Abstract
Photooxidation is one of the causes of quality deterioration in food. An antioxidant or singlet oxygen quencher is urgently needed to prevent photooxidation. γ-Oryzanol was recognized as a naturally present antioxidant in rice bran products. This research aimed to calculate the singlet oxygen quenching rate and its mechanism of γ-oryzanol to evaluate the potency of γ-oryzanol as singlet oxygen quencher. A series of linoleic acid (50 and 100 mM) or γ-oryzanol (0.7 and 1.5 mM) were prepared separately in ethanol: chloroform (96:4, v/v) containing 25 ppm of erythrosine. High-Performance Liquid Chromatography quantified the degradation of γ-oryzanol. Meanwhile, Gas Chromatography determined the changes in linoleic acid content during photooxidation. The singlet oxygen quenching rate was calculated by steady-state. The singlet oxygen quenching rate of γ-oryzanol was 3.04 × 106/M/s by physical and chemical quenching mechanism. Photooxidation caused the declined of γ-oryzanol by 0.1421 mM/h. Based on singlet oxygen quenching rate calculation, it suggests that γ-oryzanol can perform as a singlet oxygen quencher with slightly dominated by physical quenching mechanism (52.28%). The rest it performed via a chemical quenching mechanism.
Keywords: Photooxidation, Singlet oxygen quenching, γ-Oryzanol, Rice bran oil, Nanoemulsion
Photooxidation, Singlet oxygen quenching, γ-Oryzanol, Rice bran oil, Nanoemulsion.
1. Introduction
Oxidation affected food quality especially during processing and prolonged storage even at shallow temperature. The oxidation processes such as autooxidation or photooxidation, or the combination of both can cause the degradation of the food quality. Photooxidation can induce not only off flavor, but also color degradation, loss of nutrients, and potentially yield unhealthy substances. The autooxidation process needs an initiator to make radical lipids, and then it will be oxidized. Erythrosine, methylene blue, riboflavin and chlorophyll were well-known as photo-sensitizer. Erythrosine has been known can act as an effective photo-sensitizer, either in photooxidation of soybean oil in acetone or in aqueous food models containing vitamin C (Yang et al., 2002; Yang and Min, 2009; Ariviani et al., 2011). Photooxidation occurred by involving ground state triplet oxygen, photo-sensitizer, and the presence of light to produce singlet oxygen. This singlet oxygen subsequently could react with electron-rich compounds such as mono- and poly-unsaturated fatty acids, vitamins (riboflavin, vitamin D, ascorbic acid), amino acids (methionine, tyrosine and tryptophan) and produce radical compounds (Huang et al., 2004; Yettela and Min, 2008; Yang and Min, 2009). These radical compounds could further initiate autooxidation chain reactions. Therefore, in order to prevent photooxidation on the electron-rich compounds in foods, the presence of a singlet oxygen quencher or antioxidant is needed.
Ascorbic acid, α-tocopherol, and β-carotene are the most commonly used as singlet oxygen quencher by singlet oxygen or excited triplet sensitizer quenching mechanism. Singlet oxygen quenching rate of these compounds were 1.16, 4.1 and 730 × 107/M/s, respectively (Yang et al., 2002; Yettela and Min, 2008). The rate and mechanism of singlet oxygen quenching can indicate the effectiveness of antioxidants to prevent photooxidation in foods.
In this research, we focused on the analysis of γ-oryzanol as singlet oxygen quencher. γ-Oryzanol is a specific antioxidant that only be found in rice bran products, especially rice bran oil. Because rice bran oil contained relatively high (1.4% w/w) of γ-oryzanol (Dhavamani et al., 2014), it was interesting to investigate the kinetic of singlet oxygen quenching rate by γ-oryzanol to prevent photooxidation in foods. γ-Oryzanol is a mixture of triterpene alcohol and sterol ferulates (Lu et al., 2014). It is water-insoluble components and can donor one hydrogen from its phenolic group (Kochhar, 2000; Kumar and Pruthi, 2014; Zhong et al., 2017). Xu and Godber found that γ-oryzanol is a mixture of at least ten compounds. There are Δ7-stigmastenyl ferulate, stigmasteryl ferulate, cycloartenyl ferulate, 24-methylenecycloartanyl ferulate, Δ7-campestenyl ferulate, campesteryl ferulate, Δ7-sitostenyl ferulate, sitosteryl ferulate, compestanyl ferulate, and sitostanyl ferulate. Cycloartenyl ferulate, 24-methylenecycloartanyl ferulate, and campesteryl ferulate are the main components of γ-oryzanol (Xu and Godber, 1999; Dhavamani et al., 2014). The principle of singlet oxygen quenching rate involves quantification of the oxidation parameter on the oxidized substances in a model system after adding a singlet oxygen quencher or antioxidant. Then, the kinetic study can be determined by the steady-state equation (Min and Boff, 2002).
2. Materials and methods
2.1. Materials
Linoleic acid, erythrosine b, ethanol, chloroform, methanol, ammonium thiocyanate, ferrous sulphate, barium chloride were analytical grade (Merck, Germany) and γ-Oryzanol standard for HPLC (Sigma-Aldrich, Germany). Acetonitrile, methanol and 2-propanol were gradient grade for liquid chromatography; BF3 in methanol (14%) for synthesis, n-hexane GC grade (Merck, Germany). Tween 80 was analytical grade (Merck, Germany).
2.2. Methods
2.2.1. Effect of light and sensitizer on photooxidation of linoleic acid and γ-oryzanol
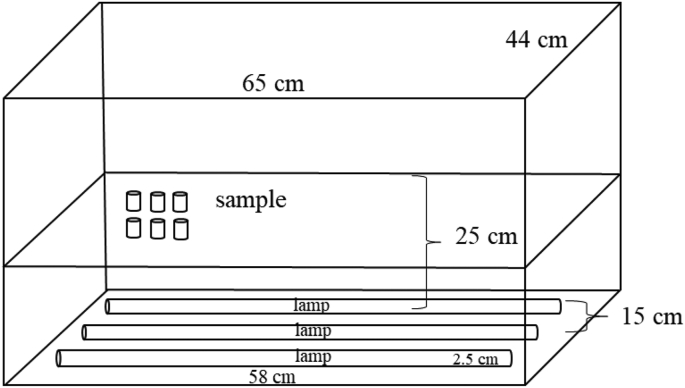
The sample solutions containing γ-oryzanol (0.7 and 1.5 mM) or linoleic acid (50 and 100 mM) and erythrosine 25 ppm was prepared in ethanol:chloroform (96:4, v/v). Chloroform was used to increase the solubility of γ-oryzanol. The 5 mL of the sample were filled into 10 mL vials. The vials were closed tightly. The ready sample in vials were put in a wooden box containing lamps and irradiated with approximately 3400 lux of light intensity, the dominant wavelength of 450–490 nm, for up to 4 h at room temperature (Figure 1). As controls, the sample solutions containing either γ-oryzanol (1.5 mM) or linoleic acid (100 mM) with or without erythrosine 25 ppm and under light or in the dark were also separately prepared. γ-Oryzanol contents were analyzed by High Performance Liquid Chromatography every hour (Sakunpak et al., 2014). Aliquots of samples were diluted in isopropanol before analysis. While linoleic acid contents were analyzed by Gas Chromatography. Peroxide values (Shantha and Decker, 1994), anisidine values (AOCS, 2004 Cd 18–90) and total oxidation (TOTOX) values were also measured. Experiments were done in duplicate.
Figure 1.
The illustration of light storage box. The lamps were Philips Lifemax (TLD 18W/840 Cool White, TIS 956–2533|TIS 236–2533).
2.2.2. Total of singlet oxygen quenching rate determination (kr + kq)
Samples of 0.03 M linoleic acid in ethanol with 25 ppm erythrosine were prepared. The duplicated samples were prepared and placed under light of approximately 3400 lux at room temperature. This radiation process was run for up to 4 h. The effect of addition of 6 ppm and 12 ppm β-carotene were also tested in photooxidation of 0.03 M linoleic acid containing 25 ppm of erythrosine in ethanol and stored under light for 4 h. Peroxide value of samples were measured by using Shantha and Decker (1994) method.
The singlet oxygen quenching rate constant by γ-oryzanol was measured according to (Suhendra, 2014) with slight modification. A 5 mL in 10 mL vial of γ-oryzanol (0–0.67 mM) containing linoleic acid (0.01–0.04 M) and 25 ppm of erythrosine was prepared in ethanol. The vials were closed tightly. The commercial antioxidants such as β-carotene or t-butylhydroquinone (TBHQ) were also employed for comparison. β-carotene (0–0.02 mM) and t-butylhydroquinone (TBHQ) (0–1.2 mM) were used as a natural and synthetic antioxidant for comparing with γ–oryzanol itself. The samples were put in a wooden storage box containing lamps and irradiated with 3400 lux of light for up to 2 h at room temperature. Peroxide value analysis (Shantha and Decker, 1994) were done after 2 h of illumination. Experiments were done in duplicate. The total rate constant of singlet oxygen quenching calculation according to (Lee et al., 1997).
2.2.3. Measurement of singlet oxygen quenching mechanism by γ-oryzanol
This section was determined based on previous work described in section 2.2.2. The 5 mL samples of γ-oryzanol (1.5 mM) and linoleic acid (100 mM) in ethanol:chloroform (96:4, v/v) were separately prepared in 10 mL vials. The samples were photo-oxidized by combination of erythrosine 25 ppm as sensitizer and stored under light (approximately 3400 lux) up to 4 h at room temperature. The decrease of linoleic acid and γ-oryzanol content were analyzed every hour. The calculation of singlet oxygen quenching mechanism by γ-oryzanol will be explained in the results and discussion section.
2.2.4. Analysis of linoleic acid
Sample preparation for linoleic acid analysis according to (Park and Goins, 1994) by slight modification. Samples (100 μL), 100 μL of dichloromethane and 1 mL of 0.5 N NaOH in methanol were poured into screw tubes. The test tubes were flushed by nitrogen gas for 30 s before were heated by water-bath at 90 °C for 10 min followed cooling down at ambient temperature. One mL of BF3 in methanol (14%) was added. After nitrogen flushing for 30 s, the test tubes were heated again at 90 °C for 10 min and cooled down. One mL of distilled water and 500 μL of n-hexane GC Grade were added. The test tubes were vortexed for 1 min before centrifugation at 1750 rpm for 5 min the upper layer was injected into Gas Chromatography (GC 2010 Plus, Shimadzu, Japan) provided with a flame ionization detector. The injector temperature was 240 °C. The oven temperature was set with 150 °C as an initial temperature, then the temperature was increased at rate 8 °C/min to achieve 200 °C for 30 min. Then, at the same rate, the temperature was increased to achieve 240 °C for 80 min. The column was used was a RTX-Wax (30 m × 0.25 mm; 0.5 μm). As the carrier gas, we used helium at 0.8 ml/min. A calibration curve was constructed to quantify of linoleic acid content in each sample.
2.2.5. Determination of γ-oryzanol
γ-Oryzanol in rice bran oils and γ-oryzanol-photooxidized (from 2.2.1 section) were quantified by High Performance Liquid Chromatography. The assay system was consisted of an isocratic pump (LC-20 AD, Shimadzu, Japan), a diode array detector (SPD-M20A, Shimadzu, Japan) and a column (Shim-Pack GIST, 5 μm C18; 4.6 × 150 mm, Shimadzu, Japan). This procedure was performed according to Sakunpak et al. (2014). Samples (1 mL) were diluted in 5 mL of isopropanol before analysis. For measurement of γ-oryzanol in rice bran oil, samples (0.5 gr) were diluted into 10 mL in isopropanol. All samples were filtered using nylon filter membrane. Acetonitrile:methanol (60:40, v/v) were used as mobile phase at a 0.8 mL/min. The temperature was set at 28 °C and the detection wavelength was set at 325 nm. The injection volume of 10–40 μL. Since γ-oryzanol is a combination of some components, there were four peaks in our standard. Peak 1–4 were cycloartenyl ferulate, 24-methylenecycloartanyl ferulate, campesteryl ferulate and β-sitosteryl ferulate, respectively. Calibration curves were constructed to quantify the amount of each component. Quantification of γ-oryzanol is calculated from the total of cycloartenyl ferulate, 24-methylenecycloartanyl ferulate, campesteryl ferulate and β-sitosteryl ferulate content, respectively, in every sample. The linear regression equation, limit of detection and limit of quantification is shown in Table 1.
Table 1.
The linear regression, limit of detection (LOD) and limit of quantification of γ-oryzanol analysis.
| No. | Compound | Linear regression equation | R2 | LOD (ppm) | LOQ (ppm) |
|---|---|---|---|---|---|
| 1. | Cycloartenyl ferulate | Y = 22453x – 26691 | 0.9971 | 5.289 | 17.63 |
| 2. | 24-methylene cycloartanyl ferulate | Y = 22435x – 34793 | 0.9970 | 7.034 | 23.44 |
| 3. | Campesteryl ferulate | Y = 22784x – 19860 | 0.9971 | 5.529 | 18.43 |
| 4. | β-sitosteryl ferulate | Y = 22453x – 7943.4 | 0.9972 | 1.617 | 5.39 |
2.2.6. Statistical analysis
The duplicate experiments were done. Regression analysis with Microsoft Excel 2013 and SPSS Statistics 24 were used to analyze the data.
3. Results and discussion
3.1. Effect of light and sensitizer on photooxidation of linoleic acid and γ-oryzanol
-
a.
Effect of light and sensitizer on linoleic acid photooxidation
The declining rate of linoleic acid at 50 and 100 mM as initial concentrations, stored under light or dark, with or without erythrosine as sensitizer, are shown in Table 2. The high degradation of linoleic acid and its oxidation products parameters was achieved by the samples containing erythrosine and held under light. Meanwhille, these parameters were relatively same during the exposure time when the samples were placed in the dark or without erythrosine. It suggested that a combination of erythrosine as sensitizer and light effectively induced photooxidation process and singlet oxygen was formed. When erythrosine got energy from light, it became a singlet erythrosine excited. By intersystem crossing, the triplet erythrosine excited can be produced. Triplet oxygen can react with triplet erythrosine excited to launch singlet oxygen.
Table 2.
The regression equation of linoleic acid photooxidation at different conditions.
| No. | Linoleic acid concentration | Eryhtrosine 25 ppm | Stored under light | Regression equation | R2 | P-value | Statistical analysis | Decreasing rate (mM/hour) |
|---|---|---|---|---|---|---|---|---|
| 1. | 100 mM | Y = −5.2858x + 103.48 | 0.905 | 0.013 | Slope is significantly different from 0 (p < 0.05) | 5.2858 | ||
| 2. | 100 mM | – | Y = −1.1518x + 104.54 | 0.2823 | 0.3568 | Slope ≈0 (p > 0.05) | ≈0 | |
| 3. | 100 mM | – | Y = −1.9391x + 111.75 | 0.2359 | 0.4069 | Slope ≈0 (p > 0.05) | ≈0 | |
| 4. | 50 mM | Y = −1.064x + 47.682 | 0.7721 | 0.0498 | Slope is significantly different from 0 (p < 0.1) | 1.064 |
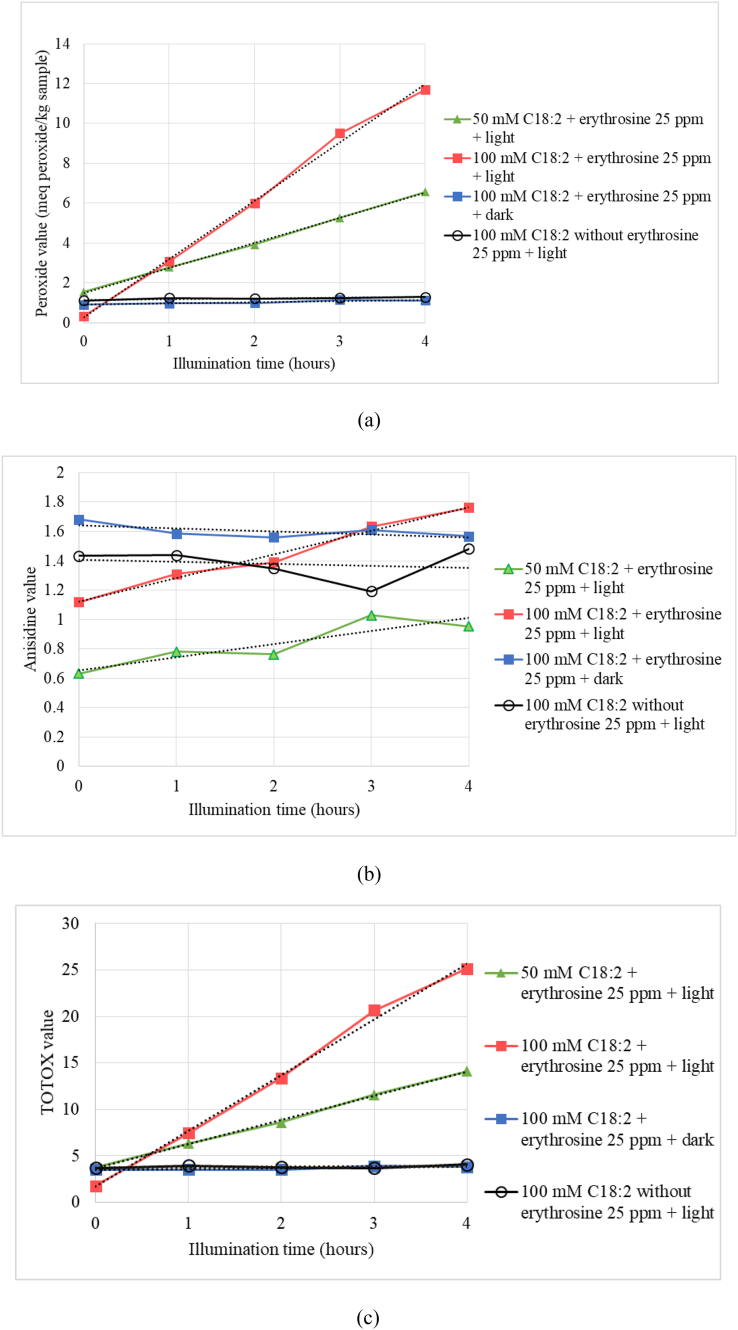
Moreover, singlet oxygen can react with linoleic acid or other target compounds, and the oxidation product formed (Min and Boff, 2002). Singlet oxygen and linoleic acid's reaction produced conjugated and non-conjugated hydroperoxide without radical lipid (Choe and Min, 2005). Therefore, linoleic acid content decreased during the illumination time. This degradation rate was followed by concentration-dependent. When 50 mM linoleic acid was initial concentration, the declining rate was 1.064 mM/h. Meanwhile, the declining rate of 100 mM linoleic acid as initial concentration up to 5.3 mM/h. Peroxide, anisidine, and total oxidation value analysis were correlated with this result. Since linoleic acid is an unsaturated fatty acid, it will be the singlet oxygen's target. Thus, the more linoleic acid, the photooxidation by singlet oxygen will be more intense. Therefore the oxidation product was formed. In this section, linoleic acid oxidation product parameters were shown by peroxide, anisidine, and total oxidation value analysis (Figure 2). These parameters were significantly increasing, while the linoleic acid content was declining during light exposure for up to 4 h.
-
b.
Effect of light and sensitizer on photooxidation of γ-oryzanol
Figure 2.
Three parameters of photo-oxidation products of linoleic acid under illumination at 3400 lux: peroxide value (a), anisidine value (b) and TOTOX value (c).
Rice bran and its derivatives containing γ-oryzanol. Rice bran oil is rich in γ-oryzanol with a range 111.7–254.8 mg/100 g oil (data not shown). Based on the previous studies, the amount of γ-oryzanol varying from 9 to 2648 mg/100 g of rice bran oil, depending on oil processing conditions (Krishna et al., 2001; Pestana et al., 2008; Dhavamani et al., 2014; Lu et al., 2014; Cuevas et al., 2017; Pokkanta et al., 2019).
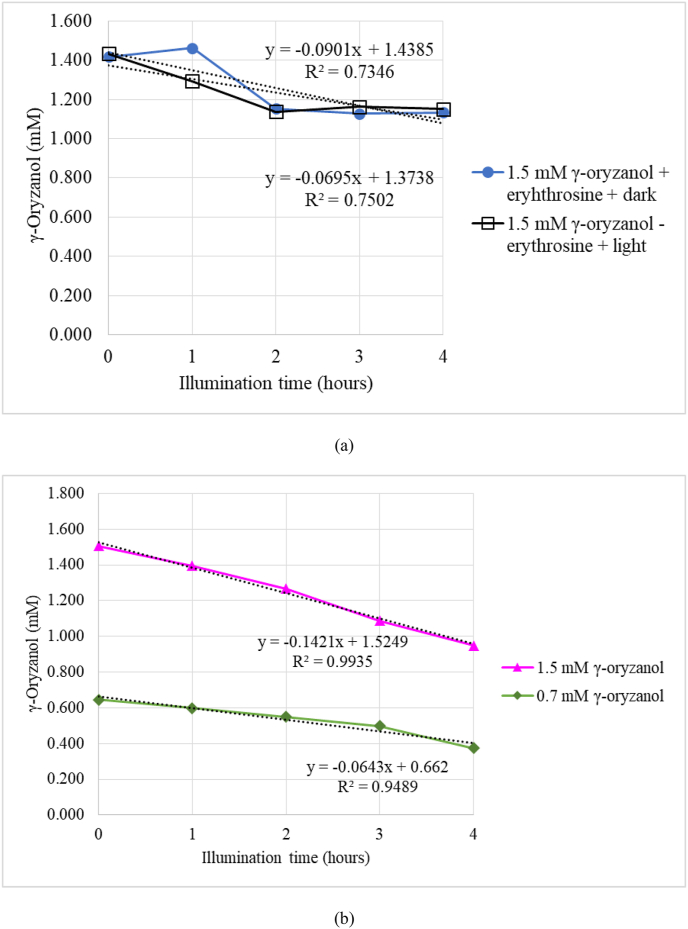
The changes of γ-oryzanol content with or without light and sensitizer are shown in Figure 3a and b samples stored in dark or without sensitizer did not significantly decrease γ-oryzanol content (p > 0.05). Meanwhile, the significant degradation of γ-oryzanol occurred when 0.7 and 1.5 mM of γ-oryzanol held under light and sensitizer (p < 0.05). When 1.5 mM of γ-oryzanol was used as initial concentration, the degradation rate was 0.1421 mM/h. Meanwhile, when the lower initial concentration of γ-oryzanol was 0.7 mM, the degradation rate was 0.0643 mM/h. Therefore, photooxidation can effectively degrade γ-oryzanol since singlet oxygen was formed. This result was similar to the previous section. γ-Oryzanol has ferulic moiety that might be targeted by singlet oxygen. To the best of our knowledge, this is the first report of degradation γ-oryzanol by photooxidation induced by erythrosine as a sensitizer.
Figure 3.
Effect of light 3400 lux and sensitizer (a) and initial concentration (b) on photooxidation of γ-oryzanol at room temperature.
3.2. Total of singlet oxygen quenching rate determination (kr + kq)
-
a.
Effect of γ-oryzanol or β-carotene on photooxidation of linoleic acid
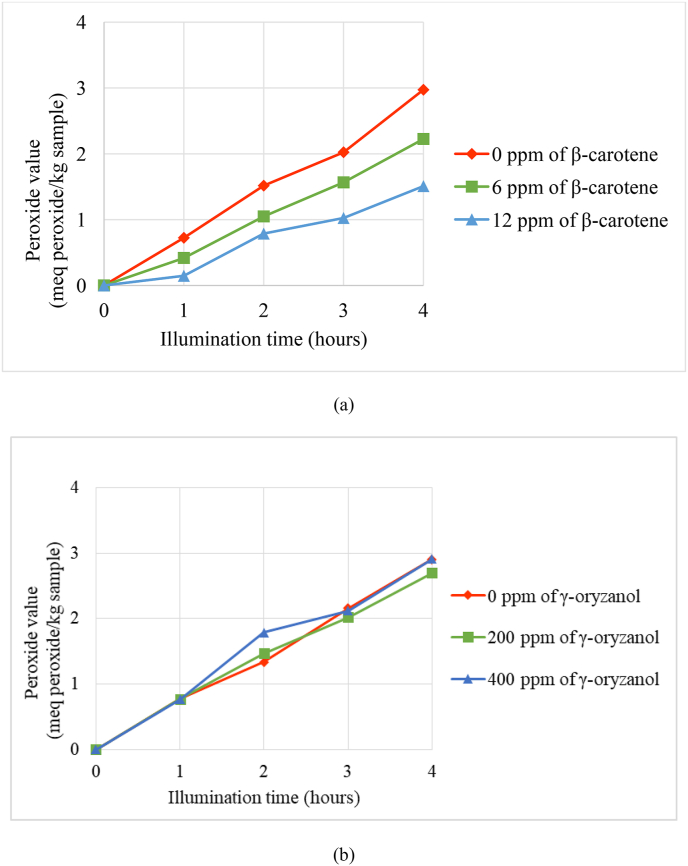
The quencher or antioxidant is urgently needed to prevent oxidation by singlet oxygen. In this experiment, we used β-carotene to prevent linoleic acid photooxidation. The peroxide value changes in samples containing 0.03 M linoleic acid and 25 ppm erythrosine as sensitizer with or without β-carotene stored under the light are shown in Figure 4. By using 6 and 12 ppm of β-carotene, the increase in peroxide value can be inhibited. β-carotene was known as an effective singlet oxygen quencher (Yang et al., 2002; Nishida et al., 2007; Ouchi et al., 2010). It can quench singlet oxygen by physical and chemical quenching mechanism. Meanwhile, γ-oryzanol less effective to prevent photooxidation in linoleic acid than β-carotene (Figure 4).
-
b.
Measurement of total singlet oxygen quenching rate constant
Figure 4.
Effect of β-carotene (0–12 ppm) (a) and γ–oryzanol (0–400 ppm) (b) on photooxidation of 0.03 M linoleic acid containing erythrosine sensitizer (25 ppm) in ethanol under light 3400 lux up to 4 h at room temperature.
The rates constants of singlet oxygen quenching by γ-oryzanol, β-carotene, and t-butylhydroquinone (TBHQ) were determined using steady-state kinetic approximation. In this system, oxidation of linoleic acid in ethanol via photooxidation involving erythrosine as sensitizer induced singlet oxygen formation. It can react with linoleic acid then the oxidized product of lipid hydroperoxide formed. The appearance of oxidized product could be prevented by singlet oxygen quencher or antioxidant. If γ-oryzanol had capability as a singlet oxygen quencher, the steady-state calculation (Eq. (1)), which was derived in previous literature (Lee et al., 1997).
| {d[AO2]/dt}−1 = K−1 {1+(kq[Q] + kox-Q[Q] + kd) / kr[A] | (1) |
K is the production rate of singlet oxygen; AO2 is oxidation product of linoleic acid or lipid hydroperoxide; kd is decomposition rate of singlet oxygen in solvent (we used ethanol); kr is singlet oxygen with linoleic acid-reaction rate; A is linoleic acid; kq and kox-Q are constant of reaction rate consists of physical and chemical singlet oxygen quenching by quencher, respectively. Q is quencher (γ-oryzanol, β-carotene and t-butylhydroquinone (TBHQ)).
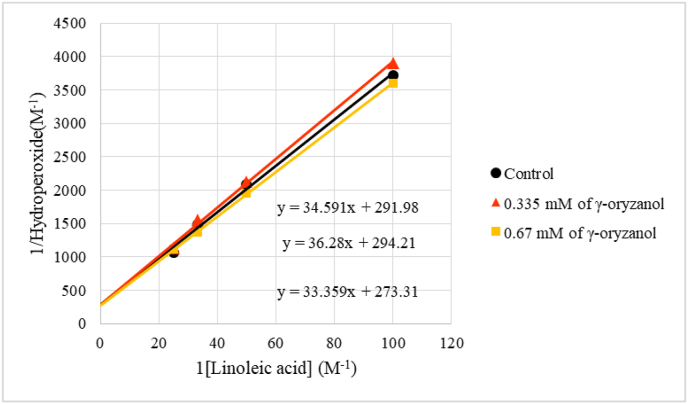
K−1 and K−1 {kd + kq[Q] + kox-Q[Q]/kr} are the intercept and slope of [AO2]−1 vs. [A]−1 ‘s plot at several levels of quencher (Q), respectively. The intercepts are unassociated of the concentration of quencher and vice versa for the slope. The [AO2]−1 vs. [A]−1's plot for individual concentrations of γ-oryzanol is figured in Figure 5. The intercepts were relatively same for individual concentration of γ-oryzanol, indicating that γ-oryzanol could be singlet oxygen quencher.
| (2) |
Figure 5.
The plot of [AO2]−1 or [lipid hydroperoxide]−1vs. [A]−1 or [linoleic acid]−1.
The linear regression equation for the [AO2]−1 vs. [A]−1 ‘s plot in the absence of γ-oryzanol was y = 34.66× + 286.33, then the slope/intercept ratio via Eq. (2) was 0.12. This value as equal as kd/kr. According to Wilkinson et al. (1995), kd, the decomposition rate of singlet oxygen in ethanol was 8.3 × 104/s. Therefore, kr or singlet oxygen with linoleic acid reactin rate was kd/slope = 7.0 × 105/M/s.
| (kq + kox-Q) = kr × slope | (3) |
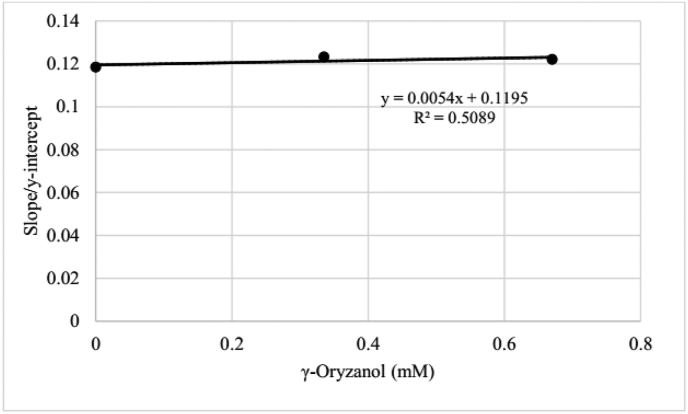
The total rate of singlet oxygen quenching by γ-oryzanol could be calculated by kr × slope (Eq. (3)). The slope/intercept ratios from Figure 5 were plotted vs concentration of γ-oryzanol into Figure 6. The slope from this regression line was calculated with kr to be total rate of singlet oxygen quenching by γ-oryzanol. Therefore, the total rate of singlet oxygen quenching (kq + kox-Q) was 5.4 × 7.0 × 105/M/s = 3.78 × 106/M/s. This value was relatively small for singlet oxygen quencher, which agrees with finding by (Mukai et al., 2015). The rate of singlet oxygen quenching by γ-oryzanol extracted from rice bran was 2.69 × 103/Lgs or 1.6 × 106/M/s (Mukai et al., 2015).
Figure 6.
The plot of slope/intercept from Figure 5 vs. the concentration of γ-oryzanol.
In this experiment, we used β-carotene and TBHQ as natural and synthetic antioxidants to compare with γ-oryzanol. The [AO2]−1 vs. [A]−1 plot for individual concentrations of β-carotene (0–0.02 mM) gave the relatively same of intercepts (52.433, 47.851, and 45.823) and the slopes were 31.126, 35.47, and 49.584 for 0, 0.01 and 0.02 mM of β-carotene, respectively. Then the ratio of slope/intercept without β-carotene was 0.59. This value was equivalent with kd/kr (Eq. (2)). Kd, the singlet oxygen decomposition rate in ethanol was 8.3 × 104/s (Wilkinson et al., 1995). As a consequence, the kr or singlet oxygen with linoleic acid reaction rate was kd/slope or 1.4 × 105/M/s. The plot of slope/intercept (0.59, 0.74, and 1.08) in y-axis and concentration of β-carotene (0–0.02 mM) in x-axis gave regression line y = 24.422× + 0.5614. Based on Eq. (3), the slope is equivalent to (kox-Q + kq)/kr, therefore the total quenching rate of singlet oxygen by β-carotene is 3.41 × 109/M/s.
Meanwhile, The [AO2]−1 vs. [A]−1 plot for individual concentrations of TBHQ (0–1.2 mM) gave the intercepts were 163.92, 173.05 and 168.74; and the slopes were 27.789, 38.902, and 43.821 for 0, 0.06 and 1.2 mM of TBHQ, respectively. Then the slope/intercept ratio were 0.17. 0.22 and 0.26, respectively. The slope/intercept ratio without TBHQ was 0.17. By using same equation that mentioned before (Eq. (2)), kr, or singlet oxygen with linoleic acid reaction rate was 4.9 × 105/M/s. The ratio of slope/intercept (0.17, 0.22 and 0.26) was plotted with concentration of TBHQ (0, 0.6 and 1.2 mM) then gave an equation y = 0.0751× + 0.1729. Calculating via Eq. (3), this slope (0.0751 × 103) × kr (4.9 × 105/M/s), so the total of singlet oxygen quenching rate by TBHQ was 3.68 × 107/M/s.
The average of total quenching rates of singlet oxygen by β-carotene, TBHQ and γ-oryzanol is shown in Table 3 β-carotene and TBHQ had singlet oxygen quenching rate about 3.6 × 109/M/s and 3.27 × 107/M/s, individually. Based on previous literature, the singlet oxygen quenching rate of β-carotene was 1.10 × 109/M/s to 1.08 × 1010/M/s by various methods were chemiluminescence detection system, singlet oxygen absorption capacity and photooxidation of soybean oil in acetone system (Yang et al., 2002; Nishida et al., 2007; Ouchi et al., 2010). Our total quenching rate of singlet oxygen by TBHQ was lower than the previous reported. In other studies, the total quenching rate of singlet oxygen by TBHQ was 1.67–1.99 × 108/M/s by photooxidation of 2,5-diphenyl-3,5-benzofuran (DPBF) and α-terpinene in methanol, respectively (Kim et al., 2009; Lee and Jung, 2010). This difference might be due to various quenching rate of singlet oxygen determination method.
Table 3.
Singlet oxygen quenching rate of γ-oryzanol, β-carotene, and TBHQ in ethanol solution.
| Antioxidants | Singlet oxygen quenching rate (M−1s−1) |
||
|---|---|---|---|
| Trial 1 | Trial 2 | Mean ± SD | |
| γ-oryzanol | 3.78 × 106 | 2.31 × 106 | 3.04 (±1.04) × 106 |
| β-carotene | 3.8 × 109 | 3.41 × 109 | 3.60 (±0.27) × 109 |
| TBHQ | 3.68 × 107 | 2.87 × 107 | 3.27 (±0.57) × 107 |
The capacity of γ-oryzanol as singlet oxygen quencher is about 1000 times lower than β-carotene and ten times lower than TBHQ. The degree of the contribution as singlet oxygen quencher by γ-oryzanol is small, maybe due to only ferulic moiety contributing to singlet oxygen's target. Meanwhile, β-carotene had many conjugated double bonds that can react with singlet oxygen to produce aldehydes (carotenals) varieties. Carotenoid endoperoxide also was formed by cycloaddition of singlet oxygen on the carotenoid molecule (Ramel et al., 2012).
Lee and Jung (2010) found that the singlet oxygen quenching mechanism can be predicted via declining of antioxidants and oxidation target. Thus, by relatively the same model, the singlet oxygen quenching mechanism by γ-oryzanol can be predicted by determining the degradation rate of γ-oryzanol and linoleic acid. Based on these data, the singlet oxygen chemical quenching rate by γ-oryzanol could be established by calculate of the relative rates of disappearance of γ-oryzanol and linoleic acid in ethanol separately irradiated under identical conditions as shown in Eq. (4).
| krγ-oryzanol / krlinoleic acid = {-d[γ-oryzanol]/dt} / {-d [linoleic acid]/dt} | (4) |
where kr γ-oryzanol and kr linoleic acid are singlet oxygen quenching rate constant of γ-oryzanol and linoleic acid, respectively. Meanwhile, -d[γ-oryzanol] or [linoleic acid] are apparent rate of γ-oryzanol or linoleic acid disappearance.
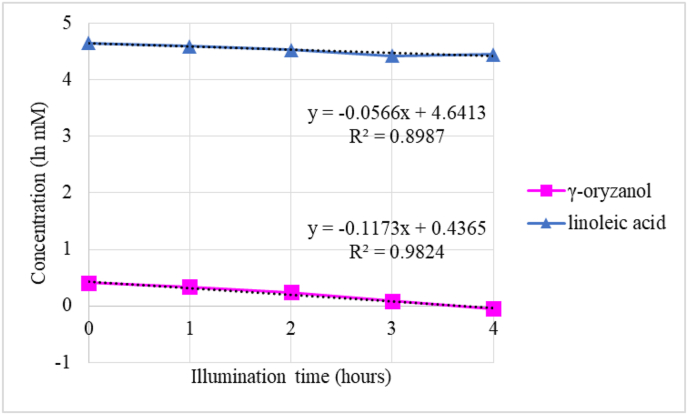
The decreasing rates of γ-oryzanol and linoleic acid is shown in Figure 7. The degradation rate of γ-oryzanol was dose-dependent manner. The higher initial concentration, the higher the degradation rate, as explained in section 3.2 (point b). The degradation rate of γ-oryzanol at initial concentration of 1.5 mM was higher (0.1421 mM/h) than that of 0.7 mM (0.0643 mM/h). The apparent rate constants of γ-oryzanol and linoleic acid were −0.1173 and −0.0566, respectively. The relative reaction rate of γ-oryzanol with linoleic acid was 2.0724. From equation above, the singlet oxygen chemical quenching rate constant for γ-oryzanol = 2.0724 × 7.0 × 105/M/s = 1.45 × 106/M/s. The ratio of chemical quenching to total quenching for γ-oryzanol was 47.72%. The results showed that the physical quenching mechanism dominated the singlet oxygen quenching mechanism of γ-oryzanol.
Figure 7.
The decreasing rate of γ-oryzanol and linoleic acid during photo-oxidation at 3400 lux.
4. Conclusion
γ-Oryzanol is specific naturally present antioxidant in rice bran oil. The γ-oryzanol content in rice bran oils varied from 111.7 to 254.8 mg γ-oryzanol/100 g of oil. The total singlet oxygen quenching rate of γ-oryzanol was 3.04 × 106/M/s with physical quenching as dominant mechanism by 52.28%.
Declarations
Author contribution statement
Yuli Perwita Sari: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Umar Santoso: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Supriyadi: Analyzed and interpreted the data; Wrote the paper.
Sri Raharjo: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Ministry of Research and Technology/National Agency for Research and Innovation, Republic of Indonesia and Universitas Gadjah Mada (RTA 2020).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- AOCS . fifth ed. American Oil Chemists Society; IL: 2004. Official Methods and Recommended Practices of the AOCS. [Google Scholar]
- Ariviani S., Raharjo S., Hastuti P. Potensi mikroemulsi β -karoten dalam menghambat fotooksidasi vitamin C sistem aqueous. Jurnal Teknologi dan Industri Pangan. 2011;XXII(1):33–39. [Google Scholar]
- 2020.Choe E., Min D.B. Chemistry and reactions of reactive oxygen species in foods. J. Food Sci. 2005;70(9):142–159. doi: 10.1080/10408390500455474. [DOI] [PubMed] [Google Scholar]
- Cuevas M.S., Souza P.T.D., Christianne E., Rodrigues C., Meirelles A.J.A. Quantification and determination of composition of steryl ferulates in refined rice bran oils using an UPLC - MS method. JAOCS (J. Am. Oil Chem. Soc.) 2017;943:375–385. [Google Scholar]
- Dhavamani S., Poorna Y., Rao C., Lokesh B.R. Total antioxidant activity of selected vegetable oils and their influence on total antioxidant values in vivo : a photochemiluminescence based analysis. Food Chem. 2014;164:551–555. doi: 10.1016/j.foodchem.2014.05.064. [DOI] [PubMed] [Google Scholar]
- Huang R., Choe E., Min D.B. Kinetics for singlet oxygen formation by riboflavin photosensitization and the reaction between riboflavin and singlet oxygen. J. Food Sci. 2004;699:C726–C732. [Google Scholar]
- Kim J.I., Lee J.H., Choi D.S., Won B.M., Jung M.Y., Park J. Kinetic study of the quenching reaction of singlet oxygen by common synthetic antioxidants tert-Butylhydroxyanisol, tert-di-Butylhydroxytoluene, and tert-Butylhydroquinone as compared with α-Tocopherol. J. Food Sci. 2009;745 doi: 10.1111/j.1750-3841.2009.01160.x. [DOI] [PubMed] [Google Scholar]
- Kochhar S.P. Stabilisation of frying oils with natural antioxidative components. Eur. J. Lipid Sci. Technol. 2000;102:552–559. [Google Scholar]
- Krishna A.G.G., Khatoon S., Shiela P.M., Sarmandal C.V., Indira T.N., Mishra A. Effect of refining of crude rice bran oil on the retention of oryzanol in the refined oil. JAOCS (J. Am. Oil Chem. Soc.) 2001;78i:127–131. [Google Scholar]
- Kumar N., Pruthi V. Potential applications of ferulic acid from natural sources. Biotechnol. Rep. 2014;4:86–93. doi: 10.1016/j.btre.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.H., Jung M.Y. Direct spectroscopic observation of singlet oxygen quenching and kinetic studies of physical and chemical singlet oxygen quenching rate constants of synthetic antioxidants BHA, BHT, and TBHQ in methanol. J. Food Sci. 2010;756:506–513. doi: 10.1111/j.1750-3841.2010.01669.x. [DOI] [PubMed] [Google Scholar]
- Lee K.H., Jung M.Y., Kim S.Y. Quenching mechanism and kinetics of ascorbyl palmitate for the reduction of the photosensitized oxidation of oils. JAOCS (J. Am. Oil Chem. Soc.) 1997;749:1053–1057. [Google Scholar]
- Lu W., Niu Y., Yang H., Sheng Y., Shi H., Lucy L. Simultaneous HPLC quantification of five major triterpene alcohol and sterol ferulates in rice bran oil using a single reference standard. Food Chem. 2014;148:329–334. doi: 10.1016/j.foodchem.2013.10.027. [DOI] [PubMed] [Google Scholar]
- Min D.B., Boff J.M. Chemistry and reaction of singlet oxygen in foods. Compr. Rev. Food Sci. Food Saf. 2002;1:58–72. doi: 10.1111/j.1541-4337.2002.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Mukai K., Ishikawa E., Abe T., Ouchi A., Nagaoka S.I., Murata K., Miyazawa T., Nakagawa K. Kinetic study of the quenching reaction of singlet oxygen by seven rice bran extracts in ethanol solution. Development of a singlet oxygen absorption capacity SOAC assay method. Biosci. Biotechnol. Biochem. 2015;7912:2063–2072. doi: 10.1080/09168451.2015.1069701. [DOI] [PubMed] [Google Scholar]
- Nishida Y., Yamashita E., Miki W. Quenching activities of common hydrophilic and lipophilic antioxidants against singlet oxygen using chemiluminescence detection system. Carotenoid Sci. 2007;11:16–20. [Google Scholar]
- Ouchi A., Aizawa K., Iwasaki Y., Inakuma T., Terao J., Nagaoka S.I., Mukai K. Kinetic study of the quenching reaction of singlet oxygen by carotenoids and food extracts in solution. Development of a singlet oxygen absorption capacity SOAC assay method. J. Agric. Food Chem. 2010;5818:9967–9978. doi: 10.1021/jf101947a. [DOI] [PubMed] [Google Scholar]
- Park P.W., Goins R.E. In situ preparation of fatty acid methyl esters for analysis of fatty acid composition in foods. J. Food Sci. 1994;596:1262–1266. [Google Scholar]
- Pestana V.R., Zambiazi R.C., Mendonça C.R.B., Bruscatto M.H., Lerma-García M.J., Ramis-ramos G. Quality changes and tocopherols and γ-orizanol concentrations in rice bran oil during the refining process. J. Am. Oil Chem. Soc. 2008;85:1013–1019. [Google Scholar]
- Pokkanta P., Sookwong P., Tanang M., Setchaiyan S. Simultaneous determination of tocols, -oryzanols, phytosterols, squalene, cholecalciferol and phylloquinone in rice bran and vegetable oil samples. Food Chem. 2019;271:630–638. doi: 10.1016/j.foodchem.2018.07.225. [DOI] [PubMed] [Google Scholar]
- Ramel F., Birtic S., Cuine S., Triantaphylide C., Ravanat J., Havaux M. Chemical quenching of singlet oxygen by carotenoids. Plant Physiol. 2012;158:1267–1278. doi: 10.1104/pp.111.182394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakunpak A., Suksaeree J., Pathompak P., Charoonratana T., Sermkaew N. Antioxidant individual γ-oryzanol screening in cold pressed rice bran oil of different Thai rice varieties by HPLC-DPPH method. Int. J. Pharm. Pharmaceut. Sci. 2014;66:2–7. [Google Scholar]
- Shantha C.N., Decker E.A. Rapid, sensitive, iron-based spectrophotometric methods for determination of peroxide values of food lipids. J. AOAC Int. 1994;772:421–424. [PubMed] [Google Scholar]
- Suhendra L. Fakultas Teknologi Pertanian, Universitas Gadjah Mada; Yogyakarta: 2014. Mekanisme Singlet Oxygen Quenching Oleh Fucoxanthin Dan Efektivitasnya Sebagai Antioksidan Dalam Mikroemulsi. [Dissertation] [Indonesian] [Google Scholar]
- Wilkinson F., Helman W.P., Ross A.B. Rate constants for the decay and reactions of the lowest electronically excited singlet state pf molecular oxygen in solution. An expanded and revised compilation. J. Phys. Chem. Ref. Data. 1995;24:663–1021. [Google Scholar]
- Xu Z., Godber J.S. Purification and identification of components of γ-oryzanol in rice bran oil. J. Agric. Food Chem. 1999;47:2724–2728. doi: 10.1021/jf981175j. [DOI] [PubMed] [Google Scholar]
- Yang T.S., Min D.B. Quenching mechanism and kinetics of ascorbic acid on the photosensitizing effects of synthetic food colorant FD&C Red Nr 3. J. Food Sci. 2009;749:718–722. doi: 10.1111/j.1750-3841.2009.01364.x. [DOI] [PubMed] [Google Scholar]
- Yang W.T., Lee J.H., Min D.B. Quenching mechanisms and kinetics of α-tocopherol and β-carotene on the photosensitizing effect of synthetic food colorant FDC Red No. 3. J. Food Sci. 2002;672:507–510. [Google Scholar]
- Yettela R.R., Min D.B. Quenching mechanisms and kinetics of trolox and ascorbic acid on the riboflavin-photosensitized oxidation of tryptophan and tyrosine. J. Agric. Food Chem. 2008;56:10887–10892. doi: 10.1021/jf8006739. [DOI] [PubMed] [Google Scholar]
- Zhong J., Liu X., Wang Y., Qin X., Li Z. γ-Oryzanol nanoemulsions produced by a low-energy emulsification method: an evaluation of process parameters and physicochemical stability. R. Soc. Chem. 2017 doi: 10.1039/c7fo00023e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.