Abstract
Epigenetic regulation disorder is important in the onset and pathogenesis of inflammatory bowel disease (IBD). SETD2, a trimethyltransferase of histone H3K36, is frequently mutated in IBD samples with a high risk of developing colorectal cancer (CRC). However, functions of SETD2 in IBD and colitis-associated CRC remain largely undefined. Here, we found that SETD2 modulates oxidative stress to attenuate colonic inflammation and tumorigenesis in mice. SETD2 expression became decreased in IBD patients and dextran sodium sulfate (DSS)-induced colitic mice. Setd2Vil-KO mice showed increased susceptibility to DSS-induced colitis, accompanied by more severe epithelial barrier disruption and markedly increased intestinal permeability that subsequently facilitated inflammation-associated CRC. Mechanistically, we found that Setd2 depletion resulted in excess reactive oxygen species (ROS) by directly down-regulating antioxidant genes, which led to defects in barrier integrity and subsequently inflammatory damage. Moreover, overexpression of antioxidant PRDX6 in Setd2Vil-KO intestinal epithelial cells (IECs) largely alleviated the overproductions of ROS and improved the cellular survival. Together, our findings highlight an epigenetic mechanism by which SETD2 modulates oxidative stress to regulate intestinal epithelial homeostasis and attenuate colonic inflammation and tumorigenesis. SETD2 might therefore be a pivotal regulator that maintains the homeostasis of the intestinal mucosal barrier.
Keywords: IBD, SETD2, Oxidative stress, ROS, Epithelial barrier
1. Introduction
Inflammatory bowel disease (IBD) is a complex and relapsing inflammatory disorder that can be sub-classified into Crohn's disease (CD) and ulcerative colitis (UC) [1,2]. Chronic inflammation frequently leads to increased risk of tumorigenesis [3], and IBD patients have higher risk of colorectal cancer (CRC) development [4,5]. IBD results from a complex interactions between the mucosal barrier, commensal bacteria, and the immune system [6]. The intestinal mucosal barrier consists of intestinal epithelial cells (IECs), which are adhered to each other via intercellular junctions, and plays a critical role in protecting the gastrointestinal tract [7]. Defects in the barrier function allow the translocation of luminal pathogens and bacteria into the intestinal lamina propria and trigger chronic inflammation and tissue injury, which occasionally causes dysplasia [8]. IBD have been associated with abnormal intestinal epithelial barrier function [9,10]. However, the underlying molecular mechanisms of IBD remain largely ambiguous.
Intestinal inflammation is usually accompanied by excessive production of reactive oxygen. High levels of reactive oxygen species (ROS) create oxidative stress within the cells and lead to a loss of homeostasis [11]. Redox imbalance has been proposed as one potential cause factor for IBD [12]. Oxidative stress plays an important role in the development and progression of IBD, and is associated with CRC pathogenesis [[13], [14], [15]]. In the gastrointestinal tract, oxidative stress leads to damage of the intestinal mucosal layer and epithelial cell apoptosis, which results in bacterial invasion in the gut and in turn stimulates the immune response [[16], [17], [18]]. Clinical studies show that the increase of ROS and biomarkers of oxidative injury contributes to tissue damage in IBD [18,19]. Our previous work has also shown that excess ROS leads to defects in barrier integrity and the subsequent inflammation [20]. In order to resist the oxidative damage, the intestinal epithelium contains an extensive system of antioxidants [21,22]. Colitis is usually associated with a decrease in the levels of antioxidants in colonic tissues [19], whereas overexpression of antioxidant enzymes results in attenuation of colitis in mice [17]. Thus, a balance between oxidant and antioxidant mechanisms is necessary to maintain intestinal epithelial homeostasis.
Epigenetic regulation disorder is important in the onset and pathogenesis of IBD [[23], [24], [25]], which may influence the maintenance of homeostasis in the intestinal epithelium. Studies have shown that DNA methylation is correlated with disease susceptibility and progression in IBD [26,27]. It is also known that inactive histone modification H3K27 trimethylation regulated by EZH2 promotes the inflammatory response and apoptosis in colitis [28]. Moreover, our previous study has also shown that BRG1, a key epigenetic regulator, attenuates colonic inflammation and tumorigenesis through autophagy-dependent oxidative stress sequestration [20]. In addition, SETD2, a trimethyltransferase of histone H3 lysine 36 (H3K36), is frequently mutated (17%) in UC samples with a high risk of developing colorectal carcinoma [29], suggesting that SETD2 plays a potential role in colitis and colitis-associated CRC.
SETD2 is the only known H3K36 methyltransferase that can alter the trimethylation status of H3K36 and regulates protein structures as well as its function [30,31]. SETD2-induced H3K36me3 is a multifunctional histone marker associated with actively transcribed regions and is critical for many biological processes, such as transcriptional regulation, DNA damage repair, chromosome segregation and alternative splicing [[32], [33], [34], [35]]. SETD2 has also been identified as a common mutation across cancer types, including glioma [36], renal cell carcinoma [34], leukemia [37,38] and lung cancer [39,40]. In addition, we have recently also reported that SETD2 is pivotal for bone marrow mesenchymal stem cells differentiation, genomic imprinting and embryonic development, initiation and metastasis of pancreatic cancer, and normal lymphocyte development [[41], [42], [43], [44], [45]]. However, functions of SETD2 in IBD and colitis -associated CRC remain largely undefined.
To investigate a possible role of SETD2 in IBD, in the present work we generated intestinal epithelium-specific Setd2-knockout mice, and found that deletion of Setd2 in intestinal tissues promotes DSS-induced epithelial damage and subsequent tumorigenesis. Mechanistically, our results highlighted SETD2 as a critical epigenetic determinant in the prevention of colitis and tumorigenesis through modulation of oxidative stress.
2. Materials and methods
2.1. Mice
All mice were bred and maintained at an animal facility under specific pathogen-free conditions. Setd2-flox mice and Villin-Cre mice were purchased from Shanghai Biomodel Organism Co.. Setd2Vil-KO mice were generated by crossing Setd2-flox mice with Villin-Cre mice. All mice were maintained on C57BL/6 J background and littermates with the same treatment were used as controls in all experiments.
2.2. Human specimen analysis
Patients with IBD and non-IBD control subjects for this study were recruited from Renji hospital (Shanghai, China). The use of pathological specimens, as well as the review of all pertinent patient records, were approved by the Ethics Review Board of Renji hospital. Immunohistochemical analyses were performed using a specific anti-SETD2 antibody (LSBio, Cat# C332416). Protein expression was scored based on a multiplicative index of the average staining intensity (0–3) and the extent of staining (0–3), which yielded a 10-point staining index that ranged from 0 to 9.
2.3. Induction of colitis and CRC
To induce acute colitis, wild-type mice were fed with 3% dextran sodium sulfate (DSS; MP Biomedicals) for 7 days, which was then followed by 5 days of regular drinking water; Setd2f/f and Setd2Vil-KO mice were fed with 2% DSS for 5 days, which was then followed by 5 days of regular drinking water. For the N-acetyl-l-cysteine (NAC) (Sigma, A7250) treatment experiments, 8-week-old mice were pretreated with NAC (100 mg/kg) every day for 4 days induced by intraperitoneal injection, and treatment was continued during DSS administration for 5 days.
In AOM/DSS experiments, mice were treated with a single dose of AOM (10 mg/kg, Sigma), and 2% DSS was given in the drinking water for 5 days, followed by two weeks of regular drinking water. The DSS treatment was repeated for two additional cycles, and mice were sacrificed 90 days after AOM injection.
2.4. Isolation of intestinal epithelial cells and culture
For RNA-Seq and ChIP-Seq analyses, IECs were isolated as follows: mice were sacrificed and colonic specimens were dissected, opened longitudinally and cleared from feces by washing extensively in cold PBS. Colons were cut in small pieces of 1 mm and incubated in 30 mM EDTA solution in PBS at 37 °C for 10 min. 10 min later, EDTA solution was replaced with ice-cold PBS and shacked vigorously for 30 s. This process was repeated once more. Supernatants were collected and combined from both incubations, then centrifuged at 1200 rpm for 5 min at 4 °C. For culture purpose, the colons were cut into pieces and washed by DMEM for three times, and then incubated with digestion buffer containing 250 μg/ml collagenase type I (Sigma, C0130) and 500 μg/ml Dispase II (Roche #04942078001) for 1.5 h in 37 °C. After the incubation, the cell suspension was passed through 100 μm cell strainers (Corning). After washes, the cells were plated in dishes coated with rat tail tendon collagen type I overnight and were cultured in DMEM with 10% FBS and 1% penicillin/streptomycin. The purities of colonic epithelial cell from the EDTA-based and enzymatic digests were 90–95% and 80–85%, respectively, using an EpCAM antibody (Invitrogen #25-5791-80) and FACS analysis.
2.5. Isolation of lamina propria cells and flow cytometry analysis
Colons were cut into small pieces and incubated in 5 mM EDTA solution in PBS at 37 °C for 30 min with gentle shaking. After removal of the epithelial layer, the remaining colon pieces were incubated at 37 °C with RPMI medium containing 1.75 mg/ml collagenase A (Roche #10103586001) and 0.05 mg/ml DNase I (Roche #104159) for 45 min. After digestion, the supernatant was passed through a 70 μm cell strainer to isolate lamina propria cells. Lamina propria cells were stained for surface markers CD4, CD11b, F4/80 and Gr-1, and subjected to flow cytometry analyses. The antibodies used are listed in Supplementary Table 2.
2.6. Organoid culture and analysis
The intestines were opened longitudinally, and villi were scraped away. The pieces were thorough washed in cold PBS, and incubated in 2 mM EDTA solution in PBS for 10 min at 4 °C. Then, EDTA solution was replaced with PBS and shacked vigorously for 45 s. Crypt fractions were purified by successive centrifugation steps. For every 500-1,000 crypts, a mixture of 100 μL Matrigel (BD Biosciences) and complete growth medium (ratio 2: 1) is added. After polymerization, 100 μl Advanced DMEM/F12 (Invitrogen) containing EGF (50 ng/ml, PeproTech), R-spondin (500 ng/ml, PeproTech), Noggin (100 ng/ml, PeproTech), N-acetyl-1-cysteine (1 μM, Sigma-Aldrich), N2 (1X, Life Technologies) and B27 (1X, Life Technologies) was added and refreshed every two or three days. On the fifth day, the organoids were stained with 7-AAD (Invitrogen, A1310) for 5 min to detect apoptosis levels, and photos were imaged by Zeiss fluorescence microscope and quantified by Image J software. (The NAC concentration in organoid culture is extremely low (1 μM), which will not affect the level of ROS.)
2.7. RNA-seq assay and data analysis
IECs were isolated from Setd2f/f and Setd2Vil−KO mice treated for 4 d with DSS by EDTA-based isolation. Each sample contained 3 independent repeated animals. NEB Next Ultra Directional RNA Library Prep Kit for Illumina (New England Biolabs, Ipswich, MA, USA) was used for the construction of sequencing libraries. The libraries were then subjected to Illumina sequencing with paired-ends 2 × 150 as the sequencing mode. The clean reads were mapped to the mouse genome (assembly GRCm38) using the HISAT2 software. Gene expression levels were estimated using FPKM (fragments per kilobase of exon per million fragments mapped) by StringTie. Gene annotation file was retrieved from Ensembl genome browser 90 databases (http://www.ensembl.org/index.html). The false discovery rate (FDR) control method was used to calculate the adjusted P-values in multiple testing in order to evaluate the significance of the differences. The statistical significance of differentially expressed genes was analyzed using a p-value threshold of <0.05 and fold change ≥1.25. We obtained 18168 differentially expressed genes (644 genes were up-regulated, and 605 genes were down-regulated). All differentially expressed mRNAs were selected for gene ontology (GO) analysis. We downloaded the GO annotations from NCBI (http://www.ncbi.nlm.nih.gov/), UniProt (http://www.uniprot.org/) and the Gene Ontology (http://www.geneontology.org/). Fisher's exact test was applied to identify the significant GO categories (P-value < 0.05).
2.8. ChIP-seq assay and data analysis
IECs were isolated from Setd2f/f and Setd2Vil−KO mice after 4 d of DSS treatment. The fragmented chromatin fragments were pre-cleared and then immunoprecipitated with Protein A + G Magnetic beads coupled with anti-H3K36me3 (Abcam, ab9050, 2–3μg/1 × 106 cells) antibody and IgG antibody (CST, 2729S, 2–3μg/1 × 106 cells). After reverse crosslinking, ChIP and input DNA fragments were end-repaired and A-tailed using the NEBNext End Repair/dA-Tailing Module (E7442, NEB) followed by adaptor ligation with the NEBNext Ultra Ligation Module (E7445, NEB). The DNA libraries were amplified for 15 cycles and sequenced using Illumina NextSeq 500 with single-end 1 × 75 as the sequencing mode. Raw reads were filtered to obtain high-quality clean reads by removing sequencing adapters, short reads (length<50 bp) and low-quality reads using Cutadapt (v1.9.1) and Trimmomatic (v0.35). Then FastQC is used to ensure high reads quality. The clean reads were mapped to the mouse genome (assembly GRCm38) using the Bowtie2 (v2.2.6) software. Peak detection was performed using the MACS (v2.1.1) peak finding algorithm with 0.01 set as the p-value cutoff. In total, 178919 and 217927 H3K36me3 peaks were identified in Setd2Vil-KO and Setd2f/f IECs over the input control, respectively. The heat maps and average profile for TSS were generated using ngsplot v2.61.
2.9. Statistics
Data were analyzed using the student's t-test and results are presented as the mean + s.e.m (SEM) unless otherwise indicated. Pearson correlation coefficients were used to evaluate the relationships between SETD2 and gene expressions, and the Wilcoxon signed rank test was used for patient analysis. Statistical analysis was performed using the GraphPad Prism software. A p value that was less than 0.05 was considered statistically significant for all data sets.
For RNA isolation, Real-time PCR, Western blotting, Immunohistochemistry, Analysis of intestinal permeability, ROS detection, ChIP-qPCR, Plasmid construction, 16S-rDNA sequencing, antibiotics treatment and histopathological analysis, can be found in Supplementary methods. All the antibodies used are listed in Supplementary Table 2.
3. Results
3.1. SETD2 expression becomes decreased in IBD patients and DSS treated mice
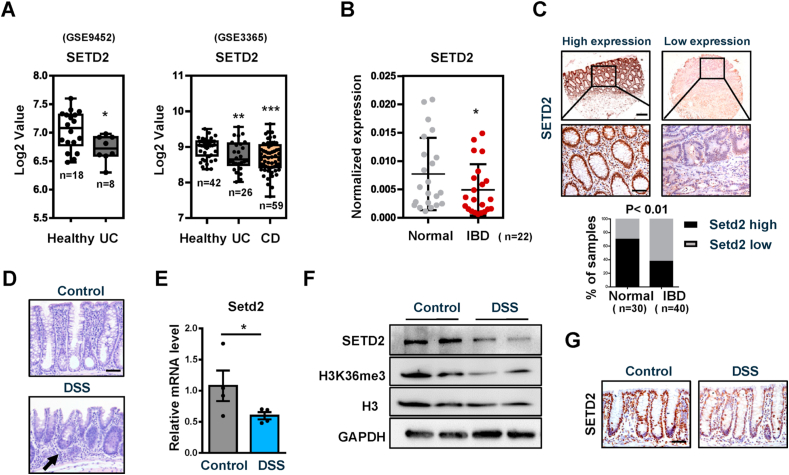
To explore a possible role of SETD2 in IBD, we analyzed SETD2 mRNA expression in public datasets of IBD samples (using datasets from NCBI's Gene Expression Omnibus: GSE9452 and GSE3365). The results indicated that the level of SETD2 mRNA was reduced in IBD specimens compared with that in healthy controls (Fig. 1A). To validate these data in IBD, we performed quantitative RT-PCR (RT-qPCR) assays and immunohistochemistry analyses to determine SETD2 expression in colonic biopsy specimens from IBD patients as well as from normal controls. Compared with the levels in biopsies from healthy specimens, we showed that SETD2 mRNA levels were significantly decreased in the IBD patients (Fig. 1B). The quantification of the immunohistochemical results revealed that the protein levels of SETD2 in the colonic epithelial cells were significantly lower in the IBD specimens relative to that in the healthy subjects (Fig. 1C).
Fig. 1.
SETD2 expression is downregulated in IBD patients and DSS treated mice.
(A) Boxplot of SETD2 expression levels in healthy controls and IBD specimens (using dataset GSE9452 and GSE3365).
(B) RT-qPCR analysis of SETD2 mRNA in IBD specimens and healthy subjects (n = 22 per group).
(C) SETD2 staining images are shown in the upper panel, and epithelial SETD2 expressions in normal and IBD biopsies are quantified in the bottom panel (χ2 test). Staining indexes use a 10-point quantification scale, and a score >4 is considered higher level. Scale bar: 100 μm (upper), 50 μm (bottom).
(D–G) Samples are all derived from 8-week-old wild-type mice with or without 3%DSS treatment (n = 4 per genotype).
(D) Representative images of H&E-stained colon sections as indicated. Arrow: immune cell infiltration. Scale Bars: 50 μm.
(E) RT-qPCR analysis of SETD2 mRNA in IECs from control and DSS-treated wild-type mice (n = 4 per genotype).
(F) Immunoblot analyses of SETD2 expression in IECs from control and DSS-treated wild-type mice are shown, and quantitation results are shown in the right.
(G) Immunohistochemical of SETD2 expression in control and DSS-treated wild-type mice are shown. Scale Bars: 50 μm.
The data represent the mean ± S.E.M, and statistical significance was determined by a two-tailed Student's t-test. *p < 0.05, **p < 0.01 and ***p < 0.001.
To further evaluate the relevance of SETD2 in IBD, we measured SETD2 expression level in a DSS-induced colitic mouse model (Supplementary Fig. 1A), which is a common mouse model that imitates the clinical pathology of IBD [46,47]. The DSS-treated wild-type mice exhibited epithelial erosions and inflammation in the colon (Fig. 1D). Consistently, the DSS-treated colons produced significantly more proinflammatory cytokines and chemokines than the colons from control littermates (Supplementary Fig. 1B). These results indicated that we successfully established a colitic mouse model. To identify cellular populations expressing SETD2 in the colon, we performed co-immunofluorescence staining of SETD2 with E-cadherin, ChgA (Enteroendocrine cells), DCLK1 (Tuft cells), F4/80 and CD4, respectively, in the colon of wild-type mice. SETD2+ cells were located along the intestinal epithelium and SETD2 was co-expressed with E-cadherin, ChgA, DCLK1, F4/80 and CD4 respectively (Supplementary Fig. 1C), indicating that SETD2 is expressed in both epithelial cells and non-epithelial cells in the colon. Subsequently, we evaluated SETD2 expression in the intestine of colitic mice. Similar to what was seen in IBD patients, SETD2 was down-regulated in the intestine of DSS-induced colitic mice (Fig. 1E–G). Together, these findings suggest a potential link between SETD2 reduction and IBD pathogenesis.
3.2. Setd2Vil-KO mice are more susceptible to DSS-induced colitis
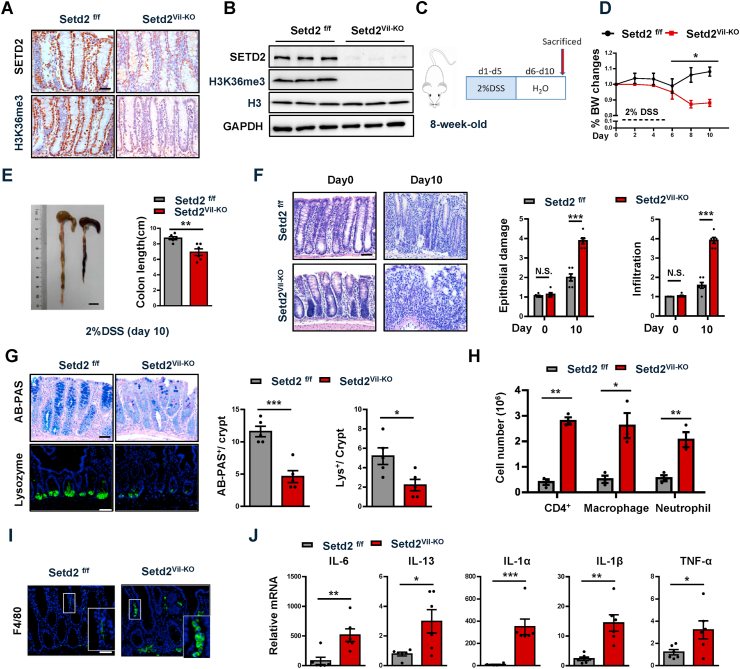
To assess a role of SETD2 in colonic inflammation, we crossed Setd2-flox mice (Setd2f/f mice) with Villin-Cre mice to obtain an intestinal epithelium-specific Setd2 knockout mouse strain (Villin-Cre; Setd2 flox/flox mice, hereinafter referred to as Setd2Vil-KO mice). As expected, Setd2 was efficiently ablated and H3K36me3 was substantially reduced in the intestinal epithelium of Setd2Vil-KO mice by immunohistochemical staining and immunoblot validation (Fig. 2A–B). Histological examination of 8-week-old Setd2Vil-KO mice revealed no obvious abnormalities in the colon crypt (Supplementary Fig. 2A). However, as compared to the Setd2f/f mice, the levels of several proinflammatory cytokines and chemokines (IL-1α, CXCL1) were significantly higher in the colonic sections of Setd2Vil-KO mice (Supplementary Fig. 2B and 2C). Meanwhile, Setd2Vil-KO mice exhibited abnormal decreases of mucus-producing goblet cells and AMP-producing Paneth cells (Supplementary Fig. 2D). The two types of cells play important roles in intestinal antibacterial defense by releasing antimicrobial factors [48,49]. These observations were further confirmed by RT-qPCR analysis (Supplementary Fig. 2E).
Fig. 2.
Loss of Setd2 in IECs aggravates DSS-induced colitis in mice.
(A) Immunohistochemical analyses of SETD2 and H3K36me3 expressions are shown.
(B) Immunoblot analyses of SETD2 and H3K36me3 expression in IECs from Setd2Vil-KO and Setd2f/f mice are shown. Scale Bars: 20 μm.
(C) Schematic representation of the DSS protocol used to induce acute colitis in Setd2Vil-KO and Setd2f/f mice.
(D, E) Setd2Vil-KO and Setd2f/f mice were fed 2% DSS in drinking water, and loss of body weights (D) and colon length (E) are recorded (n = 6 per genotype). Scale Bars: 1 cm.
(F) H&E stained sections of colon tissues collected on day 0 and 10 from 2% DSS-treated Setd2Vil-KO and Setd2f/f mice, and the quantitation of DSS-induced epithelial damage and immune cell infiltration are shown in the right (n = 6 per genotype). Scale Bars: 50 μm.
(G) Alcian blue-Periodic acid Schiff (AB-PAS; goblet cells) staining in the colon and lysozyme (Lys; Paneth cells) staining in the small intestine as indicated (n = 6 per genotype). Scale Bars: 20 μm.
(H) After 5 d of DSS treatment, colonic lamina propria cells from Setd2Vil-KO and Setd2f/fmice are analyzed by flow cytometry for CD4 + T cells, CD11b +; F4/80 + macrophages, and CD11b +; Gr-1 + neutrophils (n = 3 per genotype).
(I) Colon sections from mice treated with DSS were stained for F4/80 to detect macrophages. Scale Bars: 50 μm.
(J) Relative mRNA expression levels of inflammatory mediators in whole colon of Setd2Vil-KO and Setd2f/f mice were determined by RT-qPCR (n = 6 per genotype).
The data represent the mean ± S.E.M, and statistical significance was determined by a two-tailed Student's t-test. *p < 0.05, **p < 0.01 and ***p < 0.001. N.S., Not Significant. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
To further define the role of SETD2 in colitis, we assessed the consequence of Setd2 loss in acute colitis by challenging Setd2f/f and Setd2Vil-KO mice with 3% DSS for 7 days and then monitored their susceptibility. We noticed that Setd2Vil-KO mice exhibited hyper-susceptibility to 3% DSS-induced acute colitis, and most of the Setd2Vil-KO mice survived no longer than 10 days of observations. Therefore, the mice were switched to a lower dose of DSS (2%) and shorter treatment time (5 days) challenge (Fig. 2C) to induce acute colitis. After DSS administration, Setd2Vil-KO mice lost more body weight than Setd2f/f mice (Fig. 2D), suggesting that Setd2Vil-KO mice probably had enhanced inflammation and intestinal damage, as body weight loss is one of the characteristics for the severity of DSS-induced colitis [50,51]. In addition, macroscopic dissection revealed significantly shorter colons in the Setd2Vil-KO mice compared with the Setd2f/f mice (Fig. 2E). On further histopathological examination, the Setd2Vil-KO mice exhibited widespread damage in the colon, with more ulcerations as compared with Setd2f/f mice. At 10 days post DSS treatment, the Setd2f/f colon showed minimal to mild inflammation, while colon from Setd2Vil-KO mice displayed moderate to severe inflammation, with many areas of complete crypt loss and erosions (Fig. 2F). The excess inflammatory response observed in the Setd2Vil-KO mice was also associated with a loss of goblet cells and Paneth cells, and protein level of MUC2 secreted by goblet cells was also decreased in Setd2Vil-KO mice (Fig. 2G, Supplementary Fig. 2F). Since Setd2Vil-KO mice showed higher epithelial damage and inflammation, we then analyzed immune cell infiltration by performing flow cytometry. At 5 days post DSS treatment, we observed a higher number of CD4 + T cells, neutrophils, and macrophages in Setd2Vil-KO mice compared with control mice (Fig. 2H and Supplementary Fig. 2G). Notably, the immune cell infiltration also extended to the entire colon, as evidenced by the accumulation of F4/80-positive cells in relatively non-inflamed areas of the Setd2Vil-KO colons (Fig. 2I). It is well-known that infiltrating immune cells produce cytokines and chemokines to resolve the inflammation process [52,53]. As expected, the DSS-treated Setd2Vil-KO colons produced prominently more proinflammatory cytokines and chemokines than the colons from the Setd2f/f littermates (Fig. 2J and Supplementary Fig. 2H). Collectively, these results demonstrate that depletion of Setd2 leads to exacerbated colitis, suggesting that SETD2 plays a pivotal role in attenuating inflammatory response in experimental colitis.
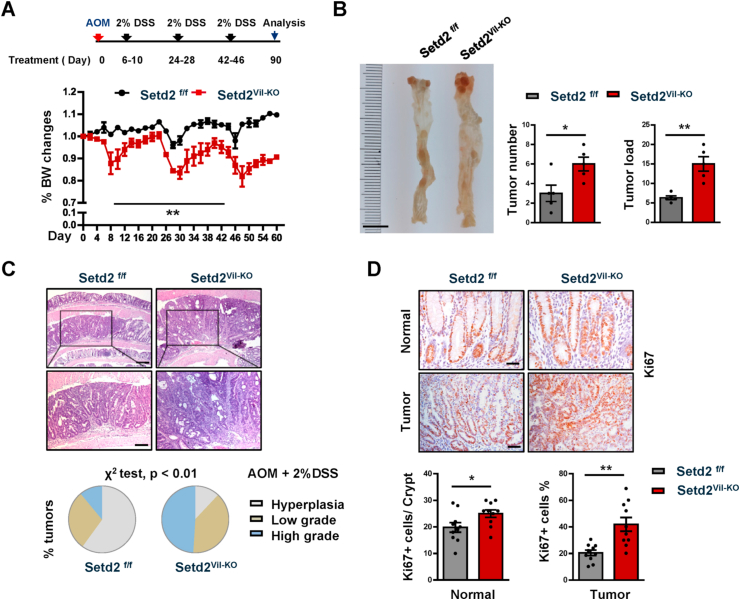
3.3. Setd2 deficiency facilitates the formation of colitis-associated CRC
The observation that Setd2Vil-KO mice suffered from the sustained inflammation, prompted us to investigate a possible role of SETD2 in colitis-associated tumorigenesis. We induced CRC by injecting the DNA-methylating agent azoxymethane (AOM), followed by three cycles of 2% DSS treatments, and each cycle consisted of 5 days of 2%DSS water followed by 14 days of water alone. We recorded the changes in body weight throughout the duration of the AOM/DSS treatment, and determined the tumor burden 90 days after the AOM injection. We found that Setd2Vil-KO mice suffered from chronic inflammation and lost noticeably more body weight compared with the control mice (Fig. 3A). Compared with the Setd2f/f mice, Setd2-deficient mice had more macroscopic polypoid lesions, which were on average threefold larger in size (Fig. 3B). Histological analysis revealed that around 50% of polyps in Setd2Vil-KO mice were classified as high-grade dysplasia. However, the lesions developed in Setd2f/f mice were mainly graded as low-grade dysplasia or hyperplasia (Fig. 3C). Accordingly, the colons of Setd2Vil-KO mice exhibited higher proliferative rates, indicated by Ki67 staining, than those in the control mice (Fig. 3D). Thus, Setd2 ablation results in increased inflammatory response, which facilitates the development and progression of colitis-associated CRC.
Fig. 3.
Setd2 loss promotes the colitis -associated CRC.
(A) Setd2Vil-KO and Setd2f/f mice were injected with AOM on day 0 (2 months of age) and treated with 2% DSS during three 5-day cycles as indicated. Body weight changes are recorded as indicated (n = 5 per genotype).
(B) At day 90 after the AOM injection, the mice were sacrificed to examine the tumor burden (n = 5 per genotype). Scale Bars: 1 cm.
(C) Representative images of colons and the overall grading of the tumors in each genotype (χ2 test) are shown (n = 5 per genotype). Scale Bars: 200 μm (upper), 100 μm (bottom).
(D) Representative images of Ki67 staining in normal colons and tumors from AOM/DSS-treated Setd2Vil-KO and Setd2f/f mice are shown (n = 5 per genotype). Scale Bars: 20 μm (upper), 50 μm (bottom). The data represent the mean ± S.E.M, and statistical significance was determined by a two-tailed Student's t-test. *p < 0.05, **p < 0.01.
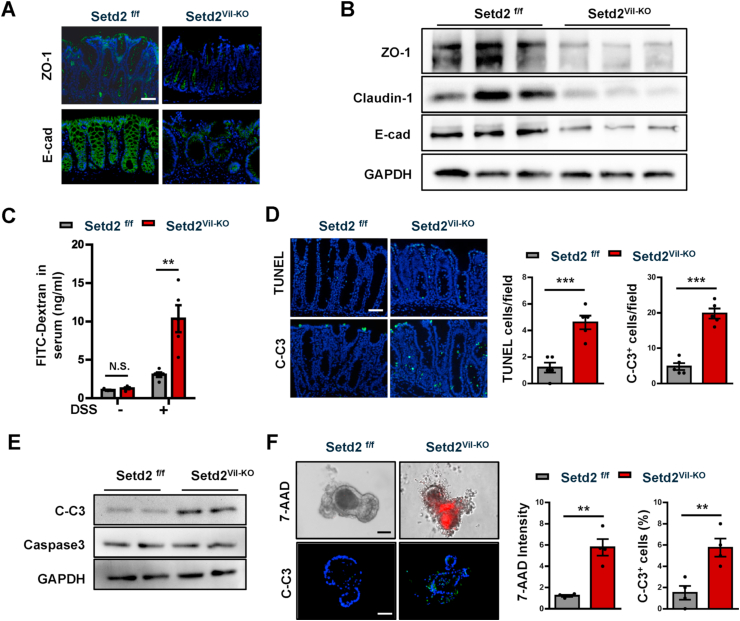
3.4. Disruption of Setd2 induces intestinal epithelium apoptosis and barrier damage in experimental colitis
Given that intestinal epithelial barrier dysfunctions could frequently contribute to gut inflammation [7,9,10], and that there were increased epithelial damage in the absence of Setd2 (Fig. 2F), we investigated whether the loss of Setd2 compromised the barrier integrity by assessing the distribution of junction proteins and permeability of intestinal colonic mucosa. We examined the distribution of the tight junction protein 1 (ZO-1), E-cadherin, Claudin-1 and Claudin-2 in Setd2f/f and Setd2Vil-KO mice, four markers to reveal the intercellular junctions of intestinal colonic mucosa. In contrast to the controls, the Setd2-deficient colons showed partially disrupted or discontinuous ZO-1, E-cadherin, Claudin-1 and Claudin-2 staining (Fig. 4A, Supplementary Fig. 3A), indicating that an intact mucosal barrier was compromised in Setd2Vil-KO mice. This observation was further strengthened by Western blot and RT-qPCR analyses showing reduced expression of ZO-1, E-cadherin, Claudin-1 and Claudin-2 in Setd2Vil-KO mice compared with control mice (Fig. 4B, Supplementary Fig. 3B). In accordance with the severe ulceration (Fig. 2F), intestinal permeability was markedly increased in Setd2Vil-KO mice, evidenced by the enhanced fluorescence in the serum of DSS-treated Setd2Vil-KO mice fed with FITC-labeled dextran (Fig. 4C). Thus, these results indicate that Setd2 deletion in colons leads to epithelial barrier damage and colonic leakage in experimental colitis.
Fig. 4.
Loss of Setd2 in IECs induces cell apoptosis and barrier damage.
(A) Representative ZO-1 and E-cadherin staining in colon sections from DSS-treated (5 d) Setd2Vil-KO and Setd2f/f mice. Scale Bars: 100 μm.
(B) Immunoblotting analysis of the indicated proteins in IECs isolated from DSS-treated (5 d) Setd2Vil-KO and Setd2f/f mice.
(C) Colonic permeability was measured by the concentration of FITC-dextran in the blood serum (n = 5 per genotype).
(D) TUNEL (Upper) and cleaved caspase 3 (Lower) staining of colon sections from DSS-treated (5 d) Setd2Vil-KO and Setd2f/f mice, and quantitation results are shown in the right (n = 5 per genotype). Scale Bars: 50 μm.
(E) Western blot analysis of the indicated proteins in IECs isolated from DSS treated (5 d) Setd2Vil-KO and Setd2f/f mice.
(F) Intestinal organoids derived from Setd2Vil-KO and Setd2f/f mice. After 5 d of differentiation, 7-AAD-stained organoids (red) were imaged after 48 h (Upper), and quantitations of the fluorescence density are shown in the right. C-Casp3 staining of the organoid sections as indicated (Lower), and quantitation results are shown in the right (n = 4 per genotype). Scale Bars: 20 μm.
The data represent the mean ± S.E.M, and statistical significance was determined by a two-tailed Student's t-test. **p < 0.01, ***p < 0.001. N.S., Not Significant. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Considering that epithelial apoptosis is one of the mechanisms by which DSS can induce intestinal inflammation or colitis, and that the epithelial barrier is disrupted in the absence of Setd2 (Fig. 4C), we examined possible defects in epithelial cell survival in the Setd2Vil-KO mice. On the histopathological examination, the numbers of TUNEL- and cleaved caspase-3-positive cells were significantly higher in the colon sections from the Setd2Vil-KO mice than in those from the Setd2f/f mice (Fig. 4D). This observation was further strengthened by western blotting showing markedly elevated signaling intensities of cleaved caspase-3 in Setd2Vil-KO mice compared with Setd2f/f mice (Fig. 4E). Besides, the expression of Annexin A1, α-tubulins and F-actin, which is related to epithelial restitution, was reduced in Setd2Vil-KO mice, suggesting that there is impairment in epithelial restitution in IECs lacking Setd2 (Supplementary Fig. 3C and 3D). We next established intestinal organoid cultures to assess whether loss of Setd2 induces apoptosis in a cell-intrinsic manner. As shown by 7-aminoactinomycin D (7-AAD) whole-mount staining and cleaved caspase-3 immunostaining, the organoids lacking SETD2 displayed a substantial increase in apoptosis relative to the controls (Fig. 4F). Besides, the expression of ZO-1, E-cadherin, Claudin-1 and Claudin-2 was decreased in organoids lacking SETD2 (Supplementary Fig. 3E–3G). These data emphasize that SETD2 is required for the survival of IECs, whose defects might cause the barrier disruption and the subsequent inflammation.
3.5. SETD2 modulates ROS homeostasis to regulate colon inflammation
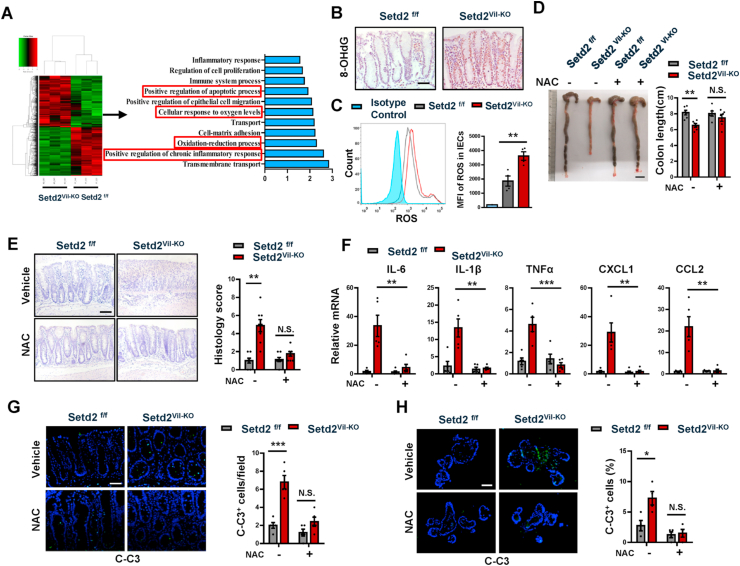
To further investigate the molecular mechanisms in which SETD2 regulates intestinal epithelial homeostasis, we performed RNA-seq analysis. Next-generation sequencing using RNA from Setd2Vil-KO and Setd2f/f IECs after 4 d of DSS treatment respectively revealed that the global transcriptome was changed dramatically in Setd2Vil-KO IECs compared to the Setd2f/f IECs, indicating a significant function of SETD2 in IECs (Fig. 5A). Among a total of 18168 genes expressed, 644 genes were up-regulated, and 605 genes were down-regulated (Fold change > 1.25) in Setd2Vil-KO IECs. Gene Ontology (GO) term analysis indicated that there was a significant enrichment of genes linked to apoptotic regulation and inflammatory response (Fig. 5A), which is consistent with previous observation (Fig. 2, Fig. 4D). Interestingly, some genes related to “cellular response to oxygen levels” and “oxidation-reduction process” were also significantly enriched (Fig. 5A). These results were validated by RT-qPCR (Supplementary Fig. 4A). Of note, the mRNA expression levels of some antioxidant genes were significantly down-regulated, suggesting increased oxidative stress in the absence of Setd2. To confirm this finding, we performed 8-oxo-2′-deoxyguanosine (8-OHdG) staining to examine oxidative stress in colon. We detected that 8-OHdG level was significantly increased in the colon and organoid of Setd2Vil-KO mice after DSS treatment (Fig. 5B, Supplementary Fig. 4B). Oxidative stress is associated with intestinal inflammation and CRC pathogenesis, and ROS plays an important role in apoptosis induction and inflammatory damage under both physiologic and pathologic conditions [13,14]. Therefore, we studied the role of ROS in SETD2-mediated inflammation by staining using 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA), which is a fluorescent probe that reacts with ROS. IECs were isolated from Setd2Vil-KO and Setd2f/f mice after DSS treatment, and flow cytometry analysis indicated that Setd2-deficency IECs produced significantly more amount of ROS relative to that in the control IEC cells (Fig. 5C).
Fig. 5.
SETD2 mediates ROS reaction to control cell apoptosis and colon inflammation.
(A) Heat map of RNA-seq data to compare the gene expression in the IECs from Setd2Vil-KO and Setd2f/f mice treated for 4 d with DSS. Go term analysis of gene expression changes in the right.
(B) 8-OHdG staining from DSS-treated (5d) Setd2Vil-KO and Setd2f/f mice. Scale Bars: 50 μm.
(C) Histograms and MFI quantifications of ROS in IECs isolated from DSS-treated (5d) Setd2Vil-KO and Setd2f/f mice (n = 4 per genotype).
(D–H) 8-week-old Setd2Vil-KO and Setd2f/f mice were pretreated with NAC (100 mg/kg) every day for 4 days induced by intraperitoneal injection, and treatment was continued during DSS administration for 5 days.
(D) The mice were euthanized on days 10 to measure colon length (n = 6 per genotype). Scale Bars: 1 cm.
(E) H&E stained sections of colon tissues collected on day 10 from DSS-treated mice as indicated, and semi-quantitative scoring of the histopathology is shown in the right (n = 6 per genotype). Scale Bars: 100 μm.
(F) Relative mRNA expression levels of inflammatory mediators in whole colon from DSS-treated (5 d) mice as indicated (n = 5 per genotype).
(G) Cleaved caspase-3 staining of colon sections from DSS-treated (5 d) Setd2Vil-KO and Setd2f/f mice with or without NAC treatment (n = 5 per genotype). Scale Bars: 50 μm.
(H) Organoids were isolated from 8-week-old Setd2Vil-KO and Setd2f/f mice with or without NAC treatment, and cleaved caspase-3 staining (n = 4 per genotype). Scale Bars: 20 μm.
The data represent the mean ± S.E.M, and statistical significance was determined by a two-tailed Student's t-test. *p < 0.05, **p < 0.01, ***p < 0.001. N.S., Not Significant. MFI, Median Fluorescence Intensity.
To provide evidence that ROS indeed contributes to the barrier defects and colitis, we performed rescue assays to determine whether blocking ROS could reverse the inflammatory phenotype caused by the Setd2 loss. Two-month-old Setd2Vil-KO and Setd2f/f mice were pretreated with an antioxidant, N-acetyl-l-cysteine (NAC) every day for 4 days, and treatment was continued during DSS administration for 5 days [13,54]. According to colon length and histopathological staining (Fig. 5D–E), the blockade of ROS production via the administration of NAC in the Setd2Vil-KO mice greatly eased the severity of inflammation, and the colonic morphology score was restored to the normal value. 8-OHdG level was significantly decreased in the colon of Setd2Vil-KO mice after NAC treatment (Supplementary Fig. 4C). As judged by the expression levels of proinflammatory cytokines and chemokines, the severe inflammatory response occurring in the Setd2Vil-KO mice was largely mitigated by the ROS inhibition (Fig. 5F). Additionally, the NAC treatment restored the numbers of Paneth and goblet cells to levels similar to those in the control mice (Supplementary Fig. 4D and 4E). Besides, NAC-treated Setd2Vil-KO mice exhibited a less intestinal permeability, barrier disruption and epithelial cell apoptosis than Setd2f/f mice (Supplementary Fig. 4F–4H and Fig. 5G). In addition, the relieved 8-OHdG level, barrier defects and epithelial apoptosis were also repeated in intestinal organoid culture experiment (Supplementary Fig. 4I-4L and Fig. 5H). These results suggest that the blockade of ROS could reverse the phenotype of epithelial barrier disruption and colon inflammation in Setd2Vil-KO mice. Altogether, ROS plays an important role in experimental colitis exacerbated by SETD2 deficiency.
3.6. SETD2-mediated H3K36me3 facilitates the transcriptions of antioxidant genes to maintain ROS homeostasis
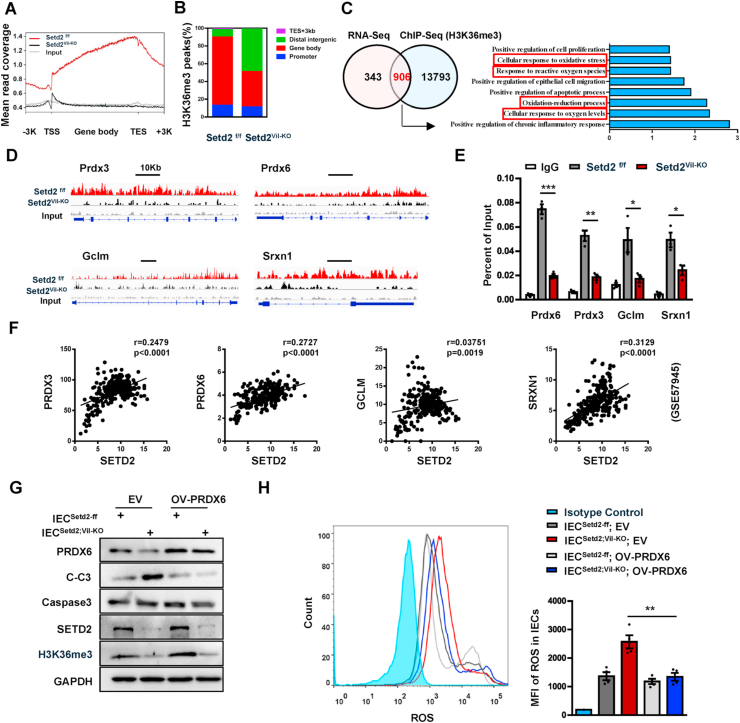
To elucidate the molecular basis by which SETD2 modulated ROS, we immunoprecipitated H3K36me3-bound chromatin in Setd2Vil-KO and Setd2f/f IECs after 4 d of DSS treatment and analyzed the precipitated DNA by deep sequencing. 178919 and 217927 H3K36me3 peaks were identified in Setd2Vil-KO and Setd2f/f IECs, respectively. The altered density of H3K36me3 intervals (Setd2Vil-KO versus Setd2f/f) were widely localized within whole genomic regions, including gene bodies, promoters, and intergenic zones (Fig. 6A–B), indicating that SETD2 is responsible for the H3K36 trimethylaiton on these regions. To correlate the chromatin binding with the transcriptional regulation, we integrated the ChIP-seq data with the expression profile. The Venn diagrams indicated that 906 genes (including downregulated and upregulated genes) showed direct H3K36me3 occupancies and expression changes upon the Setd2 ablation (Fig. 6C). GO term analysis revealed that these overlapping 906 genes were still closely related to oxidative stress pathway (Fig. 6C). As shown in Fig. 5, ablation of Setd2 increased ROS production and down-regulated the expression levels of antioxidant genes, suggesting that Setd2/H3K36me3 might positively regulate some antioxidant-coding genes related to ROS production. As expected, antioxidant genes including Prdx3, Prdx6, Gclm, and Srxn1 were listed among the 906 genes described above, and their downregulation was validated in colon epithelial cells collected from Setd2Vil-KO mice, comparing with cells from Setd2f/f mice (Supplementary Fig. 4A). Direct H3K36me3 occupancies within these candidate gene loci were also seen in Genome Browser tracks (Fig. 6D). We further validated the existence of H3K36me3 bindings at the gene bodies of Prdx3, Prdx6, Gclm and Srxn1 by the ChIP-qPCR assays, and found that the intensity of H3K36me3 bindings within these gene loci decreased along with the loss of Setd2 (Fig. 6E). Consistent with these results, there were significant positive correlations between mRNA levels of SETD2 and that of PRDX3, PRDX6, GCLM and SRXN1 respectively based on GSE57945 database (Fig. 6F). Thus, Setd2 loss could repress the transcription of antioxidant genes, which were important for antioxidant defenses. The peroxiredoxin [PRDX] family contains the most of important antioxidant proteins. PRDX6, a bifunctional 25-kDa protein, is one member of PRDXs [55]. Next, we sought to investigate whether restoration of PRDX6 in Setd2-depleted IECs would attenuate the overproduction of ROS and improve the cell survival as compared with Setd2-deleted IECs. As expected, overexpression of PRDX6 in Setd2-depleted IECs could significantly reduce the ROS production and the cleaved caspase-3 signals (Fig. 6G–H). Besides, PRDX6 overexpression could maintain the epithelial barrier and preserved cell junction proteins (Supplementary Fig. 5). Thus, ectopic Prdx6 could alleviate the overproduction of ROS and improve the cellular survival in the Setd2-deficient intestinal epithelium. Together, these results indicate that SETD2 prevents colon inflammation and epithelial barrier defects via the regulation of antioxidant to maintain ROS homeostasis.
Fig. 6.
SETD2 regulates antioxidant protein via H3K36me3.
(A) Normalized read density of H3K36me3 ChIP-seq signals in IECs isolated from Setd2Vil-KO and Setd2f/f mice treated for 4 d with DSS.
(B) Analysis of the occupancy of H3K36me3 ChIP-seq peaks in gene bodies and intergenic regions.
(C) Venn diagram showing the number of genes harboring H3K36me3 binding and displaying expression changes in Setd2Vil-KO IECs. Right panel shows the Go term analysis of the overlapping genes.
(D) Snapshot of H3K36me3 ChIP-Seq signals at the Prdx3, Prdx6, Gclm and Srxn1 gene loci in IECs isolated from DSS-treated (4 d) Setd2Vil-KO and Setd2f/f mice.
(E) ChIP-qPCR analysis of H3K36me3 binding for Prdx3, Prdx6, Gclm and Srxn1 loci in IECs from DSS-treated (4 d) Setd2Vil-KO and Setd2f/f mice, and IgG was used as the control (n = 3 per genotype).
(F) Correlation between SETD2 and PRDX3, PRDX6, GCLM and SRXN1 expression levels in IBD specimens (GSE 57945). Statistical significance was determined using the Pearson correlation coefficient.
(G) IECs were isolated from Setd2f/f and Setd2Vil-KO mice, and the corresponding are named as IECSetd2-ff and IECSetd2; VIL-KO. Western blot analysis of the indicated proteins inIECSetd2-ff and IECSetd2; VIL-KO with or without PRDX6 overexpression.
(H) Histograms and MFI quantifications of ROS in IECSetd2-ff and IECSetd2; VIL-KO with or without PRDX6 overexpression (n = 4 per genotype).
The data represent the mean ± S.E.M, and statistical significance was determined by a two-tailed Student's t-test. *p < 0.05, **p < 0.01, ***p < 0.001. MFI, Median Fluorescence Intensity.
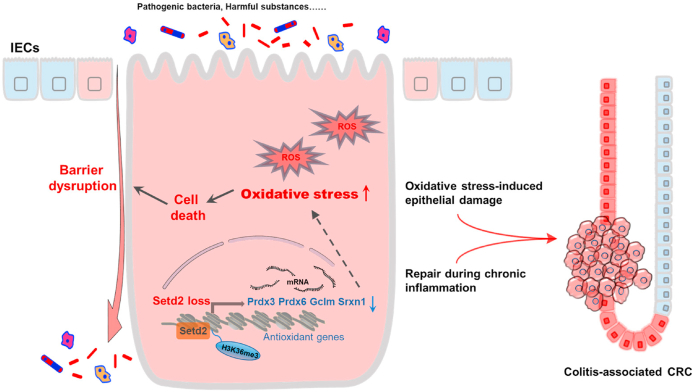
Gut microbes also play an important role in driving colonic inflammatory responses [6,56]. As compared with the Setd2f/f mice, there was less bacterial richness but unchanged gut microbiota composition in Setd2Vil-KO mice (Supplementary Fig. 6A and B). To rule out the possibility that the intestinal microbiota contributes to epithelial barrier damage caused by Setd2 deficiency, we generated gut microbiota-depleted mice by treating Setd2Vil-KO and Setd2f/f mice with drinking water containing an antibiotic cocktail for 4 weeks and then treated them with 2% DSS [57,58]. Interestingly, depletion of the gut microbiota by antibiotics eased the severity of colitis and reduced expression of proinflammatory cytokines and chemokines of Setd2Vil-KO mice comparable to those observed in the Setd2f/f mice (Supplementary Fig. 6C and D). However, antibiotic-treated Setd2Vil-KO mice still exhibited more severe barrier disruption, intestinal permeability, epithelial cell apoptosis and oxidative stress than Setd2f/f mice (Supplementary Fig. 6E-O). Moreover, the mRNA expression levels of some antioxidant genes (Prdx3, Prdx6, Gclm, and Srxn1) were still significantly down-regulated in antibiotic-treated Setd2Vil-KO mice compared with Setd2f/f mice (Supplementary Fig. 6P). These results suggested that depletion of the gut microbiota did not facilitate the recovery of intestinal barrier in Setd2Vil-KO mice. Thus, the gut microbiota does not contribute to intestinal barrier disruption caused by Setd2 deficiency. Considered together, SETD2 protects the colon epithelial barrier from inflammatory insults through a mechanism dependent on oxidative stress (Fig. 7).
Fig. 7.
Loss of Setd2 promotes oxidative stress-induced colonic inflammation and tumorigenesis. SETD2 directly controls multifaceted antioxidant genes including Prdx3, Prdx6, Gclm and Srxn1 in intestinal epithelial cells (IECs). Thus, Setd2 loss in IECs leads to insufficient antioxidant, which results in excess reactive oxygen species (ROS), and thereby compromises barrier integrity and promotes inflammation. Compensatory regeneration coupled with oxidative stress-induced epithelial damage promotes the malignant progression of CRC.
4. Discussion
Although epigenetic dysregulation is recently believed to contribute to development of intestinal inflammation [25,26,59] and epithelial barrier disruption is one of the major causes of IBD, whether epigenetic elements are drivers during the regulation of epithelial barrier integrity and colonic inflammation and tumorigenesis has not been genetically determined. In this study, we established mice with epithelium-specific deletion of Setd2 (Setd2Vil-KO mice), and then challenged with various inducing agents such as DSS and AOM to generate different types of models of colonic inflammation and tumorigenesis. We demonstrated clearly that deletion of Setd2 that is responsible for one of the major histone modifications, disturbs intestinal epithelial homeostasis and worsens barrier defects by modulating oxidative stress, which promotes colonic inflammatory response and subsequent tumorigenesis (Fig. 7). Moreover, the excess inflammatory damage occurring in the Setd2-depleted mice was largely alleviated by the ROS inhibition through antioxidant NAC treatment. Our results highlight the importance that SETD2 functions as a homeostatic regulator that integrates the intact epithelial barrier by modulating oxidative stress in the colon. Support for our findings comes from a recent report indicating that mutations of the Setd2 gene occur in up to 17% in UC samples with a high risk of developing colorectal carcinoma [29]. Thus, our study provides the first direct causal evidence that SETD2 plays a pivotal role in IBD and colitis-associated CRC.
The present study reveals a mechanism by which SETD2 inhibits ROS overreaction to maintain intestinal epithelial homeostasis against intestinal inflammation and tumorigenesis. ROS plays an important role in cell apoptosis induction and inflammation under both physiologic and pathologic conditions [13,14,20]. We found that Setd2 deficiency in the colon epithelium leads to ROS accumulations, and then enhances epithelial cell death and barrier disruption. And this finding is further supported by the experiment of antioxidant NAC treatment. Therefore, excess ROS accumulation is mainly responsible for the colon inflammation observed in the Setd2Vil-KO mice. It is well known that SETD2 exerts epigenetic regulation via promoting the transcriptions of its downstream genes by marking gene bodies with H3K36me3 and facilitating the elongation of their pre-mRNA [60,61]. At molecular level, we show that Setd2 deficiency down-regulates the mRNA levels of the members of antioxidant genes including Prdx3, Prdx6, Gclm and Srxn1 in the analysis of sequencing results. The current findings demonstrate that SETD2 attenuates colonic inflammation through maintaining the expression of antioxidant genes to suppress oxidative stress. Clinically, we also found that expression level of SETD2 in IBD samples is positively correlated with the expressions of PRDX3, PRDX6, GCLM and SRXN1, respectively. Setd2 deficiency led to epithelial barrier disruption accompanied by immune cell infiltration, such as CD4 + T cells, neutrophils, and macrophages (Fig. 2H), suggesting that immune cells might also play a role in the destruction of the epithelium. STAT signaling is involved in the proinflammatory response triggered in colitogenic macrophages and in colitic IEC [62,63]. In the present study, we also examined whether STAT signaling is altered in Setd2-depleted colons. There was no appreciable alternation of p-STAT1 expression level in the Setd2Vil-KO mice. However, p-STAT3 expression was increased in the Setd2Vil-KO mice, indicating activation of p-STAT3 (Supplementary Fig. 7). Therefore, our findings reveal for the first time the important role of SETD2-mediated H3K36me3 modification in the maintenance of intestinal epithelial homeostasis through the oxidative stress pathway, and provide insights into the molecular mechanisms underlying the intestinal inflammation and colitis-associated CRC with epigenetic disorders.
It is worth mentioning that SETD2 appears to interact with various proteins to influence transcription. Notably, our previous work reported that SETD2-mediated H3K36me3 could participate in cross-talks with other chromatin markers in oocytes, including H3K4me3 and H3K27me3 in transcribing regions [44]. In the present study, we demonstrate that expression of some antioxidant genes is regulated by SETD2-mediated H3K36me3. However, little is known about the epigenetic functions of other chromatin markers on regulating these genes in IBD. It would be interesting to study the physiological role of the cross-talk between H3K36me3 and other chromatin markers in IBD and colitis-associated CRC in the future. Moreover, recent studies have also revealed that SETD2 has the capacity to regulate cellular signaling through modification of non-histone substrates. For example, it has been reported that SETD2 could tri-methylate α-tubulin on K40 and mediate K525me1 of STAT1, which implicates a vital role in mitosis and antiviral immunity respectively [64,65]. In the present study, we show that SETD2-mediated H3K36me3 promotes the expression of some antioxidant genes. However, it remains to be determined whether SETD2 can interact with these proteins and methylate them to affect the progression of IBD and tumorigenesis.
5. Conclusions
Our findings highlight that SETD2 plays a critical role in the intestinal homeostasis and regulates barrier function and colon inflammation by modulating oxidative stress in the colon. We also established a SETD2/H3K36me3-deficient IBD mouse model that could be used for pre-clinical researches on colitis with epigenetic disorders. Given that SETD2 mutation accounts for 17% IBD patients with a high risk of developing colorectal carcinoma, our results may provide insight into our understanding of pharmaceutical investigation of these diseases associated with SETD2/H3K36me3 mutation.
Author contributions
M.L. mainly performed the experiments, analyzed the data and wrote the paper. H.R., X.L., J.L., W.F. and J.X. helped with the experiments. L.L. and W.Q.G. conceived the concept, designed the experiments and drafted the manuscript. All authors had edited and approved the final manuscript.
Data availability
All data are available from the authors upon reasonable request. RNA-Seq and ChIP-Seq raw data have been deposited in the Gene Expression Omnibus (GEO) under accession number GEO: GSE 151968.
Declaration of competing interest
The authors declare no competing interests.
Acknowledgements
This study was supported by funds from Ministry of Science and Technology of the People's Republic of China (2017YFA0102900 to W.Q.G.), National Natural Science Foundation of China (81772938 to L.L., 81872406 and 81630073 to W.Q.G.), State Key Laboratory of Oncogenes and Related Genes (KF01801 to L.L.), Science and Technology Commission of Shanghai Municipality (18140902700 and 19140905500 to L.L., 16JC1405700 to W.Q.G.), KC Wong foundation (to W.Q.G.), 111 project (B21024 to W.Q.G.) and Innovation Research Plan from Shanghai Municipal Education Commission (ZXGF082101 to L.L.), by the Shenzhen Basic Research Foundation (JCYJ20170818090044949), and the Guangdong Province Basic Research Foundation (2018A030310012). The study is also supported by Bio-ID Center, School of Biomedical Engineering, Shanghai Jiao Tong University.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2021.102004.
Contributor Information
Wei-Qiang Gao, Email: gao.weiqiang@sjtu.edu.cn.
Li Li, Email: lil@sjtu.edu.cn.
Abbreviations
- AMP
antimicrobial peptide
- AOM
azoxymethane
- 7-AAD
7-aminoactinomycin D
- CD
Crohn's disease
- CRC
colorectal cancer
- DSS
dextran sodium sulfate
- GO
gene ontology
- H2DCFDA
2′,7′-dichlorodihydrofluorescein diacetate
- H3K36
histone H3 lysine 36
- IBD
inflammatory bowel disease
- IEC
intestinal epithelial cell
- NAC
N-acetyl-l-cysteine
- 8-OHdG
8-oxo-2′-deoxyguanosine
- PRDX
peroxiredoxin
- ROS
reactive oxygen species
- RT-qPCR
Real-Time quantitative PCR
- UC
ulcerative colitis
- ZO-1
tight junction protein 1
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Maloy K.J., Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474(7351):298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 2.Kaser A., Zeissig S., Blumberg R.S. Inflammatory bowel disease. Annu. Rev. Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mantovani A. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 4.Grivennikov S.I., Greten F.R., Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillen C.D. Ulcerative colitis and Crohn's disease a comparison of the colorectal cancer risk in extensive colitis. Gut. 1994;35:1590–1592. doi: 10.1136/gut.35.11.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xavier R.J., Podolsky D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448(7152):427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 7.Rescigno M. The intestinal epithelial barrier in the control of homeostasis and immunity. Trends Immunol. 2011;32(6):256–264. doi: 10.1016/j.it.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Nowarski R. Epithelial IL-18 equilibrium controls barrier function in colitis. Cell. 2015;163(6):1444–1456. doi: 10.1016/j.cell.2015.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmitz H. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology. 1999;116:301–309. doi: 10.1016/s0016-5085(99)70126-5. [DOI] [PubMed] [Google Scholar]
- 10.Westbrook A.M., Szakmary A., Schiestl R.H. Mechanisms of intestinal inflammation and development of associated cancers: lessons learned from mouse models. Mutat. Res. 2010;705(1):40–59. doi: 10.1016/j.mrrev.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sedelnikova O.A. Role of oxidatively induced DNA lesions in human pathogenesis. Mutat. Res. 2010;704(1–3):152–159. doi: 10.1016/j.mrrev.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moura F.A. Antioxidant therapy for treatment of inflammatory bowel disease: does it work? Redox Biol. 2015;6:617–639. doi: 10.1016/j.redox.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ravindran R. The amino acid sensor GCN2 controls gut inflammation by inhibiting inflammasome activation. Nature. 2016;531(7595):523–527. doi: 10.1038/nature17186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrett C.W. Selenoprotein P influences colitis-induced tumorigenesis by mediating stemness and oxidative damage. J. Clin. Invest. 2015;125(7):2646–2660. doi: 10.1172/JCI76099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Federico A. Chronic inflammation and oxidative stress in human carcinogenesis. Int. J. Canc. 2007;121(11):2381–2386. doi: 10.1002/ijc.23192. [DOI] [PubMed] [Google Scholar]
- 16.Tian T., Wang Z., Zhang J. Pathomechanisms of oxidative stress in inflammatory bowel disease and potential antioxidant therapies. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/4535194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darnaud M. Enteric delivery of regenerating family member 3 alpha alters the intestinal microbiota and controls inflammation in mice with colitis. Gastroenterology. 2018;154(4):1009–1023 e14. doi: 10.1053/j.gastro.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Kinchen J. Structural remodeling of the human colonic mesenchyme in inflammatory bowel disease. Cell. 2018;175(2):372–386 e17. doi: 10.1016/j.cell.2018.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pei R. Aronia berry supplementation mitigates inflammation in T cell transfer-induced colitis by decreasing oxidative stress. Nutrients. 2019;11(6) doi: 10.3390/nu11061316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu M. BRG1 attenuates colonic inflammation and tumorigenesis through autophagy-dependent oxidative stress sequestration. Nat. Commun. 2019;10(1):4614. doi: 10.1038/s41467-019-12573-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okuda M. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology. 2002;122(2):366–375. doi: 10.1053/gast.2002.30983. [DOI] [PubMed] [Google Scholar]
- 22.Kruidenier L. Intestinal oxidative damage in inflammatory bowel disease: semi-quantification, localization, and association with mucosal antioxidants. J. Pathol. 2003;201(1):28–36. doi: 10.1002/path.1409. [DOI] [PubMed] [Google Scholar]
- 23.Alenghat T. Histone deacetylase 3 coordinates commensal-bacteria-dependent intestinal homeostasis. Nature. 2013;504(7478):153–157. doi: 10.1038/nature12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Theodoris C.V. Human disease modeling reveals integrated transcriptional and epigenetic mechanisms of NOTCH1 haploinsufficiency. Cell. 2015;160(6):1072–1086. doi: 10.1016/j.cell.2015.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albert-Bayo M. Intestinal mucosal mast cells: key modulators of barrier function and homeostasis. Cells. 2019;8(2) doi: 10.3390/cells8020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasler R. A functional methylome map of ulcerative colitis. Genome Res. 2012;22(11):2130–2137. doi: 10.1101/gr.138347.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooke J. Mucosal genome-wide methylation changes in inflammatory bowel disease. Inflamm. Bowel Dis. 2012;18(11):2128–2137. doi: 10.1002/ibd.22942. [DOI] [PubMed] [Google Scholar]
- 28.Wellman A.S. Intestinal epithelial sirtuin 1 regulates intestinal inflammation during aging in mice by altering the intestinal microbiota. Gastroenterology. 2017;153(3):772–786. doi: 10.1053/j.gastro.2017.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chakrabarty S. Targeted sequencing-based analyses of candidate gene variants in ulcerative colitis-associated colorectal neoplasia. Br. J. Canc. 2017;117(1):136–143. doi: 10.1038/bjc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mikkelsen T.S. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2007;448(7153):553–560. doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu M. Histone H3 lysine 36 methyltransferase Hypb/Setd2 is required for embryonic vascular remodeling. Proc. Natl. Acad. Sci. U. S. A. 2010;107(7):2956–2961. doi: 10.1073/pnas.0915033107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pfister S.X. Inhibiting WEE1 selectively kills histone H3K36me3-deficient cancers by dNTP starvation. Canc. Cell. 2015;28(5):557–568. doi: 10.1016/j.ccell.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y. H3K36 histone methyltransferase Setd2 is required for murine embryonic stem cell differentiation toward endoderm. Cell Rep. 2014;8(6):1989–2002. doi: 10.1016/j.celrep.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 34.Kanu N. SETD2 loss-of-function promotes renal cancer branched evolution through replication stress and impaired DNA repair. Oncogene. 2015;34(46):5699–5708. doi: 10.1038/onc.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li F. The histone mark H3K36me3 regulates human DNA mismatch repair through its interaction with MutSalpha. Cell. 2013;153(3):590–600. doi: 10.1016/j.cell.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Terzic J. Inflammation and colon cancer. Gastroenterology. 2010;138(6):2101–2114 e5. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 37.Parker H. Genomic disruption of the histone methyltransferase SETD2 in chronic lymphocytic leukaemia. Leukemia. 2016;30(11):2179–2186. doi: 10.1038/leu.2016.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mar B.G. Mutations in epigenetic regulators including SETD2 are gained during relapse in paediatric acute lymphoblastic leukaemia. Nat. Commun. 2014;5:3469. doi: 10.1038/ncomms4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walter D.M. Systematic in vivo inactivation of chromatin-regulating enzymes identifies Setd2 as a potent tumor suppressor in lung adenocarcinoma. Canc. Res. 2017;77(7):1719–1729. doi: 10.1158/0008-5472.CAN-16-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee J.J. Tracing oncogene rearrangements in the mutational history of lung adenocarcinoma. Cell. 2019;177(7):1842–1857 e21. doi: 10.1016/j.cell.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 41.Wang L. H3K36 trimethylation mediated by SETD2 regulates the fate of bone marrow mesenchymal stem cells. PLoS Biol. 2018;16(11) doi: 10.1371/journal.pbio.2006522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ji Z. The histone methyltransferase Setd2 is indispensable for V(D)J recombination. Nat. Commun. 2019;10(1):3353. doi: 10.1038/s41467-019-11282-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.niu n. Loss of Setd2 promotes Kras-induced acinar-to-ductal metaplasia and epithelia–mesenchymal transition during pancreatic carcinogenesis. Gut. 2019:1–12. doi: 10.1136/gutjnl-2019-318362. 0. [DOI] [PubMed] [Google Scholar]
- 44.Xu Q. SETD2 regulates the maternal epigenome, genomic imprinting and embryonic development. Nat. Genet. 2019;51(5):844–856. doi: 10.1038/s41588-019-0398-7. [DOI] [PubMed] [Google Scholar]
- 45.Zuo X. The histone methyltransferase SETD2 is required for expression of acrosin-binding protein 1 and protamines and essential for spermiogenesis in mice. J. Biol. Chem. 2018;293(24):9188–9197. doi: 10.1074/jbc.RA118.002851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wirtz S. Chemically induced mouse models of intestinal inflammation. Nat. Protoc. 2007;2(3):541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 47.Wirtz S., Neurath M.F. Mouse models of inflammatory bowel disease. Adv. Drug Deliv. Rev. 2007;59(11):1073–1083. doi: 10.1016/j.addr.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 48.Wlodarska M. NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell. 2014;156(5):1045–1059. doi: 10.1016/j.cell.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bevins C.L., Salzman N.H. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Microbiol. 2011;9(5):356–368. doi: 10.1038/nrmicro2546. [DOI] [PubMed] [Google Scholar]
- 50.Okayasu I. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 51.Gupta J. Dual function of p38alpha MAPK in colon cancer: suppression of colitis-associated tumor initiation but requirement for cancer cell survival. Canc. Cell. 2014;25(4):484–500. doi: 10.1016/j.ccr.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 52.Ramirez-Carrozzi V. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat. Immunol. 2011;12(12):1159–1166. doi: 10.1038/ni.2156. [DOI] [PubMed] [Google Scholar]
- 53.Steinke J.W., Borish L. 3. Cytokines and chemokines. J. Allergy Clin. Immunol. 2006;117(2 Suppl Mini-Primer):S441–S445. doi: 10.1016/j.jaci.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 54.Cha H. Increased susceptibility of IDH2-deficient mice to dextran sodium sulfate-induced colitis. Redox Biol. 2017;13:32–38. doi: 10.1016/j.redox.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patel P., Chatterjee S. Peroxiredoxin6 in endothelial signaling. Antioxidants. 2019;8(3) doi: 10.3390/antiox8030063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Belkaid Y., Hand T.W. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rakoff-Nahoum S. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118(2):229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 58.Morgun A. Uncovering effects of antibiotics on the host and microbiota using transkingdom gene networks. Gut. 2015;64(11):1732–1743. doi: 10.1136/gutjnl-2014-308820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ray G., Longworth M.S. Epigenetics, DNA organization, and inflammatory bowel disease. Inflamm. Bowel Dis. 2019;25(2):235–247. doi: 10.1093/ibd/izy330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fontebasso A.M. Mutations in SetD2 and genes affecting histone H3K36 methylation target hemispheric high-grade gliomas. Acta Neuropathol. 2013;125:659–669. doi: 10.1007/s00401-013-1095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Neri F. Intragenic DNA methylation prevents spurious transcription initiation. Nature. 2017;543(7643):72–77. doi: 10.1038/nature21373. [DOI] [PubMed] [Google Scholar]
- 62.Piedra-Quintero Z.L. Myosin 1F regulates M1-polarization by stimulating intercellular adhesion in macrophages. Front. Immunol. 2018;9:3118. doi: 10.3389/fimmu.2018.03118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Serrano C. Compartmentalized response of IL-6/STAT3 signaling in the colonic mucosa mediates colitis development. J. Immunol. 2019;202(4):1239–1249. doi: 10.4049/jimmunol.1801060. [DOI] [PubMed] [Google Scholar]
- 64.Park I.Y. Dual chromatin and cytoskeletal remodeling by SETD2. Cell. 2016;166(4):950–962. doi: 10.1016/j.cell.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen K. Methyltransferase SETD2-mediated methylation of STAT1 is critical for interferon antiviral activity. Cell. 2017;170(3):492–506 e14. doi: 10.1016/j.cell.2017.06.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available from the authors upon reasonable request. RNA-Seq and ChIP-Seq raw data have been deposited in the Gene Expression Omnibus (GEO) under accession number GEO: GSE 151968.