Abstract
G protein-coupled receptors (GPCRs) are integral membrane proteins that transduce a wide array of inputs including light, ions, hormones, and neurotransmitters into intracellular signaling responses which underlie complex processes ranging from vision to learning and memory. Although traditionally thought to signal primarily from the cell surface, GPCRs are increasingly being recognized as capable of signaling from intracellular membrane compartments, including endosomes, the Golgi apparatus, and nuclear membranes. Remarkably, GPCR signaling from these membranes produces functional effects that are distinct from signaling from the plasma membrane, even though often the same G protein effectors and second messengers are activated. In this review we will discuss the emerging idea of a “spatial bias” in signaling. We will present the evidence for GPCR signaling through G protein effectors from intracellular membranes, and the ways in which this signaling differs from canonical plasma membrane signaling with important implications for physiology and pharmacology. We also highlight the potential mechanisms underlying spatial bias of GPCR signaling, including how intracellular membranes and their associated lipids and proteins affect GPCR activity and signaling.
Keywords: GPCR, signaling, trafficking, compartmentalized signaling, endosomes, Golgi, nuclear membrane
1. Introduction
G protein-coupled receptor (GPCR) signaling which underlies complex processes ranging from smell and taste to immune responses to vision, learning, and memory, is being reconsidered in the context of the membranes from which GPCRs signal. GPCRs are the largest class of transmembrane signaling receptors and are activated by a wide array of inputs including light, ions, peptide and non-peptide hormones and neurotransmitters. GPCRs transduce these inputs through conformational changes in their seven transmembrane domains. Conformational changes are driven and stabilized by interaction with classical heterotrimeric G protein effectors, which modulate diverse downstream signaling pathways and second messengers, including adenylyl cyclase and cAMP, ion channels, phospholipases, GTPases, and kinase cascades (Weis and Kobilka, 2018; Wu et al., 2019). GPCRs were originally thought to activate these signaling pathways primarily from the plasma membrane. However, GPCRs also localize to other membranes in the cell and can signal from these membranes, suggesting an important spatial component of signaling.
GPCRs signaling from intracellular membranes can occur through both non-G protein and G protein effectors. The first evidence of GPCR signaling from intracellular membranes suggested that this signaling is mediated by non-G protein effectors, specifically β-arrestins. β-arrestins interact with GPCRs and scaffold kinase signaling pathways in endosomes and at the plasma membrane, in addition to their roles in G protein signaling termination and GPCR internalization from the plasma membrane (Lohse et al., 1990; Luttrell and Lefkowitz, 2002). Subsequent studies have demonstrated that a number of other non-G protein effectors could promote differential GPCR signaling at locations other than the plasma membrane (Varsano et al., 2012; Jean-Alphonse et al., 2014; Grimsey et al., 2015; Alekhina and Marchese, 2016). However, research over the past decade supports the exciting new idea that signaling by a GPCR through the same G protein effector in different locations produces distinct signaling responses. In this review we will focus on how G protein signaling from GPCRs in intracellular membrane compartments differs from classical plasma membrane signaling (Fig. 1). We will also discuss potential mechanisms by which components of different cellular membranes may shape this “spatial bias” in signaling.
Fig. 1. Examples of localization and signaling of GPCRs from intracellular membranes.
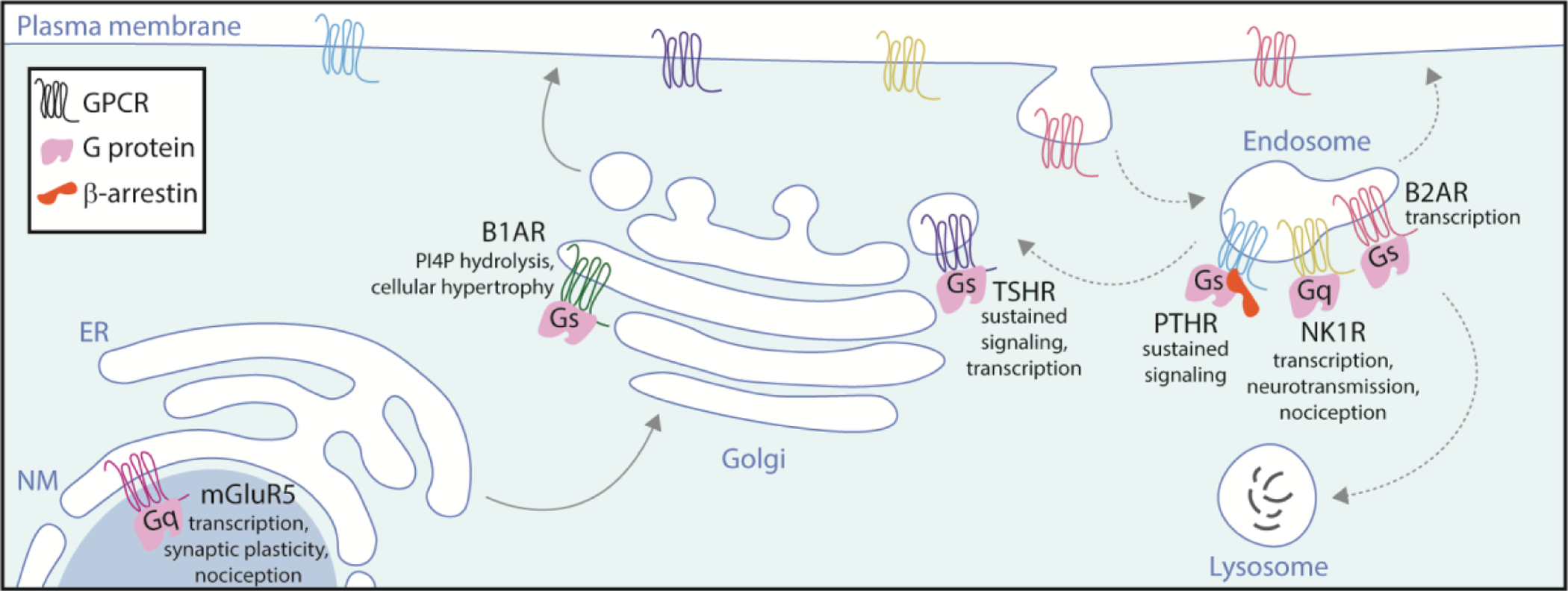
In addition to the plasma membrane GPCRs localize and signal through G proteins in compartments along the biosynthetic pathway (solid arrows) and the endolysosomal pathway (dashed arrows). Non-canonical GPCR signaling through G proteins in these membranes can proceed by distinct interactions, such as a PTHR-G protein-arrestin signaling complex, and produce distinct downstream signaling effects, including but not limited to sustained signaling, transcriptional responses, and effects on cell growth, neurotransmission, and the perception of pain.
2. GPCRs signaling in endosomes
2.1. GPCR localization to the endosomal pathway
Many GPCRs enter and cycle within the endosomal pathway after activation and internalization from the plasma membrane. GPCR internalization is predominantly mediated by clathrin and β-arrestin-1 or β-arrestin-2 which interacts with clathrin via clathrin adaptor protein AP-2 (Laporte et al., 1999; Weinberg and Puthenveedu, 2019). Within the endosomal pathway, GPCRs can enter recycling pathways which return receptors to the plasma membrane or degradative pathways which sort receptors to the lysosome. GPCR sorting to these different endosomal pathways is often an active process (Puthenveedu et al., 2010; Bowman and Puthenveedu, 2015). Sorting can be regulated by amino acid trafficking motifs on a receptor, post-translational modifications like phosphorylation or ubiquitination, and interactions with endosome-associated proteins (Hanyaloglu and von Zastrow, 2008).
Regulated trafficking of GPCRs has clear consequences for their signaling. Classically, relocation of GPCRs from the plasma membrane to endosomes was proposed to serve as a mechanism of terminating signaling from the plasma membrane and desensitizing the cell to extracellular ligands (Lefkowitz et al., 1980; Hertel and Perkins, 1984). However, the discovery of endosomal G protein signaling indicates that receptor endocytosis and localization to endosomal compartments contributes to “spatial bias” in GPCR signaling. We will now examine the early evidence for GPCR G protein signaling from endosomes, and how this signaling differs from signaling at the plasma membrane at a cellular and organismal level.
2.2. Evidence for GPCR activation and signaling from endosomes
Building on a report of endosomal G protein signaling by the yeast GPCR Ste2 (Slessareva et al., 2006), early insights into endosomal G protein signaling in mammalian cells stemmed from prolonged signaling responses even after receptors had internalized from the plasma membrane. Activation of parathyroid hormone receptor (PTHR) and the thyroid stimulating hormone receptor (TSHR), which both couple to Gαs, elevates cellular cAMP even after robust internalization of receptors from the plasma membrane (Calebiro et al., 2009; Ferrandon et al., 2009). These observations were inconsistent with the hypothesis that removal of GPCRs from the plasma membrane serves mainly to terminate G protein signaling. Indeed, prolonged cAMP signaling persists even when agonist is removed from the extracellular media, and fluorescently labeled peptide ligands colocalize with receptors in endosomal compartments, suggesting that ligands can traffic with the receptor to endosomes and continue to signal (Calebiro et al., 2009; Ferrandon et al., 2009). Critically, prolonged elevation of cellular cAMP is disrupted by inhibition of endocytosis by pharmacological or genetic approaches, indicating endocytosis is required for sustained cAMP production by these GPCRs (Calebiro et al., 2009; Ferrandon et al., 2009).
Receptor endocytosis might contribute to distinct cAMP signaling profiles for different GPCRs. For example, inhibiting endocytosis partially decreases cAMP production by the activated Gαs-coupled dopamine receptor D1 (DRD1) and the β2-adrenergic receptor (B2AR) (Kotowski et al., 2011; Irannejad et al., 2013). In the case of DRD1, inhibiting endocytosis measurably decreases cAMP as early as one to two minutes after agonist addition (Kotowski et al., 2011). In the case of B2AR, inhibiting endocytosis decreases cAMP production mainly at later time points, greater than five minutes after agonist treatment (Irannejad et al., 2013). This later time may reflect the time required for B2AR sorting to the specific endosomal domain from which it initiates endosomal G protein signaling (Bowman et al., 2016), as we discuss in more detail in section 5.2. Therefore, some GPCRs might require components of the endocytic process for acute signaling, whereas others require endocytosis for a second wave of cAMP signaling after desensitization of the initial plasma membrane cAMP signaling.
Conformational biosensors based on nanobodies have recently emerged as a powerful method that complements conventional signaling assays to study spatially restricted signaling. These nanobodies are a single protein domain derived from the antigen-binding region of heavy-chain only antibodies produced in camelid species (Manglik et al., 2017). Several generated nanobodies specifically bind the active conformation of a specific GPCR or family of GPCRs (Manglik et al., 2017). Additionally, another nanobody, referred to as Nb37, recognizes a nucleotide-free Gαs conformation as a readout of GDP exchange by the Gαs subunit of the activated G protein (Westfield et al., 2011; Irannejad et al., 2013). Nanobodies have greatly aided in vitro and in vivo studies of GPCR biology (Manglik et al., 2017). When tagged with a fluorescent protein and expressed in live cells, nanobody biosensors localize to membranes containing active GPCR or Gαs protein, providing a high spatiotemporal readout of GPCR activation (Fig. 2a). Indeed, fluorescently-tagged β-adrenergic receptor active conformation nanobody (Nb80) and nucleotide-free Gαs nanobody (Nb37) are recruited to B2AR at the plasma membrane and in endosomal compartments upon agonist treatment, highlighting the presence of both active GPCR and G protein in endosomes (Irannejad et al., 2013; Bowman et al., 2016). Similar nanobodies which recognize the active conformation of opioid receptors (Nb33 and Nb39) colocalize with internalized μ-opioid receptor (MOR), δ-opioid receptor (DOR), and κ-opioid receptors (KOR) in endosomes upon agonist treatment (Stoeber et al., 2018; Kunselman et al., 2020). Beyond the examples highlighted here, signaling assays and biosensor imaging have demonstrated signaling of internalized Gαs, Gαi, and Gαq-coupled GPCRs, including the sphingosine-1-phosphate receptor, glucagon-like peptide-1 receptor, cannabinoid receptor 1, luteinizing hormone receptor (LHR), and the calcium-sensing receptor (CaSR), across a range of endosomal compartments ranging from very early endosomes, to early endosomes marked by EEA1/Rab5, to potentially even late endosomes/lysosomes marked by Rab7 (Rozenfeld and Devi, 2008; Ferrandon et al., 2009; Mullershausen et al., 2009; Kuna et al., 2013; Sposini et al., 2017; Gorvin et al., 2018; Stoeber et al., 2018; Jimenez-Vargas et al., 2020; Kunselman et al., 2020).
Fig. 2: Tools for studying GPCR spatial signaling bias.
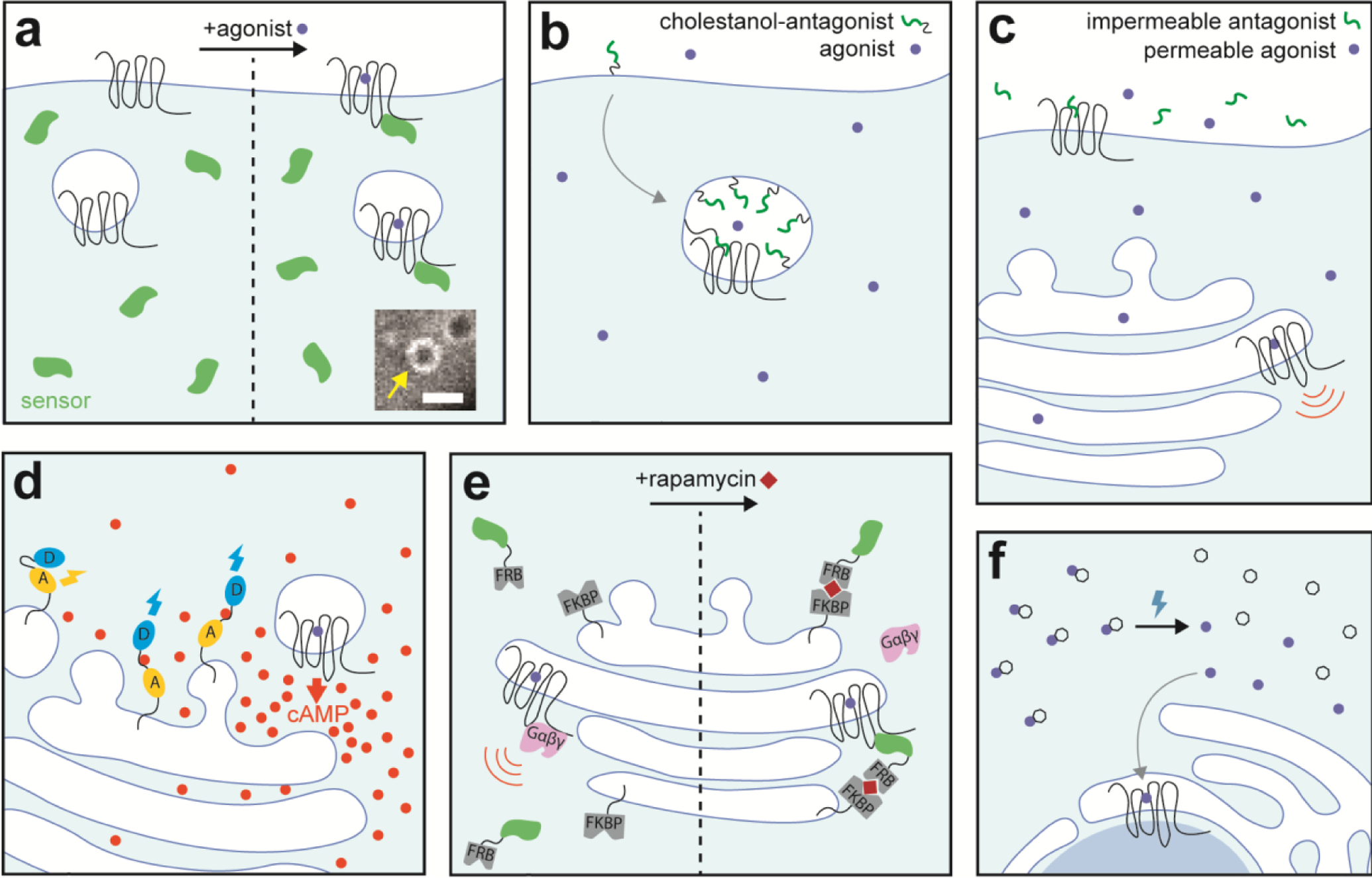
a. Conformational biosensors including nanobodies and miniG proteins recognize active GPCR or Gs conformations. When expressed in cells these sensors will translocate from the cytosol to membranes containing active GPCR or G protein. If tagged with a fluorescent protein, translocation of the sensor can be visualized by confocal microscopy. Inset: HEK cell expressing Flag-DOR and venus-miniGsi and treated with 10μM SNC80 agonist. Accumulation of the miniG sensor is visible on endosomal membranes (yellow arrow), scale bar=1.5μm. b. Cholestanol-conjugated ligands incorporate into the lipid bilayer, are internalized, and accumulate in endosomes. When concentrated in endosomes, these ligands can provide prolonged and specific agonism or antagonism of endosomal GPCRs. Whether these endosomally-targeted antagonists also inhibit plasma membrane GPCRs over prolonged time periods, and if so, to what levels is not clear. c. To isolate GPCR signaling from intracellular sites, cells can be treated with a membrane permeable agonist along with a membrane impermeable antagonist. The permeable agonist can access and activate intracellular GPCRs, while the impermeable antagonist acts only on GPCRs in the plasma membrane, preventing agonist from binding to these sites. d. Fluorescent FRET sensors for PKA phosphorylation events or second messengers like cAMP produced downstream of GPCR activation can be targeted to specific cellular membranes. Localization of these sensors to specific membranes provides a readout of local signaling events. Second messenger like cAMP or phosphorylation of a kinase motif on the sensor leads to a conformational change in the sensor. Changes in cellular levels of cAMP or kinase activity are measured as the ratio between donor fluorophore (D) and acceptor fluorophore (A) emission for a ratiometric FRET sensor, like the one depicted. e. Nanobodies which recognize the active conformation of GPCRs specifically inhibit GPCR signaling from intracellular sites. Rapamycin induces dimerization of FRB and FKBP domains fused to a nanobody or targeted to the organelle of interest, respectively. Dimerization leads to a high local concentration of nanobody which binds to active GPCRs. These nanobodies compete with endogenous G proteins for interaction with the active receptor, interfering with G protein activation and signaling in the organelle of interest. f. Caged ligands are chemically modified so that they are inactive at the target receptor until uncaging. Local uncaging of caged ligands by a specific wavelength of light leads to photolysis of the caged ligand, freeing the active ligand. The free ligand can then bind to the target receptor.
2.3. Distinct consequences of endosomal G protein signaling
Illustrating the concept of “spatial bias,” G protein signaling from GPCRs in endosomes diverges from plasma membrane signaling in a number of ways at the molecular and cellular level. Sustained G protein signaling by PTHR and the vasopressin receptor 2 (V2R) is associated with prolonged arrestin-Gβγ, GPCR-Gβγ, or GPCR-arrestin interactions on endosomes (Ferrandon et al., 2009; Feinstein et al., 2011, 2013; Wehbi et al., 2013). These data, coupled with single-particle electron microscopy structure of a chimeric GPCR simultaneously bound to β-arrestin-1 and a heterotrimeric G protein, support a hypothesis in which a subset of GPCRs which strongly bind β-arrestins sustain endosomal signaling from stabilized arrestin-Gβγ complexes which could support multiple rounds of Gαs activation (Wehbi et al., 2013; Thomsen et al., 2016). Whether this complex is uniquely stabilized in the endosomal compartment remains to be explored.
The role of arrestins in endosomal signaling has been extensively reviewed recently in more detail (Peterson and Luttrell, 2017; Ranjan et al., 2017; Weinberg and Puthenveedu, 2019). Endosomal GPCR-arrestin-G protein complexes likely function differently than GPCR-arrestin signaling complexes. The observation that some GPCRs remain associated with arrestin on the endosome and the detection of GPCR-arrestin-kinase complexes were some of the earliest evidence of potential GPCR signaling from endosomes (Déry et al., 1999; Luttrell et al., 1999; DeFea et al., 2000a; Oakley et al., 2000; Wei et al., 2003). β-arrestins bound to a GPCR can scaffold a number of kinases, and kinases, including Src, Raf, JNK, and ERK, can also be visualized on endosomes containing GPCRs and/or arrestin (DeFea et al., 2000a, 2000b; McDonald et al., 2000; Luttrell et al., 2001). GPCR signaling through arrestins is an excellent example of how GPCRs can also signal through different effectors in different locations.
Spatial bias shapes downstream transcriptional responses, either as a consequence of, or in addition to differential interactions with G protein effectors. In the case of B2AR, a prototypical Gαs-coupled receptor, endosomal cAMP production is required and sufficient for transcription of genes not upregulated by B2AR signaling from the plasma membrane (Tsvetanova and von Zastrow, 2014; Bowman et al., 2016). Similarly, CaSR and neurokinin 1 receptor (NK1R) also require endosomal signaling to induce gene transcription from serum response elements (Jensen et al., 2017; Gorvin et al., 2018). How endosomal signaling drives transcriptional responses is an ongoing area of research and may result from spatiotemporal regulation of GPCR-effector interactions and second messengers.
Endosomal GPCR signaling is linked to physiology and can be specifically modulated by new spatially-targeted pharmacology. Sustained cAMP signaling and endocytosis of the LHR are required for meiosis in the oocyte of ovarian follicles, suggesting an important link between LHR endosomal signaling and fertility (Lyga et al., 2016). Exciting new work in the context of pain neurotransmission by NK1R and the calcitonin receptor-like receptor (CLR) uses spatially-targeted ligands to specifically inhibit endosomal signaling. NK1R or CLR antagonists conjugated to the lipid cholestanol incorporate into the outer leaflet of the membrane bilayer, are internalized, and accumulate in endosomes (Fig. 2b) (Jensen et al., 2017; Yarwood et al., 2017). These endosomally-targeted antagonists inhibit sustained signaling in spinal cord neurons and provide greater and longer lasting pain relief in animal models of inflammatory pain than traditional antagonists (Jensen et al., 2017; Yarwood et al., 2017). A similar approach using lipoparticles to deliver DOR agonist to endosomes supports a role for endosomal DOR signaling in promoting sustained suppression of neuronal excitability and antinociception (Jimenez-Vargas et al., 2020). These compartment-targeted ligands demonstrate the contribution of endosomal signaling to reproductive health and pain pathologies and highlight potential therapeutic approaches which exploit spatial bias in signaling.
3. GPCR signaling in the Golgi
3.1. GPCR localization to the Golgi
GPCRs can sort retrogradely from endosomes to the Golgi after internalization from the plasma membrane or be retained in the Golgi during biosynthetic trafficking en route to the plasma membrane after synthesis at the endoplasmic reticulum (ER). Retrograde sorting from endosomes to the most distal compartment and “sorting station” of the Golgi, the trans-Golgi network (TGN), is often mediated by the retromer complex (Chen et al., 2019). GPCRs including, PTHR and TSHR, internalize and traffic retrogradely to the TGN (Feinstein et al., 2011; Godbole et al., 2017). Though not all GPCRs recycle through the TGN, as integral membrane proteins all GPCRs are expected to transit through the Golgi where they receive important modifications like glycosylation before initial insertion into the plasma membrane (Dong et al., 2007). For many GPCRs Golgi residence is transient, but some receptors like DOR and β1-adrenergic receptor (B1AR) exhibit steady-state Golgi localization (Irannejad et al., 2017; Shiwarski et al., 2017b). Golgi export of these receptors is highly regulated by amino acid trafficking motifs in receptors themselves, protein interactions, and even phospholipids (Mittal et al., 2013; Koliwer et al., 2015; Shiwarski et al., 2017a, 2019; St-Louis et al., 2017). Here we present new and emerging evidence supporting GPCR activation in the Golgi, as well as differential signaling by B1AR and TSHR in the Golgi.
3.2. Evidence for GPCR activation and signaling in the Golgi
Regardless of the mechanisms by which GPCRs localize to the Golgi, GPCRs can be activated by ligands and signal from Golgi compartments. Conformational biosensors including the nanobodies already described, as well as mini-G protein biosensors which mimic Gα subunit interactions with active GPCR conformations (Nehmé et al., 2017; Wan et al., 2018), reveal activation of GPCRs or Gαs proteins in the Golgi (Godbole et al., 2017; Irannejad et al., 2017; Stoeber et al., 2018; Nash et al., 2019). The nucleotide-free Gαs nanobody (Nb37) localizes to compartments containing both labeled TSH internalized with endogenous TSHR, and a TGN marker, as well as compartments containing a component of the retromer complex (Godbole et al., 2017). Together these data suggest TSHR signals from retrograde endocytic compartments at the TGN (Godbole et al., 2017). Activation of B1AR, DOR, MOR, and KOR by ligands in the Golgi have similarly been revealed by conformational biosensors recognizing active receptor conformations (Irannejad et al., 2017; Stoeber et al., 2018; Nash et al., 2019; Che et al., 2020).
Notably, for GPCRs not internalizing from the cell surface with their associated ligand, activation in the Golgi is limited to agonists which can readily cross multiple cell membranes either by diffusion or active transport by transporters like the organic cation transporter 3 (OCT3) catecholamine transporter (Irannejad et al., 2017; Stoeber et al., 2018). For both B1AR and DOR, membrane impermeable antagonists inhibit signaling responses induced by cell impermeable agonists, but fail to completely block signaling downstream of cell permeable agonists (Fig. 2c), with this residual signaling attributed to Golgi GPCRs (Irannejad et al., 2017; Stoeber et al., 2018). The dependence of intracellular GPCR activation on ligand permeability forms the basis of many approaches to assay signaling from both Golgi and nuclear membrane-localized GPCRs, which will be discussed in more detail later.
3.3. Distinct consequences of GPCR signaling in the Golgi
GPCR signaling from the Golgi mediates differential and spatially-restricted cellular signaling effects. Förster resonance energy transfer (FRET) sensors can be targeted to both the plasma membrane and Golgi to measure local cAMP or protein kinase A (PKA) activity (Fig. 2d) (Dipilato and Zhang, 2009; Depry et al., 2011; Godbole et al., 2017). In the case of TSHR, Golgi-localized FRET sensors reveal Golgi-localized cAMP and PKA signaling up to ten minutes after receptor internalization, which is lost when cells are treated with an endocytosis inhibitor (Godbole et al., 2017). This signaling is linked to cAMP response element-binding protein (CREB) phosphorylation and transcription of cAMP-regulated genes. Golgi membrane integrity and anchoring of PKA at the Golgi through interactions with A-kinase-anchoring proteins (AKAPs) are required for TSH-induced CREB phosphorylation, suggesting the spatial organization of these signaling components at the Golgi regulates downstream signaling effects (Godbole et al., 2017).
In the case of B1AR, activation of Golgi B1AR signaling in cardiac myocytes is required and sufficient to induce phospholipase C epsilon (PLCε)-dependent hydrolysis of phosphatidylinositol 4-phosphate (PI4P), the primary phospholipid at the Golgi (Nash et al., 2019). In an interesting parallel to TSHR, PLCε interaction with muscle specific AKAP, mAKAP, is required for B1AR-dependent PI4P hydrolysis, suggesting that localization of signaling effectors may be a major component in regulating Golgi signaling pathways (Nash et al., 2019). In addition to traditional pharmacological approaches using ligands with different membrane permeability, B1AR signaling from the Golgi was inhibited by a novel non-pharmacological approach, developed by Irannejad, et al., 2017. β-adrenergic receptor active conformation nanobody Nb80, is recruited to the Golgi via chemically induced dimerization and competes with endogenous G proteins for binding to the active receptor, effectively preventing local G protein activation (Fig. 2e) (Irannejad et al., 2017). Inhibition of B1AR Golgi signaling not only decreases PI4P hydrolysis, but also decreases cellular hypertrophy and atrial natriuretic factor (ANF) expression, both of which are hallmarks of cardiac hypertrophy often preceding heart failure (Nash et al., 2019). These data provide rationale for specifically targeting Golgi B1AR signaling for treatment of heart failure and could guide new pharmacological approaches.
4. GPCRs signaling in the nuclear membrane
4.1. GPCR localization to the nuclear membrane and endoplasmic reticulum
Similar to GPCRs localized to the Golgi, GPCRs can localize to nuclear membranes and connected ER membranes after synthesis or after internalizing from the plasma membrane. Localization to nuclear membranes can be mediated by a classical nuclear localization sequence (NLS) and/or importins. Nuclear targeting via an NLS or importins can occur after GPCR internalization, as observed for the protease-activated receptor 2 and the oxytocin receptor, or directly after synthesis, as observed for for α1-adrenergic receptors and the platelet-activating factor receptor (Wright et al., 2012; Di Benedetto et al., 2014; Joyal et al., 2014; Bhosle et al., 2016). In contrast, other GPCRs such as the metabotropic glutamate receptor 5 (mGluR5) utilize non-NLS sequences to direct nuclear localization, and may interact with chromatin to retain these receptors in the nucleus (Sergin et al., 2017). Among ER-targeted GPCRs, like the protease-activated receptor 4, ER localization can be regulated by classical RXR motifs, which are recognized by the COPI protein complex to retain receptors in the biosynthetic pathway (Brock et al., 2005; Cunningham et al., 2012). These same COPI interacting motifs also are found in some Golgi-localized GPCR, like DOR (St-Louis et al., 2017; Shiwarski et al., 2019). Here we will focus on common themes and signaling of the particularly well-studied mGluR5 from nuclear membranes, but a comprehensive review of the diverse and varied signaling from these membranes has been well reviewed recently (Jong et al., 2018).
4.2. Evidence for GPCR signaling from nuclear membranes
GPCR signaling from nuclear membranes has been demonstrated by both pharmacological approaches using membrane permeable and impermeable ligands, as well as assaying GPCR signaling in isolated nuclei. In a number of different neuronal cell types, activation of Gq-coupled mGluR5 by membrane permeable agonists promotes sustained calcium signaling (Jong et al., 2009; Purgert et al., 2014; Vincent et al., 2016). These responses persist even when surface receptors are blocked by an impermeable antagonist, suggesting surface receptor activation and internalization are not required for sustained calcium signaling (Jong et al., 2009; Purgert et al., 2014; Vincent et al., 2016). Agonist application to isolated nuclei containing mGluR5 or the endothelin type B receptor (ETBR) produces calcium release from these membranes, suggesting that nuclear membranes contain GPCRs and downstream signaling components, including Gq, PLC, and inositol triphosphate receptors (IP3Rs), sufficient to stimulate calcium release (Kumar et al., 2008; Merlen et al., 2013; Vincent et al., 2016).
In addition to traditional ligands, “caged” ligands which consist of an agonist or antagonist chemically modified to be inactive until light-induced photolysis allows local uncaging, have been used to study signaling from nuclear membranes (Fig. 2f). Uncaging of intracellular caged agonists of mGluR5, angiotensin receptor, or ETBR promotes activation of the respective intracellular and intracellular calcium release (Merlen et al., 2013; Purgert et al., 2014; Tadevosyan et al., 2015; Jong and O’Malley, 2017).
4.3. Distinct consequences of GPCR signaling from nuclear membranes by mGluR5
Signaling of intracellular mGluR5 in neurons is a particularly well studied example of how signaling from nuclear membranes diverges from signaling at the plasma membrane and influences physiology. In striatal neurons, signaling from plasma membrane-localized or intracellular mGluR5 is sufficient to activate CREB transcription factor, whereas intracellular mGluR5 signaling is required and sufficient to activate Elk1 transcription factor and transcription of associated serum-response element target genes, including several genes linked to neuronal plasticity (Jong et al., 2009; Kumar et al., 2012). In hippocampal neurons, intracellular mGluR5 mediates one form of synaptic plasticity, long term depression, whereas plasma membrane-localized mGluR5 mediates both long term depression and long term potentiation, suggesting that intracellular mGluR5 signaling may be relevant to complex processes like learning and memory (Purgert et al., 2014). Endogenous glutamate activation of intracellular mGluR5 in spinal cord neurons contributes to pain pathologies linked to neuronal hypersensitivity after nerve injury (Vincent et al., 2016). Inhibiting intracellular mGluR5 signaling with a membrane permeable antagonist or inhibiting the neuronal transporter which allows glutamate to access intracellular receptors produces greater pain relief than membrane impermeable antagonists (Vincent et al., 2016). Future approaches using ligands specifically targeted to nuclear membranes, similar to the approach described for endosomally-targeted antagonists (Jensen et al., 2017; Yarwood et al., 2017), could further clarify the role of mGluR5 in these complex neural processes and pathologies.
5. Mechanisms of GPCR spatial signaling bias
Despite evidence supporting “spatial bias” in GPCR signaling through G proteins in different cellular membranes, the mechanisms driving spatially biased signaling are not fully understood. Understanding how GPCR signaling from different cellular membranes drives distinct downstream responses while signaling through the same G protein effectors will be essential to understanding GPCR biology and designing new spatially-targeted therapeutic approaches. Temporal and spatial organization of signaling emerge as common themes differentiating GPCR signaling through G proteins in intracellular membranes from signaling in the plasma membrane. We will propose potential mechanisms of spatial bias with emphasis on how spatial bias may be shaped by the kinetics of signaling, spatial organization of signaling effectors, as well as the lipid composition of membranes.
5.1. Temporal component of intracellular signaling
Sustained signaling responses are associated with GPCR signaling from non-canonical locations. However, the factors driving sustained signaling and the consequences of “second waves” of intracellular signaling are unclear (Lohse and Calebiro, 2013; Stoeber et al., 2018). PTHR, TSHR, and mGluR5 signaling from endosomes, Golgi, and nuclear membranes, are all associated with prolonged cAMP or calcium signaling over longer time scales than those observed for plasma membrane signaling of these receptors (Calebiro et al., 2009; Ferrandon et al., 2009; Jong et al., 2009; Godbole et al., 2017). The mechanisms which terminate G protein signaling at the plasma membrane, such as GPCR phosphorylation by G protein-coupled receptor kinases (GRKs) and β-arrestin binding (Luttrell and Lefkowitz, 2002), have not yet been definitively demonstrated to terminate G protein signaling at other membranes. Indeed, other proteins present on endosomes and at the TGN can regulate and terminate G protein signaling from these membranes (see section 5.2).
Regarding the consequences of intracellular signaling, it is difficult to fully dissociate the contribution of temporal and spatial components. Signaling for several GPCRs from endosomes, the TGN/Golgi, or nuclear membranes is associated with gene transcription (Jong et al., 2009; Tsvetanova and von Zastrow, 2014; Godbole et al., 2017; Jensen et al., 2017; Yarwood et al., 2017; Gorvin et al., 2018). How intracellular signaling may drive transcriptional responses is not clear, and it is unlikely that intracellular signaling alone produces these responses. A direct consequence of sustained intracellular signaling may be a larger integrated, or total, signaling response. However, in the case of transcriptional responses downstream of B2AR signaling through Gs at endosomes, magnitude of the cAMP signaling response alone does not directly correlate with transcription of endosomally regulated genes (Tsvetanova and von Zastrow, 2014; Tsvetanova et al., 2017). Additionally, production of cAMP at the endosomal membrane is sufficient to produce the same transcriptional responses (Tsvetanova and von Zastrow, 2014). Golgi-localized PKA is also required for TSHR transcriptional responses, making a strong case for a spatial component of these signaling responses which we will now consider (Godbole et al., 2017).
5.2. Spatial component of intracellular signaling: Proteins
Spatial organization of GPCR effectors and downstream signaling components is a possible mechanism driving spatially biased signaling. Signaling effectors like G proteins and adenylyl cyclases, a common downstream effector of Gs and Gi-coupled receptors, localize to multiple membrane locations, including endosomes and the Golgi, consistent with GPCR signaling from these locations (Calebiro et al., 2009; Ferrandon et al., 2009; Kotowski et al., 2011; Cancino et al., 2014). These GPCR effectors, including G protein subunits and adenylyl cyclases, also dynamically traffic to different membranes and cellular compartments in response to GPCR signaling (Wedegaertner et al., 1996; Hynes et al., 2004; Allen et al., 2005; Saini et al., 2007; Lazar et al., 2020). In a recent example of this phenomenon, GPCR signaling promotes isoform-specific internalization of adenylyl cyclase 9 (AC9) to endosomes (Lazar et al., 2020). AC9 is also required for endosomal cAMP production by B2AR (Lazar et al., 2020), highlighting the potential link between effector localization and endosomal signaling. As illustrated for B1AR and TSHR, AKAPs are essential for mediating spatially biased signaling of these GPCRs in the Golgi (Godbole et al., 2017; Nash et al., 2019). AKAPs scaffold a variety of signaling effectors including adenylyl cyclases, phosphodiesterases, kinases, and phospholipases at multiple cellular membranes and facilitate efficient interactions between effectors to promote localized signaling responses (Welch et al., 2010; Zhang et al., 2011; Kapiloff et al., 2014).
Interestingly, spatial organization of effectors into microdomains, even within the same intracellular membrane compartment, can influence GPCR signaling. B2AR in the active conformation, visualized by an active conformation nanobody (Nb80), localizes throughout the endosomal membrane (Irannejad et al., 2013; Bowman et al., 2016). However, the nucleotide-free Gαs nanobody (Nb37) is restricted to recycling tubules on the endosome (Bowman et al., 2016). These data suggest that although B2AR is active throughout the endosome, G protein coupling and activation is restricted to a specific endosomal domain. B2AR localization to these endosomal microdomains is required for endosomal transcriptional responses (Bowman et al., 2016). The mechanisms restricting receptor localization or G protein activation to these specific domains are not yet known, and is an exciting area for future study.
Microdomains are best studied at the plasma membrane where lipid rafts, caveolae, clathrin-coated pits, and primary cilia can compartmentalize and organize GPCR signaling (Patel et al., 2008; Hilgendorf et al., 2016; Mykytyn and Askwith, 2017; Weinberg and Puthenveedu, 2019). These domains often have distinct protein and lipid compositions, including cholesterol and phospholipids, and can localize GPCRs and specific effectors to produce distinct signaling outputs or maintain specific local signaling environments (Russo et al., 2009; Marley et al., 2013; Mukhopadhyay et al., 2013; Garcia-Gonzalo et al., 2015; Singh et al., 2015; Bachmann et al., 2016; Moore et al., 2016; Weinberg et al., 2017). As we increase our understanding of how microdomains organize GPCR signaling in the plasma membrane, we will likely be able to identify analogous mechanisms that regulate GPCR signaling from microdomains in intracellular compartments.
In addition to classical signaling molecules, membrane trafficking proteins can play dual roles in regulating both GPCR trafficking and signaling. The retromer complex, which has been linked to TSHR, PTHR, and B2AR trafficking (Feinstein et al., 2011; Temkin et al., 2011; Godbole et al., 2017), plays divergent roles in regulating GPCR signaling. PTHR endosomal signaling is terminated by retromer, whereas TSHR signals from compartments containing retromer components at the TGN (Feinstein et al., 2011; Godbole et al., 2017). B2AR actively sorts to retromer-containing recycling tubules in the endosome, which are the site of G protein activation and initiation of endosomal transcriptional responses (Temkin et al., 2011; Bowman et al., 2016). APPL1, a protein associated with very early endosomes and GPCR recycling, also negatively regulates cAMP production by LHR, B1AR, and the follicle-stimulating hormone receptor (FSHR) (Sposini et al., 2017).
5.3. Spatial component of intracellular signaling: Membrane lipids
At a more fundamental level, the lipid composition of different cellular membranes may drive spatial differences in GPCR signaling. Lipids and expression of lipid modifying enzymes vary across cell types (Chattopadhyay and Paila, 2007; Balla, 2013). Cellular membranes within the same cell are heterogeneous and differ not only in protein composition, but in lipid composition, including cholesterol and phospholipids, both of which can influence GPCR signaling (Ikonen, 2008; Van Meer et al., 2008; Balla, 2013). Cholesterol is most enriched at the plasma membrane, but can also be found in endosomal compartments and the TGN (Mukherjee et al., 1998; Ikonen, 2008; Van Meer et al., 2008). Cholesterol interacts with GPCRs and modulates GPCR activity in a variety of ways including but not limited to receptor dimerization, ligand binding, G protein coupling, and signaling (Xiang et al., 2002; Pucadyil and Chattopadhyay, 2004; Cherezov et al., 2007; Hanson et al., 2008; Levitt et al., 2009; Oates et al., 2012; Prasanna et al., 2014).
Phospholipids which vary in cellular membrane distribution also directly and indirectly modulate GPCR and effector activity. Negatively charged phospholipid phosphatidylglycerol (PG) acts as an allosteric modulator at B2AR and promotes agonist binding (Dawaliby et al., 2016). PG and phosphatidylserine (PS) decrease B2AR coupling to Gi over Gs. This selectivity was attributed to a negatively charged amino acid motif present on the Gi protein. This motif may be repelled from a negatively charged membrane, and interestingly can be regulated by acute changes in local membrane charge such as through the influx of calcium ions (Strohman et al., 2019). PG similarly promotes interaction of the neurotensin receptor 1 (NTSR1) with Gq and Gβ1γ1 and increases GDP exchange by the G protein (Inagaki et al., 2012). Phosphatidylinositol-4,5-bisphosphate (PI4,5P2), which is enriched in the plasma membrane, can also stabilize G protein interactions with GPCRs and promote GPCR-stimulated GTP hydrolysis (Yen et al., 2018). Beyond their direct effects on GPCR-G protein activity and coupling, membrane lipids also bind to GPCR interacting partners and effectors, including arrestins, GRKs, adenylyl cyclases, and AKAPs, (Gaidarov et al., 1999; Ostrom et al., 2002; Vögler et al., 2008; Homan et al., 2013; Kapiloff et al., 2014). Taken together, these data suggest that spatial signaling bias may be influenced by the effect of local membrane lipids on GPCR activity.
6. Conclusion and future perspectives
Our understanding of GPCR biology and pharmacology has been transformed by the discovery that GPCRs can signal from multiple cellular locations. GPCRs can activate the same G protein effectors on intracellular membranes, including endosomes, the Golgi, and nuclear membranes, as are activated at the plasma membrane, yet produce distinct signaling effects (Fig. 1). In addition to these compartments in the membrane trafficking pathway, GPCR signaling has also been reported on unexpected cellular compartments including the mitochondria and melanosomes (reviewed in Jong et al., 2018). These discoveries, enabled by the development of new tools allowing for activation of GPCRs and measurement of conformational changes and signaling with high spatial and temporal resolution, have changed our view of GPCRs from being simple on-off switches at the plasma membrane, to being master regulators of multiple spatially and temporally distinct phases of signaling (Fig. 2).
These new ideas and advances point to an exciting time ahead for studying the cell biology of GPCRs. From a fundamental perspective, the idea that the intracellular location of GPCRs is a key determinant of signaling provides a revised appreciation for the role of membranes and trafficking in regulating GPCR function. Classically thought of as a mechanism to add or remove GPCRs from the cell surface, trafficking might also be a critical mechanism to transport GPCRs between distinct spatially separated signaling complexes. Further, selectively modulating signaling from specific compartments is an exciting and emerging prospect for developing therapeutics with greater efficacy and less adverse effects. Future quantitative analysis of GPCR localization and signaling in cell types of interest, and of how signaling events in different membranes contribute to a net signaling response will allow us to leverage GPCR location to fine-tune targeting of GPCRs for better therapeutics.
Acknowledgements
SEC and MAP would like to thank Ian Chronis, Josh Lott, Loyda Morales Rodriguez, Dr. Kasun Ratnayake, Dr. Prahatha Venkatraman, and Dr. Zara Weinberg for invaluable feedback and discussion on this manuscript. MAP was supported by National Institutes of Health GM117425 and National Science Foundation 1517776.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of interest
The authors declare no conflicts of interest.
Availability of data and material
Not applicable
Code availability
Not applicable
Ethics approval
Not applicable
Consent to participate
Not applicable
Consent for publication
Not applicable
References
- Alekhina O, and Marchese A (2016). β-arrestin1 and Signal-transducing Adaptor Molecule 1 (STAM1) cooperate to promote focal adhesion kinase autophosphorylation and chemotaxis via the chemokine receptor CXCR4. J Biol Chem 291, 26083–26097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JA, Yu JZ, Donati RJ, and Rasenick MM (2005). Beta-adrenergic receptor stimulation promotes G(alpha)s internalization through lipid rafts: A study in living cells. Mol Pharmacol 67, 1493–1504. [DOI] [PubMed] [Google Scholar]
- Bachmann VA et al. (2016). Gpr161 anchoring of PKA consolidates GPCR and cAMP signaling. Proc Natl Acad Sci U S A 113, 7786–7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balla T (2013). Phosphoinositides: Tiny lipids with giant impact on cell regulation. Physiol Rev 93, 1019–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Benedetto A et al. (2014). Osteoblast regulation via ligand-activated nuclear trafficking of the oxytocin receptor. Proc Natl Acad Sci U S A 111, 16502–16507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhosle V et al. (2016). Nuclear localization of platelet-activating factor receptor controls retinal neovascularization. Cell Discov 2, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman SL, and Puthenveedu MA (2015). Postendocytic Sorting of Adrenergic and Opioid Receptors: New Mechanisms and Functions. Prog Mol Biol Transl Sci 132, 189–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman SL, Shiwarski DJ, and Puthenveedu MA (2016). Distinct G protein-coupled receptor recycling pathways allow spatial control of downstream G protein signaling. J Cell Biol 214, 797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock C, Boudier L, Maurel D, Blahos J, and Pin J-P (2005). Assembly-dependent surface targeting of the heterodimeric GABAB Receptor is controlled by COPI but not 14-3-3. Mol Biol Cell 16, 5572–5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calebiro D, Nikolaev VO, Gagliani MC, de Filippis T, Dees C, Tacchetti C, Persani L, and Lohse MJ (2009). Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLoS Biol 7, e1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancino J et al. (2014). Control systems of membrane transport at the interface between the endoplasmic reticulum and the Golgi. Dev Cell 30, 280–294. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay A, and Paila YD (2007). Lipid-protein interactions, regulation and dysfunction of brain cholesterol. Biochem Biophys Res Commun 354, 627–633. [DOI] [PubMed] [Google Scholar]
- Che T et al. (2020). Nanobody-enabled monitoring of kappa opioid receptor states. Nat Commun 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KE, Healy MD, and Collins BM (2019). Towards a molecular understanding of endosomal trafficking by Retromer and Retriever. Traffic 20, 465–478. [DOI] [PubMed] [Google Scholar]
- Cherezov V et al. (2007). High-resolution crystal structure of an engineered human β2-adrenergic G protein-coupled receptor. Science (80-) 318, 1258–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MR, McIntosh KA, Pediani JD, Robben J, Cooke AE, Nilsson M, Gould GW, Mundell S, Milligan G, and Plevin R (2012). Novel role for proteinase-activated receptor 2 (PAR2) in membrane trafficking of proteinase-activated receptor 4 (PAR4). J Biol Chem 287, 16656–16669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawaliby R, Trubbia C, Delporte C, Masureel M, Van Antwerpen P, Kobilka BK, and Govaerts C (2016). Allosteric regulation of G protein-coupled receptor activity by phospholipids. Nat Chem Biol 12, 35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFea KA, Vaughn ZD, O’Bryan EM, Nishijima D, Déry O, and Bunnett NW (2000a). The proliferative and antiapoptotic effects of substance P are facilitated by formation of a β-arrestin-dependent scaffolding complex. Proc Natl Acad Sci U S A 97, 11086–11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFea KA, Zalevsky J, Thoma MS, Dery O, Mullins RD, and Bunnett NW (2000b). β-Arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol 148, 1267–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depry C, Allen MD, and Zhang J (2011). Visualization of PKA activity in plasma membrane microdomains. Mol Biosyst 7, 52–58. [DOI] [PubMed] [Google Scholar]
- Déry O, Thoma MS, Wong H, Grady EF, and Bunnett NW (1999). Trafficking of proteinase-activated receptor-2 and β-arrestin-1 tagged with green fluorescent protein. β-Arrestin-dependent endocytosis of a proteinase receptor. J Biol Chem 274, 18524–18535. [DOI] [PubMed] [Google Scholar]
- Dipilato LM, and Zhang J (2009). The role of membrane microdomains in shaping β 2-adrenergic receptor-mediated cAMP dynamics. Mol Biosyst 5, 832–837. [DOI] [PubMed] [Google Scholar]
- Dong C, Filipeanu CM, Duvernay MT, and Wu G (2007). Regulation of G protein-coupled receptor export trafficking. Biochim Biophys Acta 1768, 853–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein TN, Wehbi VL, Ardura JA, Wheeler DS, Ferrandon S, Gardella TJ, and Vilardaga JP (2011). Retromer terminates the generation of cAMP by internalized PTH receptors. Nat Chem Biol 7, 278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein TN, Yui N, Webber MJ, Wehbi VL, Stevenson HP, King JD, Hallows KR, Brown D, Bouley R, and Vilardaga JP (2013). Noncanonical control of vasopressin receptor type 2 signaling by retromer and arrestin. J Biol Chem 288, 27849–27860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandon S, Feinstein TN, Castro M, Wang B, Bouley R, Potts JT, Gardella TJ, and Vilardaga J-P (2009). Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat Chem Biol 5, 734–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidarov I, Krupnick JG, Falck JR, Benovic JL, and Keen JH (1999). Arrestin function in G protein-coupled receptor endocytosis requires phosphoinositide binding. [DOI] [PMC free article] [PubMed]
- Garcia-Gonzalo FR, Phua SC, Roberson EC, Garcia G, Abedin M, Schurmans S, Inoue T, and Reiter JF (2015). Phosphoinositides Regulate Ciliary Protein Trafficking to Modulate Hedgehog Signaling. Dev Cell 34, 400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbole A, Lyga S, Lohse MJ, and Calebiro D (2017). Internalized TSH receptors en route to the TGN induce local Gs-protein signaling and gene transcription. Nat Commun 8, 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorvin CM et al. (2018). AP2σ Mutations Impair Calcium-Sensing Receptor Trafficking and Signaling, and Show an Endosomal Pathway to Spatially Direct G-Protein Selectivity. Cell Rep 22, 1054–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsey NJ, Aguilar B, Smith TH, Le P, Soohoo AL, Puthenveedu MA, Nizet V, and Trejo JA (2015). Ubiquitin plays an atypical role in GPCR-induced p38 MAP kinase activation on endosomes. J Cell Biol 210, 1117–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MA, Cherezov V, Griffith MT, Roth CB, Jaakola VP, Chien EYT, Velasquez J, Kuhn P, and Stevens RC (2008). A Specific Cholesterol Binding Site Is Established by the 2.8 Å Structure of the Human β2-Adrenergic Receptor. Structure 16, 897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanyaloglu AC, and von Zastrow M (2008). Regulation of GPCRs by Endocytic Membrane Trafficking and Its Potential Implications. Annu Rev Pharmacol Toxicol 48, 537–568. [DOI] [PubMed] [Google Scholar]
- Hertel C, and Perkins JP (1984). Receptor-specific mechanisms of desensitization of β-adrenergic receptor function. Mol Cell Endocrinol 37, 245–256. [DOI] [PubMed] [Google Scholar]
- Hilgendorf KI, Johnson CT, and Jackson PK (2016). The primary cilium as a cellular receiver: Organizing ciliary GPCR signaling. Curr Opin Cell Biol 39, 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homan KT, Glukhova A, and Tesmer JJG (2013). Regulation of G Protein-Coupled Receptor Kinases by Phospholipids. Curr Med Chem 20, 39–46. [PubMed] [Google Scholar]
- Hynes TR, Mervine SM, Yost EA, Sabo JL, and Berlot CH (2004). Live cell imaging of Gs and the β2-adrenergic receptor demonstrates that both αs and β 1γ7 internalize upon stimulation and exhibit similar trafficking patterns that differ from that of the β2-adrenergic receptor. J Biol Chem 279, 44101–44112. [DOI] [PubMed] [Google Scholar]
- Ikonen E (2008). Cellular cholesterol trafficking and compartmentalization. Nat Rev Mol Cell Biol 9, 125–138. [DOI] [PubMed] [Google Scholar]
- Inagaki S, Ghirlando R, White JF, Gvozdenovic-Jeremic J, Northup JK, and Grisshammer R (2012). Modulation of the interaction between neurotensin receptor NTS1 and Gq protein by lipid. J Mol Biol 417, 95–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irannejad R et al. (2013). Conformational biosensors reveal GPCR signalling from endosomes. Nature 495, 534–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irannejad R, Pessino V, Mika D, Huang B, Wedegaertner PB, Conti M, and von Zastrow M (2017). Functional selectivity of GPCR-directed drug action through location bias. Nat Chem Biol 13, 799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean-Alphonse F, Bowersox S, Chen S, Beard G, Puthenveedu MA, and Hanyaloglu AC (2014). Spatially restricted G protein-coupled receptor activity via divergent endocytic compartments. J Biol Chem 289, 3960–3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen DD et al. (2017). Neurokinin 1 receptor signaling in endosomes mediates sustained nociception and is a viable therapeutic target for prolonged pain relief. Sci Transl Med 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Vargas NN et al. (2020). Endosomal signaling of delta opioid receptors is an endogenous mechanism and therapeutic target for relief from inflammatory pain. Proc Natl Acad Sci, 202000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong Y-JI, and O’Malley KL (2017). Mechanisms Associated with Activation of Intracellular Metabotropic Glutamate Receptor, mGluR5. Neurochem Res 42, 166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong YJI, Harmon SK, and O’Malley KL (2018). GPCR signalling from within the cell. Br J Pharmacol 175, 4026–4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong YJI, Kumar V, and O’Malley KL (2009). Intracellular metabotropic glutamate receptor 5 (mGluR5) activates signaling cascades distinct from cell surface counterparts. J Biol Chem 284, 35827–35838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyal JS et al. (2014). Subcellular localization of coagulation factor II receptor-like 1 in neurons governs angiogenesis. Nat Med 20, 1165–1173. [DOI] [PubMed] [Google Scholar]
- Kapiloff MS, Rigatti M, and Dodge-Kafka KL (2014). Architectural and functional roles of a kinase-anchoring proteins in camp microdomains. J Gen Physiol 143, 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koliwer J, Park M, Bauch C, Von Zastrow M, and Kreienkamp HJ (2015). The golgi-associated PDZ domain protein PIST/GOPC stabilizes the β1-Adrenergic receptor in intracellular compartments after internalization. J Biol Chem 290, 6120–6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotowski SJ, Hopf FW, Seif T, Bonci A, and von Zastrow M (2011). Endocytosis Promotes Rapid Dopaminergic Signaling. Neuron 71, 278–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Fahey PG, Jong YJI, Ramanan N, and O’Malley KL (2012). Activation of intracellular metabotropic glutamate receptor 5 in striatal neurons leads to up-regulation of genes associated with sustained synaptic transmission including Arc/Arg3.1 protein. J Biol Chem 287, 5412–5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Jong YJI, and O’Malley KL (2008). Activated nuclear metabotropic glutamate receptor mGlu5 couples to nuclear Gq/11 proteins to generate inositol 1,4,5-trisphosphate-mediated nuclear Ca2+ release. J Biol Chem 283, 14072–14083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuna RS, Girada SB, Asalla S, Vallentyne J, Maddika S, Patterson JT, Smiley DL, DiMarchi RD, and Mitra P (2013). Glucagon-like peptide-1 receptor-mediated endosomal cAMP generation promotes glucose-stimulated insulin secretion in pancreatic β-cells. Am J Physiol - Endocrinol Metab 305. [DOI] [PubMed] [Google Scholar]
- Kunselman JM, Gupta A, gomes I, Devi LA, and Puthenveedu MA (2020). Compartment-specific opioid receptor signaling is selectively modulated by Dynorphin subtypes. BioRxiv Cell Biol, 2020.06.21.162206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laporte SA, Oakley RH, Zhang J, Holt JA, Ferguson SSG, Caron MG, and Barak LS (1999). The β2-adrenergic receptor/βarrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proc Natl Acad Sci U S A 96, 3712–3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar AM, Irannejad R, Baldwin TA, Sundaram AB, Gutkind JS, Inoue A, Dessauer CW, and Von Zastrow M (2020). G protein-regulated endocytic trafficking of adenylyl cyclase type 9. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz RJ, Wessels MR, and Stadel JM (1980). Hormones, Receptors, and Cyclic AMP: Their Role in Target Cell Refractoriness. Curr Top Cell Regul 17, 205–230. [DOI] [PubMed] [Google Scholar]
- Levitt ES, Clark MJ, Jenkins PM, Martens JR, and Traynor JR (2009). Differential effect of membrane cholesterol removal on μ- and δ-opioid receptors. A parallel comparison of acute and chronic signaling to adenylyl cyclase. J Biol Chem 284, 22108–22122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse MJ, Benovic JL, Codina J, Caron MG, and Lefkowitz RJ (1990). β-arrestin: A protein that regulates β-adrenergic receptor function. Science (80-) 248, 1547–1550. [DOI] [PubMed] [Google Scholar]
- Lohse MJ, and Calebiro D (2013). Cell biology: Receptor signals come in waves. Nature 495, 457–458. [DOI] [PubMed] [Google Scholar]
- Luttrell LM et al. (1999). β-arrestin-dependent formation of β2 adrenergic receptor-src protein kinase complexes. Science (80-) 283, 655–661. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, and Lefkowitz RJ (2002). The role of β-arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci 115, 455–465. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Roudabush FL, Choy EW, Miller WE, Field ME, Pierce KL, and Lefkowitz RJ (2001). Activation and targeting of extracellular signal-regulated kinases by β-arrestin scaffolds. Proc Natl Acad Sci U S A 98, 2449–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyga S, Volpe S, Werthmann RC, Götz K, Sungkaworn T, Lohse MJ, and Calebiro D (2016). Persistent cAMP Signaling by Internalized LH Receptors in Ovarian Follicles. Endocrinology 157, 1613–1621. [DOI] [PubMed] [Google Scholar]
- Manglik A, Kobilka BK, and Steyaert J (2017). Nanobodies to Study G Protein-Coupled Receptor Structure and Function. Annu Rev Pharmacol Toxicol 57, 19–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marley A, Choy RWY, and von Zastrow M (2013). GPR88 Reveals a Discrete Function of Primary Cilia as Selective Insulators of GPCR Cross-Talk. PLoS One 8, 70857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald PH, Chow C-W, Miller WE, Laporte SA, Field ME, Lin F-T, Davis RJ, and Lefkowitz RJ (2000). beta -Arrestin 2: A Receptor-Regulated MAPK Scaffold for the Activation of JNK3. Science (80-) 290, 1574–1577. [DOI] [PubMed] [Google Scholar]
- Van Meer G, Voelker DR, and Feigenson GW (2008). Membrane lipids: Where they are and how they behave. Nat Rev Mol Cell Biol 9, 112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlen C et al. (2013). Intracrine endothelin signalling evokes IP3-dependent increases in nucleoplasmic Ca 2+ in adult cardiac myocytes *. J Mol Cell Cardiol 62, 189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal N et al. (2013). Select G-protein-coupled receptors modulate agonist-induced signaling via a ROCK, LIMK, and β-arrestin 1 pathway. Cell Rep 5, 1010–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BS, Stepanchick AN, Tewson PH, Hartle CM, Zhang J, Quinn AM, Hughes TE, and Mirshahi T (2016). Cilia have high cAMP levels that are inhibited by Sonic Hedgehog-regulated calcium dynamics. Proc Natl Acad Sci U S A 113, 13069–13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Zha X, Tabas I, and Maxfield FR (1998). Cholesterol distribution in living cells: Fluorescence imaging using dehydroergosterol as a fluorescent cholesterol analog. Biophys J 75, 1915–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S, Wen X, Ratti N, Loktev A, Rangell L, Scales SJ, and Jackson PK (2013). The ciliary G-protein-coupled receptor Gpr161 negatively regulates the sonic hedgehog pathway via cAMP signaling. Cell 152, 210–223. [DOI] [PubMed] [Google Scholar]
- Mullershausen F, Zecri F, Cetin C, Billich A, Guerini D, and Seuwen K (2009). Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nat Chem Biol 5, 428–434. [DOI] [PubMed] [Google Scholar]
- Mykytyn K, and Askwith C (2017). G-Protein-Coupled receptor signaling in cilia. Cold Spring Harb Perspect Biol 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash CA, Wei W, Irannejad R, and Smrcka AV. (2019). Golgi localized βi-adrenergic receptors stimulate golgi PI4P hydrolysis by PLCε to regulate cardiac hypertrophy. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehmé R, Carpenter B, Singhal A, Strege A, Edwards PC, White CF, Du H, Grisshammer R, and Tate CG (2017). Mini-G proteins: Novel tools for studying GPCRs in their active conformation. PLoS One 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Caron MG, and Barak LS (2000). Differential affinities of visual arrestin, βarrestin1, and βarrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem 275, 17201–17210. [DOI] [PubMed] [Google Scholar]
- Oates J, Faust B, Attrill H, Harding P, Orwick M, and Watts A (2012). The role of cholesterol on the activity and stability of neurotensin receptor 1. Biochim Biophys Acta - Biomembr 1818, 2228–2233. [DOI] [PubMed] [Google Scholar]
- Ostrom RS, Liu X, Head BP, Gregorian C, Seasholtz TM, and Insel PA (2002). Localization of adenylyl cyclase isoforms and G protein-coupled receptors in vascular smooth muscle cells: Expression in caveolin-rich and noncaveolin domains. Mol Pharmacol 62, 983–992. [DOI] [PubMed] [Google Scholar]
- Patel HH, Murray F, and Insel PA (2008). G-Protein-Coupled Receptor-Signaling Components in Membrane Raft and Caveolae Microdomains. In: Handbook of Experimental Pharmacology, Handb Exp Pharmacol, 167–184. [DOI] [PubMed] [Google Scholar]
- Peterson YK, and Luttrell LM (2017). The Diverse Roles of Arrestin Scaffolds in G Protein-Coupled Receptor Signaling. Pharmacol Rev 69, 256–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasanna X, Chattopadhyay A, and Sengupta D (2014). Cholesterol modulates the dimer interface of the β2- adrenergic receptor via cholesterol occupancy sites. Biophys J 106, 1290–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucadyil TJ, and Chattopadhyay A (2004). Cholesterol modulates ligand binding and G-protein coupling to serotonin1A receptors from bovine hippocampus. Biochim Biophys Acta - Biomembr 1663, 188–200. [DOI] [PubMed] [Google Scholar]
- Purgert CA, Izumi Y, Jong YJI, Kumar V, Zorumski CF, and O’Malley KL (2014). Intracellular mGluR5 can mediate synaptic plasticity in the hippocampus. J Neurosci 34, 4589–4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthenveedu MA, Lauffer B, Temkin P, Vistein R, Carlton P, Thorn K, Taunton J, Weiner OD, Parton RG, and Zastrow M Von (2010). Sequence-Dependent Sorting of Recycling Proteins by Actin-Stabilized Endosomal Microdomains. Cell 143, 761–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjan R, Dwivedi H, Baidya M, Kumar M, and Shukla AK (2017). Novel Structural Insights into GPCR–β-Arrestin Interaction and Signaling. Trends Cell Biol 27, 851–862. [DOI] [PubMed] [Google Scholar]
- Rozenfeld R, and Devi LA (2008). Regulation of CB 1 cannabinoid receptor trafficking by the adaptor protein AP-3. FASEB J 22, 2311–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo A, Soh UJK, Paing MM, Arora P, and Trejo JA (2009). Caveolae are required for protease-selective signaling by protease-activated receptor-1. Proc Natl Acad Sci U S A 106, 6393–6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini DK, Kalyanaraman V, Chisari M, and Gautam N (2007). A family of G protein βγ subunits translocate reversibly from the plasma membrane to endomembranes on receptor activation. J Biol Chem 282, 24099–24108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergin I, Jong YJI, Harmon SK, Kumar V, and O’Malley KL (2017). Sequences within the C terminus of the metabotropic glutamate receptor 5 (mGluR5) are responsible for inner nuclear membrane localization. J Biol Chem 292, 3637–3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiwarski DJ, Crilly SE, Dates A, and Puthenveedu MA (2019). Dual RXR motifs regulate nerve growth factor–mediated intracellular retention of the delta opioid receptor. Mol Biol Cell 30, 680–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiwarski DJ, Darr M, Telmer CA, Bruchez MP, and Puthenveedu MA (2017a). PI3K class II α regulates δ-opioid receptor export from the trans-Golgi network. Mol Biol Cell 28, 2202–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiwarski DJ, Tipton A, Giraldo MD, Schmidt BF, Gold MS, Pradhan AA, and Puthenveedu MA (2017b). A PTEN-regulated checkpoint controls surface delivery of σ opioid receptors. J Neurosci 37, 3741–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J, Wen X, and Scales SJ (2015). The orphan G protein-coupled receptor Gpr175 (Tpra40) enhances Hedgehog signaling by modulating cAMP levels. J Biol Chem 290, 29663–29675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slessareva JE, Routt SM, Temple B, Bankaitis VA, and Dohlman HG (2006). Activation of the Phosphatidylinositol 3-Kinase Vps34 by a G Protein α Subunit at the Endosome. Cell 126, 191–203. [DOI] [PubMed] [Google Scholar]
- Sposini S, Jean-Alphonse FG, Ayoub MA, Oqua A, West C, Lavery S, Brosens JJ, Reiter E, and Hanyaloglu AC (2017). Integration of GPCR Signaling and Sorting from Very Early Endosomes via Opposing APPL1 Mechanisms. Cell Rep 21, 2855–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Louis É, Degrandmaison J, Grastilleur S, Génier S, Blais V, Lavoie C, Parent J-L, and Gendron L (2017). Involvement of the coatomer protein complex I in the intracellular traffic of the delta opioid receptor. Mol Cell Neurosci 79, 53–63. [DOI] [PubMed] [Google Scholar]
- Stoeber M, Jullié D, Lobingier BT, Laeremans T, Steyaert J, Schiller PW, Manglik A, and von Zastrow M (2018). A Genetically Encoded Biosensor Reveals Location Bias of Opioid Drug Action. Neuron 98, 963–976.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohman MJ, Maeda S, Hilger D, Masureel M, Du Y, and Kobilka BK (2019). Local membrane charge regulates β2 adrenergic receptor coupling to Gi3. Nat Commun 10, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadevosyan A et al. (2015). Photoreleasable ligands to study intracrine angiotensin II signalling. J Physiol 593, 521–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temkin P, Lauffer B, Jäger S, Cimermancic P, Krogan NJ, and Von Zastrow M (2011). SNX27 mediates retromer tubule entry and endosome-to-plasma membrane trafficking of signalling receptors. Nat Cell Biol 13, 715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen ARB et al. (2016). GPCR-G Protein-β-Arrestin Super-Complex Mediates Sustained G Protein Signaling. Cell 166, 907–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetanova NG, Trester-Zedlitz M, Newton BW, Riordan DP, Sundaram AB, Johnson JR, Krogan NJ, and Von Zastrow M (2017). G protein-coupled receptor endocytosis confers uniformity in responses to chemically distinct ligands. Mol Pharmacol 91, 145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetanova NG, and von Zastrow M (2014). Spatial encoding of cyclic AMP signaling specificity by GPCR endocytosis. Nat Chem Biol 10, 1061–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varsano T, Taupin V, Guo L, Baterina OY, and Farquhar MG (2012). The PDZ Protein GIPC Regulates Trafficking of the LPA1 Receptor from APPL Signaling Endosomes and Attenuates the Cell’s Response to LPA. PLoS One 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent K et al. (2016). Intracellular mGluR5 plays a critical role in neuropathic pain. Nat Commun 7, 10604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vögler O, Barceló JM, Ribas C, and Escribá PV. (2008). Membrane interactions of G proteins and other related proteins. Biochim Biophys Acta - Biomembr 1778, 1640–1652. [DOI] [PubMed] [Google Scholar]
- Wan Q, Okashah N, Inoue A, Nehmé R, Carpenter B, Tate CG, and Lambert NA (2018). Mini G protein probes for active G protein-coupled receptors (GPCRs) in live cells. J Biol Chem, jbc.RA118.001975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedegaertner PB, Bourne HR, and Von Zastrow M (1996). Activation-induced subcellular redistribution of G(sα). Mol Biol Cell 7, 1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehbi VL, Stevenson HP, Feinstein TN, Calero G, Romero G, and Vilardaga JP (2013). Noncanonical GPCR signaling arising from a PTH receptor-arrestin- Gβγ complex. Proc Natl Acad Sci U S A 110, 1530–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Ahn S, Shenoy SK, Karnik SS, Hunyady L, Luttrell LM, and Lefkowitz RJ (2003). Independent β-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc Natl Acad Sci U S A 100, 10782–10787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg ZY, and Puthenveedu MA (2019). Regulation of G protein-coupled receptor signaling by plasma membrane organization and endocytosis. Traffic 20, 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg ZY, Zajac AS, Phan T, Shiwarski DJ, and Puthenveedu MA (2017). Sequence-Specific Regulation of Endocytic Lifetimes Modulates Arrestin-Mediated Signaling at the μ Opioid Receptor. Mol Pharmacol 91, 416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis WI, and Kobilka BK (2018). The Molecular Basis of G Protein–Coupled Receptor Activation. Annu Rev Biochem 87, 897–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch EJ, Jones BW, and Scott JD (2010). Network with the AKAPs context-dependent regulation of anchored Enzymes. Mol Interv 10, 86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfield GH et al. (2011). Structural flexibility of the G alpha s alpha-helical domain in the beta2-adrenoceptor Gs complex. Proc Natl Acad Sci U S A 108, 16086–16091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CD, Wu SC, Dahl EF, Sazama AJ, and O’Connell TD (2012). Nuclear localization drives α1-adrenergic receptor oligomerization and signaling in cardiac myocytes. Cell Signal 24, 794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu V, Yeerna H, Nohata N, Chiou J, Harismendy O, Raimondi F, Inoue A, Russell RB, Tamayo P, and Gutkind JS (2019). Illuminating the Onco-GPCRome: Novel G protein-coupled receptor-driven oncocrine networks and targets for cancer immunotherapy. J Biol Chem 294, 11062–11086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Rybin VO, Steinberg SF, and Kobilka B (2002). Caveolar localization dictates physiologic signaling of β2-adrenoceptors in neonatal cardiac myocytes. J Biol Chem 277, 34280–34286. [DOI] [PubMed] [Google Scholar]
- Yarwood RE et al. (2017). Endosomal signaling of the receptor for calcitonin gene-related peptide mediates pain transmission. Proc Natl Acad Sci U S A 114, 12309–12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen HY et al. (2018). PtdIns(4,5)P2 stabilizes active states of GPCRs and enhances selectivity of G-protein coupling. Nature 559, 423–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Malik S, Kelley GG, Kapiloff MS, and Smrcka AV. (2011). Phospholipase Cε scaffolds to muscle-specific A kinase anchoring protein (mAKAPβ) and integrates multiple hypertrophic stimuli in cardiac myocytes. J Biol Chem 286, 23012–23021. [DOI] [PMC free article] [PubMed] [Google Scholar]


