Abstract
Background
Sarcopenia is associated with cognitive impairment in older adults. However, the underlying mechanisms are not fully understood.
Aim
To explore the mediating role of nutritional status in the relationship between sarcopenia and cognitive impairment.
Methods
Three thousand eight hundred and ten participants (mean age 61.94 ± 8.01 years) from the West China Health and Aging Trend (WCHAT) study were included. We defined sarcopenia using the Asian Working Group for Sarcopenia (AWGS) 2019 criteria. Cognitive status and nutritional status were measured using the Short Portable Mental Status Questionnaire (SPMSQ) and the Mini Nutritional Assessment Short Form (MNA-SF). Relationships between sarcopenia, nutritional status, and cognitive function were explored using multiple linear regression. Two mediation models were generated to examine whether nutritional status mediates the association between sarcopenia and cognitive function using PROCESS macro version 3.5.
Results
The study involved 3147 (82.6%) non-sarcopenic, 387 (10.2%) sarcopenic, and 276 (7.2%) severely sarcopenic individuals. In mediation model 1, sarcopenia (β = 0.208, 95% CI 0.072 to 0.344, P = 0.0028) was significantly associated with cognitive impairment, and nutritional status mediated this association (indirect effect = 0.162, bootstrap 95% CI 0.116 to 0.212). Mediation model 2 indicated that nutritional status exhibited a full mediating effect regarding the association between sarcopenia and cognitive impairment (indirect effect = 0.131, bootstrap 95% CI: 0.08 to 0.188; direct effect = 0.046, bootstrap 95% CI − 0.115 to 0.21) and a partial mediating effect regarding the association between severe sarcopenia and cognitive impairment (indirect effect = 0.21, bootstrap 95% CI: 0.143 to 0.283; direct effect = 0.476, bootstrap 95% CI: 0.234–0.724).
Conclusions
The relationship between sarcopenia and cognitive impairment was significantly mediated by nutritional status. Early nutritional interventions may prevent cognitive decline in sarcopenic older adults.
Keywords: Sarcopenia, Cognitive function, Nutrition, Older adults
Introduction
Sarcopenia is an age-related skeletal muscle disorder involving decreased muscle mass, muscle strength, and function, and it is associated with adverse clinical outcomes including falls, frailty, disability, and mortality [1, 2]. Aging, malnutrition, sedentary lifestyle, mitochondrial dysfunction, inflammation, and oxidative stress are major risk factors for sarcopenia [3]. The prevalence of sarcopenia depends on the setting and the definition used. According to the Asian Working Group for Sarcopenia (AWGS) 2019 criteria, the prevalence of sarcopenia is approximately 14.9–32.2% in community-dwelling populations and 45.5–61.1% in clinic or hospital populations [4–7]. With aging populations, sarcopenia is likely to become a much more widespread public health concern worldwide in the future. The development of an International Classification of Diseases (ICD)-10 code for sarcopenia (M62.84) was an important step forward in recognizing sarcopenia as an independent condition, and this could lead to further research and a rapid increase in the development of sarcopenia screening, diagnosis, staging, and intervention in clinical practice [8].
Cognitive impairment is also a global public health issue. The prevalence of mild cognitive impairment (MCI) in adults aged ≥ 65 years is 10–20% [9], and approximately 50% will progress to dementia in 5 years [10]. Due to the increasing burden associated with dementia and the lack of effective treatments for cognitive impairment, identifying the most vulnerable populations and developing interventions are urgent. Studies have indicated that sarcopenic patients are vulnerable to developing cognitive impairment, and sarcopenia is common among individuals with dementia [11, 12]. Likewise, malnutrition is a risk factor for cognitive impairment, common among patients with dementia, and associated with sarcopenia [13, 14]. Therefore, understanding the complex interrelationships between sarcopenia, nutritional status, and cognitive impairment is critical because of the high prevalence of these conditions in aging populations.
In this study, we obtained baseline data from the West China Health and Aging Trend (WCHAT) study. We hypothesized that nutritional status mediates the relationship between sarcopenia and cognitive impairment, and confirming this relationship may contribute to the widespread adoption of nutrition-related interventions in sarcopenic older adults. Thus, we performed a mediation analysis to examine whether the independent variable “sarcopenia” affects the dependent variable “cognitive impairment” through the indirect effect of the mediating variable “malnutrition,” independent of relevant covariates. To identify the optimal intervention period, participants were further classified into non-sarcopenic, sarcopenic, and severely sarcopenic individuals (according to the AWGS 2019 criteria) in our mediation analysis.
Methods
Study design and participants
The WCHAT study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of West China Hospital, Sichuan University (No. 2017(445)). The study design has been described in detail elsewhere [15, 16]. Briefly, the WCHAT study is a multicenter prospective cohort study conducted in four provinces in western China to assess the health and aging status of individuals from a variety of ethnic backgrounds. This study is a cross-sectional analysis of baseline data collected between July and December 2018. Questionnaire data were collected by trained interviewers via face-to-face interviews. Anthropometric and bioimpedance measurements were conducted by trained technicians. Of the 7536 WCHAT participants, we excluded individuals who did not have sarcopenia assessment data (n = 3502), cognitive status data (n = 217), and nutritional status data (n = 7). Finally, 3810 participants were included in the analysis.
Sarcopenia assessment
For sarcopenia diagnosis, we applied the latest criteria published by the Asia Working Group for Sarcopenia, the AWGS 2019 criteria [17]. Muscle mass was measured by bioelectrical impedance analysis (Inbody 770; BioSpace, Seoul, Korea). Low muscle mass was defined as appendicular skeletal muscle mass index (ASMI, ASM/height2) < 7.0 and < 5.7 kg/m2 for men and women, respectively. Muscle strength was assessed using a handgrip strength dynamometer (EH101; Camry, Zhongshan, China). Participants performed two independent tests with the dominant hand and the higher value was defined as the handgrip strength. Low handgrip strength was defined as < 28 and < 18 kg for men and women, respectively. Physical performance was measured using the Short Physical Performance Battery (SPPB) [18]. The SPPB involves a short walk (4 m), five repeated chair stands, and a set of balance tests involving side‐by‐side, semi‐tandem, and tandem stands. The score for each item ranges from 0 to 4 (0 = unable to complete the test; 4 = highest performance level), with a total score ranging from 0 to 12. Low physical performance was defined as SPPB score ≤ 9. The AWGS 2019 criteria define sarcopenia as low muscle mass plus low muscle strength or low physical performance, while severe sarcopenia is defined as low muscle mass, low muscle strength, and low physical performance.
Nutritional and cognitive status assessment
Nutritional status was measured using the Mini Nutritional Assessment Short Form (MNA-SF), with 12–14, 8–11, and < 8 points indicating normal nutritional status, risk of malnutrition, and malnutrition, respectively [19]. Cognitive status was assessed using the Chinese version of the Short Portable Mental Status Questionnaire (SPMSQ) [20]. The total number of errors was counted and the scores were then adjusted based on each individual’s reported educational level. Scores of 0–2, 3–4, 5–7, and 8–10 were then categorized as normal cognitive function, mild cognitive function impairment, moderate cognitive function impairment, and severe cognitive function impairment, respectively.
Covariates
Age, gender (male/female), ethnicity (Han/Tibetan/Qiang/Yi/other minority ethnic group), marital status (single/married), educational level (illiterate/elementary school/middle school/high school and above), alcohol consumption (yes/no), smoking (yes/no), sleep quality, and number of chronic diseases (0 or 1/ ≥ 2) were collected at baseline. Height and weight were used to calculate body mass index. Sleep quality was evaluated using Pittsburgh sleep quality index (PSQI), a self-reported questionnaire examining seven sleep-related dimensions over the preceding month with total summed score ranging from 0 to 21 [21]. Scores > 5 indicated significant sleep disturbance. A self-reported questionnaire was used to collect data on chronic diseases, including diabetes mellitus, hypertension, coronary heart disease, chronic obstructive pulmonary disease, gastrointestinal disease, hepatic disease, kidney disease, stroke, Alzheimer's disease, arthritis, tumor, mental disorder, and other diseases. Multimorbidity was defined as having two or more chronic diseases.
Statistical analyses
The continuous variables are presented as mean ± SD, and the categorical variables are presented as frequency (percentage). Differences between groups were tested by one-way analysis of variance (ANOVA) followed by post hoc Bonferroni tests for continuous variables, and Pearson’s chi-square test for categorical variables. Multiple linear regression analyses were carried out with SPMSQ scores (cognitive status) and MNA-SF scores (nutritional status) as dependent variables, adjusted for age, gender, body mass index, educational level, and sleep quality, due to the known associations between these covariates and nutrition/cognition.
Statistical mediation models, adjusted for the aforementioned covariates, were generated to examine whether nutritional status mediates the association between sarcopenia and cognitive function using the PROCESS macro version 3.5. Two mediation models were used (model 1: sarcopenia vs non-sarcopenia; model 2: sarcopenia vs non-sarcopenia, and severe sarcopenia vs non-sarcopenia). Statistical mediation analysis was conducted using nonparametric bootstrapping with 5000 replications to calculate bias-corrected bootstrapped 95% confidence intervals (CIs). An indirect effect was considered significant if the 95% CI did not include zero.
All analyses were performed using SPSS software (version 26.0; IBM Corp., Armonk, NY, USA), and P < 0.05 was considered to be statistically significant.
Results
Baseline characteristics
We enrolled 3810 participants (1385 men and 2425 women) aged ≥ 50 years. The mean age was 61.94 ± 8.01 years. Table 1 shows the descriptive characteristics of the participants according to sarcopenia severity. There were 387 (10.2%) and 276 (7.2%) participants with sarcopenia and severe sarcopenia, respectively, according to the AWGS 2019 criteria, with a higher prevalence of sarcopenia in men than in women. Individuals with sarcopenia/severe sarcopenia were older than the non-sarcopenic individuals (P < 0.001). Non-sarcopenic individuals had higher body mass index and muscle mass compared to individuals with sarcopenia/severe sarcopenia (P < 0.001). Muscle strength (handgrip strength) decreased with increasing sarcopenia severity (P < 0.001), while physical performance (based on SPPB) was significantly different only between severe sarcopenia and the other two groups (P < 0.001).
Table 1.
Baseline characteristics of study participants
| Characteristics | Overall | Normal | Sarcopenia | Severe sarcopenia | P value |
|---|---|---|---|---|---|
| N = 3810 | N = 3147 | N = 387 | N = 276 | ||
| Age, year, mean (SD) | 61.94(8.01) | 60.84(7.45) | 64.63(7.77) * | 70.70(8.25)*a | < 0.001 |
| BMI, mean (SD) | 25.30(3.71) | 26.01(3.51) | 22.08(2.49) * | 21.74(2.80)* | < 0.001 |
| Anthropometric measures | |||||
| ASMI, mean (SD) | 6.65(0.93) | 6.83(0.86) | 5.82(0.67) * | 5.69(0.74)* | < 0.001 |
| Grip strength, mean (SD) | 22.40(8.63) | 23.30(8.72) | 19.86(7.03) * | 15.76(5.35)*a | < 0.001 |
| SPPB, mean (SD) | 10.15(1.75) | 10.36(1.63) | 10.16(1.64) | 7.72(1.30)*a | < 0.001 |
| Gender, N (%) | < 0.001 | ||||
| Male | 1385(36.4) | 1093(34.7) | 172(44.4) | 120(43.5) | |
| Female | 2425(63.6) | 2054(65.3) | 215(55.6) | 156(56.5) | |
| Ethnics | < 0.001 | ||||
| Han, N (%) | 1667(43.8) | 1311(41.7) | 206(53.2) | 150(54.3) | |
| Tibetan, N (%) | 963(25.3) | 810(25.8) | 92(23.8) | 61(22.1) | |
| Qiang, N (%) | 955(25.1) | 863(27.4) | 60(15.5) | 32(11.6) | |
| Yi, N (%) | 165(4.3) | 112(3.6) | 22(5.7) | 31(11.2) | |
| Others, N (%) | 58(1.5) | 49(1.6) | 7(1.8) | 2(0.7) | |
| Education, N (%) | < 0.001 | ||||
| Illiterate | 1099(28.8) | 857(27.2) | 116(30.0) | 126(45.7) | |
| Elementary school | 1308(34.3) | 1066(33.9) | 140(36.2) | 102(37.0) | |
| Middle school | 854(22.4) | 740(23.5) | 80(20.7) | 34(12.3) | |
| High school and above | 549(14.4) | 484(15.4) | 51(13.2) | 14(5.1) | |
| Marital status, N (%) | < 0.001 | ||||
| Married | 3229(84.8) | 2710(86.1) | 325(84.0) | 194(70.3) | |
| Single | 581(15.2) | 437(13.9) | 62(16.0) | 82(29.7) | |
| Cognitive function, N (%) | < 0.001 | ||||
| Complete cognitive function | 3316(87.0) | 2788(88.6) | 330(85.3) | 198(71.7) | |
| Mild impairment | 386(10.1) | 297(9.4) | 45(11.6) | 44(15.9) | |
| Moderate-severe impairment | 108(2.8) | 62(2.0) | 12(3.1) | 34(12.3) | |
| Nutritional status, N (%) | < 0.001 | ||||
| Malnutrition/nutritional risk | 712(18.7) | 442(14.0) | 141(36.4) | 129(46.7) | |
| Normal | 3098(81.3) | 2705(86.0) | 246(63.6) | 147(53.3) | |
| Life-styles | |||||
| Sleep quality, N (%) | < 0.001 | ||||
| PQSI > 5 | 1749(47.5) | 1419(46.6) | 171(45.7) | 159(60.5) | |
| PQSI ≤ 5 | 1933(52.5) | 1626(53.4) | 203(54.3) | 104(39.5) | |
| Smoking history, N (%) | < 0.001 | ||||
| Yes | 666(17.5) | 504(16.1) | 95(24.5) | 67(24.4) | |
| No | 3129(82.5) | 2629(83.9) | 292(75.5) | 208(75.6) | |
| Drinking alcohol, N (%) | 0.978 | ||||
| Yes | 988(25.9) | 814(25.9) | 102(26.4) | 72(26.1) | |
| No | 2821(74.1) | 2332(74.1) | 285(73.6) | 204(73.9) | |
| Number of chronic diseases, N (%) | 0.005 | ||||
| 0 or 1 | 3036(79.7) | 2529(80.4) | 308(79.6) | 199(72.1) | |
| ≥ 2 | 774(20.3) | 618(19.6) | 79(20.4) | 77(27.9) | |
SD standard deviation, BMI body mass index; ASMI appendicular skeletal muscle mass index, SPPB short physical performance battery, PQSI Pittsburgh sleep quality index
*P < 0.05 vs. normal
aP < 0.05 vs. sarcopenia
The prevalence of sarcopenia differed significantly among educational levels and among ethnic groups (P < 0.001). Participants who were single (unmarried/widowed/divorced) had a higher prevalence of sarcopenia (P < 0.001). Of all the participants, 386 (10.1%) had MCI, 108 (2.8%) had moderate/severe cognitive impairment, and 712 (18.7%) had malnutrition or risk of malnutrition. The prevalence of sarcopenia among participants with different cognitive function or nutritional status varied significantly (P < 0.001). Compared to the non-sarcopenic group, individuals with sarcopenia/severe sarcopenia had poor sleep quality and higher rates of smoking, multimorbidity, cognitive impairment, and malnutrition. The rate of alcohol consumption was 25.9% (P = 0.978), with no significant differences between sarcopenia stages.
Associations between sarcopenia, nutritional status, and cognitive function
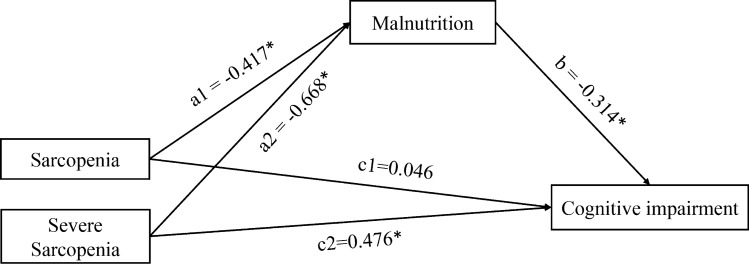
Table 2 shows the results of multiple linear regression analysis in two mediation models adjusted for age, gender, body mass index, educational level, and sleep quality. In mediation model 1, there were significant associations between sarcopenia and nutritional status (β = − 0.513, 95% CI − 0.636 to − 0.389, P < 0.001), nutritional status and cognitive status (β = − 0.317, 95% CI − 0.352 to − 0.281, P < 0.001), and sarcopenia and cognitive status (β = 0.208, 95% CI 0.072 to 0.344, P = 0.003). Multicategorical mediation model 2 involved categorizing participants into sarcopenic and severe sarcopenic individuals (according to the AWGS 2019 criteria). In model 2, both sarcopenia (β = − 0.417, 95% CI − 0.563 to − 0.272, P < 0.001) and severe sarcopenia (β = − 0.668, 95% CI − 0.844 to − 0.492, P < 0.001) were significantly associated with nutritional status. The association between nutritional status and cognitive status (β = − 0.314, 95% CI − 0.349 to − 0.279, P < 0.001) remained significant. However, only severe sarcopenia was directly associated with cognitive status (β = 0.476, 95% CI: 0.282 to 0.669, P < 0.001), not sarcopenia (β = 0.046, 95% CI: − 0.114 to 0.205, P = 0.575) (Fig. 1).
Table 2.
Multiple linear regression analysis in mediation models
| Dependent variable | Independent variable | β | SE | 95%CI | P | R2 | ||
|---|---|---|---|---|---|---|---|---|
| LLCI | ULCI | |||||||
| Model 1a | Nutritional status | Sarcopenia | − 0.513 | 0.063 | − 0.636 | − 0.389 | < 0 .001 | 0.23 |
| Cognitive function | Nutritional status | − 0.317 | 0.018 | − 0.352 | − 0.281 | < 0 .001 | 0.31 | |
| Sarcopenia | − 0.208 | 0.070 | 0.072 | 0.344 | 0.003 | |||
| Model 2a | Nutritional status | Sarcopenia | − 0.417 | 0.074 | − 0.563 | − 0.272 | < 0 .001 | 0.23 |
| Severe sarcopenia | − 0.668 | 0.090 | − 0.844 | − 0.492 | < 0 .001 | |||
| Cognitive function | Nutritional status | − 0.314 | 0.018 | − 0.349 | − 0.279 | < 0 .001 | 0.31 | |
| Sarcopenia | 0.046 | 0.081 | − 0.114 | 0.205 | 0.575 | |||
| Severe sarcopenia | 0.476 | 0.099 | 0.282 | 0.669 | < 0 .001 | |||
SE standard error, CI confidence interval
aadjusted for age, gender, body mass index, educational level, and sleep quality
Fig. 1.
Multicategorical mediation model of SPMSQ score (cognitive status). Paths a1 and a2 represent the effect of sarcopenia and severe sarcopenia on malnutrition; path b represents the effect of malnutrition on cognitive impairment; paths c1 and c2 represent the relative direct effect of sarcopenia and severe sarcopenia on cognitive impairment. Unstandardized regression coefficients are reported. *P < 0.05
Mediating effect of nutritional status on the relationship between sarcopenia and cognitive function
The mediating effect estimates in the two mediation models are shown in Table 3. In model 1, our mediation hypothesis was confirmed, because there was a significant indirect effect (mediating effect) regarding nutritional status partly mediating the association between sarcopenia and cognitive impairment (indirect effect = 0.162, bootstrap 95% CI 0.116–0.212). The proportion of the total effect mediated by nutritional status was 43.78%. In model 2, regarding the association between severe sarcopenia and cognitive impairment, the bias-corrected and accelerated bootstrap 95% CI of the indirect effect (0.143–0.283) and direct effect (0.234–0.724) excluded zero, which indicated a partial mediating effect of nutritional status. The proportion of the total effect mediated by nutritional status was 30.58%. Regarding the mediating effect of nutritional status between sarcopenia and cognitive impairment, the bootstrap 95% CI of the indirect effect (0.08 to 0.188) excluded zero, while that of the direct effect (− 0.115 to 0.21) encompassed zero. This indicates a full mediating effect of nutritional status regarding the association between sarcopenia and cognitive impairment. The proportion of the total effect mediated by nutritional status was 74.01%.
Table 3.
Mediation models: relative total, direct, and indirect effects
| Coefficient | Boot SE | 95%CI | Relative Proportion | |||
|---|---|---|---|---|---|---|
| Boot LLCI | Boot ULCI | |||||
| Model 1a | Total effects | 0.370 | 0.080 | 0.213 | 0.529 | |
| Direct effects | 0.208 | 0.075 | 0.063 | 0.357 | 56.22% | |
| Indirect effects | 0.162 | 0.025 | 0.116 | 0.212 | 43.78% | |
| Model 2a | Sarcopenia | |||||
| Total effects | 0.177 | 0.085 | 0.013 | 0.349 | ||
| Direct effects | 0.046 | 0.082 | − 0.115 | 0.210 | 25.99% | |
| Indirect effects | 0.131 | 0.027 | 0.080 | 0.188 | 74.01% | |
| Severe sarcopenia | ||||||
| Total effects | 0.685 | 0.137 | 0.419 | 0.955 | ||
| Direct effects | 0.476 | 0.126 | 0.234 | 0.724 | 69.42% | |
| Indirect effects | 0.210 | 0.036 | 0.143 | 0.283 | 30.58% | |
Boot SE bootstrap standard error, Boot CI bootstrap confidence interval
aAdjusted for age, gender, BMI, educational level and sleep quality
Discussion
The present study used mediation models to explore the mediation role of nutritional status between two important clinical conditions: sarcopenia and cognitive impairment. Our findings indicated that malnutrition is a significant contributor to cognitive impairment in sarcopenic older adults. To the best of our knowledge, there are no other studies that highlight malnutrition as a mediator of the relationship between sarcopenia and cognitive impairment in older adults. Our results indicated that improving nutritional status may ameliorate the negative impact of sarcopenia on cognitive impairment.
Although no studies have examined nutrition as a mediator between sarcopenia and cognitive impairment, studies have highlighted the relationships between sarcopenia and cognitive impairment, sarcopenia and nutrition, and nutrition and cognitive impairment. A recent systematic review of 15 studies (n = 10,410) found that sarcopenia in older adults increases the risk of cognitive impairment independent of participant characteristics or sarcopenia/cognitive impairment diagnostic criteria [11]. This meta-analysis indicated that sarcopenia was associated with an approximately twofold increased risk of MCI and dementia [11]. Similarly, sarcopenia is common among dementia patients [12]. The shared pathophysiological mechanisms and pathways between sarcopenia and cognitive impairment involve oxidative stress, chronic inflammation, hormonal changes, insulin resistance, and the gut microbiota [11, 22, 23]. Thus, the relationship between sarcopenia and cognitive impairment is complex and mediated by various internal and external factors, among which nutrition may be a major contributor. A prospective cohort study reported that malnutrition led to an approximately fourfold increased risk of sarcopenia/severe sarcopenia during a 4-year follow-up [14]. Additionally, a study showed that compared to non-sarcopenia individuals, sarcopenic individuals were more likely to have poorer nutritional status and a higher risk of malnutrition [24]. In addition, accumulating evidence suggest that nutrition is important for optimizing cognition and reducing the risk of dementia [25, 26]. Overall, our results are consistent with previous research on the relationship between sarcopenia and cognitive impairment. Thus, the mediating role of nutritional status is robust. Studies are needed to further explore the common underlying determinants of sarcopenia and cognitive impairment.
Sarcopenia and cognitive deterioration are two common geriatric syndromes with few effective treatments. Therefore, modifiable risk factors, such as nutritional status, provide an opportunity for intervention. In mediation model 1, the relationship between sarcopenia and cognitive impairment consisted of a relative direct effect and a relative indirect effect. The relative indirect effect (mediating effect) of nutrition accounted for 43.78% of the total effect. When we categorized sarcopenia into sarcopenia and severe sarcopenia in mediation model 2, both the direct effect (69.42%) and indirect effect (30.58%) of nutritional status remained significant between severe sarcopenia and cognitive impairment, while only the indirect effect (74.01%) of nutritional status remained significant between sarcopenia and cognitive impairment, which is known as a full mediating effect. This indicates that nutritional status mediates the association between sarcopenia and cognitive function, especially regarding non-severe sarcopenia (i.e., low muscle mass + low muscle strength/low physical performance). As shown in Table 3, the relative total effects of sarcopenia on cognitive impairment increased with sarcopenia severity, while the proportion of relative indirect effects (mediated by nutritional status) decreased with sarcopenia severity. This may be attributable to a higher comorbidity burden among severely sarcopenic older adults. In these cases, mediators other than nutrition may also influence the association between severe sarcopenia and cognitive impairment, which makes the interrelationships complicated. Thus, early nutritional intervention may have a better effect regarding preventing cognitive impairment in sarcopenic older adults.
Multiple findings indicate that healthy lifestyle habits protect against cognitive deterioration [27]. Dietary patterns, including nutritional interventions, have received enormous interest from researchers. Studies have indicated that various dietary patterns, including the Mediterranean diet (MedDi), Dietary Approaches to Stop Hypertension (DASH), Mediterranean-DASH diet Intervention for Neurological Delay (MIND), ketogenic diet, and caloric restriction may prevent or delay cognitive deterioration [28]. Several studies have shown that the higher the adherence to MedDi, the lower the risk of cognitive impairment and dementia [29, 30]. Although the mechanisms underlying the proposed benefits of MedDi regarding preventing cognitive deterioration are not fully understood, several pathways may explain this neuroprotective effect, including reduction in oxidative stress and inflammation, interference with amyloid aggregation, improvement of cardiovascular health, modification of the gut microbiota, and stimulation of epigenetic modification [28, 31, 32]. Notably, several studies have suggested that MedDi protects against sarcopenia [33, 34], though there is no consensus [35]. In a word, MedDi may be a beneficial choice for sarcopenic older adults to prevent or delay cognitive deterioration. In contrast to the above dietary patterns, the role of special nutritional supplements (vitamins B6, B12, C, and E; folic acid; and n-3 polyunsaturated fatty acids) in the prevention/improvement of cognitive decline is unclear [36, 37]. Additional research is needed to explore whether nutritional supplements can slow age-associated decreases in cognitive function and which nutritional supplements are suitable for sarcopenic older adults.
There are several limitations in this study. First, we could only establish associations rather than causality because of the cross-sectional design of this study. Previous studies indicated that the association between sarcopenia and cognitive impairment is complex and bidirectional [11, 23]. Only longitudinal studies that evaluate the actual temporal relationship between these factors can determine a causal link. Second, although we adjusted for several important confounders in our mediation models, residual confounding factors such as chronic diseases cannot be ruled out. Comorbid chronic diseases are common among older adults. Due to the large sample size and distribution of survey participants over a large area, a self-reported questionnaire was used to assess chronic diseases. Further studies are needed to collect accurate healthcare information based on medical records to evaluate the roles of chronic diseases in the development of malnutrition, sarcopenia, and/or cognitive impairment. Third, centralized investigation rather than a household survey may result in an underestimation of malnutrition. Fourth, we used the MNA-SF to assess nutritional status rather than a comprehensive nutritional assessment due to our large sample size. However, despite these limitations, the novelty of the study and the large sample of community-dwelling older adults with a variety of ethnic backgrounds are strengths of this study.
Conclusions
In conclusion, our study demonstrated that the relationship between sarcopenia and cognitive impairment is mediated by nutritional status. Accordingly, improving the nutritional status of sarcopenic older adults could counteract the decreased cognitive function associated with sarcopenia. Early nutritional intervention is recommended for improved prevention.
Funding
This work was supported by 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (Grant No. ZY2017201); Geriatric Health Care and Medical Research Center, Sichuan University, Chengdu, Sichuan Province, China; National Key R&D Program of China (Grant No. 2017YFC0840100 and Grant No. 2017YFC0840101); National Clinical Research Center for Geriatric (Grant No. Z2018B09); the Fundamental Research Funds for the Central University (Grant No. 20826041D4046); Post-doc Coronavirus Epidemic Prevention and Control Fund (Grant No. 0040204153349); West China Hospital Postdoctoral Fund (Grant No. 2020HXBH011). The financial sponsors had no role in the design, implementation, analyses, or reporting of the results.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Research on human and animal participants
This study was approved by the Ethics Committee of West China Hospital, Sichuan University (No.2017(445)).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. 2019;393:2636–2646. doi: 10.1016/S0140-6736(19)31138-9. [DOI] [PubMed] [Google Scholar]
- 2.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marzetti E, Calvani R, Tosato M, et al. Sarcopenia: an overview. Aging Clin Exp Res. 2017;29:11–17. doi: 10.1007/s40520-016-0704-5. [DOI] [PubMed] [Google Scholar]
- 4.Nagaura Y, Kondo H, Nagayoshi M, et al. Sarcopenia is associated with insomnia in Japanese older adults: a cross-sectional study of data from the Nagasaki Islands study. BMC Geriatr. 2020;20:256. doi: 10.1186/s12877-020-01658-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pang BWJ, Wee SL, Lau LK, et al. Prevalence and associated factors of sarcopenia in Singaporean adults-the Yishun study. J Am Med Dir Assoc. 2020 doi: 10.1016/j.jamda.2020.05.029. [DOI] [PubMed] [Google Scholar]
- 6.Kirk B, Zanker J, Bani Hassan E, et al. Sarcopenia definitions and outcomes consortium (SDOC) criteria are strongly associated with malnutrition, depression, falls, and fractures in high-risk older persons. J Am Med Dir Assoc. 2020 doi: 10.1016/j.jamda.2020.06.050. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen TN, Nguyen AT, Khuong LQ, et al. Reliability and validity of SARC-F questionnaire to assess sarcopenia among Vietnamese geriatric patients. Clin Interv Aging. 2020;15:879–886. doi: 10.2147/CIA.S254397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anker SD, Morley JE, von Haehling S. Welcome to the ICD-10 code for sarcopenia. J Cachexia Sarcopenia Muscle. 2016;7:512–514. doi: 10.1002/jcsm.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA. 2014;312:2551–2561. doi: 10.1001/jama.2014.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morley JE. An overview of cognitive impairment. Clin Geriatr Med. 2018;34:505–513. doi: 10.1016/j.cger.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Peng TC, Chen WL, Wu LW, et al. Sarcopenia and cognitive impairment: a systematic review and meta-analysis. Clin Nutr. 2020;39:2695–2701. doi: 10.1016/j.clnu.2019.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Umegaki H, Bonfiglio V, Komiya H, et al. Association between sarcopenia and quality of life in patients with early dementia and mild cognitive impairment. J Alzheimers Dis. 2020;76:435–442. doi: 10.3233/JAD-200169. [DOI] [PubMed] [Google Scholar]
- 13.Gomez-Gomez ME, Zapico SC. Frailty, cognitive decline, neurodegenerative diseases and nutrition interventions. Int J Mol Sci. 2019;20:2842. doi: 10.3390/ijms20112842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beaudart C, Sanchez-Rodriguez D, Locquet M, et al. Malnutrition as a strong predictor of the onset of sarcopenia. Nutrients. 2019;11:2883. doi: 10.3390/nu11122883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X, Hou L, Xia X, et al. Prevalence of sarcopenia in multi ethnics adults and the association with cognitive impairment: findings from West-China Health and aging trend study. BMC Geriatr. 2020;20:63. doi: 10.1186/s12877-020-1468-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Ge M, Zhao W, et al. Association between number of teeth, denture use and frailty: findings from the West China health and aging trend study. J Nutr Health Aging. 2020;24:423–428. doi: 10.1007/s12603-020-1346-z. [DOI] [PubMed] [Google Scholar]
- 17.Chen LK, Woo J, Assantachai P, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21:300–307. doi: 10.1016/j.jamda.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 19.Kaiser MJ, Bauer JM, Ramsch C, et al. Validation of the mini nutritional assessment short-form (MNA-SF): a practical tool for identification of nutritional status. J Nutr Health Aging. 2009;13:782–788. doi: 10.1007/s12603-009-0214-7. [DOI] [PubMed] [Google Scholar]
- 20.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23:433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 21.Buysse DJ, Reynolds CF, 3rd, Monk TH, et al. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 22.Low S, Ng TP, Lim CL, et al. Association between lower extremity skeletal muscle mass and impaired cognitive function in Type 2 Diabetes. Sci Rep. 2020;10:2956. doi: 10.1038/s41598-020-59914-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jia L, Zhang H. Sarcopenia and cognitive impairment. Clin Nutr. 2020;39:3207–3208. doi: 10.1016/j.clnu.2020.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Eglseer D, Eminovic S, Lohrmann C. Association between sarcopenia and nutritional status in older adults: a systematic literature review. J Gerontol Nurs. 2016;42:33–41. doi: 10.3928/00989134-20160613-03. [DOI] [PubMed] [Google Scholar]
- 25.Abbatecola AM, Russo M, Barbieri M. Dietary patterns and cognition in older persons. Curr Opin Clin Nutr Metab Care. 2018;21:10–13. doi: 10.1097/MCO.0000000000000434. [DOI] [PubMed] [Google Scholar]
- 26.Power R, Prado-Cabrero A, Mulcahy R, et al. The role of nutrition for the aging population: implications for cognition and Alzheimer's disease. Annu Rev Food Sci Technol. 2019;10:619–639. doi: 10.1146/annurev-food-030216-030125. [DOI] [PubMed] [Google Scholar]
- 27.Gill SS, Seitz DP. Lifestyles and cognitive health: what older individuals can do to optimize cognitive outcomes. JAMA. 2015;314:774–775. doi: 10.1001/jama.2015.9526. [DOI] [PubMed] [Google Scholar]
- 28.Vinciguerra F, Graziano M, Hagnas M, et al. Influence of the Mediterranean and ketogenic Diets on cognitive status and decline: a narrative review. Nutrients. 2020;12:1019. doi: 10.3390/nu12041019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor MK, Mahnken JD, Sullivan DK. NHANES 2011–2014 reveals cognition of US older adults may benefit from better adaptation to the Mediterranean Diet. Nutrients. 2020;12:1929. doi: 10.3390/nu12071929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shannon OM, Stephan BCM, Granic A, et al. Mediterranean diet adherence and cognitive function in older UK adults: the European prospective investigation into cancer and nutrition-norfolk (EPIC-Norfolk) study. Am J Clin Nutr. 2019;110:938–948. doi: 10.1093/ajcn/nqz114. [DOI] [PubMed] [Google Scholar]
- 31.Bauer JM, Morley JE. The relevance of healthy diets for the prevention of frailty and cognitive impairment. Curr Opin Clin Nutr Metab Care. 2018;21:1–3. doi: 10.1097/MCO.0000000000000438. [DOI] [PubMed] [Google Scholar]
- 32.Casamenti F, Stefani M. Olive polyphenols: new promising agents to combat aging-associated neurodegeneration. Expert Rev Neurother. 2017;17:345–358. doi: 10.1080/14737175.2017.1245617. [DOI] [PubMed] [Google Scholar]
- 33.Isanejad M, Sirola J, Mursu J, et al. Association of the Baltic Sea and Mediterranean diets with indices of sarcopenia in elderly women. OSPTRE-FPS study Eur J Nutr. 2018;57:1435–1448. doi: 10.1007/s00394-017-1422-2. [DOI] [PubMed] [Google Scholar]
- 34.Kelaiditi E, Jennings A, Steves CJ, et al. Measurements of skeletal muscle mass and power are positively related to a Mediterranean dietary pattern in women. Osteoporos Int. 2016;27:3251–3260. doi: 10.1007/s00198-016-3665-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan R, Leung J, Woo J. A prospective cohort study to examine the association between dietary patterns and sarcopenia in Chinese community-dwelling older people in Hong Kong. J Am Med Dir Assoc. 2016;17:336–342. doi: 10.1016/j.jamda.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 36.D'Cunha NM, Georgousopoulou EN, Dadigamuwage L, et al. Effect of long-term nutraceutical and dietary supplement use on cognition in the elderly: a 10-year systematic review of randomised controlled trials. Br J Nutr. 2018;119:280–298. doi: 10.1017/S0007114517003452. [DOI] [PubMed] [Google Scholar]
- 37.Rutjes AW, Denton DA, Di Nisio M, et al. Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in mid and late life. Cochrane Database Syst Rev. 2018;12:11906. doi: 10.1002/14651858.CD011906.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]