Abstract
Physical activity and social support are associated with better outcomes after surviving acute myocardial infarction (AMI), and greater walkability has been associated with activity and support. We used data from the SILVER-AMI study (November 2014–June 2017), a longitudinal cohort of community-living adults ≥ 75 years hospitalized for AMI to assess associations of neighborhood walkability with health outcomes, and to assess whether physical activity and social support mediate this relationship, if it exists. We included data from 1345 participants who were not bedbound, were discharged home, and for whom we successfully linked walkability scores (from Walk Score®) for their home census block. Our primary outcome was hospital-free survival time (HFST) at six months after discharge; secondary outcomes included physical and mental health at six months, assessed using SF-12. Physical activity and social support were measured at baseline. Covariates included cognition, functioning, comorbidities, participation in rehabilitation or physical therapy, and demographics. We employed survival analysis to examine associations between walkability and HFST, before and after adjustment for covariates; we repeated analyses using linear regression with physical and mental health as outcomes. In adjusted models, walkability was not associated with physical health (ß = 0.010; 95% CI: −0.027, 0.047), mental health (ß = −0.08; 95% CI: −0.175, −0.013), or HFST (ß = 0.008; 95% CI: −0.023, 0.009). Social support was associated with mental health in adjusted models. Neighborhood walkability was not predictive of outcomes among older adults with existing coronary disease, suggesting that among older adults, mobility limitations may supercede neighborhood walkability.
Keywords: Secondary prevention, Cardiovascular disease, Physical activity, Social support, Neighborhood environment, Walkability, Older adults
1. Introduction
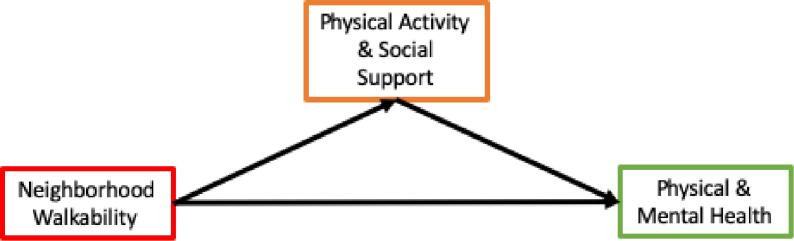
Patients discharged from the hospital after an acute myocardial infarction (AMI) are at high risk for readmission and mortality (Yan et al., 2004, Goldberg et al., 2004). These risks are especially pronounced among patients who are physically inactive and those with depression (Bush et al., 2001, Carney et al., 2003, Gerber et al., 2011). To mitigate these risks for poor outcomes, the American College of Cardiology and the American Heart Association guidelines recommend that patients engage in a minimum of 30-minutes of aerobic activity three to four times per week and increase daily activity post-AMI (Antman et al., 2004). Additionally, because higher levels of social support are associated with better mental health among older adults, improving social support has been invoked as a mechanism to protect AMI survivors from developing depression (Kawachi and Berkman, 2001, Berkman and Glass, 2000, Thoits, 2011, Blazer, 1982). It is plausible that the neighborhood environment may influence health outcomes post-AMI either directly or indirectly through physical activity and social support. Neighborhood structure can foster ease of engagement in regular daily activity and social interaction among neighbors (Fig. 1) (Matthews and Yang, 2010, Giles-Corti and Donovan, 2002, Negami et al., 2018).
Fig. 1.
Hypothesized relaionships among neighborhood walkability, physical activity and social support, and health outcomes
Walkability, defined as the ability to access basic necessities by foot (Lo, 2009), is a measure of the neighborhood environment. Walkability scores incorporate access to public transportation, quality of sidewalks, access to food, and safety. Research in urban planning suggests that walkability is modifiable and municipalities can design neighborhoods and spaces that encourage interaction between residents, thereby promoting social engagement, positive living, and community satisfaction (Negami et al., 2018, Sarracino, 2013, Cooper, 2014). For example, creating pedestrian safe crossings and neighborhood retail outlets that residents can walk to encourages daily activity and social interaction. This is important for promotion of population health, as levels of walkability are associated with higher rates of regular physical activity and active commuting, and lower rates of obesity (Lovasi et al., 2012, Tomey et al., 2013, Balfour and Kaplan, 2002, Hirsch et al., 2013, Zuniga-Teran et al., 2017). Among older adults, specifically, walkability and street connectivity are reported to have a positive influence on well-being and functioning (Balfour and Kaplan, 2002, Engel et al., 2016). As such, it is plausible that neighborhood walkability plays an important role in promoting recovery post-AMI among older adults.
We explored relationships among walkability, hospital-free survival time (HFST), and physical and mental health, among a cohort of older adults who survived AMI. We hypothesized that patients who return to a neighborhood with greater walkability post-AMI will have better health outcomes, and that this relationship is at least partially mediated by higher levels of self-reported physical activity and social support. To investigate this hypothesis, we linked data from the SILVER-AMI study, a prospective cohort of older adults discharged home after being hospitalized for AMI that includes measures of social support, physical activity, and self-reported physical and mental health, as well as incident hospitalizations and mortality at six months, with their home neighborhood’s walkability scores (Dodson et al., 2014).
2. Methods
2.1. Overview
We linked data on HFST, physical and mental health, and physical activity and social support from the SILVER-AMI cohort with data on neighborhood walkability from Walk Score®. We pre-specified analyses to investigate hypothesized associations among neighborhood walkability and health outcomes at six months, including HFST and perceived physical and mental health. We also planned to explore the effect of hypothesized mediators, physical activity and social support levels, on these relationships, if they exist (Fig. 1).
2.2. Cohort descripton
We used data from the SILVER-AMI prospective cohort (N = 3041), which included participants who were hospitalized for AMI. Participants were recruited between January 2013 through October 2016 from 94 community and academic hospitals across 27 US states. Participants were followed up for six months, with the last follow-up period ending in June 2017. The physical activity questionnaire was included starting November 2014, and as such, our sample was restricted to those recruited after this time who completed this questionnaire (N = 1595). We included data from 1557 participants who were discharged home, were not bedbound (defined as being able to complete at least one activity of daily living independently), and had completed social support and physical activity assessments at baseline during the index hospitalization, or the hospitalization during which the participant was recruited (Appendix) (Dodson et al., 2014). Of these, we were able to link neighborhood walkability scores to the home addresses of 1345 participants.
2.3. Patient and public involvement
Though patients surviving AMI were engaged when being recruited for this study before being discharged home, this study is a post-hoc analysis of their data after recruitment ended. As such, patients were not involved in the design or conduct of this study.
2.4. Independent variable: Neighborhood walkability
We obtained data on walkability from Walk Score®, a validated algorithm to assess pedestrian friendliness (e.g., population density, block length, and intersection density) and proximity of amenities of census blocks, the smallest geographic unit of the Census (Carr et al., 2010, Duncan et al., 2011). Scores range from 0 to 100, where a score between 0 and 24 describes census blocks where almost all errands require a car and a score between 90 and 100 describes census blocks where daily errands do not require a car. Amenities within 0.25 miles, or an approximately 5-minute walk for the average person, are given maximum points. A decay function is used to award points to amenities further away, with zero points given to those that are a 30-minute or greater walk for the average person. Home addresses of included participants were geocoded and used to obtain walkability scores of the census block where their home was located.
2.5. Dependent variables: Health outcomes at six months
Our pre-specified primary outcome was six-month HFST, defined as days from discharge until first readmission or death. Medical records were reviewed to ascertain hospitalization and deaths, with the latter also informed by proxy report and review of obituaries. At six months, the site research coordinator collected medical records on any hospital readmissions, and deaths from the site of the index hospitalization, as well as all other hospitals the participant reported using at the time of baseline interview. Medical records were provided to the Yale Coordinating Center where events were reconciled with the participant’s self-reported hospitalizations during the six-month phone interview. Any outstanding records were then collected. Medical records and death certificates were reviewed by physician investigators to determine whether reported events represent true hospital admissions, as well as primary discharge diagnosis and/or cause of death.
Perceived physical and mental health at six months were secondary outcomes. Physical and mental health were assessed by the physical component score (PCS) and mental component score (MCS) of the Short Form-12 (SF-12), respectively (Jenkinson et al., 1997, Ware et al., 1996).
2.6. Mediators and covariates
Hypothesized mediators in the relationship between walkability and health outcomes included physical activity and social support. Physical activity was measured by participant-reported frequency and duration of strenuous, moderate, and mild exercise, asking them to recall their activity levels one month prior to the index hospitalization (Gill et al., 2012). Physical activity was used as a continuous variable of MET-minutes/week calculated from the reported frequency and duration of these different levels of exercise. Social support was assessed at baseline using a shortened, five-item version of the Medical Outcomes Study Social Support Scale (Sherbourne and Stewart, 1991), using a continuous scale with a minimum possible score of five and a maximum possible score of 25.
Covariates included sociodemographic characteristics, characteristics of the index hospitalization, comorbid conditions, participation in rehabilitation or physical therapy post-discharge, cognition, in-hospital mobility, and pre-AMI impairment in activities of daily living. Sociodemographic characteristics included were age, education level (<high school, high school/GED, 2-year or 4-year college degree, graduate or post-graduate degree), race (white or non-white), sex, and marital status (married/living with a partner or not). We also included each participant’s home address’ census tract area deprivation index national rank. Characteristics of the index hospitalization included measures that characterize the severity of the AMI and cardiac function, including length of stay, information about revascularization procedures and ejection fraction. Comorbid conditions included were histories of prior coronary disease, arrhythmias, congestive heart failure, peripheral vascular disease, stroke, chronic obstructive pulmonary disease, chronic kidney disease, cancer, and diabetes. General cognitive ability was assessed using the Telephone Interview of Cognitive Status (Brandt et al., 1988, Fong et al., 2009). Physical functioning included in-hospital mobility and ability to perform daily tasks independently. In-hospital mobility was assessed using the Timed Up and Go test, which measured the time it takes the participant to stand from a seated position, walk three meters, and then return to the chair and sit down (Viccaro et al., 2011). The Activities of Daily Living assessment included self-reported ability to bathe, get dressed, get out of a chair, and walk (Katz, 1983).
2.7. Analysis
We first described characteristics of the sample, including means and distributions of sociodemographic characteristics, comorbid conditions, characteristics of the index hospitalization, physical functioning at baseline, levels of physical activity and social support at baseline, and levels of physical and mental health at six months. Next, we grouped the sample by quintiles of their home neighborhood walkability. We described characteristics of primary and secondary outcomes (HFST and physical and mental health at six months) and hypothesized mediators (physical activity and social support at baseline) across quintiles of neighborhood walkability. We used a linear contrast method, which treats each quintile as a categorical variable with five ordinal levels, to test for trends across these groups.
Next, we assessed whether our hypothesized mediators, physical activity and social support, were associated with our outcomes of interest, HFST, and physical (PCS) and mental (MCS) health among our cohort. We used linear regression to first assess unadjusted associations, and then adjusted for all covariates.
We then used Cox proportional hazards to assess associations with HFST and linear regression to explore associations among walkability and physical and mental health at six months. We first performed unadjusted analyses, then adjusted for social support and physical activity. Each model was run before and after further adjustment for sociodemographic characteristics and then all covariates. We planned to perform a formal mediation analysis using structural equation modeling to assess the role of our hypothesized mediators if an association between walkability and our outcomes was found.
The Yale Institutional Review Board approved this study. Statistical significance was set at α = 0.05. All analyses were performed using SAS version 9.4.
3. Results
The mean age of our study participants was 81.4 (SD: 5.0, range: 75–101) years, 43% female, and 87% white (Table 1). Approximately half were married or living with a partner. The mean area deprivation index national rank of participants’ home neighborhoods was 41.9 (26.5). Hospitalizations were a mean of 5.9 (5.1) days long and most participants had an invasive cardiac procedure performed (86.2%). Approximately 20% had an in-hospital left ventricular ejection fraction < 40%. Most participants (88%) reported impairment of at least one activity of daily living, but half was able to complete the Timed Up and Go test in under 25 s. One-third of participants rated themselves as having fair or poor health at baseline. Mean self-reported physical activity levels were 991.6 (1193.7) MET-min/week during the month prior to admission. Mean social support levels were 22.1 (4.2).
Table 1.
Baseline descriptive characteristics of all eligible SILVER-AMI participants, November 2014 – June 2017 (N = 1557).
| Characteristic | n (%) or Mean (SD) |
|---|---|
| Sociodemographics | |
| Age (mean, SD) | 81.39 (5.03) |
| Male (n, %) | 0888 (57.03%) |
| White (n, %) | 1359 (87.28%) |
| Highest level of education (n, %) | |
| Less than high school | 0184 (11.82%) |
| High school/GED | 0675 (43.35%) |
| 2-year or 4-year college degree | 0462 (29.67%) |
| Graduate or post-graduate degree | 0222 (14.26%) |
| Married or living as married/living with partner (n, %) | 0787 (50.55%) |
| Area deprivation index (ADI) national rank | 41.9 (26.47) |
| Comorbid conditions prior to AMI hospitalization | |
| Coronary artery disease (n, %) | 0837 (53.76%) |
| Arrhythmias (n, %) | 0407 (26.14%) |
| Heart failure (n, %) | 0284 (18.24%) |
| Peripheral arterial disease (n, %) | 0182 (11.69%) |
| Cerebrovascular disease (n, %) | 0232 (14.90%) |
| Chronic obstructive pulmonary disease (n, %) | 0212 (13.62%) |
| Chronic kidney disease (eGFR < 60 ml/min/1.73 m2; n, %) | 0927 (59.54%) |
| Cancer (n, %) | 0322 (20.68%) |
| Diabetes mellitus (n, %) | 0597 (38.34%) |
| Hospitalization characteristics | |
| Length of stay (mean, SD) | 5.89 (5.14) |
| Revascularization Status (n, %) | |
| No cardiac cath | 0215 (13.81%) |
| Cardiac cath without revascularization | 0262 (16.83%) |
| PCI only | 0900 (57.80%) |
| CABG | 0180 (11.56%) |
| In-hospital LVEF (n, %) | |
| ≥50% | 0790 (50.74%) |
| 40–50% | 0294 (18.88%) |
| 30–40% | 0203 (13.04%) |
| <30% | 0117 (7.51%) |
| Participated in cardiac rehabilitation after discharge (n, %) | 0491 (31.54%) |
| Received physical therapy after discharge (n, %) | 0329 (21.13%) |
| Baseline functioning | |
| TICS Total Score (mean, SD) | 30.73 (4.55) |
| Timed Up and Go (n, %) | |
| Completed in <= 15 s | 0480 (30.83%) |
| Completed in > 15 and <= 25 s | 0301 (19.33%) |
| Completed in > 25 s | 0213 (13.68%) |
| Did not complete due to short-term or long-term impairment | 0278 (17.85%) |
| Any ADL domains Impaired (n, %) | 0195 (12.52%) |
| Physical activity one month prior to admission (mean, SD) | 991.60 (1193.76) |
| Social Support Score (mean, SD) | 22.06 (4.22) |
| SF-1 General Health (n, %) | |
| Excellent or Very good | 0466 (29.93%) |
| Good | 0573 (36.80%) |
| Fair | 0370 (23.76%) |
| Poor | 0147 (09.44%) |
Abbreviations: SD = standard deviation; GED = general education diploma; eGFR = estimated glomerular filtration rate; PCI = percutaneous coronary intervention; CABG = coronary artery bypass graft; LVEF = left ventricular ejection fraction; TICS = Telephone Interview of Cognitive Status; ADL = activities of daily living.
We were able to link Walk Scores® to the home neighborhoods of 1345 participants, or 86% of our eligible sample. The mean (SD) Walk Score® of participants’ home neighborhoods in the lowest quintile was 1.44 (1.47) compared with a mean of 77.23 (11.95) in the highest quintile (Table 2). There was no difference in levels of physical activity at baseline across quintiles of neighborhood walkability. Social support scores tended to be higher among participants living in neighborhoods in the lowest quintiles of walkability. Physical health at six months did not differ across quintiles but mental health scores at six months tended to be lower among those living in neighborhoods with greater walkability. There was no difference in HFST across quintiles of neighborhood walkability.
Table 2.
Mean values of predictor, outcome, and hypothesized mediating variables across quintiles of neighborhood walkability of SILVER-AMI participants (November 2014 – June 2017; N = 1345).
| Variable | Q1 | Q2 | Q3 | Q4 | Q5 | p-trend |
|---|---|---|---|---|---|---|
| Walk Score® | 1.44 (1.47) | 10.32 (3.75) | 26.35 (4.98) | 46.69 (6.81) | 77.23 (11.95) | – |
| Physical activity | 1012.04 (1226.29) | 1109.40 (1384.07) | 839.63 (977.09) | 1027.41 (1206.51) | 940.91 (961.58) | 0.41 |
| Social support | 22.54 (3.74) | 22.05 (4.34) | 22.20 (3.85) | 22.43 (3.73) | 21.10 (5.11) | 0.002 |
| PCS | 42.90 (10.96) | 42.64 (10.83) | 41.69 (11.15) | 42.26 (10.88) | 42.24 (11.09) | 0.46 |
| MCS | 56.06 (7.25) | 57.35 (6.68) | 56.04 (7.57) | 54.99 (9.07) | 55.52 (7.28) | 0.03 |
| HFST | 168.88 (34.69) | 164.57 (42.21) | 165.70 (38.08) | 167.49 (35.16) | 164.97 (40.90) | 0.51 |
Abbreviations: PCS = physical component score of SF-12; MCS = mental component score of SF-12; HFST = hospital-free survival time (days).
Our hypothesized mediating variables, more physical activity and higher levels of social support, were mostly associated with better outcomes (Table 3). We found each unit increase of physical activity (MET-min/week) in the month prior to hospitalization was associated with longer HFST (β = 0.002; 95% CI: 0.000, 0.004), but this association was attenuated after adjustment for covariates. Higher levels of social support at baseline were also associated with longer HFST (β = 0.44; 95% CI: 0.001, 0.89), but this relationship was attenuated in fully adjusted models (β = 0.02; 95% CI: −0.06, 0.10). Each unit increase in physical activity was associated with better physical health at six months (β = 0.001; 95% CI: 0.001, 0.002), but not with mental health at six months (β = 0.000; 95% CI: −0.000, 0.001) in unadjusted models. After adjustment for all covariates, associations with physical activity were attenuated. Higher levels of social support at baseline were associated with better mental health at six months (β = 0.29; 95% CI: 0.19, 0.38), but not with physical health at six months (β = 0.12; 95% CI: −0.02, 0.26). Higher levels of social support at baseline remained associated with better mental health at six months (β = 0.26; 95% CI: 0.14, 0.38) in fully adjusted models.
Table 3.
Associations between physical activity and social support at baseline and outcomes at six months before and after adjustment for all covariates. (SILVER-AMI, November 2014 – June 2017; N = 1345).
| Physical Activity |
Social support |
|||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted |
Adjusted* |
Unadjusted |
Adjusted* |
|||||
| Outcome | ß | 95% CI | ß | 95% CI | ß | 95% CI | ß | 95% CI |
| PCS | 0.0012 | 0.001–0.002 | 0.0004 | −0.002–0.001 | 0.1239 | −0.0167–0.2644 | −0.023 | −0.1803, 0.1338 |
| MCS | 0.0002 | −0.0002–0.0006 | 0.0001 | −0.0003–0.0005 | 0.2876 | 0.1908–0.3844 | 0.259 | 0.1413, 0.3776 |
| HFST | 0.0023 | 0.0004–0.0042 | 0.0001 | −0.0003–0.0002 | 0.4435 | 0.0011–0.8860 | 0.018 | −0.0596, 0.957 |
Abbreviations: PCS = physical component score of SF-12; MCS = mental component score of SF-12; HFST = hospital-free survival time (days).
*Adjusted for age, sex, race, education, marital status, home census tract area deprivation index, comorbid conditions (coronary artery disease, arrhythmias, heart failure, peripheral arterial disease, cerebrovascular disease, chronic obstructive pulmonary disease, chronic kidney disease, any cancer, and diabetes), index hospitalization characteristics (length of stay, whether revascularization was performed, and ejection fraction), post-hospitalization cardiac rehabilitation or physical therapy, and physical functioning (TICS, timed up and go, and impairment in activities of daily living).
In unadjusted Cox proportional hazard models, walkability was not associated with HFST (Table 4). Walkability was also not associated with physical health at six months. However, each point increase in walkability was inversely associated with mental health at six months (β = -0.02; 95% CI: −0.04, −0.01). This association persisted after adjustment for social support (β = -0.02; 95% CI: −0.03, −0.00) and after further adjustment for sociodemographic characteristics (β = -0.09; 95% CI: −0.17, −0.01), but was attenuated after adjustment for all additional covariates (β = -0.08; 95% CI: −0.18, 0.01).
Table 4.
Associations between neighborhood walkability and outcomes before and after adjustment for mediators (social support and physical activity), sociodemographics, and other covariates related to clinical conditions, characteristics of the index hospitalization, and physical functioning (SILVER-AMI, November 2014 – June 2017). Unadjusted model; PA/SS model; sociodemographic model; fully adjusted model.
| Unadjusted model |
PA/SS model |
Sociodemographic model |
Fully adjusted model |
|||||
|---|---|---|---|---|---|---|---|---|
| Outcome | ß | 95% CI | ß | 95% CI | ß | 95% CI | ß | 95% CI |
| PCS | −0.0086 | −0.0315, 0.0143 | −0.0101 | −0.0365, 0.0163 | −0.0155 | −0.471, 0.0241 | 0.0098 | −0.0273, 0.0469 |
| MCS | −0.0206 | −0.0366, −0.0047 | −0.0165 | −0.0324, −0.0006 | −0.0886 | −0.1651, −0.0121 | −0.0810 | −0.1750, 0.0130 |
| HFST | −0.0151 | −0.0881, 0.0579 | −0.0487 | −0.1350, 0.0377 | −0.0112 | −0.1287, 0.1062 | −0.0075 | −0.0236, 0.0086 |
Abbreviations: PA = physical activity; SS = social support; PCS = physical component score of SF-12; MCS = mental component score of SF-12; HFST = hospital-free survival time (days).
*Associations between neighborhood walkability and PCS and HFST were adjusted for physical activity because physical activity was associated with these outcomes; the association between neighborhood walkability and MCS was adjusted for social support because social support was associated with this outcome.
**Sociodemographic model further adjusted the PA/SS model for individual-level demographic factors, including age, sex, race, education, and marital status.
***Fully adjusted model further adjusted the sociodemographic model for comorbid conditions (coronary artery disease, arrhythmias, heart failure, peripheral arterial disease, cerebrovascular disease, chronic obstructive pulmonary disease, chronic kidney disease, any cancer, and diabetes), index hospitalization characteristics (length of stay, whether revascularization was performed, and ejection fraction), post-hospitalization cardiac rehabilitation or physical therapy, and physical functioning (TICS, timed up and go, impairment in activities of daily living, and census tract area deprivation index.
Because our primary predictor variable, walkability, was not associated with HFST or with physical and mental health at six months, we did not perform a formal mediation analysis.
4. Discussion
Among a cohort of older adults discharged home after surviving AMI, we found no association among neighborhood walkability and our outcomes of HFST and perceived physical and mental health at six months. Counter to our hypothesis, greater neighborhood walkability was inversely associated with perceived mental health at six months, though this association was weak and was no longer significant in adjusted models. Neighborhood walkability was not associated with HFST, nor with physical or mental health at six months in adjusted models. Neighborhood walkability was also inconsistently associated with hypothesized mediators: walkability was inversely associated with social support and not associated with physical activity at baseline.
That greater neighborhood walkability was not associated with higher levels of physical activity among our sample was unexpected. Our findings are inconsistent with those from a prior study using data from the Multi-Ethnic Study of Atherosclerosis (MESA), which reported a 10-point increase in Walk Score® was associated with 9 more minutes of walking per week (Hirsch et al., 2013). Notably, our cohort was, on average, eleven years older than the MESA cohort and was recruited after the onset of heart disease. It is therefore possible that greater neighborhood walkability was not associated with more physical activity in our study population because mobility limitations may have superceded walkability’s influence on physical activity levels. Supporting this logic, a study among adults between 75 and 80 years of age did not find an association between Walk Scores® and physical activity (Takahashi et al., 2012). Another study found that residents of retired households performed errands less frequently by foot than the average household in a neighborhood with the same Walk Score® (Manaugh and El-Geneidy, 2011).
Though associations between neighborhood walkability and physical activity were not found, higher levels of physical activity prior to the index hospitalization were associated with better physical health at six months and longer HFST. It is possible that these participants were less frail at baseline, buffering the impact of the AMI. Further, though current clinical guidelines advise increasing physical activity post-AMI (Antman et al., 2004), we did not study the effects of change in physical activity on health outcomes. However, findings from other cohort studies of older adults with longer follow-up times report lack of moderate or vigorous exercise post-AMI to be independently associated with mortality and cardiovascular disease outcomes (Fried et al., 1998, Booth et al., 2014). Older adults may be less likely to achieve this level of activity outdoors in their neighborhoods, and instead, may be more likely to engage in physical activity in indoor environments, such as recreation centers or shopping malls (King, 2001). Taken together, while physical activity before and after AMI may be an important promoter of better outcomes, the neighborhood environment may not influence rates of physical activity among older adults.
Social support may be an important protective factor among older adults post-AMI. We found that higher levels of social support were associated with longer HFST and with better mental health at six months; the association with better mental health persisted after adjustment for all covariates. Aligned with this hypothesis, Frasure-Smith, et al., reported that high levels of social support buffered the impact of depression on mortality one-year after AMI (Frasure-Smith et al., 2000). These findings are also consistent with existing literature describing that social support improves mental health directly and indirectly, through stress-buffering mechanisms (Kawachi and Berkman, 2001, Thoits, 2011).
Though social support was associated with better mental health, both social support and mental health were inversely associated with neighborhood walkability. The inverse association between walkability and mental health persisted after adjustment for being married or living with a partner. These findings were inconsistent with our hypothesis and with existing literature. It is possible that older adults who live in more walkable neighborhoods and were perhaps more socially engaged in the community before their AMI felt relatively more socially isolated if they were unable to maintain the same level of engagement post-AMI. This relative drop in social support may be a risk factor for poor mental health. It is also possible that because more walkable neighborhoods are in urban areas, our findings signal social isolation and loneliness among urban residents that have been previously reported (Scharf and de Jong Gierveld, 2008). We do not have data on social support prior to the index hospitalization to tease out these two potential scenarios. Notably, these inverse associations were weak and the association between walkability and mental health was attenuated after adjustment for all covariates. Participants living in neighborhoods in the highest quintile of walkability reported, on average, a one-half point lower MCS than participants living in neighborhoods with the lowest walkability. This absolute difference is below the minimally clinically important difference of 4–7 points on the SF-12 among survivors of AMI, so the inverse association with walkability may not be meaningful (Soo Hoo et al., 2014).
Our study has limitations. First, our cohort was limited to a mostly white sample of older adults who survived AMI. Our findings therefore may not be generalizable to other populations. Second, though we limited our analyses to participants who were not bedbound and were discharged home, given the age and disease burden among our sample, effects of neighborhood walkability may not be strong enough to influence disease trajectory or social habits. Third, we assumed that if a patient was discharged home, they actually went to their home rather than an informal caregiver’s home. As such, walkability scores may not describe the walking environment of the post-discharge location for a portion of our sample. Fourth, our measure of neighborhood walkability was more crude than other walkability frameworks that assess important qualities of the accessibility of the pedestrian environment such as curb cuts, length of crosswalk timing, and places to rest (Zuniga-Teran et al., 2017). We acknowledge these metrics are especially important for the older adult population, however, these measures require survey or observation of the walking environment. Because our cohort was recruited from 27 states, this was not feasible for our study and we relied on a less precise, but valid proxy for walkability available for most of our participants’ home addresses; it is possible that our null findings are a result of using a relatively blunt assessment of walkability. Fifth, we used a single, general measure of social support and did not have access to measures of social networks, neighborhood cohesion, or social capital among our sample. These unmeasured factors may be more closely linked to neighborhood walkability. Sixth, our measures of social support and physical activity were performed during the baseline assessment, and we may have incorrectly assumed that these factors would not change during the post-discharge period. In addition, our measure of baseline physical activity may be influenced by recall bias, as we asked participants to recall their activity levels one month prior to hospitalization. Finally, our sample was followed for up to six months; longer follow-up time may yield different results.
5. Conclusions
We found inconsistent relationships between neighborhood walkability, HFST, and physical and mental health among a cohort of older adults surviving AMI followed for up to six months. Though higher levels of physical activity and social support at baseline were associated with better physical and mental health at six months, neighborhood walkability was not associated with either of these factors. Efforts to improve physical and mental health among older adults surviving AMI may be more effective if they incorporate indoor venues for physical activity and social support, such as group exercise programs or walking groups in malls or schools.
6. Contributorship statement
BR, CR, SIC participated in original conceptualization of the study. BR, CR, HMK, SIC, AMH, and MG contributed to the development of the study design. ST conducted analyses for this study. BR, AMH, CR, HMK, and SIC collectively contributed to interpretation of results. BR led writing of the manuscript and all authors provided critical review of the manuscript. All authors are guarantors.
7. Data sharing
The data that support the findings of this study are available from the executive body of the SILVER-AMI study at Yale University School of Medicine. Restrictions apply to the availability of SILVER-AMI data, as primary analyses are ongoing by study investigators. Data are available with the permission of the executive body of SILVER-AMI, upon request. No additional data are available.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Drs. Roy and Riley report personal fees from Heluna Health, personal fees from the Institute for Healthcare Improvement, and grant funding from the Robert Wood Johnson Foundation and the Institute for Healthcare Improvement, outside the submitted work. Dr. Roy also reports grant funding from the National Heart, Lung, and Blood Institute outside the submitted work. Dr. Krumholz reports personal fees from UnitedHealth, personal fees from IBM Watson Health, personal fees from Element Science, personal fees from Aetna, personal fees from Facebook, personal fees from Siegfried & Jensen Law Firm, personal fees from Arnold & Porter Law Firm, personal fees from Ben C. Martin Law Firm, personal fees from National Center for Cardiovascular Diseases, Beijing, ownership of HugoHealth, ownership of Refactor Health, contracts from the Centers for Medicare & Medicaid Services, grants from Medtronic and the Food and Drug Administration, grants from Medtronic and Johnson and Johnson, grants from Shenzhen Center for Health Information, outside the submitted work. The other authors report no competing interests.
Acknowledgement
We would like to thank Xuemei Song for contributing to conducting analyses for this study. This work was funded by the National Institutes of Health (National Heart, Lung, and Blood Institute) R01HL115295. The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; National Institutes of Health. This work was conducted at the Yale Program on Aging/Claude D. Pepper Older Americans Independence Center (P30AG021342). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Appendix
Flow diagram of participant eligibility for this analysis, SILVER-AMI, January 2013 – June 2017.

References
- Antman E.M., Anbe D.T., Armstrong P.W. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction—executive summary. Circulation. 2004;110(5):588–636. doi: 10.1161/01.CIR.0000134791.68010.FA. [DOI] [PubMed] [Google Scholar]
- Balfour J.L., Kaplan G.A. Neighborhood environment and loss of physical function in older adults: evidence from the Alameda County Study. Am. J. Epidemiol. 2002;155(6):507–515. doi: 10.1093/aje/155.6.507. [DOI] [PubMed] [Google Scholar]
- Berkman L.F., Glass T. Social integration, social networks, social support, and health. Soc. Epidemiol. 2000;1:137–173. [Google Scholar]
- Blazer D.G. Social support and mortality in an elderly community population. Am. J. Epidemiol. 1982;115(5):684–694. doi: 10.1093/oxfordjournals.aje.a113351. [DOI] [PubMed] [Google Scholar]
- Booth J.N., Levitan E.B., Brown T.M., Farkouh M.E., Safford M.M., Muntner P. Effect of sustaining lifestyle modifications (nonsmoking, weight reduction, physical activity, and mediterranean diet) after healing of myocardial infarction, percutaneous intervention, or coronary bypass (from the REasons for Geographic and Racial Differences in Stroke Study) Am. J. Cardiol. 2014;113(12):1933–1940. doi: 10.1016/j.amjcard.2014.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J., Spencer M., Folstein M. The telephone interview for cognitive status. Neuropsychiatry Neuropsychol. Behav. Neurol. 1988;1(2):111–117. [Google Scholar]
- Bush D.E., Ziegelstein R.C., Tayback M., Richter D., Stevens S., Zahalsky H., Fauerbach J.A. Even minimal symptoms of depression increase mortality risk after acute myocardial infarction. Am. J. Cardiol. 2001;88(4):337–341. doi: 10.1016/s0002-9149(01)01675-7. [DOI] [PubMed] [Google Scholar]
- Carney R.M., Blumenthal J.A., Catellier D., Freedland K.E., Berkman L.F., Watkins L.L., Czajkowski S.M., Hayano J., Jaffe A.S. Depression as a risk factor for mortality after acute myocardial infarction. Am. J. Cardiol. 2003;92(11):1277–1281. doi: 10.1016/j.amjcard.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Carr L.J., Dunsiger S.I., Marcus B.H. Walk score™ as a global estimate of neighborhood walkability. Am. J. Prev. Med. 2010;39(5):460–463. doi: 10.1016/j.amepre.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. Wellbeing and the Environment. Wiley Online Library; 2014.
- Dodson J.A., Geda M., Krumholz H.M., Lorenze N., Murphy T.E., Allore H.G., Charpentier P., Tsang S.W., Acampora D., Tinetti M.E., Gill T.M., Chaudhry S.I. Design and rationale of the comprehensive evaluation of risk factors in older patients with AMI (SILVER-AMI) study. BMC Health Serv. Res. 2014;14(1) doi: 10.1186/s12913-014-0506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan D.T., Aldstadt J., Whalen J., Melly S.J., Gortmaker S.L. Validation of Walk Score® for estimating neighborhood walkability: an analysis of four US metropolitan areas. Int. J. Environ. Res. Public Health. 2011;8(11):4160–4179. doi: 10.3390/ijerph8114160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel L., Chudyk A.M., Ashe M.C., McKay H.A., Whitehurst D.G.T., Bryan S. Older adults' quality of life–Exploring the role of the built environment and social cohesion in community-dwelling seniors on low income. Soc. Sci. Med. 2016;164:1–11. doi: 10.1016/j.socscimed.2016.07.008. [DOI] [PubMed] [Google Scholar]
- Fong T.G., Fearing M.A., Jones R.N., Shi P., Marcantonio E.R., Rudolph J.L., Yang F.M., Dan Kiely K., Inouye S.K. Telephone interview for cognitive status: creating a crosswalk with the Mini-Mental State Examination. Alzheimer's Dementia. 2009;5(6):492–497. doi: 10.1016/j.jalz.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasure-Smith N, Lespérance F, Gravel G, et al. Social support, depression, and mortality during the first year after myocardial infarction. Circulation. 2000;101(16):1919-1924. [DOI] [PubMed]
- Fried L.P., Kronmal R.A., Newman A.B. Risk factors for 5-year mortality in older adults: the Cardiovascular Health Study. JAMA. 1998;279(8):585–592. doi: 10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]
- Gerber Y., Myers V., Goldbourt U., Benyamini Y., Scheinowitz M., Drory Y. Long-term trajectory of leisure time physical activity and survival after first myocardial infarction: a population-based cohort study. Eur. J. Epidemiol. 2011;26(2):109–116. doi: 10.1007/s10654-010-9523-8. [DOI] [PubMed] [Google Scholar]
- Giles-Corti B., Donovan R.J. The relative influence of individual, social and physical environment determinants of physical activity. Soc. Sci. Med. 2002;54(12):1793–1812. doi: 10.1016/s0277-9536(01)00150-2. [DOI] [PubMed] [Google Scholar]
- Gill D.P., Jones G.R., Zou G., Speechley M. Using a single question to assess physical activity in older adults: a reliability and validity study. BMC Med. Res. Method. 2012;12(1):20. doi: 10.1186/1471-2288-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg R.J., Currie K., White K., Brieger D., Steg P.G., Goodman S.G., Dabbous O., Fox K.A.A., Gore J.M. Six-month outcomes in a multinational registry of patients hospitalized with an acute coronary syndrome (the Global Registry of Acute Coronary Events [GRACE]) Am. J. Cardiol. 2004;93(3):288–293. doi: 10.1016/j.amjcard.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Hirsch J.A., Moore K.A., Evenson K.R., Rodriguez D.A., Roux A.V.D. Walk score® and transit score® and walking in the multi-ethnic study of atherosclerosis. Am. J. Prev. Med. 2013;45(2):158–166. doi: 10.1016/j.amepre.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson C., Layte R., Jenkinson D., Lawrence K., Petersen S., Paice C., Stradling J. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies? J. Public Health. 1997;19(2):179–186. doi: 10.1093/oxfordjournals.pubmed.a024606. [DOI] [PubMed] [Google Scholar]
- Katz S. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J. Am. Geriatr. Soc. 1983;31(12):721–727. doi: 10.1111/j.1532-5415.1983.tb03391.x. [DOI] [PubMed] [Google Scholar]
- Kawachi I., Berkman L.F. Social ties and mental health. J. Urban Health. 2001;78(3):458–467. doi: 10.1093/jurban/78.3.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A.C. Interventions to promote physical activity by older adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001;56(Supplement 2):36–46. doi: 10.1093/gerona/56.suppl_2.36. [DOI] [PubMed] [Google Scholar]
- Lo R.H. Walkability: what is it? J. Urbanism. 2009;2(2):145–166. [Google Scholar]
- Lovasi G.S., Grady S., Rundle A. Steps forward: review and recommendations for research on walkability, physical activity and cardiovascular health. Public Health Rev. 2012;33(4):484–506. doi: 10.1007/BF03391647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manaugh K., El-Geneidy A. Validating walkability indices: How do different households respond to the walkability of their neighborhood? Transport. Res. D Transp. Environ. 2011;16(4):309–315. [Google Scholar]
- Matthews S.A., Yang T.-C. Exploring the role of the built and social neighborhood environment in moderating stress and health. Ann. Behav. Med. 2010;39(2):170–183. doi: 10.1007/s12160-010-9175-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negami H.R., Mazumder R., Reardon M., Ellard C.G. Field analysis of psychological effects of urban design: a case study in Vancouver. Cities Health. 2018;2(2):106–115. [Google Scholar]
- Sarracino F. Nova Science Publishers; Hauppauge, NY, US: 2013. The Happiness Compass: Theories, Actions and Perspectives for Well-Being. [Google Scholar]
- Scharf T., de Jong Gierveld J. Loneliness in urban neighbourhoods: an anglo-dutch comparison. Eur. J. Ageing. 2008;5(2):103–115. doi: 10.1007/s10433-008-0080-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherbourne C.D., Stewart A.L. The MOS social support survey. Soc. Sci. Med. 1991;32(6):705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- Soo Hoo S.Y., Gallagher Rs., Elliott D. Systematic review of health-related quality of life in older people following percutaneous coronary intervention. Nurs. Health Sci. 2014;16(4):415–427. doi: 10.1111/nhs.12121. [DOI] [PubMed] [Google Scholar]
- Takahashi PY, Baker MA, Cha S, Targonski PV. A cross-sectional survey of the relationship between walking, biking, and the built environment for adults aged over 70 years. Risk management and healthcare policy. 2012;5:35. [DOI] [PMC free article] [PubMed]
- Thoits P.A. Mechanisms linking social ties and support to physical and mental health. J. Health Soc. Behav. 2011;52(2):145–161. doi: 10.1177/0022146510395592. [DOI] [PubMed] [Google Scholar]
- Tomey K., Diez Roux A.V., Clarke P., Seeman T. Associations between neighborhood characteristics and self-rated health: a cross-sectional investigation in the Multi-Ethnic Study of Atherosclerosis (MESA) cohort. Health Place. 2013;24:267–274. doi: 10.1016/j.healthplace.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viccaro L.J., Perera S., Studenski S.A. Is timed up and go better than gait speed in predicting health, function, and falls in older adults? J. Am. Geriatr. Soc. 2011;59(5):887–892. doi: 10.1111/j.1532-5415.2011.03336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware John.E., Kosinski Mark, Keller Susan.D. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med. Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Yan A.T., Tan M., Fitchett D., Chow C.-M., Fowlis R.A., McAvinue T.G., Roe M.T., Peterson E.D., Tu J.V., Langer A., Goodman S.G. One-year outcome of patients after acute coronary syndromes (from the Canadian Acute Coronary Syndromes Registry) Am. J. Cardiol. 2004;94(1):25–29. doi: 10.1016/j.amjcard.2004.03.024. [DOI] [PubMed] [Google Scholar]
- Zuniga-Teran A.A., Orr B.J., Gimblett R.H., Chalfoun N.V., Marsh S.E., Guertin D.P., Going S.B. Designing healthy communities: testing the walkability model. Front. Archit. Res. 2017;6(1):63–73. [Google Scholar]