Abstract
Gastrointestinal (GI) cancers are among the main reasons for cancer death globally. The deadliest types of GI cancer include colon, stomach, and liver cancers. Multiple lines of evidence have shown that angiogenesis has a key role in the growth and metastasis of all GI tumors. Abnormal angiogenesis also has a critical role in many non-malignant diseases. Therefore, angiogenesis is considered to be an important target for improved cancer treatment. Despite much research, the mechanisms governing angiogenesis are not completely understood. Recently, it has been shown that angiogenesis-related non-coding RNAs (ncRNAs) could affect the development of angiogenesis in cancer cells and tumors. The broad family of ncRNAs, which include long non-coding RNAs, microRNAs, and circular RNAs, are related to the development, promotion, and metastasis of GI cancers, especially in angiogenesis. This review discusses the role of ncRNAs in mediating angiogenesis in various types of GI cancers and looks forward to the introduction of mimetics and antagonists as possible therapeutic agents.
Keywords: gastrointestinal cancers, angiogenesis, circular RNAs, microRNAs, non-coding RNAs, long non-coding RNAs
Graphical abstract
Deregulation of many types of ncRNAs can affect angiogenesis and metastasis in GI cancers. The rapid development of different types of mimetics or antagonists of specific ncRNAs, as well as more effective delivery technologies has raised the possibility to employ ncRNAs as targets to treat metastatic GI cancers.
Introduction
Gastrointestinal (GI) cancers are among the key reasons for cancer mortality worldwide. Colon, liver, and stomach cancer are the most common types of GI cancer, causing the most deaths.1 Although the worldwide cancer statistics show that pancreatic ductal adenocarcinoma (PDAC) and esophageal cancer are less common than other GI cancers, the incidence of pancreatic and esophageal cancer as compared to liver and stomach cancer depends on the region.1,2 Oncogenic mutations are likely to occur in GI tissues (intestines, stomach, and liver) because the epithelial cells are rapidly turned over.3,4 Unfortunately, some GI cancers do not show any symptoms in the early stages. This means that diagnosis often occurs at late stages, which reduces the effectiveness of therapy. Therefore, there is a need to generate newer and more efficient therapeutic approaches to increase patients’ survival. In recent years, many attempts to identify the main mutations in GI cancer cells have been carried out, with the aim to design more effective drugs.5,6 Despite some successes in newer therapeutic approaches, GI cancers remain life-threatening diseases.7,8 Considering its fundamental role in cancer growth and metastasis, angiogenesis has become an attractive target in cancer therapy.
Angiogenesis and GI cancers
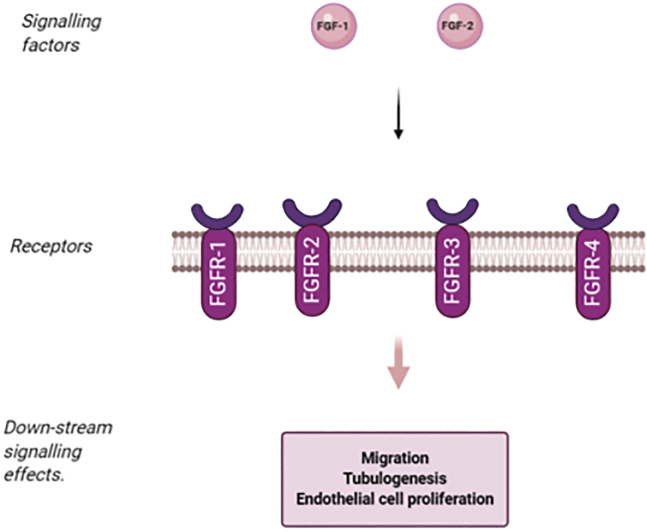
The expression of VEGF-A is upregulated in colorectal cancer and is associated with colorectal cancer metastasis9,10 and shorter patient survival.9,11,12 VEGF-A, the first VEGF member to be characterized, was the basis for the development of anti-angiogenesis as a therapeutic strategy, including the clinical development of bevacizumab, a humanized monoclonal antibody targeting VEGF-A. In a pivotal clinical trial, the use of bevacizumab in combination with irinotecan, 5-fluorouracil, and leucovorin was shown to improve the survival of patients with metastatic colorectal cancer (mCRC), resulting in its approval as the first antiangiogenic therapy.13 VEGF-A expression is associated with hematogenous and lymphatic spread,14,15 greater microvessel density (MVD),16 and a poor prognosis17 in gastric cancer (GC). Higher VEGF-A expression in pancreatic cancer is correlated with cancer progression, higher metastatic risk, and poor prognosis.18, 19, 20, 21, 22 Fibroblast growth factor (FGF) is a family composed of 20 different molecules, which have various biological functions, including the stimulation of angiogenesis.23 FGF-1 and FGF-2 are the most studied with regard to angiogenesis.24 The functioning of FGFs is mediated through tyrosine kinase receptors (FGFR1–FGFR4)25 (Figure 1). Endothelial cells express FGFR-1; however, small amounts of FGFR-2 have also been reported in endothelial cells.26 Activation of FGFR can induce the migration, growth, and tube formation of endothelial cells.27, 28, 29, 30 Many cancer cell lines secrete FGF-1 and FGF-2. It has been found that the concentration of FGF-2 is increased in the urine of patients with various cancers.31,32 FGFs were upregulated in blood samples of patients with colorectal cancer.33 FGFs can increase invasion and proliferation in colon cancer cells.34 In colorectal cancer patients, higher FGF-2 levels were associated with an elevated risk of metastasis.35 It was reported that there was a positive correlation between levels of FGF expression and stage D of colorectal cancer.36 It was also reported that FGFs play a role in cancer cell resistance to chemotherapy.37 In addition, serum FGF-2 levels had a predictive value for progression of disease in untreated metastatic colorectal cancer.38 FGF was upregulated in GC surgical samples.39 It was found that there was a positive association between high expression of FGF and invasion of GC cells40 and lymph-node metastasis.41 The expression of FGF-2 in GC could predict recurrence after resection.42,43 FGFs have a role in angiogenesis in pancreatic cancer,44, 45, 46 and it was found that the level of FGF could be used for prediction of patient survival and risk of metastasis.47 Despite much research, the mechanisms governing dysfunctional angiogenesis are not completely understood. Recently, a number of angiogenesis-related non-coding RNAs (ncRNAs) have been investigated, and they have been found to affect the development of angiogenesis in cancer cells and tumors.48
Figure 1.
Overview of FGFs and their receptors and related signaling pathways
Angiogenesis-related ncRNAs and GI cancers
ncRNAs are generated via the transcription of various sections of the genome. MicroRNAs (miRNAs or miRs) are an important group of ncRNAs, estimated to be able to regulate 40%–90% of the human genome.49 miRNAs and long non-coding RNAs (lncRNAs) can modulate gene expression at the transcriptional as well as post-transcriptional levels. They can also act as epigenetic regulators. It has been found that ncRNAs can inhibit the translation of mRNAs. Moreover, ncRNAs can regulate many biological pathways and subsequently alter the cell fate by stimulating or inhibiting the expression of specific genes.50,51 Recently, many basic research studies have attempted to identify the mechanisms of disease pathogenesis using both living organisms and also in vitro and in silico systems. These investigations have demonstrated that ncRNAs can play critical functions, for instance in pancreatic cancer development. It has been revealed that miRNAs can affect cancer cell proliferation, migration, invasion, and metastasis.49,52
Angiogenesis-related microRNAs in GI cancer
miRNA biogenesis
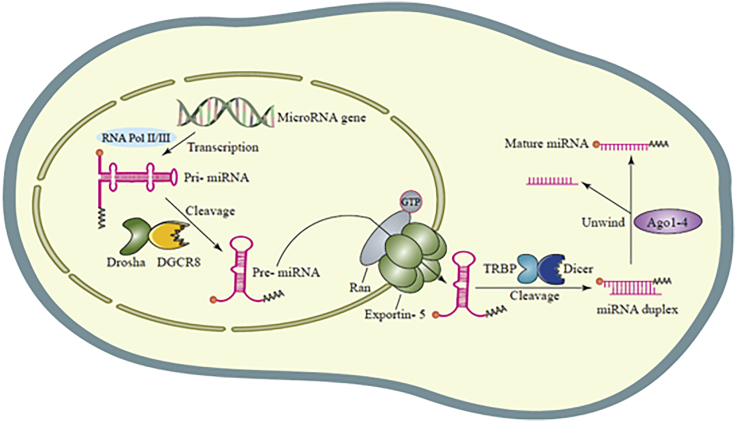
miRNAs are single-stranded non-coding RNAs, which contain 20–22 nucleotides.53 miRNAs are transcribed from their specific genes by RNA polymerase II and III, to generate pri-miRNAs (primary miRNAs), which are then cleaved by the Drosha enzyme to form pre-miRNAs (precursor miRNAs).54,55 Pre-miRNAs have a hairpin-like structure, which is cleaved when transported from inside the nucleus into the cytoplasm as a miRNA duplex by the Dicer enzyme to form the mature miRNA structure, which is the active form of miRNA.56,57 The less-stable strand of the miRNA duplex is normally incorporated into the RISC (miRNA-induced silencing complex) in order to regulate protein expression (Figure 2). This regulation is often accomplished by hybridization of the miRNA to the 3′ untranslated region (UTR) of the sequence of its target mRNA.58,59
Figure 2.
MicroRNA biogenesis
Angiogenesis-related microRNAs in colon cancer
miR-145 works in concert with the tumor suppressor p53 and is post-transcriptionally activated by upregulated p53.60 miR-145 was first found to be downregulated in colorectal cancer, and then the deregulation of this miRNA was found in lung, breast, ovarian, bladder, nasopharyngeal, prostate, and GC.61, 62, 63 Moreover, miR-145 has a role in smooth muscle cell flexibility and development.64 Xu et al.65 investigated the effects of miR-145 and its targets, p70S6K1, VEGF, and HIF-1, on angiogenesis in colon cancer. They display that miR-145 lowered in ovarian and colon cancer. The expression of p70S6K1 (ribosomal protein S6 kinase beta-1) is post-transcriptionally suppressed by miR-145. HIF-1 and VEGF, which are the downstream mediators of p70S6K1, were decreased by upregulation of miR-145. Exogenous P70S6K1 rescues the miR-145 suppression of VEGF and HIF-1 levels and restores tumor angiogenesis and tumorigenesis. There is therefore an inverse association between the level of miR-145 and p70S6K1 in colon cancer.65
miRNA-27b is located on chromosome 966 and has a role in angiogenesis by increasing endothelial sprouting.67,68 miR-27b plays a role as a tumor repressor by targeting PPARγ in neuroblastoma, although the function of miR-27b, as well as its target in colon cancer, is not completely clear. In one study, Ye et al.69 assessed the association between miR-27b and VEGF-C in angiogenesis in colorectal cancer. The expression of miR-27b was downregulated in CRC, and the high expression of miR-27b inhibited tumor growth, cell proliferation, and colony formation. They reported that miR-27b could act as a tumor repressor and inhibit angiogenesis by targeting VEGF-C in colorectal cancer. They also found that DNA hyper-methylation of the CpG islands in miR-27b reduced its expression.69
miR-590-5p is one of the newly identified microRNAs, whose functions are not completely understood. However, some studies have shown that this miRNA was downregulated in colorectal cancer. It could also act as an oncogene in cervical cancer but as a tumor suppressor in renal cell cancer.70,71 It is thought that the direct target of miR-590-5p is the mRNA of the transcription factor family NF90, which is transcribed from the ILF3 gene.72 The specific protein isoforms are NF90 and NF110. NF90 is about 90 kDa and is also known as NFAR1 or DRBP76. NF110 is about 110 kDa and is also known as NFAR2, TCP110, or ILF3.72 NF90 and NF110 are different at the C terminus but homologous in the central region and the N terminus.72 NF90 was purified as a DNA binding complex that regulated the IL2 promoter.73 NF90 has many functions, such as in protein translation from mRNAs, controlling mitosis, RNA processing, and host resistance to infection.74 It was also implicated in the occurrence of angiogenesis of breast cancer. Moreover, the NF90/NF45 complex regulated E6 expression in cervical cancer.75 Zhou et al.76 studied the effects of miR-590-5p, NF90, and VEGF on angiogenesis in colon cancer. The deletion of miR-590-5p in vivo enhanced colorectal cancer progression, while its high expression suppressed tumor growth, angiogenesis, and lung metastasis. NF90 participates in regulating VEGF protein synthesis and is a target of miR-590-5p. High expression of NF90 restored angiogenesis and VEGFA expression. NF90-shRNA reduced tumor development, and the deletion of NF90 decreased the pri-miR-590 and increased miR 590 5p.76
miR-19a and miR-19b are about 96% identical to each other and only differ by one nucleotide at position 11. They have a role in some cellular processes and tumor progression.77,78 Chen et al.79 investigated the effects of miR-19a and its target, KRAS, on angiogenesis in colon cancer. They generated a KRAS 3′ UTR-Mut by removing the predicted binding site for miR-19a within KRAS. miR-19a inhibited the expression of the gene containing the wild-type KRAS 3′ UTR in HCT116 cells, but the cells with mutant KRAS 3′ UTR were not affected by miR-19a. The high expression of miR-19a decreased the expression of KRAS. In a vascular tube formation assay, the high expression of miR-19a produced anti-angiogenesis effects, which could be rescued via expression of KRAS. They also used a nude mouse xenograft model to study the in vivo function of miR-19a in solid tumors. Findings showed that the density of blood vessels and the size of the xenograft tumors grown from HCT116 cells that overexpressed miR-19a were lower in comparison with the controls. Moreover, the levels of VEGF-A and KRAS were decreased.79
CXCR4 is a constituent of the GPCR family that is expressed in some epithelial cancers, where it increases proliferation, migration, and angiogenesis.80, 81, 82 Deletion of CXCR4 suppressed CXCL12-induced angiogenesis via downregulating PI3K-AKT, MAPK-ERK, and Wnt-β-catenin signaling pathways.83 In a recent study, Fang et al.84 assessed the effects of miR-622, CXCR4, and VEGFA on angiogenesis in colorectal cancer. In vitro studies showed that the high expression of miR-622 suppressed angiogenesis in CRC and the proliferation, migration, tube formation, and invasion of human umbilical vein endothelial cells (HUVECs). Moreover, the higher expression of miR-622 inhibited angiogenesis in CRC tumors in vivo, as detected via the quantification of VEGF-A and Ki67 levels and microvessel density. CXCR4 is a target of miR-622, and higher expression of CXCR4 reversed the VEGFA suppression by miR-622 and also restored the angiogenesis that had been inhibited by miR-622.84
Table 1 lists some different angiogenesis-related miRNAs that have been shown to participate in colorectal cancer.
Table 1.
Angiogenesis-related miRNAs in colon cancer
| microRNA | Expression change in CRC | Target | Inhibit or induce angiogenesis | Model (in vivo, in vitro, human) | Cell line | Reference |
|---|---|---|---|---|---|---|
| miR-1 | down | VEGF | inhibit | in vitro, human | HT-29, HCT-116, ClonA1, CL-187, SW-620 | Zhu et al.85 |
| miR-622 | up | CXCR4, VEGFA | inhibit | in vitro, in vivo | Caco-2, HT-29 | Fang et al.84 |
| miR-19a | up | KRAS | inhibit | in vitro | HCT116 | Chen et al.79 |
| miR-6868-5p | down | FOXM1 | inhibit | in vivo | HCT8 | Wang et al.86 |
| in vitro | HCT116 | |||||
| miR-125a-3p | up | FUT5, FUT6 | inhibit | in vitro, human | SW480, SW620 | Liang et al.87 |
| miR-590-5p | down | NF90, VEGFA | inhibit | in vitro, in vivo, in vivo | HT29, SW620, LOVO, SW480, HCT116 | Zhou et al.76 |
| miR-17∼92 | up | GFBR2, HIF1α, VEGFA | induce | in vivo, in vitro | HCT116 | Ma et al.88 |
| miR-145-5p | down | Cx43 | inhibit | in vitro | SW480 | Thuringer et al.89 |
| miR-126 | down | VEGF | inhibit | in vitro, human | LoVo, HT29, SW480, SW620, SW1116, HCT116 | Zhang et al.90 |
| miR-27b | down | VEGFC | inhibit | in vitro, human | SW620, SW480, RKO, HT29, 293T | Ye et al.69 |
| miR-885-3p | up | BMPR1A | inhibit | in vivo, in vivo, human | HT-29 | Xiao et al.91 |
| miR-150-5p | down | VEGFA | inhibit | human, in vitro, in vivo | HCT116, SW620, HCT8, HT29, SW480, DLD-1, FHC | Chen et al.92 |
| miR-143 | down | IGF-IR | inhibit | human, in vitro, in vivo | SW1116 | Qian et al.93 |
| miR-107 | up | VEGF, HIF-1b | inhibit | human, in vitro, in vivo | HeLa, HCT116 | Yamakuchi et al.94 |
| miR-145 | down | p70S6K1, VEGF, HIF-1 | inhibit | in vitro, in vivo, human | SW1116, SW480 | Xu et al.65 |
| miR-181a-5p | down | MMP-14 | inhibit | in vitro, in vivo | Li et al.95 | |
| miR-503-5p | down | VEGF-A | inhibit | in vitro, in vivo, human | HT-29, LoVo, HCT116, RKO, SW620 | Wei et al.96 |
| miR-182-5p | down | VEGF-C | inhibit | in vitro, in vivo, human | SW620, LoVo, RKO, HT-29, HCT116 | Yan et al.97 |
| miR-524-5p | up | WNK1 | inhibit | in vitro, in vivo | HT-29, COLO205 | Li et al.98 |
Angiogenesis-related microRNAs in pancreatic cancer
It has been shown that miR-139 can suppress proliferation, migration, and metastasis in some types of cancer.99 miR-139 was highly expressed in pancreatic cancer endothelial cells (CECs).
Li et al.100 studied the effects of miR-139 and CXCR4 on angiogenesis in pancreatic cancer. Quantitative polymerase chain reaction (qPCR) analysis was applied to quantify the expression pattern of miR-139. The effects of miRNA expression on CEC proliferation, migration, and tube formation were evaluated after transfecting with a specific miRNA suppressor. The expression of fourteen miRNAs was enhanced more than 20 times in the CECs obtained from pancreatic cancer patients. Among these, miR-200c and miR-139 were most overexpressed in CECs. Transfection with inhibitors of miR-200c or miR-139 decreased migration of CECs (all p < 0.05). The average tube length and proliferation were reduced after miR-200c and miR-139 inhibition in three CEC cultures (all p < 0.05).100
BCL2L1 and BCL-xL are members of the BCL-2 family101 that inhibit apoptosis and autophagy in cancer cells. BCL2L11, also known as Bim, has the opposite function and can stimulate apoptosis by inhibiting BCL2 and BCL-xL. Bim is a pro-apoptotic protein located in the outer mitochondrial membrane, where it promotes the apoptotic cascade.102 The role of Bim in pancreatic cancer remains somewhat unclear. Liu et al.103 assessed the effects of the miR-24-Bim pathway on angiogenesis in pancreatic cancer. Expression of miR-24 resulted in lower expression of Bim. miR-24 enhanced tumor development and angiogenesis in vivo by inhibiting Bim expression.103
AGTR1 is expressed in several cancers, such as ovarian carcinoma. Suppression of AGTR1 decreased angiogenesis and cell survival via reducing VEGF expression.104 AGTR1 and angiotensin II have functions in endometrial tumor development by inducing VEGF.105,106 Moreover, AGTR1 is upregulated in breast cancer. Inhibition of AGTR1 suppressed proliferation as well as causing G1/S cell cycle arrest.107 In breast cancer, AGTR1 enhanced invasion, migration, and metastasis and also stimulated angiogenesis by increasing VEGF-A expression.108 Guo et al.109 demonstrated the effects of miR-410, AGTR1, and CD31 on angiogenesis in pancreatic cancer. AGTR1 is a target of miR-410, which inhibits its expression, and, conversely, the suppression of miR-410 enhances the AGTR1 expression. Overexpression of miR-410 inhibited cell invasion and growth by reducing AGTR1. In addition, the expression of VEGF and ERK signaling activation were both blocked by miR-410. This was similar to the action of losartan, which acts as an angiotensin II inhibitor. miR-410 blocked the induction of the VEGF and ERK pathways via stimulating angiotensin II. The high expression of miR-410 suppressed angiogenesis in vivo by inhibition of CD31. The deletion of the ERK signaling pathway inhibited angiogenesis, cell invasion, and proliferation in pancreatic cancer. Downregulation of miR-410 was detected in pancreatic cancer samples, although there was high expression of AGTR1 in this cancer. Pearson correlation analysis showed an inverse relationship between AGTR1 and miR-410 expression. They concluded that miR-410 inhibited cell migration, growth, invasion, and angiogenesis by reducing AGTR1 expression in pancreatic cancer.109
SOCS5 is a member of the SOCS protein family, also known as the SSI protein family.110 SOCS5 modulates the function of STAT3 and is mediated by IL-6.111,112 SOCS5 binds to the JAK kinase domain, suppresses the auto-phosphorylation of JAK, and negatively regulates the JAK/STAT3 pathway.113
Hu et al.114 studied the effects of miR-301a and SOCS5 on angiogenesis in pancreatic cancer. Higher expression of miR-301a in pancreatic cancer was associated with low overall survival. High expression of miR-301a increased angiogenesis, migration, and invasion, while suppression of miR-301a inhibited invasion and decreased orthotopic pancreatic tumor metastasis and growth. SOCS5 has been recognized as a target of miR-301a, because miR-301a inhibited the SOCS5 expression, leading to induction of JAK/STAT3 signaling, and was linked to poor survival of pancreatic cancer patients.114
miR-454 acts as an oncogene in several cancers, such as uveal melanoma115 and non-small cell lung cancer.116 Fan et al.117 investigated the effects of miR-454 and LRP6 on angiogenesis in PDAC. Human pancreatic cancer cell lines (PANC-1 and MiaPaCa-2 cells) were transfected with a miR-454-expressing plasmid and assayed for colony formation, cell proliferation, pro-angiogenic markers, cell cycle, and invasion. The effect of miR-454 overexpression on lung metastasis of PDAC was investigated in vivo. The high expression of miR-454 suppressed colony formation, cell invasion, and proliferation and arrested the cells at the G2/M stage. miR-454-overexpressing PANC-1 cells contained low levels of VEGF and had a lower ability to induce endothelial cell tube-like formation. LRP6 is a target of miR-454, which can suppress the Wnt/β-catenin pathway activation in PDAC. Ectopic expression of LRP6 reversed the inhibitory effects of miR-454 in PDAC.117
Table 2 lists some angiogenesis-related miRNAs involved in pancreatic cancer.
Table 2.
Angiogenesis-related miRNAs in pancreatic cancer
| microRNA | Expression in pancreatic cancer | Target | Effect on angiogenesis (inhibit/induce) | Model (in vivo, in vitro, human) | Type of cell line | Reference |
|---|---|---|---|---|---|---|
| miR-139 | up | CXCR4 | inhibit | human | Li et al.100 | |
| miR200c | up | VEGFA | inhibit | human | Li et al.100 | |
| miR-24 | up | Bim | induce | in vitro, in vivo | HUVEC | Liu et al.103 |
| miR-410 | down | AGTR1 | inhibit | in vitro, in vivo, human | PANC-1, MIA-PaCa-2, AsPC-1 | Guo et al.109 |
| miR-454 | up | LRP6 | inhibit | in vitro, in vivo | PANC-1, MIA-PaCa-2 | Fan et al.117 |
| miR-301a | up | SOCS5 | induce | in vitro, in vivo | PANC-1, BXPC3 | Hu et al.114 |
Angiogenesis-related microRNAs in hepatocellular carcinoma (HCC)
miR29a/b/c are members of the miR-29 family. They are broadly similar to each other, but they have some differences in the target sequence they recognize. They are generally downregulated in HCC.118 Some articles have shown that miR-29 has an inhibitory effect on apoptosis, migration, proliferation, and invasion of non-HCC tumor types.119, 120, 121 The miR-29b levels were inversely associated with MMP-2 expression, as well as invasion, metastasis, and angiogenesis. Activation of the MMP-2 enzyme causes degradation of the extracellular matrix (ECM), which then promotes the metastasis and invasion of tumors.122 MMP-2 accelerates ECM remodeling and the secretion of ECM-bound growth factors, which can promote the proliferation and migration of ECs. Generally, the overexpression of MMP-2 is observed in HCC.123,124
Fang et al.125 investigated the effects of miRNA-29b and MMP-2 on angiogenesis in HCC. They studied the effects of miR-29b on tumor invasion, metastasis, and angiogenesis using Transwell assays and capillary tube formation. Human tumor samples, a Matrigel plug assay, and in vivo subcutaneous xenograft tumor growth were used. Loss- and gain-of-function experiments demonstrated that miR-29b inhibited the ability of tumor cells to enhance the formation of endothelial cell capillary tubes and Matrigel invasion. They showed that tumors originating from miR-29b-expressing HCC cells had a lower intrahepatic metastatic capacity and lower microvessel density in mouse models. Experiments showed that MMP-2 was a target of miR-29b. The removal of MMP-2 using a neutralizing antibody or an RNA interference approach duplicated the anti-invasion and anti-angiogenesis properties of miR-29b. They suggested that miR-29b exerted its anti-angiogenic activity by inhibiting the expression of MMP-2 in tumor cells and suppressing VEGFR-2 signaling in endothelial cells.125
Bentwich et al.126 identified miR-503 for the first time, which was confirmed by Sewer et al.127 and cloned by Landgraf et al.128 The miR-503 gene is situated on chromosome Xq26.3, and the targets of miR-503 in ECs were found to be cdc25A (cell division cycle 25 homolog) and CCNE1 (G1/S-specific cyclin-E1). miR-503 inhibits endothelial cell function in diabetes mellitus and could promote reparative angiogenesis following limb ischemia.129 However, the functions of miR-503 in angiogenesis and cancer development are not completely clear.
In 2013, Zhou et al.130 assessed the effects of miR-503, FGF2, and VEGFA on angiogenesis in HCC. miR-503 overexpression lowered angiogenesis in vitro, while in vivo the expression of miR-503 was decreased by HIF1α. VEGFA and FGF2 are both targets of miR-503 in cancer; therefore, miR-503 plays an anti-angiogenic role in tumorigenesis. The latter suggested a new mechanism for hypoxia-induced VEGFA and FGF2 via HIF1α-induced suppression of miR-503.130
Sphingosine kinase 1 (SPHK1) is an enzyme that produces sphingosine-1-phosphate (S1P) and participates in the control of sphingolipid metabolism.131 SPHK1 can enhance breast cancer tumorigenesis via increasing S1P and inducing angiogenesis.132 The SPHK1/S1P/S1P-receptor axis was confirmed to participate in the angiogenesis associated with liver fibrosis.133 Lu et al.134 investigated the effects of SPHK1 and miR-506 on angiogenesis in HCC. Database analysis demonstrated that miR-506 could target the 3′ UTR of SPHK1. Using reverse transcriptase-PCR and western blotting, they revealed that miR-506 decreased the protein and mRNA expression of SPHK1. The overexpression of miR-506 in HepG2 cells decreased the S1P content in the supernatant. This supernatant suppressed HUVEC tube formation and decreased angiogenesis. The supernatant from HepG2 cells with high expression of SPHK1 reversed the suppression of angiogenesis. The use of anti-miR-506 increased the S1P production in the supernatant, while knockdown of SPHK1 in HepG2 cells abrogated the anti-miR-506-mediated acceleration of angiogenesis. These studies showed a close relationship between miR-506 and SPHK1 levels in HCC.134
miR-146a has a role in several cancers and can regulate the immune system, such as antiviral activity and inflammatory response.135, 136, 137, 138, 139 The relatively high expression of miR-146a was observed in papillary thyroid carcinoma and could be related to loss of the KIT transcript and Kit protein.136 miR-146a could also enhance proliferation in cervical cancer.140 Zhu et al.141 studied the effects of miR-146a and PDGFRA on angiogenesis in HCC. They examined the miR-146a expression in HUVECs in the presence or absence of HCC cells. PDGFRA is a miR-146a target, and this miRNA could increase angiogenesis by enhancing PDGFRA in HCC. They also found that miR-146a increased expression of PDGFRA in HUVECs via affecting BRCA1. High expression of PDGFRA in HCC clinical samples was correlated with microvascular invasion and predicted a poor prognosis.141
miR-126 has a role in cancers by modulating migration, invasion, and proliferation.142, 143, 144 miR-126 is known to be a tumor suppressor, preventing proliferation and enhancing apoptosis in HCC cells.145 Gong et al.146 investigated the effects of EGFL7 and miR-126 on angiogenesis in HCC. Western blotting and qRT-PCR were used to determine the levels of EGFL7, miR-126, Fas/FasL, ERK, caspase mRNA, and Bcl-2 expression. TUNEL and Cell Counting Kit 8 assays were used to measure apoptosis and proliferation. Flow cytometry was applied to examine cell cycle distribution. A rat model of HCC was established, and the quantity of new blood vessels and tumor weight were measured at 3 weeks post-tumor transplantation. miR-126 was downregulated in HCC, whereas the levels of ERK mRNA and protein and EGFL7 were increased. The high expression of miR-126 inhibited P-ERK, ERK, Bcl-2, and EGFL7 and also enhanced the apoptosis-related proteins caspase-3 and Fas/FasL and suppressed proliferation. Overexpression of miR-126 in nude mice produced fewer blood vessels and reduced the tumor weight. Suppression of miR-126 reduced apoptosis and increased angiogenesis and proliferation.146
Table 3 lists some different angiogenesis-associated miRNAs in HCC.
Table 3.
Angiogenesis-associated miRNAs in hepatocellular carcinoma
| microRNA | Expression in HCC | Target | Effect on angiogenesis (inhibit/induce) | Model (in vivo, in vitro, human) | Type of cell line | Reference |
|---|---|---|---|---|---|---|
| miR-1301 | down | BCL9 | inhibit | in vitro, in vivo, human | Hep3B, HepG2, SMMC-7721, Huh-7 | Yang et al.147 |
| miR-26b-5p | down | VE-cadherin, snail, MMP2 | inhibit | in vitro, in vivo | Bel7402, SMMC7721, HepG2, PLC, LO2 | Wang et al.148 |
| miR-199a-3p | down | VEGFA, VEGFR2, VEGFR1, HGF, MMP2 | inhibit | in vitro, in vivo | HepG2, SNU449 | Ghosh et al.149 |
| miR-203a | up | HOXD3, VEGFR | inhibit | in vitro, in vivo | SMMC-7721, Hep3B | Ghosh et al.149 |
| miR-144-3p | down | SGK3 | inhibit | in vitro, in vivo, human | QGY-7703, SK-hep1 | Wu et al.150 |
| miR-497 | down | VEGFA, AEG-1 | inhibit | in vitro, human, in vivo | PLC/PRF/5, SMMC-7721, HepG2, Huh7, SK-HEP-1, Hep3B | Yan et al.151 |
| miR-142 | down | TGF-β | inhibit | in vitro, human | HepG-2, SMMC-7721 | Yu et al.152 |
| miR-126 | up | EGFL7 | inhibit | in vitro, in vivo, human | MMC-7721, MHCC-97H, HCCLM3 | Yu et al.152 |
| miR-338-3p | down | MACC1, VEGF, β-catenin | inhibit | in vitro, human | Hep3B, Huh7, HepG2, Bel-7402, HEK293T | Zhang et al.153 |
| miR-638 | down | VEGF | induce | in vitro, in vivo, human | Hep3B, SMMC-7721, HepG2, MHCC-97L, MHCC-97H | Cheng et al.154 |
| miR-126 | down | EGFL7 | inhibit | in vitro, in vivo, human | HepG2, Bet-7402, SMMC-7721 | Gong et al.146 |
| miR-210 | up | FGFRL1 | induce | in vitro, in vivo, human | HL-7702, SMMC-7721 | Yang et al.155 |
| miR-182 | up | RASA1 | induce | in vitro, human | SK-HEP-1, HCC-LM3 | Du et al.156 |
| miR-126 | down | Spred1 | inhibit | in vitro, human | Ji et al.157 | |
| miR-26a | down | HGF | inhibit | in vitro, in vivo, human | HUVECs | Yang et al.158 |
| miR-126-3p | down | LRP6, PIK3R2 | inhibit | in vitro, human | HepG2, SMMC-7721, BEL-7402 | Du et al.159 |
| miR-195 | down | VEGF, VAV2, CDC42 | inhibit | in vitro, in vivo, human | MHCC-97L, Huh-7, QGY-7703, MHCC-97H, SMMC-7721 | Wang et al.160 |
| miR-302a/b/c | down | MACC1 | inhibit | in vitro | cell line missing | Cao et al.161 |
| miR-26a | down | VEGFA | inhibit | in vitro, in vivo, human | HepG2 | Chai et al.162 |
| miR-146a | up | PDGFRA | induce | in vitro, in vivo | HCCLM3 | Zhu et al.141 |
| miR-506 | down | SPHK1 | inhibit | in vitro, human | HepG2 | Lu et al.134 |
| miR-98 and miR-214 | down | VEGF, Ang-1, MMP-2 | inhibit | in vitro | HepG2 | Yahya et al.163 |
| miR-375 | up | PDGFC | inhibit | in vitro, human | Hep3B, HepG2, Huh1, Huh7 | Li et al.164 |
| miR-503 | down | FGF2, VEGFA | inhibit | human, in vitro, in vivo | HepG2, LO2 | Zhou et al.130 |
| miR-29b | down | MMP-2 | inhibit | human, in vitro, in vivo | Fang et al.125 | |
| miR-200b | down | ERG | induce | in vitro, human | Hep3B | Moh-Moh-Aung et al.165 |
Angiogenesis-related microRNAs in GC
miR-125a is located in chromosome 19q13, and its expression has been found to be low in several cancers, such as breast166, ovarian,167 and lung cancer168 and medulloblastoma169. In GC, lower expression of miR-125a was correlated to indicators of malignancy, such as tumor invasion and size.170
Dai et al.171 assessed the effects of VEGF-A and miR-125a on angiogenesis in GC and reported that miR-125a could affect VEGF-A expression. Low expression of miR-125a enhanced the release of VEGF-A and also increased Akt phosphorylation in HUVECs, angiogenesis, EC migration, and proliferation. miR-125a expression was reduced in GC and was negatively associated with VEGF-A expression (p < 0.05). miR-125a expression was inversely correlated to the microvessel density in vivo.171
VEGF-A has an important function in the regulation of angiogenesis.17 In addition, levels of VEGF-A are correlated with enhanced tumor aggression and decreased survival in patients.172,173 Xie et al.174 investigated the effects of miR-1 and VEGF-A on angiogenesis in GC. They evaluated the expression of miR-1 in GC cell lines and the clinicopathological features in 90 paired GC and normal stomach samples. Proliferation and migration assays were used to detect the effects of miR-1 in GC cells. Protein array and bioinformatic analysis was used to identify the miR-1 target. qPCR, ELISA, EC tube formation, and western blotting were used to assess the regulation mechanisms of miR-1. A reporter assay was also used to confirm the presumed binding site of miR-1 on target genes. miR-1 was downregulated in GC specimens. Patients with lower expression of miR-1 had a poor survival compared with those with higher expression of miR-1 (p = 0.0027). miR-1 overexpression in GC suppressed proliferation, EC tube formation, and migration via inhibiting the expression of EDN1 and VEGF-A. Suppression of miR-1 by an antagonist decreased EDN1 and VEGF-A expression in low-malignant GC or non-malignant GC samples.174
The PI3K/mTOR/AKT signaling pathway is dysregulated in various cancers. Elements of the AKT/PI3K/mTOR signaling pathway can be mutated or dysregulated in cancer, causing hyper-activation of the pathway and affecting chemosensitivity, apoptosis, proliferation, and other biological processes.175
Wu et al.176 assessed the effects of miR-616-3p and the AKT/PI3K/mTOR pathway on angiogenesis in GC. They found that the miR-616-3p was overexpressed in GC and was correlated with a poor prognosis. Also, loss-of-function and gain-of-function studies showed that miR-616-3p increased angiogenesis and triggered the epithelial-mesenchymal transition (EMT) in GC.176
The mammalian GI tract secretes members of the trefoil factor family, which are a group of small-molecular-weight polypeptides.177 TFF1 is a member of the trefoil peptide family, which suppresses GI tumorigenesis. The expression of TFF1 is high in the normal human stomach, where it acts to preserve gastric epithelial function and structure.178
Shi et al.179 investigated the effects of miR-632 and TFF1 on angiogenesis in GC using serum samples and GC tissues to measure the expression of miR-632 with real-time PCR. A dual-luciferase reporter assay was performed to examine how miR-632 controlled the TFF1 expression. Endothelial cell recruitment and tube formation assays also were used with or without miR-632 treatment. Moreover, in situ hybridization assays and western blotting were used to determine markers of endothelial migration and angiogenesis. miR-632 expression was high in GC and inversely correlated with its target TFF1. miR-632 stimulated EC recruitment and tube formation, while recombinant TFF1 reversed the miR-632-induced angiogenesis.179
RUNX1, RUNX2, and RUNX3 are members of the Runt family of transcription factors, with key roles in both normal tissue and cancers.180 RUNX3 has a role in T cell differentiation, neurogenesis within the dorsal root ganglia, and GC tumorigenesis.180
Lee et al.181 studied the effects of miR-495, miR-130a, and RUNX3 on angiogenesis in GC and employed bioinformatic and microarray analysis to measure the miR-130a and miR-495 expression. miR-495 and miR-130a were both highly expressed in GC under hypoxic conditions. miR-495 and miR-130a both suppressed the expression of RUNX3 at the protein level but not at the mRNA level. miR-495 and miR-130a suppressed luciferase activity in a reporter assay for RUNX3-3′ UTR binding. miR-130a and miR-495 reduced apoptosis as shown by annexin V-fluorescein isothiocyanate (FITC)/propidium iodide staining as well as flow cytometry. In SNU484 GC cells, the expression of miR-130a and miR-495 was associated with lower levels of RUNX3, p21, and Bim. Antagonistic miRs for miR-495 and miR-130a decreased angiogenesis, as shown by a Matrigel plug assay.181
Table 4 lists some angiogenesis-related miRNAs reported to be involved in GC.
Table 4.
Angiogenesis-related miRNAs in gastric cancer
| microRNA | Expression in gastric cancer | Target | Effect on angiogenesis (inhibit/induce) | Model (in vivo, in vitro, human) | Type of cell line | Reference |
|---|---|---|---|---|---|---|
| miR-632 | up | TFF1 | induce | in vitro | BGC823, MGC803, EAhy926, MKN45 | Shi et al.179 |
| miR-612 | down | FOXM1 | inhibit | in vitro, in vivo | MKN-45, MKN-28, AGS, SGC-7901 | Wang et al.182 |
| miR-616-3p | up | PTEN/AKT/mTOR pathway | induce | in vitro | KN-28, MGC-80, GES-1, HEK293 T, AGS, SGC-7901 | Wu et al.176 |
| miR-532-5p | down | LINC01410 | inhibit | in vitro, in vivo | MNK-45, SGC-7901, AGS, HGC-27, BGC-23, GES-1 | Zhang et al.183 |
| miR-26a/b | down | HGF VEGF | inhibit | in vitro, in vivo | MKN-28, GES-1, AGS, HEK293 T, SGC-7901, MGC-80 | Si et al.184 |
| miR-1 | down | EDN1 VEGF-A | inhibit | in vitro, in vivo | SGC7901, MKN28, NCI-N87, BGC823, AGS, HGC27 | Xie et al.185 |
| miR-1228 | down | CK2A2 | inhibit | in vitro, in vivo | AGS, SGC-7901, HEK293T | Jia et al.186 |
| miR-218 | down | ROBO1 | inhibit | in vitro, in vivo | BGC-823, HUVEC-2C, HMVEC | Zhang et al.187 |
| miR-520b/e | down | EGFR | inhibit | in vitro | SGC-7901, MGC-803 | Li et al.188 |
| miR-101, miR-27b, miR-128 | down | VEGF-C | inhibit | in vitro, in vivo | MKN-45, SGC-7901, BGC-823 | Liu et al.189 |
| miR-125a | down | VEGF-A | inhibit | in vitro, human | GES-1, AGS, SGC7901, BCG-823, HUVEC | Dai et al.171 |
| miR-130a, miR-495 | up | RUNX3 | induce | in vitro | SNU5, SNU16, SNU484, MKN45, MKN1 | Lee et al.181 |
| miR-506 | down | ETS1 | inhibit | in vitro, human | AGS, SGC-7901, Kato-III, MKN45, BGC-823, HGC-27, MGC-803 | Li et al.190 |
| miR-874 | down | 3′ UTR of STAT3 | inhibit | in vitro, human | AGS, BGC823, MKN28, SGC-7901, GES-1 | Zhang et al.191 |
| miR-126 | down | VEGF-A | inhibit | in vitro, in vivo, human | SGC-7901, MKN-28, MKN-45 | Chen et al.192 |
| miR-29a/c | down | VEGF | inhibit | in vitro, human | HUVE, SGC790, HEK293T | Zhang et al.193 |
| miR-135a | down | focal adhesion kinase (FAK) | inhibit | in vitro, in vivo, human | MGC-803, MKN45, SGC-7901, BGC-823, MKN1, GES-1 | Cheng et al.194 |
| miRNA-145 | down | 3′ UTR of Ets1 | inhibit | in vitro, in vivo, human | SGC-7901, GES-1, MKN-45, HUVEC, AGS | Zheng et al.195 |
| miR-616-3p | up | PTEN | induce | in vitro, human | MGC-803, MKN-28, AGS, SGC-7901 | Wu et al.176 |
| miR-506 | up | ETS1 | inhibit | in vitro, human | Kato-III, SGC-7901, BGC-823, HGC-27, MKN45, MGC-803, AGS | Li et al.190 |
Angiogenesis-related microRNAs in other GI cancers
The expression of miR-377 was low in esophageal squamous cell carcinoma (ESCC) cell lines and also in serum and tumor samples from ESCC patients. miR-377 is located in chromosomal region 14q32, which is sometimes obliterated in ESCC.196,197 The function of miR-377 in cancer is not yet completely clear. CD133 is a marker of tumor-initiating cells (TICs) or cancer stem cells,198 but the function of CD133 in ESCC is not completely clear,199, 200, 201, 202 and it is uncertain whether CD133 is a TIC marker in ESCC. Li et al.203 investigated the effects of miR-377, CD133, and VEGF on angiogenesis in ESCC. miR-377 expression in ESCC was generally low, while the tumor tissue and serum levels of miR-377 were associated with patient survival. Upregulation of miR-377 was inversely correlated with pathologic tumor stage, distant metastasis, resistance to radiotherapy and chemotherapy, and the amount of residual tumor. In the laboratory, high expression of miR-377 suppressed angiogenesis, metastasis, and proliferation in ESCC cell lines, while knockdown of miR-377 had the opposite effects. Furthermore, miR-377 targeted both VEGF and CD133 via binding to their 3′ UTRs. Systemic delivery of a formulated miR-377 mimetic inhibited ESCC tumor development in nude mice, suppressed angiogenesis, and inhibited lung metastasis without any toxicity.203
miR-143-3p plays a role in many cancers, including HCC, prostate cancer, lung cancer, and colorectal cancer.204, 205, 206, 207 Downregulation of miR-143-3p is correlated with poor clinical outcomes. The VEGF family is composed of VEGFA, VEGFB, VEGFC, VEGFD, VEGFE, and PLGF.208 PLGF has a key role in several cancers,209 although its activity is limited in normal physiological processes. PLGF stimulates Flt-1 and also synergizes with VEGF effects.209 Several small-molecule inhibitors of VEGFR2 and VEGF, including cediranib,210 sunitinib,211 and vandetanib,212 have been approved for cancer therapy. Jin et al.213 studied the effects of miR-143-3p, ITGA6, and PLGF on angiogenesis in gallbladder carcinoma. They found that miR-143-3p was an inhibitor of tumor angiogenesis and development. ELISA, antibody arrays, and a PLGF rescue analysis showed that PLGF had a key role in the anti-angiogenic effects of miR-143-3p. Dual-luciferase assays and miRNA target-prediction software were used to demonstrate that ITGA6 functioned as a miR-143-3p target. Western blotting and ELISAs showed that PLGF expression was reduced via the ITGA6/AKT/ PI3K pathway.213
Table 5 lists some angiogenesis-related miRNAs in other GI cancers (ESCC and gallbladder cancer).
Table 5.
Angiogenesis-related miRNAs in other GI cancers.
| microRNA | Cancer type | Expression in cancer | Target | Effect on angiogenesis (inhibit/induce) | Model (in vivo, in vitro, human) | Type of cell line | Reference |
|---|---|---|---|---|---|---|---|
| miR-377 | esophageal | down | VEGF CD133 | inhibit | in vivo, in vitro, human | KYSE30, KYSE70, KYSE150, KYSE270, KYSE410 | Li et al.203 |
| miR-143-3p | gallbladder | down | ITGA6 | inhibit | in vitro, in vivo, human | GBC-SD, SGC996, NOZ, OCUG-1, EHGB-1 | Jin et al.213 |
lncRNA biogenesis
lncRNAs are over 200 nt in length and are transcribed by RNA polymerase II (Pol II). More than 10,000 different lncRNAs have been estimated to exist in humans.214,215 All mammalian lncRNAs have similar functional, structural, and mechanistic properties. They usually can be spliced and have a poly-A tail.216 In addition, they can modulate target gene expression at the transcriptional or post-transcriptional levels and thereby affect many biological and cellular processes.217, 218, 219
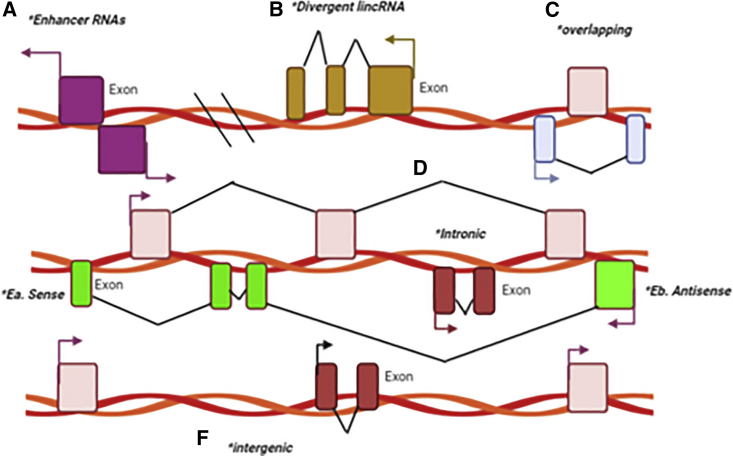
Spurlock et al.220 categorized lncRNAs according to the structural source (Figure 3). In overlapping lncRNAs, the protein-coding gene sequence is overlapped with the lncRNA intron.220,221 In divergent lncRNAs, the adjacent protein coding gene and the lncRNA are transcribed on opposite strands.222 In intronic lncRNAs, the entire sequence of the lncRNA is the same as the intron of a gene.223 In intergenic lncRNAs, the sequence of the lncRNA is distributed between two distinct genes.224 Intergenic lncRNAs can either be sense225 or antisense226 types, where the lncRNA sequence is located between the exons of a different transcript on the antisense/sense strand.227,228 Finally, enhancer RNAs can be transcribed in one sense (1D-eRNAs) or in two senses (2D-eRNAs) as genomic transcriptional enhancers, commonly situated near to protein-coding genes.229
Figure 3.
Classification of lncRNAs based on structural origin
(A) Enhancer RNAs are transcribed in one (1D-eRNAs) or two senses (2D-eRNAs) by genomic transcriptional enhancers, commonly situated near to protein-coding genes. (B) Divergent lncRNAs: the adjacent protein coding gene and the lncRNA are transcribed on opposite strands. (C) Overlapping lncRNAs: protein-coding genes are overlapped with the lncRNA intron. (D) Intronic lncRNAs: the entire sequence of the lncRNA is contained within the intron of a gene. Ea. sense or Eb. antisense types: the lncRNA is located between the exons of a different transcript on the antisense/sense strand. (F) Intergenic lncRNAs: the sequence of lncRNA is contained within two distinct genes as a single unit.
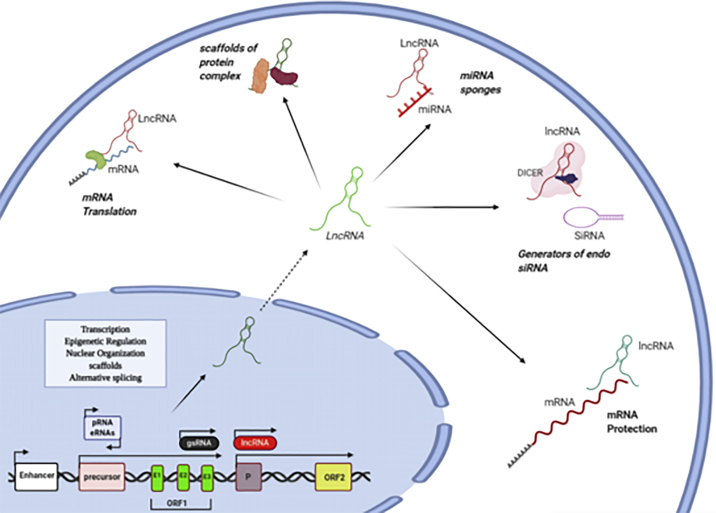
Numerous factors can regulate the function of lncRNAs. For example, RNA-binding proteins (RBPs) have been demonstrated as important regulators of lncRNAs. An important regulatory mechanism of lncRNAs is the post-transcriptional regulation by RBPs. Some cancer-associated lncRNAs have been found to be regulated by the interaction with RBPs, such as human antigen R (HuR), ARE/poly(U)-binding/degradation factor 1 (AUF1), insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1), or tristetraprolin (TTP).230 Some lncRNAs can function as scaffolds during formation of subcellular structures, such as nuclear bodies. These nuclear bodies are formed by phase separation of RNA-binding proteins with a prion-like domain, low complexity region, or intrinsic disordered region. The scaffold ncRNAs forming these nuclear bodies are also referred to as architectural RNAs (arcRNAs). They can bind to and assemble RNA-binding proteins and thereby induce liquid-liquid phase separation.231 Moreover, it has been found that the functions of lncRNAs depend on their subcellular localization.217 By using RNA fluorescence in situ hybridization in human cell lines, a broad range of subcellular localization patterns, either in the nucleus or the cytoplasm, have been demonstrated.232 Nonetheless, it is usual to list lncRNAs based on their similar mechanisms of action233 (Figure 4).
Figure 4.
lncRNA classification based on their function
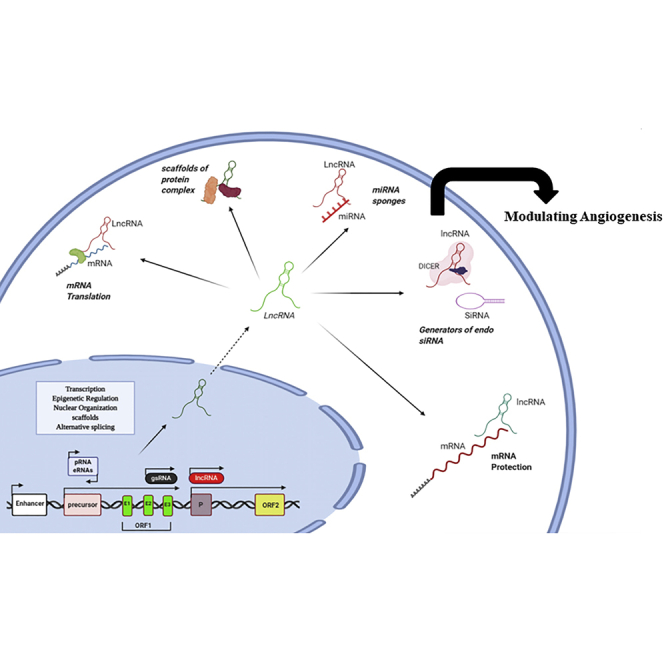
lncRNAs are involved in mRNA transcription, epigenetic modulation, nuclear organization, and altered splicing at the nuclear level. In the cytoplasm, lncRNAs can act as enhancers for mRNA translation, miRNA sponges, generators of endogenous siRNA, scaffolds for protein complexes, and protectors of mRNA.
Some lncRNAs play a role in the nuclear structure by regulating the structure of nuclear interchromatin granules, paraspeckles, and speckles.234 Some other roles of nuclear lncRNAs are the regulation of gene expression via epigenetic activities as well as chromatin-modification factors.235 In addition, some kinds of stable lncRNAs, as well as circular RNAs (circRNAs) and competing endogenous RNAs (ceRNAs), are located together within the cell, where they act as sponges or decoys to remove their target miRNAs and thereby alter gene expression.236 The ceRNA hypothesis suggests an intrinsic mechanism to regulate biological processes. However, whether the dynamic changes of ceRNAs can affect miRNA activities remains controversial.237,238 The ceRNA hypothesis postulates that RNAs that share miRNA response elements (MREs) in their 3′ UTRs can influence the expression of miRNAs and induce gene silencing. Several recent studies have demonstrated that lncRNAs can contain MREs and interact with other RNA transcripts such as ceRNAs. The complex crosstalk involving ceRNAs has been found in many different cancer types, including GC.237,238 A more updated concept that builds on this previous idea is the target-directed miRNA decay (TDMD), which involves the direct degradation of the miRNA rather than its transient binding to a complementary sequence. This mechanism is triggered by target RNAs with extensive complementarity between the miRNA and the target, especially at the 3′ end, triggering the dissociation of the miRNA from the AGO PAZ domain.239, 240, 241, 242, 243 Above all, functional interactions and disequilibrium of ceRNA networks (ceRNETs) may contribute to disease pathogenesis.
lncRNAs have a crucial role in transcription by helping the assembly of transcriptional repressors or activators to modulate transcription.244 lncRNAs can also regulate gene expression after transcription by modulating RNA-binding proteins, which affect translation and splicing, as well as altering mRNA translation or mRNA stability.245,246 Additionally, some lncRNAs can post-transcriptionally regulate protein expression and affect the fate of proteins by increasing ubiquitination.247
lncRNAs and angiogenesis in GI cancer
The lncRNA SNHG6 is situated on chromosome 8q13.1 and comprises 5 transcripts (SNHG6-201 to SNHG6-205). High expression of SNHG6 has been observed in several different cancers, such as HCC,248 CRC,249,250 lung adenocarcinoma,251 and breast cancer.252 Overexpression of SNHG6 was associated with enhanced tumor progression and poor survival in subjects with cholangiocarcinoma (CCA). SNHG6 has a role in cell apoptosis, invasion, proliferation, and migration in vitro, and enhanced tumor growth in vivo, but the role of SNHG6 in CCA remains uncertain. Wang et al.253 studied the effects of SNHG6 and E2F8 on angiogenesis in CCA. They found that the expression of SNHG6 was generally high in CCA. Moreover, ectopic expression of SNHG6 stimulated cell cycle progression, proliferation, angiogenesis, and migration in CCA, and the deletion of SNHG6 suppressed several cellular processes. Several articles have suggested that SNHG6 could compete with E2F8 to bind to miR-101-3p.253 The thrombospondin family consists of 5 members, which play a key role in several cellular processes.254 THBS1 (also known as TSP1) is an endogenous anti-angiogenic factor that inhibits tumor development by suppressing angiogenesis.255,256 The high expression of THBS1 on T cells also suppressed angiogenesis and inhibited tumor development.257 Wang et al.258 assessed the effects of BZRAP1-AS1 and THBS1 on angiogenesis in HCC. Screening of the lncRNA genes that were expressed in HCC suggested a candidate was BZRAP1-AS1. Microarray-based data analysis and qRT-PCR were employed to measure the expression of THBS1 and BZRAP1-AS1 in HCC. Bisulfite sequencing and methylation-specific PCR were used to measure the gene methylation level. Chromatin immunoprecipitation (ChIP) assays and ribosome-inactivating protein RNA pull-down were performed to assess the interactions between BZRAP1-AS1, DNMT3b, and THBS1. The in vitro role of DNMT3b, THBS1, and BZRAP1-AS1 in angiogenesis and in vivo tumorigenesis were assessed via gain- and loss-of-function tests. BZRAP1-AS1 was found to be overexpressed in HCC. Under-expression of BZRAP1-AS1 in HCC suppressed angiogenesis, proliferation, and migration of HUVECs. Knockdown of BZRAP1-AS1 suppressed angiogenesis and HCC tumor development in vivo, by upregulating THBS1.258
The lncRNA OR3A4 was recently discovered to be a regulator of GC and breast cancer.259,260 Guo et al.259 reported that OR3A4 could enhance invasion, migration, HUVEC tube formation, and angiogenesis in GC. Because the function of OR3A4 in HCC was unclear, Li et al.261 studied the effects of OR3A4 and the mTOR/AGGF1/Akt pathway on angiogenesis in HCC. OR3A4 was overexpressed in HCC cell lines and tissues. Loss-of-function assays confirmed OR3A4 as a promoter for HCC development and angiogenesis. They used Kaplan-Meier analysis and qRT-PCR to find a correlation between AGGF1 and OR3A4 expression in tumor samples and poor prognostic outcomes in HCC patients. Spearman’s correlation curve and western blotting demonstrated a positive correlation between OR3A4 and the AGGF1 level. Rescue assays showed that OR3A4 enhanced cancer development and angiogenesis in HCC by affecting AGGF1/Akt/mTOR.261
One member of the trypsin-like serine protease family is human kallikrein-related peptidase 4 (KLK4).262,263 The level of KLK4 expression was associated with the development and progression of prostate cancer.264 Cui et al.265 showed that KLK4 could control the Wnt/β-catenin pathway to enhance oral squamous cell carcinoma. Tang et al.266 studied the effects of LINC01314 and KLK4 on angiogenesis in GC. Data analysis and microarrays were used to screen for expression of several lncRNAs in GC, and they detected a binding relationship between KLK4 and LINC01314. The human GC cell line SGC-7901 was modified by overexpression or silencing of LINC01314 or KLK4 to examine how LINC01314 could affect cellular processes in GC. Wnt-1, KLK4 cyclin D1, β-catenin, E-cadherin, and N-cadherin levels were measured, and cell invasion and migration were assessed. Then, microvessel density, tumor weight, and VEGFR-3 and VEGF-C expression in tumors were evaluated. KLK4 was found to be a target gene of LINC01314. Silencing of KLK4 or overexpression of LINC01314 led to less invasion and migration of GC cells and correlated with lower expression of β-catenin, Wnt-1, N-cadherin, and cyclin D1, while E-cadherin was increased. Moreover, the MVD and tumor weight of the transplanted tumors were lower, and angiogenesis was inhibited, accompanied by downregulation of VEGFR-3 and VEGF-C.266
Table 6 lists some lncRNAs related to angiogenesis in GI cancers.
Table 6.
Angiogenesis-related lncRNAs in GI cancers
| Cancer | lncRNA | Expression in cancer | Target | Effect on angiogenesis (inhibit/induce) | Model (in vivo, in vitro, human) | Type of cell line | Reference |
|---|---|---|---|---|---|---|---|
| Colon cancer | HNF1A-AS1 | up | OTX1 | induce | in vivo, in vitro | HCT116, SW620 | Wu et al.267 |
| SUMO1P3 | up | VEGFA | induce | in vitro, in vivo, human | HT29, HCT116, SW480, SW620, LoVo | Zhang et al.268 | |
| Pancreatic cancer | JHDM1D-AS1 | up | HGF, FGF1 | induce | in vitro, in vivo | PANC-1 | Kondo et al.269 |
| Gastric cancer | LINC01410 | up | NF-κB | induce | in vitro, in vivo, human | HGC-27, BGC-23, AGS, MNK-45, SGC-7901 | Zhang et al.183 |
| LINC01314 | down | KLK4 | inhibit | in vitro, in vivo | SGC-7901 | Tang et al.266 | |
| PVT1 | up | VEGFA | induce | in vitro, in vivo, human | BGC-823, MNK-45, GES-1, SGC-7901, HGC-27, HUVEC, AGS, SUN-638 | Zhao et al.270 | |
| Hepatocellular carcinoma | LINC00488 | up | TLN1 | inhibit | in vitro, in vivo, human | Huh-7, Hep3B, HCCLM3, MHCC97 | Gao et al.271 |
| lncRNA-OR3A4 | up | AGGF1 | induce | in vitro, human | Huh7, SMMC-7721, HepG2, Hep3B | Li et al.261 | |
| BZRAP1-AS1 | up | AS1, BZRAP1 | inhibit | in vivo, in vitro, human | HuH-7, BEL-7405, SK-HEP-1, HCCLM3, LI7, BCLC-9 | Wang et al.258 | |
| UBE2CP3 | up | ERK1/2/HIF-1α/VEGFA | induce | in vitro, in vivo, human | HepG2, SMMC-7721 | Lin et al.272 | |
| lncRNA MVIH | up | PGK1 | induce | human | Yuan et al.273 | ||
| MALAT1 | up | VEGF-A | induce | Hou et al.274 | |||
| Cholangiocarcinoma | SNHG6 | up | E2F8 | induce | in vitro, in vivo, human | HCCC-9810, RBE | Wang et al.253 |
circRNA biogenesis
circRNAs are non-coding RNAs that can be produced by two possible mechanisms for closed-loop generation. These models were suggested by Jeck et al.:275 (1) circularization carried out by intron-pairing, and (2) lariat-driven circularization. Both of these have been broadly accepted to occur. The generation of intron-pairing-driven circularization results from the pairing between complementary bases within the sequence of various introns. This brings adjacent exons to close proximity, and then the spliceosome cuts away the neighboring exons and the introns combine to form the circRNA configuration. The lariat-driven circularization is based on a covalent binding between the donor and splice acceptor. It results in a circRNA that includes the exon lariat.275 Another subtype of circRNA is called intron circRNA, which has recently been discovered.276 The 7-nucleotide G-rich element and 11-nucleotide C-rich element within the parent gene of the intron circRNA are merged to create a circular structure, which is then spliced by the spliceosome.276 The spliceosome plays a key role in circRNA biogenesis, relying on trans-acting factors and cis-regulatory elements.277
In one study by Zhang et al.,278 four thiopurines were used to tag newly created RNA to demonstrate that low levels of circRNA could be a byproduct of imperfect pre-RNA splicing. Transcription mediated by RNA polymerase II (pol II) occurred simultaneously with the generation of circRNAs, showing that rapid extension of the strand may improve the reverse splicing of the complementary paired sequences. Moreover, the activity of pol II is rigorously regulated by cis-regulatory elements.278 It was suggested that circRNA biogenesis may be post-transcriptional in nature.279 In addition to pol II and pre-RNA, circRNA biogenesis is also controlled by a variety of enzymes, proteins, active elements, and intron sequences. Previous research indicated that RNA-binding proteins could also play a role in circRNA generation, as a distinct inhibitory mechanism noted in tumors during the EMT.280 The RNA helicase DExH-box helicase 9 (DHX9) is specific for the reverse repeat Alu element, which is required for the modulation of RNA post-transcriptional splicing.281 Alu elements are a group of functional sequences commonly observed in primates and are closely involved with circRNA biogenesis.281 The levels of DHX9 expression influenced the generation of splicing products, and its deletion was found to promote circRNA biogenesis.281 Furthermore, exon circularization is a dynamic process that is controlled by adjacent introns. Indeed, flanking and individual introns can regulate circRNA biogenesis via base-pairing.282 Alterations to the mRNA structure have been found to modulate alternative splicing, transcription, advanced structure, translation, and stability. Moreover, Tang et al.283 discovered that N6-methyladenosine (m6A) could improve circRNA generation in open reading frames within mouse male germ cells. Moreover, the tissue levels of other circRNAs could also unfavorably affect circRNA biogenesis284.
circRNAs and angiogenesis in GI cancers
Hox genes can regulate cell differentiation and development, receptor signaling, apoptosis, and angiogenesis in cancer.285 The function of HOXC6 in PDAC is not clear, but high expression of HOXC6 in prostate cancer was correlated with disease development.286 Shi et al.287 assessed the effects of hsa_circ_001653 and the human homeobox on angiogenesis in PDAC. Hsa-circ-001653 expression was evaluated in 83 paired tumor and normal tissue samples. Assays for cell cycle, cell viability, apoptosis, and invasion were used to examine phenotypic changes in PDAC cells. HUVEC tube-like formation was evaluated in the presence of PDAC cells. In addition, crosstalk between miR-377 and hsa_circ_001653 HOXC6 was investigated using Ago2 immunoprecipitation, northern blot analysis, and a dual-luciferase reporter assay. Human PDAC cells were inoculated into nude mice for analysis of in vivo tumor growth. Hsa_circ_001653 expression was high in PDAC samples. Hsa_circ_001653 knock-down in PDAC cells using RNA interference suppressed cell-cycle progression, cell viability, invasion, and in vitro angiogenesis and showed a pro-apoptotic effect. When human PDAC cells were inoculated into nude mice, suppression of hsa_circ_001653 had a therapeutic effect on tumor growth.287
HN1 (hematological and neurological expressed 1) protein was first identified in mouse embryonic tissues288 and has a role in several diseases.289 HN1 was correlated with a poor prognosis in HCC patients.290 Pu et al.291 investigated the hsa_circ_0000092 and HN1 effects on angiogenesis in HCC. The levels of miR-338-3p, HN1, and hsa_circ_0000092 expression in HCC were examined. RNA pull-down, dual-luciferase reporter, and northern blot assays were used to detect the relationship between miR-338-3p, hsa_circ_0000092, and HN1. A group of mimetics, suppressors, or small interfering RNA (siRNA) plasmids were delivered into HCC cells to confirm the ability of HN1, miR-338-3p, and hsa_circ_0000092 to regulate in vitro angiogenesis, cell migration, proliferation, and invasion. The function of hsa_circ_0000092 in HCC tumor development was investigated by silencing hsa_circ_0000092 with siRNA. The expression levels of hsa_circ_0000092 and HN1 in HCC were high, while the expression of miR-338-3p was downregulated. Hsa_circ_0000092 could bind to miR-338-3p in order to increase HN1 expression. Both the downregulation of hsa_circ_0000092 or the upregulation of miR-338-3p were observed to inhibit angiogenesis, cell invasion, proliferation, and migration in HCC cells in vitro, by downregulating HN1. Knock-down of hsa_circ_0000092 inhibited HCC tumor development in vivo.291
Table 7 lists some circRNAs reported to be related to angiogenesis in GI cancers.
Table 7.
Angiogenesis-related circRNAs in GI cancers
| Cancer | CircRNA | Expression in gastric cancer | Target | Effect on angiogenesis (inhibit/induce) | Model (in vivo, in vitro, human) | Type of cell line | Reference |
|---|---|---|---|---|---|---|---|
| Pancreatic ductal adenocarcinoma | hsa_circ_001653 | up | HOXC6 | inhibit | in vitro, in vivo, human | Capan-2 (ZY-H431), SW1990(ZY-H338), PANC1 (ZY-H147), BxPC3 (ZY-H145) | Shi et al.287 |
| Hepatocellular carcinoma | hsa_circ_0000092 | up | HN1 | induce | in vitro, in vivo, human | Hep3B, LM3, MHCC97L, SK-hep1, HepG2 | Pu et al.291 |
| ircRNA-100338 | up | MMP2, MMP9 | induce | in vitro, in vivo, human | Hep3B, MHCC97L, MHCC97H, HLE, Huh7, BEL7402, SMCC7721 HCCLM3 and HCCLM6 | Huang et al.292 | |
| CircGFRA1 | up | miR-149 | induce | in vitro, human | SK-HEP-1, Huh6, Huh7, HCCLM3 | Yu et al.293 |
Future for therapeutic ncRNAs in GI cancer
ncRNAs are involved in the regulation of almost all physiological processes, such as cell development, differentiation, proliferation, and apoptosis. In this context, ncRNAs could have great potential as new biomarkers for diagnosis and prognosis and in therapeutic approaches for cancers, including GI cancer. Recently, new methods and tools have been developed to detect and quantify cancer-regulated ncRNAs. For example, the use of multiplexed qRT-PCR, microarrays, or next-generation sequencing (NGS)-based genome-wide approaches have helped to provide a complete picture of the expression level of many ncRNAs.294 Moreover, bioinformatic approaches and various databases have enabled the discovery of thousands of novel ncRNAs in cancers, including GI cancer. These databases include CRlncRNA, Lnc2Cancer, and LncRNADisease.295, 296, 297
The ncRNAs could be an attractive new type of therapeutics, especially against undruggable targets for the treatment of GI cancer.298 Several oncogenic ncRNAs can promote adverse drug reaction resistance in GC. These include miRNAs, such as miR-27a, miR-19a/b, and miR-135a-5p; lncRNAs, such as HOTAIR, CASC9, MRUL, UCA1, D63785, NEAT1, and HULC; and circRNAs, such as circAKT3 and circFN1.299 Therefore, ncRNAs could provide a new approach for better clinical decision making. For example, miRNAs can mediate potent and specific gene silencing, making them attractive therapeutic tools. To date, the greatest efforts in this setting have been to explore the potential application of ncRNA-based therapeutics for cancer.300
Despite the potential of ncRNAs in cancer therapy, many challenges still remain, including rapid degradation and clearance, poor cellular uptake, off-target effects, and immunogenicity. Rational design, chemical modifications, and improved delivery carriers offer significant opportunities to overcome these challenges.301 Moreover, the accurate mechanisms of ncRNAs in particular processes, such as angiogenesis, have not been completely characterized. Despite these limitations, future research could improve ncRNA properties to overcome these challenges. For example, miRNA delivery is being addressed in many different ways, such as with chemical modifications and nanotechnology-based delivery vehicles.302
The expression patterns of ncRNAs and mRNAs using in silico approaches are always used before wet-lab validation experiments. However, it is worth noting that the gene expression profiles in the human population (particularly in processes such as angiogenesis) are extremely heterogenous, due to different genetic backgrounds, environmental exposure, dietary considerations, and overall health status of the tissue donors. A weak association or a non-significant association does not necessarily mean that the expression of a particular ncRNA and an mRNA are not related.303
In addition to in silico prediction and correlation analysis of the relationship between the expression of a miRNA and the expression of its target mRNA, in vitro experiments employing the transfection of ectopic miRNA mimics or inhibitors into cells is a commonly used approach to validate the function of a particular miRNA. However, in some circumstances, the ectopic miRNA mimics or inhibitors in cells are over-expressed to a level that is often much higher than the endogenous level of the miRNA, suggesting the possibility of an exaggerated effect in the study. On the other hand, the concentration of a miRNA may not reach the level that is necessary within an intracellular compartment to allow its function to suppress target genes (in reality, subcellular concentrations of specific miRNAs are very difficult to measure), which could lead to the effect of the miRNA in vitro being underestimated or completely ignored. Furthermore, almost all in vitro studies have demonstrated the direct regulation of drug-metabolizing enzymes and transporters by miRNAs; however, potential indirect regulation cannot be ruled out, since off-target effects of miRNAs have been somewhat overlooked. These artificial results from in vitro studies may partially explain discrepancies between in vitro and in vivo results.303
Conclusions
Angiogenesis is required for tumors to grow to more than 2 mm in diameter, since the simple diffusion of nutrients and oxygen can no longer supply the rapid proliferation of cancer cells. Angiogenesis is a delicate balance between inhibitory and stimulatory factors. Inhibiting angiogenesis could be a new approach for GI cancer treatment. It is known that in primary tumors, angiogenesis is a continuous and highly intricate series of molecular events that eventually leads to the exponential growth of the tumor. Recently, substantial evidence has accumulated about the function of the miRNAs in both angiogenesis and metastasis of cancers. Studies have demonstrated that deregulation of many types of ncRNAs can affect angiogenesis and metastasis in GI cancers. Furthermore, the rapid development of different types of mimetics or antagonists of specific ncRNAs, as well as more effective delivery technologies, has raised the possibility to employ ncRNAs as targets to treat metastatic GI cancers. Direct links between the role of ncRNAs in angiogenesis and GI cancer metastasis remain to be fully determined. Therefore, ncRNA-based therapy is still not available in clinical settings. Even so, with further advances in technology, ncRNA-based therapeutic approaches will likely be applied to combat angiogenesis in GI cancer in the coming years.
Acknowledgments
Author contributions
H.M. and M.R.H. contributed to conception, design, statistical analysis, and drafting of the manuscript. Z.S.R., K.A., S.R., M.M.T., H.K., and M.K.S. contributed to data collection and manuscript drafting. All authors approved the final version for submission.
Declaration of interests
M.R.H. declares the following potential conflicts of interest. Scientific Advisory Boards: Transdermal Cap, Inc., Cleveland, OH, USA; BeWell Global, Inc., Wan Chai, Hong Kong; Hologenix, Inc., Santa Monica, CA, USA; LumiThera, Inc., Poulsbo, WA, USA; Vielight, Toronto, ON, Canada; Bright Photomedicine, Sao Paulo, Brazil; Quantum Dynamics, LLC, Cambridge, MA, USA; Global Photon, Inc., Bee Cave, TX, USA; Medical Coherence, Boston, MA, USA; NeuroThera, Newark, DE, USA; JOOVV, Inc., Minneapolis-St. Paul, MN, USA; AIRx Medical, Pleasanton, CA, USA; FIR Industries, Inc., Ramsey, NJ, USA; UVLRx Therapeutics, Oldsmar, FL, USA; Ultralux UV, Inc., Lansing, MI, USA; Illumiheal & Petthera, Shoreline, WA, USA; MB Lasertherapy, Houston, TX, USA; ARRC LED, San Clemente, CA, USA; Varuna Biomedical Corp., Incline Village, NV, USA; Niraxx Light Therapeutics, Inc., Boston, MA, USA. Consulting; Lexington Int., Boca Raton, FL, USA; USHIO Corp., Japan; Merck KGaA, Darmstadt, Germany; Philips Electronics Nederland B.V., Eindhoven, Netherlands; Johnson & Johnson, Inc., Philadelphia, PA, USA; Sanofi-Aventis Deutschland GmbH, Frankfurt am Main, Germany. Stockholdings: Global Photon Inc., Bee Cave, TX, USA; Mitonix, Newark, DE, USA. All other authors declare no competing interests.
Contributor Information
Mohammad Karim Shahrzad, Email: mk_shahrzad@yahoo.com.
Michael R. Hamblin, Email: hamblin.lab@gmail.com.
Hamed Mirzaei, Email: mirzaei-h@kaums.ac.ir.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Saluja A., Maitra A. Pancreatitis and pancreatic cancer. Gastroenterology. 2019;156:1937–1940. doi: 10.1053/j.gastro.2019.03.050. [DOI] [PubMed] [Google Scholar]
- 3.Lasota J., Miettinen M. Clinical significance of oncogenic KIT and PDGFRA mutations in gastrointestinal stromal tumours. Histopathology. 2008;53:245–266. doi: 10.1111/j.1365-2559.2008.02977.x. [DOI] [PubMed] [Google Scholar]
- 4.Li X., Nadauld L., Ootani A., Corney D.C., Pai R.K., Gevaert O., Cantrell M.A., Rack P.G., Neal J.T., Chan C.W.M. Oncogenic transformation of diverse gastrointestinal tissues in primary organoid culture. Nat. Med. 2014;20:769–777. doi: 10.1038/nm.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bang Y.J., Van Cutsem E., Feyereislova A., Chung H.C., Shen L., Sawaki A., Lordick F., Ohtsu A., Omuro Y., Satoh T. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 6.Marano L., Chiari R., Fabozzi A., De Vita F., Boccardi V., Roviello G., Petrioli R., Marrelli D., Roviello F., Patriti A. c-Met targeting in advanced gastric cancer: An open challenge. Cancer Lett. 2015;365:30–36. doi: 10.1016/j.canlet.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham D., Humblet Y., Siena S., Khayat D., Bleiberg H., Santoro A., Bets D., Mueser M., Harstrick A., Verslype C. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N. Engl. J. Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 8.Russo M., Crisafulli G., Sogari A., Reilly N.M., Arena S., Lamba S., Bartolini A., Amodio V., Magrì A., Novara L. Adaptive mutability of colorectal cancers in response to targeted therapies. Science. 2019;366:1473–1480. doi: 10.1126/science.aav4474. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi Y., Tucker S.L., Kitadai Y., Koura A.N., Bucana C.D., Cleary K.R., Ellis L.M. Vessel counts and expression of vascular endothelial growth factor as prognostic factors in node-negative colon cancer. Arch. Surg. 1997;132:541–546. doi: 10.1001/archsurg.1997.01430290087018. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi Y., Kitadai Y., Bucana C.D., Cleary K.R., Ellis L.M. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res. 1995;55:3964–3968. [PubMed] [Google Scholar]
- 11.Kang S.M., Maeda K., Onoda N., Chung Y.S., Nakata B., Nishiguchi Y., Sowa M. Combined analysis of p53 and vascular endothelial growth factor expression in colorectal carcinoma for determination of tumor vascularity and liver metastasis. Int. J. Cancer. 1997;74:502–507. doi: 10.1002/(sici)1097-0215(19971021)74:5<502::aid-ijc4>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 12.Ishigami S.I., Arii S., Furutani M., Niwano M., Harada T., Mizumoto M., Mori A., Onodera H., Imamura M. Predictive value of vascular endothelial growth factor (VEGF) in metastasis and prognosis of human colorectal cancer. Br. J. Cancer. 1998;78:1379–1384. doi: 10.1038/bjc.1998.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrara N., Hillan K.J., Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem. Biophys. Res. Commun. 2005;333:328–335. doi: 10.1016/j.bbrc.2005.05.132. [DOI] [PubMed] [Google Scholar]
- 14.Konno S., Takebayashi Y., Aiba M., Akiyama S., Ogawa K. Clinicopathological and prognostic significance of thymidine phosphorylase and proliferating cell nuclear antigen in gastric carcinoma. Cancer Lett. 2001;166:103–111. doi: 10.1016/s0304-3835(01)00432-3. [DOI] [PubMed] [Google Scholar]
- 15.Kimura H., Konishi K., Nukui T., Kaji M., Maeda K., Yabushita K., Tsuji M., Miwa A. Prognostic significance of expression of thymidine phosphorylase and vascular endothelial growth factor in human gastric carcinoma. J. Surg. Oncol. 2001;76:31–36. doi: 10.1002/1096-9098(200101)76:1<31::aid-jso1006>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi Y., Bucana C.D., Akagi Y., Liu W., Cleary K.R., Mai M., Ellis L.M. Significance of platelet-derived endothelial cell growth factor in the angiogenesis of human gastric cancer. Clin. Cancer Res. 1998;4:429–434. [PubMed] [Google Scholar]
- 17.Maeda K., Chung Y.S., Ogawa Y., Takatsuka S., Kang S.M., Ogawa M., Sawada T., Sowa M. Prognostic value of vascular endothelial growth factor expression in gastric carcinoma. Cancer. 1996;77:858–863. doi: 10.1002/(sici)1097-0142(19960301)77:5<858::aid-cncr8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 18.Karayiannakis A.J., Bolanaki H., Syrigos K.N., Asimakopoulos B., Polychronidis A., Anagnostoulis S., Simopoulos C. Serum vascular endothelial growth factor levels in pancreatic cancer patients correlate with advanced and metastatic disease and poor prognosis. Cancer Lett. 2003;194:119–124. doi: 10.1016/s0304-3835(03)00047-8. [DOI] [PubMed] [Google Scholar]
- 19.Liu C.D., Tilch L., Kwan D., McFadden D.W. Vascular endothelial growth factor is increased in ascites from metastatic pancreatic cancer. J. Surg. Res. 2002;102:31–34. doi: 10.1006/jsre.2001.6307. [DOI] [PubMed] [Google Scholar]
- 20.Niedergethmann M., Hildenbrand R., Wostbrock B., Hartel M., Sturm J.W., Richter A., Post S. High expression of vascular endothelial growth factor predicts early recurrence and poor prognosis after curative resection for ductal adenocarcinoma of the pancreas. Pancreas. 2002;25:122–129. doi: 10.1097/00006676-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Ellis L.M., Takahashi Y., Fenoglio C.J., Cleary K.R., Bucana C.D., Evans D.B. Vessel counts and vascular endothelial growth factor expression in pancreatic adenocarcinoma. Eur. J. Cancer. 1998;34:337–340. doi: 10.1016/s0959-8049(97)10068-5. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda N., Adachi M., Taki T., Huang C., Hashida H., Takabayashi A., Sho M., Nakajima Y., Kanehiro H., Hisanaga M. Prognostic significance of angiogenesis in human pancreatic cancer. Br. J. Cancer. 1999;79:1553–1563. doi: 10.1038/sj.bjc.6690248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Auguste P., Javerzat S., Bikfalvi A. Regulation of vascular development by fibroblast growth factors. Cell Tissue Res. 2003;314:157–166. doi: 10.1007/s00441-003-0750-0. [DOI] [PubMed] [Google Scholar]
- 24.Montesano R., Vassalli J.-D., Baird A., Guillemin R., Orci L. Basic fibroblast growth factor induces angiogenesis in vitro. Proc. Natl. Acad. Sci. USA. 1986;83:7297–7301. doi: 10.1073/pnas.83.19.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson D.E., Lu J., Chen H., Werner S., Williams L.T. The human fibroblast growth factor receptor genes: a common structural arrangement underlies the mechanisms for generating receptor forms that differ in their third immunoglobulin domain. Mol. Cell. Biol. 1991;11:4627–4634. doi: 10.1128/mcb.11.9.4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura T., Mochizuki Y., Kanetake H., Kanda S. Signals via FGF receptor 2 regulate migration of endothelial cells. Biochem. Biophys. Res. Commun. 2001;289:801–806. doi: 10.1006/bbrc.2001.6046. [DOI] [PubMed] [Google Scholar]
- 27.Javerzat S., Auguste P., Bikfalvi A. The role of fibroblast growth factors in vascular development. Trends Mol. Med. 2002;8:483–489. doi: 10.1016/s1471-4914(02)02394-8. [DOI] [PubMed] [Google Scholar]
- 28.Arbeit J.M., Olson D.C., Hanahan D. Upregulation of fibroblast growth factors and their receptors during multi-stage epidermal carcinogenesis in K14-HPV16 transgenic mice. Oncogene. 1996;13:1847–1857. [PubMed] [Google Scholar]
- 29.Kwan C.-P., Venkataraman G., Shriver Z., Raman R., Liu D., Qi Y., Varticovski L., Sasisekharan R. Probing fibroblast growth factor dimerization and role of heparin-like glycosaminoglycans in modulating dimerization and signaling. J. Biol. Chem. 2001;276:23421–23429. doi: 10.1074/jbc.M010786200. [DOI] [PubMed] [Google Scholar]
- 30.Schlessinger J., Plotnikov A.N., Ibrahimi O.A., Eliseenkova A.V., Yeh B.K., Yayon A., Linhardt R.J., Mohammadi M. Crystal structure of a ternary FGF-FGFR-heparin complex reveals a dual role for heparin in FGFR binding and dimerization. Mol. Cell. 2000;6:743–750. doi: 10.1016/s1097-2765(00)00073-3. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen M., Watanabe H., Budson A.E., Richie J.P., Hayes D.F., Folkman J. Elevated levels of an angiogenic peptide, basic fibroblast growth factor, in the urine of patients with a wide spectrum of cancers. J. Natl. Cancer Inst. 1994;86:356–361. doi: 10.1093/jnci/86.5.356. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen M., Watanabe H., Budson A.E., Richie J.P., Folkman J. Elevated levels of the angiogenic peptide basic fibroblast growth factor in urine of bladder cancer patients. J. Natl. Cancer Inst. 1993;85:241–242. doi: 10.1093/jnci/85.3.241. [DOI] [PubMed] [Google Scholar]
- 33.Landriscina M., Cassano A., Ratto C., Longo R., Ippoliti M., Palazzotti B., Crucitti F., Barone C. Quantitative analysis of basic fibroblast growth factor and vascular endothelial growth factor in human colorectal cancer. Br. J. Cancer. 1998;78:765–770. doi: 10.1038/bjc.1998.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galzie Z., Fernig D.G., Smith J.A., Poston G.J., Kinsella A.R. Invasion of human colorectal carcinoma cells is promoted by endogenous basic fibroblast growth factor. Int. J. Cancer. 1997;71:390–395. doi: 10.1002/(sici)1097-0215(19970502)71:3<390::aid-ijc15>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 35.George M.L., Tutton M.G., Abulafi A.M., Eccles S.A., Swift R.I. Plasma basic fibroblast growth factor levels in colorectal cancer: a clinically useful assay? Clin. Exp. Metastasis. 2002;19:735–738. doi: 10.1023/a:1021322201816. [DOI] [PubMed] [Google Scholar]
- 36.Grotowski M., Piechota W. Receptors of selected cytokines and angiokine bFGF in patients with colorectal cancer (a preliminary study) Pol. Merkur. Lekarski. 2001;11:398–401. [PubMed] [Google Scholar]
- 37.Dirix L.Y., Vermeulen P.B., Pawinski A., Prové A., Benoy I., De Pooter C., Martin M., Van Oosterom A.T. Elevated levels of the angiogenic cytokines basic fibroblast growth factor and vascular endothelial growth factor in sera of cancer patients. Br. J. Cancer. 1997;76:238–243. doi: 10.1038/bjc.1997.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dirix L.Y., Vermeulen P.B., Hubens G., Benoy I., Martin M., De Pooter C., Van Oosterom A.T. Serum basic fibroblast growth factor and vascular endothelial growth factor and tumour growth kinetics in advanced colorectal cancer. Ann. Oncol. 1996;7:843–848. doi: 10.1093/oxfordjournals.annonc.a010764. [DOI] [PubMed] [Google Scholar]
- 39.Tanimoto H., Yoshida K., Yokozaki H., Yasui W., Nakayama H., Ito H., Ohama K., Tahara E. Expression of basic fibroblast growth factor in human gastric carcinomas. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1991;61:263–267. doi: 10.1007/BF02890427. [DOI] [PubMed] [Google Scholar]
- 40.Noda M., Hattori T., Kimura T., Naitoh H., Kodama T., Kashima K., Pignatelli M. Expression of fibroblast growth factor 2 mRNA in early and advanced gastric cancer. Acta Oncol. 1997;36:695–700. doi: 10.3109/02841869709001339. [DOI] [PubMed] [Google Scholar]
- 41.Ueki T., Koji T., Tamiya S., Nakane P.K., Tsuneyoshi M. Expression of basic fibroblast growth factor and fibroblast growth factor receptor in advanced gastric carcinoma. J. Pathol. 1995;177:353–361. doi: 10.1002/path.1711770405. [DOI] [PubMed] [Google Scholar]
- 42.Yoshikawa T., Tsuburaya A., Kobayashi O., Sairenji M., Motohashi H., Yanoma S., Noguchi Y. Plasma concentrations of VEGF and bFGF in patients with gastric carcinoma. Cancer Lett. 2000;153:7–12. doi: 10.1016/s0304-3835(99)00426-7. [DOI] [PubMed] [Google Scholar]
- 43.Anzai H., Kitadai Y., Bucana C.D., Sanchez R., Omoto R., Fidler I.J. Expression of metastasis-related genes in surgical specimens of human gastric cancer can predict disease recurrence. Eur. J. Cancer. 1998;34:558–565. doi: 10.1016/s0959-8049(97)10075-2. [DOI] [PubMed] [Google Scholar]
- 44.Estival A., Durand S., Clerc P., Louvel D., Vaysse N., Valdiguié P., Clemente F. Pancreatic cancer cell regulation by lipids and by basic fibroblast growth factor expression. Cancer Detect. Prev. 1997;21:546–552. [PubMed] [Google Scholar]
- 45.Ghaneh P., Kawesha A., Evans J.D., Neoptolemos J.P. Molecular prognostic markers in pancreatic cancer. J. Hepatobiliary Pancreat. Surg. 2002;9:1–11. doi: 10.1007/s005340200000. [DOI] [PubMed] [Google Scholar]
- 46.Fujioka S., Yoshida K., Yanagisawa S., Kawakami M., Aoki T., Yamazaki Y. Angiogenesis in pancreatic carcinoma: thymidine phosphorylase expression in stromal cells and intratumoral microvessel density as independent predictors of overall and relapse-free survival. Cancer. 2001;92:1788–1797. doi: 10.1002/1097-0142(20011001)92:7<1788::aid-cncr1695>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 47.Ohta T., Yamamoto M., Numata M., Iseki S., Tsukioka Y., Miyashita T., Kayahara M., Nagakawa T., Miyazaki I., Nishikawa K., Yoshitake Y. Expression of basic fibroblast growth factor and its receptor in human pancreatic carcinomas. Br. J. Cancer. 1995;72:824–831. doi: 10.1038/bjc.1995.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jin K.-T., Yao J.-Y., Fang X.-L., Di H., Ma Y.-Y. Roles of lncRNAs in cancer: Focusing on angiogenesis. Life Sci. 2020;252:117647. doi: 10.1016/j.lfs.2020.117647. [DOI] [PubMed] [Google Scholar]
- 49.Cech T.R., Steitz J.A. The noncoding RNA revolution-trashing old rules to forge new ones. Cell. 2014;157:77–94. doi: 10.1016/j.cell.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 50.Peng J.-F., Zhuang Y.-Y., Huang F.-T., Zhang S.-N. Noncoding RNAs and pancreatic cancer. World J. Gastroenterol. 2016;22:801–814. doi: 10.3748/wjg.v22.i2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vorvis C., Hatziapostolou M., Mahurkar-Joshi S., Koutsioumpa M., Williams J., Donahue T.R., Poultsides G.A., Eibl G., Iliopoulos D. Transcriptomic and CRISPR/Cas9 technologies reveal FOXA2 as a tumor suppressor gene in pancreatic cancer. Am. J. Physiol. Gastrointest. Liver Physiol. 2016;310:G1124–G1137. doi: 10.1152/ajpgi.00035.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chandra Gupta S., Nandan Tripathi Y. Potential of long non-coding RNAs in cancer patients: From biomarkers to therapeutic targets. Int. J. Cancer. 2017;140:1955–1967. doi: 10.1002/ijc.30546. [DOI] [PubMed] [Google Scholar]
- 53.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 54.Rossbach M. Small non-coding RNAs as novel therapeutics. Curr. Mol. Med. 2010;10:361–368. doi: 10.2174/156652410791317048. [DOI] [PubMed] [Google Scholar]
- 55.Nelson K.M., Weiss G.J. MicroRNAs and cancer: past, present, and potential future. Mol. Cancer Ther. 2008;7:3655–3660. doi: 10.1158/1535-7163.MCT-08-0586. [DOI] [PubMed] [Google Scholar]
- 56.Macfarlane L.-A., Murphy P.R. MicroRNA: biogenesis, function and role in cancer. Curr. Genomics. 2010;11:537–561. doi: 10.2174/138920210793175895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith H.C. The role of microRNAs in gastric cancer. Am. J. Dig. Dis. 2016;3:29–37. [Google Scholar]
- 58.Selbach M., Schwanhausser B., Thierfelder N., Fang Z., Khanin R., Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455:58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- 59.Baek D., Villén J., Shin C., Camargo F.D., Gygi S.P., Bartel D.P. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suzuki H.I., Yamagata K., Sugimoto K., Iwamoto T., Kato S., Miyazono K. Modulation of microRNA processing by p53. Nature. 2009;460:529–533. doi: 10.1038/nature08199. [DOI] [PubMed] [Google Scholar]
- 61.Iorio M.V., Ferracin M., Liu C.-G., Veronese A., Spizzo R., Sabbioni S., Magri E., Pedriali M., Fabbri M., Campiglio M. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 62.Ichimi T., Enokida H., Okuno Y., Kunimoto R., Chiyomaru T., Kawamoto K., Kawahara K., Toki K., Kawakami K., Nishiyama K. Identification of novel microRNA targets based on microRNA signatures in bladder cancer. Int. J. Cancer. 2009;125:345–352. doi: 10.1002/ijc.24390. [DOI] [PubMed] [Google Scholar]
- 63.Xu X., Wu X., Jiang Q., Sun Y., Liu H., Chen R., Wu S. Downregulation of microRNA-1 and microRNA-145 contributes synergistically to the development of colon cancer. Int. J. Mol. Med. 2015;36:1630–1638. doi: 10.3892/ijmm.2015.2364. [DOI] [PubMed] [Google Scholar]
- 64.Cordes K.R., Sheehy N.T., White M.P., Berry E.C., Morton S.U., Muth A.N., Lee T.H., Miano J.M., Ivey K.N., Srivastava D. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu Q., Liu L.-Z., Qian X., Chen Q., Jiang Y., Li D., Lai L., Jiang B.H. MiR-145 directly targets p70S6K1 in cancer cells to inhibit tumor growth and angiogenesis. Nucleic Acids Res. 2012;40:761–774. doi: 10.1093/nar/gkr730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee J.-J., Drakaki A., Iliopoulos D., Struhl K. MiR-27b targets PPARγ to inhibit growth, tumor progression and the inflammatory response in neuroblastoma cells. Oncogene. 2012;31:3818–3825. doi: 10.1038/onc.2011.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Urbich C., Kaluza D., Frömel T., Knau A., Bennewitz K., Boon R.A., Bonauer A., Doebele C., Boeckel J.N., Hergenreider E. MicroRNA-27a/b controls endothelial cell repulsion and angiogenesis by targeting semaphorin 6A. Blood. 2012;119:1607–1616. doi: 10.1182/blood-2011-08-373886. [DOI] [PubMed] [Google Scholar]
- 68.Biyashev D., Veliceasa D., Topczewski J., Topczewska J.M., Mizgirev I., Vinokour E., Reddi A.L., Licht J.D., Revskoy S.Y., Volpert O.V. miR-27b controls venous specification and tip cell fate. Blood. 2012;119:2679–2687. doi: 10.1182/blood-2011-07-370635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ye J., Wu X., Wu D., Wu P., Ni C., Zhang Z., Chen Z., Qiu F., Xu J., Huang J. miRNA-27b targets vascular endothelial growth factor C to inhibit tumor progression and angiogenesis in colorectal cancer. PLoS ONE. 2013;8:e60687. doi: 10.1371/journal.pone.0060687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xiao X., Tang C., Xiao S., Fu C., Yu P. Enhancement of proliferation and invasion by MicroRNA-590-5p via targeting PBRM1 in clear cell renal carcinoma cells. Oncol. Res. 2013;20:537–544. doi: 10.3727/096504013X13775486749335. [DOI] [PubMed] [Google Scholar]
- 71.Chu Y., Ouyang Y., Wang F., Zheng A., Bai L., Han L., Chen Y., Wang H. MicroRNA-590 promotes cervical cancer cell growth and invasion by targeting CHL1. J. Cell. Biochem. 2014;115:847–853. doi: 10.1002/jcb.24726. [DOI] [PubMed] [Google Scholar]
- 72.Wandrey F., Montellese C., Koos K., Badertscher L., Bammert L., Cook A.G., Zemp I., Horvath P., Kutay U. The NF45/NF90 heterodimer contributes to the biogenesis of 60S ribosomal subunits and influences nucleolar morphology. Mol. Cell. Biol. 2015;35:3491–3503. doi: 10.1128/MCB.00306-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shim J., Lim H., R Yates J., Karin M. Nuclear export of NF90 is required for interleukin-2 mRNA stabilization. Mol. Cell. 2002;10:1331–1344. doi: 10.1016/s1097-2765(02)00730-x. [DOI] [PubMed] [Google Scholar]
- 74.Patiño C., Haenni A.-L., Urcuqui-Inchima S. NF90 isoforms, a new family of cellular proteins involved in viral replication? Biochimie. 2015;108:20–24. doi: 10.1016/j.biochi.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 75.Shamanna R.A., Hoque M., Pe’ery T., Mathews M.B. Induction of p53, p21 and apoptosis by silencing the NF90/NF45 complex in human papilloma virus-transformed cervical carcinoma cells. Oncogene. 2013;32:5176–5185. doi: 10.1038/onc.2012.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou Q., Zhu Y., Wei X., Zhou J., Chang L., Sui H., Han Y., Piao D., Sha R., Bai Y. MiR-590-5p inhibits colorectal cancer angiogenesis and metastasis by regulating nuclear factor 90/vascular endothelial growth factor A axis. Cell Death Dis. 2016;7:e2413. doi: 10.1038/cddis.2016.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yamamoto K., Ito S., Hanafusa H., Shimizu K., Ouchida M. Uncovering direct targets of miR-19a involved in lung cancer progression. PLoS ONE. 2015;10:e0137887. doi: 10.1371/journal.pone.0137887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lu W.-D., Zuo Y., Xu Z., Zhang M. MiR-19a promotes epithelial-mesenchymal transition through PI3K/AKT pathway in gastric cancer. World J. Gastroenterol. 2015;21:4564–4573. doi: 10.3748/wjg.v21.i15.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen M., Lin M., Wang X. Overexpression of miR-19a inhibits colorectal cancer angiogenesis by suppressing KRAS expression. Oncol. Rep. 2018;39:619–626. doi: 10.3892/or.2017.6110. [DOI] [PubMed] [Google Scholar]
- 80.Cai C., Rodepeter F.R., Rossmann A., Teymoortash A., Lee J.-S., Quint K., DI Fazio P., Ocker M., Werner J.A., Mandic R. SIVmac239-Nef down-regulates cell surface expression of CXCR4 in tumor cells and inhibits proliferation, migration and angiogenesis. Anticancer Res. 2012;32:2759–2768. [PubMed] [Google Scholar]
- 81.Milligan G. G protein-coupled receptor dimerization: function and ligand pharmacology. Mol. Pharmacol. 2004;66:1–7. doi: 10.1124/mol.104.000497.. [DOI] [PubMed] [Google Scholar]
- 82.Rosenbaum D.M., Rasmussen S.G., Kobilka B.K. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Song Z.-Y., Wang F., Cui S.-X., Qu X.-J. Knockdown of CXCR4 inhibits CXCL12-induced angiogenesis in HUVECs through downregulation of the MAPK/ERK and PI3K/AKT and the Wnt/β-catenin pathways. Cancer Invest. 2018;36:10–18. doi: 10.1080/07357907.2017.1422512. [DOI] [PubMed] [Google Scholar]
- 84.Fang Y., Sun B., Wang J., Wang Y. miR-622 inhibits angiogenesis by suppressing the CXCR4-VEGFA axis in colorectal cancer. Gene. 2019;699:37–42. doi: 10.1016/j.gene.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 85.Zhu D., Sun Y., Zhang D., Dong M., Jiang G., Zhang X., Zhou J. miR-1 inhibits the progression of colon cancer by regulating the expression of vascular endothelial growth factor. Oncol. Rep. 2018;40:589–598. doi: 10.3892/or.2018.6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang Y., Wu M., Lei Z., Huang M., Li Z., Wang L., Cao Q., Han D., Chang Y., Chen Y. Dysregulation of miR-6868-5p/FOXM1 circuit contributes to colorectal cancer angiogenesis. J. Exp. Clin. Cancer Res. 2018;37:292. doi: 10.1186/s13046-018-0970-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liang L., Gao C., Li Y., Sun M., Xu J., Li H., Jia L., Zhao Y. miR-125a-3p/FUT5-FUT6 axis mediates colorectal cancer cell proliferation, migration, invasion and pathological angiogenesis via PI3K-Akt pathway. Cell Death Dis. 2017;8:e2968. doi: 10.1038/cddis.2017.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ma H., Pan J.S., Jin L.X., Wu J., Ren Y.D., Chen P., Xiao C., Han J. MicroRNA-17∼92 inhibits colorectal cancer progression by targeting angiogenesis. Cancer Lett. 2016;376:293–302. doi: 10.1016/j.canlet.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 89.Thuringer D., Jego G., Berthenet K., Hammann A., Solary E., Garrido C. Gap junction-mediated transfer of miR-145-5p from microvascular endothelial cells to colon cancer cells inhibits angiogenesis. Oncotarget. 2016;7:28160–28168. doi: 10.18632/oncotarget.8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang Y., Wang X., Xu B., Wang B., Wang Z., Liang Y., Zhou J., Hu J., Jiang B. Epigenetic silencing of miR-126 contributes to tumor invasion and angiogenesis in colorectal cancer. Oncol. Rep. 2013;30:1976–1984. doi: 10.3892/or.2013.2633. [DOI] [PubMed] [Google Scholar]
- 91.Xiao F., Qiu H., Cui H., Ni X., Li J., Liao W., Lu L., Ding K. MicroRNA-885-3p inhibits the growth of HT-29 colon cancer cell xenografts by disrupting angiogenesis via targeting BMPR1A and blocking BMP/Smad/Id1 signaling. Oncogene. 2015;34:1968–1978. doi: 10.1038/onc.2014.134. [DOI] [PubMed] [Google Scholar]
- 92.Chen X., Xu X., Pan B., Zeng K., Xu M., Liu X., He B., Pan Y., Sun H., Wang S. miR-150-5p suppresses tumor progression by targeting VEGFA in colorectal cancer. Aging (Albany NY) 2018;10:3421–3437. doi: 10.18632/aging.101656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Qian X., Yu J., Yin Y., He J., Wang L., Li Q., Zhang L.Q., Li C.Y., Shi Z.M., Xu Q. MicroRNA-143 inhibits tumor growth and angiogenesis and sensitizes chemosensitivity to oxaliplatin in colorectal cancers. Cell Cycle. 2013;12:1385–1394. doi: 10.4161/cc.24477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yamakuchi M., Lotterman C.D., Bao C., Hruban R.H., Karim B., Mendell J.T., Huso D., Lowenstein C.J. P53-induced microRNA-107 inhibits HIF-1 and tumor angiogenesis. Proc. Natl. Acad. Sci. USA. 2010;107:6334–6339. doi: 10.1073/pnas.0911082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li Y., Kuscu C., Banach A., Zhang Q., Pulkoski-Gross A., Kim D., Liu J., Roth E., Li E., Shroyer K.R. miR-181a-5p inhibits cancer cell migration and angiogenesis via downregulation of matrix metalloproteinase-14. Cancer Res. 2015;75:2674–2685. doi: 10.1158/0008-5472.CAN-14-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wei L., Sun C., Zhang Y., Han N., Sun S. miR-503-5p inhibits colon cancer tumorigenesis, angiogenesis, and lymphangiogenesis by directly downregulating VEGF-A. Gene Ther. 2020 doi: 10.1038/s41434-020-0167-3. Published online June 12, 2020. [DOI] [PubMed] [Google Scholar]
- 97.Yan S., Wang H., Chen X., Liang C., Shang W., Wang L., Li J., Xu D. MiR-182-5p inhibits colon cancer tumorigenesis, angiogenesis, and lymphangiogenesis by directly downregulating VEGF-C. Cancer Lett. 2020;488:18–26. doi: 10.1016/j.canlet.2020.04.021. [DOI] [PubMed] [Google Scholar]
- 98.Li X., Li Z., Zhu Y., Li Z., Yao L., Zhang L., Yuan L., Shang Y., Liu J., Li C. miR-524-5p inhibits angiogenesis through targeting WNK1 in colon cancer cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2020;318:G827–G839. doi: 10.1152/ajpgi.00369.2019. [DOI] [PubMed] [Google Scholar]
- 99.Luo H.-N., Wang Z.-H., Sheng Y., Zhang Q., Yan J., Hou J., Zhu K., Cheng Y., Xu Y.L., Zhang X.H. MiR-139 targets CXCR4 and inhibits the proliferation and metastasis of laryngeal squamous carcinoma cells. Med. Oncol. 2014;31:789. doi: 10.1007/s12032-013-0789-z. [DOI] [PubMed] [Google Scholar]
- 100.Li L., Li B., Chen D., Liu L., Huang C., Lu Z., Lun L., Wan X. miR-139 and miR-200c regulate pancreatic cancer endothelial cell migration and angiogenesis. Oncol. Rep. 2015;34:51–58. doi: 10.3892/or.2015.3945. [DOI] [PubMed] [Google Scholar]
- 101.Boise L.H., González-García M., Postema C.E., Ding L., Lindsten T., Turka L.A., Mao X., Nuñez G., Thompson C.B. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 102.Wei M.C., Zong W.-X., Cheng E.H.-Y., Lindsten T., Panoutsakopoulou V., Ross A.J., Roth K.A., MacGregor G.R., Thompson C.B., Korsmeyer S.J. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu R., Zhang H., Wang X., Zhou L., Li H., Deng T., Qu Y., Duan J., Bai M., Ge S. The miR-24-Bim pathway promotes tumor growth and angiogenesis in pancreatic carcinoma. Oncotarget. 2015;6:43831–43842. doi: 10.18632/oncotarget.6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Park Y.-A., Choi C.H., Do I.-G., Song S.Y., Lee J.K., Cho Y.J., Choi J.J., Jeon H.K., Ryu J.Y., Lee Y.Y. Dual targeting of angiotensin receptors (AGTR1 and AGTR2) in epithelial ovarian carcinoma. Gynecol. Oncol. 2014;135:108–117. doi: 10.1016/j.ygyno.2014.06.031. [DOI] [PubMed] [Google Scholar]
- 105.Shibata K., Kikkawa F., Mizokami Y., Kajiyama H., Ino K., Nomura S., Mizutani S. Possible involvement of adipocyte-derived leucine aminopeptidase via angiotensin II in endometrial carcinoma. Tumour Biol. 2005;26:9–16. doi: 10.1159/000084181. [DOI] [PubMed] [Google Scholar]
- 106.Watanabe Y., Shibata K., Kikkawa F., Kajiyama H., Ino K., Hattori A., Tsujimoto M., Mizutani S. Adipocyte-derived leucine aminopeptidase suppresses angiogenesis in human endometrial carcinoma via renin-angiotensin system. Clin. Cancer Res. 2003;9:6497–6503. [PubMed] [Google Scholar]
- 107.Du N., Feng J., Hu L.-J., Sun X., Sun H.-B., Zhao Y., Yang Y.P., Ren H. Angiotensin II receptor type 1 blockers suppress the cell proliferation effects of angiotensin II in breast cancer cells by inhibiting AT1R signaling. Oncol. Rep. 2012;27:1893–1903. doi: 10.3892/or.2012.1720. [DOI] [PubMed] [Google Scholar]
- 108.Chen X., Meng Q., Zhao Y., Liu M., Li D., Yang Y., Sun L., Sui G., Cai L., Dong X. Angiotensin II type 1 receptor antagonists inhibit cell proliferation and angiogenesis in breast cancer. Cancer Lett. 2013;328:318–324. doi: 10.1016/j.canlet.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 109.Guo R., Gu J., Zhang Z., Wang Y., Gu C. MicroRNA-410 functions as a tumor suppressor by targeting angiotensin II type 1 receptor in pancreatic cancer. IUBMB Life. 2015;67:42–53. doi: 10.1002/iub.1342. [DOI] [PubMed] [Google Scholar]
- 110.Zhang J.-G., Farley A., Nicholson S.E., Willson T.A., Zugaro L.M., Simpson R.J., Moritz R.L., Cary D., Richardson R., Hausmann G. The conserved SOCS box motif in suppressors of cytokine signaling binds to elongins B and C and may couple bound proteins to proteasomal degradation. Proc. Natl. Acad. Sci. USA. 1999;96:2071–2076. doi: 10.1073/pnas.96.5.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nicholson S.E., Hilton D.J. The SOCS proteins: a new family of negative regulators of signal transduction. J. Leukoc. Biol. 1998;63:665–668. doi: 10.1002/jlb.63.6.665. [DOI] [PubMed] [Google Scholar]
- 112.Subramanian A., Kuehn H., Gould J., Tamayo P., Mesirov J.P. GSEA-P: a desktop application for Gene Set Enrichment Analysis. Bioinformatics. 2007;23:3251–3253. doi: 10.1093/bioinformatics/btm369. [DOI] [PubMed] [Google Scholar]
- 113.Feng Z.P., Chandrashekaran I.R., Low A., Speed T.P., Nicholson S.E., Norton R.S. The N-terminal domains of SOCS proteins: a conserved region in the disordered N-termini of SOCS4 and 5. Proteins. 2012;80:946–957. doi: 10.1002/prot.23252. [DOI] [PubMed] [Google Scholar]
- 114.Hu H., Zhang Q., Chen W., Wu T., Liu S., Li X., Luo B., Zhang T., Yan G., Lu H., Lu Z. MicroRNA-301a promotes pancreatic cancer invasion and metastasis through the JAK/STAT3 signaling pathway by targeting SOCS5. Carcinogenesis. 2020;41:502–514. doi: 10.1093/carcin/bgz121. [DOI] [PubMed] [Google Scholar]
- 115.Sun L., Wang Q., Gao X., Shi D., Mi S., Han Q. MicroRNA-454 functions as an oncogene by regulating PTEN in uveal melanoma. FEBS Lett. 2015;589(19 Pt B):2791–2796. doi: 10.1016/j.febslet.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 116.Zhu D.-Y., Li X.-N., Qi Y., Liu D.-L., Yang Y., Zhao J., Zhang C.Y., Wu K., Zhao S. MiR-454 promotes the progression of human non-small cell lung cancer and directly targets PTEN. Biomed. Pharmacother. 2016;81:79–85. doi: 10.1016/j.biopha.2016.03.029. [DOI] [PubMed] [Google Scholar]
- 117.Fan Y., Shi C., Li T., Kuang T. microRNA-454 shows anti-angiogenic and anti-metastatic activity in pancreatic ductal adenocarcinoma by targeting LRP6. Am. J. Cancer Res. 2017;7:139–147. [PMC free article] [PubMed] [Google Scholar]
- 118.Xiong Y., Fang J.H., Yun J.P., Yang J., Zhang Y., Jia W.H., Zhuang S.M. Effects of microRNA-29 on apoptosis, tumorigenicity, and prognosis of hepatocellular carcinoma. Hepatology. 2010;51:836–845. doi: 10.1002/hep.23380. [DOI] [PubMed] [Google Scholar]
- 119.Cortez M.A., Nicoloso M.S., Shimizu M., Rossi S., Gopisetty G., Molina J.R., Carlotti C., Jr., Tirapelli D., Neder L., Brassesco M.S. miR-29b and miR-125a regulate podoplanin and suppress invasion in glioblastoma. Genes Chromosomes Cancer. 2010;49:981–990. doi: 10.1002/gcc.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Muniyappa M.K., Dowling P., Henry M., Meleady P., Doolan P., Gammell P., Clynes M., Barron N. MiRNA-29a regulates the expression of numerous proteins and reduces the invasiveness and proliferation of human carcinoma cell lines. Eur. J. Cancer. 2009;45:3104–3118. doi: 10.1016/j.ejca.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 121.Zhao J.-J., Lin J., Lwin T., Yang H., Guo J., Kong W., Dessureault S., Moscinski L.C., Rezania D., Dalton W.S. microRNA expression profile and identification of miR-29 as a prognostic marker and pathogenetic factor by targeting CDK6 in mantle cell lymphoma. Blood. 2010;115:2630–2639. doi: 10.1182/blood-2009-09-243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kessenbrock K., Plaks V., Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ogasawara S., Yano H., Momosaki S., Nishida N., Takemoto Y., Kojiro S., Kojiro M. Expression of matrix metalloproteinases (MMPs) in cultured hepatocellular carcinoma (HCC) cells and surgically resected HCC tissues. Oncol. Rep. 2005;13:1043–1048. [PubMed] [Google Scholar]
- 124.Giannelli G., Bergamini C., Marinosci F., Fransvea E., Quaranta M., Lupo L., Schiraldi O., Antonaci S. Clinical role of MMP-2/TIMP-2 imbalance in hepatocellular carcinoma. Int. J. Cancer. 2002;97:425–431. doi: 10.1002/ijc.1635. [DOI] [PubMed] [Google Scholar]
- 125.Fang J.H., Zhou H.C., Zeng C., Yang J., Liu Y., Huang X., Zhang J.P., Guan X.Y., Zhuang S.M. MicroRNA-29b suppresses tumor angiogenesis, invasion, and metastasis by regulating matrix metalloproteinase 2 expression. Hepatology. 2011;54:1729–1740. doi: 10.1002/hep.24577. [DOI] [PubMed] [Google Scholar]
- 126.Bentwich I., Avniel A., Karov Y., Aharonov R., Gilad S., Barad O., Barzilai A., Einat P., Einav U., Meiri E. Identification of hundreds of conserved and nonconserved human microRNAs. Nat. Genet. 2005;37:766–770. doi: 10.1038/ng1590. [DOI] [PubMed] [Google Scholar]
- 127.Sewer A., Paul N., Landgraf P., Aravin A., Pfeffer S., Brownstein M.J., Tuschl T., van Nimwegen E., Zavolan M. Identification of clustered microRNAs using an ab initio prediction method. BMC Bioinformatics. 2005;6:267. doi: 10.1186/1471-2105-6-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Landgraf P., Rusu M., Sheridan R., Sewer A., Iovino N., Aravin A., Pfeffer S., Rice A., Kamphorst A.O., Landthaler M. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129:1401–1414. doi: 10.1016/j.cell.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Caporali A., Meloni M., Völlenkle C., Bonci D., Sala-Newby G.B., Addis R., Spinetti G., Losa S., Masson R., Baker A.H. Deregulation of microRNA-503 contributes to diabetes mellitus-induced impairment of endothelial function and reparative angiogenesis after limb ischemia. Circulation. 2011;123:282–291. doi: 10.1161/CIRCULATIONAHA.110.952325. [DOI] [PubMed] [Google Scholar]
- 130.Zhou B., Ma R., Si W., Li S., Xu Y., Tu X., Wang Q. MicroRNA-503 targets FGF2 and VEGFA and inhibits tumor angiogenesis and growth. Cancer Lett. 2013;333:159–169. doi: 10.1016/j.canlet.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 131.Rosa R., Marciano R., Malapelle U., Formisano L., Nappi L., D’Amato C., D’Amato V., Damiano V., Marfè G., Del Vecchio S. Sphingosine kinase 1 overexpression contributes to cetuximab resistance in human colorectal cancer models. Clin. Cancer Res. 2013;19:138–147. doi: 10.1158/1078-0432.CCR-12-1050. [DOI] [PubMed] [Google Scholar]
- 132.Nagahashi M., Ramachandran S., Kim E.Y., Allegood J.C., Rashid O.M., Yamada A., Zhao R., Milstien S., Zhou H., Spiegel S., Takabe K. Sphingosine-1-phosphate produced by sphingosine kinase 1 promotes breast cancer progression by stimulating angiogenesis and lymphangiogenesis. Cancer Res. 2012;72:726–735. doi: 10.1158/0008-5472.CAN-11-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yang L., Yue S., Yang L., Liu X., Han Z., Zhang Y., Li L. Sphingosine kinase/sphingosine 1-phosphate (S1P)/S1P receptor axis is involved in liver fibrosis-associated angiogenesis. J. Hepatol. 2013;59:114–123. doi: 10.1016/j.jhep.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 134.Lu Z., Zhang W., Gao S., Jiang Q., Xiao Z., Ye L., Zhang X. MiR-506 suppresses liver cancer angiogenesis through targeting sphingosine kinase 1 (SPHK1) mRNA. Biochem. Biophys. Res. Commun. 2015;468:8–13. doi: 10.1016/j.bbrc.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 135.Curtale G., Citarella F., Carissimi C., Goldoni M., Carucci N., Fulci V., Franceschini D., Meloni F., Barnaba V., Macino G. An emerging player in the adaptive immune response: microRNA-146a is a modulator of IL-2 expression and activation-induced cell death in T lymphocytes. Blood. 2010;115:265–273. doi: 10.1182/blood-2009-06-225987. [DOI] [PubMed] [Google Scholar]
- 136.He H., Jazdzewski K., Li W., Liyanarachchi S., Nagy R., Volinia S., Calin G.A., Liu C.G., Franssila K., Suster S. The role of microRNA genes in papillary thyroid carcinoma. Proc. Natl. Acad. Sci. USA. 2005;102:19075–19080. doi: 10.1073/pnas.0509603102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bhaumik D., Scott G.K., Schokrpur S., Patil C.K., Campisi J., Benz C.C. Expression of microRNA-146 suppresses NF-kappaB activity with reduction of metastatic potential in breast cancer cells. Oncogene. 2008;27:5643–5647. doi: 10.1038/onc.2008.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hurst D.R., Edmonds M.D., Scott G.K., Benz C.C., Vaidya K.S., Welch D.R. Breast cancer metastasis suppressor 1 up-regulates miR-146, which suppresses breast cancer metastasis. Cancer Res. 2009;69:1279–1283. doi: 10.1158/0008-5472.CAN-08-3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li Y., Vandenboom T.G., 2nd, Wang Z., Kong D., Ali S., Philip P.A., Sarkar F.H. miR-146a suppresses invasion of pancreatic cancer cells. Cancer Res. 2010;70:1486–1495. doi: 10.1158/0008-5472.CAN-09-2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang X., Tang S., Le S.-Y., Lu R., Rader J.S., Meyers C., Zheng Z.M. Aberrant expression of oncogenic and tumor-suppressive microRNAs in cervical cancer is required for cancer cell growth. PLoS ONE. 2008;3:e2557. doi: 10.1371/journal.pone.0002557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhu K., Pan Q., Zhang X., Kong L.-Q., Fan J., Dai Z., Wang L., Yang X.R., Hu J., Wan J.L. MiR-146a enhances angiogenic activity of endothelial cells in hepatocellular carcinoma by promoting PDGFRA expression. Carcinogenesis. 2013;34:2071–2079. doi: 10.1093/carcin/bgt160. [DOI] [PubMed] [Google Scholar]
- 142.Ebrahimi F., Gopalan V., Smith R.A., Lam A.K.-Y. miR-126 in human cancers: clinical roles and current perspectives. Exp. Mol. Pathol. 2014;96:98–107. doi: 10.1016/j.yexmp.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 143.Wang S., Wang X., Guo Q., Wang G., Han X., Li X., Shi Z.W., He W. MicroRNA-126 overexpression inhibits proliferation and invasion in osteosarcoma cells. Technol. Cancer Res. Treat. 2016;15:NP49–NP59. doi: 10.1177/1533034615601563. [DOI] [PubMed] [Google Scholar]
- 144.Guo C., Sah J.F., Beard L., Willson J.K., Markowitz S.D., Guda K. The noncoding RNA, miR-126, suppresses the growth of neoplastic cells by targeting phosphatidylinositol 3-kinase signaling and is frequently lost in colon cancers. Genes Chromosomes Cancer. 2008;47:939–946. doi: 10.1002/gcc.20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhao C., Li Y., Zhang M., Yang Y., Chang L. miR-126 inhibits cell proliferation and induces cell apoptosis of hepatocellular carcinoma cells partially by targeting Sox2. Hum. Cell. 2015;28:91–99. doi: 10.1007/s13577-014-0105-z. [DOI] [PubMed] [Google Scholar]
- 146.Gong C., Fang J., Li G., Liu H.-H., Liu Z.-S. Effects of microRNA-126 on cell proliferation, apoptosis and tumor angiogenesis via the down-regulating ERK signaling pathway by targeting EGFL7 in hepatocellular carcinoma. Oncotarget. 2017;8:52527–52542. doi: 10.18632/oncotarget.17283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yang C., Xu Y., Cheng F., Hu Y., Yang S., Rao J., Wang X. miR-1301 inhibits hepatocellular carcinoma cell migration, invasion, and angiogenesis by decreasing Wnt/β-catenin signaling through targeting BCL9. Cell Death Dis. 2017;8:e2999. doi: 10.1038/cddis.2017.356. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 148.Wang Y., Sun B., Sun H., Zhao X., Wang X., Zhao N., Zhang Y., Li Y., Gu Q., Liu F. Regulation of proliferation, angiogenesis and apoptosis in hepatocellular carcinoma by miR-26b-5p. Tumour Biol. 2016;37:10965–10979. doi: 10.1007/s13277-016-4964-7. [DOI] [PubMed] [Google Scholar]
- 149.Ghosh A., Dasgupta D., Ghosh A., Roychoudhury S., Kumar D., Gorain M., Butti R., Datta S., Agarwal S., Gupta S. MiRNA199a-3p suppresses tumor growth, migration, invasion and angiogenesis in hepatocellular carcinoma by targeting VEGFA, VEGFR1, VEGFR2, HGF and MMP2. Cell Death Dis. 2017;8:e2706. doi: 10.1038/cddis.2017.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wu M., Huang C., Huang X., Liang R., Feng Y., Luo X. MicroRNA-144-3p suppresses tumor growth and angiogenesis by targeting SGK3 in hepatocellular carcinoma. Oncol. Rep. 2017;38:2173–2181. doi: 10.3892/or.2017.5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yan J.J., Zhang Y.N., Liao J.Z., Ke K.P., Chang Y., Li P.Y., Wang M., Lin J.S., He X.X. MiR-497 suppresses angiogenesis and metastasis of hepatocellular carcinoma by inhibiting VEGFA and AEG-1. Oncotarget. 2015;6:29527–29542. doi: 10.18632/oncotarget.5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yu Q., Xiang L., Yin L., Liu X., Yang D. and Zhou, J. Loss-of-function of miR-142 by hypermethylation promotes TGF-β-mediated tumour growth and metastasis in hepatocellular carcinoma. Cell Prolif. 2017;50:e12384. doi: 10.1111/cpr.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang T., Liu W., Zeng X.C., Jiang N., Fu B.S., Guo Y., Yi H.M., Li H., Zhang Q., Chen W.J., Chen G.H. Down-regulation of microRNA-338-3p promoted angiogenesis in hepatocellular carcinoma. Biomed. Pharmacother. 2016;84:583–591. doi: 10.1016/j.biopha.2016.09.056. [DOI] [PubMed] [Google Scholar]
- 154.Cheng J., Chen Y., Zhao P., Liu X., Dong J., Li J., Huang C., Wu R., Lv Y. Downregulation of miRNA-638 promotes angiogenesis and growth of hepatocellular carcinoma by targeting VEGF. Oncotarget. 2016;7:30702–30711. doi: 10.18632/oncotarget.8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yang Y., Zhang J., Xia T., Li G., Tian T., Wang M., Wang R., Zhao L., Yang Y., Lan K., Zhou W. MicroRNA-210 promotes cancer angiogenesis by targeting fibroblast growth factor receptor-like 1 in hepatocellular carcinoma. Oncol. Rep. 2016;36:2553–2562. doi: 10.3892/or.2016.5129. [DOI] [PubMed] [Google Scholar]
- 156.Du C., Weng X., Hu W., Lv Z., Xiao H., Ding C., Gyabaah O.A.K., Xie H., Zhou L., Wu J., Zheng S. Hypoxia-inducible MiR-182 promotes angiogenesis by targeting RASA1 in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2015;34:67. doi: 10.1186/s13046-015-0182-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ji J.S., Xu M., Song J.J., Zhao Z.W., Chen M.J., Chen W.Q., Tu J.F., Yang X.M. Inhibition of microRNA-126 promotes the expression of Spred1 to inhibit angiogenesis in hepatocellular carcinoma after transcatheter arterial chemoembolization: in vivo study. OncoTargets Ther. 2016;9:4357–4367. doi: 10.2147/OTT.S106513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yang X., Zhang X.F., Lu X., Jia H.L., Liang L., Dong Q.Z., Ye Q.H., Qin L.X. MicroRNA-26a suppresses angiogenesis in human hepatocellular carcinoma by targeting hepatocyte growth factor-cMet pathway. Hepatology. 2014;59:1874–1885. doi: 10.1002/hep.26941. [DOI] [PubMed] [Google Scholar]
- 159.Du C., Lv Z., Cao L., Ding C., Gyabaah O.A., Xie H., Zhou L., Wu J., Zheng S. MiR-126-3p suppresses tumor metastasis and angiogenesis of hepatocellular carcinoma by targeting LRP6 and PIK3R2. J. Transl. Med. 2014;12:259. doi: 10.1186/s12967-014-0259-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang R., Zhao N., Li S., Fang J.H., Chen M.X., Yang J., Jia W.H., Yuan Y., Zhuang S.M. MicroRNA-195 suppresses angiogenesis and metastasis of hepatocellular carcinoma by inhibiting the expression of VEGF, VAV2, and CDC42. Hepatology. 2013;58:642–653. doi: 10.1002/hep.26373. [DOI] [PubMed] [Google Scholar]
- 161.Cao Y.P., Pan M., Song Y.L., Zhang H.L., Sui H.T., Shan B.C., Piao H.X. MiR-302 a/b/c suppresses tumor angiogenesis in hepatocellular carcinoma by targeting MACC1. Eur. Rev. Med. Pharmacol. Sci. 2019;23:7863–7873. doi: 10.26355/eurrev_201909_18996. [DOI] [PubMed] [Google Scholar]
- 162.Chai Z.T., Kong J., Zhu X.D., Zhang Y.Y., Lu L., Zhou J.M., Wang L.R., Zhang K.Z., Zhang Q.B., Ao J.Y. MicroRNA-26a inhibits angiogenesis by down-regulating VEGFA through the PIK3C2α/Akt/HIF-1α pathway in hepatocellular carcinoma. PLoS ONE. 2013;8:e77957. doi: 10.1371/journal.pone.0077957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yahya S.M.M., Yahya S.M.M. The Effect of miR-98 and miR-214 on Apoptotic and Angiogenic Pathways in Hepatocellular Carcinoma HepG2 Cells. Indian J. Clin. Biochem. 2020;35:353–358. doi: 10.1007/s12291-019-00824-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Li D., Wang T., Sun F.F., Feng J.Q., Peng J.J., Li H., Wang C., Wang D., Liu Y., Bai Y.D. MicroRNA-375 represses tumor angiogenesis and reverses resistance to sorafenib in hepatocarcinoma. Cancer Gene. Ther. 2020;28:126–140. doi: 10.1038/s41417-020-0191-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Moh-Moh-Aung A., Fujisawa M., Ito S., Katayama H., Ohara T., Ota Y., Yoshimura T., Matsukawa A. Decreased miR-200b-3p in cancer cells leads to angiogenesis in HCC by enhancing endothelial ERG expression. Sci. Rep. 2020;10:10418. doi: 10.1038/s41598-020-67425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.O’Day E., Lal A. MicroRNAs and their target gene networks in breast cancer. Breast Cancer Res. 2010;12:201. doi: 10.1186/bcr2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nam E.J., Yoon H., Kim S.W., Kim H., Kim Y.T., Kim J.H., Kim J.W., Kim S. MicroRNA expression profiles in serous ovarian carcinoma. Clin. Cancer Res. 2008;14:2690–2695. doi: 10.1158/1078-0432.CCR-07-1731. [DOI] [PubMed] [Google Scholar]
- 168.Jiang L., Huang Q., Zhang S., Zhang Q., Chang J., Qiu X., Wang E. Hsa-miR-125a-3p and hsa-miR-125a-5p are downregulated in non-small cell lung cancer and have inverse effects on invasion and migration of lung cancer cells. BMC Cancer. 2010;10:318. doi: 10.1186/1471-2407-10-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ferretti E., De Smaele E., Po A., Di Marcotullio L., Tosi E., Espinola M.S.B., Di Rocco C., Riccardi R., Giangaspero F., Farcomeni A. MicroRNA profiling in human medulloblastoma. Int. J. Cancer. 2009;124:568–577. doi: 10.1002/ijc.23948. [DOI] [PubMed] [Google Scholar]
- 170.Hashiguchi Y., Nishida N., Mimori K., Sudo T., Tanaka F., Shibata K., Ishii H., Mochizuki H., Hase K., Doki Y., Mori M. Down-regulation of miR-125a-3p in human gastric cancer and its clinicopathological significance. Int. J. Oncol. 2012;40:1477–1482. doi: 10.3892/ijo.2012.1363. [DOI] [PubMed] [Google Scholar]
- 171.Dai J., Wang J., Yang L., Xiao Y., Ruan Q. miR-125a regulates angiogenesis of gastric cancer by targeting vascular endothelial growth factor A. Int. J. Oncol. 2015;47:1801–1810. doi: 10.3892/ijo.2015.3171. [DOI] [PubMed] [Google Scholar]
- 172.Jüttner S., Wissmann C., Jöns T., Vieth M., Hertel J., Gretschel S., Schlag P.M., Kemmner W., Höcker M. Vascular endothelial growth factor-D and its receptor VEGFR-3: two novel independent prognostic markers in gastric adenocarcinoma. J. Clin. Oncol. 2006;24:228–240. doi: 10.1200/JCO.2004.00.3467. [DOI] [PubMed] [Google Scholar]
- 173.Suzuki S., Dobashi Y., Hatakeyama Y., Tajiri R., Fujimura T., Heldin C.H., Ooi A. Clinicopathological significance of platelet-derived growth factor (PDGF)-B and vascular endothelial growth factor-A expression, PDGF receptor-β phosphorylation, and microvessel density in gastric cancer. BMC Cancer. 2010;10:659. doi: 10.1186/1471-2407-10-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Xie M., Dart D.A., Guo T., Xing X.-F., Cheng X.-J., Du H., Jiang W.G., Wen X.Z., Ji J.F. MicroRNA-1 acts as a tumor suppressor microRNA by inhibiting angiogenesis-related growth factors in human gastric cancer. Gastric Cancer. 2018;21:41–54. doi: 10.1007/s10120-017-0721-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Will M., Qin A.C.R., Toy W., Yao Z., Rodrik-Outmezguine V., Schneider C., Huang X., Monian P., Jiang X., de Stanchina E. Rapid induction of apoptosis by PI3K inhibitors is dependent upon their transient inhibition of RAS-ERK signaling. Cancer Discov. 2014;4:334–347. doi: 10.1158/2159-8290.CD-13-0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wu Z.-H., Lin C., Liu C.-C., Jiang W.-W., Huang M.-Z., Liu X., Guo W.J. MiR-616-3p promotes angiogenesis and EMT in gastric cancer via the PTEN/AKT/mTOR pathway. Biochem. Biophys. Res. Commun. 2018;501:1068–1073. doi: 10.1016/j.bbrc.2018.05.109. [DOI] [PubMed] [Google Scholar]
- 177.Taupin D., Podolsky D.K. Trefoil factors: initiators of mucosal healing. Nat. Rev. Mol. Cell Biol. 2003;4:721–732. doi: 10.1038/nrm1203. [DOI] [PubMed] [Google Scholar]
- 178.Kim J.H., Kim M.A., Lee H.S., Kim W.H. Comparative analysis of protein expressions in primary and metastatic gastric carcinomas. Hum. Pathol. 2009;40:314–322. doi: 10.1016/j.humpath.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 179.Shi Y., Huang X., Chen G., Wang Y., Liu Y., Xu W., Tang S., Guleng B., Liu J., Ren J. miR-632 promotes gastric cancer progression by accelerating angiogenesis in a TFF1-dependent manner. BMC Cancer. 2019;19:14. doi: 10.1186/s12885-018-5247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Li Q.-L., Ito K., Sakakura C., Fukamachi H., Inoue K.-i., Chi X.-Z., Lee K.Y., Nomura S., Lee C.W., Han S.B. Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell. 2002;109:113–124. doi: 10.1016/s0092-8674(02)00690-6. [DOI] [PubMed] [Google Scholar]
- 181.Lee S.H., Jung Y.D., Choi Y.S., Lee Y.M. Targeting of RUNX3 by miR-130a and miR-495 cooperatively increases cell proliferation and tumor angiogenesis in gastric cancer cells. Oncotarget. 2015;6:33269–33278. doi: 10.18632/oncotarget.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wang L., Bo X., Zheng Q., Ge W., Liu Y., Li B. Paired box 8 suppresses tumor angiogenesis and metastasis in gastric cancer through repression of FOXM1 via induction of microRNA-612. J. Exp. Clin. Cancer Res. 2018;37:159. doi: 10.1186/s13046-018-0830-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhang J.X., Chen Z.H., Chen D.L., Tian X.P., Wang C.Y., Zhou Z.W., Gao Y., Xu Y., Chen C., Zheng Z.S. LINC01410-miR-532-NCF2-NF-kB feedback loop promotes gastric cancer angiogenesis and metastasis. Oncogene. 2018;37:2660–2675. doi: 10.1038/s41388-018-0162-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 184.Si Y., Zhang H., Ning T., Bai M., Wang Y., Yang H., Wang X., Li J., Ying G., Ba Y. miR-26a/b Inhibit Tumor Growth and Angiogenesis by Targeting the HGF-VEGF Axis in Gastric Carcinoma. Cell Physiol. Biochem. 2017;42:1670–1683. doi: 10.1159/000479412. [DOI] [PubMed] [Google Scholar]
- 185.Xie M., Dart D.A., Guo T., Xing X.F., Cheng X.J., Du H., Jiang W.G., Wen X.Z., Ji J.F. MicroRNA-1 acts as a tumor suppressor microRNA by inhibiting angiogenesis-related growth factors in human gastric cancer. Gastric Cancer. 2018;21:41–54. doi: 10.1007/s10120-017-0721-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jia L., Chen J., Xie C., Shao L., Xu Z., Zhang L. microRNA-1228⁎ impairs the pro-angiogenic activity of gastric cancer cells by targeting macrophage migration inhibitory factor. Life Sci. 2017;180:9–16. doi: 10.1016/j.lfs.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 187.Zhang X., Dong J., He Y., Zhao M., Liu Z., Wang N., Jiang M., Zhang Z., Liu G., Liu H. miR-218 inhibited tumor angiogenesis by targeting ROBO1 in gastric cancer. Gene. 2017;615:42–49. doi: 10.1016/j.gene.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 188.Li S., Zhang H., Ning T., Wang X., Liu R., Yang H., Han Y., Deng T., Zhou L., Zhang L. MiR-520b/e Regulates Proliferation and Migration by Simultaneously Targeting EGFR in Gastric Cancer. Cell Physiol. Biochem. 2016;40:1303–1315. doi: 10.1159/000453183. [DOI] [PubMed] [Google Scholar]
- 189.Liu H.T., Xing A.Y., Chen X., Ma R.R., Wang Y.W., Shi D.B., Zhang H., Li P., Chen H.F., Li Y.H., Gao P. MicroRNA-27b, microRNA-101 and microRNA-128 inhibit angiogenesis by down-regulating vascular endothelial growth factor C expression in gastric cancers. Oncotarget. 2015;6:37458–37470. doi: 10.18632/oncotarget.6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Li Z., Liu Z., Dong S., Zhang J., Tan J., Wang Y., Ge C., Li R., Xue Y., Li M. miR-506 Inhibits Epithelial-to-Mesenchymal Transition and Angiogenesis in Gastric Cancer. Am. J. Pathol. 2015;185:2412–2420. doi: 10.1016/j.ajpath.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 191.Zhang X., Tang J., Zhi X., Xie K., Wang W., Li Z., Zhu Y., Yang L., Xu H., Xu Z. miR-874 functions as a tumor suppressor by inhibiting angiogenesis through STAT3/VEGF-A pathway in gastric cancer. Oncotarget. 2015;6:1605–1617. doi: 10.18632/oncotarget.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chen H., Li L., Wang S., Lei Y., Ge Q., Lv N., Zhou X., Chen C. Reduced miR-126 expression facilitates angiogenesis of gastric cancer through its regulation on VEGF-A. Oncotarget. 2014;5:11873–11885. doi: 10.18632/oncotarget.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhang H., Bai M., Deng T., Liu R., Wang X., Qu Y., Duan J., Zhang L., Ning T., Ge S. Cell-derived microvesicles mediate the delivery of miR-29a/c to suppress angiogenesis in gastric carcinoma. Cancer Lett. 2016;375:331–339. doi: 10.1016/j.canlet.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 194.Cheng Z., Liu F., Zhang H., Li X., Li Y., Li J., Liu F., Cao Y., Cao L., Li F. miR-135a inhibits tumor metastasis and angiogenesis by targeting FAK pathway. Oncotarget. 2017;8:31153–31168. doi: 10.18632/oncotarget.16098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zheng L., Pu J., Qi T., Qi M., Li D., Xiang X., Huang K., Tong Q. miRNA-145 targets v-ets erythroblastosis virus E26 oncogene homolog 1 to suppress the invasion, metastasis, and angiogenesis of gastric cancer cells. Mol. Cancer Res. 2013;11:182–193. doi: 10.1158/1541-7786.MCR-12-0534. [DOI] [PubMed] [Google Scholar]
- 196.Ihara Y., Kato Y., Bando T., Yamagishi F., Minamimura T., Sakamoto T., Tsukada K., Isobe M. Allelic imbalance of 14q32 in esophageal carcinoma. Cancer Genet. Cytogenet. 2002;135:177–181. doi: 10.1016/s0165-4608(01)00654-9. [DOI] [PubMed] [Google Scholar]
- 197.Haller F., Von Heydebreck A., Zhang J.D., Gunawan B., Langer C., Ramadori G., Wiemann S., Sahin O. Localization-and mutation-dependent microRNA (miRNA) expression signatures in gastrointestinal stromal tumours (GISTs), with a cluster of co-expressed miRNAs located at 14q32.31. J. Pathol. 2010;220:71–86. doi: 10.1002/path.2610. [DOI] [PubMed] [Google Scholar]
- 198.Valent P., Bonnet D., De Maria R., Lapidot T., Copland M., Melo J.V., Chomienne C., Ishikawa F., Schuringa J.J., Stassi G. Cancer stem cell definitions and terminology: the devil is in the details. Nat. Rev. Cancer. 2012;12:767–775. doi: 10.1038/nrc3368. [DOI] [PubMed] [Google Scholar]
- 199.Hang D., Dong H.C., Ning T., Dong B., Hou D.L., Xu W.G. Prognostic value of the stem cell markers CD133 and ABCG2 expression in esophageal squamous cell carcinoma. Dis. Esophagus. 2012;25:638–644. doi: 10.1111/j.1442-2050.2011.01298.x. [DOI] [PubMed] [Google Scholar]
- 200.Nakajima T.E., Yoshida H., Okamoto N., Nagashima K., Taniguchi H., Yamada Y., Shimoda T., Masutomi K. Nucleostemin and TWIST as predictive markers for recurrence after neoadjuvant chemotherapy for esophageal carcinoma. Cancer Sci. 2012;103:233–238. doi: 10.1111/j.1349-7006.2011.02142.x. [DOI] [PubMed] [Google Scholar]
- 201.Lu C., Xu F., Gu J., Yuan Y., Zhao G., Yu X., Ge D. Clinical and biological significance of stem-like CD133(+)CXCR4(+) cells in esophageal squamous cell carcinoma. J. Thorac. Cardiovasc. Surg. 2015;150:386–395. doi: 10.1016/j.jtcvs.2015.05.030. [DOI] [PubMed] [Google Scholar]
- 202.Okamoto K., Ninomiya I., Ohbatake Y., Hirose A., Tsukada T., Nakanuma S., Sakai S., Kinoshita J., Makino I., Nakamura K. Expression status of CD44 and CD133 as a prognostic marker in esophageal squamous cell carcinoma treated with neoadjuvant chemotherapy followed by radical esophagectomy. Oncol. Rep. 2016;36:3333–3342. doi: 10.3892/or.2016.5133. [DOI] [PubMed] [Google Scholar]
- 203.Li B., Xu W.W., Han L., Chan K.T., Tsao S.W., Lee N.P.Y., Law S., Xu L.Y., Li E.M., Chan K.W. MicroRNA-377 suppresses initiation and progression of esophageal cancer by inhibiting CD133 and VEGF. Oncogene. 2017;36:3986–4000. doi: 10.1038/onc.2017.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Liu X., Gong J., Xu B. miR-143 down-regulates TLR2 expression in hepatoma cells and inhibits hepatoma cell proliferation and invasion. Int. J. Clin. Exp. Pathol. 2015;8:12738–12747. [PMC free article] [PubMed] [Google Scholar]
- 205.Su J., Liang H., Yao W., Wang N., Zhang S., Yan X., Feng H., Pang W., Wang Y., Wang X. MiR-143 and MiR-145 regulate IGF1R to suppress cell proliferation in colorectal cancer. PLoS ONE. 2014;9:e114420. doi: 10.1371/journal.pone.0114420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wei J., Ma Z., Li Y., Zhao B., Wang D., Jin Y., Jin Y. miR-143 inhibits cell proliferation by targeting autophagy-related 2B in non-small cell lung cancer H1299 cells. Mol. Med. Rep. 2015;11:571–576. doi: 10.3892/mmr.2014.2675. [DOI] [PubMed] [Google Scholar]
- 207.Wu D., Huang P., Wang L., Zhou Y., Pan H., Qu P. MicroRNA-143 inhibits cell migration and invasion by targeting matrix metalloproteinase 13 in prostate cancer. Mol. Med. Rep. 2013;8:626–630. doi: 10.3892/mmr.2013.1501. [DOI] [PubMed] [Google Scholar]
- 208.Hicklin D.J., Ellis L.M. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J. Clin. Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 209.De Falco S. The discovery of placenta growth factor and its biological activity. Exp. Mol. Med. 2012;44:1–9. doi: 10.3858/emm.2012.44.1.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lee J.-M., Cimino-Mathews A., Peer C.J., Zimmer A., Lipkowitz S., Annunziata C.M., Cao L., Harrell M.I., Swisher E.M., Houston N. Safety and clinical activity of the programmed death-ligand 1 inhibitor durvalumab in combination with poly (ADP-ribose) polymerase inhibitor olaparib or vascular endothelial growth factor receptor 1-3 inhibitor cediranib in women’s cancers: a dose-escalation, phase I study. J. Clin. Oncol. 2017;35:2193–2202. doi: 10.1200/JCO.2016.72.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Faivre S., Niccoli P., Castellano D., Valle J.W., Hammel P., Raoul J.-L., Vinik A., Van Cutsem E., Bang Y.J., Lee S.H. Sunitinib in pancreatic neuroendocrine tumors: updated progression-free survival and final overall survival from a phase III randomized study. Ann. Oncol. 2017;28:339–343. doi: 10.1093/annonc/mdw561. [DOI] [PubMed] [Google Scholar]
- 212.Hatem R., Labiod D., Château-Joubert S., de Plater L., El Botty R., Vacher S., Bonin F., Servely J.L., Dieras V., Bièche I., Marangoni E. Vandetanib as a potential new treatment for estrogen receptor-negative breast cancers. Int. J. Cancer. 2016;138:2510–2521. doi: 10.1002/ijc.29974. [DOI] [PubMed] [Google Scholar]
- 213.Jin Y.P., Hu Y.P., Wu X.S., Wu Y.S., Ye Y.Y., Li H.F., Liu Y.C., Jiang L., Liu F.T., Zhang Y.J. miR-143-3p targeting of ITGA6 suppresses tumour growth and angiogenesis by downregulating PLGF expression via the PI3K/AKT pathway in gallbladder carcinoma. Cell Death Dis. 2018;9:182. doi: 10.1038/s41419-017-0258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Jia H., Osak M., Bogu G.K., Stanton L.W., Johnson R., Lipovich L. Genome-wide computational identification and manual annotation of human long noncoding RNA genes. RNA. 2010;16:1478–1487. doi: 10.1261/rna.1951310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Cabili M.N., Trapnell C., Goff L., Koziol M., Tazon-Vega B., Regev A., Rinn J.L. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 2011;25:1915–1927. doi: 10.1101/gad.17446611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Hombach S., Kretz M. Non-coding RNAs: classification, biology and functioning. Adv. Exp. Med. Biol. 2016;937:3–17. doi: 10.1007/978-3-319-42059-2_1. [DOI] [PubMed] [Google Scholar]
- 217.Chen L.-L. Linking long noncoding RNA localization and function. Trends Biochem. Sci. 2016;41:761–772. doi: 10.1016/j.tibs.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 218.Jarroux J., Morillon A., Pinskaya M. History, discovery, and classification of lncRNAs. Adv. Exp. Med. Biol. 2017;1008:1–46. doi: 10.1007/978-981-10-5203-3_1. [DOI] [PubMed] [Google Scholar]
- 219.Kashi K., Henderson L., Bonetti A., Carninci P. Discovery and functional analysis of lncRNAs: Methodologies to investigate an uncharacterized transcriptome. Biochim. Biophys. Acta. 2016;1859:3–15. doi: 10.1016/j.bbagrm.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 220.Spurlock C.F., 3rd, Crooke P.S., 3rd, Aune T.M. Biogenesis and transcriptional regulation of long noncoding RNAs in the human immune system. J. Immunol. 2016;197:4509–4517. doi: 10.4049/jimmunol.1600970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Clark M.B., Amaral P.P., Schlesinger F.J., Dinger M.E., Taft R.J., Rinn J.L., Ponting C.P., Stadler P.F., Morris K.V., Morillon A. The reality of pervasive transcription. PLoS Biol. 2011;9:e1000625. doi: 10.1371/journal.pbio.1000625. discussion e1001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Hangauer M.J., Vaughn I.W., McManus M.T. Pervasive transcription of the human genome produces thousands of previously unidentified long intergenic noncoding RNAs. PLoS Genet. 2013;9:e1003569. doi: 10.1371/journal.pgen.1003569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Yu A.D., Wang Z., Morris K.V. Long noncoding RNAs: a potent source of regulation in immunity and disease. Immunol. Cell Biol. 2015;93:277–283. doi: 10.1038/icb.2015.2. [DOI] [PubMed] [Google Scholar]
- 224.Khachane A.N., Harrison P.M. Mining mammalian transcript data for functional long non-coding RNAs. PLoS ONE. 2010;5:e10316. doi: 10.1371/journal.pone.0010316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Moran V.A., Perera R.J., Khalil A.M. Emerging functional and mechanistic paradigms of mammalian long non-coding RNAs. Nucleic Acids Res. 2012;40:6391–6400. doi: 10.1093/nar/gks296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Aune T.M., Crooke P.S., 3rd, Spurlock C.F., 3rd Long noncoding RNAs in T lymphocytes. J. Leukoc. Biol. 2016;99:31–44. doi: 10.1189/jlb.1RI0815-389R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Mattick J.S., Rinn J.L. Discovery and annotation of long noncoding RNAs. Nat. Struct. Mol. Biol. 2015;22:5–7. doi: 10.1038/nsmb.2942. [DOI] [PubMed] [Google Scholar]
- 228.Guttman M., Rinn J.L. Modular regulatory principles of large non-coding RNAs. Nature. 2012;482:339–346. doi: 10.1038/nature10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lam M.T., Li W., Rosenfeld M.G., Glass C.K. Enhancer RNAs and regulated transcriptional programs. Trends Biochem. Sci. 2014;39:170–182. doi: 10.1016/j.tibs.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Jonas K., Calin G.A., Pichler M. RNA-Binding Proteins as Important Regulators of Long Non-Coding RNAs in Cancer. Int. J. Mol. Sci. 2020;21:2969. doi: 10.3390/ijms21082969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ito K.K., Watanabe K., Kitagawa D. The Emerging Role of ncRNAs and RNA-Binding Proteins in Mitotic Apparatus Formation. Noncoding RNA. 2020;6:13. doi: 10.3390/ncrna6010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Cabili M.N., Dunagin M.C., McClanahan P.D., Biaesch A., Padovan-Merhar O., Regev A., Rinn J.L., Raj A. Localization and abundance analysis of human lncRNAs at single-cell and single-molecule resolution. Genome Biol. 2015;16:20. doi: 10.1186/s13059-015-0586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Hombach S., Kretz M. Non-coding RNAs: Classification, Biology and Functioning. Adv. Exp. Med. Biol. 2016;937:3–17. doi: 10.1007/978-3-319-42059-2_1. [DOI] [PubMed] [Google Scholar]
- 234.Naganuma T., Hirose T. Paraspeckle formation during the biogenesis of long non-coding RNAs. RNA Biol. 2013;10:456–461. doi: 10.4161/rna.23547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Quinodoz S., Guttman M. Long noncoding RNAs: an emerging link between gene regulation and nuclear organization. Trends Cell Biol. 2014;24:651–663. doi: 10.1016/j.tcb.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Tay Y., Rinn J., Pandolfi P.P. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505:344–352. doi: 10.1038/nature12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Guo L.-L., Song C.-H., Wang P., Dai L.-P., Zhang J.-Y., Wang K.-J. Competing endogenous RNA networks and gastric cancer. World J. Gastroenterol. 2015;21:11680–11687. doi: 10.3748/wjg.v21.i41.11680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Denzler R., Agarwal V., Stefano J., Bartel D.P., Stoffel M. Assessing the ceRNA hypothesis with quantitative measurements of miRNA and target abundance. Mol. Cell. 2014;54:766–776. doi: 10.1016/j.molcel.2014.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Yang A., Shao T.-J., Bofill-De Ros X., Lian C., Villanueva P., Dai L., Gu S. AGO-bound mature miRNAs are oligouridylated by TUTs and subsequently degraded by DIS3L2. Nat. Commun. 2020;11:2765. doi: 10.1038/s41467-020-16533-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Sheu-Gruttadauria J., Pawlica P., Klum S.M., Wang S., Yario T.A., Schirle Oakdale N.T., Steitz J.A., MacRae I.J. Structural Basis for Target-Directed MicroRNA Degradation. Mol. Cell. 2019;75:1243–1255.e7. doi: 10.1016/j.molcel.2019.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Fuchs Wightman F., Giono L.E., Fededa J.P., de la Mata M. Target RNAs Strike Back on MicroRNAs. Front. Genet. 2018;9:435. doi: 10.3389/fgene.2018.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.de la Mata M., Gaidatzis D., Vitanescu M., Stadler M.B., Wentzel C., Scheiffele P., Filipowicz W., Großhans H. Potent degradation of neuronal miRNAs induced by highly complementary targets. EMBO Rep. 2015;16:500–511. doi: 10.15252/embr.201540078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kleaveland B., Shi C.Y., Stefano J., Bartel D.P. A Network of Noncoding Regulatory RNAs Acts in the Mammalian Brain. Cell. 2018;174:350–362.e17. doi: 10.1016/j.cell.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Bonasio R., Shiekhattar R. Regulation of transcription by long noncoding RNAs. Annu. Rev. Genet. 2014;48:433–455. doi: 10.1146/annurev-genet-120213-092323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Xu Y., Wu W., Han Q., Wang Y., Li C., Zhang P., Xu H. 2019. New Insights into the Interplay between Non-Coding RNAs and RNA-Binding Protein HnRNPK in Regulating Cellular Functions Cells 8, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Ferrè F., Colantoni A., Helmer-Citterich M. Revealing protein-lncRNA interaction. Brief. Bioinform. 2016;17:106–116. doi: 10.1093/bib/bbv031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Yoon J.H., Abdelmohsen K., Kim J., Yang X., Martindale J.L., Tominaga-Yamanaka K., White E.J., Orjalo A.V., Rinn J.L., Kreft S.G. Scaffold function of long non-coding RNA HOTAIR in protein ubiquitination. Nat. Commun. 2013;4:2939. doi: 10.1038/ncomms3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Chang L., Yuan Y., Li C., Guo T., Qi H., Xiao Y., Dong X., Liu Z., Liu Q. Upregulation of SNHG6 regulates ZEB1 expression by competitively binding miR-101-3p and interacting with UPF1 in hepatocellular carcinoma. Cancer Lett. 2016;383:183–194. doi: 10.1016/j.canlet.2016.09.034. [DOI] [PubMed] [Google Scholar]
- 249.Wang X., Lai Q., He J., Li Q., Ding J., Lan Z., Gu C., Yan Q., Fang Y., Zhao X., Liu S. LncRNA SNHG6 promotes proliferation, invasion and migration in colorectal cancer cells by activating TGF-β/Smad signaling pathway via targeting UPF1 and inducing EMT via regulation of ZEB1. Int. J. Med. Sci. 2019;16:51–59. doi: 10.7150/ijms.27359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Zhu Y., Xing Y., Chi F., Sun W., Zhang Z., Piao D. Long noncoding RNA SNHG6 promotes the progression of colorectal cancer through sponging miR-760 and activation of FOXC1. OncoTargets Ther. 2018;11:5743–5752. doi: 10.2147/OTT.S170246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Liang R., Xiao G., Wang M., Li X., Li Y., Hui Z., Sun X., Qin S., Zhang B., Du N. SNHG6 functions as a competing endogenous RNA to regulate E2F7 expression by sponging miR-26a-5p in lung adenocarcinoma. Biomed. Pharmacother. 2018;107:1434–1446. doi: 10.1016/j.biopha.2018.08.099. [DOI] [PubMed] [Google Scholar]
- 252.Lv P., Qiu X., Gu Y., Yang X., Xu X., Yang Y. Long non-coding RNA SNHG6 enhances cell proliferation, migration and invasion by regulating miR-26a-5p/MAPK6 in breast cancer. Biomed. Pharmacother. 2019;110:294–301. doi: 10.1016/j.biopha.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 253.Wang H., Wang L., Tang L., Luo J., Ji H., Zhang W., Zhou J., Li Q., Miao L. Long noncoding RNA SNHG6 promotes proliferation and angiogenesis of cholangiocarcinoma cells through sponging miR-101-3p and activation of E2F8. J. Cancer. 2020;11:3002–3012. doi: 10.7150/jca.40592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Sipes J.M., Murphy-Ullrich J.E., Roberts D.D. Thrombospondins: Purification of human platelet thrombospondin-1. Methods Cell Biol. 2018;143:347–369. doi: 10.1016/bs.mcb.2017.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Kumar M.M., Goyal R. LncRNA as a therapeutic target for angiogenesis. Curr. Top. Med. Chem. 2017;17:1750–1757. doi: 10.2174/156802661766616111644744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Zaslavsky A., Baek K.-H., Lynch R.C., Short S., Grillo J., Folkman J., Italiano J.E., Jr., Ryeom S. Platelet-derived thrombospondin-1 is a critical negative regulator and potential biomarker of angiogenesis. Blood. 2010;115:4605–4613. doi: 10.1182/blood-2009-09-242065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Schadler K.L., Crosby E.J., Zhou A.Y., Bhang D.H., Braunstein L., Baek K.H., Crawford D., Crawford A., Angelosanto J., Wherry E.J., Ryeom S. Immunosurveillance by antiangiogenesis: tumor growth arrest by T cell-derived thrombospondin-1. Cancer Res. 2014;74:2171–2181. doi: 10.1158/0008-5472.CAN-13-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Wang W., Chen G., Wang B., Yuan Z., Liu G., Niu B., Chen Y., Zhou S., He J., Xue H. Long non-coding RNA BZRAP1-AS1 silencing suppresses tumor angiogenesis in hepatocellular carcinoma by mediating THBS1 methylation. J. Transl. Med. 2019;17:421. doi: 10.1186/s12967-019-02145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Guo X., Yang Z., Zhi Q., Wang D., Guo L., Li G., Miao R., Shi Y., Kuang Y. Long noncoding RNA OR3A4 promotes metastasis and tumorigenicity in gastric cancer. Oncotarget. 2016;7:30276–30294. doi: 10.18632/oncotarget.7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Liu G., Hu X., Zhou G. Long non-coding RNA OR3A4 promotes proliferation and migration in breast cancer. Biomed. Pharmacother. 2017;96:426–433. doi: 10.1016/j.biopha.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 261.Li W., Fu Q., Man W., Guo H., Yang P. LncRNA OR3A4 participates in the angiogenesis of hepatocellular carcinoma through modulating AGGF1/akt/mTOR pathway. Eur. J. Pharmacol. 2019;849:106–114. doi: 10.1016/j.ejphar.2019.01.049. [DOI] [PubMed] [Google Scholar]
- 262.Seiz L., Kotzsch M., Grebenchtchikov N.I., Geurts-Moespot A.J., Fuessel S., Goettig P., Gkazepis A., Wirth M.P., Schmitt M., Lossnitzer A. Polyclonal antibodies against kallikrein-related peptidase 4 (KLK4): immunohistochemical assessment of KLK4 expression in healthy tissues and prostate cancer. Biol. Chem. 2010;391:391–401. doi: 10.1515/BC.2010.033. [DOI] [PubMed] [Google Scholar]
- 263.Dong Y., Stephens C., Walpole C., Swedberg J.E., Boyle G.M., Parsons P.G., McGuckin M.A., Harris J.M., Clements J.A. Paclitaxel resistance and multicellular spheroid formation are induced by kallikrein-related peptidase 4 in serous ovarian cancer cells in an ascites mimicking microenvironment. PLoS ONE. 2013;8:e57056. doi: 10.1371/journal.pone.0057056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Wilkinson R., Woods K., D’Rozario R., Prue R., Vari F., Hardy M.Y., Dong Y., Clements J.A., Hart D.N.J., Radford K.J. Human kallikrein 4 signal peptide induces cytotoxic T cell responses in healthy donors and prostate cancer patients. Cancer Immunol. Immunother. 2012;61:169–179. doi: 10.1007/s00262-011-1095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Cui Z., Cui Y., Yang S., Luo G., Wang Y., Lou Y., Sun X. KLK4 silencing inhibits the growth of oral squamous cell carcinoma through Wnt/β-catenin signaling pathway. Cell Biol. Int. 2017;41:392–404. doi: 10.1002/cbin.10736. [DOI] [PubMed] [Google Scholar]
- 266.Tang L., Wen J.-B., Wen P., Li X., Gong M., Li Q. Long non-coding RNA LINC01314 represses cell migration, invasion, and angiogenesis in gastric cancer via the Wnt/β-catenin signaling pathway by down-regulating KLK4. Cancer Cell Int. 2019;19:94. doi: 10.1186/s12935-019-0799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Wu J., Meng X., Jia Y., Chai J., Wang J., Xue X., Dang T. Long non-coding RNA HNF1A-AS1 upregulates OTX1 to enhance angiogenesis in colon cancer via the binding of transcription factor PBX3. Exp. Cell Res. 2020;393:112025. doi: 10.1016/j.yexcr.2020.112025. [DOI] [PubMed] [Google Scholar]
- 268.Zhang L.M., Wang P., Liu X.M., Zhang Y.J. LncRNA SUMO1P3 drives colon cancer growth, metastasis and angiogenesis. Am. J. Transl. Res. 2017;9:5461–5472. [PMC free article] [PubMed] [Google Scholar]
- 269.Kondo A., Nonaka A., Shimamura T., Yamamoto S., Yoshida T., Kodama T., Aburatani H., Osawa T. Long Noncoding RNA JHDM1D-AS1 Promotes Tumor Growth by Regulating Angiogenesis in Response to Nutrient Starvation. Mol. Cell. Biol. 2017;37:e00125-17. doi: 10.1128/MCB.00125-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Zhao J., Du P., Cui P., Qin Y., Hu C., Wu J., Zhou Z., Zhang W., Qin L., Huang G. LncRNA PVT1 promotes angiogenesis via activating the STAT3/VEGFA axis in gastric cancer. Oncogene. 2018;37:4094–4109. doi: 10.1038/s41388-018-0250-z. [DOI] [PubMed] [Google Scholar]
- 271.Gao J., Yin X., Yu X., Dai C., Zhou F. Long noncoding RNA LINC00488 functions as a ceRNA to regulate hepatocellular carcinoma cell growth and angiogenesis through miR-330-5. Dig. Liver Dis. 2019;51:1050–1059. doi: 10.1016/j.dld.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 272.Lin J., Cao S., Wang Y., Hu Y., Liu H., Li J., Chen J., Li P., Liu J., Wang Q., Zheng L. Long non-coding RNA UBE2CP3 enhances HCC cell secretion of VEGFA and promotes angiogenesis by activating ERK1/2/HIF-1α/VEGFA signalling in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2018;37:113. doi: 10.1186/s13046-018-0727-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Yuan S.X., Yang F., Yang Y., Tao Q.F., Zhang J., Huang G., Yang Y., Wang R.Y., Yang S., Huo X.S. Long noncoding RNA associated with microvascular invasion in hepatocellular carcinoma promotes angiogenesis and serves as a predictor for hepatocellular carcinoma patients’ poor recurrence-free survival after hepatectomy. Hepatology. 2012;56:2231–2241. doi: 10.1002/hep.25895. [DOI] [PubMed] [Google Scholar]
- 274.Hou Z.H., Xu X.W., Fu X.Y., Zhou L.D., Liu S.P., Tan D.M. Long non-coding RNA MALAT1 promotes angiogenesis and immunosuppressive properties of HCC cells by sponging miR-140. Am. J. Physiol. Cell Physiol. 2020;318:C649–C663. doi: 10.1152/ajpcell.00510.2018. [DOI] [PubMed] [Google Scholar]
- 275.Jeck W.R., Sorrentino J.A., Wang K., Slevin M.K., Burd C.E., Liu J., Marzluff W.F., Sharpless N.E. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19:141–157. doi: 10.1261/rna.035667.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Zhang Y., Zhang X.-O., Chen T., Xiang J.-F., Yin Q.-F., Xing Y.-H., Zhu S., Yang L., Chen L.L. Circular intronic long noncoding RNAs. Mol. Cell. 2013;51:792–806. doi: 10.1016/j.molcel.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 277.Zhang X.-O., Wang H.-B., Zhang Y., Lu X., Chen L.-L., Yang L. Complementary sequence-mediated exon circularization. Cell. 2014;159:134–147. doi: 10.1016/j.cell.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 278.Zhang Y., Xue W., Li X., Zhang J., Chen S., Zhang J.L., Yang L., Chen L.L. The biogenesis of nascent circular RNAs. Cell Rep. 2016;15:611–624. doi: 10.1016/j.celrep.2016.03.058. [DOI] [PubMed] [Google Scholar]
- 279.Liang D., Wilusz J.E. Short intronic repeat sequences facilitate circular RNA production. Genes Dev. 2014;28:2233–2247. doi: 10.1101/gad.251926.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Conn S.J., Pillman K.A., Toubia J., Conn V.M., Salmanidis M., Phillips C.A., Roslan S., Schreiber A.W., Gregory P.A., Goodall G.J. The RNA binding protein quaking regulates formation of circRNAs. Cell. 2015;160:1125–1134. doi: 10.1016/j.cell.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 281.Aktaş T., Avşar Ilık İ., Maticzka D., Bhardwaj V., Pessoa Rodrigues C., Mittler G., Manke T., Backofen R., Akhtar A. DHX9 suppresses RNA processing defects originating from the Alu invasion of the human genome. Nature. 2017;544:115–119. doi: 10.1038/nature21715. [DOI] [PubMed] [Google Scholar]
- 282.Ashwal-Fluss R., Meyer M., Pamudurti N.R., Ivanov A., Bartok O., Hanan M., Evantal N., Memczak S., Rajewsky N., Kadener S. circRNA biogenesis competes with pre-mRNA splicing. Mol. Cell. 2014;56:55–66. doi: 10.1016/j.molcel.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 283.Tang C., Xie Y., Yu T., Liu N., Wang Z., Woolsey R.J., Tang Y., Zhang X., Qin W., Zhang Y. m6A-dependent biogenesis of circular RNAs in male germ cells. Cell Res. 2020;30:211–228. doi: 10.1038/s41422-020-0279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Panda A.C., Grammatikakis I., Munk R., Gorospe M., Abdelmohsen K. Emerging roles and context of circular RNAs. Wiley Interdiscip. Rev. RNA. 2017;8:e1386. doi: 10.1002/wrna.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Zhang Q., Jin X.S., Yang Z.Y., Wei M., Liu B.Y., Gu Q.L. Upregulated Hoxc6 expression is associated with poor survival in gastric cancer patients. Neoplasma. 2013;60:439–445. doi: 10.4149/neo_2013_057. [DOI] [PubMed] [Google Scholar]
- 286.Zhou J., Yang X., Song P., Wang H., Wang X. HOXC6 in the prognosis of prostate cancer. Artif. Cells Nanomed. Biotechnol. 2019;47:2715–2720. doi: 10.1080/21691401.2019.1635136. [DOI] [PubMed] [Google Scholar]
- 287.Shi H., Li H., Zhen T., Dong Y., Pei X., Zhang X. hsa_circ_001653 Implicates in the Development of Pancreatic Ductal Adenocarcinoma by Regulating MicroRNA-377-Mediated HOXC6 Axis. Mol. Ther. Nucleic Acids. 2020;20:252–264. doi: 10.1016/j.omtn.2019.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 288.Varisli L., Gonen-Korkmaz C., Debelec-Butuner B., Erbaykent-Tepedelen B., Muhammed H.S., Bogurcu N., Saatcioglu F., Korkmaz K.S. Ubiquitously expressed hematological and neurological expressed 1 downregulates Akt-mediated GSK3β signaling, and its knockdown results in deregulated G2/M transition in prostate cells. DNA Cell Biol. 2011;30:419–429. doi: 10.1089/dna.2010.1128. [DOI] [PubMed] [Google Scholar]
- 289.Li L., Zeng T.-T., Zhang B.-Z., Li Y., Zhu Y.-H., Guan X.-Y. Overexpression of HN1L promotes cell malignant proliferation in non-small cell lung cancer. Cancer Biol. Ther. 2017;18:904–915. doi: 10.1080/15384047.2017.1385678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Chen J.J., Sun X., Mao Q.Q., Jiang X.Y., Zhao X.G., Xu W.J., Zhong L. Increased expression of hematological and neurological expressed 1 (HN1) is associated with a poor prognosis of hepatocellular carcinoma and its knockdown inhibits cell growth and migration partly by down-regulation of c-Met. Kaohsiung J. Med. Sci. 2020;36:196–205. doi: 10.1002/kjm2.12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Pu J., Wang J., Li W., Lu Y., Wu X., Long X., Luo C., Wei H. hsa_circ_0000092 promotes hepatocellular carcinoma progression through up-regulating HN1 expression by binding to microRNA-338-3p. J. Cell. Mol. Med. 2020 doi: 10.1111/jcmm.15010. Published online February 20, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Huang X.Y., Huang Z.L., Huang J., Xu B., Huang X.Y., Xu Y.H., Zhou J., Tang Z.Y. Exosomal circRNA-100338 promotes hepatocellular carcinoma metastasis via enhancing invasiveness and angiogenesis. J. Exp. Clin. Cancer Res. 2020;39:20. doi: 10.1186/s13046-020-1529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Yu Y.X., Ge T.W., Zhang P. Circular RNA circGFRA1 promotes angiogenesis, cell proliferation and migration of hepatocellular carcinoma by combining with miR-149. Eur. Rev. Med. Pharmacol. Sci. 2020;24:11058–11064. doi: 10.26355/eurrev_202011_23591. [DOI] [PubMed] [Google Scholar]
- 294.Girardi E., López P., Pfeffer S. On the Importance of Host MicroRNAs During Viral Infection. Front. Genet. 2018;9:439. doi: 10.3389/fgene.2018.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Chen G., Wang Z., Wang D., Qiu C., Liu M., Chen X., Zhang Q., Yan G., Cui Q. LncRNADisease: a database for long-non-coding RNA-associated diseases. Nucleic Acids Res. 2013;41:D983–D986. doi: 10.1093/nar/gks1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Wang J., Zhang X., Chen W., Li J., Liu C. CRlncRNA: a manually curated database of cancer-related long non-coding RNAs with experimental proof of functions on clinicopathological and molecular features. BMC Med. Genomics. 2018;11(Suppl 6):114. doi: 10.1186/s12920-018-0430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Gao Y., Wang P., Wang Y., Ma X., Zhi H., Zhou D., Li X., Fang Y., Shen W., Xu Y. Lnc2Cancer v2.0: updated database of experimentally supported long non-coding RNAs in human cancers. Nucleic Acids Res. 2019;47(D1):D1028–D1033. doi: 10.1093/nar/gky1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Hu G., Drescher K.M., Chen X.M. Exosomal miRNAs: Biological Properties and Therapeutic Potential. Front. Genet. 2012;3:56. doi: 10.3389/fgene.2012.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Wei L., Sun J., Zhang N., Zheng Y., Wang X., Lv L., Liu J., Xu Y., Shen Y., Yang M. Noncoding RNAs in gastric cancer: implications for drug resistance. Mol. Cancer. 2020;19:62. doi: 10.1186/s12943-020-01185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Pourhanifeh M.H., Mahjoubin-Tehran M., Shafiee A., Hajighadimi S., Moradizarmehri S., Mirzaei H., Asemi Z. MicroRNAs and exosomes: Small molecules with big actions in multiple myeloma pathogenesis. IUBMB Life. 2020;72:314–333. doi: 10.1002/iub.2211. [DOI] [PubMed] [Google Scholar]
- 301.Liu Y., Wang J. Therapeutic Potentials of Noncoding RNAs: Targeted Delivery of ncRNAs in Cancer Cells. Adv. Exp. Med. Biol. 2016;927:429–458. doi: 10.1007/978-981-10-1498-7_16. [DOI] [PubMed] [Google Scholar]
- 302.Segal M., Slack F.J. Challenges identifying efficacious miRNA therapeutics for cancer. Expert Opin. Drug Discov. 2020;15:987–992. doi: 10.1080/17460441.2020.1765770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Ning B., Yu D., Yu A.-M. Advances and challenges in studying noncoding RNA regulation of drug metabolism and development of RNA therapeutics. Biochem. Pharmacol. 2019;169:113638. doi: 10.1016/j.bcp.2019.113638. [DOI] [PMC free article] [PubMed] [Google Scholar]