Abstract
Background: Haemonchus contortus is an important pathogenic nematode parasite and major economic constraint of small ruminants in tropics and subtropics regions. This review is an attempt to systematically address the; (a) efficacy of different plants against H. contortus by in vitro and in vivo proof; (b) toxicology, mechanism of action, and active phyto-compounds involve in anti-haemonchiasis activity; (c) and comparative analysis of plant species evaluated both in vitro and in vivo.
Methods: Online databases (Google Scholar, PubMed, Scopus, and ScienceDirect) were searched and published research articles (1980–2020) were gathered and reviewed.
Results: A total of 187 plant species were reported belonging to 59 families and 145 genera with Asteraceae and Fabaceae being frequently used. Out of the total plant species, 171 species were found to be evaluated in vitro and only 40 species in vivo. Twenty-four species were commonly evaluated for in vitro and in vivo anti-haemonchiasis activity. Among the reported assays, egg hatching test (EHT) and fecal egg count reduction (FECR) were the most widely used assays in vitro and in vivo, respectively. Moreover, sheep were the frequently used experimental model in vivo. After comparative analysis, Lachesiodendron viridiflorum, Corymbia citriodora, Calotropis procera, and Artemisia herba-alba were found highly effective both in vitro and in vivo. L. viridiflorum inhibited enzymatic activities and metabolic processes of the parasite and was found to be safe without toxic effects. C. citriodora was moderately toxic in vivo, however, the plant extract produced promising nematicidal effects by causing muscular disorganization and changes in the mitochondrial profile. Additionally, C. procera and A. herba -alba despite of their high anti-haemonchiasis activity were found to be highly toxic at the tested concentrations. C. procera caused perforation and tegumental disorganization along with adult worm paralysis. Nineteen compounds were reported, among which anethole and carvone completely inhibited egg hatching in vitro and significantly reduced fecal egg count, decreased male length, and reproductive capacity of female in vivo.
Conclusion: This review summarized different medicinal plants owing to nematicidal activities against H. contortus eggs, larvae, and adult worms. Plants like L. viridiflorum, C. citriodora, C. procera, and A. herba-alba, while compounds anethole and carvone having promising nematicidal activities and could be an alternative source for developing novel drugs after further investigation.
Keywords: medicinal plants, pharmacology, nematicidal activity, Haemonchus contortus, toxicology, ethnoveterinary, antiparasitic, anthelmintic
Introduction
Haemonchus contortus is the causative agent of haemonchiasis usually known as “twisted barber” pole worm or stomach worm (Saminathan et al., 2015), and a common blood feeder of small ruminants. The parasite is present throughout the tropical and subtropical regions of the world where it is a major constraint for profitable production of sheep and goats (Zenebe et al., 2017). Haemonchiasis is characterized by severe anemia, leading to a serious impairment of the animal, severe economic losses, and acute disease outbreaks with high death rate particularly in young animals (Selemon, 2018). Among the parasitic diseases, gastrointestinal nematode infections remain one of the main causes of impaired production in small ruminants (Hounzangbe-Adote et al., 2005). According to the pharmaceutical companies the annual cost of antiparasitic compounds is proposed to be tens of billions of dollars worldwide (Wolstenholme et al., 2004). However, the annual treatment cost of H. contortus has been estimated to be 26 million USD in Kenya, 46 million USD in South Africa, and 103 million USD in India (Peter and Chandrawathani, 2005).
To overcome the major economic losses in agriculture, it is essential to enhance the control of key parasitic diseases (Gilleard, 2006). For this purpose, various approaches are being in use to control parasitism, including biological control, pasture management, dietary management, vaccination, and the use of anthelmintic chemicals. Most widely used practice being followed nowadays is the use of anthelmintic chemicals. Unfortunately, regular and indiscriminate administration has posed a variety of problems including emergence of resistance in nematode parasites, e.g. multi resistant H. contortus has been reported. Furthermore, the commercially available anthelmintics drugs are somewhat costly and smallholder farmers are unable to expend meager income for purchasing of drugs to carry on regular treatment (Irum et al., 2015).
As a result, there is a dire need to develop alternative anthelmintic approaches from natural flora which can be less toxic, biodegradable, environmental friendly, and to cover the most challenging problem of parasite resistance issue (Carvalho et al., 2012). Worldwide, different plant species have been reported and evaluated for natural bio-products to control the parasitic infections and reduce the dependency on conventional chemotherapy. Testing for biological activity in vitro and in vivo has to be done after a purification process in order to exclude interference with accompanying compounds and reference standards for quality control of herbal medicines largely depend on isolated compounds with documented purity (Bucar et al., 2013). The compounds from plants have also been found to be synergistic enhancers in that though they may not possess any anthelmintic properties alone, but when used concurrently with standard drugs they enhance the activity of the drug. The synergistic effect of the association of an anthelmintic drug and plant extracts against resistant pathogens leads to new choices for the treatment of infectious diseases. Also synergy between bioactive plant product and antiparasitic will confront problems of toxicity and overdose since lesser concentrations of two agents in combination are required, due to these reasons, there is need for continuous exploration of multidrug resistance modulating principles from plants sources (Aiyegoro and Okoh, 2009). The herbal medicines however, suffer from lack of standardization parameters. The main limitation is the lack of standardization of raw materials, processing methods and the final products, dosage formulation, and the non-existence of criteria for quality control (Sachan et al., 2016).
Recently, the interest of researchers in exploring the antiparasitic properties of ethnoveterinary medicinal plants is increasing and this field of research is inundated with ethnopharmacological studies. This review is aimed to gather fragmented literature about the; (a) efficacy of different plants against H. contortus by in vitro and in vivo proof; (b) toxicology, mechanism of action, and active phyto-compounds involve in anti-haemonchiasis activity; (c) and comparative analysis of plant species evaluated both in vitro and in vivo. Moreover, the study also highlights existing knowledge gaps in the present research and provides future recommendations to fulfill those gaps.
Methodology
The systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher et al., 2009). No protocol was followed for conducting this systematic review. The PRISMA check list is provided in the supporting information section (Supplementary Table S1).
Databases and Searching Criteria
To find the published literature, a systematic search was performed using different databases, including Google Scholar, PubMed, Scopus, and ScienceDirect. Research articles published in English language from 1980 to 2020 were gathered for this systematic review. Key words such as: anthelmintic activity, nematicidal activity of plants, medicinal plants used for H. contortus, in vitro/in vivo efficacy of plants against H. contortus, active compounds in plants, mechanism of plant extract inhibition and toxicity of plants. “Anthelmintic AND Hemonchus contortus”, “Natural nematicidal OR anti-haemonchiasis NOT synthetic”, “Natural in vitro OR in vivo anthelmintic”. Bibliographies of research articles were also searched and relevant references were extracted and downloaded. Moreover, to support the findings of the review further literature search was conducted and relevant articles were included.
Inclusion/Exclusion Criteria
Research articles describing (a) in vitro/in vivo efficacy of medicinal plants against H. contortus, (b) containing full information regarding plant name, country name, extract, concentration, inhibition, time exposure, and assay type, (c) original research articles, (d) and published in English language were included in this systematic review. While articles with (a) epidemiological and molecular dataset of H. contortus, (b) antiparasitic activities other than H. contortus, (c) synthetic drugs/chemicals tested against H. contortus, and (d) language other than English were excluded.
Data Extraction
Endnote (Thomson Reuters, San Francisco, CA, United States) was used to compile the articles. Researchers very carefully extracted all the data from the selected articles including author (s) name, country name, plant name, family name, plant part used, plants’ life form, extract used, concentration, time exposure, inhibition, and year of publication. Data were arranged into tables and figures. Chemical structures of the compounds were drawn using MarvinSketch (18.24.0) (https://chemaxon.com/products/marvinn) and Inkscape (0.92) (https://inkscape.org/) was used to further refine and improve the resolution of each chemical structure. PubChem (https://pubchem.ncbi.nlm.nih.gov) was also used to attain the IUPAC name (s) of pure compounds reported in this review.
Taxonomic Clarification
Plant scientific names, synonyms, and families were searched and corrected using “the plant list” (http://www.theplantlist.org), “tropicos” (http://www.tropicos.org), “world flora online” (http://www.worldfloraonline.org), and “Medicinal Plant Name Services-KEW” (https://mpns.science.kew.org/mpns-portal).
Quantitative Analysis
Jaccard Similarity Index (JI)
Jaccard similarity index was calculated to determine the similarity between the two sets of studies reported in this review. One set of study is the “in vitro pharmacological validation of medicinal plants” and the other one is the “in vivo pharmacological validation of medicinal plants”. JI was calculated by using the formula (Kayani et al., 2015):
Where “a” is the total number of plant species used in vitro, “b” is the total number of plant species used in vivo as anthelmintic against H. contortus, and “c” is the number of plant species common to both in vitro and in vivo studies.
Results
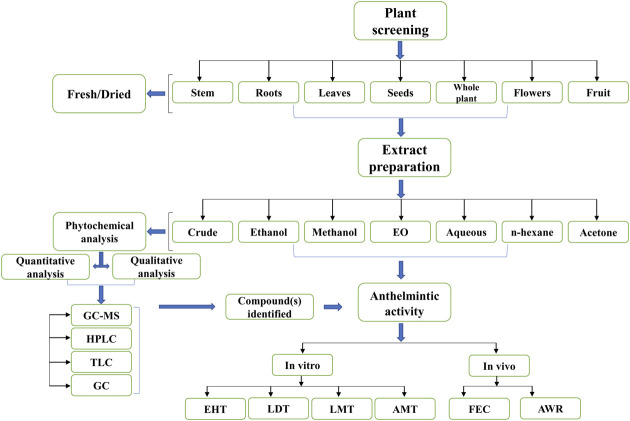
We identified a total of 1,480 published articles through literature search. After removing duplicates, and irrelevant articles, a total of 108 articles were selected for this review (Figure 1). Quality assessment of the selected articles was performed and summarized as author name, species/compound(s) stated in the article, plant source, species authentication, quality control as well as chemical analysis reported (Table 1).
FIGURE 1.
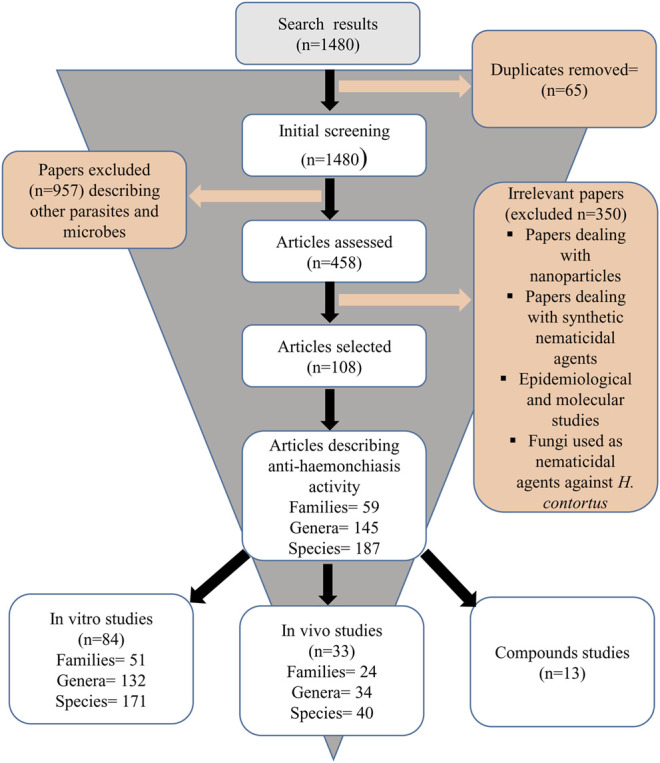
Flow chart of screening process of articles.
TABLE 1.
Quality assessment of the articles selected for this systematic review.
| Study | Species/compound stated in the article | Plant source | Authenticated species | Quality control reported? | Chemical analysis reported? |
|---|---|---|---|---|---|
| Cala et al. (2012) | Melia azedaracha L | Not stated | − | Yes | Yes-liquid-liquid chromatography |
| Trichilia claussenii C | |||||
| Irum et al. (2015) | Artemisia vestita Wall ex Besser | Collected from northern areas of Pakistan | + | No | No |
| Seriphidium maritimum (L.) Poljakov (=Artemisia maritima L.) | |||||
| Jaheed et al. (2019) | Balanites egyptiaca (L.) Delile | Purchased from local market, upper Egypt | − | Yes | Yes-gas chromatography–mass spectrometry (GC–MS) analysis |
| Katiki et al. (2019) | Anethole and carvone | Appalachian farming systems research left | − | Yes | No |
| Lone et al. (2012) | Euphorbia helioscopia L | Kashmir valley, India | + | No | No |
| Lopes et al. (2018) | Anacardium occidentale L | NatVita in eusébio, ceará | − | Yes | Yes-liquid Chromatography-Mass spectroscopy (LC-MS) analysis |
| Maphosa et al. (2010) | Elephantorrhiza elephantina (Burch.) Skeels | Ntselamanzi, nkonkobe muncipality, eastern cape province, South Africa | + | Yes | No |
| Aloe ferox Mill. | |||||
| Leonotis leonurus (L.) R. Br | |||||
| Marie-Magdeleine et al. (2014) | Musa x paradisiaca L | Guadeloupe French west indies | − | No | No |
| Minho et al. (2008) | Acacia decurrens (J.C.Wendl.) Willd. (=Acacia molissima Willd.) | Not stated | − | Yes | No |
| Monteiro et al. (2011) | Jatropha curcas L | Obtained from embrapa eastern amazon, don eliseu | + | Yes | No |
| Njoku and Asuzu (1998) | Ocimum gratissimum L | Collected from nsukka in enugu state Nigeria | + | Yes | No |
| Nsereko et al. (2019) | Senna occidentalis (L.) Link. (=Cassia occidentalis L.) | Uganda | + | Yes | No |
| Euphorbia hirta L | |||||
| Oliveira et al. (2009) | Cocos nucifera L | Agroindustria tropical located in fortaleza, ceara state university | - | Yes | No |
| Ademola and Eloff (2011) | Senna alata (L.) Roxb. (=Cassia alata) | Collected from zaria, Nigeria | + | No | No |
| Martínez-Ortiz-de-Montellano et al. (2019) | Lysiloma latisiliquum (L.) Benth. | Harvested from tropical forest in merida, yucatan, Mexico | − | Yes | No |
| Onobrychis viciifolia Scop | |||||
| Pessoa et al. (2002) | Ocimum gratissimum L | Fortaleza, ceará, northeast of Brazil | + | Yes | Yes-method not stated |
| Iqbal et al. (2006b) | Nicotiana tabacum L | Purchased from local market in faisalabad, Pakistan | + | Yes | No |
| Sirama et al. (2015) | Gymnanthemum amygdalinum (Delile) Sch.Bip. (=Vernonia amygdalina Del.) | Not stated | − | Yes | No |
| Squires et al. (2010) | Artemisia annua L | Appalachian farming systems research left | − | No | No |
| Artemisia absinthium L | |||||
| Maphosa and Masika (2012) | Elephantorrhiza elephantina (Burch.) Skeels | Matatiele district, eastern cape province, South Africa | − | Yes | No |
| Maciel et al. (2006) | Azadirachta indica A. Juss. (=Melia azedarach L.) | Not stated | − | Yes | No |
| Macedo et al. (2010) | Eucalyptus staigeriana F. Muell. Ex F.M. Bailey | Dierberguer óleos essenciais ltda (barra bonita, são paulo state, Brazil | − | Yes | Yes-GC-MS analysis |
| Lone et al. (2013) | Euphorbia helioscopia L | Kashmir valley, India | + | No | No |
| Kamaraj et al. (2010) | Azadirachta indica A. Juss. (=Melia azedarach L.) | Amman nagar, dharamapuri district, Tamil nadu, India | + | Yes | No |
| Iqbal et al. (2006c) | Swertia chirayita (Roxb.) H.Karst. (=Swertia chirata Buch-Ham) | Procured from local market faisalabad, Pakistan | + | Yes | No |
| Zhu et al. (2013b) | Artemisia lancea Vaniot | Collected from hunan, China | + | Yes | Yes-GC-MS analysis |
| Idris et al. (1982) | Artemisia herba-alba Asso (=Seriphidium herba-alba (Asso) Soják) | Not stated | − | Yes | No |
| Silva et al. (2019) | Parkia platycephala Benth | Chapadinha, maranhao, Brazil | − | Yes | No |
| Qi et al. (2015) | Zanthoxylum bungeanum Maxim. (=Zanthoxylum simulans Hance) | Collected from hunan, China | + | No | Yes-GC–MS analysis |
| Katiki et al. (2017b) | Essential oils and other compounds | Grasp Ind. Com. Ltda (curitiba, parana, Brazil) | − | No | Yes-gas chromatography (GC) analysis |
| Katiki et al. (2017a) | Terminalia catappa L | Instituto de zootecnia-nova odessa, sao polo, Brazil | + | Yes | Yes-method not stated |
| Ferreira et al. (2013) | Annona muricata L | Collected from terra de ismael grange located in the municipality of jurucê | − | No | Yes-high performance liquid chromatography and (HPLC) thin-layer chromatography (TLC) analysis |
| São paulo, Brazil | |||||
| Váradyová et al. (2018) | Althaea officinalis L | Commercial sources (AGROKARPATY, plavnica, Slovak republic and BYLINY mikeš s.r.o., cíčenice, Czech republic) | − | No | Yes-liquid chromatography-Mass spectrometry assay |
| Petasites hybridus (L.) G.Gaertn., B.Mey. and Scherb | |||||
| Inula helenium L | |||||
| Malva sylvestris L | |||||
| Foeniculum vulgare Mill. | |||||
| Solidago virgaurea L | |||||
| Fumaria officinalis L | |||||
| Hyssopus officinalis L | |||||
| Melisa officinalis L | |||||
| Artemisia absinthium L | |||||
| Vargas-Magaña et al. (2014) | Lysiloma latisiliqum (L.) Benth. | Faculty of veterinary Medicine-UADY, in mérida, méxico collected from nearby coastal area of mérida, méxico | − | No | No |
| Laguncularia racemose (L.) C.F. Gaertn | |||||
| Rhizophora mangle L | |||||
| Avicenna germinans (L.) L | |||||
| Mondal et al. (2015) | Alternanthera sessilis (L.) R.Br. ex DC. | Gopalgonj, Bangladesh | + | No | Yes-HPLC |
| Al-Shaibani et al. (2008) | Justicia adhatoda L. (=Adhatoda vasica Nees) | Harvested from sindh agriculture university (SAU), tandojam, Pakistan | + | Yes | No |
| Hussien et al. (2011) | Corriandrum sativum L | Collected from jimma town and also purchased from local market | + | No | No |
| Thymus schimperi Ronniger | |||||
| Ocimum gratissimum L | |||||
| Ocimum lamiifolium Hochst. ex Benth | |||||
| Ruta chalepensis L | |||||
| Echinops kebericho Mesfin | |||||
| Monglo et al. (2006) | Annona senegalensis Pers | Northern region of Cameroon | + | Yes | No |
| Terminalia leiocarpa (DC.) Baill. (=Anogeissus leiocarpus (DC) Guil and Perrot.) | |||||
| Lippia rugosa A.Chev | |||||
| Stereospermum kunthianum Cham. | |||||
| Vernonia noveboracensis (L.) Michx. (=Vernonia tonoteana L.) | |||||
| Ahmed et al. (2020) | Artemisia herba-alba Asso (=Seriphidium herba-alba (Asso) Soják) | Midaga-tola district | + | No | Yes-method not stated |
| Punica granatum L | |||||
| Acharya et al. (2014) | Rhus aromatica Aiton | Collected from South Dakota, North Dakota, Wyoming Montana | + | No | No |
| Ericameria nauseosa (Pursh) G.L. Nesom and G.I. Baird | |||||
| Perideridia gairdneri (Hook. and Arn.) Mathias | |||||
| Chrysothamnus viscidiflorus Nutt. | |||||
| Geranium viscosissimum Fisch. and C.A. Mey | |||||
| Melilotus albus Medik | |||||
| Liatris punctata Hook. | |||||
| Melilotus officinalis (L.) Lam. | |||||
| Sanguinaria canadensis L | |||||
| Lotus corniculatus L | |||||
| Arctostaphylos uva-ursi (L.) Spreng | |||||
| Rhus glabra L | |||||
| Wyethia sagittata (Pursh) Mabb | |||||
| (=Balsamorhiza sagittata (Pursh) Nutt.) | |||||
| Enothera sp | |||||
| Caltha palustris L | |||||
| Cynoglossum officinale L | |||||
| Solidago canadensis L. (=Solidago mollis Bartl.) | |||||
| Centaurea stoebe L | |||||
| Glycyrrhiza lepidota Pursh | |||||
| Lycopus americanus Muhl. Ex W.P.C. Barton | |||||
| Pedicularis racemose Douglas ex Benth. | |||||
| Stachys palustris L | |||||
| Agastache foeniculum (Pursh) Kuntze | |||||
| Pediomelum argophyllum (Pursh.) J.W.Grimes | |||||
| Monarda fistulosa L | |||||
| Clematis ligusticifolia Nutt. | |||||
| Allium cernuum Roth | |||||
| Erigeron canadensis L. (=Conyza canadensis (L.) Cronquist) | |||||
| Cornus Sericea L | |||||
| Rubus idaeus L | |||||
| Actaea rubra (Aiton) Willd. | |||||
| Symphoricarpos occidentalis (R.Br.) Hook | |||||
| Artemisia ludoviciana Nutt. | |||||
| Artemisia frigida Willd. | |||||
| Tanacetum vulgare L | |||||
| Cleomella serrulata (Pursh) Roalson and J.C.Hall | |||||
| (=Cleome serrulata Pursh) | |||||
| Epilobium angustifolium L | |||||
| Quercus macrocarpa Michx. | |||||
| Salix interior Rowlee (=Salix exigua Nutt.) | |||||
| Lithospermum molle (Michx.) Muhl | |||||
| (=Onosmodium molle Michx.) | |||||
| Cavalcante et al. (2016) | Calotropis procera (Aiton.) W.T.Aiton | Universidade estadual do ceará | + | No | Yes-method not stated |
| Ferreira et al. (2018) | Citrus × aurantiifolia (Christm.) Swingle Anthemis nobilis L. Lavandula angustifolia subsp. angustifolia (=Lavandula officinalis Chaix) | Purchased from kampo de ervas Ind. and com. Ltda-ME (ribeirão preto, SP, Brazil) | + | Yes | Yes-GC-MS |
| Fouche et al. (2016) | Aloe rupestris Baker | Collected from different locations in South Africa | + | No | No |
| Antizoma angustifolia (Burch.) Miers ex Harv. | |||||
| Calpurnia aurea (Aiton) Benth. | |||||
| Senna italica Mill. | |||||
| Cissus quadrangularis L | |||||
| Clematis brachiata Thunb. | |||||
| Cleome gynandra L | |||||
| Ficus sycomorus L | |||||
| Hypoxis rigidula Baker | |||||
| Maerua angolensis DC. | |||||
| Monsonia angustifolia E. Mey. Ex A.Rich | |||||
| Pelargonium luridum (Andrews) Sweet | |||||
| Schkuhria pinnata (lam.) Kuntze ex Thell. | |||||
| Sclerocarya birrea (A.Rich.) Hochst | |||||
| Tabernaemontana elegans Stapf | |||||
| Ahmed et al. (2013) | Allium sativum L | Collected from the university of KwaZulu-Natal (UKZN) botanical garden, pietermaritzburg campus, UKZN research farm (ukulinga). Ficus spp. were from a private garden (pietermaritzburg) and garlic and ginger samples were purchased from a commercial supermarket. | + | No | No |
| Aloe ferox Mill | |||||
| Ananas comosus (L.) Merr. | |||||
| Carica papaya L | |||||
| Ficus benjamina L | |||||
| Ficus ingens (miq.) Miq | |||||
| Ficus carica L | |||||
| Ficus benghalensis L. (=Ficus indica L.) | |||||
| Ficus lutea Vahl | |||||
| Ficus elastica Roxb. Ex Hornem | |||||
| Ficus natalensis Hochst | |||||
| Ficus sur Forssk | |||||
| Ficus sycomorus L | |||||
| Leonotis leonurus (L.) R.Br | |||||
| Azadirachta indica A. Juss. (=Melia azedarach L.) | |||||
| Peltophorum africanum Sond | |||||
| Scadoxus puniceus (L.) Friis and Nordal | |||||
| Lespedeza cuneata (Dum. Cours.) G. Don | |||||
| Tephrosia inandensis H.M.L. Forbes | |||||
| Warburgia ugandensis v | |||||
| Warburgia salutaris (G. Bertol.) Chiov. | |||||
| Cucumis myriocarpus Naudin | |||||
| Zingiber officinale Roscoe | |||||
| Iqbal et al. (2001b) | Allium sativum L | Not stated | - | No | No |
| Zingiber officinale Roscoe | |||||
| Cucurbita ficifolia Bouché (=Curcurbita mexicana Dammann) | |||||
| Ficus religiosa L | |||||
| Getachew et al. (2012) | Foeniculum vulgare Mill | Collected from addis ababa | + | No | No |
| Acokanthera schimperi (A.DC.) Schweinf | |||||
| Searsia pyroides (Burch.) Moffett | |||||
| (=Rhus vulgaris Meikle) | |||||
| Rhus glabra L | |||||
| Jasminum abyssinicum Hochst. ex DC. Myrsine africana L | |||||
| Zamilpa et al. (2019) | Dysphania ambrosioides (L.) Mosyakin and Clemants (=Chenopodium ambrosioides L.) Castela tortuosa Liebm | Acquired at a local market in the town left of cuernavaca city, morelos, Mexico | + | Yes | No |
| Iqbal et al. (2005) | Calotropis procera (Aiton) W.T.Aiton | Cholistan rangeland, district bahawalpur (Pakistan) | + | Yes | No |
| Jabbar et al. (2007) | Chenopodium album L | Procured from local market in faisalabad (Pakistan) | + | No | No |
| Caesalpinia crista L | |||||
| Kamaraj and Rahuman (2011) | Annona squamosa L | Collected from the tropical region hills, Tamil nadu, India | + | No | Yes-lieberman–Burchard test |
| Eclipta prostrata (L.) L | |||||
| Solanum torvum Sw | |||||
| Terminalia chebula Retz | |||||
| Catharanthus roseus (L.) G. Don | |||||
| Kamaraj et al. (2011) | Andrographis paniculata (Burm.f.) Nees | Collected from javadhu hills, tiruvannamalai district and dharmapuri district Tamil nadu, India | - | Yes | No |
| Anisomeles malabarica (L.) Kuntze (=Anisomeles malabarica (L.) R.Br.) | |||||
| Annona squamosa L | |||||
| Datura metel L | |||||
| Solanum torvum Sw | |||||
| Marie-Magdeleine et al. (2010) | Tabernaemontana citrifolia L | Collected in Guadeloupe, French west indies | - | No | Yes-TLC |
| Nery et al. (2010) | Anacardium humile A.St.-Hil | Collected in the cerrado of a rural region of montes claros city, Brazil | + | No | Yes-phytochemical analysis |
| Veerakumari and Chitra (2016) | Allium sativum L | Not stated | - | Yes | No |
| de Oliveira et al. (2011) | Myracrodruon urundeuva Allemao | Collected in December 2009 in fortaleza, Ceará,Brazil | + | Yes | Yes-method not stated |
| Malik et al. (2019) | Artemisia vulgaris L | Collected from botanical garden at the federal university of maranhão, sao luís, maranhão, Brazil | + | No | Yes-GC-MS analysis |
| Soares et al. (2018) | Myracrodruon urundeuva Allemao | Purchased from arboleft seed trade (birigui, sao paulo, Brazil | + | No | Yes-proteomic analysis (LC-ESI-MS/MS) |
| Tariq et al. (2009) | Artemisia absinthium L | Collected from the aharbal area of southern kashmir valley | + | No | No |
| Zhu et al. (2013a) | Arisaema franchetianum Engl. | Collected from yunnan province, China | + | No | Yes-GC–MS analysis |
| Arisaema lobatum Engl. | |||||
| Carvalho et al. (2012) | Lippia origanoides Kunth (=Lippia sidoides Cham.) | Institute of chemistry of paulista state | - | No | Yes- GC-MS analysis |
| Mentha × piperita L | Embrapa western. Amazon research station, Brazil | ||||
| Hura crepitans L | Acquired in the local market of porto velho | ||||
| Couroupita guianensis Aubl. | |||||
| Katiki et al. (2012) | Cymbopogon schoenanthus (L.) Spreng | Purchased from WNF Ind. and com. Ltda (sao Paulo-SP, Brazil) | - | No | Yes- GC analysis |
| Macedo et al. (2012) | Lantana camara L | Collected in the horto of medicinal plants of the universidade federal do ceará in plots, state of ceará, Brazil | + | No | Yes-GC analysis |
| Alpinia zerumbet (Pers.) B.L.Burtt and R.M.Sm | |||||
| Mentha arvensis L. (=Mentha villosa Becker) | |||||
| Tagetes minuta L | |||||
| Ribeiro et al. (2014) | Corymbia citriodora (Hook.) K.D.Hill and L.A.S.Johnson (=Eucalyptus citriodora Hook.) | Purchased from FERQUIMA (vargem grande paulista, são paulo, Brazil) | - | No | Yes- infrared spectroscopy (FTIR) |
| Andre et al. (2016) | Carvacrol | Obtained via the acetylation of carvacrol (Sigma–Aldrich®, st. Louis, United States | - | No | Yes-FTIR |
| Morais-Costa et al. (2016) | Lachesiodendron viridiflorum (Kunth) P.G.Ribeiro, L.P.Queiroz and Luckow (=Piptadenia viridiflora (Kunth) Benth.) | Cerrado vegetationnear montes claros city in north minas gerais state, Brazil | - | Yes | Yes-HPLC-DAD |
| Féboli et al. (2016) | Opuntia ficus-indica (L.) Mill. | Collected in the municipality of ilha solteirain the state of são paulo, Brazil in September 2014 | - | No | No |
| Tadesse et al. (2009) | Maesa lanceolate Forssk | Collected along the addis ababa-butajira road and Maesa laceolata | + | No | No |
| Coleus maculosus subsp. Maculosus (=Plectranthus punctatus (L.f.) L’Her.) | |||||
| Herath et al. (2019) | Cryptocarya massoy (Oken) Kosterm. (=Cryptocarya ovoguineensis Teschner) | Not stated | - | Yes | Yes-HPLC |
| Piper methysticum G.Forst | |||||
| Gaínza et al. (2015) | Citrus × aurantium L. (=Citrus × sinensis (L.) Osbeck) | Not stated | − | Yes | Yes-GC–MS analysis |
| Melaleuca quinquenervia (Cav.) S.T.Blake | |||||
| de Araújo-Filho et al. (2018) | Corymbia citriodora (Hook.) K.D.Hill and L.A.S.Johnson (=Eucalyptus citriodora hook.) | Purchased from ferquima (são paulo, Brazil) | − | Yes | Yes-GC–MS analysis |
| Hördegen et al. (2006) | Azadirachta indica A. Juss | Obtained from alfred galke GmbH, gittelde (Germany). Purchased from S.V.S. Medicinal crops dealers pvt. Ltd., guntur (India) | − | No | No |
| Caesalpinia crista L | |||||
| Fumaria parviflora Lam | |||||
| Embelia ribes Burm. f | |||||
| Baccharoides anthelmintica (L.) Moench (=Vernonia anthelmintica (L.) Willd.) | |||||
| Ananas comosus (L.) Merr. | |||||
| Camurça-Vasconcelos et al. (2007) | Croton grewioides Baill. (=Croton zehntneri Pax and K.Hoffm.) | Collected in vicosa, ceara state, Brazil | + | No | Yes-GC–MS analysis |
| Lippia origanoides Kunth. (=Lippia sidoides Cham.) | Purchased from PRONAT (produtos naturais) in the state of ceara | ||||
| Hernández-Villegas et al. (2011) | Phytolacca icosandra L | Collected in yaxcabá, yucatan, Mexico | + | No | Yes-method not stated |
| Eguale et al. (2011) | Croton macrostachyus Hochst. ex Delile | Collected from their natural habitat | + | No | Yes-method not stated |
| Ekebergia capensis Sparrm. | |||||
| Vachellia nilotica (L.) P.J.H.Hurter and Mabb. (=Acacia nilotica (L.) willd. Ex. Delile) | |||||
| Terminalia schimperiana Hochst. (=Terminalia glaucescens Planch. ex Benth.) | |||||
| Hamad et al. (2014) | Nicotiana tabacum L | Purchased from the local market of faisalabad, Pakistan | − | Yes | No |
| Azadirachta indica A. Juss | |||||
| De Jesús-Gabino et al. (2010) | Prosopis laevigata (Humb. and Bonpl. Ex Willd.) M.C.Johnst | Collected from the sierra de huautla, ecological reserve of the biosphere, in morelos state, Mexico | − | No | No |
| Tariq et al. (2008) | Achillea millefolium L | Collected from the aharbal area of southern kashmir valley | + | No | No |
| Piza et al. (2019) | Psidium cattleyanum Sabine | Glaucilândia, Brazil | + | Yes | No |
| André et al. (2020) | Carvacryl acetate | Not stated | − | Yes | Yes-GC- MS analysis |
| Macedo et al. (2019) | Cymbopogon citratus (DC.) Stapf | Not stated | − | Yes | Yes-GC-MS analysis |
| Araújo-Filho et al. (2019) | Corymbia citriodora (Hook.) K.D.Hill and L.A.S.Johnson (=Eucalyptus citriodora Hook.) | Not stated | − | Yes | Yes-GC-MS analysis |
| Iqbal et al. (2001a) | Sorghum bicolor (L.) Moench | Not stated | − | Yes | No |
| Lara TF et al. (2009) | Eucalyptus globulus Labill. | Not stated | − | Yes | Yes-GC-MS analysis |
| Ademola et al. (2004) | Khaya senegalensis (Desv.) A. Juss | Ibadan, Nigeria | + | Yes | No |
| Ademola et al. (2007) | Spigelia anthelmia L | Ibadan, Nigeria | + | Yes | No |
| Alowanou et al. (2019) | Bridelia ferruginea Benth | Abomey-calavi, kandi and comé, Benin | + | Yes | No |
| Combretum glutinosum Perr. ExDC. | |||||
| Mitragyna inermis (Willd.) Kuntz | |||||
| Al-Qarawi et al. (2001) | Calotropis procera (Aiton.) W.T.Aiton | Purchased from local market in almoznib, king saud university at buraydah | − | Yes | No |
| Iqbal et al. (2006a) | Baccharoides anthelmintica (L.) Moench | Faisalabad, Punjab | + | Yes | No |
| (=Vernonia anthelmintica (L.) Willd.) | |||||
| Ademola and Idowu (2006) | Leucaena leococephala (Lam.) de Wit | Ibadan, Nigeria | + | Yes | No |
| Barone et al. (2018) | Vaccinium macrocarpon Aiton | Not stated | − | Yes | Yes-Mass spectra method |
| Castillo-Mitre et al. (2017) | Vachellia campeachiana (Mill.) Seigler and Ebinger (=Acacia cochliacantha humb. and bonpl. Ex willd.) | Salitre palmarillos village, Mexico | + | Yes | Yes-Mass spectrometry and HPLC analysis |
| Domingues et al. (2013) | Ananas comosus (L.) Merr. | São paulo state, Brazil | + | Yes | No |
| Dixit et al. (2019) | Annona squamosa L | Local market, jabalpur India | − | Yes | No |
| Azadirachta indica A. Juss | |||||
| Nicotiana tabacum L | |||||
| Ferreira et al. (2016) | Thymus vulgaris L | Ferquima Ind e com ltda (Brazil) | + | Yes | Yes-GC-MS analysis |
| Githiori et al. (2004) | Hagenia abyssinica (Bruce) J.F.Gmel | Kenya and east africa | − | Yes | No |
| Dodonaea viscosa subsp. angustifolia (L.f.) J.G.West (=Dodonaea angustifolia L.f.) | |||||
| Olea europaea L | |||||
| Annona squamosa L | |||||
| Hildebrandtia sepalosa Rendle | |||||
| Azadirachta indica A. Juss | |||||
| Ananas comosus (L.) Merr. | |||||
| Heckendorn et al. (2006) | Onobrychis viciifolia Scop | Not stated | − | Yes | No |
| Palacios-Landín et al. (2015) | Allium sativum L | Local market of the city of cuernavaca and rural area of tixtla in the state of morelos, Mexico | − | Yes | No |
| Tagetes erecta L | |||||
| Hassan et al. (2019) | Abutilon theophrasti Medik | “Lower-munda” District, Qazigund,India | + | Yes | No |
| Iqbal et al. (2004) | Seriphidium brevifolium (Wall. ex DC.) Ling and Y.R.Ling (=Artemisia brevifolia Wall. ex. DC.) | Faisalabad, Pakistan | + | Yes | No |
| Cabardo and Portugaliza (2017) | Moringa oleifera Lam | Collected from brgy. Sto. Rosario, baybay city, leyte | - | No | No |
| Assis et al. (2003) | Spigelia anthelmia L | Not stated | − | No | No |
| Hajaji et al. (2018) | Matricaria recutita L | Obtained from beja, north-west of tunitia | − | Yes | Yes-method not stated |
| Costa et al. (2008) | Azadirachta indica A. Juss | Collected in eusebio, ceara, located in northeast Brazil | + | No | Yes-method not stated |
| Cortes-Morales et al. (2019) | Baccharis conferta Kunth | Collected from the iztaccíhuatl- popocatépetl national park | + | Yes | Yes-TLC |
| Eguale et al. (2007a) | Hedera helix L | Collected from addis ababa, Ethiopia | + | Yes | Yes-method not stated |
| Eguale et al. (2007b) | Coriandrum sativum L | Purchased from debre birhan, Ethiopia | + | Yes | Yes-method not stated |
| Oliveira et al. (2014) | Piper aduncum L | Galley forest of the angico river, bocaiúva site, minas gerais state, Brazil | + | Yes | Yes-GC analysis |
| Karim et al. (2019) | Artemisia vulgaris L | BAU campus | − | Yes | No |
Taxonomic Clarification
According to the modern botanical nomenclature, 53 reported plant species have a synonym issue. Plant accepted names are mentioned in table (Table 1; Supplementary Tables S2, S3), while the synonym mentioned in the original articles were put into brackets. In addition, taxonomic corrections regarding the author of those plants and their family names were also revised.
Pharmacological Validation of Medicinal Plants Against H. contortus
Total of 187 plant species belonging to 59 families and 145 genera were tested against different life stages of H. contortus (Supplementary Tables S2, S3). Major contributed families with their species were Asteraceae (n = 29), Fabaceae (n = 19), Lamiaceae (n = 12), and Euphorbiaceae (n = 6). Different life forms of plants reported were herbs, trees, and shrubs 40, 31.4, and 27% in accordance of their order. Leaves were the most frequently used part (50%) followed by seeds (11.3%), roots (8%), and whole plants (7%). Among other plant parts stems, flowers, barks, shoots, fruits, bulbs, peel, fibers, pulp, and latex were included (Supplementary Table S2).
Different solvents including n-hexane, aqueous, ethanolic, hydro-alcoholic, methanolic, and others were used in extracts preparation (Figure 2). Among all, the methanol extract was the predominant one. Bioassays reported in this review included, egg hatching test (EHT), larval development test (LDT), larval motility test (LMT), adult worm motility test (AWMT), adult parasite mortality test (APMT), and larval artificial exsheathment assay (LAEA) in in vitro studies, while fecal egg count reduction (FECR), egg count per gram of feces (EPG), total warm count reduction (TWC/WCR) in in vivo studies. Furthermore, among the above mentioned assays, EHT was the most widely used assay for evaluation of medicinal plants against H. contortus in vitro, while FECR was common in vivo. Sheep were the commonly used experimental model in vivo (Supplementary Table S3).
FIGURE 2.
Selection and processing of plant material(s) for anthelmintic evaluation.
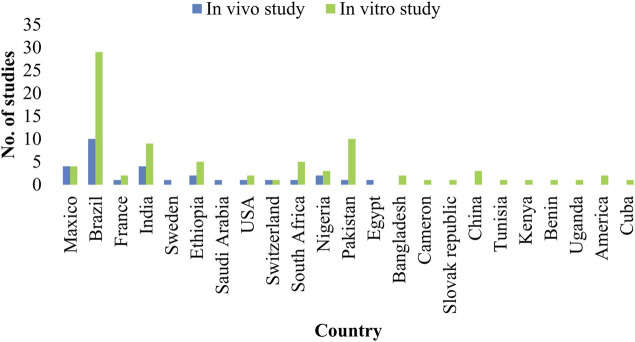
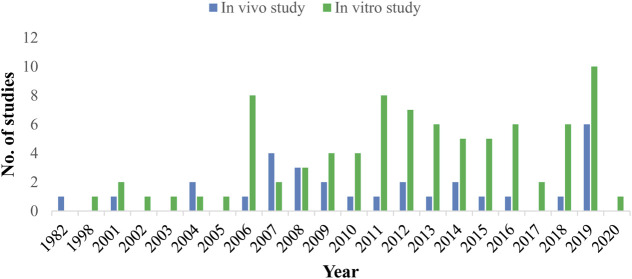
Out of the total reported plant species 171 plant species were evaluated in vitro and only 40 plant species in vivo against H. contortus. It is evident that in vitro studies were almost five times greater than in vivo studies. Mostly, in vitro studies were carried out in Brazil (n = 29 studies), then in Pakistan (n = 10 studies), and India (n = 9 studies) among others. Similarly, in vivo studies were also mostly reported from Brazil and India, 10 and 4 studies, respectively, (Figure 3). Most of the pharmacological studies were reported in the year 2019 (n = 15) and 2006, 2011, 2012 (n = 9) in each (Figure 4).
FIGURE 3.
Country-wise comparison of in vitro and in vivo studies.
FIGURE 4.
Year-wise comparison of in vitro and in vivo studies.
Comparative Analysis and Toxicity of Common Plant Species
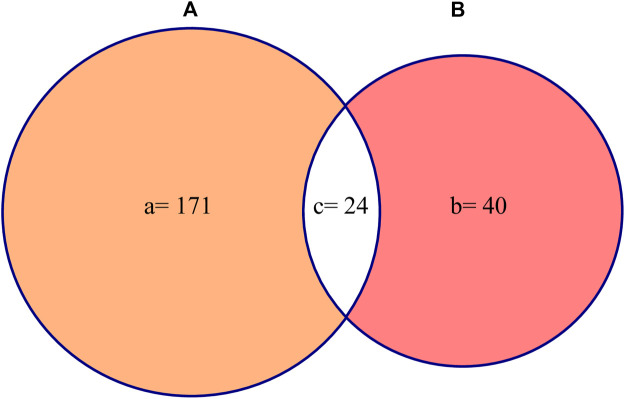
Plant species, which were common in both in vitro and in vivo studies, were compared to discriminate their efficacy against H. contortus. A total of 24 plant species were found to be commonly used both in vitro and in vivo (Table 2).
TABLE 2.
Comparative analysis, toxicology, and mechanism of action of medicinal plants.
| Plant names | In vitro | In vivo | Toxicology | Mechanism of action | References | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ext | Conc. (mg/ml) | Eff. (%) | Ext | Dos. (mg/kg) | Eff. (%) | Dos. (mg/kg) | T. level | |||
| Allium sativum L | Ethanol | 0.5 | 88.5–100 | n-hexane | 40 | 68.7 | NA | Moderate | Destructive and inhibitive effect on acetylcholinesterase causing paralysis | Krstin et al. (2018); Palacios-Landín et al. (2015); Veerakumari and Chitra (2016) |
| Annona squamosa L | Methanol | 25 | 100 | Aqueous | 1500 | No effect | NA | NA | NA | Dixit et al. (2019); Kamaraj et al. (2011) |
| Achillea millefolium L | Crude aqueous | 25 | 94.4 | Crude aqueous | 2 | 88.4 | 0.01 | Nil | Alteration of cell shape, restrict growth, collapsing cell membrane, and arrest cell division | Cavalcanti et al. (2006); Tariq et al. (2008) |
| 0.003 | ||||||||||
| Artemisia absinthium L | Methanol | 1.024 | 100 | Crude ethanol | 2000 | 90.46 | NA | Toxic | Inhibit vital metabolic enzymes, disrupt mitochondrial membrane potential, release of cytochrome c into cytoplasm and activation of caspase-3-mediated apoptosis | Lachenmeier (2010); Tariq et al. (2009); Váradyová et al. (2018) |
| 25 | 85 | |||||||||
| Artemisia herba-alba Asso | Methanol | 1.25–10 | 100 | NA | 0.003 | 100 | 300 | Toxic | NA | Ahmed et al. (2020); Almasad et al. (2007); Idris et al. (1982) |
| Artemisia capillaris Thunb. (=Artemisia vestita Wall. ex Besser) | Methanol | 50 | 95 | Methanol | 50 | 86.35 | NA | Nil | NA | Irum et al. (2015) |
| 25 | 100 | |||||||||
| Artemisia maritima L.(=Seriphidium maritimum (L.) Poljakov | Methanol | 25 | 100 | Methanol | 50 | 82.22 | NA | Nil | NA | Irum et al. (2015) |
| Ananas comosus (L.) Merr. | Ethanol | 200 | 100 | Aqueous | 2000 | No effect | 750 | Nil | Remove and digest the cuticle layer causing immobility and death | Ahmed et al. (2013); Domingues et al. (2013); Maurer (2001) |
| 5000 | ||||||||||
| Azadirachta indica A. Juss | Aqueous | 12.5 | 97.8 | Aqueous | 4000 | 85.24 | 18.4–45 | Toxic | Inhibit secretion of key enzymes, intracellular instability, neuromuscular disorganization, paralysis, and death | Deng et al. (2013); Hamad et al. (2014); Kamaraj et al. (2010); Maciel et al. (2006) |
| Hydro-alcoholic | 12.5 | 98.4 | Methano | |||||||
| Ethanol | 50 | 100 | l | |||||||
| Calotropis procera (Aiton) W.T.Aiton | Aqueous | 25 | 70 | Aqueous | 0.003 | 88.4 | 0.001–6 | Toxic | Tegumental disorganization and paralysis | Cavalcante et al. (2016); Iqbal et al. (2005); Mahmoud et al. (1979) |
| Ethyl acetate | 4 | 91 | ||||||||
| Castela tortuosa Liebm. | n-hexane | 40 | 70 | n-hexane | 40 | 27.1 | NA | NA | NA | (Zamilpa et al., 2019) |
| Coriandrum sativum L | Essential oil | 10 | 88.63 | Aqueous | 450 | 25 | 1000–5000 | Nil | Inhibit vital functions, interfere metabolic processes, and destruction of nervous system | Eguale et al. (2007b); Hussien et al. (2011); Patel et al. (2012) |
| Cocos nucifera L | Ethyl acetate | 5 | 100 | Ethyl acetate | 400 | Not effective | 2000 | Toxic | Induces chemical and physical damage by binding to proteins of cuticle, oral cavity, esophagus, and cloaca | Oliveira et al. (2009); Tayler et al. (2020) |
| 80 | 99.77 | |||||||||
| Corymbia citriodora (Hook.) K.D.Hill and L.A.S. Johnson | Essential oil | 2 | 100 | Essential oil | 0.125, 0.25, 0.5 | 100 | 5000 | Moderate | Formation of vacuoles, muscular disorganization, and changes in mitochondrial profile | Araújo-Filho et al. (2019); Ribeiro et al. (2014) |
| 8 | 100 | |||||||||
| Cymbopogon citratus (DC.) Stapf | Essential oil | 1.25 | 98.4 | Essential oil | 50 | 23.9 | 31.2 μg/ml | Nil | Alter the permeability, depolarization of membrane, and disrupt lipids, polysaccharides, and phospholipids | Macedo et al. (2019); Santoro et al. (2007) |
| Essential oil nano-emulsion | 97.1 | |||||||||
| Cymbopogon schoenanthus (L.) Spreng. | Essential oil | 0.18 | 98.6 | Essential oil | 360 | No activity | 180; 360 | Nil | Inhibit vital functions, interfere metabolic processes, and destruction of nervous system | Katiki et al. (2012) |
| 96.8 | ||||||||||
| Eucalyptus staigeriana F. Muell. Ex F.M. Bailey | Essential oil | 1.75 | 100 | Essential oil | 500 | 46.44 | 1000–5000 | Nil | Alter the permeability, depolarization of membrane, and disrupt lipids, polysaccharides, and phospholipids | Macedo et al. (2010); Ribeiro et al. (2014) |
| 200–600 | ||||||||||
| Euphorbia helioscopia L | Methanol | 25 | 98 | Methanol | 5 | 86 | 2000 | Nil | Induce expansion, increase permeability, and disturb chemical structure of membrane | Lone et al. (2012); Saleem et al. (2016) |
| 82 | ||||||||||
| Lippia origanoides Kunth (=Lippia sidoides cham.) | Essential oil | 0.625 | 100 | Essential oil | 283 | 56.9 | NA | Moderate | Inhibit vital functions, interfere metabolic processes, nervous system destruction | Camurça-Vasconcelos et al. (2007); de Melo et al. (2020) |
| Lysiloma latisiliquum (L.) Benth. | Acetone | 3.6 | Not effective | NA | 0.8 | Highly effective | NA | NA | Cytoplasmic vacuolization, disturb muscular cells, and tissues | Martínez-Ortiz-de-Montellano et al. (2019); Vargas-Magaña et al. (2014) |
| Nicotiana tabacum L | Aqueous | 25 | 77 | Aqueous | 4000 | 86.6 | 5000 | Nil | Inhibit acetylcholine, and paralysis | Andjani et al. (2019); Hamad et al. (2014); Iqbal et al. (2006b) |
| Methanol | Methano | |||||||||
| Lachesiodendron viridiflorum (Kunth) P.G.Ribeiro, L.P.Queiroz and Luckow (=Piptadenia viridiflora (Kunth) Benth.) | Aqueous | 0.075 | 100 | Aqueous | 283 | Highly effective | 203 | Nil | Inhibit enzymatic activities and metabolic processes | Morais-Costa et al. (2016) |
| Methanol | ||||||||||
| Seriphidium brevifolium (Wall. Ex DC.) Ling and Y.R.Ling | Methanol | 25 | 80 | Aqueous | 0.003 | 67.2 | NA | NA | NA | Iqbal et al. (2004) |
| Spigelia anthelmia L | Ethyl acetate | 50 | Highly effective | Aqueous | 500 | Significantly effective | 5000 | Nil | Destruct cuticle layer, degrade egg membrane and chitin of egg shell, inhibit development and death | Ademola et al. (2007); Assis et al. (2003); Ribeiro et al. (2017) |
| Methanol | ||||||||||
NA, Data not available.
After comparative analysis based on minimum concentration and maximum efficacy, the identified plant species with promising anthelmintic activity in vitro against H. contortus were Lachesiodendron viridiflorum (Kunth) P.G.Ribeiro, L.P.Queiroz and Luckow (syn. Piptadenia viridiflora (Kunth) Benth.), Cymbopogon schoenanthus (L.) Spreng., Allium sativum L., Lippia origanoides Kunth (syn. Lippia sidoides Chem.), Artemisia absinthium L., Cymbopogon citratus (DC.) Stapf, Eucalyptus staigeriana F. Muell. ex F.M. Bailey, Artemisia herba-alba Asso, and Corymbia citriodora (Hook.) K.D. Hill and L.A.S. Johnson in order of their appearance. While A. herba-alba, C. procera, C. citriodora, Lysiloma latisiliqum (L.) Benth., and Euphorbia helioscopia L. were reported with high efficacy in vivo. Among the plants, L. viridiflorum was found highly effective both in vitro and in vivo with no observed toxic effects. C. citriodora was moderately toxic in vivo, but with promising nematicidal activity in vitro and in vivo. Additionally, C. procera and A. herba-alba despite of their high anti-haemonchiasis activity were found to be highly toxic at the tested concentrations (Supplementary Table S4). However, five species namely; Annona squamosa L., Artemisia maritima L., Artemisia capillaris Thunb., Castela tortuosa Liebm., L. latisiliquum, and Seriphidium brevifolium (Wall. ex DC.) Ling and Y.R. Ling were not evaluated for their toxicological effects. Mostly non-toxic and low-toxic extracts were orally administered. The LC50 value of most plant species was missing and was not calculated.
From the results, it is evident that the effects of different plant extracts were dose and time dependent. Moreover, the comparative analysis also revealed that plant species were more effective in vitro than in vivo against various stages of the parasite.
Phyto-Compounds With Anti-Haemonchiasis Activity
In this review, 19 compounds were reported to be assessed for in vitro activity against different life stages of H. contortus. While only 3 compounds were found to be evaluated for in vivo activity using gerbil (Meriones unguiculatus) as an animal model. Based on minimum concentration and maximum nematicidal activity, Cinnamaldehyde, anethole, and carvone were highly active and completely inhibited egg hatching of the parasite at the tested concentrations of 0.085, 0.085, and 0.366 mg/ml, respectively. Carvacrol inhibited the larval development at a minimum concentration of 1 mg/ml, though the effectiveness of thymol and anethole against larvae development was also significant, but relatively at high concentrations i.e., 10 and 20 mg/ml, correspondingly.
Carvacrol and carvacryl acetate at 2 mg/ml showed 100% larval motility inhibition as compared to other compounds. Similarly, carvacryl acetate (0.2 mg/ml) and citronellal (2 mg/ml) totally reduced the motility of adult parasites in vitro. However, citronellal was moderately toxic in mice. Anethole and carvone significantly reduced fecal egg count, decreased male length, and reproductive capacity of female at 50 mg/kg concentration in vivo (Table 3).
TABLE 3.
Plant compounds efficacy against H. contortus.
| Compound name | IUPAC name | Chemical structure | Plant name/family | Extract | Concentration (mg/ml) | Assays | Inhibition (%) | References |
|---|---|---|---|---|---|---|---|---|
| 1,8-Cineole | 1,3,3-Trimethyl-2-oxabicyclo [2.2.2] octan |
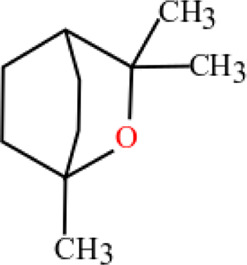
|
Artemisia lancea Vaniot/Asteraceae | Essential oil | 0.63 | EHT | 3.4 | Zhu et al. (2013b) |
| 1.25 | 10.0 | |||||||
| 2.5 | 26.6 | |||||||
| 5.0 | 56.6 | |||||||
| 10.0 | 74.8 | |||||||
| 0.63 | LDT | 10.6 | ||||||
| 1.25 | 23.4 | |||||||
| 2.5 | 33.6 | |||||||
| 5.0 | 49.2 | |||||||
| 10.0 | 65.2 | |||||||
| 0.63 | LMT | 5.5 | ||||||
| 1.25 | 10.4 | |||||||
| 2.5 | 30.2 | |||||||
| 5.0 | 48.6 | |||||||
| 10.0 | 60.3 | |||||||
| Produced synthetically | Essential oil | 1,787 | EHT | 99 | Katiki et al. (2017b) | |||
| Anethole | 1-Methoxy-4-(prop-1-en-1-yl)benzene |
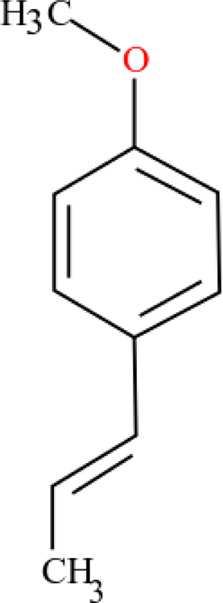
|
Croton zehntneri Pax and K.Hoffm./Euphorbiaceae | Essential oil | 0.31 | EHT | 6.7 | Camurça-Vasconcelos et al. (2007) |
| 0.62 | 26.6 | |||||||
| 1.25 | 99.9 | |||||||
| 1.25 | LDT | 35.8 | ||||||
| 2.5 | 52.1 | |||||||
| 5.0 | 87.7 | |||||||
| 10.0 | 96.7 | |||||||
| 20.0 | 100 | |||||||
| Supplied by GRASP Ind. E com. (Curitibia-PR, Brazil) | Encapsulated oil | 50 | FEC* | Fecal egg count was significantly reduced, decreased male length, and reproductive capacity of female after 45 days in santa ines lambs | Katiki et al. (2019) | |||
| 20 | The dose did not affect acquisition of parasites after pasture access and as FEC raised and body weight decreased of morada nova lambs | |||||||
| Produced synthetically | Essential oil | 0.085 | EHT | 99 | Katiki et al. (2017b) | |||
| Borneol | 1,7,7-Trimethylbicyclo [2.2.1]heptan-2-ol |
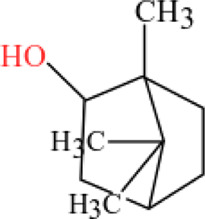
|
Zanthoxylum bungeanum Maxim. (=Zanthoxylum simulans Hance)/Rutaceae | Essential oil | 1.25 | EHT | 48.6 | Qi et al. (2015) |
| 2.5 | 62.1 | |||||||
| 5.0 | 80.0 | |||||||
| 10.0 | 92.2 | |||||||
| 20.0 | 98.8 | |||||||
| 40.0 | 100 | |||||||
| 1.25 | LDT | 38.2 | ||||||
| 2.5 | 52.2 | |||||||
| 5.0 | 80.2 | |||||||
| 10.0 | 91.2 | |||||||
| 20.0 | 98.0 | |||||||
| 40.0 | 100 | |||||||
| 1.25 | LMT | 52.8 | ||||||
| 2.5 | 37.2 | |||||||
| 5.0 | 23.2 | |||||||
| 10.0 | 9.6 | |||||||
| 20.0 | 2.6 | |||||||
| 40.0 | 1.8 | |||||||
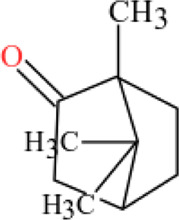
| Camphor | 1,7,7-trimethylbicyclo [2.2.1]heptan-2-one |
|
Artemisia lancea Vaniot/Asteraceae | Essential oil | 0.63 | EHT | - | Zhu et al. (2013b) |
| 1.25 | - | |||||||
| 2.5 | 2.8 | |||||||
| 5.0 | 9.0 | |||||||
| 10.0 | 12.8 | |||||||
| 0.63 | LDT | 4.4 | ||||||
| 1.25 | 15.8 | |||||||
| 2.5 | 23.4 | |||||||
| 5.0 | 37.2 | |||||||
| 10.0 | 57.0 | |||||||
| 0.63 | LMT | 5.7 | ||||||
| 1.25 | 13.2 | |||||||
| 2.5 | 17.4 | |||||||
| 5.0 | 13.0 | |||||||
| 10.0 | 18.1 | |||||||
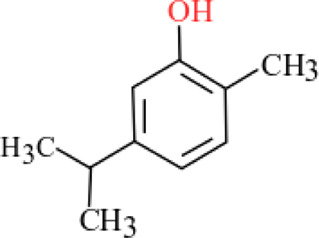
| Carvacrol | 2-Methyl-5-(propan-2-yl)phenol |
|
Arisaema franchetianum Engl., A. lobatum Engl./Araceae | Essential oil | 0.32 | EHT | 52.6 | Zhu et al. (2013a) |
| 0.63 | 65.6 | |||||||
| 1.25 | 83.4 | |||||||
| 2.5 | 93.2 | |||||||
| 5.0 | 100 | |||||||
| 10.0 | 100 | |||||||
| 0.32 | LDT | 38.4 | ||||||
| 0.63 | 54.2 | |||||||
| 1.25 | 77.2 | |||||||
| 2.5 | 89.2 | |||||||
| 5.0 | 98.0 | |||||||
| 10.0 | 100 | |||||||
| Produced synthetically | Essential oil | 5.517 | EHT | 99 | Katiki et al. (2017b) | |||
| Essential oil | 1 | EHT | 97.7 | Andre et al. (2016) | ||||
| 2 | LMT | 100 | ||||||
| 0.2 | AWMT | 58.3 | ||||||
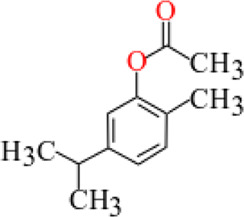
| Carvacryl acetate* | Phenol, 2-methyl-5-(1-Methylethyl)-, Acetate |
|
NA | NA | 250 | EGP | The compound reduced 57.7% of eggs per Gram of gastrointestinal parasites including H. contortus | André et al. (2020) |
| Essential oil | 8 | EHT | 89.3 | Andre et al. (2016) | ||||
| 2 | LMT | 100 | ||||||
| 0.2 | AWMT | 100 | ||||||
| Eugenol | 4-Allyl-2-methoxyphenol |
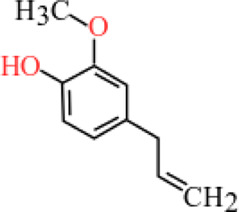
|
Ocimum gratissimum L./Lamiaceae | Essential oil | 0.625 | EHT | 58.49 | Pessoa et al. (2002) |
| 1.25 | 76.02 | |||||||
| 2.5 | 94.56 | |||||||
| 5 | 100 | |||||||
| 10 | 100 | |||||||
| Produced synthetically | Essential oil | 51.65 | EHT | 99 | Katiki et al. (2017b) | |||
| Linalool | 3,7-Dimethyl-1,6-octadien-3-ol |
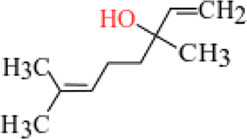
|
Arisaema franchetianum Engl., A. lobatum Engl./Araceae | Essential oil | 0.32 | EHT | 2.0 | Zhu et al. (2013a) |
| 0.63 | 6.4 | |||||||
| 1.25 | 12.6 | |||||||
| 2.5 | 29.6 | |||||||
| 5.0 | 46.6 | |||||||
| 10.0 | 65.8 | |||||||
| 0.32 | LDT | 1.8 | ||||||
| 0.63 | 4.4 | |||||||
| 1.25 | 12.0 | |||||||
| 2.5 | 25.2 | |||||||
| 5.0 | 37.6 | |||||||
| 10.0 | 48.2 | |||||||
| Produced synthetically | Essential oil | 17.47 | EHT | 99 | Katiki et al. (2017b) | |||
| Thymol | 5-Methyl-2-(propan-2-yl)phenol |
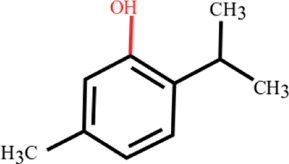
|
Lippia origanoides Kunth (=Lippia sidoides Cham.)/Verbenaceae | Essential oil | 0.31 | EHT | 9.9 | Camurça-Vasconcelos et al. (2007) |
| 0.62 | 93.6 | |||||||
| 1.25 | 98.2 | |||||||
| 1.25 | LDT | 33.0 | ||||||
| 2.5 | 54.8 | |||||||
| 5.0 | 73.9 | |||||||
| 10.0 | 99.2 | |||||||
| 20.0 | 99.7 | |||||||
| Produced synthetically | Essential oil | 5.0 | EHT | 99 | Katiki et al. (2017b) | |||
| Citronellal | 3,7-Dimethyloct-6-enal |
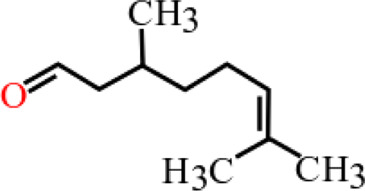
|
Corymbia citriodora (Hook.) K.D. Hill and L.A.S. Johnson (=Eucalyptus citriodora Hook.)/Myrtaceae | Essential oil | 0.75 | AWMT | 37.50 | Araújo-Filho et al. (2019) |
| 1 | 54.16 | |||||||
| 1.25 | 70.83 | |||||||
| 1.5 | 83.33 | |||||||
| 1.75 | 95.83 | |||||||
| 2 | 100 | |||||||
| Lectin | 9-Benzyl-3-methylidene-1,5-bis-(4-methylphenyl)sulfonyl-1,5,9-triazacyclododecane |
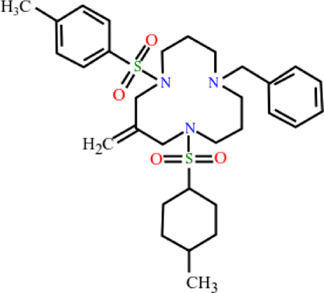
|
Parkia platycephala Benth/Leguminosae | Protein | 1.2 | LDT | - | Silva et al. (2019) |
| LET | - | |||||||
| 0.31 | LDT | 50 | ||||||
| Goniothalamin | (2R)-2-[(E)-2-phenylethenyl]-2,3-dihydropyran-6-one |
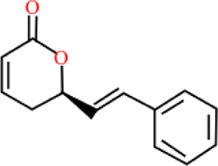
|
Cryptocarya massoy (Oken) Kosterm. (=Cryptocarya novoguineensis Teschner)/Lauraceae | NA | 200–300 μM | LDT | IC50 | Herath et al. (2019) |
| 6.25 μM | LMT | IC50 | ||||||
| Dihydrokavain | (2 S)-4-methoxy-2-(2-phenylethyl)-2,3-dihydropyran-6-one |
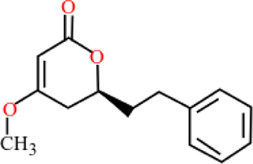
|
Piper methysticum G. Forst./Piperaceae | NA | 207 μM | LDT | IC50 | Herath et al. (2019) |
| LMT | - | |||||||
| Desmethoxyyangonin | 4-Methoxy-6-[(E)-2-phenylethenyl]pyran-2-one |
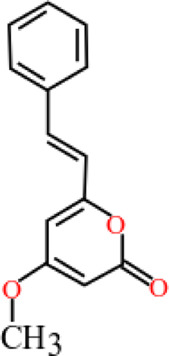
|
P. methysticum G. Forst./Piperaceae | NA | 31.7 μM | LDT | IC50 | Herath et al. (2019) |
| LMT | - | |||||||
| Yangonin | 4-Methoxy-6-[(E)-2-(4-methoxyphenyl) ethenyl] pyran-2-one |
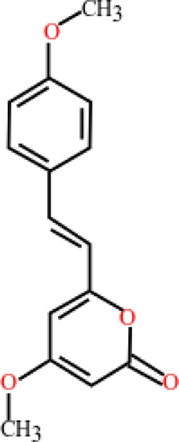
|
P. methysticum G. Forst./Piperaceae | NA | 23.7 μM | LDT | IC50 | Herath et al. (2019) |
| LMT | - | |||||||
| Carvone* | 2-Methyl-5-prop-1-en-2-ylcyclohex-2-en-1-one |
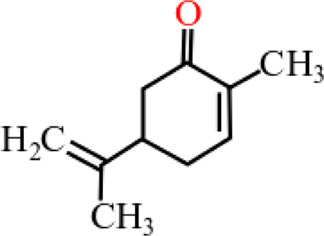
|
Supplied by GRASP Ind. E com. (Curitibia-PR, Brazil) | Encapsulated oil | 50 | FEC | Fecal egg count was significantly reduced, decreased male and reproductive capacity of female after 45 days in santa ines lambs | Katiki et al. (2019) |
| 20 | The dose did not affect acquisition of parasites after pasture access and as FEC raised and body weight decreased of morada nova lambs | |||||||
| Produced synthetically | Essential oil | 0.366 | EHT | 99 | Katiki et al. (2017b) | |||
| Cinnamaldehyde | (E)-3-phenylprop-2-enal |
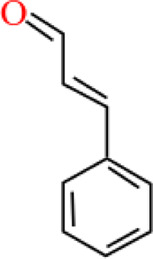
|
Produced synthetically | Essential oil | 0.085 | EHT | 99 | Katiki et al. (2017b) |
| Vanillin | 4-Hydroxy-3-methoxybenzaldehyde |
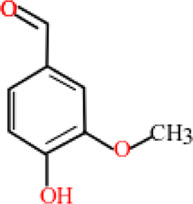
|
Produced synthetically | Essential oil | 815.16 | EHT | 99 | Katiki et al. (2017b) |
| Limonene | 1-Methyl-4-prop-1-en-2-ylcyclohexene |
|
Obtained from citrus peel | Essential oil | 207.56 | EHT | 50 | Katiki et al. (2017b) |
AWMT, Adult Worm Motility Test; LET, Larval Exsheathment Test; LMT, Larval Motility Test.
Quantitative Analysis
Jaccard Similarity Index
The JI was used to compare the two sets of data (i.e., in vitro and in vivo studies) to determine the similarity of the studies reported in this review. The result revealed 12.8% similarity between the two data sets (Figure 5).
FIGURE 5.
Jaccard similarity index (A) represents plants used in vitro and (B) represents plants used in vivo.
Discussion
Anti-haemonchiasis Medicinal Plants, Their Families, and Habit
Extensive use of Asteraceae, Fabaceae, Lamiaceae, and Euphorbiaceae, users’ reliability, and antiparasitic activity could be attributed to the presence of potent phytochemicals such as saponins, tannins, flavonoids in Asteraceae (Carvalho et al., 2013), saponins, essential oils in Lamiaceae (Raja, 2012), phenolic compounds, alkaloids, and hemagglutinins in Fabaceae (Żarnowski et al., 2001), and alkaloids and terpenoids in Euphorbiaceae (Mwine and Van Damme, 2011). Mechanism of action of these compounds against different developmental stages of H. contortus is still unknown, however, probably these may inhibit both egg embryonation and direct effects on the larvae (Al-Shaibani et al., 2008). Athanasiadou et al. (2001) attributed the antiparasitic activity of condensed tannins to their ability to bind with the cellular membrane proteins, which results in the unavailability of nutrients to the larvae, causing starvation, and death (Athanasiadou et al., 2001). It seems that complexities of these compounds enable them to interrupt various molecular targets of different developmental stages of the parasite. Saponoids usually act by binding to surface molecules (proteins/sterols) inducing inhibition of the protein expression, and/or lysis of the cell (Bruneton, 1993). The expression of surface proteins of nematodes is stage specific (Rhoads and Fetterer, 1994).
Furthermore, Asteraceae is being the first and Fabaceae is the third largest terrestrial plant families all over the world and this could be another possible reason for such an extensive utilization of these families (Tariq et al., 2017). Plant species of Fabaceae are able to fix nitrogen, which leads to protein deposition in leaves and seeds (Molares and Ladio, 2012). Species of Lamiaceae could be easily cultivated and propagated, moreover, they are mostly utilized due to their strong aroma and ability to survive in severe hot weather because of their essential oils (Raja, 2012). Euphorbiaceae extensive use for different medicinal purposes may be attributed to its global distribution and mode of adaptation in the worst dry conditions because of the succulent nature of its species and crassulacean acid metabolism (CAM) pathway ability. Plants of this family possess a wide array of secondary metabolites and tendency of mutation load due to their exposure to a wide range of environmental conditions (Mwine and Van Damme, 2011).
Herbs were frequently used form of life against H. contortus eggs, larvae, and adult worms as compared to trees and shrubs. The dominancy of herbs over other forms of life could be attributed to their easy availability and high efficacy against different ailments as compared to shrubs and trees (Ahmad et al., 2009). Herbs are widely used in folk medicines all over the globe and contain a large number of active compounds responsible for their high efficacy and, therefore, are preferred by the scientists and traditional healers (Tariq et al., 2017).
Leaves were reported as the widely used part during pharmacological validation of medicinal plants against H. contortus. Leaves contain a variety of chemical compounds and due to their easy harvesting and less harmful effect on plant life, make them as the first choice of herbalists (Bhat et al., 2013; Tariq et al., 2017).
Medicinal plants owing to their potential of having a significant source of bioactive compounds that may lead to the development of novel drugs (Azwanida, 2015; Ali et al., 2020). Scientists have analyzed and evaluated the effect of various kinds of solvents, for the purpose to extract these bioactive compounds from various plant parts (Altemimi et al., 2017). Extraction is the separation of medicinally active portions of a plant, using selective solvents through standard procedures (Azwanida, 2015). The purpose of extraction is to separate the soluble plant metabolites, leaving behind the insoluble cellular marc (residue) (Azwanida, 2015). Methanol was the most preferred solvent for plant extraction possibly owing to its polar nature that ensures the release of several bioactive compounds from plants. It has been scientifically proven that highly polar solvents should be used to extract different bioactive compounds with high accuracy (Altemimi et al., 2017). Fruitful results of active compound in plants mainly depend upon the solvent used for herbal formulation.
The results revealed that more studies were conducted to evaluate in vitro anthelmintic activities of medicinal plants as compared to in vivo. In vitro validation of medicinal plants provides the proof of reliability of these plants against H. contortus. In veterinary parasitology several in vitro techniques are broadly used for analysis of nematicidal activity of drugs/plant extracts prior to in vivo testing (Sangster and Gill, 1999). There are several positive aspects of in vitro assays prior to in vivo including less time consuming, less expensive, need for a smaller number of animals, and permitting the evaluation of the efficacy of different anthelmintic compounds throughout the life cycle of the parasite (Demeler et al., 2013). Based on the reliable results obtained from in vitro analysis further selection of extract/pure compound for in vivo evaluation can be carried-out (Zips et al., 2005). In vivo studies are mainly conducted to evaluate the mechanism of action of the desired extract/compound, the immune response of the host animal, toxicity levels, as well as the in vivo effectiveness. Although there are many advantages of in vivo studies, but there are also some shortcomings including more time consuming, expensive, and lower precision and reproducibility (Lacey et al., 1990). These limitations should be taken into account and highlight the significance of pharmaceutical/pharmacokinetic studies for the industrial development of new anthelmintic products against H. contortus. The research to find effective and natural anthelmintics has been highly inundated with in vitro studies, hence, it is suggested to evaluate the plant extracts/compounds in vivo in future.
Pharmacological Action
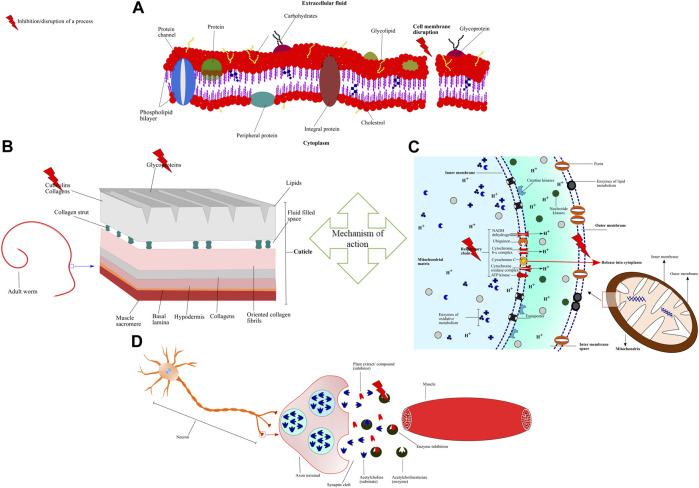
Pharmacological activity of an extract/compounds/drug depends on how the candidate interacts with enzymes, proteins, nucleic acids, biomolecules, and different types of receptors (Roy, 2011). Pharmacological activity is an important phenomenon to know the precise target of the drug/extract/compound with anthelmintic efficacy against the parasite or other organism/pathogen under observation (Figure 6).
FIGURE 6.
Schematic representation of mechanism of action and different pharmacological targets of plant extracts/compounds (A) Essential oils alter the permeability and cause depolarization of cytoplasmic membrane by interacting and disrupting the chemical structures of lipids, polysaccharides, and phospholipids (B) Condensed tannins (CT) bind to the cuticle proteins thus, inducing chemical and physical damage (C) Artemisinin disrupts the mitochondrial membrane potential and releases cytochrome c into the cytoplasm leading to inhibition of electron transfer and oxidative phosphorylation (D) Allium sativum inhibits Acetylcholinesterase (AChE) that hydrolyzes the neurotransmitter acetylcholine. Inhibition of ACheE leads to the accumulation of acetylcholine at the synaptic junction and disrupting the neuromuscular transmission which causes muscle paralysis.
A. sativum ethanolic extract inhibits the motility of the H. contortus through a destructive and inhibitive effect on the enzyme acetylcholinesterase (AChE). The enzyme rapidly hydrolyzes acetylcholine (neurotransmitter) and thus, limits and terminates the cholinergic synaptic transmission (Taylor, 1990; Lee, 1996). Inhibition of ACheE leads to the accumulation of acetylcholine, thereby interrupting the neuromuscular transmission causes paralysis of musculature. Due to muscular discoordination/paralysis food swallowing and movement through the digestive system is stopped. The parasites enter the state of starvation and energy deprivation and thus, unable to survive inside the host (Kaur and Sood, 1982; Opperman and Chang, 1992). The antiparasitic activity of A. sativum may also be attributed to the sulfur containing compounds (e.g., ajoene and allicin) which can possibly form disulphide bonds with free thiol groups, and thus, inhibit enzymes or other proteins, which are important for survival of the parasite (Krstin et al., 2018). Crude aqueous extract of Achillea millefolium L. has profound anthelmintic activity, this could be due to the presence of several key chemical constituents one of which is eugenol. It is reported that eugenol can cause alteration of cell shape, membrane blebs (Machado et al., 2011), restrict cell growth, swelling, and collapsing cell membrane and arrest cell division (Ueda-Nakamura et al., 2006). The compound artemisinin of A. absinthium can inhibit vital enzymes of metabolic cascade by forming covalent bonds and resulting in irreversible inhibition of the enzyme (s) activities. The enzymes include S-adenosyl-methionine synthesase (SAMS), spermidine synthase (SpdSyn), L-lactate dehydrogenase (LDH), pyruvate kinase, and ornithine aminotransferase (OAT) (Wang et al., 2015). Artemisinin is also reported to disrupt mitochondrial membrane potential, cause cytochrome c release into the cytoplasm (Jia et al., 2016), and inhibit the electron transfer and oxidative phosphorylation of mitochondria, with final activation of caspase-3-mediated apoptosis (Li et al., 2005). Additionally, crude aqueous extract of A. absinthium can induce ultrastructural changes such as tegumental damage, nephridial canal epithelium lining and intrauterine eggs destruction, lipid accumulation, glycogen depletion, and finally worm paralysis and death (Beshay, 2018). Similar anthelmintic effects of tegumental disorganization/perforation and adult worm paralysis were also observed for C. procera aqueous and ethanolic extracts (Khalil et al., 2016). Bromelain extracted from Ananas comosus (L.) Merr. stem has been found as potent anthelmintic against gastrointestinal nematodes (Stepek et al., 2004; Domingues et al., 2013). Bromelain removes and digests the cuticle layer of nematodes resulting in immobility and death of the parasites (Stepek et al., 2004; Stepek et al., 2006).
Azadirachta indica A. Juss. leaves contain condensed tannins (CT) (Sakti et al., 2018), which facilitate diffusion of flavonoids by binding to the cuticle proteins (Kerboeuf et al., 2008). Flavonoids and CT inhibit secretion of key enzymes (e.g., esterase, tyrosin kinase, and nonspecific cholinesterase) that may cause fatal intracellular instability, neuromuscular disorganization, energy depletion, paralysis and death of parasites (Hoste et al., 2006; Kerboeuf et al., 2008).
Essential oils of Corriandrum sativum L., C. citratus, C. schoenanthus, E. staigeriana and L. origanoides were found highly effective against H. contortus in vitro. Essential oils may acquire this efficacy owing to a mixture of different chemical constituents whose interaction can result in compounds that inhibit or disorganize vital functions from the initial stages of development onward, interrupt with parasite metabolic activities, and interfere with drive mechanisms due to possible destructuring of the nervous system (Oka et al., 2000). Furthermore, essential oils can alter the permeability and cause depolarization of cytoplasmic membrane by interacting and disrupting the chemical structures of lipids, polysaccharides, and phospholipids (Bakkali et al., 2008). Phytochemical profile of Cocos nucifera L. revealed the presence of alkaloids, flavonoids, phenols, triterpenes, and condensed tannins among others (Lima et al., 2015). The compounds like flavonoids have antioxidant activities, while condensed tannins have shown antiparasitic activities by binding to proteins present in the cuticle, oral cavity, esophagus, and cloaca, thus inducing chemical and physical damage in the parasite (Costa et al., 2010). The efficacy of plant compounds may be attributed to the fact that they inhibit or retard the growth, maturation damage, suppress appetite or reduce procreative ability, which are all the causes of mortality. Moreover, the considerable activity of plants extracts may be due to the additive or synergistic relationship among different major components which can interact with multiple molecular targets in various developmental stages of the parasite to produce a pharmacological effect (Marie-Magdeleine et al., 2009).
H. contortus exposed to C. citriodora essential oils demonstrated ultrastructural changes, such as formations of vacuoles, disorganization of muscular layer, and changes in the mitochondrial profile. These changes suggest the loss of homeostasis and loss of motility due to muscular disorganization of the parasite (Araújo-Filho et al., 2019). E. helioscopia has monoterpens, which are lipophilic and can penetrate through cell membrane, induce expansion, increase permeability and disturb membrane structure and membrane embedded enzymes (Cox et al., 2000; Samy and Ignacimuthu, 2000; Cox et al., 2001). The nematicidal activity may also be attributed to the presence of tannins, which on the surface of nematodes can form complexes with proteins and result in alteration of metabolic pathways (Min et al., 2004; Ahmed, 2010) and enzymes (cysteine proteinases), which can damage the cuticle and kill the nematode parasites (Stepek et al., 2004; Stepek et al., 2006). Similarly, saponins, amino acids, and sterols can disturb proteins structure (Mabusela et al., 1990) therefore, affecting growth and reparation of nematode body. While compound muzigadial and ajoene has anti-feedant activity and inhibit proliferation of arterial smooth muscle cells and protein prenylation (Ferri et al., 2003; Mohanlall and Odhav, 2009). L. latisiliquum leaves forage utilization presented ultrastructural changes; for instance, disturbance of intestinal muscular cells/tissues and cytoplasmic vacuolization, suggesting that different secondary metabolites of leaves may provoke these changes. The alterations to the intestinal cells may be due to the ingestion of active compounds by the parasite, and the resulting direct contact between the bioactive compounds and the intestinal cells. The cytoplasmic vacuolization described can be interpreted as signs of disturbances in cellular functions, possibly due to imbalance of fluid exchanges between the intestinal and pseudocoelomic space and/or between the muscle and the pseudocoelomic space (Martínez-Ortiz-de-Montellano et al., 2019).
The high efficacy of Nicotiana tabacum L. against H. contortus could be attributed to the presence of nicotine, a ganglion stimulant (Bowman and Rand, 1980). Nematode muscles are known to contain excitatory neuromuscular junctions containing ganglion type nicotinic receptors with acetylcholine as their neurotransmitter (Neal, 2020). Any ganglion stimulant would tend to activate theses neuromuscular junctions causing a spastic paralysis in the worms leading to their death and expulsion from the host (Nouri et al., 2016). Among the reported plants with promising anti-haemonchiasis activity, L. viridiflorum leaves are rich in flavonoids and its anthelmintic action of egg hatching inhibition could be due to the effect on enzymatic activity and metabolic processes in helminthes (Kerboeuf et al., 2008).
Different protein contents e.g., proteases, ribosomal proteins, chitinases etc. isolated from Spigelia anthelmia L. were effective against different life stages of H. contortus (Araujo et al., 2017). Proteases can cause severe damage to the cuticle by disrupting cuticular proteins of the parasite (Liang et al., 2010), in larvae the proteases may hydrolyze/digest the proteins necessary for larval migration (Araujo et al., 2017), and also degrade the egg membrane during egg hatching (Mansfield et al., 1992). Chitinases were also identified which can degrade chitin present in the egg shell (Rogers and Brooks, 1977) and larva, inhibiting their development and leading to death (Rocha et al., 2015).
Comparative analysis also revealed that plant extracts/essential oils were more effective in vitro than in vivo against various stages of the parasite. Similar differences between in vitro and in vivo results with plant treatments have been previously reported (Peneluc et al., 2009; Nogueira et al., 2012) and might be related with bioavailability of plant chemical constituents in different parts of the ruminant gastrointestinal tract (Athanasiadou et al., 2007; Eguale et al., 2007b). Furthermore, adult nematodes may also be more resistant to the active components, or rumen microbiota may reduce the activity of metabolites (Nogueira et al., 2012), and other aspects, such as ruminal pH. Mostly, in gerbils the efficacy of plant extracts was reported to be comparatively low than the activity observed in sheep. Using rodents for evaluation of plants anthelmintic activity has some drawbacks, firstly the habitat for nematodes is quite different in rodents and small ruminants, hence, association between habitat and drug site absorption define the higher or lower drug activity (Hennessy, 1997). Secondly, different efficacy obtained in rodents and sheep can be described by mechanism of distribution and biotransformation of the drug in a monogastric and polygastric animal species. However, efficacy test on rodent nematodes can help researchers deduce the prescriptions to be used on sheep and goats (Camurça-Vasconcelos et al., 2007). The studies concerning isolation and purification of plant compounds responsible for antiparasitic acitivities are few and insufficient. Most of the studies reported the presence of key components of the plants which do not provide any information about the effective antiparasitic compounds and their mechanism of action. Therefore, pure comounds isolated from plants should be given focus for in vitro, in vivo evaluation, toxicology, and pharmacology studies in future research, this will provide base-line information for developing new ecofriendly and cost effective drugs with lesser side effects.
Toxicity Evaluation
Safety issues of herbal medicines have been remained a big question and scientists are being interested in herbal medicines for decades. The notion that “natural” equals “safe” is apparently deceptive, since natural products comprise pharmacologically active compounds which, when taken in high doses or in specific conditions, can be detrimental to health. Bromelain of A. comosus is non-toxic and considered safe without any adverse effects and has shown good absorption and therapeutic benefits (Maurer, 2001). Moreover, no alteration in body weight, food, and water consumption was observed. The enzymes, urea, and creatinine levels of serum were also unaltered and no significant difference was observed (Dutta and Bhattacharyya, 2013). A. millefolium aqueous extract oral and intraperitoneal administration produced no significant biochemical and histopathological changes in Wistar rats (Cavalcanti et al., 2006). No relevant signs of toxicity were observed for longer periods of exposure, however, slight changes in blood glucose and cholesterol levels, and liver weight were detected, neither correlated with dose or period of exposure nor suggestive of toxicity (Cavalcanti et al., 2006). C. sativum was safe and no effects on hematological profile, histology, relative organ weights, and plasma markers of damage vital organs were found. However, a significant body weight loss was observed due to reduction in food intake (Patel et al., 2012), which is suggestive of the disturbances in carbohydrates, proteins, and fats (Ecobichon and Klaassen, 2001). C. citratus was safe and produced no toxic effects when tested against mice peritoneal macrophages (Santoro et al., 2007). Similarly, C. schoenanthus depicted no toxicological effects on hepatic and renal parameters in lambs (Katiki et al., 2012).
The ethyl acetate extract of C. nucifera presented no acute oral toxicity at the tested doses. However, the intraperitoneal and intramuscular administration was toxic (Tayler et al., 2020). Moreover, the hemoglobin level fallen below the normal limit after 8 days, suggesting that the extract could have negative effects if used for longer periods of time (Tayler et al., 2020). Similarly, E. staigeriana essential oil when administered orally was non-toxic, while intraperitoneal administration did not depict similar results (Macedo et al., 2010). Traditionally, when a substance administered orally and show LD50 value equal to 1000 mg/kg is considered to be safe or less toxic (Garner et al., 1961). The observed difference in toxicity may be attributed to the fact that after oral administration, the extract may be poorly absorbed, detoxified by the liver (Hayes and Loomis, 1996) or degraded by the stomach and gut digestive enzymes, however, during intraperitoneal administration the absorption is systematic and toxicity is stronger and appear earlier (Hayes and Loomis, 1996; Obici et al., 2008).
E. helioscopia was safe and produced no physiological alternations of vital organs and the biochemical parameters were also unchanged at the tested doses (Saleem et al., 2016). S. anthelmia and N. tabacum did not affect the body weight and animal behavior and were considered to be non-toxic (Ribeiro et al., 2017; Andjani et al., 2019).
Low toxicity of A. sativum and L. viridiflorum was observed on human HaCat and mammalian macrophages cells, respectively, (Krstin et al., 2018; de Melo et al., 2020). Mice became dead after oral administration of C. citriodora essential oil and citronellal suggesting toxicity of the plant species (Araújo-Filho et al., 2019). Prolonged use of A. absinthium and A. herba-alba lead to neurotoxicity and infertility by affecting the reproductive system, respectively (Almasad et al., 2007; Lachenmeier, 2010). A. indica poisoning affect was dose and time dependent and histopathological analysis showed that the testicles, liver, and kidneys were the organs affected (Deng et al., 2013). C. procera latex was found to be toxic at the tested doses, animals developed signs of nervousness, salivation, urination, dyspnea, tachycardia, and loss of condition. Severe pathological changes in intestines, heart, liver, kidneys, lungs, and brain were also observed (Mahmoud et al., 1979). A slight change (decrease) in the serum biochemical profile was also observed. This decrease in serum zinc, iron, and copper concentration (Al-Qarawi et al., 2001) might be due to continue (i) interference of adult parasites in the abomasum with digestibility and absorption of nutritive substances as a results of existing damage to the abomasal mucosa and its digestive function, or (ii) effects of unknown toxic principles elaborated by the worm.
Conclusion and Future Recommendations
Mostly, in vitro studies have been performed to evaluate the anti-haemonchiasis activity of plants. In vitro studies have a key role in initial screening however, these studies provide no information of bioavailability, toxicity, and in vivo efficacy of tested extract/compound. Hence, in future in vivo studies by using suitable animal models should be carried out to understand the pharmacokinetics and pharmacodynamics of the tested extract/compound. Most of the in vivo studies provide no evidence about toxicity and mechanism of action of the medicinal plant/compound, this is the most neglected aspect and strongly suggested to researchers to evaluate toxicity levels and pharmacological action of the tested plant/compound. Mentha x vilosa Huds., Anthemis nobilis L (syn. Chamaemelum nobilis (L.) All.), Lantana camara L., Trichilia claussenii C. DC., Croton macrostachyus Hochst ex Delile, Lavandula officinalis (Chaix and Kitt.), Coleus maculosus subsp. edulis (Vatke) A.J.Paton (Plectranthus punctatus (L.f) L’Her.), Maesa lanceolata Forssk., and Foeniculum vulgare Mill. among others were highly effective in vitro against different life stages of the parasite, however, these plant species are not tested for in vivo efficacy. These plants should be evaluated for in vivo anti-haemonchiasis activity along with phytochemical profile, toxicological effects, and pharmacological activity. Most of the reported studies provide no information regarding time exposure and LC50 values of medicinal plant/compound used for in vitro evaluation. Time exposure and LC50 are very important parameters to understand the accurate efficacy of medicinal plants, therefore, it is recommended to provide this information in future studies. Plants contain a number of different compounds, which act synergistically to perform an activity, only few compounds have been isolated and tested in vitro/in vivo against H. contortus. It is recommended to identify/isolate individual compounds and evaluate their activity, this will provide more precise and in depth information of anti-haemonchiasis potential of the medicinal plant under observation. Plant compounds cinnamaldehyde, thymol, and carvacrol have revealed high efficacy in in vitro studies, it is recommended to further investigate these compounds for in vivo activity. Carvone and anethole have shown promising anti-haemonchiasis potential in vitro and in vivo, however, their toxicity levels and pharmacological effects are unknown and should be investigated in future studies. L. viridiflorum has revealed high efficacy both in vitro and in vivo and has no adverse/toxic effects after oral administration and considered to be safe, it is recommended to pharmaceutical industries to further investigate this plant species because it could be an alternative candidate for drug development against H. contortus.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
MA and RA conceptualized the idea. RA, MR, and SM searched the literature and drafted the manuscript. RA, MR, SM, SN, and SA drafted the figures and tables. MA, SNK, and SM supervised the whole process, provided comments on the initial draft and helped in editing. All authors critically revised the manuscript and approved the final version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.644027/full#supplementary-material.
References
- Acharya J., Hildreth M. B., Reese R. N. (2014). In vitro screening of forty medicinal plant extracts from the United States Northern Great Plains for anthelmintic activity against Haemonchus contortus . Vet. Parasitol. 201 (1-2), 75–81. 10.1016/j.vetpar.2014.01.008 [DOI] [PubMed] [Google Scholar]
- Ademola I. O., Eloff J. N. (2011). Ovicidal and larvicidal activity of Cassia alata leaf acetone extract and fractions on Haemonchus contortus: in vitro studies. Pharm. Biol. 49 (5), 539–544. 10.3109/13880209.2010.526948 [DOI] [PubMed] [Google Scholar]
- Ademola I. O., Fagbemi B. O., Idowu S. O. (2007). Anthelmintic activity of Spigelia anthelmia extract against gastrointestinal nematodes of sheep. Parasitol. Res. 101 (1), 63–69. 10.1007/s00436-006-0444-0 [DOI] [PubMed] [Google Scholar]
- Ademola I. O., Fagbemi B. O., Idowu S. O. (2004). Evaluation of the anthelmintic activity of Khaya senegalensis extract against gastrointestinal nematodes of sheep: in vitro and in vivo studies. Vet. Parasitol. 122 (2), 151–164. 10.1016/j.vetpar.2004.04.001 [DOI] [PubMed] [Google Scholar]
- Ademola I. O., Idowu S. O. (2006). Anthelmintic activity of Leucaena leucocephala seed extract on Haemonchus contortus -infective larvae. Vet. Rec. 158 (14), 485. 10.1136/vr.158.14.485 [DOI] [PubMed] [Google Scholar]
- Ahmad H., Khan S. M., Ghafoor S., Ali1 N. (2009). Ethnobotanical study of upper Siran. J. Herbs, Spices Med. Plants 15 (1), 86–97. [Google Scholar]
- Ahmed A. H., Ejo M., Feyera T., Regassa D., Mummed B., Huluka S. A. (2020). In Vitro anthelmintic activity of crude extracts of Artemisia herba-alba and Punica granatum against Haemonchus contortus . J. Parasitol. Res. 2020, 1. 10.1155/2020/4950196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed M. A. A. (2010). Gastrointestinal (nematode) infections in small ruminants: epidemiology, anthelmintic efficacy and the effect of wattle tannins. [Google Scholar]
- Ahmed M., Laing M. D., Nsahlai I. V. (2013). In vitro anthelmintic activity of crude extracts of selected medicinal plants against Haemonchus contortus from sheep. J. Helminthol. 87 (2), 174–179. 10.1017/s0022149x1200020x [DOI] [PubMed] [Google Scholar]
- Aiyegoro O., Okoh A. (2009). Use of bioactive plant products in combination with standard antibiotics: implications in antimicrobial chemotherapy. J. Med. Plants Res. 3 (13), 1147–1152. [Google Scholar]
- Ali R., Khan S., Khan M., Adnan M., Ali I., Khan T. A. et al. (2020). A systematic review of medicinal plants used against Echinococcus granulosus. PLoS One 15 (10), e0240456. 10.1371/journal.pone.0240456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Qarawi A. A., Mahmoud O., Sobaih M., Haroun E., Adam S. (2001). A preliminary study on the anthelmintic activity of Calotropis procera latex against Haemonchus contortus infection in Najdi sheep. Vet. Res. Commun. 25 (1), 61–70. 10.1023/a:1026762002947 [DOI] [PubMed] [Google Scholar]
- Al-Shaibani I., Phulan M., Arijo A., Qureshi T. (2008). Ovicidal and larvicidal properties of Adhatoda vasica (L.) extracts against gastrointestinal nematodes of sheep in vitro . Pak Vet. J. 28 (2), 79–83. [Google Scholar]
- Almasad M. M., Qazan W. S., Daradka H. (2007). Reproductive toxic effects of Artemisia herba alba ingestion in female Spague-Dawley rats. Pak J. Biol. Sci. 10 (18), 3158–3161. 10.3923/pjbs.2007.3158.3161 [DOI] [PubMed] [Google Scholar]
- Alowanou G. G., Olounladé P. A., Akouèdegni G. C., Faihun A. M. L., Koudandé D. O., Hounzangbé-Adoté S. (2019). In vitro anthelmintic effects of Bridelia ferruginea, Combretum glutinosum, and Mitragyna inermis leaf extracts on Haemonchus contortus, an abomasal nematode of small ruminants. Parasitol. Res. 118 (4), 1215–1223. 10.1007/s00436-019-06262-5 [DOI] [PubMed] [Google Scholar]
- Altemimi A., Lakhssassi N., Baharlouei A., Watson D., Lightfoot D. (2017). Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 6 (4), 42. 10.3390/plants6040042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andjani H., Sentosa Y., Yati K., Fauzantoro A., Gozan M., Yoo Y. (2019). Acute oral toxicity test of nicotiana tabacum L. Bio-oil against female winstar rats. Paper presented at the IOP Conference Series: Earth and Environmental Science. [Google Scholar]
- André W. P. P., Paiva Junior J. R. d., Cavalcante G. S., Ribeiro W. L. C., Araújo Filho J. V. d., Santos J. M. L. d., et al. (2020). Anthelmintic activity of nanoencapsulated carvacryl acetate against gastrointestinal nematodes of sheep and its toxicity in rodents. Rev. Bras. Parasitol. Vet. 29 (1), e013119. 10.1590/s1984-29612019098 [DOI] [PubMed] [Google Scholar]
- Andre W. P. P., Ribeiro W. L. C., Cavalcante G. S., Santos J. M. L. d., Macedo I. T. F., Paula H. C. B. d., et al. (2016). Comparative efficacy and toxic effects of carvacryl acetate and carvacrol on sheep gastrointestinal nematodes and mice. Vet. Parasitol. 218, 52–58. 10.1016/j.vetpar.2016.01.001 [DOI] [PubMed] [Google Scholar]
- Araujo S. A., Soares A. M. d. S., Silva C. R., Almeida Júnior E. B., Rocha C. Q., Ferreira A. T. d. S., et al. (2017). In vitro anthelmintic effects of Spigelia anthelmia protein fractions against Haemonchus contortus . PLoS One 12 (12), e0189803. 10.1371/journal.pone.0189803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo-Filho J. V. d., Ribeiro W. L. C., André W. P. P., Cavalcante G. S., Rios T. T., Schwinden G. M., et al. (2019). Anthelmintic activity of Eucalyptus citriodora essential oil and its major component, citronellal, on sheep gastrointestinal nematodes. Rev. Bras. Parasitol. Vet. 28 (4), 644–651. 10.1590/s1984-29612019090 [DOI] [PubMed] [Google Scholar]
- Assis L., Bevilaqua C., Morais S., Vieira L., Costa C., Souza J. (2003). Ovicidal and larvicidal activity in vitro of Spigelia anthelmia Linn. extracts on Haemonchus contortus . Vet. Parasitol. 117 (1-2), 43–49. 10.1016/j.vetpar.2003.07.021 [DOI] [PubMed] [Google Scholar]
- Athanasiadou S., Githiori J., Kyriazakis I. (2007). Medicinal plants for helminth parasite control: facts and fiction. Animal 1 (9), 1392–1400. 10.1017/s1751731107000730 [DOI] [PubMed] [Google Scholar]
- Athanasiadou S., Kyriazakis I., Jackson F., Coop R. L. (2001). Direct anthelmintic effects of condensed tannins towards different gastrointestinal nematodes of sheep: in vitro and in vivo studies. Vet. Parasitol. 99 (3), 205–219. 10.1016/s0304-4017(01)00467-8 [DOI] [PubMed] [Google Scholar]
- Azwanida N. (2015). A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med. Aromat Plants 4 (196), 2167. 10.4172/2167-0412.1000196 [DOI] [Google Scholar]
- Bakkali F., Averbeck S., Averbeck D., Idaomar M. (2008). Biological effects of essential oils - a review. Food Chem. Toxicol. 46 (2), 446–475. 10.1016/j.fct.2007.09.106 [DOI] [PubMed] [Google Scholar]
- Barone C. D., Zajac A. M., Manzi-Smith L. A., Howell A. B., Reed J. D., Krueger C. G., et al. (2018). Anthelmintic efficacy of cranberry vine extracts on ovine Haemonchus contortus . Vet. Parasitol. 253, 122–129. 10.1016/j.vetpar.2018.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beshay E. V. N. (2018). Therapeutic efficacy of Artemisia absinthium against Hymenolepis nana: in vitro and in vivo studies in comparison with the anthelmintic praziquantel. J. Helminthol. 92 (3), 298–308. 10.1017/s0022149x17000529 [DOI] [PubMed] [Google Scholar]
- Bhat J. A., Kumar M., Bussmann R. W. (2013). Ecological status and traditional knowledge of medicinal plants in kedarnath wildlife sanctuary of garhwal himalaya, India. J. Ethnobiol. Ethnomedicine 9 (1), 1. 10.1186/1746-4269-9-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman W. C., Rand M. J. (1980). Textbook of pharmacology. Blackwell Scientific Publications. [Google Scholar]
- Bruneton J. (1993). Pharmacognosie: phytochimie plantes médicinales.
- Bucar F., Wube A., Schmid M. (2013). Natural product isolation - how to get from biological material to pure compounds. Nat. Prod. Rep. 30 (4), 525–545. 10.1039/c3np20106f [DOI] [PubMed] [Google Scholar]
- Cabardo D. E., Jr, Portugaliza H. P. (2017). Anthelmintic activity of Moringa oleifera seed aqueous and ethanolic extracts against Haemonchus contortus eggs and third stage larvae. Int. J. Vet. Sci. Med. 5 (1), 30–34. 10.1016/j.ijvsm.2017.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cala A. C., Chagas A. C. S., Oliveira M. C. S., Matos A. P., Borges L. M. F., Sousa L. A. D., et al. (2012). In vitro anthelmintic effect of Melia azedarach L. and Trichilia claussenii C. against sheep gastrointestinal nematodes. Exp. Parasitol. 130 (2), 98–102. 10.1016/j.exppara.2011.12.011 [DOI] [PubMed] [Google Scholar]
- Camurça-Vasconcelos A., Bevilaqua C., Morais S., Maciel M., Costa C., Macedo I., et al. (2007). Anthelmintic activity of Croton zehntneri and Lippia sidoides essential oils. Vet. Parasitol. 148 (3-4), 288–294. 10.1016/j.vetpar.2007.06.012 [DOI] [PubMed] [Google Scholar]
- Carvalho C. C. d., Machado K. N., Ferreira P. M. P., Pessoa C., Fonseca T. H. S., Gomes M. A., et al. (2013). Biological screening of extracts of Brazilian Asteraceae plants.
- Carvalho C. O., Chagas A. C. S., Cotinguiba F., Furlan M., Brito L. G., Chaves F. C., et al. (2012). The anthelmintic effect of plant extracts on Haemonchus contortus and Strongyloides venezuelensis . Vet. Parasitol. 183 (3-4), 260–268. 10.1016/j.vetpar.2011.07.051 [DOI] [PubMed] [Google Scholar]
- Castillo-Mitre G. F., Olmedo-Juárez A., Rojo-Rubio R., González-Cortázar M., Mendoza-de Gives P., Hernández-Beteta E. E., et al. (2017). Caffeoyl and coumaroyl derivatives from Acacia cochliacantha exhibit ovicidal activity against Haemonchus contortus . J. Ethnopharmacology 204, 125–131. 10.1016/j.jep.2017.04.010 [DOI] [PubMed] [Google Scholar]
- Cavalcante G. S., de Morais S. M., Andre W. P. P., Ribeiro W. L. C., Rodrigues A. L. M., De Lira F. C. M. L., et al. (2016). Chemical composition and in vitro activity of Calotropis procera (Ait.) latex on Haemonchus contortus . Vet. Parasitol. 226, 22–25. 10.1016/j.vetpar.2016.06.012 [DOI] [PubMed] [Google Scholar]
- Cavalcanti A. M., Baggio C. H., Freitas C. S., Rieck L., de Sousa R. S., Da Silva-Santos J. E., et al. (2006). Safety and antiulcer efficacy studies of Achillea millefolium L. after chronic treatment in Wistar rats. J. Ethnopharmacology 107 (2), 277–284. 10.1016/j.jep.2006.03.011 [DOI] [PubMed] [Google Scholar]
- Cortes-Morales J. A., Olmedo-Juárez A., Trejo-Tapia G., González-Cortazar M., Domínguez-Mendoza B. E., Mendoza-de Gives P., et al. (2019). In vitro ovicidal activity of Baccharis conferta Kunth against Haemonchus contortus . Exp. Parasitol. 197, 20–28. 10.1016/j.exppara.2019.01.003 [DOI] [PubMed] [Google Scholar]
- Costa C., Bevilaqua C., Camurça-Vasconcelos A., Maciel M., Morais S., Castro C., et al. (2008). In vitro ovicidal and larvicidal activity of Azadirachta indica extracts on Haemonchus contortus . Small Rumin Res. 74 (1-3), 284–287. 10.1016/j.smallrumres.2007.09.003 [DOI] [Google Scholar]
- Costa C. T. C., Bevilaqua C. M. L., Morais S. M., Camurça-Vasconcelos A. L. F., Maciel M. V., Braga R. R., et al. (2010). Anthelmintic activity of Cocos nucifera L. on intestinal nematodes of mice. Res. Vet. Sci. 88 (1), 101–103. 10.1016/j.rvsc.2009.05.008 [DOI] [PubMed] [Google Scholar]
- Cox S. D., Mann C. M., Markham J. L., Bell H. C., Gustafson J. E., Warmington J. R., et al. (2000). The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). J. Appl. Microbiol. 88 (1), 170–175. 10.1046/j.1365-2672.2000.00943.x [DOI] [PubMed] [Google Scholar]
- Cox S., Mann C., Markham J., Gustafson J., Warmington J., Wyllie S. (2001). Determining the antimicrobial actions of tea tree oil. Molecules 6 (2), 87–91. 10.3390/60100087 [DOI] [Google Scholar]
- de Araújo-Filho J. V., Ribeiro W. L. C., André W. P. P., Cavalcante G. S., Guerra M. d. C. M., Muniz C. R., et al. (2018). Effects of Eucalyptus citriodora essential oil and its major component, citronellal, on Haemonchus contortus isolates susceptible and resistant to synthetic anthelmintics. Ind. Crops Prod. 124, 294–299. 10.1016/j.indcrop.2018.07.059 [DOI] [Google Scholar]
- De Jesús-Gabino A. F., Mendoza-de Gives P., Salinas-Sánchez D. O., López-Arellano M. E., Liébano-Hernández E., Hernández-Velázquez V. M., et al. (2010). Anthelmintic effects of Prosopis laevigatan-hexanic extract against Haemonchus contortus in artificially infected gerbils (Meriones unguiculatus). J. Helminthol. 84 (1), 71–75. 10.1017/s0022149x09990332 [DOI] [PubMed] [Google Scholar]
- de Melo A. R. B., Maciel Higino T. M., da Rocha Oliveira A. D. P., Fontes A., da Silva D. C. N., de Castro M. C. A. B., et al. (2020). Lippia sidoides and Lippia origanoides essential oils affect the viability, motility and ultrastructure of Trypanosoma cruzi . Micron 129, 102781. 10.1016/j.micron.2019.102781 [DOI] [PubMed] [Google Scholar]
- de Oliveira L. M. B., Bevilaqua C. M. L., Macedo I. T. F., de Morais S. M., Machado L. K. A., Campello C. C., et al. (2011). Effects of Myracrodruon urundeuva extracts on egg hatching and larval exsheathment of Haemonchus contortus . Parasitol. Res. 109 (3), 893. 10.1007/s00436-011-2331-6 [DOI] [PubMed] [Google Scholar]
- Demeler J., Gill J. H., von Samson-Himmelstjerna G., Sangster N. C. (2013). The in vitro assay profile of macrocyclic lactone resistance in three species of sheep trichostrongyloids. Int. J. Parasitol. Drugs Drug Resist. 3, 109–118. 10.1016/j.ijpddr.2013.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y.-x., Cao M., Shi D.-x., Yin Z.-q., Jia R.-y., Xu J., et al. (2013). Toxicological evaluation of neem (Azadirachta indica) oil: acute and subacute toxicity. Environ. Toxicol. Pharmacol. 35 (2), 240–246. 10.1016/j.etap.2012.12.015 [DOI] [PubMed] [Google Scholar]
- Dixit A. K., Das G., Dixit P., Sharma R. L. (2019). Efficacy of herbal extracts and closantel against fenbendazole-resistant Haemonchus contortus . J. Helminthol. 93 (5), 529–532. 10.1017/s0022149x18000627 [DOI] [PubMed] [Google Scholar]
- Domingues L. F., Giglioti R., Feitosa K. A., Fantatto R. R., Rabelo M. D., de Sena Oliveira M. C., et al. (2013). In vitro and in vivo evaluation of the activity of pineapple (Ananas comosus) on Haemonchus contortus in Santa Inês sheep. Vet. Parasitol. 197 (1-2), 263–270. 10.1016/j.vetpar.2013.04.031 [DOI] [PubMed] [Google Scholar]
- Dutta S., Bhattacharyya D. (2013). Enzymatic, antimicrobial and toxicity studies of the aqueous extract of Ananas comosus (pineapple) crown leaf. J. Ethnopharmacology 150 (2), 451–457. 10.1016/j.jep.2013.08.024 [DOI] [PubMed] [Google Scholar]
- Ecobichon D. J., Klaassen C. (2001). Casarett and Doull’s Toxicology: the basic science of poisons Toxic effects of pesticides. New York: McGraw-Hill, 769–774. [Google Scholar]
- Eguale T., Tadesse D., Giday M. (2011). In vitro anthelmintic activity of crude extracts of five medicinal plants against egg-hatching and larval development of Haemonchus contortus . J. Ethnopharmacology 137 (1), 108–113. 10.1016/j.jep.2011.04.063 [DOI] [PubMed] [Google Scholar]
- Eguale T., Tilahun G., Debella A., Feleke A., Makonnen E. (2007a). Haemonchus contortus: in vitro and in vivo anthelmintic activity of aqueous and hydro-alcoholic extracts of Hedera helix . Exp. Parasitol. 116 (4), 340–345. 10.1016/j.exppara.2007.01.019 [DOI] [PubMed] [Google Scholar]
- Eguale T., Tilahun G., Debella A., Feleke A., Makonnen E. (2007b). In vitro and in vivo anthelmintic activity of crude extracts of Coriandrum sativum against Haemonchus contortus. J. Ethnopharmacology 110 (3), 428–433. 10.1016/j.jep.2006.10.003 [DOI] [PubMed] [Google Scholar]
- Féboli A., Laurentiz A. C., Soares S. C. S., Augusto J. G., Anjos L. A., Magalhães L. G., et al. (2016). Ovicidal and larvicidal activity of extracts of Opuntia ficus-indica against gastrointestinal nematodes of naturally infected sheep. Vet. Parasitol. 226, 65–68. 10.1016/j.vetpar.2016.06.030 [DOI] [PubMed] [Google Scholar]
- Ferreira L. E., Benincasa B. I., Fachin A. L., Contini S. H. T., França S. C., Chagas A. C. S., et al. (2018). Essential oils of Citrus aurantifolia, Anthemis nobile and Lavandula officinalis: in vitro anthelmintic activities against Haemonchus contortus . Parasite. Vector 11 (1), 269. 10.1186/s13071-018-2849-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira L. E., Benincasa B. I., Fachin A. L., França S. C., Contini S. S. H. T., Chagas A. C. S., et al. (2016). Thymus vulgaris L. essential oil and its main component thymol: anthelmintic effects against Haemonchus contortus from sheep. Vet. Parasitol. 228, 70–76. 10.1016/j.vetpar.2016.08.011 [DOI] [PubMed] [Google Scholar]
- Ferreira L. E., Castro P. M. N., Chagas A. C. S., França S. C., Beleboni R. O. (2013). In vitro anthelmintic activity of aqueous leaf extract of Annona muricata L. (Annonaceae) against Haemonchus contortus from sheep. Exp. Parasitol. 134 (3), 327–332. 10.1016/j.exppara.2013.03.032 [DOI] [PubMed] [Google Scholar]
- Ferri N., Yokoyama K., Sadilek M., Paoletti R., Apitz-Castro R., Gelb M. H., et al. (2003). Ajoene, a garlic compound, inhibits protein prenylation and arterial smooth muscle cell proliferation. Br. J. Pharmacol. 138 (5), 811–818. 10.1038/sj.bjp.0705126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouche G., Sakong B. M., Adenubi O. T., Pauw E., Leboho T., Wellington K. W., et al. (2016). Anthelmintic activity of acetone extracts from South African plants used on egg hatching of Haemonchus contortus . Onderstepoort J. Vet. Res. 83 (1), 1–7. 10.4102/ojvr.v83i1.1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaínza Y. A., Domingues L. F., Perez O. P., Rabelo M. D., López E. R., Chagas A. C. d. S. (2015). Anthelmintic activity in vitro of Citrus sinensis and Melaleuca quinquenervia essential oil from Cuba on Haemonchus contortus . Ind. Crops Prod. 76, 647–652. 10.1016/j.indcrop.2015.07.056 [DOI] [Google Scholar]
- Garner R. J., Clarke E. G. C., Clarke M. L. (1961). Veterinary toxicology: baillière. Tindall and Cox London. 10.1002/jps.3030470329 [DOI] [Google Scholar]
- Getachew S., Ibrahim N., Abebe B., Eguale T. (2012). In vitro evaluation of Anthelmintic activities of crude extracts of selected medicinal plants against Haemonchus contortus in AlemgenaWereda, Ethiopia. APG 3, 20–27. 10.5829/idosi.apg.2012.3.2.64177 [DOI] [Google Scholar]
- Gilleard J. S. (2006). Understanding anthelmintic resistance: the need for genomics and genetics. Int. J. Parasitol. 36 (12), 1227–1239. 10.1016/j.ijpara.2006.06.010 [DOI] [PubMed] [Google Scholar]
- Githiori J. B., Höglund J., Waller P. J., Baker R. L. (2004). Evaluation of anthelmintic properties of some plants used as livestock dewormers against Haemonchus contortus infections in sheep. Parasitology 129 (2), 245–253. 10.1017/s0031182004005566 [DOI] [PubMed] [Google Scholar]
- Hajaji S., Alimi D., Jabri M. A., Abuseir S., Gharbi M., Akkari H. (2018). Anthelmintic activity of Tunisian chamomile (Matricaria recutita L.) against Haemonchus contortus . J. Helminthol. 92 (2), 168–177. 10.1017/s0022149x17000396 [DOI] [PubMed] [Google Scholar]
- Hamad K. K., Iqbal Z., Abbas R. Z., Khan A., Muhammad G., Epperson B. (2014). Combination of Nicotiana tabacum and Azadirachta indica: a novel substitute to control levamisole and ivermectin-resistant Haemonchus contortus in ovine. Pak Vet. J. 8318(1):2074–7764. [Google Scholar]
- Hassan M., Ra M., Joshi T., Yatoo F. A., Habib H. (2019). In vitro anthelmintic activity of Abutilon theophrasti Medik.(malvaceae) against eggs and l3 larvae of Haemonchus contortus . Vitro Mol Toxicol Asian Journal of Pharmaceutical and Clinical Research(3). [Google Scholar]
- Hayes A. W., Loomis T. A. (1996). Loomis's essentials of toxicology. Elsevier. [Google Scholar]
- Heckendorn F., Häring D. A., Maurer V., Zinsstag J., Langhans W., Hertzberg H. (2006). Effect of sainfoin (Onobrychis viciifolia) silage and hay on established populations of Haemonchus contortus and Cooperia curticei in lambs. Vet. Parasitol. 142 (3-4), 293–300. 10.1016/j.vetpar.2006.07.014 [DOI] [PubMed] [Google Scholar]
- Hennessy D. (1997). Modifying the formulation or delivery mechanism to increase the activity of anthelmintic compounds. Vet. Parasitol. 72 (3-4), 367–390. 10.1016/s0304-4017(97)00106-4 [DOI] [PubMed] [Google Scholar]
- Herath H. M. P. D., Preston S., Jabbar A., Garcia-Bustos J., Addison R. S., Hayes S., et al. (2019). Selected α-pyrones from the plants Cryptocarya novoguineensis (Lauraceae) and Piper methysticum (Piperaceae) with activity against Haemonchus contortus in vitro . Int. J. Parasitol. Drugs Drug Resist. 9, 72–79. 10.1016/j.ijpddr.2018.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Villegas M., Borges-Argáez R., Rodriguez-Vivas R., Torres-Acosta J., Méndez-González M., Caceres-Farfan M. (2011). Ovicidal and larvicidal activity of the crude extracts from Phytolacca icosandra against Haemonchus contortus . Vet. Parasitol. 179 (1-3), 100–106. 10.1016/j.vetpar.2011.02.019 [DOI] [PubMed] [Google Scholar]
- Hördegen P., Cabaret J., Hertzberg H., Langhans W., Maurer V. (2006). In vitro screening of six anthelmintic plant products against larval Haemonchus contortus with a modified methyl-thiazolyl-tetrazolium reduction assay. J. Ethnopharmacology 108 (1), 85–89. 10.1016/j.jep.2006.04.013 [DOI] [PubMed] [Google Scholar]
- Hoste H., Jackson F., Athanasiadou S., Thamsborg S. M., Hoskin S. O. (2006). The effects of tannin-rich plants on parasitic nematodes in ruminants. Trends Parasitol. 22 (6), 253–261. 10.1016/j.pt.2006.04.004 [DOI] [PubMed] [Google Scholar]
- Hounzangbe-Adote M. S., Paolini V., Fouraste I., Moutairou K., Hoste H. (2005). In vitro effects of four tropical plants on three life-cycle stages of the parasitic nematode, Haemonchus contortus . Res. Vet. Sci. 78 (2), 155–160. 10.1016/j.rvsc.2004.05.009 [DOI] [PubMed] [Google Scholar]
- Hussien J., Urgessa K., Regassa F., Jemal A., Abajebel S., Hussien N. (2011). Antihelmentic effects of the essential oil extracts of selected medicinal plants against Haemonchus contortus . Int. J. Agric. Res. 6 (3), 290–298. 10.3923/ijar.2011.290.298 [DOI] [Google Scholar]
- Idris U. E., Adam S. E., Tartour G. (1982). The anthelmintic efficacy of Artemisia herba-alba against Haemonchus contortus infection in goats. Natl. Inst. Anim. Health Q. (Tokyo) 22 (3), 138–143. [PubMed] [Google Scholar]
- Iqbal Z., Lateef M., Ashraf M., Jabbar A. (2004). Anthelmintic activity of Artemisia brevifolia in sheep. J. Ethnopharmacol 93 (2-3), 265–268. 10.1016/j.jep.2004.03.046 [DOI] [PubMed] [Google Scholar]
- Iqbal Z., Lateef M., Jabbar A., Akhtar M. S., Khan M. N. (2006a). Anthelmintic activity ofVernonia anthelmintica. Seeds against trichostrongylid nematodes of sheep. Pharm. Biol. 44 (8), 563–567. 10.1080/13880200600896512 [DOI] [Google Scholar]
- Iqbal Z., Lateef M., Jabbar A., Ghayur M. N., Gilani A. H. (2006b). In vitro andIn vivo anthelmintic activity ofNicotiana tabacum L. leaves against gastrointestinal nematodes of sheep. Phytother. Res. 20 (1), 46–48. 10.1002/ptr.1800 [DOI] [PubMed] [Google Scholar]
- Iqbal Z., Lateef M., Jabbar A., Muhammad G., Khan M. N. (2005). Anthelmintic activity of Calotropis procera (Ait.) Ait. F. flowers in sheep. J. Ethnopharmacology 102 (2), 256–261. 10.1016/j.jep.2005.06.022 [DOI] [PubMed] [Google Scholar]
- Iqbal Z., Lateef M., Khan M. N., Jabbar A., Akhtar M. S. (2006c). Anthelmintic activity of Swertia chirata against gastrointestinal nematodes of sheep. Fitoterapia 77 (6), 463–465. 10.1016/j.fitote.2006.05.010 [DOI] [PubMed] [Google Scholar]
- Iqbal Z., Munir M. A., Khan M., Akhtar M. S., Javed I., Of O. (2001a). In vitro inhibitory effects of Sorghum bicolor on hatching and moulting of Haemonchus contortus eggs. Prospects 3, 451–453. [Google Scholar]
- Iqbal Z., Nadeem Q. K., Khan M., Akhtar M., Waraich F. N. (2001b). In vitro anthelmintic activity of Allium sativum, Zingiber officinale, Curcurbita mexicana and Ficus religiosa . Int. J. Agric. Biol. 3 (4), 454–457. [Google Scholar]
- Irum S., Ahmed H., Mukhtar M., Mushtaq M., Mirza B., Donskow-Łysoniewska K., et al. (2015). Anthelmintic activity of Artemisia vestita Wall ex DC. and Artemisia maritima L. against Haemonchus contortus from sheep. Vet. Parasitol. 212 (3-4), 451–455. 10.1016/j.vetpar.2015.06.028 [DOI] [PubMed] [Google Scholar]
- Jabbar A., Zaman M. A., Iqbal Z., Yaseen M., Shamim A. (2007). Anthelmintic activity of Chenopodium album (L.) and Caesalpinia crista (L.) against trichostrongylid nematodes of sheep. J. Ethnopharmacology 114 (1), 86–91. 10.1016/j.jep.2007.07.027 [DOI] [PubMed] [Google Scholar]
- Jaheed E., Mohamed A. H., Hassan N. M. F., Mahran K. M. A., Nasr S. M., Abou-Zeina H. A. A. (2019). Evaluation of the curative effect of Balanites aegyptiaca fruits ethanolic extract on Haemonchosis experimentally induced in Egyptian Baladi goats: phytoanalytical, parasitological and hematological studies. J. Parasit Dis. 43 (4), 638–650. 10.1007/s12639-019-01143-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J., Qin Y., Zhang L., Guo C., Wang Y., Yue X., et al. (2016). Artemisinin inhibits gallbladder cancer cell lines through triggering cell cycle arrest and apoptosis. Mol. Med. Rep. 13 (5), 4461–4468. 10.3892/mmr.2016.5073 [DOI] [PubMed] [Google Scholar]
- Kamaraj C., Rahuman A. A., Bagavan A., Mohamed M. J., Elango G., Rajakumar G., et al. (2010). Ovicidal and larvicidal activity of crude extracts of Melia azedarach against Haemonchus contortus (Strongylida). Parasitol. Res. 106 (5), 1071–1077. 10.1007/s00436-010-1750-0 [DOI] [PubMed] [Google Scholar]
- Kamaraj C., Rahuman A. A. (2011). Efficacy of anthelmintic properties of medicinal plant extracts against Haemonchus contortus . Res. Vet. Sci. 91 (3), 400–404. 10.1016/j.rvsc.2010.09.018 [DOI] [PubMed] [Google Scholar]
- Kamaraj C., Rahuman A. A., Elango G., Bagavan A., Zahir A. A. (2011). Anthelmintic activity of botanical extracts against sheep gastrointestinal nematodes, Haemonchus contortus . Parasitol. Res. 109 (1), 37–45. 10.1007/s00436-010-2218-y [DOI] [PubMed] [Google Scholar]
- Karim M. A., Islam M. R., Lovelu M. A., Nahar S. F., Dutta P. K., Talukder M. H. (2019). In vitro evaluation of anthelmintic activity of tannin-containing plant Artemisia extracts against Haemonchus contortus from goat. J. Bangladesh Agric. Univ. 17 (3), 363–368. 10.3329/jbau.v17i3.43216 [DOI] [Google Scholar]
- Katiki L., Chagas A., Takahira R. K., Juliani H., Ferreira J., Amarante A. F. T. d. (2012). Evaluation of Cymbopogon schoenanthus essential oil in lambs experimentally infected with Haemonchus contortus . Vet. Parasitol. 186 (3-4), 312–318. 10.1016/j.vetpar.2011.12.003 [DOI] [PubMed] [Google Scholar]
- Katiki L. M., Araujo R. C., Ziegelmeyer L., Gomes A. C. P., Gutmanis G., Rodrigues L., et al. (2019). Evaluation of encapsulated anethole and carvone in lambs artificially- and naturally-infected with Haemonchus contortus . Exp. Parasitol. 197, 36–42. 10.1016/j.exppara.2019.01.002 [DOI] [PubMed] [Google Scholar]
- Katiki L. M., Barbieri A. M. E., Araujo R. C., Veríssimo C. J., Louvandini H., Ferreira J. F. S. (2017b). Synergistic interaction of ten essential oils against Haemonchus contortus in vitro . Vet. Parasitol. 243, 47–51. 10.1016/j.vetpar.2017.06.008 [DOI] [PubMed] [Google Scholar]
- Katiki L. M., Gomes A. C. P., Barbieri A. M. E., Pacheco P. A., Rodrigues L., Veríssimo C. J., et al. (2017a). Terminalia catappa : chemical composition, in vitro and in vivo effects on Haemonchus contortus . Vet. Parasitol. 246, 118–123. 10.1016/j.vetpar.2017.09.006 [DOI] [PubMed] [Google Scholar]
- Kaur R., Sood M. (1982). In vitro effect of anthelmintics on malic enzyme and cholinesterase of Haemonchus contortus (Nematoda: trichostrongylidae). Indian J. Parasit 6, 267–268. 10.1007/BF02213962 [DOI] [Google Scholar]
- Kayani S., Ahmad M., Sultana S., Khan Shinwari Z., Zafar M., Yaseen G., et al. (2015). Ethnobotany of medicinal plants among the communities of Alpine and Sub-alpine regions of Pakistan. J. Ethnopharmacology 164, 186–202. 10.1016/j.jep.2015.02.004 [DOI] [PubMed] [Google Scholar]
- Kerboeuf D., Riou M., Guegnard F. (2008). Flavonoids and related compounds in parasitic disease control. Mrmc 8 (2), 116–128. 10.2174/138955708783498168 [DOI] [PubMed] [Google Scholar]
- Khalil L. M., Azzam A. M., Mohamed H. A., Aboueldahab M. M., Taha H. A., Soliman M. I. (2016). In Vitro effects of the stem extracts of the plant Calotropis Procera on Schistosoma mansoni adult worms. Egypt. J. Zool 174 (4083), 1–14. [Google Scholar]
- Krstin S., Sobeh M., Braun M., Wink M. (2018). Anti-parasitic activities of Allium sativum and Allium cepa against Trypanosoma b. brucei and Leishmania tarentolae . Medicines 5 (2), 37. 10.3390/medicines5020037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey E., Redwin J., Gill J., Demargheriti V., Waller P. (1990). A larval development assay for the simultaneous detection of broad spectrum anthelmintic resistance. Resistance of Parasites to Antiparasitic Drugs. Round Table Conference held at the 7th International Congress of Parasitology, Paris, France, August ,1990 . [Google Scholar]
- Lachenmeier D. W. (2010). Wormwood (Artemisia absinthium L.)-A curious plant with both neurotoxic and neuroprotective properties?. J. Ethnopharmacology 131 (1), 224–227. 10.1016/j.jep.2010.05.062 [DOI] [PubMed] [Google Scholar]
- Lara Tf M., Ml Bevilaqua C., Mb de Oliveira L., Lf Camurca-Vasconcelos A., Viera da. S. L., et al. (2009). In vitro ovicidal and larvicidal activity of Eucalyptus globulus essential oil on Haemonchus contortus . Rev. Bras Parasitol. Vet. 18, 62–66. 10.4322/rbpv.01803011 [DOI] [PubMed] [Google Scholar]
- Lee D. L. (1996). Why do some nematode parasites of the alimentary tract secrete acetylcholinesterase?. Int. J. Parasitol. 26 (5), 499–508. 10.1016/0020-7519(96)00040-9 [DOI] [PubMed] [Google Scholar]
- Li W., Mo W., Shen D., Sun L., Wang J., Lu S., et al. (2005). Yeast model uncovers dual roles of mitochondria in the action of artemisinin. Plos Genet. 1 (3), e36. 10.1371/journal.pgen.0010036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L., Meng Z., Ye F., Yang J., Liu S., Sun Y., et al. (2010). The crystal structures of two cuticle-degrading proteases from nematophagous fungi and their contribution to infection against nematodes. FASEB j. 24 (5), 1391–1400. 10.1096/fj.09-136408 [DOI] [PubMed] [Google Scholar]
- Lima E. B. C., Sousa C. N. S., Meneses L. N., Ximenes N. C., Santos Júnior M. A., Vasconcelos G. S., et al. (2015). Cocos nucifera (L.) (Arecaceae): a phytochemical and pharmacological review. Braz. J. Med. Biol. Res. 48 (11), 953–964. 10.1590/1414-431x20154773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lone B. A., Bandh S. A., Chishti M. Z., Bhat F. A., Tak H., Nisa H. (2013). Anthelmintic and antimicrobial activity of methanolic and aqueous extracts of Euphorbia helioscopia L. Trop. Anim. Health Prod. 45 (3), 743–749. 10.1007/s11250-012-0283-1 [DOI] [PubMed] [Google Scholar]
- Lone B. A., Chishti M., Bhat F. A., Tak H., Bandh S. A. (2012). In vitro and in vivo anthelmintic activity of Euphorbia helioscopia L. Vet. Parasitol. 189 (2-4), 317–321. 10.1016/j.vetpar.2012.04.023 [DOI] [PubMed] [Google Scholar]
- Lopes L. G., Silva M. H., Figueiredo A., Canuto K. M., Brito E. S., Ribeiro P. R. V., et al. (2018). The intake of dry cashew apple fiber reduced fecal egg counts in Haemonchus contortus-infected sheep. Exp. Parasitol. 195, 38–43. 10.1016/j.exppara.2018.10.004 [DOI] [PubMed] [Google Scholar]
- Mabusela W. T., Stephen A. M., Botha M. C. (1990). Carbohydrate polymers from Aloe ferox leaves. Phytochemistry 29 (11), 3555–3558. 10.1016/0031-9422(90)85275-k [DOI] [Google Scholar]
- Macedo I. T., Bevilaqua C. M., de Oliveira L. M., Camurça-Vasconcelos A. L., Morais S. M., Machado L. K., et al. (2012). In vitro activity of Lantana camara, Alpinia zerumbet, Mentha villosa and Tagetes minuta decoctions on Haemonchus contortus eggs and larvae. Vet. Parasitol. 190 (3-4), 504–509. 10.1016/j.vetpar.2012.07.001 [DOI] [PubMed] [Google Scholar]
- Macedo I. T., Bevilaqua C. M., de Oliveira L. M., Camurça-Vasconcelos A. L., Vieira L. d. S., Oliveira F. R., et al. (2010). Anthelmintic effect of Eucalyptus staigeriana essential oil against goat gastrointestinal nematodes. Vet. Parasitol. 173 (1-2), 93–98. 10.1016/j.vetpar.2010.06.004 [DOI] [PubMed] [Google Scholar]
- Macedo I. T. F., Oliveira L. M. B. d., André W. P. P., Araújo Filho J. V. d., Santos J. M. L. d., Rondon F. C. M., et al. (2019). Anthelmintic effect of Cymbopogon citratus essential oil and its nanoemulsion on sheep gastrointestinal nematodes. Rev. Bras. Parasitol. Vet. 28 (3), 522–527. 10.1590/s1984-29612019065 [DOI] [PubMed] [Google Scholar]
- Machado M., Dinis A. M., Salgueiro L., Custódio J. B. A., Cavaleiro C., Sousa M. C. (2011). Anti-Giardia activity of Syzygium aromaticum essential oil and eugenol: effects on growth, viability, adherence and ultrastructure. Exp. Parasitol. 127 (4), 732–739. 10.1016/j.exppara.2011.01.011 [DOI] [PubMed] [Google Scholar]
- Maciel M., Morais S. M., Bevilaqua C., Camurça-Vasconcelos A., Costa C., Castro C. (2006). Ovicidal and larvicidal activity of Melia azedarach extracts on Haemonchus contortus . Vet. Parasitol. 140 (1-2), 98–104. 10.1016/j.vetpar.2006.03.007 [DOI] [PubMed] [Google Scholar]
- Mahmoud O. M., Adam S. E. I., Tartour G. (1979). The effects of Calotropis procera on small ruminants. J. Comp. Pathol. 89 (2), 251–263. 10.1016/0021-9975(79)90064-1 [DOI] [PubMed] [Google Scholar]
- Malik S., de Mesquita L., Silva C., de Mesquita J., de Sá Rocha E., Bose J., et al. (2019). Chemical profile and biological activities of essential oil from Artemisia vulgaris L. Cultivated in Brazil. Pharmaceuticals 12 (2), 49. 10.3390/ph12020049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield L. S., Gamble H. R., Fetterer R. H. (1992). Characterization of the eggshell of Haemonchus contortus-I. Structural components. Comp. Biochem. Physiol. B: Comp. Biochem. 103 (3), 681. 10.1016/0305-0491(92)90390-d [DOI] [PubMed] [Google Scholar]
- Maphosa V., Masika P. J. (2012). Anthelmintic screening of fractions of Elephantorrhiza elephantina root extract against Haemonchus contortus . Trop. Anim. Health Prod. 44 (1), 159–163. 10.1007/s11250-011-9903-4 [DOI] [PubMed] [Google Scholar]
- Maphosa V., Masika P. J., Bizimenyera E. S., Eloff J. N. (2010). In-vitro anthelminthic activity of crude aqueous extracts of Aloe ferox, Leonotis leonurus and Elephantorrhiza elephantina against Haemonchus contortus . Trop. Anim. Health Prod. 42 (2), 301–307. 10.1007/s11250-009-9421-9 [DOI] [PubMed] [Google Scholar]
- Marie-Magdeleine C., Hoste H., Mahieu M., Varo H., Archimede H. (2009). In vitro effects of Cucurbita moschata seed extracts on Haemonchus contortus . Vet. Parasitol. 161 (1-2), 99–105. 10.1016/j.vetpar.2008.12.008 [DOI] [PubMed] [Google Scholar]
- Marie-Magdeleine C., Mahieu M., D’Alexis S., Philibert L., Archimede H. (2010). In vitro effects of Tabernaemontana citrifolia extracts on Haemonchus contortus . Res. Vet. Sci. 89 (1), 88–92. 10.1016/j.rvsc.2010.01.002 [DOI] [PubMed] [Google Scholar]
- Marie-Magdeleine C., Udino L., Philibert L., Bocage B., Archimede H. (2014). In vitro effects of Musa x paradisiaca extracts on four developmental stages of Haemonchus contortus . Res. Vet. Sci. 96 (1), 127–132. 10.1016/j.rvsc.2013.12.004 [DOI] [PubMed] [Google Scholar]
- Martínez-Ortiz-de-Montellano C., de Jesús Torres-Acosta J. F., Fourquaux I., Sandoval-Castro C. A., Hoste H. (2019). Ultrastructural study of adult Haemonchus contortus exposed to polyphenol-rich materials under in vivo conditions in goats. Parasite 26. 10.1051/parasite/2019065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer H. R. (2001). Bromelain: biochemistry, pharmacology and medical use. Cmls, Cel. Mol. Life Sci. 58 (9), 1234–1245. 10.1007/pl00000936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min B. R., Pomroy W. E., Hart S. P., Sahlu T. (2004). The effect of short-term consumption of a forage containing condensed tannins on gastro-intestinal nematode parasite infections in grazing wether goats. Small Ruminant Res. 51 (3), 279–283. 10.1016/s0921-4488(03)00204-9 [DOI] [Google Scholar]
- Minho A., Bueno I., Louvandini H., Jackson F., Gennari S., Abdalla A. (2008). Effect of Acacia molissima tannin extract on the control of gastrointestinal parasites in sheep. Anim. Feed Sci. Technol. 147 (1-3), 172–181. 10.1016/j.anifeedsci.2007.09.016 [DOI] [Google Scholar]
- Mohanlall V., Odhav B. (2009). Furans and furanones with antimycotoxigenic activity isolated from Warburgia salutaris (Canellaceae). J. Med. Plants Res. 3 (4), 231–240. [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. G., Group a. t. P. (2009). Preferred reporting Items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 151 (4), 264–269. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- Molares S., Ladio A. (2012). The usefulness of edible and medicinal Fabaceae in Argentine and Chilean Patagonia: environmental availability and other sources of supply. Evid. Based Complement. Alternat Med. 2012, 901918. 10.1155/2012/901918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal H., Hossain H., Awang K., Saha S., Mamun-Ur-Rashid S., Islam M. K., et al. (2015). Anthelmintic activity of ellagic acid, a major constituent of Alternanthera sessilis against Haemonchus contortus . Pak Vet. J. 35 (1). 58–62. [Google Scholar]
- Monglo D., Njongmeta L. M., Musongong G., Ngassoum M., Nukenine E. N. (2006). Evaluation of anthelmintic potential of ethanolic plant extracts from northern Cameroon against eggs and infective larvae of Haemonchus contortus . J. Biol. Sci. 6 (2), 426–433. 10.3923/jbs.2006.426.433 [DOI] [Google Scholar]
- Monteiro M. V. B., Bevilaqua C. M. L., Palha M. d. D. C., Braga R. R., Schwanke K., Rodrigues S. T., et al. (2011). Ethnoveterinary knowledge of the inhabitants of marajó island, eastern amazonia, Brazil. Acta Amaz. 41 (2), 233–242. 10.1590/s0044-59672011000200007 [DOI] [Google Scholar]
- Morais-Costa F., Bastos G. A., Soares A. C. M., Costa E. G. L., Vasconcelos V. O., Oliveira N. J. F., et al. (2016). In vitro and in vivo action of Piptadenia viridiflora (Kunth) Benth against Haemonchus contortus in sheep. Vet. Parasitol. 223, 43–49. 10.1016/j.vetpar.2016.04.002 [DOI] [PubMed] [Google Scholar]
- Mwine J. T., Van Damme P. (2011). Why do Euphorbiaceae tick as medicinal plants? A review of Euphorbiaceae family and its medicinal features. J. Med. Plants Res. 5 (5), 652–662. [Google Scholar]
- Neal M. J. (2020). Medical pharmacology at a glance. John Wiley & Sons. [Google Scholar]
- Nery P., Nogueira F., Martins E., Duarte E. (2010). Effects of Anacardium humile leaf extracts on the development of gastrointestinal nematode larvae of sheep. Vet. Parasitol. 171 (3-4), 361–364. 10.1016/j.vetpar.2010.03.043 [DOI] [PubMed] [Google Scholar]
- Njoku C. J., Asuzu I. U. (1998). The anthelmintic effects of the leaf extract of Ocimum gratissimum (L.). Phytomedicine 5 (6), 485–488. 10.1016/s0944-7113(98)80047-0 [DOI] [PubMed] [Google Scholar]
- Nogueira F. A., Fonseca L. D., da Silva R. B., de Paiva Ferreira A. V., Nery P. S., Geraseev L. C., et al. (2012). In vitro and in vivo efficacy of aqueous extract of Caryocar brasiliense Camb. to control gastrointestinal nematodes in sheep. Parasitol. Res. 111 (1), 325–330. 10.1007/s00436-012-2843-8 [DOI] [PubMed] [Google Scholar]
- Nouri F., Nourollahi-Fard S. R., Foroodi H. R., Sharifi H. (2016). In vitro anthelmintic effect of Tobacco (Nicotiana tabacum) extract on parasitic nematode, Marshallagia marshalli . J. Parasit Dis. 40 (3), 643–647. 10.1007/s12639-014-0550-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nsereko G., Emudong P., Omujal J., Acai J., Kungu J. M., Kabi F., et al. (2019). Comparison of the efficacy of crude methanolic extracts of Cassia occidentalis and Euphorbia hirta with levamisole-HCL against gastrointestinal nematodes of economic importance to goat production in Uganda. Trop. Anim. Health Prod. 51 (8), 2269–2278. 10.1007/s11250-019-01939-6 [DOI] [PubMed] [Google Scholar]
- Obici S., Otobone F. J., Sela V. R. d. S., Ishida K., Silva J. C. d., Nakamura C. V., et al. (2008). Preliminary toxicity study of dichloromethane extract of Kielmeyera coriacea stems in mice and rats. J. Ethnopharmacology 115 (1), 131–139. 10.1016/j.jep.2007.09.013 [DOI] [PubMed] [Google Scholar]
- Oka Y., Nacar S., Putievsky E., Ravid U., Yaniv Z., Spiegel Y. (2000). Nematicidal activity of essential oils and their components against the root-knot nematode. Phytopathology 90 (7), 710–715. 10.1094/phyto.2000.90.7.710 [DOI] [PubMed] [Google Scholar]
- Oliveira G. L., Vieira T. M., Nunes V. F., Ruas M. d. O., Duarte E. R., Moreira D. d. L., et al. (2014). Chemical composition and efficacy in the egg-hatching inhibition of essential oil of Piper aduncum against Haemonchus contortus from sheep. Revista Brasileira de Farmacognosia 24 (3), 288–292. 10.1016/j.bjp.2014.07.004 [DOI] [Google Scholar]
- Oliveira L. M. B., Bevilaqua C. M. L., Costa C. T. C., Macedo I. T. F., Barros R. S., Rodrigues A. C. M., et al. (2009). Anthelmintic activity of Cocos nucifera L. against sheep gastrointestinal nematodes. Vet. Parasitol. 159 (1), 55–59. 10.1016/j.vetpar.2008.10.018 [DOI] [PubMed] [Google Scholar]
- Opperman C. H., Chang S. (1992). Nematode acetylcholinesterases: molecular forms and their potential role in nematode behavior. Parasitol. Today 8 (12), 406–411. 10.1016/0169-4758(92)90189-9 [DOI] [PubMed] [Google Scholar]
- Palacios-Landín J., Mendoza-de Gives P., Salinas-Sánchez D. O., López-Arellano M. E., Liébano-Hernández E., Hernández-Velázquez V. M., et al. (2015). In vitro and in vivo nematocidal activity of Allium sativum and Tagetes erecta extracts against Haemonchus contortus . Turk Parazitol Derg 39 (4), 260–264. 10.5152/tpd.2015.4523 [DOI] [PubMed] [Google Scholar]
- Patel D., Desai S., Devkar R., Ramachandran A. V. (2012). Acute and sub-chronic toxicological evaluation of hydro-methanolic extract of Coriandrum Sativum L. seeds. EXCLI J. 11, 566–575. [PMC free article] [PubMed] [Google Scholar]
- Peneluc T., Domingues L. F., de Almeida G. N., Ayres M. C. C., Moreira E. L. T., da Cruz A. C. F., et al. (2009). Atividade anti-helmíntica do extrato aquoso das folhas de Zanthoxylum rhoifolium Lam.(Rutaceae). Rev. Bras Parasitol. Vet. 18 (1), 43–48. 10.4322/rbpv.018e1008 [DOI] [PubMed] [Google Scholar]
- Pessoa L., Morais S., Bevilaqua C., Luciano J. (2002). Anthelmintic activity of essential oil of Ocimum gratissimum Linn. and eugenol against Haemonchus contortus . Vet. Parasitol. 109 (1-2), 59–63. 10.1016/s0304-4017(02)00253-4 [DOI] [PubMed] [Google Scholar]
- Peter J. W., Chandrawathani P. (2005). Haemonchus contortus: parasite problem No. 1 from tropics - polar Circle. Problems and prospects for control based on epidemiology. Trop. Biomed. 22 (2), 131–137. [PubMed] [Google Scholar]
- Piza M., Féboli A., Augusto J., Anjos L., Laurentiz A., Royo V., et al. (2019). In vitro ovicidal and larvicidal activity of Psidium cattleianum Sabine leaves against gastrointestinal nematodes of naturally infected sheep. Bol Ind. Anim. 76, 1–8. 10.17523/bia.2019.v76.e1450 [DOI] [Google Scholar]
- Qi H., Wang W., Dai J., Zhu L. (2015). In vitro anthelmintic activity of Zanthoxylum simulans essential oil against Haemonchus contortus . Vet. Parasitol. 211 (3-4), 223–227. 10.1016/j.vetpar.2015.05.029 [DOI] [PubMed] [Google Scholar]
- Raja R. R. (2012). Medicinally potential plants of Labiatae (Lamiaceae) family: an overview. Res. J. Med. Plant 6 (3), 203–213. 10.3923/rjmp.2012.203.213 [DOI] [Google Scholar]
- Rhoads M. L., Fetterer R. H. (1994). Purification and characterization of surface-associated proteins from adult Haemonchus contortus . J. Parasitol. 80, 756–763. 10.2307/3283254 [DOI] [PubMed] [Google Scholar]
- Ribeiro J., Ribeiro W., Camurça-Vasconcelos A., Macedo I., Santos J., Paula H., et al. (2014). Efficacy of free and nanoencapsulated Eucalyptus citriodora essential oils on sheep gastrointestinal nematodes and toxicity for mice. Vet. Parasitol. 204 (3-4), 243–248. 10.1016/j.vetpar.2014.05.026 [DOI] [PubMed] [Google Scholar]
- Ribeiro W. L. C., Andre W. P. P., Cavalcante G. S., de Araújo-Filho J. V., Santos J. M. L., Macedo I. T. F., et al. (2017). Effects of Spigelia anthelmia decoction on sheep gastrointestinal nematodes. Small Ruminant Res. 153, 146–152. 10.1016/j.smallrumres.2017.06.001 [DOI] [Google Scholar]
- Rocha R. O., Morais J. K. S., Oliveira J. T. A., Oliveira H. D., Sousa D. O. B., Souza C. E. A., et al. (2015). Proteome of soybean seed exudates contains plant defense-related proteins active against the root-knot nematode Meloidogyne incognita . J. Agric. Food Chem. 63 (22), 5335–5343. 10.1021/acs.jafc.5b01109 [DOI] [PubMed] [Google Scholar]
- Rogers W. P., Brooks F. (1977). The mechanism of hatching of eggs of Haemonchus contortus . Int. J. Parasitol. 7 (1), 61–65. 10.1016/0020-7519(77)90026-1 [DOI] [PubMed] [Google Scholar]
- Roy J. (2011). “Bulk drugs or active pharmaceutical ingredients,” in Woodhead Publishing Series in Biomedicine, An Introduction to Pharmaceutical Sciences, Woodhead Publishing, 69–109. 10.1533/9781908818041.69 [DOI] [Google Scholar]
- Sachan A. K., Vishnoi G., Kumar R. (2016). Need of standardization of herbal medicines in modern era. ijpm 8 (3), 300–307. 10.5138/09750185.1847 [DOI] [Google Scholar]
- Sakti A. A., Kustantinah K., Nurcahyo R. W. (2018). In Vitro and in Vivo anthelmintic activities of aqueous leaf infusion of Azadirachta indica against Haemonchus contortus . Trop. Anim. Sci. J. 41 (3), 185–190. 10.5398/tasj.2018.41.3.185 [DOI] [Google Scholar]
- Saleem U., Ahmad B., Ahmad M., Erum A., Hussain K., Irfan Bukhari N. (2016). Is folklore use ofEuphorbia helioscopiadevoid of toxic effects? Drug Chem. Toxicol. 39 (2), 233–237. 10.3109/01480545.2015.1092040 [DOI] [PubMed] [Google Scholar]
- Saminathan M., Gopalakrishnan A., Latchumikanthan A., Milton A., Aravind M., Dhama K., et al. (2015). Histopathological and parasitological study of blood-sucking Haemonchus contortus infection in sheep. Adv. Anim. Vet. Sci. 3 (2), 99–108. 10.14737/journal.aavs/2015/3.2.99.108 [DOI] [Google Scholar]
- Samy R. P., Ignacimuthu S. (2000). Antibacterial activity of some folklore medicinal plants used by tribals in Western Ghats of India. J. Ethnopharmacol 69 (1), 63–71. 10.1016/s0378-8741(98)00156-1 [DOI] [PubMed] [Google Scholar]
- Sangster N. C., Gill J. (1999). Pharmacology of anthelmintic resistance. Parasitol. Today 15 (4), 141–146. 10.1016/s0169-4758(99)01413-1 [DOI] [PubMed] [Google Scholar]
- Santoro G. F., Cardoso M. G., Guimarães L. G. L., Freire J. M., Soares M. J. (2007). Anti-proliferative effect of the essential oil ofCymbopogon citratus(DC) Stapf (lemongrass) on intracellular amastigotes, bloodstream trypomastigotes and culture epimastigotes ofTrypanosoma cruzi(Protozoa: kinetoplastida). Parasitology 134 (11), 1649–1656. 10.1017/s0031182007002958 [DOI] [PubMed] [Google Scholar]
- Selemon M. (2018). Review on control of Haemonchus contortus in sheep and goat. J. Vet. Med. Res. 5 (5). [Google Scholar]
- Silva R. R. S., Silva C. R., Santos V. F., Barbosa C. R. S., Muniz D. F., Santos A. L. E., et al. (2019). Parkia platycephala lectin enhances the antibiotic activity against multi-resistant bacterial strains and inhibits the development of Haemonchus contortus . Microb. Pathogenesis 135, 103629. 10.1016/j.micpath.2019.103629 [DOI] [PubMed] [Google Scholar]
- Sirama V., Kokwaro J., Owuor B., Yusuf A., Kodhiambo M. (2015). In-vitro anthelmintic activity of Vernonia amygdalina Del.(asteraceae) roots using adult Haemonchus contortus worms. Int. J. Pharmacol. Res. 5 (1), 1–2. [Google Scholar]
- Soares A. M., Oliveira J. T., Rocha C. Q., Ferreira A. T., Perales J., Zanatta A. C., et al. (2018). Myracrodruon urundeuva seed exudates proteome and anthelmintic activity against Haemonchus contortus . PLoS One 13 (7), e0200848. 10.1371/journal.pone.0200848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires J. M., Foster J. G., Lindsay D. S., Caudell D. L., Zajac A. M. (2010). Efficacy of an orange oil emulsion as an anthelmintic against Haemonchus contortus in gerbils (Meriones unguiculatus) and in sheep. Vet. Parasitol. 172 (1-2), 95–99. 10.1016/j.vetpar.2010.04.017 [DOI] [PubMed] [Google Scholar]
- Stepek G., Behnke J. M., Buttle D. J., Duce I. R. (2004). Natural plant cysteine proteinases as anthelmintics? Trends Parasitol. 20 (7), 322–327. 10.1016/j.pt.2004.05.003 [DOI] [PubMed] [Google Scholar]
- Stepek G., Lowe A., Buttle D. J., Duce I., Behnke J. M. (2006). In vitro and in vivo anthelmintic efficacy of plant cysteine proteinases against the rodent gastrointestinal nematode, Trichuris muris . Parasitology 132 (5), 681–689. 10.1017/s003118200500973x [DOI] [PubMed] [Google Scholar]
- Tadesse D., Eguale T., Giday M., Mussa A. (2009). Ovicidal and larvicidal activity of crude extracts of Maesa lanceolata and Plectranthus punctatus against Haemonchus contortus . J. Ethnopharmacology 122 (2), 240–244. 10.1016/j.jep.2009.01.014 [DOI] [PubMed] [Google Scholar]
- Tariq A., Sadia S., Pan K., Ullah I., Mussarat S., Sun F., et al. (2017). A systematic review on ethnomedicines of anti-cancer plants. Phytother. Res. 31 (2), 202–264. 10.1002/ptr.5751 [DOI] [PubMed] [Google Scholar]
- Tariq K. A., Chishti M. Z., Ahmad F., Shawl A. S. (2008). Anthelmintic efficacy ofAchillea millifoliumagainst gastrointestinal nematodes of sheep:in vitroandin vivostudies. J. Helminthol. 82 (3), 227–233. 10.1017/s0022149x08972515 [DOI] [PubMed] [Google Scholar]
- Tariq K., Chishti M., Ahmad F., Shawl A. (2009). Anthelmintic activity of extracts of Artemisia absinthium against ovine nematodes. Vet. Parasitol. 160 (1-2), 83–88. 10.1016/j.vetpar.2008.10.084 [DOI] [PubMed] [Google Scholar]
- Tayler N. M., De Jesús R., Spadafora R., Coronado L. M., Spadafora C. (2020). Antiplasmodial activity of Cocos nucifera leaves in Plasmodium berghei-infected mice. J. Parasit Dis., 44, 1–9. 10.1007/s12639-020-01207-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. A. (1990). A larval development test for the detection of anthelmintic resistance in nematodes of sheep. Res. Vet. Sci. 49 (2), 198–202. 10.1016/s0034-5288(18)31077-4 [DOI] [PubMed] [Google Scholar]
- Ueda-Nakamura T., Mendonça-Filho R. R., Morgado-Díaz J. A., Korehisa Maza P., Prado Dias Filho B., Aparício Garcia Cortez D., et al. (2006). Antileishmanial activity of Eugenol-rich essential oil from Ocimum gratissimum . Parasitol. Int. 55 (2), 99–105. 10.1016/j.parint.2005.10.006 [DOI] [PubMed] [Google Scholar]
- Váradyová Z., Pisarčíková J., Babják M., Hodges A., Mravčáková D., Kišidayová S., et al. (2018). Ovicidal and larvicidal activity of extracts from medicinal-plants against Haemonchus contortus . Exp. Parasitol. 195, 71–77. 10.1016/j.exppara.2018.10.009 [DOI] [PubMed] [Google Scholar]
- Vargas-Magaña J., Torres-Acosta J., Aguilar-Caballero A., Sandoval-Castro C., Hoste H., Chan-Pérez J. (2014). Anthelmintic activity of acetone–water extracts against Haemonchus contortus eggs: interactions between tannins and other plant secondary compounds. Vet. Parasitol. 206 (3-4), 322–327. 10.1016/j.vetpar.2014.10.008 [DOI] [PubMed] [Google Scholar]
- Veerakumari L., Chitra N. (2016). Effect of Allium sativum on the motility and acetylcholinesterase of Haemonchus contortus . IJSR 5 (1), 883–887. 10.21275/v5i1.nov152809 [DOI] [Google Scholar]
- Wang J., Zhang C.-J., Chia W. N., Loh C. C., Li Z., Lee Y. M., et al. (2015). Haem-activated promiscuous targeting of artemisinin in Plasmodium falciparum . Nat. Commun. 6 (1), 1–11. 10.1038/ncomms10111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme A. J., Fairweather I., Prichard R., von Samson-Himmelstjerna G., Sangster N. C. (2004). Drug resistance in veterinary helminths. Trends Parasitol. 20 (10), 469–476. 10.1016/j.pt.2004.07.010 [DOI] [PubMed] [Google Scholar]
- Zamilpa A., García-Alanís C., López-Arellano M. E., Hernández-Velázquez V. M., Valladares-Cisneros M. G., Salinas-Sánchez D. O., et al. (2019). In vitro nematicidal effect of Chenopodium ambrosioides and Castela tortuosa n-hexane extracts against Haemonchus contortus (Nematoda) and their anthelmintic effect in gerbils. J. Helminthol. 93 (4), 434–439. 10.1017/s0022149x18000433 [DOI] [PubMed] [Google Scholar]
- Żarnowski R., Żarnowska E. D., Kozubek A. (2001). Alkylresorcinols in the family Fabaceae. Acta Soc. Bot. Pol. 70 (1), 25–29. 10.5586/asbp.2001.004 [DOI] [Google Scholar]
- Zenebe S., Feyera T., Assefa S. (2017). In vitro anthelmintic activity of crude extracts of aerial parts of Cissus quadrangularis L. and leaves of Schinus molle L. against Haemonchus contortus . Biomed. Res. Int. 2017. 1905987. 10.1155/2017/1905987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., Dai J., Yang L., Qiu J. (2013a). Anthelmintic activity of Arisaema franchetianum and Arisaema lobatum essential oils against Haemonchus contortus . J. Ethnopharmacology 148 (1), 311–316. 10.1016/j.jep.2013.04.034 [DOI] [PubMed] [Google Scholar]
- Zhu L., Dai J., Yang L., Qiu J. (2013b). In vitro ovicidal and larvicidal activity of the essential oil of Artemisia lancea against Haemonchus contortus (Strongylida). Vet. Parasitol. 195 (1-2), 112–117. 10.1016/j.vetpar.2012.12.050 [DOI] [PubMed] [Google Scholar]
- Zips D., Thames H. D., Baumann M. (2005). New anticancer agents: in vitro and in vivo evaluation. In Vivo 19 (1), 1–7. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.