10 and Lenti-NC (approximately 5 × 108 TU/mL) were slowly injected into the punctured discs. X-ray was performed at 0, 4 and 8 weeks after injection and X-ray results indicated that the DHI index has become smaller gradually in all the punctured groups. Moreover, at 4 and 8 weeks, the DHI index of IVDD+KLF10 group was higher than that of IVDD+NC (Lenti-NC) group (P<0.05) (Fig. 7B-C). At 8 weeks after injection, all groups of rats were sacrificed for MRI scanning and pathological staining. Sagittal and transverse planes of T2-weighted MRI results demonstrated that the transfection of KLF10 has led to higher signals and lower degenerative scores than IVDD+NC group (P<0.01) (Fig. 7D and E). In contrast to IVDD+NC group, the histological score was remarkably lower in IVDD+KLF10 group (P<0.01) (Fig. 7F and G). Immunohistochemical staining results revealed that the injection of lentivirus KLF10 resulted in high levels of TGF-β1 expression, indicating that TGF-β pathway was activated in vivo. Furthermore, the levels of cleaved caspase 3 and MMP3 were shown to be reduced, whereas the levels of collagen II were increased in IVDD+KLF10 group when compared with IVDD+NC group. These implied that KLF10 alleviated IVDD through anti-apoptosis mechanism, enhancing anabolism and reducing catabolism in vivo (Fig. 7H). Taken together, these results suggested that KLF10 exerts its therapeutic function on rat IVDD model via activating TGF-β signaling pathway.
Figure 7.
KLF10 overexpression alleviates IVDD in a rat model. (A) A flowchart of the experiments in rats. (B, C) X-ray examination was performed at 0, 4 and 8 weeks after needle puncture. A significant decrease of the DHI% was observed in all puncture groups at 4 and 8 weeks after surgery. A significant increase in DHI% was indicated at 4 and 8 weeks after surgery in rat treated by Lenti-KLF10. Light blue arrow indicated the target disc. DHI: disc height index. n = 5. (D, E) T2-weighted MRI of the indicated groups were obtained 8 weeks after needle puncture. The degree of disc degeneration by MRI grade was significantly lower in the IVDD+KLF10 group than in the IVDD+NC group. n = 5. (F, G) Hematoxylin–eosin (HE) and safranin-O/fast green staining of the intervertebral discs in the indicated groups at 8 weeks after injection. Histological score showed a significant decrease in rats treated by KLF10. n = 5. Scale bars:250 μm. (H) Immunohistochemical staining of KLF10, TGF-β1, caspase3, MMP3 and collagen II in the disc samples of each group. n = 3. Scale bars:50 μm. Data are presented as the mean ± SD. ∗, P<0.05; ∗∗, P<0.01; ∗∗∗, P<0.001.
4. Discussion
KLF10/TIEG was originally discovered in the human osteoblast cells following TGF-β treatment [7]. KLF10 protein includes three zinc finger motifs at the C-terminal end and several proline-rich Src homology-3 (SH3) binding domains at the N-terminal end, rendering KLF10 to bind to the GC rich Sp1-like sequences for transcriptional regulation. Therefore, KLF10 is categorized as one of the Krüppel-like family of transcription factors [30]. An accumulation study revealed that KLF10 plays a critical role in regulating cell proliferation, cell apoptosis and inflammatory reaction in human carcinoma cells [31,32]. Except that, KLF10 could enhance Wnt signaling in bone through β-catenin and in developing skeleton, suggesting it as a mediator of chondrocyte hypertrophy [12,33]. Researchers have reported that KLF10 has enhanced TGF-β/Smad2 signaling pathway via downregulation of Smad7 expression, leading to increased collagen production, promoting cell proliferation and invasion in keloid pathogenesis [14]. As KLF10 plays multiple functional roles in different kinds of diseases, it might also have an important role in the development of IVDD.
In our study, by detecting the levels of KLF10 in large-scale human degenerative disc, we discovered that KLF10 was downregulated in degenerative NP tissues and KLF10 expression was negatively correlated with the Pfirrmann grades of IVDD patients. Moreover, the expressed pattern of KLF10 was also verified in the rat IVDD models. These data implied that KLF10 probably has an important role in IVDD. Inflammatory response plays a tremendous role in the process of disc degeneration [4]. Inflammatory cytokines, for example IL-1β, secreted by IVDD cells could cause ECM degradation and changes of IVDD cell phenotype [34]. To mimic the circumstances of IVDD in vitro, human NP cells were treated with IL-1β in our experiment. Interestingly, the mRNA and protein levels of KLF10 were significantly decreased after IL-1β treatment, meaning that inflammatory cascade inhibits the expression of KLF10 of NP cells in IVDD. To further explore the function of KLF10 in the process of IVDD, a stably overexpressed or knocked down KLF10 in NP cells via lentivirus infection was constructed. Loss or gain of function experiments have demonstrated that KLF10 overexpression has dominantly improved the viability and functions such as anti-apoptosis, enhanced proliferation and migration of IVDD cells. During the development of IVDD, at least two kind of ECM-degrading enzymes including the MMPs and a ADAMTS were excessively activated [[35], [36], [37]], accelerating the degradation of ECM. Our data indicated that the overexpression of KLF10 not only decreased the level of MMPs and ADAMTS that are induced by IL-1β, but also enhanced NP cells to produce the components of ECM such as collagen II and aggrecan. These results have prompted us to further investigate the underlying mechanism as higher levels of KLF10 have a positive effect on IVDD. Interestingly, western blotting analysis demonstrated that TGF-β pathway could be remarkably activated by KLF10 in NP cells. The blockade of TGF-β pathway could reverse the protective effects on anti-apoptosis, enhanced proliferation and migration of IVDD cells. All these results suggest that KLF10 mediates IVDD progression by regulating TGF-β signaling pathway. Moreover, our animal experiment has demonstrated that degenerative disc with lentivirus KLF10 injection has lower MRI degeneration score and histological score. IHC staining analysis in rat discs also indicated that KLF10 exerted a protective role in degenerative NP tissues by TGF-β signaling pathway.
However, recent years studies demonstrated that excessive activation of TGF-β signaling pathway may accelerate the progression of IVDD [38,39]. The question that TGF-β signaling pathway is beneficial or detrimental to IVDD function is still debatable [40]. It is necessary for us to further explore the relationship between KLF10 and TGF-β signaling pathway and the underlying molecular mechanism of the protective role of KLF10 in IVDD. In our study, we found that LY364947 could significantly suppress the expression of P-Smad3, which exacerbated the dysfunction of NP cells. Thus, the question whether KLF10 enhances TGF-β signaling pathway to exert its protective role in IVDD via Smad3 requires answer. In the future, we will use the RNA-sequence method to analyze upregulated genes upon overexpression or inactivation of KLF10 in NP cells, which may help us to find out potential downstream targets influencing NP function or NP cell differentiation.
This is the first study to reveal that KLF10 was dysregulated in IVDD and overexpressed KLF10 could alleviate IVDD by regulating TGF-β signaling pathway both in vitro and in vivo, which were involved in prohibiting apoptosis, promoting proliferation and migration of NP cells. Furthermore, KLF10 also plays a vital role in ameliorating the remodeling of ECM in the development of IVDD through inhibition of catabolism and enhancement of anabolism. Therefore, our research indicated that KLF10 has a great potential to become an effective therapeutic target of IVDD.
Funding
The present study was funded by the National Natural Science Foundation of China (No. 81672199 and NO. 81972106), Key Discipline Construction Project of Pudong Health Bureau of Shanghai (No. PWZxk2017-08) and Shanghai Natural Science Foundation (No. 19ZR1441700).
Declaration of competing interest
The authors declare no competing financial interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jot.2021.04.003.
Abbreviations
- KLF10
Krüppel like factor 10
- IVDD
Intervertebral disc degeneration
- NP
Nucleus pulposus
- ECM
Extracellular matrix
- TGF-β
Transforming growth factor beta
- DH
Disc height index
- HE
Hematoxylin–eosin
- TU
Transduction Units
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
References
- 1.Vos T., Barber R.M., Bell B., Bertozzi-Villa A., Biryukov S., Bolliger I. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gore M., Sadosky A., Stacey B.R., Tai K.S., Leslie D. The burden of chronic low back pain: clinical comorbidities, treatment patterns, and health care costs in usual care settings. Spine (Phila Pa 1976. 2012;37(11):E668–E677. doi: 10.1097/BRS.0b013e318241e5de. [DOI] [PubMed] [Google Scholar]
- 3.Vos T., Flaxman A.D., Naghavi M., Lozano R., Michaud C., Ezzati M. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Risbud M.V., Shapiro I.M. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10(1):44–56. doi: 10.1038/nrrheum.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang J., Tian Y., Phillips K.L., Chiverton N., Haddock G., Bunning R.A. Tumor necrosis factor α- and interleukin-1β-dependent induction of CCL3 expression by nucleus pulposus cells promotes macrophage migration through CCR1. Arthritis Rheum. 2013;65(3):832–842. doi: 10.1002/art.37819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakai D., Nakamura Y., Nakai T., Mishima T., Kato S., Grad S. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat Commun. 2012;3:1264. doi: 10.1038/ncomms2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Subramaniam M., Harris S.A., Oursler M.J., Rasmussen K., Riggs B.L., Spelsberg T.C. Identification of a novel TGF-beta-regulated gene encoding a putative zinc finger protein in human osteoblasts. Nucleic Acids Res. 1995;23(23):4907–4912. doi: 10.1093/nar/23.23.4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Memon A., Lee W.K. KLF10 as a tumor suppressor gene and its TGF-β signaling. Cancers. 2018;10(6) doi: 10.3390/cancers10060161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou M., Chen J., Zhang H., Liu H., Yao H., Wang X. KLF10 inhibits cell growth by regulating PTTG1 in multiple myeloma under the regulation of microRNA-106b-5p. Int J Biol Sci. 2020;16(12):2063–2071. doi: 10.7150/ijbs.45999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kammoun M., Piquereau J., Nadal-Desbarats L., Même S., Beuvin M., Bonne G. Novel role of Tieg1 in muscle metabolism and mitochondrial oxidative capacities. Acta Physiol. 2020;228(3) doi: 10.1111/apha.13394. [DOI] [PubMed] [Google Scholar]
- 11.Bos J.M., Subramaniam M., Hawse J.R., Christiaans I., Rajamannan N.M., Maleszewski J.J. TGFβ-inducible early gene-1 (TIEG1) mutations in hypertrophic cardiomyopathy. J Cell Biochem. 2012;113(6):1896–1903. doi: 10.1002/jcb.24058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee J.M., Ko J.Y., Park J.W., Lee W.K., Song S.U., Im G.I. KLF10 is a modulatory factor of chondrocyte hypertrophy in developing skeleton. J Orthop Res. 2020;38(9):1987–1995. doi: 10.1002/jor.24653. [DOI] [PubMed] [Google Scholar]
- 13.Johnsen S.A., Subramaniam M., Janknecht R., Spelsberg T.C. TGFbeta inducible early gene enhances TGFbeta/Smad-dependent transcriptional responses. Oncogene. 2002;21(37):5783–5790. doi: 10.1038/sj.onc.1205681. [DOI] [PubMed] [Google Scholar]
- 14.Hu Z.C., Shi F., Liu P., Zhang J., Guo D., Cao X.L. TIEG1 represses smad7-mediated activation of TGF-β1/smad signaling in keloid pathogenesis. J Invest Dermatol. 2017;137(5):1051–1059. doi: 10.1016/j.jid.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 15.Johnsen S.A., Subramaniam M., Katagiri T., Janknecht R., Spelsberg T.C. Transcriptional regulation of Smad2 is required for enhancement of TGFbeta/Smad signaling by TGFbeta inducible early gene. J Cell Biochem. 2002;87(2):233–241. doi: 10.1002/jcb.10299. [DOI] [PubMed] [Google Scholar]
- 16.Yang H., Liu H., Li X., Pan H., Li Z., Wang J. TNF-α and TGF-β1 regulate Syndecan-4 expression in nucleus pulposus cells: role of the mitogen-activated protein kinase and NF-κB pathways. Connect Tissue Res. 2015;56(4):281–287. doi: 10.3109/03008207.2014.996702. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J., Li Z., Chen F., Liu H., Wang H., Li X. TGF-β1 suppresses CCL3/4 expression through the ERK signaling pathway and inhibits intervertebral disc degeneration and inflammation-related pain in a rat model. Exp Mol Med. 2017;49(9):e379. doi: 10.1038/emm.2017.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakai T., Mochida J., Sakai D. Synergistic role of c-Myc and ERK1/2 in the mitogenic response to TGF beta-1 in cultured rat nucleus pulposus cells. Arthritis Res Ther. 2008;10(6):R140. doi: 10.1186/ar2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfirrmann C.W., Metzdorf A., Zanetti M., Hodler J., Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26(17):1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 20.Guo J., Shao M., Lu F., Jiang J., Xia X. Role of Sirt1 plays in nucleus pulposus cells and intervertebral disc degeneration. Spine (Phila Pa 1976. 2017;42(13) doi: 10.1097/BRS.0000000000001954. E757-e66. [DOI] [PubMed] [Google Scholar]
- 21.Li Y., Yu S., Li L., Chen J., Quan M., Li Q. KLF4-mediated upregulation of CD9 and CD81 suppresses hepatocellular carcinoma development via JNK signaling. Cell Death Dis. 2020;11(4):299. doi: 10.1038/s41419-020-2479-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu M.H., Yang K.C., Chen Y.J., Sun Y.H., Lin F.H., Yang S.H. Optimization of puncture injury to rat caudal disc for mimicking early degeneration of intervertebral disc. J Orthop Res. 2018;36(1):202–211. doi: 10.1002/jor.23628. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H., La Marca F., Hollister S.J., Goldstein S.A., Lin C.Y. Developing consistently reproducible intervertebral disc degeneration at rat caudal spine by using needle puncture. J Neurosurg Spine. 2009;10(6):522–530. doi: 10.3171/2009.2.SPINE08925. [DOI] [PubMed] [Google Scholar]
- 24.Masuda K., Aota Y., Muehleman C., Imai Y., Okuma M., Thonar E.J. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine. 2005;30(1):5–14. doi: 10.1097/01.brs.0000148152.04401.20. [DOI] [PubMed] [Google Scholar]
- 25.Cheng X., Zhang L., Zhang K., Zhang G., Hu Y., Sun X. Circular RNA VMA21 protects against intervertebral disc degeneration through targeting miR-200c and X linked inhibitor-of-apoptosis protein. Ann Rheum Dis. 2018;77(5):770–779. doi: 10.1136/annrheumdis-2017-212056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han B., Zhu K., Li F.C., Xiao Y.X., Feng J., Shi Z.L. A simple disc degeneration model induced by percutaneous needle puncture in the rat tail. Spine. 2008;33(18):1925–1934. doi: 10.1097/BRS.0b013e31817c64a9. [DOI] [PubMed] [Google Scholar]
- 27.Chen J., Xie J.-J., Jin M.-Y., Gu Y.-T., Wu C.-C., Guo W.-J. Sirt6 overexpression suppresses senescence and apoptosis of nucleus pulposus cells by inducing autophagy in a model of intervertebral disc degeneration. Cell Death Dis. 2018;9(2) doi: 10.1038/s41419-017-0085-5. 56-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ji M.L., Jiang H., Zhang X.J., Shi P.L., Li C., Wu H. Preclinical development of a microRNA-based therapy for intervertebral disc degeneration. Nat Commun. 2018;9(1):5051. doi: 10.1038/s41467-018-07360-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tam V., Chan W.C.W., Leung V.Y.L., Cheah K.S.E., Cheung K.M.C., Sakai D. Histological and reference system for the analysis of mouse intervertebral disc. J Orthop Res. 2018;36(1):233–243. doi: 10.1002/jor.23637. [DOI] [PubMed] [Google Scholar]
- 30.Gunther M., Laithier M., Brison O. A set of proteins interacting with transcription factor Sp1 identified in a two-hybrid screening. Mol Cell Biochem. 2000;210(1–2):131–142. doi: 10.1023/a:1007177623283. [DOI] [PubMed] [Google Scholar]
- 31.He Q., Yan D., Dong W., Bi J., Huang L., Yang M. circRNA circFUT8 upregulates krüpple-like factor 10 to inhibit the metastasis of bladder cancer via sponging miR-570-3p. Mol Ther Oncolytics. 2020;16:172–187. doi: 10.1016/j.omto.2019.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang N., Chen J., Zhang H., Wang X., Yao H., Peng Y. LncRNA OIP5-AS1 loss-induced microRNA-410 accumulation regulates cell proliferation and apoptosis by targeting KLF10 via activating PTEN/PI3K/AKT pathway in multiple myeloma. Cell Death Dis. 2017;8(8) doi: 10.1038/cddis.2017.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subramaniam M., Cicek M., Pitel K.S., Bruinsma E.S., Nelson Holte M.H., Withers S.G. TIEG1 modulates β-catenin sub-cellular localization and enhances Wnt signaling in bone. Nucleic Acids Res. 2017;45(9):5170–5182. doi: 10.1093/nar/gkx118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillips K.L., Cullen K., Chiverton N., Michael A.L., Cole A.A., Breakwell L.M. Potential roles of cytokines and chemokines in human intervertebral disc degeneration: interleukin-1 is a master regulator of catabolic processes. Osteoarthritis Cartilage. 2015;23(7):1165–1177. doi: 10.1016/j.joca.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 35.Hatano E., Fujita T., Ueda Y., Okuda T., Katsuda S., Okada Y. Expression of ADAMTS-4 (aggrecanase-1) and possible involvement in regression of lumbar disc herniation. Spine. 2006;31(13):1426–1432. doi: 10.1097/01.brs.0000219954.67368.be. [DOI] [PubMed] [Google Scholar]
- 36.Nemoto O., Yamagishi M., Yamada H., Kikuchi T., Takaishi H. Matrix metalloproteinase-3 production by human degenerated intervertebral disc. J Spinal Disord. 1997;10(6):493–498. [PubMed] [Google Scholar]
- 37.Wang J., Markova D., Anderson D.G., Zheng Z., Shapiro I.M., Risbud M.V. TNF-α and IL-1β promote a disintegrin-like and metalloprotease with thrombospondin type I motif-5-mediated aggrecan degradation through syndecan-4 in intervertebral disc. J Biol Chem. 2011;286(46):39738–39749. doi: 10.1074/jbc.M111.264549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zieba J., Forlenza K.N., Khatra J.S., Sarukhanov A., Duran I., Rigueur D. TGFβ and BMP dependent cell fate changes due to loss of filamin B produces disc degeneration and progressive vertebral fusions. PLoS Genet. 2016;12(3) doi: 10.1371/journal.pgen.1005936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bian Q., Jain A., Xu X., Kebaish K., Crane J.L., Zhang Z. Excessive activation of TGFβ by spinal instability causes vertebral endplate sclerosis. Sci Rep. 2016;6:27093. doi: 10.1038/srep27093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen S., Liu S., Ma K., Zhao L., Lin H., Shao Z. TGF-β signaling in intervertebral disc health and disease. Osteoarthritis Cartilage. 2019;27(8):1109–1117. doi: 10.1016/j.joca.2019.05.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.