Abstract
Nontuberculous mycobacterial (NTM) pulmonary disease (PD) is an emerging condition with heterogeneous manifestations from both the microbiological and the clinical point of view. Diagnostic and therapeutic guidelines are available but there are still unmet patients' and physicians' needs, including therapy-related adverse events, symptom control, management of comorbidities, risk of re-exposure to the pathogen and unfavourable outcomes.
In the present review, we provide currently available evidence for an integrated approach to NTM-PD beyond antibiotic therapy. This includes 1) avoiding exposure to environments where mycobacteria are present and careful evaluation of lifestyle and habits; 2) implementing a personalised pulmonary rehabilitation plan and airway clearance techniques to improve symptoms, exercise capacity, health-related quality of life (QoL) and functional capacity in daily living activities; 3) a nutritional evaluation and intervention to improve health-related QoL and to control gastrointestinal side-effects during antimicrobial therapy, particularly in those with low body mass index and history of weight loss; and 4) managing comorbidities that affect disease outcomes, including structural lung diseases, immune status evaluation and psychological support when appropriate.
Short abstract
An integrated approach, including risk factor prevention, management of comorbidities, nutritional evaluation and intervention and pulmonary rehabilitation, should be considered in the optimal management of nontuberculous mycobacterial pulmonary disease https://bit.ly/2YEqvQg
Introduction
Nontuberculous mycobacterial (NTM) pulmonary disease (PD) is an emerging condition that affects not only immunocompromised patients but also immunocompetent subjects, usually with underlying structural lung disease [1]. The radiological spectrum varies from cavitations similar to tuberculosis (TB) infection to a bronchiectasis/bronchiolitic pattern (typically expressed as tree-in-bud, bronchial wall thickening and centrilobular nodules with undefined margins). These alterations can be caused by mycobacterial infection in the first instance or can be the consequence of a pre-existing anatomical remodelling that favours mycobacterial colonisation and later infection. Radiological extent can also vary from diffuse forms to disease located in the upper lobes or apical segments of lower lobes and those that preferentially affect the middle lobe and lingula (typically the “Lady Windermere” phenotype). The clinical manifestations of the disease can also be very heterogeneous and largely depend on the pathogenicity of the mycobacterium and on the patient's immune status. Presentation can range from paucisymptomatic forms to chronic symptoms such as cough and sputum production, particularly associated with bronchiectasis, and chronic fatigue with weight loss and haemoptysis in the most advanced forms.
Because of the interaction between pathogen-related factors (NTM-specific virulence, bacterial load and exposure) and host-related factors (patient's immune status, pre-existing anatomical changes and comorbidities) (table 1), the spectrum of mycobacterial infections may vary from silent colonisation to incipient and overt PD when bacterial load increases and dissemination occurs. In this scenario, an integrated approach should be considered that includes avoiding exposure to environments where mycobacteria are present, implementing respiratory physiotherapy and airway clearance techniques (ACTs), a nutritional evaluation, particularly in the case of low body mass index (BMI) and weight loss, and managing comorbidities, including psychological support when appropriate.
TABLE 1.
Risk factors/predisposing factors for nontuberculous mycobacterial pulmonary disease
| Risk factor | Examples |
| Exposure | |
| Domestic | Household plumbing fixtures |
| Showerheads | |
| Taps | |
| Humidifiers | |
| Heating/ventilation systems | |
| Homelessness, incarceration or institutionalisation | |
| Occupational | Mining |
| Healthcare | |
| Steel industry | |
| Farming and breeding | |
| Veterinary | |
| Waste collection | |
| Fish farming | |
| Geology and speleology | |
| Recreational | Gardening |
| Camping | |
| Horse-riding | |
| Fishing | |
| Swimming | |
| Hot tubs | |
| Damage substrate | |
| Pre-existing lung disease | CF |
| Non-CF bronchiectasis | |
| Primary ciliary dyskinesia | |
| Previous pulmonary tuberculosis | |
| Asthma | |
| COPD | |
| Alpha-1 antitrypsin deficiency | |
| Pneumoconiosis (especially silicosis) | |
| ILDs (especially pulmonary alveolar proteinosis) | |
| ABPA | |
| Williams–Campbell syndrome | |
| Congenital tracheobronchomegaly (Mounier–Kuhn syndrome) | |
| Demographic and constitutional factors (Lady Windermere syndrome) | Older age (>40–70 years) |
| Female sex | |
| Non-smoking history | |
| Slender body habitus | |
| Low BMI/low body fat | |
| Scoliosis | |
| Pectus excavatum | |
| Flattened thoracic cage (platythorax) | |
| “Straight back syndrome” | |
| Low vitamin D | |
| GORD | |
| Defective immunity | |
| Primary immunodeficiency | Mutations in the IFN-γ/IL-12 pathway (e.g. IFNγ-R1, IFN-γ-R2, IL-12R, STAT, tyrosine kinase 2) |
| Deficiency in NF-κB essential modulator | |
| Defective macrophage and dendritic cell function (e.g. chronic granulomatous disease) | |
| Defective T-cells (e.g. CVID, SCID) | |
| Complement C4 deficiency | |
| Acquired immunodeficiency | HIV-AIDS |
| Acquired neutralising anti-IFN-γ antibodies | |
| Diabetes mellitus | |
| Chronic kidney disease | |
| Malignancy | |
| Alcohol abuse | |
| Medications | Oral corticosteroids |
| Inhaled corticosteroids | |
| TNF-α antagonists | |
| Solid organ transplantation (particularly lung transplant) | |
| Haematopoietic stem cell transplantation | |
| Cancer chemotherapy |
CF: cystic fibrosis; COPD: chronic obstructive pulmonary disease; ILD: interstitial lung disease; ABPA: allergic bronchopulmonary aspergillosis; BMI: body mass index; GORD: gastro-oesophageal reflux disease; IFN: interferon; IL: interleukin; STAT: signal transducer and activator of transcription 1; CVID: common variable immunodeficiency; SCID: severe combined immune deficiency; TNF: tumour necrosis factor.
In this review, we focus on NTM-PD management, considering those aspects that go beyond antibiotic therapy and concentrating on an integrated approach from the early stages of the disease (figure 1).
FIGURE 1.
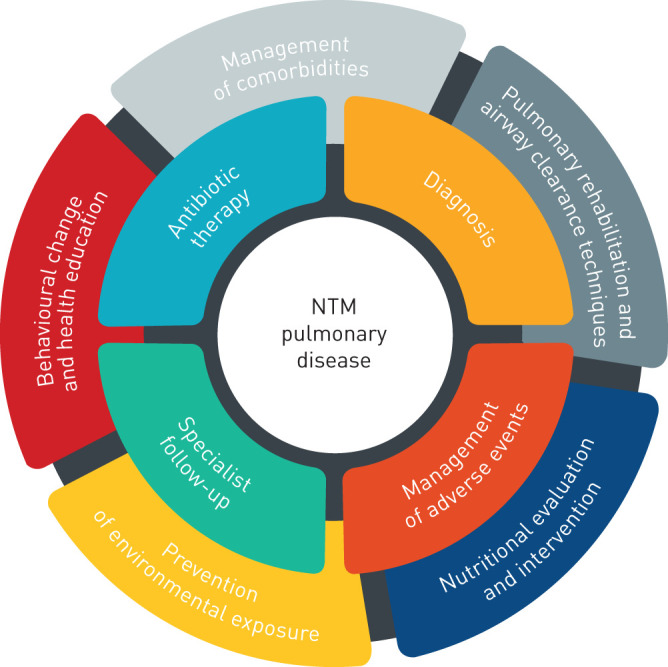
First level (inner ring) and second level (outer ring) interventions for patients with nontuberculous mycobacterial (NTM) pulmonary disease.
Literature search strategy
We conducted a literature search in the English language using the PubMed/MEDLINE and EMBASE databases, including all types of studies up until the end of July 2020. Keywords used to perform the research are reported in table 2. Conference abstracts were excluded.
TABLE 2.
Keywords used to perform the research
| (Non-tuberculous Mycobacteria OR Non-tuberculous Mycobacteria Pulmonary Disease) AND (Nutritional status OR Nutritional evaluation OR Nutritional intervention), |
| (Non-tuberculous Mycobacteria OR Non-tuberculous Mycobacteria Pulmonary Disease) AND (Vitamin D OR Vitamin), |
| (Non-tuberculous Mycobacteria OR Non-tuberculous Mycobacteria Pulmonary Disease) AND (Nutraceutical OR Probiotics), |
| (Non-tuberculous Mycobacteria OR Non-tuberculous Mycobacteria Pulmonary Disease) AND (Behavioral change OR Health education OR Environmental exposure OR Risk Factors), |
| (Non-tuberculous Mycobacteria OR Non-tuberculous Mycobacteria Pulmonary Disease) AND (Pulmonary Rehabilitation OR Exercise Training OR Respiratory Physiotherapy OR Airway Clearing Techniques OR Airway Clearance Techniques), |
| (Non-tuberculous Mycobacteria OR Non-tuberculous Mycobacteria Pulmonary Disease) AND (Comorbidities OR Structural Lung Disease OR Asthma OR Alpha-1 Antitrypsin Deficiency OR Idiopathic Pulmonary Fibrosis OR Interstitial Lung Disease OR Bronchiectasis), |
| (Non-tuberculous Mycobacteria OR Non-tuberculous Mycobacteria Pulmonary Disease) AND (Systemic Diseases OR Gastroesophageal Reflux Disease OR Immunodeficiency OR Immune deficits OR Immune defects) |
Behavioural change and education in NTM-PD
NTM are ubiquitous environmental organisms, commonly found in soil and water biofilms. The significant geographic differences in the distribution of NTM species in human isolates depends on several factors, such as the geochemical characteristics of the environment, the climate, the population density and the presence of specific environmental niches. Soils serve as a large natural source of mycobacteria, and certain soil properties are major determinants of NTM growth and persistence in the environment. Countries at high risk of NTM-PD have a higher content of copper, sodium and silt but low manganese and clay content in the soil [2–4]. High atmospheric water content may also promote NTM growth in soil. In fact, environments with high humidity levels, greater levels of precipitation, warmer temperatures, high daily rates of evapotranspiration (i.e. movement of water from land to the atmosphere by evaporation from the soil and other surfaces and by transpiration from plants) and a high percentage cover by surface water are associated with an increased risk of NTM infection [2, 5]. NTM growth and persistence in surface waters is favoured by low pH, water temperature up to 55°C, low dissolved oxygen content and a high content of salt, soluble zinc, humic acid and fulvic acid [6–8].
Epidemiological studies have demonstrated that countries at high risk of NTM-PD are significantly larger, have greater population densities and have higher education and income levels [2]. Living in an urban versus a rural setting has been associated with altered rates and patterns of NTM infection, e.g. living in an area of higher population density is associated with Mycobacterium kansasii infection, whereas rural areas are associated with Mycobacterium avium complex (MAC) [9]. A recent epidemiological study found a correlation between the incidence of NTM-PD and higher air concentration of particulate matter and benzo[a]pyrene, a polycyclic aromatic hydrocarbon, suggesting that airborne pollution in urban and industrial areas represents a potential risk factor for NTM-PD, probably through the production of reactive oxygen species causing airway chronic inflammation and pulmonary tissue damage [10].
Well-known NTM environmental niches include pine forest (boreal-rich) and peat-rich soils, water bodies (including lakes, rivers and streams), brackish marshes and potable waters [6, 7, 11]. Furthermore, NTM can survive and persist in household and industrial plumbing systems, despite ozonation and filtration, owing to the ability of the bacteria to grow at a low nutrient concentration and associate in biofilms, as well as their chlorine resistance [12, 13]. Studies of the homes of NTM-PD patients have found NTM isolates in showerheads, bathtub water, drain outlets, humidifiers, heating/ventilation systems, bathroom inlets, bathroom and kitchen faucets and refrigerator taps [3, 14, 15].
NTM infection can be acquired through inhalation of aerosolised droplets containing NTM, from natural surface water or hot water systems, or ingestion with subsequent aspiration. Therefore, activities potentially increasing domestic, occupational or recreational exposure to NTM in water (e.g. swimming), soils (e.g. gardening, mining, coal working, agriculture, landscaping, construction, tunnel work) and aerosols (e.g. showering, hot tubs) are associated with increased NTM-PD prevalence [5, 14, 16–20]. In particular, in a recent case–control study, NTM isolation from shower aerosols was associated with a higher risk of NTM-PD [21]. Maekawa et al. [22] found that engaging in soil-related activities more than twice per week was strongly associated with NTM lung disease in a bronchiectasis patient population.
Based on the global increase in the incidence and prevalence of NTM-PD, especially in geographic areas hit by natural disasters, it has been suggested that natural disaster survivors might be at increased risk of NTM-PD after inhaling or aspirating contaminated water–soil mixtures [23]. During and after natural disasters such as tsunamis, hurricanes, earthquakes and tornados, the ecosystem that is normally inhabited by NTM is disrupted by large-scale mixing of ocean water with fresh water and of water with soil, causing widespread water–soil NTM aerosolisation [23]. Long-term prospective epidemiological and microbiological studies in disaster-prone areas are needed to validate this hypothesis of natural disaster-associated NTM-PD, a new potential public health issue.
Given the wide diffusion and the high variety of environments in which NTM can proliferate and the large number of situations in which NTM can be inhaled, it is difficult to devise practical and effective strategies for avoiding NTM disease; to date, no guidelines specifically address NTM disease prevention [24].
Soils are a large natural source of mycobacteria, but there are few strategies available for decreasing NTM infections from soils, primarily because it is impossible to change soil composition. Possible strategies are to drain the marshlands and to combat global warming to decrease the evapotranspiration rate.
By contrast, several strategies may be adopted to decrease NTM contamination of household and industrial plumbing systems. The water network should be renovated, with old and corroded pipelines replaced and water stagnation time in pipelines and household plumbing systems reduced. Disinfectant choice seems to be crucial because it not only influences the frequency of NTM detection at the tap, but also increases NTM persistence in the system and may select for specific pathogenic NTM species [15]. Chloramine is less efficient than chlorine at controlling NTM colonisation [15]. Last but not least, given NTM growth and persistence in warm water with temperatures up to 55°C [4], the most appropriate set-point for household water heaters should be defined to decrease NTM survival while preventing scalding injuries.
In addition, drinking water systems using surface water are more likely to have NTM than systems using a groundwater source; therefore, drinking water supply networks should be reconsidered [25].
Finally, given the high rates of NTM relapse or re-infection (up to 50% of patients who completed treatment [26–29]), patients with known NTM-PD or who are at higher risk of NTM-PD should avoid exposure to potentially contaminated environments. Possible sources of exposure to NTM in water, soils and aerosols, including domestic, occupational and recreational activities, are summarised in table 1.
Nutritional evaluation and intervention
Typical characteristics of patients with NTM-PD are a low BMI and history of weight loss [30–32]. Such features are among those that make the clinician suspect a NTM infection and are so typical of middle-aged women that a specific phenotype, “Lady Windermere syndrome”, has been identified [33]. Most studies on altered nutritional status in respiratory diseases have focused their attention on chronic obstructive pulmonary diseases (COPDs), in which systemic inflammation represents one of the most relevant factors in the development of the nutritional abnormalities [5]. Much less is known about the nutritional implications of other chronic respiratory diseases, e.g. bronchiectasis, that are characterised by chronic lung infections that cause persistent inflammation. In specific types of respiratory infections, such as NTM-PD, weight loss and low BMI are not only characteristic elements of the clinical picture but are also associated with disease spread, severity of radiological scores [34] and negative outcomes [35–37].
Prospective studies on NTM-PD patients found that female patients were significantly thinner than control subjects, with a lower self-reported BMI before NTM disease [37–41]. A plausible mechanism by which individuals with slender body habitus and low body fat content may be predisposed to NTM infections is the altered expression of adipokines, such as leptin and adiponectin, with known immunomodulatory functions [42]. Leptin is not only a satiety hormone, it also promotes both the adaptive immune response, by activating T-helper 1 cells and stimulating the release of interferon-γ (IFN-γ), and the innate immune response, by increasing phagocytic function, chemotaxis, and proliferation and activation of natural killer cells [43]. Leptin is produced mainly by white adipose tissue and, thus, thin individuals have lower leptin levels; a relative deficiency in leptin levels has been observed in NTM-PD patients [42]. Conversely, adiponectin, whose levels are inversely related to the amount of body fat, decreases the production of the host-protective cytokine tumour necrosis factor-α (TNF-α) [43].
In chronic inflammatory lung diseases such as COPD, there is increased evidence of vitamin D deficiency and alterations in calcium metabolism, often associated with negative outcomes [44]. Vitamin D seems to have an immunomodulatory effect, stimulating cell-mediated immunity by favouring the proliferation and activity of T-helper 2 over T-helper 1 cells and enhancing phagocytosis and granuloma formation. In in vitro studies on TB, vitamin D supported the innate immune response and promoted an anti-inflammatory effect with reduced tissue damage [45]. Furthermore, 25(OH)D restricted Mycobacterium tuberculosis growth through cytokine production, particularly LL-37, in macrophages and epithelial cells [45].
Severe vitamin D deficiency was initially found to have a high prevalence in NTM-PD, representing an independent risk factor for the disease [46], but this observation has been disproved by subsequent studies [32, 47]. Furthermore, several studies have suggested an association between vitamin D receptor gene polymorphism and the risk of pulmonary TB, but they were not conclusive in regard to NTM-PD. Fujita et al. [32] found that bone mineral density was decreased in patients with MAC-PD without relation to serum vitamin D level.
An altered vitamin status was also evaluated in patients with NTM-PD. Oh et al. [47] measured concentrations of vitamins A, D and E along with homocysteine and methylmalonic acid as indicators of vitamin B12 deficiency in a case–control study including 150 patients with NTM-PD and 150 healthy controls. The serum concentrations of vitamins A and E were significantly lower in patients with NTM-PD than in healthy controls; in particular, vitamin A deficiency was associated with an 11-fold increase in risk of NTM-PD [47].
Oh et al. [48] also measured the serum concentrations of seven trace elements (cobalt, copper, chromium, manganese, molybdenum, selenium and zinc) and observed higher serum concentrations of copper and molybdenum and lower serum concentrations of selenium and zinc in patients with NTM lung disease compared to healthy controls. None of the seven trace elements was associated with treatment outcomes [48]. Therefore, the exact impact of these alterations in vitamins and micronutrients on disease pathogenicity and progression is still unknown and requires further study.
Physical deconditioning is frequent in patients with COPD as well as bronchiectasis [49]; however, the presence of sarcopenia and the evaluation of muscle mass in patients with NTM-PD have only been marginally evaluated. Morimoto et al. [30] described decreased muscle mass in patients with MAC-PD and found that the percentage of triceps skinfold and mid-upper arm muscle circumference were negatively correlated with the severity of radiological scores.
To date, no studies have fully evaluated the nutritional status of NTM-PD patients or the impact of initiating antibiotic therapy on the nutritional parameters of NTM-PD patients. From the limited evidence available, the prevalence of nutritional and metabolic disorders is not yet clear. Therefore, it is not possible to give any precise nutritional indication with the exception of supplementation in case of vitamin deficiencies and specific nutritional assessment in case of cachexia. Sarcopenia may require joint nutritional and rehabilitative intervention. Probiotics and dietary supplements do not currently have a clear indication either. Prospective studies that fully evaluate the nutritional status of these patients are necessary.
Pulmonary rehabilitation, exercise training and respiratory physiotherapy
Pulmonary rehabilitation (PR) is defined by the American Thoracic Society (ATS) and European Respiratory Society (ERS) as a “comprehensive intervention based on a thorough patient assessment followed by patient-tailored therapies that include, but are not limited to, exercise training and educational and behavioural changes, designed to improve the physical and psychological condition of people with chronic respiratory disease and to promote the long-term adherence of health-enhancing behaviours” [50, 51]. PR is the ideal setting in which an interdisciplinary team that includes pulmonary physicians, physiotherapists, respiratory therapists, nurses, psychologists, nutritionists and occupational therapists can cooperate to improve symptoms and exercise performance, to promote autonomy, to enhance quality of life (QoL) and to effect long-term health-enhancing behavioural changes. The role and benefits of PR have been well defined in patients with COPD [50, 52] and emerging evidence suggests that these benefits could be extended to other chronic respiratory conditions, such as interstitial lung disease (ILD), alpha-1 antitrypsin (AAT) deficiency, asthma, bronchiectasis and lung transplantation. NTM-PD typically occurs in the setting of these pre-existing structural lung diseases (table 1) [53–56]. The benefits of PR can be summarised as follows: reduces hospitalisation and symptoms of dyspnoea; improves exercise capacity, health-related QoL and functional capacity in the activities of daily living; and enhances self-efficacy, knowledge and collaborative self-management [53–59].
However, not all these benefits are reached together and maintained for the same time. In patients with progressive ILDs, such as idiopathic pulmonary fibrosis (IPF), the benefits in exercise capacity and dyspnoea reduction are rarely sustained 6 months following the intervention [53]. In diseases with a more favourable prognosis, results can be maintained for longer periods. To the best of our knowledge, there are no studies specifically assessing the role of PR and respiratory physiotherapy in patients with NTM-PD. However, part of the evidence can be derived from other diseases that show similarities with NTM-PD, such as bronchiectasis and TB [60]. The main objective of PR in patients with bronchiectasis is to encourage ACTs, while improving ventilatory capacity and exercise tolerance. In this scenario, two different interventions play a fundamental role: 1) exercise training, which is associated with short-term improvement in exercise capacity, dyspnoea and fatigue and fewer exacerbations over 12 months [55, 61]; and 2) ACTs, which are used regularly to reduce the respiratory symptoms related to cough and, thus, improve health-related QoL [62].
Because no technique currently shows superiority over another, a personalised approach is recommended for all interventions and strategies to promote behavioural change. Adherence is needed to ensure successful implementation in clinical practice. Therefore, according to current evidence, ACTs and PR should be offered to all patients with bronchiectasis, including those with NTM-PD and a bronchiectasis/bronchiolitic pattern [56, 63].
PR also plays a key role in the treatment of pulmonary TB sequelae and it is recommended in all patients with pulmonary impairment after TB treatment and as preparation in candidates for surgery, including those with localised bronchiectasis and aspergillomas [64]. Patients with TB, as well as NTM-PD, may have different types of lung impairment: the most prevalent pattern is airflow limitation due to sequelae affecting the airways, including bronchiectasis and tracheobronchial stenosis, while a restrictive pattern due to lung parenchyma destruction (cavitations and pulmonary fibrosis) and pleural alterations (fibrothorax) is less frequent [64]. Obstructive and restrictive patterns may coexist. In this scenario, PR comprehending pulmonary physiotherapy and aerobic exercise, even in short-term programmes, produces a significant improvement in aerobic capacity, symptoms control and QoL [65, 66].
Despite the absence of trials specifically assessing the role of PR and ACTs in patients with NTM-PD, the experience obtained from bronchiectasis and TB patients and the guidelines recommendation [67] suggest the importance of offering these personalised non-pharmacological treatment options to all patients to improve QoL and treatment outcomes and to prevent disease recurrence.
Management of comorbidities
Comorbidities may play a fundamental role in increasing subject susceptibility to NTM-PD; therefore, structural lung diseases and immunological defects should be suspected and investigated every time a NTM isolate is found in respiratory samples. Systemic diseases may also be both a cause (e.g. minor immune deficiencies such as diabetes mellitus or nutrient malabsorption causing nutritional deficiencies) and a consequence (e.g. anxiety and depression) of NTM-PD.
Structural lung disease
NTM-PD typically occurs in the setting of pre-existing structural lung disease, because of impaired mucociliary clearance, abnormal composition of sputum and airway damage caused by persistent inflammation [5].
Bronchiectasis and NTM-PD frequently coexist, raising the question of whether NTM infection is a cause or a consequence (or both) of bronchiectasis. In some diseases, such as cystic fibrosis (CF) or post-TB bronchiectasis, it seems reasonable that anatomic bronchial alterations precede NTM infection and that stagnant secretions provide an important medium for NTM proliferation. However, a few reports have described cases in which pulmonary NTM lesions preceded the development of bronchiectasis by cartilage and smooth muscle destruction [68].
The prevalence of COPD in NTM-PD patients is also high [69]. The association of AAT deficiency with NTM-PD is likely due not only to underlying emphysema or bronchiectasis, but also to AAT deficiency itself, as shown in a recent study [70]. In fact, AAT itself may enhance host immunity against microbial pathogens, and Bai et al. [70] observed that macrophages obtained after a session of AAT infusion were significantly better able to control Mycobacterium intracellulare infection compared to those pre-AAT infusion [38].
With regards to ILDs, Park and Olivier [71] described a higher incidence of NTM-PD in IPF patients. Although many IPF patients were still treated with immunosuppressive drugs at the time the study was conducted, a higher incidence of NTM-PD compared with the general population was also found in immunosuppressant-naïve patients, probably due to the anatomical honeycombing alterations caused by the disease [71].
Rare associations between NTM-PD and other respiratory diseases such as silicosis [72], Kartagener syndrome [73] and Pneumocystis pneumonia [74] have also been described. Silicosis, in particular, seems to be associated with a higher incidence of environmental mycobacterial exposure and lower cure rates after treatment [72].
It is increasingly recognised that NTM-PD also occurs in patients without underlying lung disease. This condition was first described in a cohort of white, postmenopausal, thin, tall women with pectus excavatum and mitral valve prolapse who developed predominantly middle lobe and lingula bronchiectasis: the cluster of features described as Lady Windermere syndrome [75]. At first, voluntary cough suppression was considered a potential explanation for this condition. A recent whole exome-sequencing study defined a genetic predisposition for this disease phenotype, identifying minor mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, unassociated with a diagnosis of CF, and mutations in genes involved in immune regulation, ciliary function and connective tissue [37, 76, 77].
Furthermore, NTM-PD patients without underlying structural lung disease present some typical demographic and constitutional features. Epidemiological data indicate that older age and female sex are risk factors for NTM-PD [1]. Because oestrogen binds macrophages and augments their phagocytic function, it is postulated that the observed low postmenopausal oestrogen levels in female NTM-PD patients may increase disease susceptibility [78].
Smoking seems to be a protective factor for NTM infection [79, 80], as similarly reported in other granulomatous lung diseases such as sarcoidosis and hypersensitivity pneumonitis, suggesting that smoking might inhibit granuloma formation [81].
In addition, a substantial proportion of patients with NTM-PD also have thoracic cage abnormalities such as scoliosis, flattened thoracic cage (“platythorax”), pectus excavatum and a nearly vertical vertebral column in lateral chest X-ray (“straight back syndrome”), a condition associated with mitral valve prolapse [37, 43, 82].
Immune defects
Inherited or acquired immunodeficiencies should be considered particularly in recurrent, persistent or severe pulmonary infections and with NTM species that are normally non-pathogenic, as well as in young patients or patients presenting with NTM disease in the absence of structural lung disease [5].
Primary immunodeficiencies include very rare disorders characterised by defective macrophage and dendritic cell function (e.g. chronic granulomatous disease), defective T-cells (e.g. common variable immunodeficiency), severe combined immune deficiency and the so-called Mendelian susceptibility to mycobacterial disease [83], which are caused by mutations in 11 genes encoding cytokines, receptors and downstream signal-transducing proteins of the IFN-γ/interleukin-12 pathway [84].
Acquired immunodeficiencies are far more frequent and result from untreated AIDS/HIV infection, presence of anti-IFN-γ autoantibodies, active solid or haematological malignancy, diabetes mellitus, chronic renal failure and alcohol abuse [5]. Although disseminated NTM infection is a well-known complication of AIDS, isolated NTM-PD is uncommon among HIV-positive patients and may occur when the CD4+ T-cell count is very low (<50 cells·mm−3) [85].
An increased risk of NTM disease has also been reported with the use of immunosuppressant medications, including biological agents, especially TNF-α antagonists, inhaled and oral corticosteroids and other immunosuppressive treatments for haematopoietic stem cell and solid organ transplantation. Lung transplantation is the strongest risk factor for NTM disease after organ transplantation because of a combination of factors, including immune suppression, local defects in the transplanted allograft resulting in abnormal ciliary function, bronchial devascularisation, denervation and lymphatic insufficiency post-transplant. Most NTM infections occur in the first 8 months post-transplant and are associated with high mortality [86, 87].
The increasingly widespread use of TNF-α antagonists as an effective treatment of many inflammatory and autoimmune disorders, e.g. rheumatoid arthritis, vasculitis, inflammatory bowel disease and psoriasis, has led to an increased incidence of NTM-PD, suggesting that anti-TNF-α intrinsically predisposes to new NTM infections and exacerbates indolent infections [88, 89].
Similarly, the increasing incidence rate of NTM-PD in the last years has been attributed to the rise in inhaled corticosteroid (ICS) use for the treatment of several chronic pulmonary diseases, e.g. asthma and COPD. In fact, several case–control studies have shown that ICS current use or ICS use within the past year is associated with an increased risk of NTM-PD compared with non-use [90–93]. Furthermore, the same studies have shown a strong dose-response relationship between higher ICS doses and the risk of NTM-PD [90–93], and a greater risk for fluticasone compared with budesonide [91, 93], likely due to differences in pharmacokinetic and pharmacodynamic properties.
Other systemic diseases
There are conflicting data regarding an increased prevalence of gastro-oesophageal reflux disease (GORD) and proton pump inhibitor (PPI) prescription in patients with NTM lung infection [5, 41]. It has been speculated that PPI promotes gastrointestinal survival of NTM, and, subsequently, reflux delivers NTM into airways through chronic gastric micro-aspiration [94]. Data from the US Bronchiectasis Research Registry indicated a higher prevalence of GORD in patients with NTM compared to those without [41]. No data are available with regards to psychological eating disorders and malabsorption, which might influence the high prevalence of low BMI and weight loss in these patients.
Conclusions
Initial suggestions for the holistic management of patients with NTM-PD beyond antibiotic therapy should comprehend four main domains: 1) a careful evaluation of lifestyle and habits that could favour mycobacterial exposure in order to promote disease prevention; 2) PR and ACTs, which may be effective, low-budget therapeutic interventions, to improve symptoms such as dyspnoea and exercise capacity, health-related QoL and functional capacity in the activities of daily living, and to enhance self-efficacy; 3) a nutritional evaluation, which may be required for preventative purposes but may also improve QoL and control gastrointestinal side-effects during antimicrobial therapy; and 4) investigation of comorbidities, because they are fundamental to disease pathogenesis and, therefore, acting on these factors may improve disease outcome and prevent relapses or re-infections.
Considering the complexity of NTM-PD, which requires multiple expertise, the involvement of a multidisciplinary team may favour the optimisation of patient management (figure 2). Multidisciplinary discussion has been effectively introduced in the diagnostic examination and management of other respiratory diseases including lung tumours and ILDs [95, 96]. In particular, respiratory medicine and infectious disease specialists should prescribe antibiotic therapy and monitor possible adverse events, but they should also evaluate and treat comorbidities and predisposing factors that may affect disease prognosis. Nutritionists should be involved to evaluate nutritional status and to implement a nutritional intervention as needed. Physiotherapists and respiratory therapists are a fundamental part of the therapeutic and prevention process with ACT and PR programmes. Finally, pharmacists might also be included to discuss the best treatment options.
FIGURE 2.
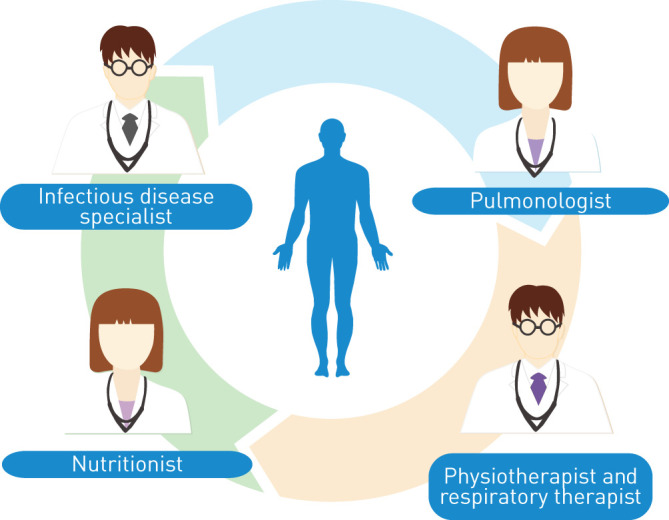
Clinical figures involved in the multidisciplinary team for the holistic management of patients with nontuberculous mycobacteria pulmonary disease.
Multidisciplinary discussion should be organised periodically with the aim of discussing new cases to promptly introduce an integrated approach. However, clinical cases may also be re-discussed as needed to optimise management and follow-up.
Footnotes
Conflict of interest: P. Faverio has nothing to disclose.
Conflict of interest: F. De Giacomi has nothing to disclose.
Conflict of interest: B.D. Bodini has nothing to disclose.
Conflict of interest: A. Stainer has nothing to disclose.
Conflict of interest: A. Fumagalli has nothing to disclose.
Conflict of interest: F. Bini has nothing to disclose.
Conflict of interest: F. Luppi has nothing to disclose.
Conflict of interest: S. Aliberti has nothing to disclose.
References
- 1.Daley CL, Iaccarino JM, Lange C, et al. . Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J 2020; 56: 2000535. doi: 10.1183/13993003.00535-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adjemian J, Olivier KN, Seitz AE, et al. . Spatial clusters of nontuberculous mycobacterial lung disease in the United States. Am J Respir Crit Care Med 2012; 186: 553–558. doi: 10.1164/rccm.201205-0913OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lipner EM, Knox D, French J, et al. . A geospatial epidemiologic analysis of nontuberculous mycobacterial infection: an ecological study in Colorado. Ann Am Thorac Soc 2017; 14: 1523–1532. doi: 10.1513/AnnalsATS.201701-081OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parikh A, Vinnard C, Fahrenfeld N, et al. . Revisiting John Snow to meet the challenge of nontuberculous mycobacterial lung disease. Int J Environ Res Public Health 2019; 16: 4250. doi: 10.3390/ijerph16214250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haworth CS, Banks J, Capstick T, et al. . British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax 2017; 72: Suppl. 2, ii1–ii64. doi: 10.1136/thoraxjnl-2017-210927 [DOI] [PubMed] [Google Scholar]
- 6.Kirschner RA, Parker BC, Falkinham JO. Epidemiology of infection by nontuberculous mycobacteria. Mycobacterium avium, Mycobacterium intracellulare, and Mycobacterium scrofulaceum in acid, brown-water swamps of the southeastern United States and their association with environmental variables. Am Rev Respir Dis 1992; 145: 271–275. doi: 10.1164/ajrccm/145.2_Pt_1.271 [DOI] [PubMed] [Google Scholar]
- 7.Falkinham JO. Surrounded by mycobacteria: nontuberculous mycobacteria in the human environment. J Appl Microbiol 2009; 107: 356–367. doi: 10.1111/j.1365-2672.2009.04161.x [DOI] [PubMed] [Google Scholar]
- 8.Falkinham JO. Ecology of nontuberculous mycobacteria – where do human infections come from? Semin Respir Crit Care Med 2013; 34: 95–102. doi: 10.1055/s-0033-1333568 [DOI] [PubMed] [Google Scholar]
- 9.Marras TK, Daley CL. Epidemiology of human pulmonary infection with nontuberculous mycobacteria. Clin Chest Med 2002; 23: 553–567. doi: 10.1016/S0272-5231(02)00019-9 [DOI] [PubMed] [Google Scholar]
- 10.Modrá H, Ulmann V, Caha J, et al. . Socio-economic and environmental factors related to spatial differences in human non-tuberculous mycobacterial diseases in the Czech Republic. Int J Environ Res Public Health 2019; 16: 3969. doi: 10.3390/ijerph16203969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ulmann V, Kracalikova A, Dziedzinska R. Mycobacteria in water used for personal hygiene in heavy industry and collieries: a potential risk for employees. Int J Environ Res Public Health 2015; 12: 2870–2877. doi: 10.3390/ijerph120302870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Revetta RP, Gomez-Alvarez V, Gerke TL, et al. . Changes in bacterial composition of biofilm in a metropolitan drinking water distribution system. J Appl Microbiol 2016; 121: 294–305. doi: 10.1111/jam.13150 [DOI] [PubMed] [Google Scholar]
- 13.Vaerewijck MJM, Huys G, Palomino JC, et al. . Mycobacteria in drinking water distribution systems: ecology and significance for human health. FEMS Microbiol Rev 2005; 29: 911–934. doi: 10.1016/j.femsre.2005.02.001 [DOI] [PubMed] [Google Scholar]
- 14.Honda JR, Virdi R, Chan ED. Global environmental nontuberculous mycobacteria and their contemporaneous man-made and natural niches. Front Microbiol 2018; 9: 2029. doi: 10.3389/fmicb.2018.02029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donohue MJ, Mistry JH, Donohue JM, et al. . Increased frequency of nontuberculous mycobacteria detection at potable water taps within the United States. Environ Sci Technol 2015; 49: 6127–6133. doi: 10.1021/acs.est.5b00496 [DOI] [PubMed] [Google Scholar]
- 16.Falkinham JO. Current epidemiologic trends of the nontuberculous mycobacteria (NTM). Curr Environ Health Rep 2016; 3: 161–167. doi: 10.1007/s40572-016-0086-z [DOI] [PubMed] [Google Scholar]
- 17.Lee J-W, Myong J-P. Association between occupational and radiological factors and nontuberculous mycobacteria lung infection in workers with prior dust exposure. Int J Environ Res Public Health 2019; 16: 1966. doi: 10.3390/ijerph16111966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sonnenberg P, Murray J, Glynn JR, et al. . Risk factors for pulmonary disease due to culture-positive M. tuberculosis or nontuberculous mycobacteria in South African gold miners. Eur Respir J 2000; 15: 291–296. doi: 10.1034/j.1399-3003.2000.15b12.x [DOI] [PubMed] [Google Scholar]
- 19.Corbett EL, Churchyard GJ, Clayton T, et al. . Risk factors for pulmonary mycobacterial disease in South African gold miners. A case–control study. Am J Respir Crit Care Med 1999; 159: 94–99. doi: 10.1164/ajrccm.159.1.9803048 [DOI] [PubMed] [Google Scholar]
- 20.De Groote MA, Pace NR, Fulton K, et al. . Relationships between Mycobacterium isolates from patients with pulmonary mycobacterial infection and potting soils. Appl Environ Microbiol 2006; 72: 7602–7606. doi: 10.1128/AEM.00930-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tzou CL, Dirac MA, Becker AL, et al. . Association between Mycobacterium avium complex pulmonary disease and mycobacteria in home water and soil. Ann Am Thorac Soc 2020; 17: 57–62. doi: 10.1513/AnnalsATS.201812-915OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maekawa K, Ito Y, Hirai T, et al. . Environmental risk factors for pulmonary Mycobacterium avium-intracellulare complex disease. Chest 2011; 140: 723–729. doi: 10.1378/chest.10-2315 [DOI] [PubMed] [Google Scholar]
- 23.Honda JR, Bernhard JN, Chan ED. Natural disasters and nontuberculous mycobacteria: a recipe for increased disease? Chest 2015; 147: 304–308. doi: 10.1378/chest.14-0974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griffith DE, Aksamit T, Brown-Elliott BA, et al. . An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007; 175: 367–416. doi: 10.1164/rccm.200604-571ST [DOI] [PubMed] [Google Scholar]
- 25.Falkinham JO, Norton CD, LeChevallier MW. Factors influencing numbers of Mycobacterium avium, Mycobacterium intracellulare, and other mycobacteria in drinking water distribution systems. Appl Environ Microbiol 2001; 67: 1225–1231. doi: 10.1128/AEM.67.3.1225-1231.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallace RJ, Brown-Elliott BA, McNulty S, et al. . Macrolide/azalide therapy for nodular/bronchiectatic Mycobacterium avium complex lung disease. Chest 2014; 146: 276–282. doi: 10.1378/chest.13-2538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallace RJ, Zhang Y, Brown-Elliott BA, et al. . Repeat positive cultures in Mycobacterium intracellulare lung disease after macrolide therapy represent new infections in patients with nodular bronchiectasis. J Infect Dis 2002; 186: 266–273. doi: 10.1086/341207 [DOI] [PubMed] [Google Scholar]
- 28.Field SK, Fisher D, Cowie RL. Mycobacterium avium complex pulmonary disease in patients without HIV infection. Chest 2004; 126: 566–581. doi: 10.1378/chest.126.2.566 [DOI] [PubMed] [Google Scholar]
- 29.Xu H-B, Jiang R-H, Li L. Treatment outcomes for Mycobacterium avium complex: a systematic review and meta-analysis. Eur J Clin Microbiol Infect Dis 2014; 33: 347–358. doi: 10.1007/s10096-013-1962-1 [DOI] [PubMed] [Google Scholar]
- 30.Morimoto K, Yoshiyama T, Kurashima A, et al. . Nutritional indicators are correlated with the radiological severity score in patients with Mycobacterium avium complex pulmonary disease: a cross-sectional study. Intern Med Tokyo Jpn 2014; 53: 397–401. doi: 10.2169/internalmedicine.53.1277 [DOI] [PubMed] [Google Scholar]
- 31.Faverio P, Stainer A, Bonaiti G, et al. . Characterizing non-tuberculous mycobacteria infection in bronchiectasis. Int J Mol Sci 2016; 17: 1913. doi: 10.3390/ijms17111913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujita K, Ito Y, Oguma T, et al. . Association between Mycobacterium avium complex lung disease and serum vitamin D status, antimicrobial peptide levels, and bone mineral density. Medicine (Baltimore) 2018; 97: e12463. doi: 10.1097/MD.0000000000012463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan ED, Iseman MD. Slender, older women appear to be more susceptible to nontuberculous mycobacterial lung disease. Gend Med 2010; 7: 5–18. doi: 10.1016/j.genm.2010.01.005 [DOI] [PubMed] [Google Scholar]
- 34.Ikegame S, Maki S, Wakamatsu K, et al. . Nutritional assessment in patients with pulmonary nontuberculous mycobacteriosis. Intern Med Tokyo Jpn 2011; 50: 2541–2546. doi: 10.2169/internalmedicine.50.5853 [DOI] [PubMed] [Google Scholar]
- 35.Gochi M, Takayanagi N, Kanauchi T, et al. . Retrospective study of the predictors of mortality and radiographic deterioration in 782 patients with nodular/bronchiectatic Mycobacterium avium complex lung disease. BMJ Open 2015; 5: e008058. doi: 10.1136/bmjopen-2015-008058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayashi M, Takayanagi N, Kanauchi T, et al. . Prognostic factors of 634 HIV-negative patients with Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 2012; 185: 575–583. doi: 10.1164/rccm.201107-1203OC [DOI] [PubMed] [Google Scholar]
- 37.Kim RD, Greenberg DE, Ehrmantraut ME, et al. . Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med 2008; 178: 1066–1074. doi: 10.1164/rccm.200805-686OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kartalija M, Ovrutsky AR, Bryan CL, et al. . Patients with nontuberculous mycobacterial lung disease exhibit unique body and immune phenotypes. Am J Respir Crit Care Med 2013; 187: 197–205. doi: 10.1164/rccm.201206-1035OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee SJ, Ryu YJ, Lee JH, et al. . The impact of low subcutaneous fat in patients with nontuberculous mycobacterial lung disease. Lung 2014; 192: 395–401. doi: 10.1007/s00408-014-9565-x [DOI] [PubMed] [Google Scholar]
- 40.Wakamatsu K, Nagata N, Maki S, et al. . Patients with mac lung disease have a low visceral fat area and low nutrient intake. Pulm Med 2015; 2015: 218253. doi: 10.1155/2015/218253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aksamit TR, O'Donnell AE, Barker A, et al. . Adult patients with bronchiectasis: a first look at the US Bronchiectasis Research Registry. Chest 2017; 151: 982–992. doi: 10.1016/j.chest.2016.10.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tasaka S, Hasegawa N, Nishimura T, et al. . Elevated serum adiponectin level in patients with Mycobacterium avium-intracellulare complex pulmonary disease. Respir Int Rev Thorac Dis 2010; 79: 383–387. [DOI] [PubMed] [Google Scholar]
- 43.Chan ED, Iseman MD. Underlying host risk factors for nontuberculous mycobacterial lung disease. Semin Respir Crit Care Med 2013; 34: 110–123. doi: 10.1055/s-0033-1333573 [DOI] [PubMed] [Google Scholar]
- 44.Graumam RQ, Pinheiro MM, Nery LE, et al. . Increased rate of osteoporosis, low lean mass, and fragility fractures in COPD patients: association with disease severity. Osteoporos Int 2018; 29: 1457–1468. doi: 10.1007/s00198-018-4483-z [DOI] [PubMed] [Google Scholar]
- 45.Brighenti S, Bergman P, Martineau AR. Vitamin D and tuberculosis: where next? J Intern Med 2018; 284: 145–162. [ 10.1111/joim.12777]. [DOI] [PubMed] [Google Scholar]
- 46.Jeon K, Kim S-Y, Jeong B-H, et al. . Severe vitamin D deficiency is associated with non-tuberculous mycobacterial lung disease: a case–control study. Respirology 2013; 18: 983–988. doi: 10.1111/resp.12109 [DOI] [PubMed] [Google Scholar]
- 47.Oh J, Park H-D, Kim S-Y, et al. . Assessment of vitamin status in patients with nontuberculous mycobacterial pulmonary disease: potential role of vitamin A as a risk factor. Nutrients 2019; 11: 343. doi: 10.3390/nu11020343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oh J, Shin SH, Choi R, et al. . Assessment of 7 trace elements in serum of patients with nontuberculous mycobacterial lung disease. J Trace Elem Med Biol 2019; 53: 84–90. doi: 10.1016/j.jtemb.2019.02.004 [DOI] [PubMed] [Google Scholar]
- 49.Gale NS, Bolton CE, Duckers JM, et al. . Systemic comorbidities in bronchiectasis. Chron Respir Dis 2012; 9: 231–238. doi: 10.1177/1479972312459973 [DOI] [PubMed] [Google Scholar]
- 50.Spruit MA, Singh SJ, Garvey C, et al. . An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013; 188: e13–e64. doi: 10.1164/rccm.201309-1634ST [DOI] [PubMed] [Google Scholar]
- 51.Rochester CL, Vogiatzis I, Holland AE, et al. . An official American Thoracic Society/European Respiratory Society policy statement: enhancing implementation, use, and delivery of pulmonary rehabilitation. Am J Respir Crit Care Med 2015; 192: 1373–1386. doi: 10.1164/rccm.201510-1966ST [DOI] [PubMed] [Google Scholar]
- 52.Gloeckl R, Halle M, Kenn K. Interval versus continuous training in lung transplant candidates: a randomized trial. J Heart Lung Transplant 2012; 31: 934–941. doi: 10.1016/j.healun.2012.06.004 [DOI] [PubMed] [Google Scholar]
- 53.Holland AE, Hill CJ, Conron M, et al. . Short term improvement in exercise capacity and symptoms following exercise training in interstitial lung disease. Thorax 2008; 63: 549–554. doi: 10.1136/thx.2007.088070 [DOI] [PubMed] [Google Scholar]
- 54.Mendes FAR, Gonçalves RC, Nunes MPT, et al. . Effects of aerobic training on psychosocial morbidity and symptoms in patients with asthma: a randomized clinical trial. Chest 2010; 138: 331–337. doi: 10.1378/chest.09-2389 [DOI] [PubMed] [Google Scholar]
- 55.Newall C, Stockley RA, Hill SL. Exercise training and inspiratory muscle training in patients with bronchiectasis. Thorax 2005; 60: 943–948. doi: 10.1136/thx.2004.028928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nishiyama O, Kondoh Y, Kimura T, et al. . Effects of pulmonary rehabilitation in patients with idiopathic pulmonary fibrosis. Respirol Carlton Vic 2008; 13: 394–399. doi: 10.1111/j.1440-1843.2007.01205.x [DOI] [PubMed] [Google Scholar]
- 57.Langer D, Burtin C, Schepers L, et al. . Exercise training after lung transplantation improves participation in daily activity: a randomized controlled trial. Am J Transplant 2012; 12: 1584–1592. doi: 10.1111/j.1600-6143.2012.04000.x [DOI] [PubMed] [Google Scholar]
- 58.Lee AL, Hill CJ, Cecins N, et al. . The short and long term effects of exercise training in non-cystic fibrosis bronchiectasis – a randomised controlled trial. Respir Res 2014; 15: 44. doi: 10.1186/1465-9921-15-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ochmann U, Kotschy-Lang N, Raab W, et al. . Long-term efficacy of pulmonary rehabilitation in patients with occupational respiratory diseases. Respir Int Rev Thorac Dis 2012; 84: 396–405. [DOI] [PubMed] [Google Scholar]
- 60.O'Neill K, O'Donnell AE, Bradley JM. Airway clearance, mucoactive therapies and pulmonary rehabilitation in bronchiectasis. Respirology 2019; 24: 227–237. doi: 10.1111/resp.13459 [DOI] [PubMed] [Google Scholar]
- 61.Liaw M-Y, Wang Y-H, Tsai Y-C, et al. . Inspiratory muscle training in bronchiectasis patients: a prospective randomized controlled study. Clin Rehabil 2011; 25: 524–536. doi: 10.1177/0269215510391682 [DOI] [PubMed] [Google Scholar]
- 62.Moran F, Piper A, Elborn JS, et al. . Respiratory muscle pressures in non-CF bronchiectasis: repeatability and reliability. Chron Respir Dis 2010; 7: 165–171. doi: 10.1177/1479972310375595 [DOI] [PubMed] [Google Scholar]
- 63.Severiche-Bueno D, Gamboa E, Reyes LF, et al. . Hot topics and current controversies in non-cystic fibrosis bronchiectasis. Breathe 2019; 15: 286–295. doi: 10.1183/20734735.0261-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muñoz-Torrico M, Cid-Juárez S, Galicia-Amor S, et al. . Tuberculosis sequelae assessment and rehabilitation. In: Migliori GB, Bothamley G, Duarte R, et al., eds. Tuberculosis (ERS Monograph). Sheffield, European Respiratory Society, 2018; pp. 326–342. [Google Scholar]
- 65.Rivera JA, Wilches-Luna EC, Mosquera R, et al. . Pulmonary rehabilitation on aerobic capacity and health-related quality of life in patients with sequelae of pulmonary TB. Physiotherapy 2015; 101: e1288. doi: 10.1016/j.physio.2015.03.1203 [DOI] [Google Scholar]
- 66.Ando M, Mori A, Esaki H, et al. . The effect of pulmonary rehabilitation in patients with post-tuberculosis lung disorder. Chest 2003; 123: 1988–1995. doi: 10.1378/chest.123.6.1988 [DOI] [PubMed] [Google Scholar]
- 67.Polverino E, Goeminne PC, McDonnell MJ, et al. . European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J 2017; 50: 1700629. doi: 10.1183/13993003.00629-2017 [DOI] [PubMed] [Google Scholar]
- 68.Bonaiti G, Pesci A, Marruchella A, et al. . Nontuberculous mycobacteria in noncystic fibrosis bronchiectasis. BioMed Res Int 2015; 2015: 197950. doi: 10.1155/2015/197950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adjemian J, Olivier KN, Seitz AE, et al. . Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med 2012; 185: 881–886. doi: 10.1164/rccm.201111-2016OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bai X, Bai A, Honda JR, et al. . Alpha-1-antitrypsin enhances primary human macrophage immunity against non-tuberculous mycobacteria. Front Immunol 2019; 10: 1417. doi: 10.3389/fimmu.2019.01417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Park IK, Olivier KN. Nontuberculous mycobacteria in cystic fibrosis and non-cystic fibrosis bronchiectasis. Semin Respir Crit Care Med 2015; 36: 217–224. doi: 10.1055/s-0035-1546751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blanco Pérez JJ, Pérez González A, Morano Amado LE, et al. . Clinical significance of environmental mycobacteria isolated from respiratory specimens of patients with and without silicosis. Arch Bronconeumol 2016; 52: 145–150. doi: 10.1016/j.arbres.2015.07.007 [DOI] [PubMed] [Google Scholar]
- 73.Kim JH, Song WJ, Jun JE, et al. . Mycobacterium abscessus lung disease in a patient with Kartagener syndrome. Tuberc Respir Dis 2014; 77: 136–140. doi: 10.4046/trd.2014.77.3.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen F, Sethi G, Goldin R, et al. . Concurrent granulomatous Pneumocystis carinii and Mycobacterium xenopi pneumonia: an unusual manifestation of HIV immune reconstitution disease. Thorax 2004; 59: 997–999. doi: 10.1136/thx.2003.012567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reich JM, Johnson RE. Mycobacterium avium complex pulmonary disease presenting as an isolated lingular or middle lobe pattern. The Lady Windermere syndrome. Chest 1992; 101: 1605–1609. doi: 10.1378/chest.101.6.1605 [DOI] [PubMed] [Google Scholar]
- 76.Szymanski EP, Leung JM, Fowler CJ, et al. . Pulmonary nontuberculous mycobacterial infection. A multisystem, multigenic disease. Am J Respir Crit Care Med 2015; 192: 618–628. doi: 10.1164/rccm.201502-0387OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cowman SA, Jacob J, Hansell DM, et al. . Whole-blood gene expression in pulmonary nontuberculous mycobacterial infection. Am J Respir Cell Mol Biol 2018; 58: 510–518. doi: 10.1165/rcmb.2017-0230OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Uwamino Y, Nishimura T, Sato Y, et al. . Low serum estradiol levels are related to Mycobacterium avium complex lung disease: a cross-sectional study. BMC Infect Dis 2019; 19: 1055. doi: 10.1186/s12879-019-4668-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wickremasinghe M, Ozerovitch LJ, Davies G, et al. . Non-tuberculous mycobacteria in patients with bronchiectasis. Thorax 2005; 60: 1045–1051. doi: 10.1136/thx.2005.046631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shteinberg M, Stein N, Adir Y, et al. . Prevalence, risk factors and prognosis of nontuberculous mycobacterial infection among people with bronchiectasis: a population survey. Eur Respir J 2018; 51: 1702469. doi: 10.1183/13993003.02469-2017 [DOI] [PubMed] [Google Scholar]
- 81.Maier LA. Is smoking beneficial for granulomatous lung diseases? Am J Respir Crit Care Med 2004; 169: 893–895. doi: 10.1164/rccm.2402023 [DOI] [PubMed] [Google Scholar]
- 82.Iseman MD, Buschman DL, Ackerson LM. Pectus excavatum and scoliosis. Thoracic anomalies associated with pulmonary disease caused by Mycobacterium avium complex. Am Rev Respir Dis 1991; 144: 914–916. doi: 10.1164/ajrccm/144.4.914 [DOI] [PubMed] [Google Scholar]
- 83.Rosain J, Kong X-F, Martinez-Barricarte R, et al. . Mendelian susceptibility to mycobacterial disease: 2014–2018 update. Immunol Cell Biol 2019; 97: 360–367. doi: 10.1111/imcb.12210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mortaz E, Moloudizargari M, Varahram M, et al. . What immunological defects predispose to non-tuberculosis mycobacterial infections? Iran J Allergy Asthma Immunol 2018; 17: 100–109. [PubMed] [Google Scholar]
- 85.Horsburgh CR, Gettings J, Alexander LN, et al. . Disseminated Mycobacterium avium complex disease among patients infected with human immunodeficiency virus, 1985–2000. Clin Infect Dis 2001; 33: 1938–1943. doi: 10.1086/324508 [DOI] [PubMed] [Google Scholar]
- 86.Rao M, Silveira FP. Non-tuberculous mycobacterial infections in thoracic transplant candidates and recipients. Curr Infect Dis Rep 2018; 20: 14. doi: 10.1007/s11908-018-0619-8 [DOI] [PubMed] [Google Scholar]
- 87.Longworth SA, Blumberg EA, Barton TD, et al. . Non-tuberculous mycobacterial infections after solid organ transplantation: a survival analysis. Clin Microbiol Infect 2015; 21: 43–47. doi: 10.1016/j.cmi.2014.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wallis RS, Schluger NW. Pulmonary infectious complications of tumor necrosis factor blockade. Infect Dis Clin North Am 2010; 24: 681–692. doi: 10.1016/j.idc.2010.04.010 [DOI] [PubMed] [Google Scholar]
- 89.Winthrop KL, Chang E, Yamashita S, et al. . Nontuberculous mycobacteria infections and anti-tumor necrosis factor-α therapy. Emerg Infect Dis 2009; 15: 1556–1561. doi: 10.3201/eid1510.090310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hojo M, Iikura M, Hirano S, et al. . Increased risk of nontuberculous mycobacterial infection in asthmatic patients using long-term inhaled corticosteroid therapy. Respirology 2012; 17: 185–190. doi: 10.1111/j.1440-1843.2011.02076.x [DOI] [PubMed] [Google Scholar]
- 91.Andréjak C, Nielsen R, Thomsen VØ, et al. . Chronic respiratory disease, inhaled corticosteroids and risk of non-tuberculous mycobacteriosis. Thorax 2013; 68: 256–262. doi: 10.1136/thoraxjnl-2012-201772 [DOI] [PubMed] [Google Scholar]
- 92.Liu VX, Winthrop KL, Lu Y, et al. . Association between inhaled corticosteroid use and pulmonary nontuberculous mycobacterial infection. Ann Am Thorac Soc 2018; 15: 1169–1176. doi: 10.1513/AnnalsATS.201804-245OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brode SK, Campitelli MA, Kwong JC, et al. . The risk of mycobacterial infections associated with inhaled corticosteroid use. Eur Respir J 2017; 50: 1700037. doi: 10.1183/13993003.00037-2017 [DOI] [PubMed] [Google Scholar]
- 94.Thomson RM, Armstrong JG, Looke DF. Gastroesophageal reflux disease, acid suppression, and Mycobacterium avium complex pulmonary disease. Chest 2007; 131: 1166–1172. doi: 10.1378/chest.06-1906 [DOI] [PubMed] [Google Scholar]
- 95.Luppi F, Faverio P, Wuyts WA. Multidisciplinary approach to systemic diseases: benefits for diagnosis and management of complex disorders. In: Wuyts WA, Cottin V, Spagnolo P, et al., eds. Pulmonary Manifestations of Systemic Diseases (ERS Monograph). Sheffield, European Respiratory Society, 2019; pp. 1–13. doi:10.1183/2312508X.10013719. [Google Scholar]
- 96.Ung KA, Campbell BA, Duplan D, et al. . Impact of the lung oncology multidisciplinary team meetings on the management of patients with cancer. Asia Pac J Clin Oncol 2016; 12: e298–e304. doi: 10.1111/ajco.12192 [DOI] [PubMed] [Google Scholar]


