Abstract
Interferon-γ autoantibodies increase the risk of disseminated nontuberculous mycobacterial infections. Addition of rituximab to antibiotics accelerates and improves outcomes, but refractory infections can occur due to persistent production of autoantibodies. We combined bortezomib with rituximab to reduce autoantibodies leading to clinical and radiographic improvement in infection.
Keywords: Nontuberculous mycobacteria, Interferon gamma, Autoantibodies, Rituximab, Bortezomib
Highlights
-
•
IFNγ autoantibodies increase the risk of disseminated infections with intracellular pathogens.
-
•
Rituximab combined with antibiotics improves outcomes, but infections can become refractory.
-
•
The addition of bortezomib is safe with close monitoring and can improve clinical outcomes.
1. Introduction
Disseminated infections with nontuberculous mycobacteria (NTM) occur exclusively in the setting of immune deficiency. Defects in the IFNγ-IL-12 axis are major risks for severe mycobacterial infection [1]. Autoantibodies to IFNγ can inhibit this pathway and are a well-described etiology of adult-onset immunodeficiency. This syndrome occurs primarily in individuals born in Southeast Asia and leads to an increased risk of disseminated infections with NTM and other intracellular pathogens [2]. Production of high titer, neutralizing anti-IFNγ antibodies can cause chronic, progressive infections which are often refractory to antibiotic therapy alone.
Recent evidence has supported the use of rituximab in the management of patients with anti-IFNγ autoantibodies and disseminated opportunistic infections [1]. Rituximab decreases autoantibody titers, which improves IFNγ signaling, leading to better clinical outcomes [3]. Serum anti-IFNγ autoantibody titers often correlate with disease activity [1]. However, progressive infection despite improvement in titers has also been described [3]. Prolonged rituximab therapy is often required and there is risk for relapse after discontinuation despite secondary antibiotic prophylaxis [1]. Refractory infections in the setting of rituximab may reflect the presence of long-lived autoantibody producing plasma cells which are not targeted by CD20+ B-cell depletion. We report a patient with IFNγ autoantibodies and recrudescent disseminated Mycobacterium intracellulare (MAC) infection which progressed despite antibiotics and continued B cell suppression with rituximab. Bortezomib, a proteosome inhibitor, was added to target plasma cells with clinical and radiologic improvement.
2. Case description
A 38-year-old woman originally from the Philippines with a history of anti-IFNγ autoantibodies and disseminated MAC presented with progressive infection despite antimycobacterials. She was initially diagnosed at 32-years after presenting with back pain, diffuse lymphadenopathy, and myalgias. These symptoms improved with steroids but recurred when they were tapered. A PET scan showed diffuse hypermetabolic bone lesions in the bilateral femora, left acetabulum, left pubic ramus, sacrum, and both iliac bones. There was also involvement of the eighth rib, thoracic vertebrae, manubrium, and both clavicles with extension into the surrounding musculature. Multiple bone biopsies yielded no growth. However, DNA sequencing identified Mycobacterium intracellulare, prompting referral to the National Institutes of Health (NIH) where she was found to have anti-IFNγ autoantibodies (see Supplementary Methods and Figure).
She underwent surgical debridement of the epidural abscess with internal fixation and C3 corpectomy with fusion. Despite therapy with multiple antibiotics including azithromycin, ethambutol, amikacin, rifampin and linezolid, she had clinical, laboratory and radiographic progression. The patient was signed onto 13-I-0082 (ClinicalTrials.gov Identifier: NCT01842386), and rituximab was started at 1g on D0, D14, D42, and monthly for a total of 8 doses over 7 months. During rituximab therapy she had clinical and radiographic improvement. The patient was maintained on all oral antibiotics for 1 additional year and gradually tapered to secondary prophylaxis with azithromycin alone.
There was no clinical or radiologic evidence of active infection until two years later when she presented with new left hip pain and an MRI demonstrated left iliac osteomyelitis with sacroiliac joint involvement and enhancement of the surrounding musculature. Repeat biopsy of the left posterior iliac grew MAC, and rituximab was restarted along with rifampin, tedizolid, clofazimine, moxifloxacin and azithromycin was continued. She remained on monthly rituximab; bedaquiline was added after four months due lack of clinical improvement. Despite no detectable CD20+ B-cells, her infection continued to progress radiographically with increased left pelvic osteomyelitis with involvement of the surrounding musculature and new right femur and ischium osteomyelitis. Her CRP increased to 256 mg/L with WBC count 14.4 K/microL. She also developed a draining sinus tract at the left posterior hip biopsy site.
Cultures from the sinus tract continued to grow MAC. Anti-IFNγ autoantibody titers had slightly declined from initial presentation, but functional testing still showed marked inhibition of STAT-1 phosphorylation indicating persistent IFNγ neutralization, which was felt to cause her progressive disease. No CD20+ B-cells were detected in the peripheral blood, suggesting that persistent autoantibody production was most likely from long-lived plasma cells. Therefore, bortezomib, a proteosome inhibitor, was added to her treatment regimen in order to target this long-lived population. She was dosed at 1.3mg/m2 subcutaneously on days 1, 4, 8, and 11 per cycle repeated every 23-days, a regimen derived from multiple myeloma treatment [4]. Valacyclovir prophylaxis was added due to the known increased risk of herpes virus reactivations with bortezomib.
After the addition of bortezomib, her inflammatory markers decreased over the next 2–3 months and clinical signs of infection stabilized. She continued to have slow clinical and radiologic improvement throughout the subsequent 6–8 months of treatment (Fig. 1). Her back and hip pain became manageable, and she had improved ambulation, eventually returning to work. Repeated MRIs showed decreasing left and right pelvic osteomyelitis with less surrounding inflammation and closure of the sinus tract. She completed 13-months of bortezomib while continuing maintenance rituximab and antimycobacterials. There was a small further decrease in her anti-IFNγ autoantibody titers over this time with detectable improvements in STAT-1 phosphorylation (Fig. 2). Bortezomib was self-discontinued after one year due to injection site irritation and intermittent gastrointestinal discomfort. She remains on maintenance rituximab and antibiotics. She is well 6 months after her last dose of bortezomib but will need continual clinical monitoring for signs of disease recurrence.
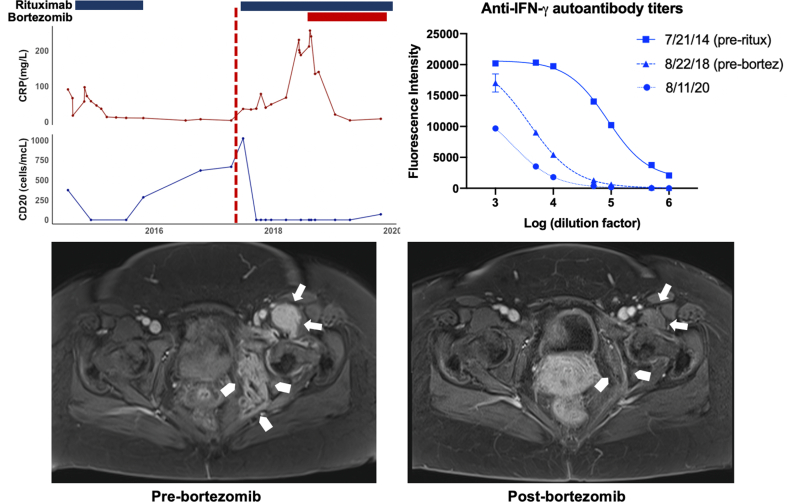
Fig. 1.
– Summary of laboratory studies and radiography: Trends in CRP, CD20+ B-cells, anti-IFNγ antibody levels, and changes in magnetic resonance imaging (MRI) of the pelvis pre- and post-bortezomib. C-reactive protein (solid red line) and CD20+ B-cells (solid blue line) changes over time and their relationship to rituximab (blue bars) and bortezomib (red bar) initiation. The vertical, red-dotted line represents time of clinical disease relapse (top-left). Anti-IFNγ autoantibody titers were highest on initial diagnosis as depicted by an increased fluorescence intensity throughout multiple Log10 dilutions (squares – top right). These titers decreased with rituximab but overall remained elevated pre-bortezomib (triangles). They decreased further after bortezomib was added to the rituximab regimen (circles). See supplementary materials for detailed methodology. Representative image of MRI pelvis post-contrast demonstrates significant soft tissue inflammation throughout the left obturator internus and pelvic sidewall (arrowheads) and the left biceps femoris (arrows) prior to bortezomib despite multiple antimycobacterial antibiotics and rituximab (bottom-left). Repeat MRI pelvis with contrast after one year of bortezomib shows resolution of soft tissue inflammation in these areas (bottom-right).
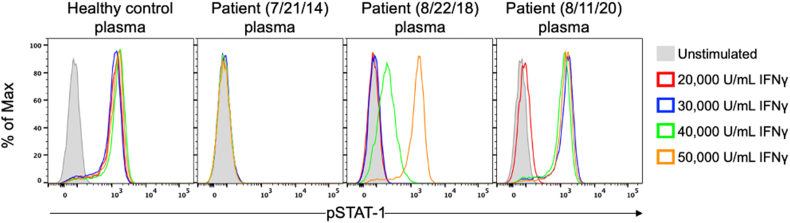
Figure-2.
STAT-1 phosphorylation assay: Functional STAT-1 phosphorylation (pSTAT-1) flow assay using stimulation with different concentrations of IFNγ. Healthy control cells in the presence of 10% healthy control plasma shows maximal STAT-1 phosphorylation at 100 U/mL (not shown). Patient plasma (7/21/14) prior to rituximab blocks STAT-1 phosphorylation in healthy control cells at the highest dose of 50,000 U/mL IFNγ. In 8/22/18 after treatment with rituximab, partial STAT-1 phosphorylation is present at 40,000 U/mL IFNγ and is fully restored at 50,000 U/mL IFNγ. Repeat assay on 8/11/20 after completing one-year of bortezomib combined with rituximab, STAT-1 phosphorylation is restored, but only at IFNγ concentrations of 30,000 U/mL and above, indicating significant and continued pSTAT1 blockade compared to healthy control plasma.
3. Discussion
Bortezomib is a proteosome inhibitor, initially approved for the treatment of refractory multiple myeloma in 2003 [4]. Plasma cells are exquisitely sensitive to proteosome inhibitors due to their constant production of antibodies. Inhibition leads to apoptosis and impaired differentiation of new plasma cells [5,6]. In vivo models in mice have shown bortezomib can deplete both short-lived and long-lived plasma cells through the induction of the unfolded protein response [7]. Human trials using bortezomib in kidney transplant patients with antibody-mediated rejection demonstrated decreased production of alloantibodies from plasma cells and lower numbers of plasma cells in the bone marrow [6]. Serum immunoglobulin levels often did not significantly decrease in these patients, suggesting that B-cell production of immunoglobulins is less affected, and new plasma cell differentiation continues as the bortezomib effects wane [8].
Our patient had progressive MAC refractory to combined rituximab and antibiotic therapy. Rituximab targets CD20+ B-cells and has proven efficacy in the treatment of autoantibody-mediated diseases including anti-IFNγ autoantibodies. However, it does not have a direct effect on mature, long-lived plasma cells [7]. Although our patient initially responded well to rituximab, she eventually relapsed, and her infection became refractory to rituximab and augmentation of antibiotics. Due to the suspicion for long-lived plasma cells continually producing anti-IFNγ autoantibodies, bortezomib was added to her treatment regimen to target these cells.
There is significant experimental and clinical evidence regarding the use of bortezomib in refractory autoantibody-mediated diseases [5,9]. It has been successfully used in the treatment of anti-NMDA encephalitis, thrombotic thrombocytopenic purpura, ANCA-associated vasculitis, and immune thrombocytopenic purpura [5,[9], [10], [11], [12]]. Notably, bortezomib in these cases was given in conjunction with or after previous failed treatments, which often included rituximab. These reports posit that clinical improvement may have depended on the combination of both medications [10].
The dynamics of plasma cell differentiation from B-cells remain poorly characterized. In the setting of plasma cell depletion with bortezomib there could be subsequent rapid differentiation of memory B-cells into new plasma cells leading to a loss of therapeutic benefit [13]. Therefore, in our patient, maintenance rituximab was continued throughout the course of bortezomib. This memory B-cell ‘tick over’ effect has also been considered important in the design of organ transplant protocols to prevent antibody-mediated rejection in which bortezomib and rituximab have also been combined [13].
Bortezomib has associated risks and most commonly can increase risk of herpes virus reactivation and cause peripheral neuropathy [5]. Our patient was maintained on valacyclovir prophylaxis; she had no progression of neuropathy over her one-year treatment course. Her IgG levels remained normal throughout treatment, suggesting that depletion of her antibody-producing plasma cells was not complete, consistent with previous reports in patients on similar regimens [6,13].
The addition of bortezomib led to dramatic clinical, laboratory, and radiologic improvement. She had a significant decrease in her back and hip pain and was able to return to work. The decrease in anti-IFNγ autoantibody titers on bortezomib was associated with improved IFNγ induced STAT-1 phosphorylation. Targeting plasma cells with bortezomib should be considered as an alternative, supplemental therapy for persistent IFNγ autoantibodies and refractory infections despite rituximab. Daratumumab, an anti-CD38 monoclonal antibody also targeting plasma cells, has recently been reported as effective salvage therapy in this clinical setting as well, providing multiple options for patients with refractory disease [14]. Due to the rarity of IFNγ autoantibodies, it is unlikely that randomized controlled trials will ever be performed. Bortezomib or daratumumab can be used safely in this setting with the potential for improved clinical outcomes.
Funding
Funding for this study was provided in part by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank our patient and her family for their willingness to participate in research.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtauto.2021.100102.
Author contributions
All authors attest that they meet the current International Committee of Medical Journal Editors (ICMJE) criteria for Authorship.
Informed consent
The authors declare that informed consent was obtained.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Browne S.K., Zaman R., Sampaio E.P., Jutivorakool K., Rosen L.B., Ding L. Anti-CD20 (rituximab) therapy for anti-IFN-gamma autoantibody-associated nontuberculous mycobacterial infection. Blood. 2012;119:3933–3939. doi: 10.1182/blood-2011-12-395707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Browne S.K., Burbelo P.D., Chetchotisakd P., Suputtamongkol Y., Kiertiburanakul S., Shaw P.A. Adult-onset immunodeficiency in Thailand and Taiwan. N. Engl. J. Med. 2012;367:725–734. doi: 10.1056/NEJMoa1111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hong G.H., Ortega-Villa A.M., Hunsberger S., Chetchotisakd P., Anunnatsiri S., Mootsikapun P. Natural history and evolution of anti-interferon-gamma autoantibody-associated immunodeficiency syndrome in Thailand and the United States. Clin. Infect. Dis. 2020;71:53–62. doi: 10.1093/cid/ciz786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bross P.F., Kane R., Farrell A.T., Abraham S., Benson K., Brower M.E. Approval summary for bortezomib for injection in the treatment of multiple myeloma. Clin. Canc. Res. 2004;10:3954–3964. doi: 10.1158/1078-0432.CCR-03-0781. [DOI] [PubMed] [Google Scholar]
- 5.Kohler S., Marschenz S., Grittner U., Alexander T., Hiepe F., Meisel A. Bortezomib in antibody-mediated autoimmune diseases (TAVAB): study protocol for a unicentric, non-randomised, non-placebo controlled trial. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-024523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perry D.K., Burns J.M., Pollinger H.S., Amiot B.P., Gloor J.M., Gores G.J. Proteasome inhibition causes apoptosis of normal human plasma cells preventing alloantibody production. Am. J. Transplant. 2009;9:201–209. doi: 10.1111/j.1600-6143.2008.02461.x. [DOI] [PubMed] [Google Scholar]
- 7.Everly J.J., Walsh R.C., Alloway R.R., Woodle E.S. Proteasome inhibition for antibody-mediated rejection. Curr. Opin. Organ Transplant. 2009;14:662–666. doi: 10.1097/MOT.0b013e328330f304. [DOI] [PubMed] [Google Scholar]
- 8.Ravetch J.V., Nussenzweig M. Killing some to make way for others. Nat. Immunol. 2007;8:337–339. doi: 10.1038/ni0407-337. [DOI] [PubMed] [Google Scholar]
- 9.Ratnasingam S., Walker P.A., Tran H., Kaplan Z.S., McFadyen J.D., Tran H. Bortezomib-based antibody depletion for refractory autoimmune hematological diseases. Blood Adv. 2016;1:31–35. doi: 10.1182/bloodadvances.2016001412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patriquin C.J., Thomas M.R., Dutt T., McGuckin S., Blombery P.A., Cranfield T. Bortezomib in the treatment of refractory thrombotic thrombocytopenic purpura. Br. J. Haematol. 2016;173:779–785. doi: 10.1111/bjh.13993. [DOI] [PubMed] [Google Scholar]
- 11.Novikov P., Moiseev S., Bulanov N., Shchegoleva E. Bortezomib in refractory ANCA-associated vasculitis: a new option? Ann. Rheum. Dis. 2016;75:e9. doi: 10.1136/annrheumdis-2015-207947. [DOI] [PubMed] [Google Scholar]
- 12.Vinayek N., Sharma V. A combination of bortezomib and rituximab yields a dramatic response in a woman with highly refractory immune thrombocytopenic purpura- a case report. J. Med. Case Rep. 2014;8:1–3. doi: 10.1186/1752-1947-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woodle E.S., Shields A.R., Ejaz N.S., Sadaka B., Girnita A., Walsh R.C. Prospective iterative trial of proteasome inhibitor-based desensitization. Am. J. Transplant. 2015;15:101–118. doi: 10.1111/ajt.13050. [DOI] [PubMed] [Google Scholar]
- 14.Ochoa S., Ding L., Kreuzburg S., Treat J., Holland S.M., Zerbe C.S. Daratumumab (anti-CD38) for treatment of disseminated nontuberculous mycobacteria in a patient with anti-IFN-gamma autoantibodies. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.