Abstract
Eight new 4-substituted 1-amino-9,10-anthraquinones containing a primary amino group were synthesized by nucleophilic substitution of bromine in 1-amino-4-bromo-9,10-anthraquinones. 1-Amino-4-[2-(hydroxyethyl)amino]-9,10-dioxo-9,10-dihydroanthracene-2-sulfonic acid containing a biogenic amine fragment (2-aminoethanol) was converted into the corresponding 1-triazenyl derivatives. The structure of the synthesized compounds was determined on the basis of the LC/MS and 13C and 1H NMR data, and their drug likeness was estimated in silico. Compounds with a good drug likeness score were analyzed by DIGEP-Pred, their possible interactions with proteins were simulated using STRING, and their biological activity was interpreted using the Kyoto Encyclopedia of Genes and Genomes.
Keywords: bromaminic acid; Ullmann reaction; LC/MS; 4-substituted 9,10-anthraquinones; triazenes; genes
INTRODUCTION
Formerly, anthraquinone derivatives were historically important natural dyes. Later, it was found that the planar tricyclic anthraquinone system gives rise to a broad spectrum of biologically important properties (Fig. 1) [1–2]. At present, anthraquinone derivatives are extensively studied as therapeutic agents against COVID-19, specifically acting against 3CLpro and PLpro proteases [3].
Fig. 1.
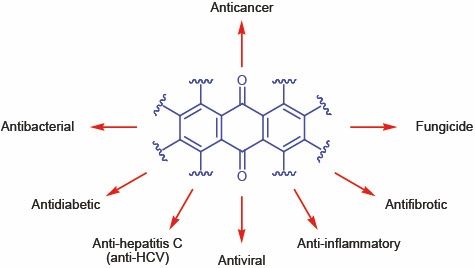
Biological activity of anthraquinone derivatives.
A large number of anthraquinone derivatives contain a sulfonate group in the 2-position. These compounds can be synthesized from bromaminic acid sodium salt 1 (Fig. 2) which is the basic starting material for the preparation of biologically active anthraquinone derivatives and numerous dyes [4–6]. In fact, bromaminic acid and its salts are widely used as intermediate products for the synthesis of anthraquinone derivatives, including acid dyes, via substitution of the 4-bromine atom by (aryl)alkylamino group [7–9].
Fig. 2.
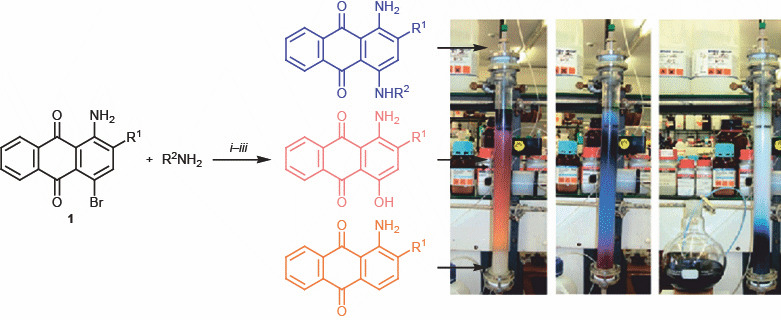
Synthesis of 4-substituted 1-aminoanthraquinones.
There are almost no published data on chemical properties of triazenyl-substituted anthraquinones [10, 11]. Triazene moiety is a known alkylating carcinolytic group. It was introduced into anthraquinone molecule by azo coupling of anthraquinone-1-diazonium salt with various aliphatic and aromatic amines.
RESULTS AND DISCUSSION
While developing an optimal procedure for the substitution of bromine in bromaminic acid 1, we tried different known nucleophilic substitution methods [12, 13] and performed a series of reactions of bromaminic acid 1 and its 2-methyl analog 2 with 2-aminoethanol under different conditions; however, the yields were not always satisfactory (Scheme 1). We found that the most efficient procedure was to react compound 1 or 2 with 2-aminoethanol in aqueous medium in the presence of a mixture of copper(II) and iron(II) salts [14]. In this case, the yield of target 1-amino-4-[(2-hydroxyethyl)amino]-9,10-dioxo-9,10-dihydroanthracene-2-sulfonic acid (5) was 96% (m/z 362.0 [M + H]+). The structure of 5 was confirmed by 1H and 13C NMR spectra and elemental analysis. Compound 6 was obtained in 65% yield, and its 1H NMR spectrum showed signals of the ethylene moiety at δ 3.50 and 3.67 ppm. Under these conditions, broaminic acid 1 was reacted with other primary aliphatic amines to obtain 1,4-diaminoanthraquinone derivatives 7–12 (Scheme 2).
Scheme.
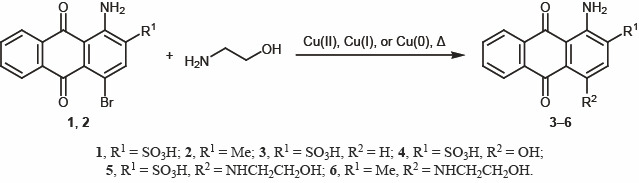
1.
Scheme.
2.
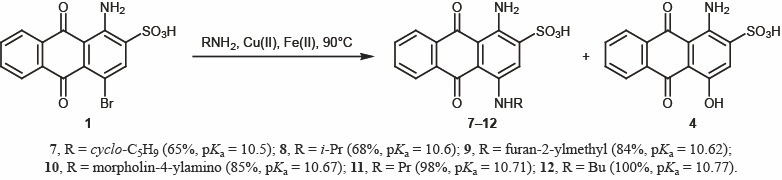
Almost all these reactions were accompanied by side formation of 1-amino-4-hydroxy-9,10-dioxo-9,10-dihydroanthracene-2-sulfonic acid (4) due to concurrent attack of hydroxy nucleophile (Table 1). Furthermore, there was a clear relation between the pKa value of the amine and the purity of the product (Scheme 2).
Table 1.
Synthesis of compounds 7–12
| Compound no. | Yield, % | Impurity of 4, % |
|---|---|---|
| 7 | 65 | 25 |
| 8 | 68 | 20 |
| 9 | 84 | 15 |
| 10 | 85 | 5 |
| 11 | 98 | 1 |
| 12 | 100 | – |
Synthesis of triazenes. In the next step of our study, aminoanthraquinone 5 was subjected to diazotization with sodium nitrite in aqueous medium in the presence of HCl at 0–5°C [15–16] (Scheme 3). The subsequent azo coupling of diazonium salts 13 with secondary and primary amines afforded triazenes 14–17 (Scheme 4). This reaction was not always smooth, depending on the properties of the initial amine and stability of the diazo coupling products. The structure of 14–17 was confirmed by spectral data. The 1H NMR spectra of 14–17 showed aromatic protons signals in the region δ 7.50–8.70 ppm.
Scheme.
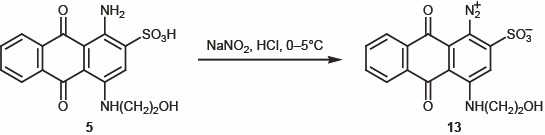
3.
Scheme.
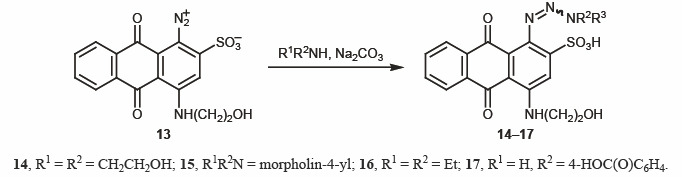
4.
Biological activity and drug likeness. Among the 12 synthesized compounds, we identified triazene derivatives with good drug likeness scores. In particular, 4-aminobenzoic acid derivative 17 (M 510.08) showed the highest drug likeness score (+0.06). Table 2 contains the detailed drug likeness parameters of the synthesized compounds.
Table 2.
Drug likeness parameters of compounds 5–17a
| Compd. no. | Formula | Molecular weight | NHBA | NHBD | LogP | TPSA, Å2 | V, Å3 | DLS |
|---|---|---|---|---|---|---|---|---|
| 5 | C16H14N2O6S | 362.06 | 6 | 5 | –0.57 | 114.29 | 307.29 | –0.73 |
| 6 | C17H16N2O3 | 296.12 | 3 | 4 | 2.76 | 71.87 | 288.83 | –0.16 |
| 7 | C19H18N2O5S | 386.09 | 5 | 5 | 1.40 | 97.61 | 340.44 | –0.19 |
| 8 | C17H16N2O5S | 360.08 | 5 | 4 | 0.72 | 96.76 | 317.25 | –0.66 |
| 9 | C19H18N2O6S | 402.09 | 6 | 4 | 0.24 | 106.48 | 355.56 | –0.17 |
| 10 | C18H17N3O6S | 403.08 | 7 | 4 | –0.53 | 110.07 | 353.50 | –0.27 |
| 11 | C17H16N2O5S | 360.08 | 5 | 4 | 0.82 | 97.63 | 317.22 | –0.57 |
| 12 | C18H18N2O5S | 374.09 | 5 | 4 | 1.34 | 97.63 | 335.12 | –0.69 |
| 14 | C18H18N4O8S | 450.08 | 10 | 5 | –0.74 | 150.24 | 380.77 | –0.79 |
| 15 | C20H22N4O6S | 446.13 | 8 | 3 | 1.18 | 117.64 | 399.55 | –0.60 |
| 16 | C20H20N4O7S | 460.11 | 9 | 3 | 0.04 | 126.10 | 402.62 | –0.23 |
| 17 | C23H18N4O8S | 510.08 | 10 | 5 | 1.01 | 155.40 | 434.50 | 0.06 |
a NHBA is the number of hydrogen bond acceptor centers, NHBD is the number of hydrogen bond donor centers, LogP is the lipophilicity coefficient, TPSA is the topological polar surface area, V is the molecular volume, and DLS is the drug likeness score.
Triazene 14 modulated the largest number of genes (10). The effect on the CHEK1 gene responsible for the p53 signaling pathway directly involved in immune strengthening was revealed. Furthermore, the gene set enrichment analysis showed modulation of 15 different biological pathways, among which cancer pathways are modulated mainly by regulation of three genes (SP1, IL23A, NFE2L2). The results of gene set enrichment analysis of protein modulation by anthraquinone derivatives and the corresponding gene codes are collected in Table 3. Figure 3 shows interactions between modulated proteins.
Table 3.
Analysis of proteins modulated by anthraquinone derivatives
| Pathway ID | Description | Number of genes | Matching genes |
|---|---|---|---|
| hsa05200 | Pathways in cancer | 3 | SP1, IL23A, NFE2L2 |
| hsa05164 | Influenza A | 2 | NXT2, SP1 |
| hsa05133 | Pertussis | 2 | SP1, IL23A |
| ko04625 | C-Type lectin receptor signaling pathway | 2 | SP1, IL23A |
| hsa05166 | HTLV-I infection | 1 | CHEK1 |
| hsa05014 | Amyotrophic lateral sclerosis (ALS) | 1 | SP1 |
| hsa05152 | Tuberculosis | 1 | IL23A |
| hsa05224 | Breast cancer | 1 | SP1 |
| hsa05204 | Chemical carcinogenesis | 1 | CBR1 |
| hsa05225 | Hepatocellular carcinoma | 1 | NFE2L2 |
| hsa05203 | Viral carcinogenesis | 1 | CHEK1 |
| hsa04216 | Ferroptosis | 1 | GSS |
| hsa05323 | Rheumatoid arthritis | 1 | IL23A |
| hsa01524 | Platinum drug resistance | 1 | TOP2A |
| hsa04115 | p53 signaling pathway | 1 | CHEK1 |
Fig. 3.
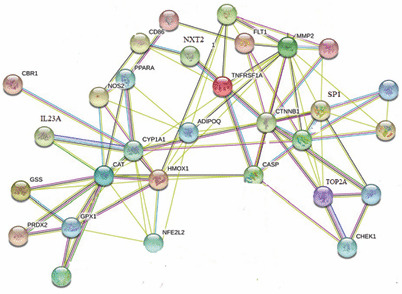
Interactions between regulated proteins.
EXPERIMENTAL
All chemicals were obtained from commercial sources and were used without further purification. The melting points were measured in open capillary tubes. The 1H NMR spectra were recorded on a Varian 400 spectrometer at 400 MHz using DMSO-d6 as solvent unless otherwise stated. The mass spectra were run on an Agilent 1100 Series high-performance liquid chromatograph equipped with a diode array detector and an Agilent mass-selective detector with the possibility of rapidly switching between positive and negative ionization modes. The progress of reactions was monitored by TLC on DC-Fertigfolien Alugram Xtra Sil G/UV254 silica gel plates (Germany).
Anthraquinone derivatives with good drug likeness scores were tested by DIGEP-Pred [17] to identify target proteins (upregulated and downregulated proteins) with a probable activity of 0.4. The list of regulated proteins was loaded to STRING [18] to identify biological process, cellular function, and combined gene set. In addition, possible modulation pathways were identified using the Kyoto Encyclopedia of Genes and Genomes.
General procedure for the synthesis of anthraquinone derivatives 5–12. Bromaminic acid 1 (4.04 g, 0.01 mol) was dissolved in 40 mL of hot water (70–80°C), the corresponding amine (0.015 mol) and sodium hydrogen carbonate (0.02 mol) were added in succession, and copper(II) sulfate (0.05 g) and iron(II) sulfate (0.05 g) were then added. The mixture was stirred, heated to 90°C, and kept at that temperature for 4 h. The progress of the reaction was monitored by the disappearance of bromaminic acid 1 (o-xylene–acetone, 4:6). After completion of the reaction, the mixture was cooled to room temperature and acidified with concentrated aqueous HCl, and the precipitate was filtered off and washed with 20% aqueous sodium chloride (60 mL). The blue moist product was dissolved in hot water (50 mL) and precipitated with concentrated aqueous HCl (3 mL).
1-Amino-4-[(2-hydroxyethyl)amino]-9,10-dioxo-9,10-dihydroanthracene-2-sulfonic acid (5). Yield 96%, blue solid, mp 287–289°C. 1H NMR spectrum, δ, ppm: 3.49 d (2H, CH2), 3.69 d (2H, CH2), 7.73 s (1H, 3-H), 7.85 t (2H, Harom, J = 7.7 Hz), 8.25 d (2H, Harom, J = 8.0 Hz). 13C NMR spectrum, δC, ppm: 45.29 (CH2), 60.24 (CH2OH), 109.38, 109.67, 121.16, 126.19, 126.36, 132.88, 133.03, 134.43, 134.47, 143.5, 143.79, 145.84 (Carom), 181.17, 182.12 (C=O). Mass spectrum: m/z 364.0 [M + H]+. Found, %: C 53.10; H 4.40; N 6.90; S 7.80. C18H18N2O7S. Calculated, %: C 53.07; H 4.42; N 6.87; S 7.86. M 364.
1-Amino-4-[(2-hydroxyethyl)amino]-2-methylantracene-9,10-dione (6). Yield 59%, blue solid, mp 300°C. 1H NMR spectrum, δ, ppm 2.30 s (3H, CH3), 3.50–3.60 m (2H, CH2), 3.67 d (2H, CH2, J = 5.2 Hz), 7.34 s (1H, 3-H), 7.68–7.86 m (4H, Harom). Mass spectrum: m/z 297.0 [M + H]+. C17H17N2O3. M 297.
1-Amino-4-[(propan-2-yl)amino]-9,10-dioxo-9,10-dihydroanthracene-2-sulfonic acid (8). Yield 68%, blue solid, mp 260–262°C. 1H NMR spectrum, δ, ppm: 1.10 s (3H, CH3), 2.20–2.25 m (6H, CH2), 7.70 t (3H, Harom), 8.00 s (2H, Harom), 10.50 s (1H, NH). Mass spectrum: m/z 361.2 [M + H]+. C17H16N2O5S. M 361.
1-Amino-4-[(morpholin-4-yl)amino]-9,10-dioxo-9,10-dihydroanthracene-2-sulfonic acid (10). Yield 85%, blue solid, mp 282°C. 1H NMR spectrum, δ, ppm: 2.00–2.10 m (4H, CH2), 3.30–4.00 m (4H, CH2), 7.70 s (3H, Harom), 8.20 s (3H, Harom, NH). Mass spectrum: m/z 406 [M + H]+. C18H17N3O6S. M 402.
1-Amino-4-(propylamino)-9,10-dioxo-9,10-dihydroanthracene-2-sulfonic acid (11). Yield 98%, blue solid, mp 262°C. 1H NMR spectrum, δ, ppm: 1.00 s (3H, CH3), 1.30–1.35 m (6H, CH2), 7.80 t (3H, Harom), 8.20 s (2H, Harom), 10.80 s (1H, NH). Mass spectrum: m/z 362.2 [M + H]+. C17H16N2O5S. M 361.
1-Amino-4-(butylamino)-9,10-dioxo-9,10-dihydroanthracene-2-sulfonic acid (12). Yield 100%, blue solid, mp 290–292°C. 1H NMR spectrum, δ, ppm: 1.00 s (3H, CH3), 1.60 s (2H, CH2), 7.80 s (3H, Harom), 8.20 s (2H, Harom), 10.70 s (1H, NH). Mass spectrum: m/z: 375.5 [M + H]+. C18H18N2O5S. M 375.
1-(Diazyn-1-ium-l-yl)-4-[(2-hydroxyethyl)amino]-9,10-dioxo-9,10-dihydroanthracene-2-sulfonic acid (13). A 50-mL round-bottom flask equipped with a magnetic stirrer was charged with a solution of anthraquinone 5 (0.1 mmol) in 1 M aqueous HCl (5.0 mL). The solution was cooled to 0–5°C in an ice bath, a solution of sodium nitrite (0.2 mmol) in 0.5 mL of distilled water was added dropwise, maintaining the temperature at 0–5°C, and the mixture was stirred for 5 min at that temperature.
Triazene derivatives 14–17 (general procedure). The mixture containing diazonium salt 13 was allowed to warm up to room temperature, a solution of the corresponding amine (0.15 mmol) in 5 mL of ethanol was added, and the mixture was stirred for ~30 s at room temperature. The progress of the reaction was monitored by change of the color of the reaction mixture (the color changed from blue to red after diazotization, and the final product was purple), as well as by RP-TLC using acetone–water (2:3) as eluent. The product was purified by reversed phase column chromatography (RP-18) using water as eluent.
1-[3,3-Bis(2-hydroxyethyl)triaz-1-en-1-yl]-4-[(2-hydroxyethyl)amino]-9,10-dioxo-9,10-dihydroanthracene-2-sulfonic acid (14). Yield 80%. 1H NMR spectrum, δ, ppm: 2.99 s (4H, CH2), 3.40 d (4H, CH2, J = 11.2 Hz), 3.65 s (4H, CH2), 5.08 s (OH), 5.25 s (2H, OH), 7.85 s (2H, Harom), 8.15–8.20 m (2H, Harom), 8.69 s (2H, Harom), 9.87 s (1H, NH). Mass spectrum: m/z 360.9 [M + H]+. C16H12N2O6S. M 361.
4-[(2-Hydroxyethyl)amino]-1-[(E)-(morpholin-4-yl)diazenyl]-9,10-dioxo-9,10-dihydroanthracene-2-sulfonic acid (15). Yield 90%. mp >300°C. 1H NMR spectrum, δ, ppm: in DMSO-d6: 3.37 t (1H, CH2, morpholine), 3.43 t (2H, CH2, morpholine), 3.70– 3.75 m (3H, CH2, morpholine), 4.95 s (5H, CH2), 7.62 s (1H, 3-H), 7.86 s (1H, Harom), 7.92 t (1H, Harom, J = 8.0 Hz), 8.17 d (1H, Harom, J = 6.4 Hz), 8.23 d (1H, Harom, J = 7.6 Hz), 9.89 s (1H, OH); in DMSO-d6–CCl4: 3.46 s (2H, CH2), 3.73 s (1H, CH2), 7.67 d (1H, 3-H, J = 6.8 Hz), 7.87–7.90 m (2H, Harom), 8.18 t (1H, Harom, J = 6.0 Hz), 8.28 d (1H, Harom, J = 4.8 Hz), 9.90 s (1H, OH). Mass spectrum: m/z 460 [M]+.
1-(3,3-Diethyltriaz-1-en-1-yl)-4-[(2-hydroxyethyl)amino]-9,10-dioxo-9,10-dihydroanthracene-2-sulfonic acid (16). Yield 52%. 1H NMR spectrum, δ, ppm: 1.18 s (3H, CH3), 2.86 d (3H, CH3, J = 4.8 Hz), 3.40 s (2H, CH2), 7.66–7.70 m (1H, 3-H), 8.04–8.30 m (8H, Harom), 9.01 s (1H, OH). Mass spectrum: m/z 425.9 [M]+.
4-(3-{4-[(2-Hydroxyethyl)amino]-2-sulfo-9,10-dioxo-9,10-dihydroanthracen-1-yl}triaz-2-en-1-yl)benzoic acid (17). Yield 95%, mp >300°C. 1H NMR spectrum, δ, ppm: 1.10 s (1H, CH2), 3.46–3.50 m (2H, CH2, J = 16.0 Hz), 7.20 d (8H, Harom, J = 7.6 Hz), 7.80 d (3H, Harom, J = 7.6 Hz), 7.90 s (4H, NH, OH). Mass spectrum: m/z 466 [M – C22H17O6CH4]+.
CONCLUSIONS
The results of computer simulation suggest the possibility of therapeutic effect of the synthesized substituted anthraquinone derivatives, which needs to be verified using experimental protocols.
Supplementary information
FUNDING
This study was performed under financial support by the Ministry of Education and Science of Ukraine (project no. 0119U103131).
CONFLICT OF INTEREST
The authors declare the absence of conflict of interest.
Supplementary information
The online version contains supplementary material available at 10.1134/S1070428021040126.
REFERENCES
- 1.Hussain H., Al-Harrasi A., Al-Rawahi A., Green I., Csuk R., Ahmed I., Shan A., Abbas G., Rehman N., Ullah R. Expert Opin. Ther. Pat. 2015;25:1053. doi: 10.1517/13543776.2015.1050793. [DOI] [PubMed] [Google Scholar]
- 2.Malik E., Muller C. Med. Res. Rev. 2016;36:705. doi: 10.1002/med.21391. [DOI] [PubMed] [Google Scholar]
- 3.Khanal P., Patil B.M., Chand J., Naaz Y. Nat. Prod. Bioprospect. 2020;10:325. doi: 10.1007/s13659-020-00260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baqi Y., Muller C.E. Org. Lett. 2007;9:1271. doi: 10.1021/ol070102v. [DOI] [PubMed] [Google Scholar]
- 5.Baqi Y., Muller C.E. Nat. Protoc. 2010;5:945. doi: 10.1038/nprot.2010.63. [DOI] [PubMed] [Google Scholar]
- 6.Baqi Y., Alzeler K., Koze M., Muller C.E. J. Med. Chem. 2009;52:3784. doi: 10.1021/jm9003297. [DOI] [PubMed] [Google Scholar]
- 7.Malik E.M., Baqi Y., Müller C.E. Beilstein J. Org. Chem. 2015;11:2326. doi: 10.3762/bioc.11.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roy S., Large J.R., Akande A.M., Kshatri A., Webb T.I., Domene C., Sergeant G.P., Mchale N.G., Thornbury K.D., Hollywood M.A. Eur. J. Med. Chem. 2004;75:426. doi: 10.1016/j.ejmech.2014.01.035. [DOI] [PubMed] [Google Scholar]
- 9.Fiene A., Baqi Y., Malik E.M., Newton P., Li W., Lee S.-Y., Hartland L.E., Muller C.E. Bioorg. Med. Chem. 2016;24:4363. doi: 10.1016/j.bmc.2016.07.027. [DOI] [PubMed] [Google Scholar]
- 10.Sabadakh O.P., Taras T.N., Luchkevich E.R., Novikov V.P. Russ. J. Org. Chem. 2015;51:277. doi: 10.1134/S1070428015020244. [DOI] [Google Scholar]
- 11.Bulgakova N.A., Gornostaev L.M. J. Org. Chem. 2001;37:1351. doi: 10.1023/A:1013164528653. [DOI] [Google Scholar]
- 12.Topanov A.P., Mashevskaya I.V., Dmitriev M.V., Maslivets A.N. Russ. J. Org. Chem. 2020;56:719. doi: 10.1134/S1070428020040247. [DOI] [Google Scholar]
- 13.Kaur G., Utreja D., Jain N., Dhillon N.K. Russ. J. Org. Chem. 2020;56:113. doi: 10.1134/S1070428020010182. [DOI] [Google Scholar]
- 14.Shupenyuk V.I., Taras T.N., Sabadakh O.P., Luchkevich E.R., Kornii Y. Fr.-Ukr. J. Chem. 2020;8:58. doi: 10.17721/fujcV8I1P58-65. [DOI] [Google Scholar]
- 15.Baqi Y., Muller C.E. Tetrahedron Lett. 2012;53:6739. doi: 10.1016/j.tetlet.2012.09.011. [DOI] [Google Scholar]
- 16.Glushkova M.A., Popkov S.V., Burdeinyi M.L. Russ. J. Org. Chem. 2020;56:390. doi: 10.1134/S1070428020030045. [DOI] [Google Scholar]
- 17.Lagunin A., Ivanov S., Rudik A., Filimonov D., Poroikov V. Bioinformatics. 2013;29:2062. doi: 10.1093/bioinformatics/btt322. [DOI] [PubMed] [Google Scholar]
- 18.Szklarczyk, D., Morris, J.H., Cook, H., Kuhn, M., Wyder, S., Simonovic, M., Santos, A., Doncheva, N.T., Roth, A., Bork, P., Jensen, L.J., and von Mering, C., Nucleic Acids Res., 2017, vol. 45, p. D362. 10.1093/nar/gkw937 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


