Abstract
Chlamydial infections in humans are widely distributed and are responsible for a variety of acute and chronic diseases. Both Chlamydia trachomatis and Chlamydia pneumoniae can lead to chronic conditions that have been linked to complications and sequelae.
This study aimed to develop a culture method in order to detect in vitro antichlamydial activity of different extracts obtained from native Argentinian plants used as antimicrobials in local ethnomedicine and to evaluate their inhibitory activity over Chlamydia trachomatis and Chlamydia pneumoniae growth. The inhibitory activity over different stages of the chlamydial life cycle on cell culture was assessed: the entry, the inclusion developing after entry, and the exponential growth stage. Also, the capability of rendering the cell refractory to chlamydial infection by pre-incubation with the extracts was assayed.
Inhibitory activity of water-based and organic-based extracts obtained from Hydrocotyle bonariensis Lam. (Araliaceae), Lithraea molleoides (Vell.) Engl. (Anacardiaceae) and Hybanthus parviflorus (Mutis ex L.f.) Baill. (Violaceae) were tested against five strains of Chlamydia trachomatis (L2/434/BU and four clinical isolates form both neonatal conjunctivitis and adult genital infections, genotypes D, E, and K) and against Chlamydia pneumoniae AR39.
The Hydrocotyle bonariensis dichloromethane extract showed a broad inhibitory activity over the exponential growth stage of Chlamydia trachomatis and Chlamydia pneumoniae independently from the chlamydial strain and the cell line. These results suggest a high inhibitory potential on both Chlamydiae species.
In order to characterize the Hydrocotyle bonariensis dichloromethane active extract, an 1H-NMR was performed. The 1H-NMR characterization showed a spectrum with characteristic signals of the fatty acid moiety of lipids or cerebrosides, volatile phenolics, phytosterols, methyl triterpenes signals, and glucose moiety of the cerebrosides.
Keywords: Chlamydia trachomatis, Chlamydia pneumoniae, Antichlamydial activity, Argentinian medicinal plant, Hydrocotyle bonariensis
Chlamydia trachomatis; Chlamydia pneumoniae; Antichlamydial activity; Argentinian medicinal plant; Hydrocotyle bonariensis.
1. Introduction
Members of the Chlamydiaceae family are obligate intracellular pathogens with a unique developmental cycle involving two well-differentiated forms: the infectious and extracellular Elementary Body (EB) and the non-infectious and intracellular replicative Reticulate Body (RB).
Chlamydial infections in humans are widely distributed and are responsible for a variety of acute and chronic diseases. Chlamydia trachomatis causes the most prevalent sexually transmitted bacterial infections in the world, with 131 million new cases among adults and adolescents per year, according to the World Health Organization (WHO, 2016) and is also the leading cause of preventable blindness by causing trachoma in endemic areas. Following vertical transmission, through an infected birth canal, this Chlamydia species can cause neonatal conjunctivitis and pneumonia. Chlamydia pneumoniae is a human respiratory pathogen circulating worldwide. It is estimated to be responsible for 10% of community-acquired pneumonia and 5% of bronchitis and sinusitis cases in developed countries (Kuo et al., 1995).
In addition to these acute diseases, both species can lead to chronic conditions. If C. trachomatis genital infections remain untreated, they can ascend to the upper genital tract producing pelvic inflammatory disease, chronic pelvic pain, and related complications such as ectopic pregnancy and tubal factor infertility (Morré et al., 2002). On the other hand, C. pneumoniae unresolved respiratory diseases may contribute to asthma onset (Webley and Hahn, 2017).
Currently recommended antibiotic treatment for chlamydial infections is a single dose of azithromycin or a 7-day course of doxycycline, which is longer in the case of severe pneumonia or lymphogranuloma venereum (CDC 2015; WHO 2016). Although these drugs can successfully eradicate most acute infections, there is an increasing number of reports of failure of the treatments (Kissinger et al., 2016; Sherrard and Jensen, 2019) and their unsatisfactory efficacy in chronic infections (Wyrick and Knight, 2004; Kohlhoff and Hammerschlag, 2015). Also, macrolides' massive use has raised resistance markers among other bacterial species (Bojang et al., 2017; Mohammadzadeh et al., 2019). All this evidence supports the need for constant efforts to continue searching for new antichlamydial agents (Alvesalo et al., 2006; Salin et al., 2010; Yamazaki et al., 2005; Osaka et al., 2012; Kahru et al., 2017; Petyaev et al., 2017).
According to the WHO, between 65 and 80% of the population living in developing countries base their primary health care on different plant species that have reported widespread use in local ethnomedicine (WHO, 2014). Thus, these plants constitute a rich source of bioactive compounds that can be investigated to develop new antimicrobial drugs. The diversity of plants growing in Argentina and their known ethnopharmacological uses offer vast potential for discovering novel structures with antimicrobial properties.
Hydrocotyle bonariensis Lam. (Araliaceae), also named “paragüitas” or “sombrilla de sapo”, is a perennial plant used in traditional medicine in South America. The leaves have been used in poultices to heal wounds, inflammatory processes, and skin rashes. Infusions prepared with leaves, flowering tops, and stems have been used as a diuretic, stimulant, emmenagogue, and antiseptic (Hieronymus, 1882; Toursarkissian, 1980a; Marzocca, 1997).
Lithraea molleoides (Vell.) Engl. (Anacardiaceae), commonly known as “chichita” or “molle de Córdoba”, is a tree that grows in Argentina, Brazil, Uruguay, and Paraguay. L. molleoides is well known by rural people of these countries as antiarthritic, hemostatic, diuretic, tonic, and useful for treating respiratory diseases (Toursarkissian, 1980b). Previous investigations on different extracts of L. molleoides have reported antiviral (Kott et al., 1999) antimicrobial (Penna et al., 2001), anti-inflammatory (Gorzalczany et al., 2011), and antinociceptive (Morucci et al., 2012) activities.
Hybanthus parviflorus (Mutis ex L.f.) Baill (Violaceae) is a perennial shrub widely distributed in America's tropical and subtropical regions, known as 'violetilla' (Zuloaga and Morrone, 1999). In Argentina, Chile, Peru, and Colombia, it has been used as emetic and purgative. H. parviflorus has been reported as insecticidal and have shown antiviral, antibacterial, and antifungal in vitro activity (Broussalis et al., 2010).
This study aimed to detect in vitro antichlamydial activity of different extracts obtained from native Argentinian plants used as antimicrobials in local ethnomedicine and evaluate their inhibitory activity over Chlamydia trachomatis and Chlamydia pneumoniae different life cycle stages.
2. Materials and methods
2.1. Plant material, plant extraction, and extract fractionation
Infusion: An infusion of each plant material, aerial parts of H. bonariensis, and leaves of L. molleoides were prepared as follows: 5 g of dried plant material in 100 mL of boiling water for 20 min. After vacuum-filtration and volume adjustment (5 % W/V), the resulting extract was freeze-dried. Two powder aqueous extracts were obtained, L. molleoides infusion (Lm Inf), and H. bonariensis infusion (Hb Inf).
Dichloromethane extracts: A dichloromethane extracts with aerial parts of H. bonariensis, leaves of L. molleoides, and aerial parts of H. parviflorus were prepared by maceration of dried plant material with different portions of dichloromethane for 24 h. After filtration, the extracts were taken to dryness under reduced pressure. Three dried dichloromethane extracts were obtained. Only H. bonariensis dichloromethane extract (Hb Cl2CH2) was tested for inhibitory activity, while the other plant material underwent the further extractive process.
Methanol and ethanol/water extracts: Following the dichloromethane extraction, the powdered plant material was air-dried and extracted with methanol or a mixture of equal parts of ethanol and water, obtaining three extracts that were taken to dryness under reduced pressure. The H. bonariensis methanol extract (Hb MeOH) and the H. parviflorus ethanol extract (Hp EtOH) were tested for inhibitory activity.
The dichloromethane extract of L. molleoides was separated on a Sephadex LH-20 column using dichloromethane and methanol as solvents yielding a resorcinol derivatives fraction (López et al., 2005). The dried methanolic extract was suspended in water and partitioned successively with ethyl ether and ethyl acetate yielding their respective fractions and remaining water-soluble fraction. During the extraction a dark brown precipitate in the interphase was formed. The precipitate was filtered, air-dried, and tested for inhibitory activity as the Lithrea molleoides insoluble fraction of methanol extract (Lm insol). The resorcinol-enriched fraction (Lm res) was separated for inhibitory testing. Further details about extraction and fractionation on Supplementary File 1.
Since all extracts and fractions were dried, to carry out the experiments, dilutions of a portion of each extract or fraction were made to add them to the culture media. The water-based extracts were diluted with distilled water, and the organic-based extracts were diluted with DMSO. DMSO's final concentration in cell cultures used for both cytotoxicity and anti-chlamydial tests was never higher than 1%.
2.2. Qualitative 1H-NMR analysis
The dried dichloromethane extract (11,1 mg) of H. bonariensis was dissolved in 1 mL of deuterated methanol (CD3OD) (Sigma-Aldrich, St. Louis, MO, USA). The Proton Nuclear Magnetic Resonance (1H-NMR) spectra were recorded at 25 °C on a 600 MHz Bruker DMX-600 spectrometer (Bruker, Karlsruhe, Germany) operating at a proton NMR frequency of 600.13 MH equipped with TCI cryoprobe and Z-gradient system. CD3OD was used for internal lock purposes. For 1H-NMR spectra, 32768 data points were recorded, covering a spectral window of 9615 Hz. One hundred twenty-eight scans of a standard one-pulse sequence with 30° flip angle for excitation and presaturation during 1.5 s relaxation delay with an effective field of γB1 = 50 Hz for suppressing the residual H2O signal was employed. An exponential window function with a line-broadening factor of 0.3 Hz was applied before Fourier transformation. The resulting spectra were manually phased, and baseline corrected and referenced to internal residual CD3OD at 3.30 ppm. A library from then Section Plant Ecology and Metabolomics, Institute of Biology, Leiden University, Leiden, The Netherlands, was used to compare the dichloromethane extract signals.
2.3. Extracts, Chlamydiae strains, cell lines, and culture media
Seven extracts described before were assayed in this study: Lm Inf, Lm insol, Lm res, Hb Cl2CH2, Hb MeOH, Hb Inf, and Hp EtOH.
Dilutions of extracts were made in order to be added to the culture media.
Chlamydiae strains assayed in this study were: Chlamydia pneumoniae AR39 strain and five strains of Chlamydia trachomatis: the ATCC strain C. trachomatis L2/434/BU, serovar L2, two clinical isolates from neonatal conjunctivitis (strain OC7405, genotype K and strain OC15205, genotype E), a clinical isolate from endocervical infection (strain EC17807 serovar D) and a clinical isolate from male urethral infection (HU18208, genotype E). C. pneumoniae and C. trachomatis ATCC strains and LLC-MK2 cell line were kindly provided by Sezione di Microbiologia DMCSS, Universitá Degli Studi di Bologna, Italy. The clinical isolates were obtained at the laboratory during previous clinical studies and were identified as C. trachomatis and then genotyped by PCR-RFLP as previously described (Gallo Vaulet et al., 2010), and later confirmed by ompA sequencing.Cell culture was performed on LLC-MK2 and HeLa (kindly provided by the Instituto de Investigaciones biomédicas y Sida INBIRS, Universidad de Buenos Aires and CONICET, Argentina), in 10 % FCS supplemented minimal Eagle's medium (MEM) with 2 mM glutamine, 1.5 g/L sodium bicarbonate, 1 mM non-essential amino acids. Infection media were the same for culturing the cell line, but with the addition of 0.56 M glucose and 1 mg/mL cycloheximide. Chlamydial elementary bodies of each strain were purified from LLC-MK2 cells by saccharose gradient ultracentrifugation, suspended in culture medium supplemented with 30 % FCS, divided into 0.5 mL aliquots, and preserved as stock in liquid nitrogen. IFU per mL was determined for each stock by infecting LLC-MK2 confluent monolayers on shell vials with 10-fold serial dilutions of the stock and counting inclusions stained with immunofluorescent labeled antibodies as described below, after 48 h incubation for C. trachomatis biovar LGV, 72 h for C. trachomatis biovar TRIC, and five days for C. pneumoniae.
2.4. Cytotoxicity
Extracts cytotoxicity: A cytotoxicity assay was used using cell culture (Ruffa et al., 2002) for each extract in concentrations up to 600 ug/mL as previously described, using the MTT [3–(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay following the manufacturer instructions. The maximum non-cytotoxic concentrations were determined as the first concentration assayed without inhibition when cells showed no cytopathogenic effects.
Extracts toxicity over EB: direct cytotoxicity of the insoluble fraction of methanolic extract of L. molleoides (LM insol) and the H. bonariensis dichloromethane extract (Hb Cl2CH2) was tested over Chlamydia elementary bodies as described in Supplementary Material. The infectivity of both treated elementary bodies was compared to non-treated controls by IFU counts comparison.
Dichloromethane direct effect: concentrations from 0.3 % to 30 % of dichloromethane in culture medium were added onto cell monolayers for the whole five conditions inoculating plates with C. trachomatis L2/434/BU. The cytopathologic effect and the reduction in the inclusion number were compared to inoculated control by immunofluorescence staining—Further details about Cytotoxicity assays and their results on Supplementary File 2.
2.5. Antichlamydial activity assays for vegetal extracts
A methodology for antichlamydial activity testing was developed based on the technical considerations described for MIC assays. LLC-MK2 or HeLa cells were seeded onto 12 mm circle coverslips in 24 well plates and incubated at 37 °C and 5 % CO2 24 h to obtain confluent cell monolayers that were then used for Chlamydiae infection. The dilution of chlamydial EB's stock was calculated to inoculate the plates with an MOI of 0,5 IFU/cell for each chlamydial strain.
Five conditions were assayed for each extract and each strain by quadruplicate: A: 2 h pre-incubation of cell culture with the extract before chlamydial infection. This condition was performed to assess the ability of the extract to render the cells refractory to infection. B: was a combination of conditions A and C to assess any possible additive effect. C: only inoculation performed with the extract D: a combination of conditions C and E. E: only 48 hs incubation with the extract after inoculation step.
Every plate included mock-infected control wells, and extract-free Chlamydia infected control wells.
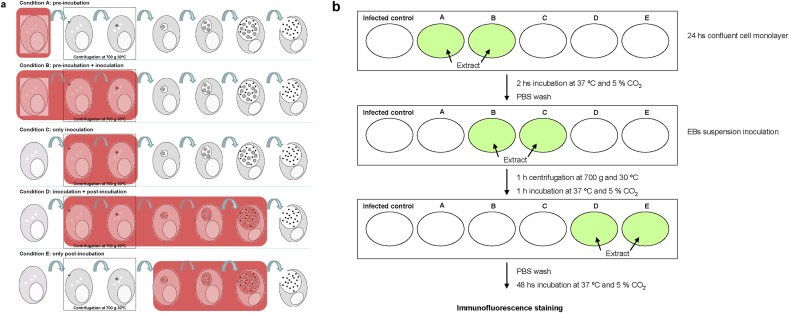
Inhibition on chlamydial life cycle steps assessed in each condition and how the assays were performed are illustrated in Figure 1a and 1b.
Figure 1.
(a) Scheme of the different stages of the in vitro chlamydial life cycle for which antichlamydial activity was evaluated by the methodology developed for this study. Black dots represent Chlamydia EBs. Shaded areas indicate the point of the chlamydial cycle where the extract is added to the culture media. Condition A: extracts were added over uninfected cells before Chlamydia inoculation and then removed before infection. Condition C: indicates the extract being in culture media during Chlamydia inoculation step (entry-stage and early events after entry). Condition E: extracts were added 2 h after Chlamydia inoculation step and remain in contact with culture for 48 h, in order to test their effect over Chlamydia exponential growth phase. (b) Cells were seeded onto circle coverslips in 24 well plates and incubated at 37 °C and 5 % CO2 24 h to obtain confluent cell monolayers. First line (pre incubation step): The culture medium was aspirated and fresh culture medium containing the assayed extract or fraction in a selected concentration according to the cytotoxicity assays was added to wells A and B, while fresh culture medium was added to the other wells. The plates were incubated at 37 °C and 5% CO2 for 2 h. The medium was aspirated, and every well was washed twice with sterile PBS. Second Line (inoculation step): Extract-free Chlamydia EBs suspension was inoculated into Control, A and E wells (Mock infected controls were also included in the experiment, not on the picture). EB suspension containing the assayed extract was inoculated to wells labeled as B, C and D. The plates were centrifugated at 700 xg and 30 °C and then were incubated at 37 °C and 5 % CO2 for one hour. Infection medium was aspirated, and the wells were washed twice with sterile PBS. Third Line: Fresh infection medium was added to infected and mock-infected controls and wells A, B and C while fresh infection medium containing the assayed extract was added to wells D and E. The plates were incubated at 37 °C and 5 % CO2 for 48 h. After immunofluorescence staining, 30 fields under 400 X magnification were counted for each coverslip and total count were estimated considering that a 12 mm diameter coverslip has 300 fields observed at a 400 X. When infection resulted in 1 inclusion/10 cells or less, the whole coverslip was examined under 400 X and all the inclusions were counted.
When organic-based extracts were assayed, DMSO was added to the culture and infection medium to maintain similar conditions to the culture on every well.
After immunofluorescence staining, thirty fields under 400 X magnification were counted for each coverslip, and the total count was estimated considering that a 12 mm diameter coverslip has 300 fields observed at a 400 X. When infection resulted in 1 inclusion/10 cells or less, the whole coverslip was examined under 400 X, and all the inclusions were counted. Results are expressed as a percentage of infection inhibition calculated comparing the number of inclusions forming units for each condition to infected non-treated controls. For C. pneumoniae, the reduction in the number of infected cells was considered given that this species can sometimes have more than one inclusion per cell.
2.6. Statistics
Basic statistics and unpaired t-test with a 95 % confidence interval and two-tailed P value were performed using GraphPad Prism 5. Figures were plot including standard error of the mean (mean ± SEM) for IFU counts and standard deviation (mean ± SD) for inhibition percentage, using the same software.
2.7. Ethical considerations
The C. trachomatis strains used for this study were isolated from clinical samples collected during previous studies conducted under ethical approval obtained from the Hospital de Clínicas “José de San Martín”, (Universidad de Buenos Aires) Ethical Committee.
3. Results
A final concentration of 100 ug/mL was chosen to test all the extracts on C. trachomatis and C. pneumoniae, except for the Lm res, which was used at a final concentration of 15 ug/mL (Cytotoxicity results available as Supplementary File 2).
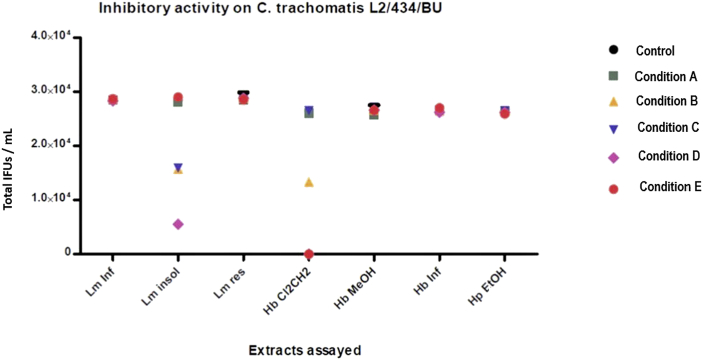
The first screening of the seven plant extracts was performed for each condition described above against C. trachomatis L2/434/Bu and OC7405 (clinical isolation from neonatal conjunctivitis) strains. Most of the assayed extracts showed no inhibitory activity in any condition tested; only Hb Cl2CH2 and Lm ins showed antichlamydial activity. None of the assayed extracts showed significant differences between the two tested strains, so Figure 2 shows the screening results for C. trachomatis L2/434/BU (results for C. trachomatis OC7405 on Figure S8 at Supplementary File 3).
Figure 2.
Screening of antichlamydial activity for L. molleoides, H. bonariensis, and H. parviflorus over C. trachomatis L2/434/BU, showing total IFU counts per mL (mean ± SEM, n = 4) for each condition assayed. Error bars are not visible to the scale used for Y-axis (for instance, Lm insol on condition A: 29130 ± 504.45). Lm Inf: L.molleoides infusion, Lm insol: L. molleoides insoluble fraction of methanol extract, Lm res: resorcinol-enriched fraction, Hb Cl2CH2: H.bonariensis dichloromethane extract, Hb MeOH: H. bonariensis methanol extract, Hb Inf: H. bonariensis infusion, Hp EtOH: H. parviflorus ethanol extract.
As Hb Cl2CH2 showed to be highly active, and it was the only one that showed inhibitory activity once the inoculation had been achieved by the EBs (condition E), further studies and assessments were thus made on this dichloromethane extract only.
As the fractions and extracts employed in this study could have extraction solvent residues, an inhibition assay using dichloromethane in the culture media was performed to ensure that this extract's activity was not due to residues. The test showed that the presence of up to 3 % dichloromethane in the inoculated cell cultures showed no inhibitory activity on the growth of C. trachomatis in all five conditions assayed. Therefore, no inhibition in the number of chlamydial inclusions in cell culture could be due to dichloromethane itself at the concentration of H. bonariensis extracts assayed. Also, no direct cytotoxic activity over Chlamydia elementary bodies was shown by neither of the two active extracts.
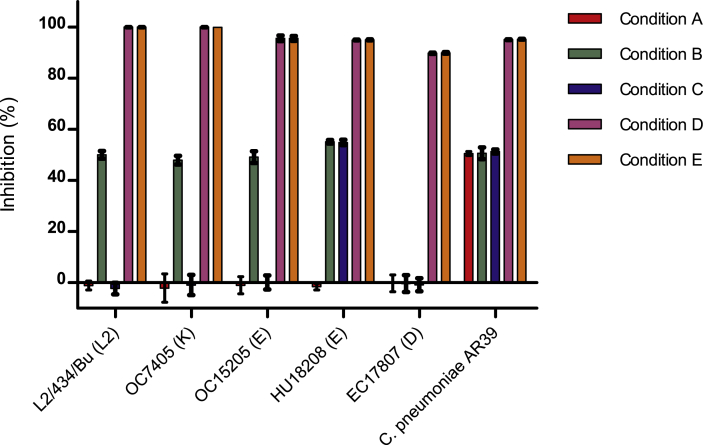
The H. bonariensis extract was tested against different strains of C. trachomatis, either LGV or TRIC strains, including clinical isolations obtained from genital and ocular infection sites. Results revealed no significant difference in the extract's inhibitory activity when left in contact with the culture during the full incubation period (48 h), independently of whether it had been present or not during the inoculation stage. Additionally, some variability in this extract's inhibitory capacity over certain strains was observed when in contact with cells and during the inoculation stage (condition B). Simultaneously, no inhibition occurred when the extract was only pre-incubated with cells, or only added during Chlamydia inoculation (conditions A and C alone). C. pneumoniae seemed to be more susceptible than C. trachomatis in any stage of the development cycle but consistently showed the maximum inhibition when the extracts remained in contact after inoculation. The detailed inhibition values are illustrated in Figure 3.
Figure 3.
Inhibition values of H. bonariensis dichloromethane extract for C. trachomatis ATCC strain, clinical isolates strains, and C. pneumoniae AR39 on every condition assayed. Condition A is preincubation with extract, Condition C is Chlamydiae inoculation with extract, Condition B equals A + C, Condition E is extract added after Chlamydiae inoculation, and Condition D equals C + E. C. trachomatis strain is followed by its genotype between parentheses. Inhibition is shown as mean%, error bars show standard deviation (t-test, compared to non-treated control).
The inhibitory capacity of the H. bonariensis extract was tested against C. trachomatis strains also on cell culture of HeLa cell line. The inhibition of chlamydial development slightly differed according to each cell line, but the higher inhibition effect was consistently observed for conditions D and E and LLC-MK2 cells. Tables with IFU counts for each cell line and condition tested are available as Supplementary File 4. This result showed that the inhibitory activity of H. bonariensis remains independent of the cell line used on the culture model.
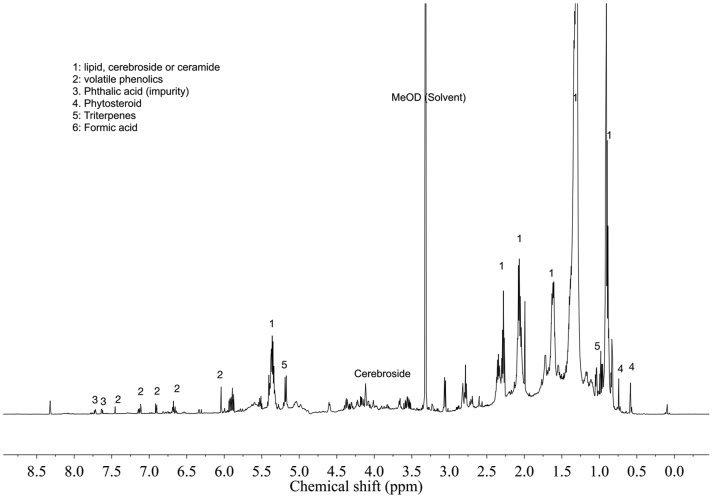
Given the interesting results obtained with the dichloromethane extract, it was analyzed by 1H-NMR to characterize the major potentially bioactive metabolites (Figure 4).
Figure 4.
Typical 1H-NMR spectrum (600 MHz, CH3OH-d4) of H. bonariensis dichlorometane extract in the range of δ 0.5 – δ 9.0. MeOD: Residual sCH3OH-d4.
The obtained spectra showed characteristic signals of the fatty acid moiety of lipids or cerebrosides (1), volatile phenolics (2), phthalates (Impurities) (3), H-18 of phytosterols (4), methyl triterpenes signals (5), formic acid (6), and glucose moiety of the cerebrosides (7). The signal (MeOD) is due to residual solvent CH3OH-d4. The identification of these metabolites was based on a metabolomic analysis of the dichloromethane extract of H. bonariensis, which allowed characterized the secondary metabolites present in it. For this purpose, a library of 1H-NMR spectra of the most common plant metabolites was used, so the major potential bioactive compounds were detected by comparing the signals obtained on the 1H-NMR spectra with the signals recorded in the library. The development of such an extensive database has contributed to the development of NMR into a fast, convenient, and effective metabolomic tool (Verpoorte et al., 2008) largely used to characterize and construct fingerprinting of extracts (Mattoli et al., 2016).
4. Discussion
A culture-based methodology to explore antichlamydial activity was designed and assessed in this study. Culture-based methods have been replaced mostly by molecular biology techniques over the years, given their simpleness and rapidness compared to chlamydiae cell culture. Focusing on the antichlamydial searching field, some high-throughput image-based screening methods (Osaka et al., 2012) or immunoassay methods (Tammela et al., 2004) have been developed to avoid time-consuming culture methods and to allow the screening of a high number of compounds at once. Nevertheless, to date, most studies still use culture-based methods as these are a more reliable tool to determine the actual effect of natural or synthetic compounds over the chlamydial growth (Bao et al., 2020; Kahru et al., 2017). However, the innovative feature of the methodology described in this study is that it allows identifying the stage at the chlamydial life cycle on which the assessed product is being active. As a screening method, it cannot be used as a high-throughput method. However, it offers valuable information in a single experiment in a cost-effective manner, allowing to focus on some possible mechanisms or targets and ruling out the others, and therefore redirect the further investigations.
Different extracts and their fractions of three native medicinal plants were evaluated in this study to assess their antichlamydial activity. The plants were selected based on their ethnomedical use and previous evidence of proven antimicrobial activity or similar effects. For instance, antibacterial and antiviral activity have been reported for the Lm inf (Kott et al., 1999; Penna et al., 2001); while L. molleoides extracts and fractions have shown nematicidal and antifungal activity (Valcic et al., 2002; Muschietti et al., 2005). H. parviflorus has been described to have a particular cyclotide (Broussalis et al., 2001) with proposed immunomodulating and antimicrobial activity (Gustafson et al., 2004). H. bonariensis leaves are used as an antiseptic for wounds and facial skin (Marzocca, 1997). In this study, the Hb Cl2CH2 showed the strongest antichlamydial activity among all the studied vegetal species.
Strains of C. trachomatis of biovar TRIC have been reported to exhibit different behavior regarding internalization routes or inclusion development, even having the same in vitro conditions (Taraktchoglou et al., 2001; Moelleken and Hegemann, 2008). Besides, differences in infectivity and productivity have been observed in cell cultures between different genotypes among biovar TRIC strains (Guseva et al., 2007; Dessus-Babus et al., 2008). Based on this evidence, C. trachomatis strains of the two biovars, with genotypes of high frequency (E and D) and low frequency (K), and different infection sites of origin, has been selected to perform the assays. All the strains selected were not resistant to commonly used antibiotics assayed (levofloxacin, azithromycin, tetracycline, doxycycline, and erythromycin), so the lack of inhibitory activity of most of the assayed extract was not due to known antibiotic resistance pathways (data available at Supplementary File 5).
The Hb Cl2CH2 showed a maximum level of inhibition on all tested C. trachomatis strain and C. pneumoniae, when added to the cell culture during all the incubation period (identified as conditions D and E in this study). This high activity could be due to the presence of one or more compounds within the extract that could interfere with the mechanisms of growth and development of the inclusion once the entry of the EBs into the host cell has been achieved. Similar results have been reported for a molecule that inhibits the Type III Secretion System (T3SS) of Yersinia that also inhibits the development after infection but showed no effect on the entry mechanism of the bacteria (Muschiol et al., 2009). More recently, some new antichlamydial agents have been designed by combining pharmacophores of C. trachomatis inhibitors with T3SS inhibitors and also showed high inhibition activity over the inclusion development (Sunduru et al., 2015). These results suggest a high inhibitory potential on both Chlamydiae species and justify efforts to characterize the tested extract's chemical composition to detect one or more bioactive compounds developed, in a long-term project, into a new antichlamydial drug.
The 1H-NMR analysis of the active fraction of H. bonariensis (Hb Cl2CH2) showed triterpenes, phenolics, and cerebrosides as main components. The signal corresponding to formic acid was considered an artifact that is sometimes generated during the extraction process, the storage of the extracts and, therefore, it is not considered a phytochemical compound present in the extract. Concerning phytosterols, they are a group of molecules derived from sterol, of low polarity, and therefore it is expected that they are found in the dichloromethane extract tested. Phytosterols are widely distributed in nature, their main reported activities are related to their cholesterol-lowering effect, and their anti-chlamydial activity has not been yet described. Oleanane-type triterpenoids and flavonoids have been reported on this plant species (Maulidiani et al., 2014), but there is no previous report of cerebrosides in H. bonariensis. However, cerebrosides from different Euphorbia species, E. peplis, and E. platyphyllos have shown antifungal and antituberculosis activity (Cateni et al., 2003, 2008).
The most frequent chemical nature of natural antichlamydial compounds studied so far is polyphenolic, such as the catechins and its derivatives, flavonoids, flavones, and gallates (reviewed by Brown et al., 2016). These compounds have shown a variety of inhibitory activity over C. trachomatis and C. pneumoniae, but often their toxicity has not been adequately assessed. Also, lipidic compounds, including terpenoids, proteinaceous compounds, and probiotics, have been studied as potential antichlamydial agents by different authors. Still, the inhibiting activity was low (50% at best) or was only demonstrated by pretreatment of EBs (Brown et al., 2016; Salin et al., 2010; Bergsson et al., 1998). On the other hand, sphingolipids, or glycolipids such as cerebrosides, are compound families that have been less studied regarding their activity over Chlamydiae. These are promising because they are involved in a variety of Chlamydia host cell interactions, so a natural origin analog could have antichlamydial activity by interference with any of these pathways (Saied et al., 2015).
Thus, further work will be done with the H. bonariensis active fraction to isolate and identify the metabolites responsible for the activity. Meanwhile, additional experiments on L. molleoides are being conducted to characterize the activity of pure compounds isolated from the insoluble fraction of the methanol extract. Both are strong candidates to develop new antichlamydial drugs that could address the need for new antimicrobials for treating chlamydial infections to avoid antimicrobial resistance among other bacterial species and treat chronic infections effectively.
The broad activity observed for H. bonariensis both on C. trachomatis and C. pneumoniae is promising. Given that it showed activity over the inclusion growing stage of the chlamydial life cycle, it could be useful as a base to develop a regular antimicrobial drug to treat acquired chlamydial infections. L. molleoides extract, on the other hand, showed inhibition during the EB entry stage, so it could be useful to develop a product to be used as a preventive to the infection, for example, as additive to condom lubricant. Further studies will be performed to reveal the mechanisms of inhibition and the chlamydicidal activity once the phytochemical studies to identify the active compound will be completed.
5. Conclusions
This manuscript describes an original culture methodology developed to assess antichlamydial activity over different Chlamydiae life cycle stages. As a result of this, extracts of Hydrocotyle bonariensis, an Argentinian medicinal plant, were shown to inhibit chlamydial growth in vitro for both Chlamydia trachomatis TRIC and LGV strains and Chlamydia pneumoniae. We consider that the methodology described is promising as a useful tool to assess new antichlamydial agents. Also, the high inhibitory activity of H. bonariensis is worthy of being further investigated in order to reveal the mechanisms of inhibition and the chlamydicidal activity once the phytochemical studies will be completed.
Declarations
Author contribution statement
Andrea Carolina Entrocassi, Alejandra Vanina Catalano: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Adriana Graciela Ouviña: Performed the experiments; Analyzed and interpreted the data.
Erica Georgina Wilson: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Paula Gladys López: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Marcelo Rodríguez Fermepin: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by the Universidad de Buenos Aires, Argentina (UBACyT grants number B049, B810, 20020130220017BA, and 20020170200222BA).
Data availability statement
Data included in article/supp. material/referenced in article.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors thank María Lucía Gallo Vaulet for her priceless help on the isolation of some of the clinical strains used in this study.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Alvesalo J., Vuorela H.J., Tammela P., Leinonen M., Saikku P., Vuorela P.M. Inhibitory effect of dietary phenolic compounds on Chlamydia pneumoniae in cell cultures. Biochem. Pharmacol. 2006;71:735–741. doi: 10.1016/j.bcp.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Bao X., Liu Z., Ni M. Synthesis and Assessment of 3-Substituted Phenazines as Novel Antichlamydial Agents. Med. Chem. 2020;16(3):413–421. doi: 10.2174/1573406415666190708145639. [DOI] [PubMed] [Google Scholar]
- Bergsson G., Arnfinnsson J., Karlsson S.M., Steingrímsson O., Thormar H. In vitro inactivation of Chlamydia trachomatis by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 1998;42(9):2290–2294. doi: 10.1128/aac.42.9.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojang E., Jafali J., Perreten V., Hart J., Harding-Esch E.M., Sillah A. Short-term increase in prevalence of nasopharyngeal carriage of macrolide-resistant Staphylococcus aureus following mass drug administration with azithromycin for trachoma control. BMC Microbiol. 2017;17:75. doi: 10.1186/s12866-017-0982-x. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussalis A.M., Göransson U., Coussio J.D., Ferraro G., Martino V., Claeson P. First cyclotide from Hybanthus (violaceae) Phytochemistry. 2001;58:47–51. doi: 10.1016/s0031-9422(01)00173-x. [DOI] [PubMed] [Google Scholar]
- Broussalis A., Clemente S., Ferraro G. Hybanthus parviflorus (violaceae): insecticidal activity of a South American plant. Crop Protect. 2010;29(9):953–956. September 2010. [Google Scholar]
- Brown M.A., Potroz M.G., Teh S.W., Cho N.J. Natural products for the treatment of Chlamydiaceae infections. Microorganisms. 2016;4:39. doi: 10.3390/microorganisms4040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cateni F., Zilic J., Falsone G., Scialino G., Banfi E. New cerebrosides from Euphorbia peplis L.: antimicrobial activity evaluation. Bioorg. Med. Chem. Lett. 2003;15(24):4345–4350. doi: 10.1016/j.bmcl.2003.09.044. 13. [DOI] [PubMed] [Google Scholar]
- Cateni F., Zilic J., Zacchigna M. Isolation and structure elucidation of cerebrosides from Euphorbia platyphyllos L. Sci. Pharm. 2008;76:451–469. [Google Scholar]
- CDC - Division of STD Prevention . STD Treatment Guidelines – Chlamydial Infections. 2015. National center for HIV/AIDS, viral hepatitis, STD, and TB prevention, centers for disease control and prevention.https://www.cdc.gov/std/tg2015/tg-2015-print.pdf (June 4, 2015) [Google Scholar]
- Dessus-Babus S., Moore C.G., Whittimore J.D., Wyrick P.B. Comparison of Chlamydia trachomatis serovar L2 growth in polarized genital epithelial cells grown in three-dimensional culture with non-polarized cells. Microb. Infect. 2008;10(5):563–570. doi: 10.1016/j.micinf.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo Vaulet M.L., Entrocassi A.C., Corominas A.I., Rodriguez Fermepin M. Distribution study of Chlamydia trachomatis genotypes in symptomatic patients in Buenos Aires, Argentina: association between genotype E and neonatal conjunctivitis. BMC Res. Notes. 2010;3:34. doi: 10.1186/1756-0500-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorzalczany S., López P., Acevedo C., Ferraro G. Anti-inflammatory effect of Lithraea molleoides extracts and isolated active compounds. J. Ethnopharmacol. 2011;6(3):994–998. doi: 10.1016/j.jep.2010.11.031. 133. [DOI] [PubMed] [Google Scholar]
- Guseva N.V., Dessus-Babus S., Moore C.G., Whittimore J.D., Wyrick P.B. Differences in Chlamydia trachomatis serovar E growth rate in polarized endometrial and endocervical epithelial cells grown in three-dimensional culture. Infect. Immun. 2007;75(2):553–564. doi: 10.1128/IAI.01517-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson K.R., McKee T.C., Bokesch H.R. Anti-HIVcyclotides. Curr. Protein Pept. Sci. 2004;5(5):331–340. doi: 10.2174/1389203043379468. [DOI] [PubMed] [Google Scholar]
- Hieronymus J. Boletín de la Academia Nacional de Ciencias en Córdoba; Tomo IV: 1882. Plantae Diaphoricae; pp. 323–324. [Google Scholar]
- Kahru E., Isojärvi J., Vuorela P., Hanski L., Fallarero A. Identification of privileged antichlamydial natural products by a ligand-based strategy. J. Nat. Prod. 2017;80:2602–2608. doi: 10.1021/acs.jnatprod.6b01052. [DOI] [PubMed] [Google Scholar]
- Kissinger P.J., White S., Manhart L.E., Schwebke J., Taylor S.N., Mena L., Khosropour C.M., Wilcox L., Schmidt N., Martin D.H. Azithromycin treatment failure for Chlamydia trachomatis among heterosexual men with nongonococcal urethritis. Sex. Transm. Dis. 2016;43(10):599–602. doi: 10.1097/OLQ.0000000000000489. 2016 Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhoff S.A., Hammerschlag M.R. Treatment of Chlamydial infections: 2014 update. Expet Opin. Pharmacother. 2015;16(2):205–212. doi: 10.1517/14656566.2015.999041. [DOI] [PubMed] [Google Scholar]
- Kott V., Barbini L., Cruañes M., Muñoz J.D., Vivot E., Cruañes J., Martino V., Ferraro G., Cavallaro L., Campos R. Antiviral activity in Argentine medicinal plants. J. Ethnopharmacol. 1999;64(1):79–84. doi: 10.1016/s0378-8741(98)00098-1. [DOI] [PubMed] [Google Scholar]
- Kuo C.C., Jackson L.A., Campbell L.A., Grayston J.T. Chlamydia pneumoniae (TWAR) Clin. Microbiol. Rev. 1995;8:451–461. doi: 10.1128/cmr.8.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López P., Ruffa M.J., Cavallaro L., Campos R., Martino and V., Ferraro G. 1,3-dihydroxy-5-(tridec-4',7'-dienyl) benzene: a new cytotoxic compound from Lithraea molleoides. Phytomedicine. 2005;12(1-2):108–111. doi: 10.1016/j.phymed.2003.07.013. 2005 Jan. [DOI] [PubMed] [Google Scholar]
- Marzocca A. Orientación Gráfica Editora; Buenos Aires: 1997. Vademecum de Malezas Medicinales de la Argentina. Indígenas y exóticas; pp. 225–226. [Google Scholar]
- Mattoli L., Burico M., Fodaroni G., Tamimi S., Bedont S., Traldi P., Stocchero M. New frontiers in pharmaceutical analysis: a metabolomic approach to check batch compliance of complex products based on natural substances. J. Pharmaceut. Biomed. Anal. 2016;126(2016):156–162. doi: 10.1016/j.jpba.2016.04.010. [DOI] [PubMed] [Google Scholar]
- Maulidiani, Abasa F., Khatib A., Shaaria K., Lajisa N.H. Chemical characterization and antioxidant activity of three medicinal Apiaceae species. Ind. Crop. Prod. 2014;55:238–247. [Google Scholar]
- Moelleken K., Hegemann J.H. The Chlamydia outer membrane protein OmcB is required for adhesion and exhibits biovar-specific differences in glycosaminoglycan binding. Mol. Microbiol. 2008;67(2):403–419. doi: 10.1111/j.1365-2958.2007.06050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadzadeh F., Dolatian M., Jorjani M., Afrakhteh M., Majd H.A., Abdi F., Pakzad R. Urogenital Chlamydia trachomatis treatment failure with azithromycin: a meta-analysis. Int. J. Reprod. Biomed. 2019;17(9):603–620. doi: 10.18502/ijrm.v17i9.5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morucci F., Lopez P., Miño J., Ferraro G., Gorzalczany S. Antinociceptive activity of aqueous extract and isolated compounds of Lithrea molleoides. J. Ethnopharmacol. 2012;13(2):401–406. doi: 10.1016/j.jep.2012.05.009. 142. [DOI] [PubMed] [Google Scholar]
- Morré S.A., Brule van den A.J., Rozendaal L., Boeke A.J., Voorhorst F.J., de Blok S., Meijer C.J. The natural course of asymptomatic Chlamydia trachomatis infections: 45% clearence and no development of clinical PID after one-year follow-up. Int. J. STD AIDS. 2002;13(2):12–18. doi: 10.1258/095646202762226092. [DOI] [PubMed] [Google Scholar]
- Muschietti L., Derita M., Sülsen V., de Dios Muñoz J., Ferraro G., Zacchino S., Martino V. In vitro antifungal assay of traditional Argentine medicinal plants. J. Ethnopharmacol. 2005;102:233–238. doi: 10.1016/j.jep.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Muschiol S., Normark S., Henriques-Normark B., Subtil A. Small molecule inhibitors of the Yersinia type III secretion system impair the development of Chlamydia after entry into host cells. BMC Microbiol. 2009;21(9):75. doi: 10.1186/1471-2180-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaka I., Hills J.M., Kieweg S.L., Shinogle H.E., Moore D.S., Hefty P.S. An automated image-based method for rapid analysis of Chlamydia infection as a tool for screening antichlamydial agents. Antimicrob. Agents Chemother. 2012;56(8):4184–4188. doi: 10.1128/AAC.00427-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penna C., Marino S., Vivot E., Cruañes M.C., de D Muñoz J., Cruañes J., Ferraro G., Gutkind G., Martino V. Antimicrobial activity of Argentine plants used in the treatment of infectious diseases. Isolation of active compounds from Sebastiania brasiliensis. J. Ethnopharmacol. 2001;77(1):37–40. doi: 10.1016/s0378-8741(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Petyaev I.M., Zigangirova N.A., Morgunova E.Y., Kyle N.H., Fedina E.D., Bashmakov Y.K. Resveratrol inhibits propagation of Chlamydia trachomatis in McCoy cells. BioMed Res. Int. 2017 doi: 10.1155/2017/4064071. Article ID 4064071, 7 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffa M.J., Ferraro G., Wagner M.L., Calcagno M.L., Campos R.H., Cavallaro L. Cytotoxic effect of Argentine medicinal plant extracts on human hepatocellular carcinoma cell line. J. Ethnopharmacol. 2002;79:335–339. doi: 10.1016/s0378-8741(01)00400-7. [DOI] [PubMed] [Google Scholar]
- Saied E.M., Banhart S., Bürkle S.E., Heuer D., Arenz C. A series of ceramide analogs modified at the 1-position with potent activity against the intracellular growth of Chlamydia trachomatis. Future Med. Chem. 2015;7(15):1971–1980. doi: 10.4155/fmc.15.126. [DOI] [PubMed] [Google Scholar]
- Salin O., Alakurtti S., Pohjala L., Siiskonen A., Maass V., Maass M., Yli-Kauhaluoma J., Vuorela P. Inhibitory effect of the natural product betulin and its derivatives against the intracellular bacterium Chlamydia penumoniae. Biochem. Pharmacol. 2010;80(8):1141–1151. doi: 10.1016/j.bcp.2010.06.051. 2010. [DOI] [PubMed] [Google Scholar]
- Sherrard J., Jensen J.S. Chlamydia treatment failure after repeat courses of azithromycin and doxycycline. Int. J. STD AIDS. 2019;30(10):1025–1027. doi: 10.1177/0956462419857303. 2019 Sep. [DOI] [PubMed] [Google Scholar]
- Sunduru N., Salin O., Gylfe A., Elofsson M. Design, synthesis and evaluation of novel polypharmacological antichlamydial agents. Eur. J. Med. Chem. 2015;101:595–603. doi: 10.1016/j.ejmech.2015.07.019. [DOI] [PubMed] [Google Scholar]
- Tammela P., Alvesalo J., Riihimäki L. Development and validation of a time-resolved fluorometric immunoassay for screening of antichlamydial activity using a genus-specific europium-conjugated antibody. Anal. Biochem. 2004;333(1):39–48. doi: 10.1016/j.ab.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Taraktchoglou M., Pacey A.A., Turnbull J.E., Eley A. Infectivity of Chlamydia trachomatis serovar LGV but not E is dependent on host cell heparan sulfate. Infect. Immun. 2001;69:968–976. doi: 10.1128/IAI.69.2.968-976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toursarkissian M. Hemisferio Sur; Buenos aires: 1980. Plantas Medicinales de la Argentina; p. 131. [Google Scholar]
- Toursarkissian M. Hemisferio Sur; Buenos Aires: 1980. Plantas Medicinales de la Argentina; p. 4. [Google Scholar]
- Valcic S., Wachter G.A., Eppler C.M., Timmermann B.N. Nematicidal alkylene resorcinols from Lithraea molleoides. J. Nat. Prod. 2002;65:1270–1273. doi: 10.1021/np020068n. [DOI] [PubMed] [Google Scholar]
- Verpoorte R., Choi Y.H., Mustafa N.R., Kim H.K. Metabolomics: back to basics. Phytochemistry Rev. 2008;7:525–537. [Google Scholar]
- Webley W.C., Hahn D.L. Infection-mediated asthma: etiology, mechanisms and treatment options, with focus on Chlamydia pneumoniae and macrolides. Respir. Res. 2017;18:98. doi: 10.1186/s12931-017-0584-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . WHO Press; Geneva, Switzerland: 2016. WHO Guidelines for the Treatment of Chlamydia trachomatis.http://apps.who.int/iris/bitstream/handle/10665/246165/9789241549714-eng.pdf;jsessionid=0D78E889D4F06BA93CE67E646173E1FE?sequence=1 [PubMed] [Google Scholar]
- World Health Organization . WHO Press; Geneva, Switzerland: 2014. Traditional Medicine Strategy 2014-2023.https://www.who.int/medicines/publications/traditional/trm_strategy14_23/en/ [Google Scholar]
- Wyrick P.B., Knight S.T. Pre-exposure of infected human endometrial epithelial cells to penicillin in vitro renders Chlamydia trachomatis refractory to azithromycin. J. Antimicrob. Chemother. 2004;54:79–85. doi: 10.1093/jac/dkh283. 2004. [DOI] [PubMed] [Google Scholar]
- Yamazaki T., Kishimoto T., Shiga S., Sato K., Hagiwara T., Inoue M., Sasaki N., Ouchi K., Hara Y. Biosynthesized tea polyphenols inactivate Chlamydia trachomatis in vitro. Antimicrob. Agents Chemother. 2005;9(6):2501–2503. doi: 10.1128/AAC.49.6.2501-2503.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuloaga F., Morrone O. Monographs in Systematic Botany from the Missouri Botanical Garden. Vol. 74. Missouri Botanical Garden Press; St. Louis: 1999. Catálogo de las plantas vasculares de la República Argentina II. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.