The development of effective but very expensive therapies presents special problems for health care policy-makers, who are committed to ensuring access to new therapies but who are also under pressure to control overall health care spending. This pressure has spawned widespread interest in the economic assessment of new therapies.1,2,3 In Canada, guidelines have been proposed for this type of economic analysis, with particular emphasis on new pharmaceuticals.4,5 The process involves assessing the quality of the evidence for effectiveness and quantifying the incremental cost–utility ratio, in dollars per quality- adjusted life-year gained.6 Although ways of classifying new therapies on this basis have been proposed, translating cost–utility ratios into acceptable policy has proved contentious.7
Cost-effectiveness studies of enzyme replacement therapy for Gaucher's disease have consistently shown that the treatment is effective and safe,8,9 and this type of therapy is associated with a significant improvement in quality of life.8 However, it is also extremely expensive.10,11 Estimates of the cost of the enzyme alone range from US$70 000 to US$550 000 per year for a typical adult with Gaucher's disease, depending on the dosage.11,12 Laupacis and associates6 have suggested that a cost per quality-adjusted life-year of US$100 000 is beyond the limit of acceptable cost-effectiveness, whereas the US Public Health Service Expert Panel on Cost-Effectiveness in Health and Medicine13 has recommended against any preset threshold. The same investment of public funds in any one of many other programs would generate a greater “bang for the buck,” at least in terms of the number of patients who might be treated.
Another approach to economic evaluation, suggested by one of us (R.B.D.),14 classifies new health care technologies according to “adoption zones” on the basis of the relation between costs and benefits relative to alternatives. Whereas some policy recommendations are clear-cut (e.g., adopt the technology if there is greater benefit at the same or lower cost, reject the technology if there is the same or lower benefit at greater cost), other situations represent tough choices (e.g., when there is greater benefit at greater cost or lower benefit at lower cost). This approach highlights the fact that a cost-effectiveness analysis can be used to identify the adoption zone for a particular technology, but it cannot help in determining whether the added benefits are worth the additional costs.
Because the cost per quality-adjusted life-year of enzyme replacement therapy for Gaucher's disease is above the level usually deemed cost-effective, in late 1992 the Ontario minister of health rejected appeals for public payment for the therapy. However, that decision was followed, in early 1993, by a widely publicized attack led by the National Gaucher Foundation of Canada. Policy-makers ran headlong into the deontological imperative of rescuing endangered life — what Jonsen called the “rule of rescue.”15 The foundation argued that the minister of health could not stand by and watch a very sick man suffer terribly, when the means to relieve his suffering were at her fingertips. In consultation with advisors in the Ministry of Health, she eventually approved a province-wide program of selective reimbursement for enzyme replacement therapy for Gaucher's disease. Although similar “rescues” of people with Gaucher's disease, especially children, have occurred in other provinces,16 Ontario is the only one with a program of reimbursement open to all patients with the disease in the province, with the level of reimbursement depending on clinical criteria of disease severity (these criteria are listed in an Appendix on eCMAJ at www.cma.ca/cmaj/vol-165/issue-5/clarkeappendix.pdf).
Policy-makers are reluctant to make decisions on the basis of the rule of rescue because of the potential for serious inequity: such decisions tend to be driven by emotional appeals, rather than by objective need. The outcomes of these decisions are also inherently unpredictable, and there is considerable potential for loss of control of expenditures.
The identification of clinical criteria for reimbursement, based on actual or anticipated severity of the disease, was therefore a critical element in the government's policy. Most patients with Gaucher's disease appear to be minimally symptomatic. The reimbursement program is intended to focus on those with severely disabling, if not life-threatening, complications, such as severe anemia or thrombocytopenia, severe skeletal complications, or pulmonary hypertension. According to the policy, decisions regarding reimbursement are to be made on the basis of objective indicators of disease severity, as assessed by an advisory committee of medical experts.
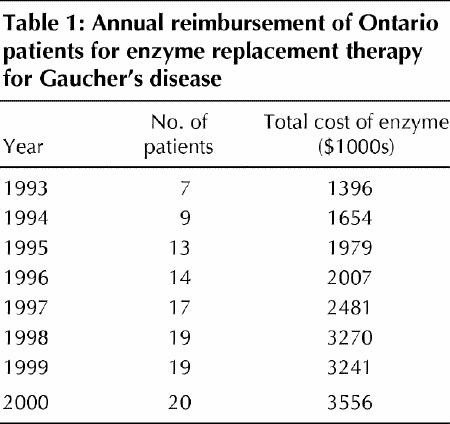
The number of patients receiving enzyme replacement therapy for Gaucher's disease increased rapidly, but not exponentially, from 1993 to the end of 2000. As of December 2000, 20 Ontario patients were receiving reimbursement for this therapy from the Ministry of Health. However, over the past 3 years, the unit cost of the enzyme (imiglucerase) has remained relatively constant. The total drug costs have levelled off at approximately $3.2 million per year (Table 1), principally because the number of patients receiving reimbursement for treatment has stabilized.
Table 1
Some problems remain with the reimbursement policy. First, the objective indicators and clinical criteria used in the assessment of disease severity are based on our current understanding of the natural history of Gaucher's disease, which is widely acknowledged to be incomplete. More research is needed on this aspect of the disease and on the predictors of disease severity. Second, the medical advisors need reassurance that the overall assignment of resources for the reimbursement program is adequate to treat all patients who meet the criteria for disease severity. The system is working, for now, primarily because the amount set aside is adequate for reasonable reimbursement of all patients meeting the criteria, but problems could arise if the number of patients were to increase substantially.
It is tempting to consider this approach as a model that might be applied in other situations. However, its success derives in part from the nature of Gaucher's disease, which may be extremely debilitating but is rarely fatal; thus, denying reimbursement to minimally symptomatic patients is not likely to raise serious ethical concerns. Furthermore, the nonfinancial costs to the patient, such as the inconvenience and discomfort of biweekly intravenous infusions, are not trivial and hence act to control the demand for the therapy. Ontario is a rich province, and the total number of patients with symptomatic disease is small, especially relative to conditions such as ischemic heart disease. Therefore, the total cost of treatment is relatively low and represents a very small proportion of the province's budget, even though the cost of treating an individual patient is high.
Despite these caveats, Ontario's policy on reimbursement for enzyme replacement therapy for Gaucher's disease is a good example of successful resource allocation for the treatment of a high-cost, low-prevalence disease.
Footnotes
Competing interests: Dr. Clarke is a paid consultant for Transkaryotic Therapies, Inc., and has received speaker fees and travel assistance from Genzyme Corporation and Transkaryotic Therapies, Inc. None declared for Drs. Amato and Deber.
Correspondence to: Dr. Joe T.R. Clarke, Department of Pediatrics, Hospital for Sick Children, 555 University Ave., Toronto ON M5G 1X8; fax 416 813–5345; jtrc@sickkids.on.ca
References
- 1.Torrance GW. Measurement of health state utilities for economic appraisal. J Health Econ 1986;5:1-30. [DOI] [PubMed]
- 2.Kaplan RM, Anderson JP. A general health policy model: update and applications. Health Serv Res 1988;23:203-35. [PMC free article] [PubMed]
- 3.Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-effectiveness in health and medicine. Toronto: Oxford University Press; 1996.
- 4.Detsky AS. Guidelines for economic analysis of pharmaceutical products: a draft document for Ontario and Canada. Pharmacoeconomics 1993;3:354-61. [DOI] [PubMed]
- 5.Torrance GW, Blaker D, Detsky AS, Kennedy W, Schubert F, Menon D, et al. Canadian guidelines for economic evaluation of pharmaceuticals. Pharmacoeconomics 1996;9:535-59. [DOI] [PubMed]
- 6.Laupacis A, Feeny D, Detsky AS, Tugwell PX. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ 1992;146:473-81. [PMC free article] [PubMed]
- 7.Hadorn DC. Setting health care priorities in Oregon: Cost-effectiveness meets the rule of rescue. JAMA 1991;265:2218-25. [PubMed]
- 8.Damiano AM, Pastores GM, Ware JE Jr. The health-related quality of life of adults with Gaucher's disease receiving enzyme replacement therapy: results from a retrospective study. Qual Life Res 1998;7:373-86. [DOI] [PubMed]
- 9.Whittington R, Goa KL. Alglucerase: a review of its therapeutic use in Gaucher's disease. Drugs 1992;44:72-93. [DOI] [PubMed]
- 10.Whittington R, Goa KL. Alglucerase: a pharmacoeconomic appraisal of its use in the treatment of Gaucher disease. Pharmacoeconomics 1995;7:63-90. [DOI] [PubMed]
- 11.Beutler E, Garber AM. Alglucerase for Gaucher's disease: dose, costs and benefits. Pharmacoeconomics 1994;5:453-9. [DOI] [PubMed]
- 12.Garber AM, Clarke AE, Goldman DP, Gluck ME. Federal and private roles in the development and provision of alglucerase therapy for Gaucher disease. Washington: US Congress, Office of Technology Assessment; 1992. Rep no. OTA-BP-H-104. Available: www.wws.princeton.edu/~ota/ (accessed 2001 July 6).
- 13.Siegel JE, Weinstein MC, Torrance GW. Reporting cost-effectiveness studies and results. In: Gold MR, Siegel JE, Russell LB, Weinstein MD, editors. Cost-effectiveness in health and medicine. Toronto: Oxford University Press; 1996. p. 276-303.
- 14.Deber RB. Translating technology assessment into policy. conceptual issues and tough choices. Int J Technol Assess Health Care 1992;8:131-7. [DOI] [PubMed]
- 15.Jonsen AR. Bentham in a box: technology assessment and health care allocation. Law Med Health Care 1986;14:172-4. [DOI] [PubMed]
- 16.MacKenzie JJ, Amato D, Clarke JTR. Enzyme replacement therapy for Gaucher's disease: the early Canadian experience. CMAJ 1998;159(10):1273-8. Abstract available: www.cma.ca/cmaj/vol-159/issue-10/1273.htm [PMC free article] [PubMed]


