Abstract
Background:
Bedaquiline has not been extensively studied among HIV drug-resistant tuberculosis (DR-TB) co-infected patients. We compared treatment outcomes in DR-TB patients treated with bedaquiline- and linezolid-containing regimens to historic controls treated with second-line injectable-containing regimens.
Methods:
Retrospective cohort study of consecutive DR-TB patients initiated on bedaquiline- and linezolid-containing regimens at a TB referral hospital in KwaZulu-Natal, South Africa. Participants were prospectively followed through 24 months for treatment outcome, and adverse events. Outcomes were compared to a historic control cohort of DR-TB HIV patients enrolled at the same facility prior to bedaquiline introduction.
Results:
Adult DR-TB patients initiating bedaquiline between January 2014 and November 2015 were enrolled (N=151). The majority of patients were female (52%), HIV co-infected (77%) and on antiretroviral therapy (100%). End of treatment outcomes included cure (63%), TB culture conversion (83%), completion (0.7%), loss-to-follow-up (15%), treatment failure (5%), and death (17%). Compared to historic controls (N=105), patients treated with bedaquiline experienced significantly higher TB culture conversion and cure, with significantly lower mortality. Adverse effects were common (92%) and most frequently attributed to linezolid (24.1%). QT segment prolongation was common but without clinical sequelae.
Conclusion:
Treatment with bedaquiline- and linezolid-containing regimens was associated with improved treatment outcomes and survival in DR-TB HIV patients.
Keywords: Bedaquiline, linezolid, drug resistant tuberculosis, HIV
INTRODUCTION
Although global tuberculosis (TB) control is slowly improving, drug-resistant TB (DR-TB) remains unacceptably high. In 2018, there were approximately 484,000 rifampin-resistant TB cases worldwide, of which ~378,000 were multi-drug resistant TB (MDR-TB), defined as resistance to at least rifampicin and isoniazid.1 In a patient-level meta-analysis, aggregate MDR-TB cure rates were 61% with 14% mortality and outcomes were substantially worse among patients with HIV co-infection.2
Bedaquiline, the first new antimycobacterial in 40 years, was approved in 2012 for treatment of DR-TB.3 Initial in vivo studies showed sterilizing capacity of bedaquiline similar to isoniazid, one of the most potent antimycobacterial drugs and subsequent phase II/III clinical trials showed exciting efficacy and effectiveness in the treatment of MDR-TB.4–7 Linezolid, a repurposed oxazolidinone, was recently categorized as a Group A antimycobacterial drug by the World Health Organization (WHO) for treatment of MDR‐TB and extensively drug‐resistant tuberculosis (XDR‐TB).8 Two randomized controlled trials demonstrated increased TB culture conversion rates and cure with linezolid but participants experienced substantial adverse events and frequent medication discontinuation.9, 10
The majority of DR-TB patients, in South Africa, are HIV co-infected (~70%).1 In 2012, the South African Department of Health created the Bedaquiline Clinical Access Programme (BCAP) for DR-TB patients.11 To characterize adverse effects, treatment outcomes, and overall survival in the context of HIV, we performed a retrospective cohort study of consecutively enrolled DR-TB patients initiated on bedaquiline- and linezolid-containing regimens [(Bedaquiline and Linezolid in XDR-TB) BLIX cohort]. We compared the BLIX cohort to a historic control cohort of DR-TB patients from the same facility enrolled prior to the introduction of bedaquiline.12 We hypothesized that bedaquiline- and linezolid-containing treatment regimens would improve treatment outcomes and mortality and that treatment outcomes would not be modified by HIV status.
METHODS
Participants
Consecutive DR-TB patients initiating bedaquiline- and linezolid-containing treatment regimens for DR-TB (BLIX cohort) were retrospectively enrolled at a TB referral hospital in KwaZulu-Natal, South Africa from January 2014 to November 2015 and prospectively followed for 24 months or until a final treatment outcome could be assigned, unless death or loss to retention in care occurred. Eligible patients were ≥ 18 years of age, had not been previously treated with bedaquiline, nor in receipt of clofazimine in the previous 60 days due to concerns of cross-resistance, were diagnosed with active pulmonary TB disease according to South African/WHO guidelines,13 and had culture-proven DR-TB with isolates showing resistance to at least isoniazid, rifampin, and either fluoroquinolone antibiotics or second-line injectables. Indications for bedaquiline referral included: (1) pre-XDR-TB (resistance to isoniazid, rifampin, and either fluoroquinolone antibiotics or second-line injectables) or XDR-TB (resistance to isoniazid, rifampin, fluoroquinolone antibiotics and a second-line injectable); (2) MDR-TB with poor response to treatment despite good reported medication adherence; or (3) MDR-TB with adverse events attributed to one or more of the drugs in the standard MDR-TB regimen, although only patients with pre-XDR and XDR-TB were included in the BLIX cohort. Bedaquiline was initiated through the BCAP. While awaiting approval though the committee, patients were initiated on standardized second-line treatment in the interim. All patients were admitted to the facility for approximately two weeks during which bedaquiline initiation was monitored. Thereafter patients were followed up as either inpatients or outpatients. All management decisions were made by hospital clinical staff. Bedaquiline was administered at a dose of 400 mg once daily for two weeks, followed by 200 mg three times per week for 22 weeks in combination with clinician-selected background regimen. Linezolid was administered at 600 mg once daily; unless poorly tolerated, then dose reduced to 300 mg.13 Clinical, demographic and adverse event data were collected retrospectively, and treatment outcomes assigned according to standard definitions at 24 months after treatment initiation.
As a comparator, we used a DR-TB cohort previously enrolled at the same hospital from August 2009 to July 2011, prior to the introduction of bedaquiline (Historical control cohort).12 Eligibility and enrolment procedures for this comparator cohort have been previously described. In this prospective cohort, all patients had culture-proven DR-TB with isolates showing resistance to at least isoniazid, rifampin, fluoroquinolone antibiotics and second-line injectables. Eligibility criteria was otherwise identical in both groups. All comparator group patients received standardized DR-TB treatment regimens and all management decisions were made by hospital clinical staff.
Clinical Outcomes
Updated WHO drug-resistant TB treatment outcome definitions were used to assess treatment outcome at the end of therapy.14 Culture conversion was defined as two consecutive negative sputum culture results taken at least 26 days apart. Unfavorable outcomes included death, treatment failure, and loss of retention in care defined as treatment interruption ≥2 months.14 Patients in the bedaquiline group had baseline and monthly ECGs while receiving bedaquiline and QTcF values were calculated for each ECG. All QT values were corrected using Fridericia’s formula, and standard definitions were used for QTcF prolongation and clinically relevant prolongation.15, 16 Treatment outcomes were defined at 24 months (cure, completion, failure) or at time of occurrence (loss to retention, death).14
Laboratory Methods
Sputum for microscopy and MTB (Mycobacterium tuberculosis) culture was obtained monthly as is routine clinical practice. MTB culture positivity was determined using the BACTEC MGIT 960 fluorometric system (Becton Dickinson Diagnostics, Sparks, MD, USA). Drug susceptibility testing for first-line and second-line drugs was performed at a regional TB reference laboratory in Durban, South Africa. Drug susceptibility testing for isoniazid, rifampin, ethambutol, streptomycin, ethionamide, ofloxacin, and kanamycin was by the modified proportional growth method using standard techniques.17, 18
Statistical Analysis
Baseline characteristics were summarized using descriptive statistics expressed as medians with interquartile ranges (IQR) for continuous variables and proportions for categorical variables. Associations between categorical variables were compared using the Fisher’s exact test, while the Wilcoxon-Mann-Whitney test was used to compare two medians. Time to culture conversion was calculated as the period from initiation of bedaquiline to the date of the first negative after two consecutive negatives at least 26 days apart. Those who did not culture convert were censored at their last visit or at TB treatment outcome. Survival analysis for mortality was calculated from time of bedaquiline initiation until time of death or censoring. Kaplan-Meier curves were stratified by cohort and by risk factors for mortality. Tests between strata were assessed using the log-rank test. Cox proportional hazards models were used to estimate hazards ratios (HR) and 95% confidence intervals (CIs) for time to mortality and culture conversion between the two groups. Univariable and multivariable logistic regression was used to identify independent risk factors associated with an unfavourable outcome. Odds ratios and hazard ratios were adjusted for age, gender, HIV status, type of TB, and history of any previous TB. Statistical analysis was performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Ethical approval
The study protocol was approved through University of KwaZulu-Natal (BE 341/15) and Columbia University, NY.
RESULTS
Patient enrolment
194 DR-TB patients were referred for initiation of bedaquiline and/or linezolid during the study period (January 2014 to November 2015); 6 were excluded for not meeting drug-resistance eligibility criteria; 5 were excluded because they initiated bedaquiline but not linezolid; and 38 were excluded because they did not have evaluable clinical or follow-up data. The remaining 151 patients (BLIX cohort) were included in the study and followed prospectively through end of treatment using routine data including clinical notes and microbiologic data.
Historical controls were enrolled from August 2009 to July 2011 as previously described.12 110 patients presented at the same facility with DR-TB for initiation of treatment; 5 did not meet study eligibility criteria (one was incarcerated and ineligible by protocol, three did not meet eligibility criteria, and one was lost to initial treatment). The remaining 105 patients were enrolled and outcome data collected.
Patient Characteristics
Demographic and clinical characteristics were similar between the 2 groups (Table 1). Patients were mostly young adults; mostly female (52.3% vs. 53.3%), TB treatment-experienced, and HIV co-infected (76.8% vs. 76.2%) in the BLIX and Historical control cohorts, respectively. In the BLIX cohort all patients were on antiretroviral therapy (ART) compared with 82.5% in the Historical control cohort. In the BLIX cohort the median CD4 count was 365 cells/mm3 (IQR 199–535) compared to 192 cells/mm3 (IQR 117–390) in the Historical control cohort. ART regimen included lamivudine (89.8%), nevirapine (92.3%), and tenofovir (84.7%) in the BLIX cohort (to avoid drug-drug interactions between efavirenz and bedaquiline) while the primary ART in the Historical control cohort was efavirenz, emtricitabine, and tenofovir.
Table 1:
Patient Characteristics
| Characteristics | BLIX cohort N=151 | Historical control cohort N=105 |
|---|---|---|
| Median age, years (IQR) | 33 (27–41) | 35 (27–43) |
| Gender, n (%) | ||
| Male | 72 (47.7) | 49 (46.7) |
| Female | 79 (52.3) | 56 (53.3) |
| Drug resistance pattern, n (%) | ||
| Pre-XDR-TB | 45 (29.8) | 0 |
| XDR-TB | 106 (70.2) | 105 (100.0) |
| HIV Status, n (%) | ||
| Positive | 116 (76.8) | 80 (76.2) |
| Negative | 35 (23.2) | 25 (23.8) |
| ART if HIV-infected, n (%) | ||
| Yes | 116 (76.8) | 66 (82.5) |
| No | - | 14 (17.5) |
| Median CD4 count | 365 (199–535)a | 192 (117–390)b |
| Cotrimoxazole, n (%) | ||
| Yes | 14/116 (12.1) | 40 (50.6) |
| No | 44/116 (37.9) | 6 (7.6) |
| Unknown/not documented | 59/116 (50.9) | 33 (41.8) |
| Previous TB, n (%) | ||
| Yes | 109 (72.2) | 101 (96.2) |
| No | 42 (27.8) | 4 (3.8) |
| Previous MDR-TB, n (%) | ||
| Yes | 46/109 (42.2) | 61/101 (60.4) |
| No | 63/109 (57.8) | 44 (43.6) |
| Cavitary lesions, n (%) | ||
| Yes | 96/144 (66.7) | 29/103 (28.2) |
| No | 48/144 (33.3) | 74/103 (71.8) |
| Median weight (kg) (IQR) | 53.4 (47.7–60.3)c | 49.9 (43.6–56.9)d |
N=82
N=77
N=136
N=104
The proportion of patients with cavitary lesions on chest radiograph was more than two-fold greater in the BLIX cohort (66.7% vs. 28.2%). Most patients in both groups had a previous episode of TB, although this was greater in the Historical control cohort (96.2% versus 72.2%). Commonly used antimycobacterials in the BLIX cohort were: bedaquiline (100%), linezolid (100%), moxifloxacin/levofloxacin (97.4%), para-aminosalicylic acid (96.7%), pyrazinamide (96.7%), clofazamine (92.7%), and terizidone/cycloserine (86.8%). In contrast, in the Historical control cohort, the most common medications included: capreomycin/kanamycin/amikacin (97.1%), moxifloxacin/levofloxacin (96.2%), pyrazinamide (96.2%), ethionamide (96.2%), para-aminosalicylic acid (90.5%), ethambutol (91.4%), terizidone/cycloserine (91.4%) and clofazimine (52.4%) (Table S1).
Culture Conversion
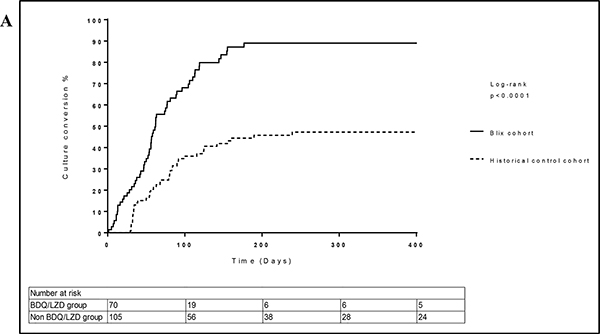
A higher proportion of the BLIX cohort achieved culture conversion compared to control patients (Table 3) (aHR 3.03, 95% CI 1.95–4.71, p<0.001). Kaplan-Meier curves for the two groups are shown in Figures 1a and 1c. Seventy BLIX patients (46.4%) initiated bedaquiline with a positive sputum culture, of whom 82.9% achieved culture conversion by the study endpoint of 24 months. The median time to culture conversion was 60 days (IQR 36–106). Amongst control group patients, 42.9% achieved culture conversion and the median time to conversion was 124 days (IQR 54–347). Importantly, patients in the BLIX cohort were also less likely than control group patients to be culture-positive at the time of hospital discharge (46.7% vs 22.7%).
Table 3:
Cox regression model for time to culture conversion (N= 175)
| Univariate | Multivariable | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | 95% Hazard Ratio Confidence | 95% Hazard Ratio Confidence | |||||||
| Hazard Ratio | Lower | Upper | P value | Adj Hazard Ratio | Lower | Upper | P value | ||
| Group | Non BDQ/LZD group | Ref | Ref | ||||||
| BDQ/LZD group | 3.14 | 2.11 | 4.69 | <0.001 | 3.03 | 1.95 | 4.71 | <0.001 | |
| Age | 1.01 | 0.99 | 1.03 | 0.469 | 1.01 | 0.99 | 1.03 | 0.298 | |
| Gender | Male | Ref | |||||||
| Female | 1.05 | 0.71 | 1.54 | 0.815 | 1.37 | 0.91 | 2.06 | 0.134 | |
| HIV Status | HIV non-infected | Ref | Ref | ||||||
| HIV-infected on ART | 0.80 | 0.051 | 1.23 | 0.304 | 0.65 | 0.41 | 1.03 | 0.068 | |
| HIV-infected not on ART | 0.28 | 0.09 | 0.92 | 0.036 | 0.32 | 0.10 | 1.09 | 0.069 | |
| Previous TB/MDR TB | MDR TB | Ref | Ref | ||||||
| Any TB (No MDR) | 1.70 | 1.12 | 2.58 | 0.013 | 1.59 | 1.02 | 2.46 | 0.039 | |
| No Previous TB | 2.02 | 1.11 | 3.70 | 0.022 | 1.18 | 0.62 | 2.24 | 0.621 | |
Figure 1a:
Kaplan Meier estimates of time to sputum culture conversion stratified by study group (N=175). Median time to culture conversion for BLIX cohort is 60 (36 – 106) days. Median time to culture conversion for Historical control cohort is 124 (54 – 347) days.
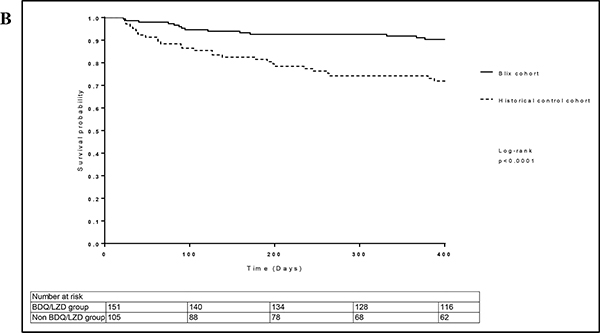
Figure 1c:
Kaplan Meier estimates of time to sputum culture conversion stratified by study group and HIV status (N=175).
Treatment Outcomes
The majority of BLIX cohort were cured (62.9%) by 24 months (Table 2). Fifty-five (36.4%) of patients in the BLIX cohort experienced an unfavourable outcome; 26 (17.2%) patients died and the median time to death was 349 days (IQR 87–473). The most common known cause of death in the BLIX cohort was respiratory failure (Table S2). Many patients were lost to care (14.6%), and there were no statistically significant predictors of treatment interruption. In the Historical control cohort, the commonest outcome at 24 months was death (41.0%) and 25.7% of patients were cured. There were 43 deaths by 24 months with a median time to death of 201 days (IQR 62 -502). The most common reported cause of death was respiratory failure (39.5% of deaths), with 17 unknown causes and 16.2% of patients interrupted treatment without medical approval (lost to retention in care). Treatment failure was reduced more than two-fold (4.6% vs 11.4%). However, only marginal improvements in retention in care were noted in our study following the addition of bedaquiline (14.6% vs 16.2%).
Table 2:
Primary Outcomes
| Outcomes | BLIX cohort N=151 n (%) | Historical control cohort N=105 n (%) |
|---|---|---|
| Treatment success | 96 (63.6) | 33 (31.4) |
| Cured | 95 (62.9) | 27 (25.7) |
| Completed treatment | 1 (0.7) | 6 (5.7) |
| Loss to retention in care | 22 (14.6) | 17 (16.2) |
| Treatment failure | 7 (4.6) | 12 (11.4) |
| Death* | 26 (17.2) | 43 (41.0) |
p<0.001
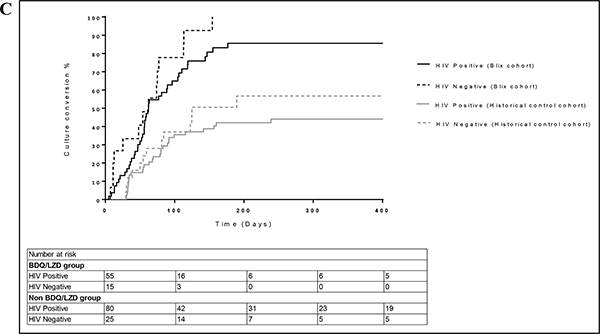
The risk of death was significantly lower in BLIX cohort compared with Historical control cohort (Table 4) (aHR 0.56, 95% CI 0.33–0.96, p=0.033). Kaplan-Meier curves for these groups are shown in Figures 1b and 1d. In multivariable analysis of all patients in the study (N=256), female gender was associated with lower odds of an unfavourable outcome (Table S3) (aOR 0.49, 95% CI 0.27–0.86, p=0.014) and patients with no previous history of TB had a significantly lower odds of having an unfavourable outcome (aOR 0.39, 95% CI 0.17–0.90, p=0.027). Cox regression with all patients showed an increased risk of death amongst HIV-infected patients not on ART (Table 4) (aHR 4.37, 95% CI 1.62–11.74, p=0.004) and patients with a previous history of TB had a lower risk of death (aHR 0.49, 95% CI 0.28–0.84, p=0.020).
Table 4:
Cox regression model for time to death (N=256)
| Univariate | Multivariable | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | 95% Hazard Ratio Confidence | 95% Hazard Ratio Confidence | |||||||
| Hazard Ratio | Lower | Upper | P value | Adj Hazard Ratio | Lower | Upper | P value | ||
| Group | Historical control cohort | Ref | Ref | ||||||
| BLIX cohort | 0.35 | 0.22 | 0.58 | <0.001 | 0.56 | 0.33 | 0.96 | 0.033 | |
| Age | 1.02 | 0.99 | 1.04 | 0.155 | 1.02 | 0.99 | 1.04 | 0.215 | |
| Gender | Male | Ref | Ref | ||||||
| Female | 0.75 | 0.45 | 1.20 | 0.233 | 0.68 | 0.41 | 1.12 | 0.133 | |
| HIV Status | HIV non-infected | Ref | Ref | ||||||
| HIV-infected on ART | 1.56 | 0.81 | 3.00 | 0.181 | 1.62 | 0.83 | 3.17 | 0.155 | |
| HIV-infected not on ART | 4.99 | 2.00 | 12.46 | 0.001 | 4.37 | 1.62 | 11.74 | 0.004 | |
| Previous TB/MDR TB | MDR TB | Ref | Ref | ||||||
| Any TB (No MDR) | 0.55 | 0.34 | 0.91 | 0.020 | 0.49 | 0.28 | 0.84 | 0.020 | |
| No Previous TB | 0.09 | 0.02 | 0.37 | 0.001 | 0.12 | 0.03 | 0.49 | 0.003 | |
Figure 1b:
Kaplan Meier estimates of time death stratified by study group (N=256). Median time to death for BLIX cohort is 651 (427 – 735) days. Median time to death for Historical control cohort is 550 (198 – 731) days.
Figure 1d:
Kaplan Meier estimates of time to time to death stratified by study group and HIV status (N=256).
Adverse Events
Most patients in the BLIX cohort (92.3%) experienced at least one adverse event during their drug-resistant treatment course however only one adverse effect was attributable to bedaquiline. Overall, 449 adverse events reported (Table 5), the commonest being gastrointestinal disturbances (N=58, 38.4%), hypothyroidism (N=41, 27.2%), peripheral neuropathy (N=56, 37.1%). Gastrointestinal disturbances included nausea, vomiting, and diarrhoea. Patients also experienced visual disturbances (N=34, 22.5%); anaemia (N=38, 25.2%); ototoxicity (N=24, 15.9%); electrolyte abnormalities including hypomagnesemia, hyponatremia, and/or hypokalemia (N=19, 12.6%); arthralgia (N=25, 16.6%); psychosis (N=22, 14.6%); and dermatological changes (N=17, 11.3%), and 22 patients had non-specific rashes. Linezolid accounted for the largest number of adverse events (24.1%). There were 214 adverse events that resulted in medication changes.
Table 5.
Reported adverse events
| Adverse Events | BLIX cohort N=151 n (%) | Historical control cohort N=105 n (%) |
|---|---|---|
| Hypothyroidism | 41 (27.2) | 17 (16.2) |
| Gastrointestinal disturbances | 58 (38.4) | 100 (95.2) |
| Arthralgias | 25 (16.6) | 5(4.8) |
| Drug-induced liver injury | 6 (4.0) | 0 (0) |
| Cardiac changes | ||
| QTcFprolongationa | 49 (32.5) | - |
| Palpitations | 2 (1.3) | 0 (0) |
| Neurological changes | ||
| Peripheral neuropathy | 56 (37.1) | 1 (0.9) |
| Visual disturbances | 34 (22.5) | 16 (15.2) |
| Ototoxicity | 24 (15.9) | 61 (58.1) |
| Psychosis | 22 (14.6) | 6 (5.7) |
| Sleep disorders | 4 (2.6) | 0 (0) |
| Seizures | 4 (2.6) | 1 (0.9) |
| Hematological changes | ||
| Anemia | 38 (25.2) | 1 (0.9) |
| Leukopenia/neutropenia | 26 (17.2) | 0 (0) |
| Thrombocytopenia | 6 (4.0) | 0 (0) |
| Renal changes | ||
| Electrolyte abnormalities | 28 (18.5) | 35 (33.3) |
| Nephrotoxicity (unspecified) | 9 (6.0) | 6 (5.7) |
| Dermatological changes | ||
| Skin discoloration/Acne | 15 (9.9) | 2 (1.9) |
| Drug rash | 2 (1.3) | 13 (12.4) |
Seven of these patients had clinically relevant QTcF prolongation defined as any ECG-reported QTcF value >500 ms
There were 112 prolonged QTcF values reported among 41 patients after bedaquiline initiation. Seven patients had at least one clinically relevant prolonged QTcF value (>500 ms) with 9 prolonged values in total during or after completion of bedaquiline treatment. There were no reported clinical dysrhythmias or changes in medication due to QTcF prolongation. The use of clofazimine in addition to bedaquiline in the treatment regimen was not associated with QTcF prolongation.
DISCUSSION
In this pragmatic study of DR-TB treatment outcomes among patients with a high HIV co-infection rate within the public health care system in South Africa, the addition of bedaquiline and linezolid to an optimized background regimen for DR-TB generated a ~two-fold improvement in cure, culture conversion, and survival compared to the Historical control cohort. When considered as an overall cohort, receipt of a bedaquiline- and linezolid-containing regimen was an independent predictor of outcome even after adjusting for multiple potential confounders. Of concern, despite improved effectiveness of bedaquiline- and linezolid-based regimens, loss to retention in care during treatment remained very high.
Several studies have demonstrated the operational effectiveness of bedaquiline-based regimens in the treatment of DR-TB.19–21 Many either reported short-term outcomes, or were conducted in non-endemic TB regions or regions with a low HIV prevalence. Our findings are important in the context of the dual TB/HIV epidemic representing long-term data in an HIV endemic setting. Furthermore, our outcome data are consistent with a similar study conducted in South Africa albeit within a considerably lower HIV prevalence setting, in which treatment success was found to be 66.2% amongst patients treated with bedaquiline.19
DR-TB is historically associated with poor treatment outcomes and mortality with HIV co-infection, is exceptionally high. The addition of bedaquiline and linezolid to the treatment regimen improved mortality greater than two-fold (17.2% vs 41.0%). Our findings are consistent with those of Schnippel and Olayanju where mortality rates of 14.7% were reported in both studies following the addition of bedaquiline for the treatment of XDR-TB patients in South Africa.19, 20 Similarly, in a patient-level meta-analysis of DR-TB, the overall mortality in patients treated with bedaquiline-containing regimens was 10.6% (range 6.6%−20%).22 The mortality rate reported in our study was only marginally higher despite substantially higher HIV co-infection rates. We postulate that the 100% concurrent ART and relatively high median CD4+ T-cell count (365 cells/mm3,IQR 199–535) in our study, possibly due to improved ART implementation, likely contributed to the improved survival. Notably, in our study, mortality amongst HIV-negative patients in the Historical control cohort was higher than the mortality amongst HIV-infected patients (on ART) in the BLIX cohort. This supports our previous study reporting poor treatment outcomes amongst patients with XDR-TB irrespective of HIV status and provides compelling evidence for the use of bedaquiline and linezolid in treatment of XDR-TB even amongst patients co-infected with HIV.23
Bedaquiline and linezolid also reduced time to TB culture conversion and resulted in fewer patients being discharged still sputum TB culture positive into the community. This may have important public health significance in reducing community driven transmission of DR-TB particularly as decentralization of TB care has resulted in a higher proportion of patients receiving ambulatory treatment especially as fewer patients received injectable agents and there was less need for admission to administer supervised injectable agents.
The side effect profiles of conventional drug resistant TB regimens are well documented,24 but the significantly greater number of deaths in patients receiving bedaquiline (13% vs 2%) in the phase IIb clinical trial has raised concerns of the toxicity of bedaquiline, particularly a prolonged QTc interval. Forty-nine (32.5%) patients in our study were found to have a clinically relevant QTcF prolongation, but none required a treatment modification due to this. This is substantially greater than the findings of Olayanju et al. where only 10% of individuals had a prolonged QT interval.19 When considering potential cardiotoxicity of this treatment regimen it is important to realize that our overall cohort was young and generally without cardiac disease, or other comorbid conditions. Therefore, the low rate of clinical arrhythmia seen in our study may not be generalizable to older or sicker cohorts. Neurological and haematological adverse effects were likely attributable to linezolid. Anaemia and peripheral neuropathy was 28 and 41 times greater amongst patients treated with bedaquiline- and linezolid-containing regimens compared to the Historical control cohort. These did not differ between patients receiving 600 mg vs 300 mg of linezolid. Recently published data from the Nix-TB study evaluating treatment of drug-resistant TB with bedaquiline, pretomanid, and linezolid (total daily dose of 1200 mg with dose adjustment depending on the toxic effects) found that peripheral neuropathy and haematological toxic effects were common, occurring in 81% and 48% of patients respectively, however they were manageable, often resulting in dose reductions or linezolid treatment interruption.25
Importantly, ototoxicity is one of the most documented adverse effects in conventional drug-resistant TB regimens. Only 15.9% of patients in the bedaquiline group (31.8% received an injectable agent) experienced ototoxicity compared with 58.1% of patients in the Historical control cohort. This finding has enormous clinical significance and impact on patients’ quality of life further supporting the withdrawal of injectables in the reduction of associated morbidity with drug-resistant TB infection.
We acknowledge several important limitations of our study. As this was a retrospective review, there is an associated bias with the selection of cases. We tried to limit this by selecting consecutive cases during the study period. Some patients that were switched from an injectable agent to bedaquiline for toxicity and had already culture converted were included in the study but excluded from some analyses. Adjustment for confounders did not change the effect of improved time to culture conversion nor the reduction in mortality in the bedaquiline group. Using all-cause mortality as the primary endpoint may underestimate the potential impact of bedaquiline and linezolid on treatment outcomes and mortality in this cohort. Because we were not able to obtain reliable information on the causes of all deaths in the study, we were not able to estimate the impact on deaths related only to DR-TB. A further limitation is the use of a historical control group, but the advantage this confers is that it allows a direct comparison between populations in the same region with similar disease profiles for DR-TB, HIV and other demographic factors.
CONCLUSIONS
In summary, the findings from this study provide compelling evidence of the benefit of initiation of bedaquiline and linezolid as part of an optimized treatment regimen including moxifloxacin and clofazimine, integrated with ART during TB treatment in patients with HIV disease. They support recommendations by the WHO and others for the integration of TB and HIV care and the omission of an injectable agent in our setting.
Supplementary Material
Table S1: Medications used in treatment regimens
Table S2: Causes of death
Table S3: Risk factors associated with unfavourable outcome
ACKNOWLEDGMENTS
The authors would like to acknowledge the invaluable contributions of Radhamoney Narasimmulu, CAPRISA staff, patients, and hospital staff.
Conflicts of Interest and Source of Funding
This work was supported by the South African Medical Research Council. N Padayatchi, N Naidu and K Naidoo are supported by the Centre for the AIDS Programme of Research in South Africa (CAPRISA). M. O’Donnell was supported by the Herbert and Florence Irving Foundation and by the U.S. National Institutes of Health (R01AI124413). J. Brust was supported by the U.S. National Institutes of Health (R01AI114304) and the Einstein-Rockefeller-CUNY CFAR (P30AI124414).
REFERENCES
- 1.World Health Organization. Global Tuberculosis Report 2019. Geneva: 2019. [Google Scholar]
- 2.Collaborative Group for the Meta-Analysis of Individual Patient Data in MDRTBt et al. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet. 2018;392(10150):821–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahajan R Bedaquiline: First FDA-approved tuberculosis drug in 40 years. Int J Appl Basic Med Res. 2013;3(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charan J, Reljic T, Kumar A. Bedaquiline versus placebo for management of multiple drug-resistant tuberculosis: A systematic review. Indian J Pharmacol. 2016;48(2):186–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diacon AH et al. Multidrug-resistant tuberculosis and culture conversion with bedaquiline. N Engl J Med. 2014;371(8):723–32. [DOI] [PubMed] [Google Scholar]
- 6.Ndjeka N et al. Treatment of drug-resistant tuberculosis with bedaquiline in a high HIV prevalence setting: an interim cohort analysis. Int J Tuberc Lung Dis. 2015;19(8):979–85. [DOI] [PubMed] [Google Scholar]
- 7.Pym AS et al. Bedaquiline in the treatment of multidrug- and extensively drug-resistant tuberculosis. Eur Respir J. 2016;47(2):564–74. [DOI] [PubMed] [Google Scholar]
- 8.Singh B et al. Linezolid for drug-resistant pulmonary tuberculosis. Cochrane Database Syst Rev. 2019;3:CD012836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sotgiu G et al. Efficacy, safety and tolerability of linezolid containing regimens in treating MDR-TB and XDR-TB: systematic review and meta-analysis. Eur Respir J. 2012;40(6):1430–42. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X et al. Systematic review and meta-analysis of the efficacy and safety of therapy with linezolid containing regimens in the treatment of multidrug-resistant and extensively drug-resistant tuberculosis. J Thorac Dis. 2015;7(4):603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conradie F et al. Clinical access to Bedaquiline Programme for the treatment of drug-resistant tuberculosis. S Afr Med J. 2014;104(3):164–6. [DOI] [PubMed] [Google Scholar]
- 12.Yuengling KA et al. Effect of Antiretroviral Therapy on Treatment Outcomes in a Prospective Study of Extensively Drug-Resistant Tuberculosis (XDR-TB) HIV Coinfection Treatment in KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr. 2018;79(4):474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Department of Health. Introduction of new drugs and drug regimens for the management of drug-resistant tuberculosis in South Africa: Policy framework. Republic of South Africa: 2015. [Google Scholar]
- 14.World Health Organization. Definitions and reporting framework for tuberculosis – 2013 revision. Geneva: 2014. [Google Scholar]
- 15.Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350(10):1013–22. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. 2014. [PubMed]
- 17.Reisner BS, Gatson AM, Woods GL. Evaluation of mycobacteria growth indicator tubes for susceptibility testing of Mycobacterium tuberculosis to isoniazid and rifampin. Diagn Microbiol Infect Dis. 1995;22(4):325–9. [DOI] [PubMed] [Google Scholar]
- 18.Rusch-Gerdes S et al. Multicenter laboratory validation of the BACTEC MGIT 960 technique for testing susceptibilities of Mycobacterium tuberculosis to classical second-line drugs and newer antimicrobials. J Clin Microbiol. 2006;44(3):688–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olayanju O et al. Long-term bedaquiline-related treatment outcomes in patients with extensively drug-resistant tuberculosis from South Africa. Eur Respir J. 2018;51(5). [DOI] [PubMed] [Google Scholar]
- 20.Schnippel K et al. Effect of bedaquiline on mortality in South African patients with drug-resistant tuberculosis: a retrospective cohort study. Lancet Respir Med. 2018;6(9):699–706. [DOI] [PubMed] [Google Scholar]
- 21.Zhao Y et al. Improved treatment outcomes with bedaquiline when substituted for second-line injectable agents in multidrug resistant tuberculosis: a retrospective cohort study. Clin Infect Dis. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. Report of the Guideline Development Group Meeting on the use of bedaquiline in the treatment of multidrug-resistant tuberculosis: a review of available evidence. Geneva: 2017. [Google Scholar]
- 23.O’Donnell MR et al. Treatment outcomes for extensively drug-resistant tuberculosis and HIV co-infection. Emerg Infect Dis. 2013;19(3):416–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torun T et al. Side effects associated with the treatment of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2005;9(12):1373–7. [PubMed] [Google Scholar]
- 25.Conradie F et al. Treatment of Highly Drug-Resistant Pulmonary Tuberculosis. N Engl J Med. 2020;382(10):893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Medications used in treatment regimens
Table S2: Causes of death
Table S3: Risk factors associated with unfavourable outcome