Clinical Implications.
-
•
Patients with mastocytosis and monoclonal mast cell activation syndrome tolerate COVID-19 vaccination.
Mastocytosis is characterized by abnormal clonal mast cell expansion and accumulation in one or more organ systems. Symptoms of mast cell activation in these patients include pruritus, flushing, and recurrent anaphylaxis. Lifetime risk for anaphylaxis in patients with mastocytosis is greatly increased over the general population and has been reported in up to 49% of some cohorts.1 Eliciting factors of anaphylaxis include venom stings, drugs, and food.2 There are limited data on the tolerance of vaccinations in patients with mastocytosis. Prior work predominantly focused on children with mastocytosis who were shown to have a higher rate of adverse reactions to the first administration of vaccine compared with that of the general population.3 The COVID-19 pandemic has led to the development of multiple vaccines, but early rollout of these vaccines has been plagued by reports of allergic reactions including anaphylaxis.4 This has led to concern about whether patients with a propensity for anaphylaxis (such as patients with mastocytosis) can safely tolerate COVID-19 vaccines,5 , 6 and a National Institutes of Health–sponsored multicenter trial to examine this question.7 We present a series of 18 patients with a history of mastocytosis who underwent COVID-19 vaccination (full series).
We performed a retrospective chart review of 259 patients with a diagnosis of cutaneous mastocytosis, systemic mastocytosis, mastocytosis in skin, or monoclonal mast cell activation syndrome (MMAS) who received care through the University of Michigan Health system from January 1, 2018 to March 17, 2021. Patients may have been evaluated by Allergy-Immunology, Hematology-Oncology, Dermatology, or Primary Care. All charts of patients with mastocytosis were reviewed to validate the diagnosis based on World Health Organization criteria. Patients with MMAS were reviewed based on symptoms of mast cell activation with either KIT D816V mutation or CD25+ mast cells in the bone marrow. Individual charts were reviewed to confirm a history of anaphylaxis, allergic reactions to food, medications, venom, contrast, and vaccines (including COVID-19 vaccines).
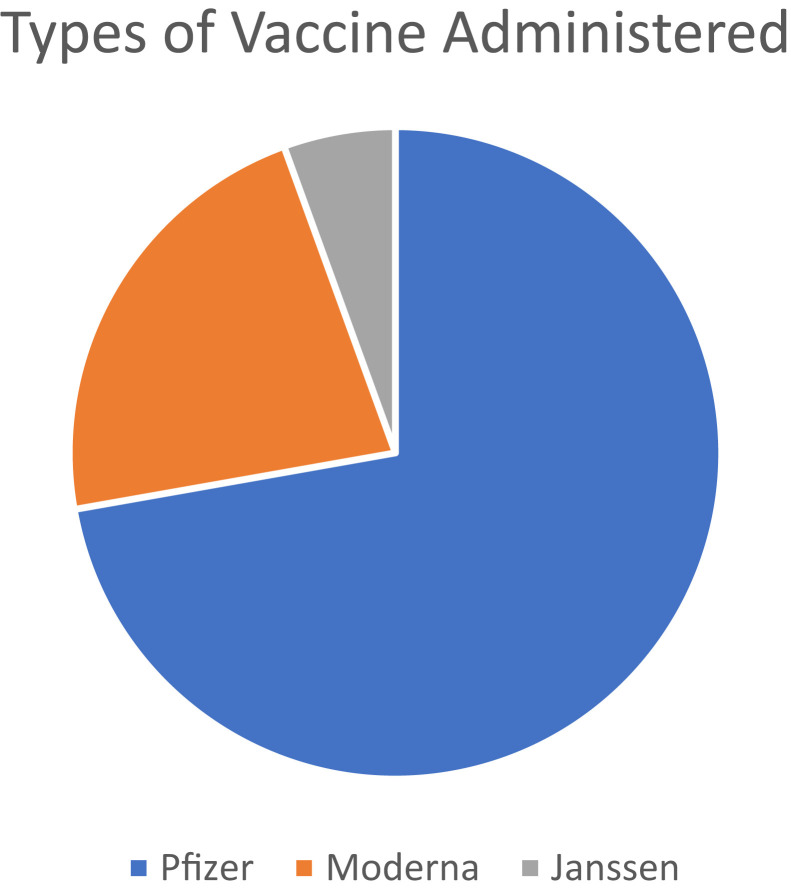
Eighteen patients underwent COVID-19 vaccination. Thirteen patients received the Pfizer vaccine, four received Moderna vaccine, and one received the Janssen vaccine (Figure 1 ). Table I lists demographic characteristics. A total of 55.56% of patients were female. Of the 18 patients, 11 had indolent systemic mastocytosis (61.1%), three had cutaneous mastocytosis (16.67%), two had systemic mastocytosis with hematologic neoplasm (11.11%), one had MMAS (5.56%), and one had mastocytosis in skin (5.56%). Of the 18 patients, 17 were White (94.44%) and one was Middle Eastern. One patient had a history of anaphylaxis to venom and food, another had idiopathic anaphylaxis as well as anaphylaxis to exercise and heat, and two reported anaphylaxis to venom. Average tryptase level was 44.56 ng/mL (range, 2-136 ng/mL). Regarding allergy history, one patient had a history of contrast allergies (reported difficulty breathing) and 10 had adverse drug reactions and a history of medication allergies. Reported drug allergies included penicillins, nonsteroidal anti-inflammatory drugs, aspirin, opioids, sulfa drugs, and anesthetics. From an atopic standpoint, six patients had a history of allergic rhinitis, five had asthma, and two had atopic dermatitis. We attempted to contact all patients by phone call to confirm whether they took an antihistamine before vaccination and to confirm documentation regarding whether they had no allergic reactions or anaphylaxis. None of the 18 patients had an allergic reaction or anaphylaxis after vaccination according to documentation. Nine patients answered our calls and provided confirmation. Four patients confirmed taking an antihistamine 30 to 60 minutes before vaccination. Three patients had documented that they had taken an antihistamine 30 to 60 minutes before vaccination, but they were unable to be reached. Four patients had no information regarding whether they had documentation that they had taken antihistamines, and were unavailable to be contacted (however, they were prescribed daily antihistamines). Three patients confirmed that they did not premedicate with an antihistamine 30 to 60 minutes before the vaccination, but took the prescribed antihistamines (two patients took the antihistamine in the evening and the other patient took it twice daily). Two patients were advised to premedicate with an antihistamine in addition to the home antihistamine, but were unable to be reached. Two patients were not prescribed antihistamines and took no doses before vaccination.
Figure 1.
Types of vaccine administered.
Table I.
Demographic characteristics of cohort
| Characteristic | Patients, n |
|---|---|
| Cutaneous mastocytosis | 3 |
| Indolent systemic mastocytosis | 11 |
| Systemic mastocytosis with an associated hematologic neoplasm | 2 |
| Monoclonal mast cell activation syndrome | 1 |
| Mastocytosis in skin | 1 |
| Sex | |
| Female | 10 |
| Male | 8 |
| Age range, y | 38-83 |
| Race | |
| White | 17 |
| Middle Eastern | 1 |
| Tryptase range, ng/mL | 2-136 |
| Atopic comorbid condition | |
| Allergic rhinitis | 6 |
| Asthma | 5 |
| Atopic dermatitis | 2 |
| History of anaphylaxis | 4 |
| Reported allergies | |
| Drug allergy | 10 |
| Contrast | 1 |
| Venom | 4 |
| Food | 1 |
Our data suggest that COVID-19 vaccines are well-tolerated in a diverse population of mastocytosis patients, most of whom had other concurrent allergies and some of whom (22%) had anaphylactic reactions to other triggers. This is consistent with the case report of two patients with mastocytosis and a history of anaphylaxis, who tolerated COVID-19 vaccination.5 This cohort contained no patients with a history of vaccine, polyethylene glycol, or polysorbate allergy. Although most patients were receiving an antihistamine, not all patients took the home regimen before vaccination or premedicated and still tolerated vaccination. The cohort also contained patients with an atopic history including asthma, allergic rhinitis, atopic dermatitis, venom, food, and drug allergies. This suggests that patients with atopy and mastocytosis should not be excluded from vaccination owing to the fear of allergic reactions. This study does not rule out mastocytosis as a risk factor in amplifying the severity of anaphylaxis in those who have allergic reactions. Therefore, caution still needs to be practiced with continuing to prescribe antihistamines to patients in addition to premedication, maintaining an observation period for at least 30 minutes after vaccination, and having necessary medications to treat anaphylaxis if it occurs. Other limitations of our study include its retrospective nature and small sample size. Our population is also mostly White, which limits generalizability. Another important factor is that only one patient received the Janssen vaccine. The COVID-19 landscape is rapidly evolving with many new vaccines being developed. As additional vaccines are authorized and more patients undergo vaccination, new findings in the mastocytosis population may emerge. More studies are needed to ensure the global safety of COVID-19 vaccines in this population.
Our findings suggest that most patients with mastocytosis, even those with an allergic or anaphylactic history, can be safely vaccinated. Patients should be encouraged to undergo vaccination, continue daily medications in addition to premedication with an antihistamine, and carry an epinephrine autoinjector.
Footnotes
Research reported in this publication was supported by the University of Michigan, Department of Internal Medicine funds.
Conflicts of interest: The authors declare that they have no relevant conflicts of interest.
References
- 1.Brockow K., Jofer C., Behrendt H. Anaphylaxis in patients with mastocytosis: a study on history, clinical features and risk factors in 120 patients. Allergy. 2008;63:226–232. doi: 10.1111/j.1398-9995.2007.01569.x. [DOI] [PubMed] [Google Scholar]
- 2.Brockow K. Epidemiology, prognosis, and risk factors in mastocytosis. Immunol Allergy Clin North Am. 2014;34:283–295. doi: 10.1016/j.iac.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Parente R., Pucino V., Magliacane D., Petraroli A., Loffredo S., Marone G., et al. Evaluation of vaccination safety in children with mastocytosis. Pediatr Allergy Immunol. 2017;28:93–95. doi: 10.1111/pai.12647. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) COVID-19 Response Team; Food and Drug Administration Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine - United States, December 14-23, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:46–51. doi: 10.15585/mmwr.mm7002e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rama T., Moreira A., Castells M. mRNA COVID-19 vaccine is well tolerated in patients with cutaneous and systemic mastocytosis with mast cell activation symptoms and anaphylaxis. J Allergy Clin Immunol. 2021;147:877–878. doi: 10.1016/j.jaci.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azenha Rama T., Álvarez-Twose I. Delving into COVID-19 vaccination-induced anaphylaxis: are mRNA vaccines safe in mast cell disorders? J Investig Allergol Clin Immunol. 2021;31:193–195. doi: 10.18176/jiaci.0680. [DOI] [PubMed] [Google Scholar]
- 7.National Institute of Allergy and Infectious Disease COVID-19:SARS vaccination (SARS) https://www.clinicaltrials.gov/ct2/show/NCT04761822?cond=covid-19+vaccine+allergy&draw=2&rank=1 Available from: