Summary
Background: Fluorescence angiography (FA) assesses anastomotic perfusion during esophagectomy with gastric conduit reconstruction, but its interpretation is subjective. This study evaluated time to fluorescent enhancement in the gastric conduit, with the aim to determine a threshold to predict postoperative anastomotic complications.
Methods: In a prospective cohort study, all consecutive patients undergoing esophagectomy with gastric conduit reconstruction from July 2018 to October 2019 were included. FA was performed before anastomotic reconstruction following injection of indocyanine green (ICG). During FA, the following time points were recorded: ICG injection, first fluorescent enhancement in the lung, at the base of the gastric conduit, at the planned anastomotic site, and at ICG watershed or in the tip of the gastric conduit. Anastomotic complications including anastomotic leakage and clinically relevant strictures were documented.
Results: Eighty-four patients were included, the majority (67 out of 84, 80%) of which underwent an Ivor Lewis procedure. After a median follow-up of 297 days, anastomotic leakage was observed in 12 out of 84 (14.3%) and anastomotic stricture in 12 out of 82 (14.6%). Time between ICG injection and enhancement in the tip was predictive for anastomotic leakage (P = 0.174, area under the curve = 0.731), and a cut-off value of 98 seconds was derived (specificity: 98%). All times to enhancement at the planned anastomotic site and ICG watershed were significantly predictive for the occurrence of a stricture, however area under the curves were <0.7.
Conclusions: The identified fluorescent threshold can be used for intraoperative decision making or to identify potentially high-risk patients for anastomotic leakage after esophagectomy with gastric conduit reconstruction.
Keywords: near-infrared fluorescence, fluorescence angiography, indocyanine green (ICG), esophagectomy, gastric conduit, esophageal cancer
INTRODUCTION
Treatment of esophageal cancer is based on a multidisciplinary strategy, in which surgery remains the cornerstone for treatment with curative intent. After esophagectomy for esophageal cancer, continuity can be restored by connecting the proximal esophagus to a gastric conduit. For the construction and pull-up of the gastric conduit for anastomosis, ligation of some of its supplying vessels is necessary. This might lead to inadequate arterial perfusion or venous congestion at the anastomotic site, which is a risk factor for anastomotic complications.1–3 Severe anastomotic complications include leakage, graft necrosis and strictures. Postoperatively, 2–25% of patients are diagnosed with anastomotic leakage with or without graft necrosis leading to a complicated, prolonged postoperative course, often including intensive care unit stay, reinterventions and a high mortality risk.4,5 In the long term, up to 42% of patients are diagnosed with an anastomotic stricture and often require multiple endoscopic interventions and intensive nutritional support, affecting their overall quality of life.6,7
To aid surgeons’ decision making on a well-perfused anastomotic site, different innovative modalities have been described to evaluate perfusion of the gastric conduit intraoperatively.8 Of those, fluorescence angiography (FA) using indocyanine green (ICG) is an emerging technique.9 Early observations support that FA use lowers anastomotic leakage rates after esophagectomy with gastric conduit reconstruction.10–12 However, leakages still occur even when management is determined according to FA. Besides the multifactorial etiology of anastomotic leakage, this could be explained by the subjective interpretation of FA. Up to now, no perfusion related cut-off point has been described for FA. Furthermore, in case of venous congestion FA displays a ‘green’ gastric conduit as arterial flow is intact. These drawbacks can lead to either an insufficient or unnecessarily excessive resection.
To overcome current limitations of FA, quantification of the time dependent change of the fluorescent signal is a promising method to provide objective judgment of tissue perfusion.13 Ideally a quantitative threshold for the fluorescence signal can be identified to predict adequate perfusion and be used in predicting patient outcomes. Quantification of fluorescence can be achieved by measuring the time to fluorescence either manually or software-derived.13 Various studies have investigated manually assessed time until fluorescent enhancement in the gastric conduit during FA and showed that this is a promising method of FA quantification, indicating both arterial and venous deficiency, with the ability to predict patient outcomes.14–17
This study (IDEAL phase 2S) evaluates the manually assessed time until fluorescent enhancement in the gastric conduit as a quantitative fluorescent value for FA and aims to determine a threshold to predict anastomotic complications.
METHODS
This was a prospective cohort study. All consecutive patients undergoing elective esophagectomy with gastric conduit reconstruction since the introduction of FA in June 2018 to October 2019 were approached. Eligible patients were 18 years of age or older and were scheduled for esophagectomy with primary continuity restoration by means of a gastric conduit. Patients with a history of esophageal and/or gastric surgery were excluded, or when FA was not, or could not, be performed due to contraindications to ICG (e.g. iodine allergy). Data from the electronic patient record system were prospectively collected.
The Institutional Review Board of the Amsterdam UMC, location AMC, approved the study protocol and confirmed that the Medical Research lnvolving Human Subjects Act (WMO) did not apply. This study was submitted retroactively to the trialregister.nl database (NL8527). Informed consent for use of data was obtained from all included patients in compliance to the General Data Protection Regulation.
Surgical procedures
Before surgery, patients standardly received neoadjuvant treatment according to CROSS or FLOT schemes.18,19
Based on the tumor location, an Ivor Lewis or McKeown procedure was performed as previously described.20,21 To summarize, a 3–4 cm wide gastric conduit was constructed by use of an endoscopic linear stapler during the abdominal phase. For gastric conduit reconstruction the left gastric artery, some branches of the right gastric artery, the left gastroepiploic artery, the short gastric vessels and, if present, the posterior gastric artery were ligated.
During the thoracic phase of an Ivor Lewis procedure, gastric conduit pull-up was performed. An intrathoracic anastomosis was created using a circular stapler, and the end of the gastric conduit was stapled using an endoscopic linear stapler. The anastomosis was covered by an omental wrap and mediastinal pleuraflap.
During the abdominal phase of the McKeown procedure, the gastric conduit was constructed through a small accessory incision when a minimally invasive approach was followed. Consequently, a left cervical incision was made, the gastric conduit was brought up to the cervical region through the prevertebral route and a hand-sewn or stapled cervical anastomosis was created wrapped with omentum.
Fluorescence angiography
FA was performed before the creation of the anastomosis, after the gastric conduit was brought up into the thorax (Ivor Lewis procedure) or exteriorly through the accessory abdominal incision and placed onto the thorax before delivery to the cervical region (McKeown). Before FA, the planned anastomotic site of the gastric conduit was determined by visual inspection and was marked by the surgeon using a surgical instrument. Subsequently, FA was performed after administration of ICG (0.05 mg/kg/bolus) through a peripheral infusion cannula. The laparoscopic PINPOINT or hand-held Spy-phi fluorescence imaging system (Stryker, Kalamazoo, MI, USA) was used to detect ICG. Surgical management was determined by subjective FA interpretation, which was based on presence or absence of ICG fluorescence.
Outcomes
The primary outcome measure was time to fluorescent enhancement. During FA, time to initial fluorescent enhancement was recorded using a digital clock and reported in a case-report form. The following time points were recorded during FA: ICG injection at a peripheral infusion site (ICGi) and the first fluorescent enhancement in the right lung (lung) in case of an Ivor Lewis procedure, at the base of the gastric conduit (base), at the planned anastomotic site (planned anastomosis), at the ICG watershed (watershed) or in the tip of the gastric conduit (tip) (Fig. 1). Time duration until watershed included both the time to tip and, when the tip was not fluorescently enhanced, to the ICG watershed. If the planned anastomotic site was changed due to subjective FA assessment, the time point was adjusted accordingly. Time values were the difference between the time points in seconds, with ICGi, lung, or base as t = 0.
Fig. 1.
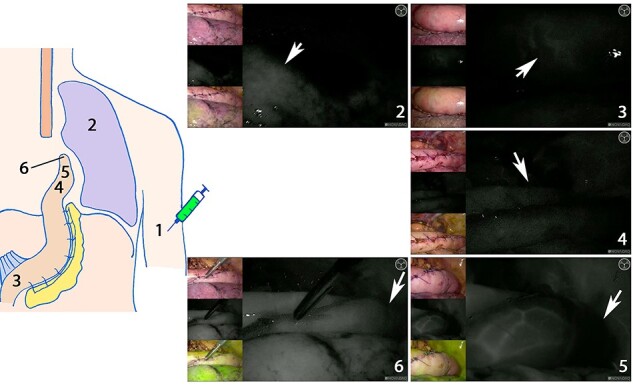
Time to fluorescent enhancement: time points measured. (1) Time of indocyanine green injection. Time of first fluorescent enhancement: (2) in the right lung; white arrow points at fluorescence in the lung. (3) At the base of the gastric conduit; white arrow points at the first signal. (4) At the planned anastomotic site; white arrow points at the planned anastomotic site. (5) At the ICG watershed; white arrow points at the tip that is not fluorescently enhanced. (6) In the tip of the gastric conduit; white arrow points at a well-perfused tip. Images (2)–(6) were derived by the laparoscopic system. For each image, visual assessment is shown on the upper left, near infrared fluorescence in white in the middle left and the larger image on the right, and merge between the two images with the fluorescent signal in pseudo-green in the lower left. Images (2)–(5) display time assessment in one patient. Image 6 is from another patient with fluorescent enhancement in the tip of the gastric conduit.
Secondary outcome measures included other FA details, including change in management and additional operative time, hemodynamic parameters during FA and postoperative anastomotic complications. Change in management due to FA was either a change of the anastomotic site or an additional resection of omentum. Additional resection of omentum was scored when resection of the omental wrap in the anastomotic region was performed according to poorly perfused omental areas depicted by FA. Additional surgical time was measured as the duration from start to end of the fluorescent mode. Hemodynamic parameters included mean arterial pressure (MAP), heart rate and the use of noradrenaline. Postoperative anastomotic complications included anastomotic leakage, graft necrosis and anastomotic stricture. Anastomotic leakage and graft necrosis were defined according to the Esophagectomy Complications Consensus Group classification,22 and reinterventions were classified according to the Clavien–Dindo (CD) score. Clinically relevant benign strictures were defined as a score for dysphagia ≥2 and treatment by ≥1 dilatation. End of follow-up was the 2020 January 1.
Statistics
All categorical data were presented as number of cases and percentages, whereas continuous data were reported in means (standard deviation [SD]) or in medians (interquartile range [IQR]) depending on the data distribution. Time values were reported in median (IQR) and were compared between patients with or without anastomotic complications using the Mann–Whitney U test. A P-value <0.05 was considered statistically significant.
Univariate logistic regression was performed to define a predictive value for time values for complications. Multivariate logistic regression was not performed due to a low absolute number of anastomotic complications. When time values had a P-value <0.2,23 a receiver operating characteristic (ROC)-curve was generated. When the ROC-curve yielded an area under the curve (AUC) above 0.7, a cut-off value was produced with high specificity and positive predictive value. Specificity was calculated using the Youden’s statistics, after which the positive predictive value was calculated for every specificity. Correlation between MAP, heart rate or noradrenaline dosage and time values was evaluated using the Spearman’s rank correlation coefficient ρ. A P-value <0.05 was considered statistically significant.
Data were analyzed using the Statistical Package for Social Sciences (SPSS) of IBM Statistics, version 26.0.
RESULTS
Ninety-eight patients with esophageal cancer underwent esophagectomy with primary gastric conduit reconstruction. Eighty-four of these patients underwent FA and were included in this analysis (Fig. 2).
Fig. 2.
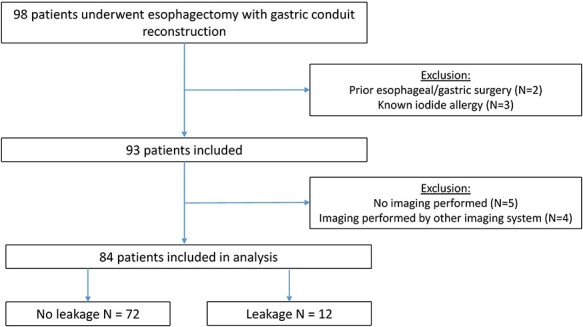
Flowchart of patient inclusion.
Baseline characteristics are shown in Table 1 and surgical details in Table 2. The mean age was 64 ± 9.4 years. The majority of patients was male (83%), received neoadjuvant chemoradiation (84%) and had an adenocarcinoma (76%). The anastomosis was constructed in the thorax in 67 out of 84 patients (80%). All procedures followed a minimally invasive approach and no conversion was required.
Table 1.
Baseline characteristics
| Cohort (n = 84) | |
|---|---|
| Gender male | 70 (83) |
| Age (years) mean ± SD | 64 ± 9.4 |
| BMI (kg/m2) mean ± SD | 26 ± 4.3 |
| ASA ≥ 3 | 19 (23) |
| Smoker active | 15 (18) |
| Comorbidity | 29 (35) |
| Pulmonary | 9 (11) |
| Cardiac | 7 (8) |
| Vascular | 9 (11) |
| Diabetes | 14 (17) |
| Tumor histology | |
| Adenocarcinoma | 64 (76) |
| Squamous cell carcinoma | 18 (21) |
| Other* | 2 (2) |
| Tumor stage | |
| cT3 | 68 (81) |
| cN+ | 56 (67) |
| cM0 or cMx | 82 (98) |
| Neoadjuvant treatment | |
| Chemoradiation | 71 (84) |
| Chemotherapy | 11 (13) |
| None | 2 (2) |
| Prior procedures | |
| Endoscopic submucosal dissection | 2 (2) |
Data shown in n (%) unless otherwise stated.
ASA, American Society of Anesthesiologists classification; BMI, body mass index; ICG, indocyanine green.
*NET or poor differentiated carcinoma.
Table 2.
Surgical procedures
| Cohort (n = 84) | |
|---|---|
| Surgical procedure | |
| Ivor Lewis | 67 (80) |
| McKeown | 17 (20) |
| Approach* | |
| Minimally invasive abdominal | 2 (2) |
| Minimally invasive thorax | 5 (6) |
| Minimally invasive abdominal and thorax | 77 (92) |
| Construction method anastomosis | |
| Stapled circular/linear | 72 (86) |
| Hand-sewn | 12 (14) |
| Configuration anastomosis | |
| End-to-end | 8 (10) |
| End-to-side | 71 (85) |
| Side-to-side | 5 (6) |
| Intraoperative blood loss (mL) median IQR | 200 (100–338) |
| Operative time (min) mean ± SD | 432 ± 54.0 |
Data shown in n (%) unless otherwise stated.
*Minimally invasive included laparoscopic or robot-assisted.
Graft perfusion at the initially planned anastomotic site was judged to be adequate after visual inspection in all cases. Based on FA, the surgical team opted for a change of anastomotic site to a clearer fluorescent region in 2 out of 84 (2.4%) cases, requiring an extra excision of 2–5 cm of the gastric conduit (Table 3). In one case this led to a change in cervical anastomotic configuration from end-to-side to end-to-end. In 5 out of 84 cases (6.0%) change in management was desired, but no additional resection was possible owing to a clear imbalance with anastomotic tension. FA use added a mean of 3 ± 1 minutes to the operativetime.
Table 3.
Change in management due to fluorescence angiography
| Cohort (n = 84) | Anastomotic leakage | |
|---|---|---|
| No change in management | 73 (87) | 7/73 (10) |
| Change in management | 11 (13) | 5/11 (45) |
| Change in anastomotic site | 2 (2) | 2/2 (100) |
| Change desired, but not possible* | 5 (6) | 2/5 (40) |
| Change in omentum | 4 (5) | 1/4 (25) |
Data shown in n (%) or n/n (%).
*Gastric conduit at maximal acceptable tension.
After a median follow-up of 297 days (IQR 167–399), anastomotic complications occurred in 23 out of 84 patients (27%). Anastomotic leakage was observed in 12 out of 84 patients (14.3%), which in one patient was secondary to graft necrosis. Anastomotic leakage rates were 9 out of 67 patients (13.4%) for intrathoracic and 3 out of 17 (17.6%) for cervical anastomoses separately. The leakage rates for change in management groups are summarized in Table 3. For 8 out of 12 (66.7%) patients with anastomotic leakage, the CD score was ≥4. Reoperation was required for 4 out of 12 patients (33.3%). In two out of four cases, reoperation included gastric conduit break down due to gastric conduit necrosis in the first case and persistent leakage after reconstruction in the second case. An anastomotic stricture was observed in 12 out of 82 patients (14.6%).
Time to fluorescence enhancement
Of the total quantitative FA assessments, 3 out of 84 were performed after the anastomosis to the proximal esophagus was constructed. Of those measurements, all assessed times were included, but this led to missing values for the times to watershed and tip. For 23 out of 81 assessments (28.4%) the tip was not fluorescently enhanced, however this was not associated with the occurrence of anastomotic leakage (P = 0.462) or stricture (P = 1.000).
Overall, the time from ICGi to base was 22 (17–31) seconds. Times were not correlated to MAP, heart rate, noradrenaline use or noradrenaline dosage (P > 0.05). Time values for patients with or without anastomotic leakage or a stricture are summarized in Table 4. Although not significant, time values were elongated in patients with an anastomotic leakage (Table 4A). Time between ICGi and tip was nonsignificantly prolonged in patients with an anastomotic leakage (P = 0.066). Time from base to the planned anastomosis significantly differed between patients with or without a stricture (P = 0.027) (Table 4B).
Table 4.
Time values for anastomotic leakage and anastomotic stricture
| Time value (s) | ||||
|---|---|---|---|---|
| T = 0 | Time point | No leakage (n = 72) | Leakage (n = 12) | P-value |
| A. Anastomotic leakage | ||||
| ICGi | Lung | 13 (9–21) | 15 (10–19) | 0.683 |
| Base | 22 (17–33) | 25 (18–27) | 0.808 | |
| Planned anastomosis | 31 (23–44) | 32 (30–36) | 0.540 | |
| Watershed | 44 (31–60) | 46 (34–65) | 0.395 | |
| Tip | 45 (31–61) | 63 (45–78) | 0.066 | |
| Lung | Base | 10 (8–12) | 12 (9–12) | 0.222 |
| Planned anastomosis | 17 (13–22) | 19 (17–22) | 0.436 | |
| Watershed | 30 (24–41) | 29 (21–58) | 0.960 | |
| Tip | 30 (23–42) | 52 (23–69) | 0.272 | |
| Base | Planned anastomosis | 7 (5–12) | 7 (5–18) | 0.387 |
| Watershed | 20 (13–29) | 28 (16–48) | 0.111 | |
| Tip | 22 (13–31) | 35 (9–50) | 0.254 | |
| B. Anastomotic stricture | ||||
| ICGi | Lung | 13 (9–20) | 13 (9–19) | 0.883 |
| Base | 22 (17–31) | 21 (16–30) | 0.901 | |
| Planned anastomosis | 31 (23–42) | 31 (27–66) | 0.372 | |
| Watershed | 44 (31–59) | 44 (37–94) | 0.273 | |
| Tip | 46 (31–63) | 44 (42–71) | 0.463 | |
| Lung | Base | 10 (8–13) | 10 (7–11) | 0.453 |
| Planned anastomosis | 17 (14–21) | 22 (14–43) | 0.186 | |
| Watershed | 30 (22–38) | 36 (29–69) | 0.077 | |
| Tip | 31 (21–42) | 36 (28–79) | 0.182 | |
| Base | Planned anastomosis | 7 (5–9) | 13 (6–16) | 0.027 |
| Watershed | 20 (12–29) | 21 (18–32) | 0.410 | |
| Tip | 22 (11–31) | 22 (18–50) | 0.235 | |
ICG, indocyanine green.
Regarding the one case of graft necrosis; 17 seconds was measured between ICGi and base, 15 seconds between the base and planned anastomosis and 27 seconds between base and watershed. Fluorescence was not observed in the tip of the gastric conduit.
Time between ICGi and tip was predictive for anastomotic leakage (measurements n = 55, anastomotic leakage n = 6 out of 55, P = 0.174, AUC = 0.731) (Supplementary Table 1A). A cut-off value of 98 seconds was derived with a specificity of 98%, sensitivity of 17%, positive predictive value of 50% and negative predictive value of 91%. All time values, except time to lung and base, were predictive with a P-value <0.2 for occurrence of a stricture and for combined anastomotic complications (Supplementary Table 1B,C). However all corresponding AUCs were <0.7.
DISCUSSION
This study manually assessed the time to fluorescent enhancement and evaluated if this differed between patients with and without anastomotic complications. Time between ICG injection and enhancement at the tip of the gastric conduit allowed distinction for anastomotic leakage and might be used as a threshold to predict anastomotic leakage after esophagectomy with a gastric conduit reconstruction.
Measuring time to fluorescent enhancement in the gastric conduit is described as a feasible method for FA quantification and is potentially predictive for occurrence of anastomotic complications.13 Absence of fluorescence after a certain time point might depict arterial insufficiency of the right gastroepiploic artery,24 and fluorescent delay could indicate arterial diffusion or venous congestion leading to ischemia.25 In this study, all the time values that were significantly associated with anastomotic complications included ICG travel time through the gastric conduit. Time values between different organs (time to lung and base) were not predictive for patient outcomes.
The time values found in this study are to some extent comparable to first reports of fluorescent thresholds in literature.15,16 Koyanagi et al. identified a threshold of 1.76 cm/s for speed in the gastric conduit predicting anastomotic leakage, which corresponds to our definition of time between the base and watershed of ~19 seconds.15 Kumagai et al. proposed anastomotic reconstruction in an area that shows ICG enhancement within 90 seconds after initial enhancement of the root of the right gastroepiploic artery, and observed anastomotic leakage in only 1 out of 77 patients (1.3%) when all anastomoses were constructed in this area.16 In the current study all time values from base to planned anastomosis were <90 seconds, many even <60 seconds. This difference might be explained by differences in anatomical landmark; e.g. the root of the right gastroepiploic artery is more proximal compared to the base of the gastric conduit, leading to longer times in the study of Kumagai et al.
In the current study, time values were not correlated to MAP, heart rate or use of noradrenaline. In current literature, MAP is also not correlated with the occurrence of anastomotic leakage.26 FA and time assessment might therefore be independent of the patient’s hemodynamic status. Relation of FA to hemodynamic parameters should be further investigated and other potentially important parameters could include cardiac output. This is an area for a subsequent research project.
The clinical consequence of a fluorescent threshold can be debated. According to meta-analyses of early observations, management based on subjective FA interpretation seems to lower anastomotic leakage rate.10–12 However, current surgical management during esophagectomy with gastric conduit reconstruction usually leaves little room for an additional resection. Importantly, in case of vascular deficiency, the balance with anastomotic tension is even more important. An excessive resection due to FA interpretation might therefore lead to a higher risk of anastomotic leakage as the tension becomes undervalued. This might be reported in this study as anastomotic leakage occurred in two out of two patients when part of the gastric conduit was additionally resected due to FA findings. Another explanation for the high anastomotic leakage rate in patients with a change in management is that the vascularization of the gastric conduit in those cases is inadequate and anastomotic leakage is unavoidable.10 The cut-off value is therefore not to be used as a simple instrument to decide on an additional shortening of the gastric conduit. However, a cut-off value with high specificity and positive predictive value might provide additional information on the anastomotic viability of the graft. Interpreting the threshold found in this study, a gastric conduit that fluoresces in the tip >98 seconds after ICG injection, has an increased probability from an a priori 14.3–50% to develop anastomotic leakage. When fluorescent enhancement is within the found threshold, the chance of leakage lowers from an a priori 14.3–9%. Based on these numbers surgical considerations can decide on bowel continuity using the gastric conduit, perform additional resection and mobilization of the gastric conduit, to choose a different graft (jejunostomy or colonic interposition), or to create an esophagostomy with continuity restoration in a second phase. For a clinical interpretation, a patient’s preference is also important. For patients, primary bowel continuity is important factor for their quality of life. In future studies, thresholds for different grades of anastomotic leakage might therefore be helpful. If the threshold predicts an increased risk of leakage with a low grade which might be treated by endoscopic stent or endosponge therapy, the choice for connection to the gastric conduit would be the most straightforward way to achieve bowel continuity. Furthermore, these high-risk patients can be monitored more closely by serial C-reactive protein measurements at day 3, 5 and 7, or a standard computed tomography scan or endoscopy at day 3 postoperatively. If endoscopy at day 3 postoperatively shows a small leakage, endosponge therapy can be started early in the postoperative pathway. Moreover, these high-risk patients could even be offered prophylactic treatment with antibiotics, stent and/or an endosponge, or may be treated with a more conservative postoperative feeding pathway.
The identified threshold is a quantitative fluorescent value but is still depending on subjective interpretation of fluorescent intensity appearance. Fluorescence intensity is, among others, dependent on ICG dose, patient characteristics, optical properties of the tissue of interest and sensitivity of the imaging device.27 It is therefore not clear how these times differ for different imaging systems. To evaluate fluorescent intensity objectively, software-derived fluorescent-time curves are promising for future research.13 For this method of quantification calibration of fluorescent systems is of paramount importance to allow data comparison and use of cut-off values in the future.
This study includes a number of limitations. Firstly, this study was not based on a sample size estimation and was limited by a low absolute number of anastomotic complications and number of missing values for the identified threshold. The identified threshold can only be used when the tip fluoresces, which in this study occurred in 72% of the cases. Secondly, anastomotic perfusion is a predictor for patient outcomes, but does not account for all anastomotic complications. Unfortunately, a correction in confounding factors for patient outcomes was not possible due to the low absolute number of anastomotic complications. However, the absolute number of anastomotic leakages in this study is relatively high compared to other studies of fluorescent thresholds in literature.14–17 Future studies should be carried out in a larger cohort that would allow for the correction of risk factors associated with anastomotic complications. Additionally, bias might be introduced by using ICG injection as t = 0 owing to the location of the peripheral cannula, and length of the gastric conduit.
In conclusion, time to fluorescent enhancement of the gastric conduit seems a feasible method to achieve quantitation of FA and is easy to assess, requiring no additional software. The identified fluorescent threshold can be used to identify potentially high-risk patients for anastomotic leakage and these patients can subsequently be monitored vigorously and pre-emptive measures, including prophylactic antibiotics, stent placement or early endosponge treatment could be considered. A larger cohort needs to be investigated to confirm this threshold, after which the identified thresholds should be established through external validation.
Supplementary Material
ACKNOWLEDGMENT
The authors would like to thank Tania Sluckin for language editing.
Contributor Information
M D Slooter, Amsterdam UMC, University of Amsterdam, Department of Surgery, Cancer Center Amsterdam, Amsterdam, the Netherlands.
D M de Bruin, Amsterdam UMC, University of Amsterdam, Department of Biomedical Engineering and Physics, Amsterdam, the Netherlands.
W J Eshuis, Amsterdam UMC, University of Amsterdam, Department of Surgery, Cancer Center Amsterdam, Amsterdam, the Netherlands.
D P Veelo, Amsterdam UMC, University of Amsterdam, Department of Anesthesiology, Amsterdam, the Netherlands.
S van Dieren, Amsterdam UMC, University of Amsterdam, Department of Surgery, Cancer Center Amsterdam, Amsterdam, the Netherlands.
S S Gisbertz, Amsterdam UMC, University of Amsterdam, Department of Surgery, Cancer Center Amsterdam, Amsterdam, the Netherlands.
M I van Berge Henegouwen, Amsterdam UMC, University of Amsterdam, Department of Surgery, Cancer Center Amsterdam, Amsterdam, the Netherlands.
FUNDING
MIvBH: unrestricted grant and materials Stryker European Operations B.V.
CONFLICTS OF INTEREST
MIvBH: unrestricted grant Olympus, consultant for Mylan, Johnson & Johnson and Medtronic. Remaining authors declared that they have no conflicts of interest.
References
- 1. Urschel J D. Esophagogastrostomy anastomotic leaks complicating esophagectomy: a review. Am J Surg 1995; 169(6): 634–40. [DOI] [PubMed] [Google Scholar]
- 2. Briel J W, Tamhankar A P, Hagen J A et al. Prevalence and risk factors for ischemia, leak, and stricture of esophageal anastomosis: gastric pull-up versus colon interposition. J Am Coll Surg 2004; 198(4): 536–41 discussion 41-2. [DOI] [PubMed] [Google Scholar]
- 3. Athanasiou A, Hennessy M, Spartalis E, Tan B H L, Griffiths E A. Conduit necrosis following esophagectomy: an up-todate literature review. World J Gastrointest Surg 2019; 11(3): 155–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Hagen P, Hulshof M C, van Lanschot J J et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012; 366(22): 2074–84. [DOI] [PubMed] [Google Scholar]
- 5. Wormuth J K, Heitmiller R F. Esophageal conduit necrosis. Thorac Surg Clin 2006; 16(1): 11–22. [DOI] [PubMed] [Google Scholar]
- 6. Helminen O, Kyto V, Kauppila J H, Gunn J, Lagergren J, Sihvo E. Population-based study of anastomotic stricture rates after minimally invasive and open oesophagectomy for cancer. BJS open 2019; 3(5): 634–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Heijl M, Gooszen J A, Fockens P, Busch O R, van Lanschot J J, van Berge Henegouwen M I. Risk factors for development of benign cervical strictures after esophagectomy. Ann Surg 2010; 251(6): 1064–9. [DOI] [PubMed] [Google Scholar]
- 8. Jansen S M, de Bruin D M, van Berge Henegouwen M I et al. Optical techniques for perfusion monitoring of the gastric tube after esophagectomy: a review of technologies and thresholds. Dis Esophagus 2018; 31(6): 1–11. [DOI] [PubMed] [Google Scholar]
- 9. van Manen L, Handgraaf H J M, Diana M et al. A practical guide for the use of indocyanine green and methylene blue in fluorescence-guided abdominal surgery. J Surg Oncol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Slooter M D, Eshuis W J, Cuesta M A, Gisbertz S S, van Berge Henegouwen M I. Journal of thoracic disease. Fluorescent imaging using indocyanine green during esophagectomy to prevent surgical morbidity: a systematic review and metaanalysis 2019; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ladak F, Dang J T, Switzer N et al. Indocyanine green for the prevention of anastomotic leaks following esophagectomy: a meta-analysis. Surg Endosc 2019; 33(2): 384–94. [DOI] [PubMed] [Google Scholar]
- 12. Van Daele E, Van Nieuwenhove Y, Ceelen W et al. Nearinfrared fluorescence guided esophageal reconstructive surgery: a systematic review. World J Gastrointest Oncol 2019; 11(3): 250–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Slooter MD, Mansvelders MS E, Bloemen P R et al. Defining indocyanine green fluorescence to assess anastomotic perfusion during gastrointestinal surgery: systematic review. In: press; BJS Open. 2020. [DOI] [PMC free article] [PubMed]
- 14. Yukaya T, Saeki H, Kasagi Y et al. Indocyanine green fluorescence angiography for quantitative evaluation of gastric tube perfusion in patients undergoing Esophagectomy. J Am Coll Surg 2015; 221(2): e37–42. [DOI] [PubMed] [Google Scholar]
- 15. Koyanagi K, Ozawa S, Oguma J et al. Blood flow speed of the gastric conduit assessed by indocyanine green fluorescence: new predictive evaluation of anastomotic leakage after esophagectomy. Medicine 2016; 95(30): e4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kumagai Y, Hatano S, Sobajima J et al. Indocyanine green fluorescence angiography of the reconstructed gastric tube during esophagectomy: efficacy of the 90-second rule. Dis Esophagus 2018; 1–4. [DOI] [PubMed] [Google Scholar]
- 17. Ishige F, Nabeya Y, Hoshino I et al. Quantitative assessment of the blood perfusion of the gastric conduit by Indocyanine green imaging. J Surg Res 2019; 234: 303–10. [DOI] [PubMed] [Google Scholar]
- 18. Shapiro J, van Lanschot J J B, Hulshof M et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015; 16(9): 1090–8. [DOI] [PubMed] [Google Scholar]
- 19. Al-Batran S E, Hofheinz R D, Pauligk C et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol 2016; 17(12): 1697–708. [DOI] [PubMed] [Google Scholar]
- 20. Anderegg M C, Gisbertz S S, van Berge Henegouwen M I. Minimally invasive surgery for oesophageal cancer. Best Pract Res Clin Gastroenterol 2014; 28(1): 41–52. [DOI] [PubMed] [Google Scholar]
- 21. Veelo D P, Gisbertz S S, Hannivoort R A et al. The effect of ondemand vs deep neuromuscular relaxation on rating of surgical and anaesthesiologic conditions in patients undergoing thoracolaparoscopic esophagectomy (DEPTH trial): study protocol for a randomized controlled trial. Trials 2015; 16: 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Low D E, Alderson D, Cecconello I et al. International consensus on standardization of data collection for complications associated with Esophagectomy: Esophagectomy complications consensus group (ECCG). Ann Surg 2015; 262(2): 286–94. [DOI] [PubMed] [Google Scholar]
- 23. Heinze G, Dunkler D. Five myths about variable selection. Transpl Int 2017; 30(1): 6–10. [DOI] [PubMed] [Google Scholar]
- 24. Zehetner J, DeMeester S R, Alicuben E T et al. Intraoperative assessment of perfusion of the gastric graft and correlation with anastomotic leaks after Esophagectomy. Ann Surg 2015; 262(1): 74–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kamiya K, Unno N, Miyazaki S et al. Quantitative assessment of the free jejunal graft perfusion. J Surg Res 2015; 194(2): 394–9. [DOI] [PubMed] [Google Scholar]
- 26. Goense L, van Rossum P S, Tromp M et al. Intraoperative and postoperative risk factors for anastomotic leakage and pneumonia after esophagectomy for cancer. Dis Esophagus 2017; 30(1): 1–10. [DOI] [PubMed] [Google Scholar]
- 27. Keereweer S, Van Driel P B, Snoeks T J et al. Optical imageguided cancer surgery: challenges and limitations. Clin Cancer Res 2013; 19(14): 3745–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


