Abstract
Background & Aims
Primary sclerosing cholangitis (PSC) is a rare cholangiopathy of unknown aetiopathogenesis. The aim of this study was to evaluate cellular senescence (CS) marker expression in cholangiocytes of patients with PSC and their correlation with clinical–pathological features and prognosis.
Methods
Thirty-five patients with PSC with at least 1 available liver sampling were included. Clinical laboratory data at the time of liver sampling were collected. The endpoints were survival without liver transplantation (LT), time to LT, and survival without LT or cirrhosis decompensation. Histological grading and staging were assessed according to Nakanuma. Immunohistochemical stains for CS markers, p16INK4A (p16) and p21WAF1/Cip1 (p21), were performed and scored by a 3-tier scale based on positivity extent in native bile duct (NBD) and ductular reaction (DR).
Results: p16 expression in NBD and DR was directly correlated with fibrosis (p ≤0.001 for both) and stage (p = 0.006 and p <0.001, respectively). Moreover, p16 in NBD was positively correlated with hepatitis activity (HA) (p = 0.026), whereas p16 in DR was directly correlated with bile duct loss (BDL) (p = 0.005) and metaplastic hepatocytes (MH) (p <0.01). p21 expression in NBD and DR was directly correlated with HA (p = 0.004 and p = 0.043, respectively), fibrosis (p = 0.006 and p <0.001, respectively), stage (p = 0.006 and p = 0.001, respectively), BDL (p = 0.002 and p = 0.03, respectively), and DR and MH (p ≤0.004 for all). By multivariate analysis, p16 expression in DR was independently associated with stage (p = 0.001), fibrosis (p = 0.001), and BDL (p = 0.011). p21 expression in NBD was independently associated with HA (p = 0.012), BDL (p = 0.04), and DR (p = 0.014). Finally, p21 expression in DR was independently associated with LT-free survival, time to LT, and adverse outcome-free survival (p = 0.001, p = 0.017, and p = 0.001, respectively).
Conclusions
Cholangiocyte senescence is detectable in all stages of PSC and is associated with histological and clinical disease severity, potentially representing a new prognostic and therapeutic target.
Lay summary
In this study, we showed that cholangiocyte senescence (CS), previously demonstrated in liver of patients with end-stage primary sclerosing cholangitis (PSC), is an early event and is detectable in all disease stages. Moreover, we observed that CS is associated with histological and clinical disease severity and patients’ outcome. Thus, we suggest that CS may represent a new prognostic tool and a potential therapeutic target in PSC.
Clinical trial number
Protocol number 0034435, 08/06/2020.
Keywords: PSC, p16, p21, Senescent cholangiocytes, Fibrosing cholangiopathy, Prognosis
Abbreviations: γGT, γ-glutamyltranspeptidase; AIH, autoimmune hepatitis; ALP, alkaline phosphatase; ALT, alanine transaminase; BDL, bile duct loss; CA, cholangitis activity; CCA, cholangiocarcinoma; CK7, cytokeratin 7; CS, cellular senescence; DR, ductular reaction; GBCA, gallbladder carcinoma; HA, hepatitis activity; HCC, hepatocellular carcinoma; HR, hazard ratio; IBD, inflammatory bowel disease; IHC, immunohistochemical; INR, international normalized ratio; LT, liver transplantation; MH, metaplastic hepatocytes; NBD, native bile duct; OR, odds ratio; p16, p16INK4A; p21, p21WAF1/Cip1; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; PT, portal tract; SASP, senescence-associated secretory phenotype; SMA, smooth muscle actin; TGFβ, transforming growth factor beta; UDCA, ursodeoxycholic acid
Graphical abstract
Highlights
-
•
Cholangiocyte senescence was previously described in end-stage PSC.
-
•
Cholangiocyte senescence is present in all stages of PSC and may represent an early pathogenic event.
-
•
Cholangiocyte senescence is associated with histological and clinical severity in patients with PSC.
-
•
Cholangiocyte senescence is independently associated with patients’ outcome in PSC.
Introduction
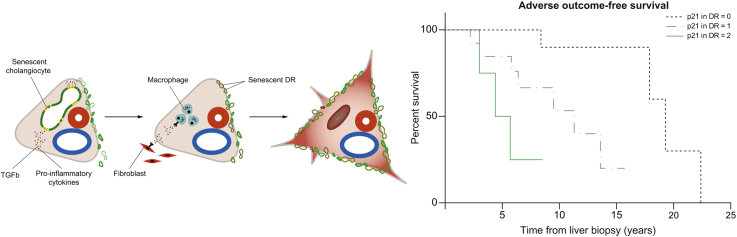
Primary sclerosing cholangitis (PSC) is a rare chronic cholestatic liver disease of unknown aetiology, characterised by inflammatory damage of intrahepatic and/or extrahepatic bile ducts, leading to strictures, fibrosis, and liver cirrhosis.1 The hallmark of the condition is the close association with inflammatory bowel diseases (IBDs) in about two-thirds of patients.2 Patients with PSC have a high risk of hepatobiliary malignancies, mainly cholangiocarcinoma (CCA), gallbladder carcinoma (GBCA), and colorectal cancer.2,3 Malignancies, liver failure, and transplant-related complications represent the most frequent causes of PSC-related deaths. No effective pharmacotherapy exists to delay disease progression, and liver transplantation (LT) is the only effective treatment to extend survival in these patients. The pathogenesis of the disease is not completely understood. Currently, PSC is considered a complex disorder accounting for multiple pathogenic components, including interaction between genetic predisposition and environmental factors, engagement of innate immunity by gut-derived microbial products, activation of adaptive immune responses by gut-derived antigens followed by clonal expansion of T cells and migration to the liver, disturbances of bile acid homeostasis, and genetic alterations of immune cell function.1 A current hypothesis, supported by various observations, suggests that cholangiocytes have a crucial role in initiating and perpetuating biliary damage in PSC. In fact, cholangiocytes seem to respond to endogenous or exogenous insults by acquiring the ability to proliferate and secrete different pro-inflammatory mediators acting in a paracrine and/or autocrine manner. This determines the recruitment and stimulation of immune cells and fibroblasts, which induce the development of the typical PSC fibro-inflammatory lesions.4,5 In case of persisting chronic injury, the reparative process becomes ineffective, and the inflammatory response perseveres, promoting ductular reaction (DR) and inducing cellular senescence (CS) in chronic injured cholangiocytes.4,5 CS is a cell state triggered by stressful insults and certain physiological processes, characterised by a prolonged and generally irreversible cell-cycle arrest in the G1 phase, associated with the acquisition of secretory features, the so-called senescence-associated secretory phenotype (SASP), macromolecular damage, and altered metabolism.6 CS may be triggered by different stimuli, including strong mitogenic or oncogenic signals, telomere shortening, and/or non-telomeric DNA damage, and it is aimed to prevent the uncontrolled replication of injured cells.6 In the recent years, CS has been recognised to play a role in the pathogenesis of different chronic conditions, including atherosclerosis, neurodegenerative disorders, chronic obstructive pulmonary disease, and a wide range of chronic liver disorders.7,8 Biliary epithelial senescence in small bile ducts and bile ductules is a characteristic of primary biliary cholangitis (PBC),[9], [10], [11] where CS correlates with disease activity and stage. Increased expression of the senescence markers SA-b-GAL, p16INK4A (p16), and p21WAF1/Cip1 (p21) has been observed in cholangiocytes in PSC,12,13 suggesting CS may have a pathogenic role in this disease. However, data regarding CS in PSC are scant, mainly from end-stage disease, and nothing is known about its clinical meaning. Interestingly, recent data on mouse models of chronic cholestatic liver disease showed CS involvement in cholestatic damage and fibrosis, which can ameliorate after specific interventions targeting CS.14,15
Therefore, the aims of our study were to evaluate the hepatic tissue expression of CS markers in liver samples from patients with PSC in different histological stages and analyse the correlation of CS with clinical-pathological features and patients’ prognosis.
Patients and methods
Design and study population
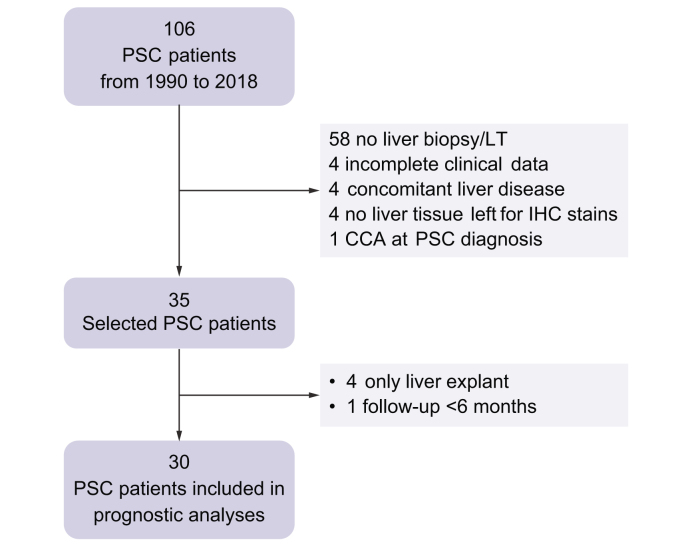
We designed a longitudinal retrospective study based on a cohort of patients who were recruited prospectively. The study was conformed to the ethical guidelines of the 1975 Declaration of Helsinki and approved by the Ethic Committee of the University Hospital of Padova, and written informed consent was obtained from each patient included in the study. Inclusion criteria were as follows: (i) age at the time of liver biopsy or LT ≥18 years; (ii) definite diagnosis of PSC according to international guidelines16; (iii) presence of at least 1 liver biopsy performed at diagnosis and/or during follow-up and/or liver specimens from explanted liver; and (iv) presence of available liver tissue for histological revaluation and immunohistochemical (IHC) stains. Exclusion criteria were as follows: (i) concomitant hepatic comorbidities (viral and non-viral hepatitis or secondary sclerosing cholangitis); and (ii) detection of primary or secondary liver cancer and/or biliary malignancies at the time of liver biopsy and/or LT, or in the following 6 months. Patients were all diagnosed and prospectively followed up over time by the same clinician (AF) in a tertiary care centre, the Centre for Rare and Cholestatic Liver Disease of the University Hospital of Padova, from 1990 to 2018. Selected cases were collected according to the inclusion and exclusion criteria, after a complete revision of imaging, laboratory data, and histology reports for each case. For prognostic evaluation, only patients with at least 6 months of follow-up after liver biopsy were considered, whereas patients in whom only liver specimens from explanted livers were available were excluded; if multiple biopsies from the same patient were available, we only considered the first one performed. The flowchart of case inclusion is reported in Fig. 1. Appropriate informed consent was routinely obtained for any procedure performed during patient’s follow-up. No donor organs were obtained from executed prisoners or other institutionalised people.
Fig. 1.
Flowchart of case selection.
CCA, cholangiocarcinoma; IHC, immunohistochemical; LT, liver transplantation; PSC, primary sclerosing cholangitis.
Clinical data collection
Clinical–biological, radiological, and laboratory data were retrospectively collected from patients’ charts. Recorded data included sex, age, date of PSC diagnosis, liver biopsy and/or LT, the subtype of PSC, the localisation of biliary changes (intrahepatic, extrahepatic, or both), the association with IBDs (and the type of IBD), and the presence of cirrhosis and ursodeoxycholic acid (UDCA) treatment at the time of liver biopsy. Results of blood tests closest to the date of liver biopsy or LT (±1 month) were also collected, including bilirubin, alanine transaminase (ALT), γ-glutamyltranspeptidase (γGT), alkaline phosphatase (ALP), serum albumin, and platelet levels, and international normalised ratio (INR). The occurrence and date of any event (including cirrhosis decompensation, listing for LT, LT, and death) developed during patients’ follow-up were recorded. The development of any hepatobiliary neoplasia (hepatocellular carcinoma [HCC], CCA, and GBCA), liver metastasis, or colorectal cancer and the occurrence of acute bacterial cholangitis during patients’ follow-up (before and after liver biopsy) were also reported.
Histological study
All liver tissue samples were formalin fixed, paraffin embedded, and routinely stained with H&E and Masson’s trichrome stain to evaluate liver fibrosis. Each case was blindly and contemporarily reviewed by an experienced (MG) and a trainee (SS) liver pathologist. Histological grading and staging were performed according to Nakanuma’s system,17 which has demonstrated the strongest prognostic value for major outcomes also in PSC.18,19 According to Nakanuma’s system, grading is determined by the extent of chronic cholangitis activity (CA) and hepatitis activity (HA). For stage assessment, we applied the 2-criteria method and evaluated the presence and extent of fibrosis and bile duct loss (BDL), excluding the evaluation of orcein-positive granules, since it is nonessential to obtain an accurate and reproducible staging.17 In addition, DR and metaplastic hepatocytes (MH) were assessed by means of immunohistochemistry.
IHC analysis
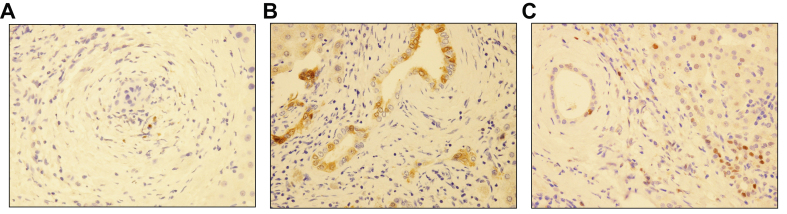
We immunohistochemically examined CS-associated markers, p16 and p21, and cytokeratin 7 (CK7). We also evaluated the amount of macrophage and myofibroblast infiltration in the portal tracts (PTs) by using IHC markers for macrophages (CD163) and myofibroblasts (smooth muscle actin [SMA]). Immunostaining was performed in liver samples obtained from needle biopsies and in selected slides from explanted livers, by using the following antibodies: anti-p16 (E6H4 clone, Ventana Medical Systems Inc., Tucson, AZ, USA; working dilution 1:1,000; mouse monoclonal), anti-p21 (SX118 clone, Dako, Glostrup, Denmark; working dilution 1:50; mouse monoclonal), anti-CK7 (OV-TL 12/30 clone, Cell Marque, Rocklin, CA, USA; working dilution 1:200; mouse monoclonal), anti-CD163 (MRQ-26 clone, Cell Marque; working dilution 1:50; mouse monoclonal), and anti-SMA (1A4 clone, Dako; working dilution 1:100; mouse monoclonal) (Rev 1, Q4). IHC staining was conducted according to standard techniques by using a Leica Microsystems Bond-Max autostainer (Leica Biosystems, Newcastle Upon Tyne, UK), and all the slides were counterstained with haematoxylin. Appropriate positive and negative controls were used for each run. CK7 stain was used to better evaluate the extent of DR and the presence and extent of MH. DR was semiquantitatively scored on a 4-tier scale, as follows: score 0, no DR; score 1, DR present in less than 50% of PTs; score 2, DR present in greater than or equal to 50% of PTs; and score 3, DR present in greater than or equal to 50% of PTs and presence of ductular buds into the acinar parenchyma (Fig. S1). MH were evaluated and scored as follows: score 0, no MH; score 1, MH present around less than 50% of PTs; and score 2, MH present around greater than or equal to 50% of PTs. In the evaluation of p21, only nuclear staining was considered, whereas p16 expression was both nuclear and cytoplasmic. p16 and p21 expression was semiquantitatively scored on a 3-tier scale based on the positivity extent in native bile duct (NBD) and in DR, as previously reported11: score 0, negative; score 1, focal expression, in less than one-third of PTs; and score 2, diffuse expression, in greater than or equal to one-third of PTs11 (Fig. 2).
Fig. 2.
IHC evaluation of CS markers in PSC cases.
(A) Examples of a negative stain for p16 (original magnification 40×), (B) a diffuse nuclear and cytoplasmic p16 expression in both NBD and DR (original magnification 40×), and (C) a diffuse p21 nuclear expression, in both NBD and DR (original magnification 40×). CS, cellular senescence; DR, ductular reaction; IHC, immunohistochemical; NBD, native bile duct; p16, p16INK4A; p21, p21WAF1/Cip1; PSC, primary sclerosing cholangitis.
The amount of macrophage and myofibroblast infiltration in the PTs was semiquantitatively scored as follows: score 0, no macrophages/myofibroblasts in the PTs; score 1, very few macrophages/myofibroblasts in some PTs; score 2, numerous macrophages/myofibroblasts in most PTs; and score 3, clusters of macrophages/myofibroblasts in most PTs (Fig. S2). In each case, for both markers, a positive internal control was present, confirming the good performance of the staining, and was represented by Kupffer cells in the sinusoids for CD163 and the muscular layer of vessel walls for SMA.
Statistical analysis
Continuous variables were summarised as median and IQR, whereas categorical variables as frequency and percentage. To overcome potential assay variability over time, biochemical variables were expressed as ratios of upper limits of normal. For clinical–pathological correlations, Student’s t test, the 1-way ANOVA test, the Spearman rank correlation test, and the Fisher exact probability test were used when appropriate. To assess the prognostic value of cholangiocyte CS, we considered 3 endpoints: (1) LT-free survival (time elapsed from the first liver biopsy to last visit or occurrence of LT or PSC-related death, including death for end-stage liver disease, CCA, or HCC); (2) time to LT; and (3) adverse outcome-free survival (time elapsed from the first liver biopsy to last visit or occurrence of an adverse event, defined as (i) development of cirrhosis decompensation (i.e. the first occurrence of ascites, variceal bleeding, hepatic encephalopathy, or hepatorenal syndrome); (ii) LT or listing for LT; and (iii) PSC-related death, as previously defined.18 The Kaplan–Meier method was used to calculate survival and the log-rank test was applied to compare survival between different groups. The univariate Cox regression model was performed to first assess the individual prognostic value of CS markers, histological findings, and biochemistry. Variables that were identified as significant on univariate analyses were then included in the multivariate Cox backward stepwise regression analysis. Hazard ratios (HRs) and their 95% CIs were also calculated. All analyses were performed by using Statistical Package for the Social Science (version 25, IBM SPSS Statistics, Chicago, IL, USA) and GraphPad (version 6, GraphPad Software, San Diego, CA, USA) statistical software products. Values of p <0.05 were considered significant.
Results
Baseline characteristics of the patients
Overall, 35 patients with PSC who had 44 liver biopsies/explants were included in the study. The 44 liver samples were distributed as follows: 24 patients underwent only 1 liver biopsy at diagnosis or during follow-up, 2 patients underwent 2 liver biopsies during follow-up, 3 patients underwent 1 liver biopsy at diagnosis or during follow-up and subsequently underwent transplantation (1 liver biopsy and 1 LT tissue for each patient), and 2 patients underwent 2 liver biopsies at diagnosis or during follow-up and also subsequently underwent transplantation (2 liver biopsy and 1 LT tissue for each patient). Finally, as already mentioned, in 4 patients, only tissue from the explanted liver was available. However, 30 patients were considered for prognostic analyses, as 4 cases with only tissue from the explanted liver and 1 patient with less than 6 months of follow-up were excluded. All patients were treated with UDCA after PSC diagnosis. Baseline clinical features of the patients at the time of PSC diagnosis are summarised in Table 1.
Table 1.
Clinical features of the 35 patients with PSC at the time of diagnosis.
| Feature | Value∗ |
|---|---|
| Male sex | 14 (40.0) |
| Age† | 27 (22–38) |
| Type of PSC | |
| Large-duct PSC | 28 (80.0) |
| Small-duct PSC | 3 (8.6) |
| PSC-AIH variant | 4 (11.4) |
| Localisation of bile duct changes in large-duct PSC | |
| Intrahepatic only | 8 (26.7) |
| Intrahepatic and extrahepatic | 16 (53.3) |
| Extrahepatic only | 6 (20.0) |
| Cirrhosis at diagnosis | 2 (5.7) |
| Inflammatory bowel disease | |
| Ulcerative colitis | 13 (37.1) |
| Crohn disease | 5 (14.3) |
| Absence of IBD | 17 (48.6) |
AIH, autoimmune hepatitis; IBD, inflammatory bowel disease; PSC, primary sclerosing cholangitis.
Categorical variables are described as frequency (%).
Age is reported as median (IQR).
Histological analysis and clinical–pathological correlations
Detailed histological findings are summarised in Table 2. According to Nakanuma’s system, stage 1 was observed in 6.8% of specimens, stage 2 in 31.8%, stage 3 in 34.1%, and stage 4 in 27.3%. DR and MH were found in all liver samples, both being present in ≥50% of PTs in most of the cases. The stage of the disease strongly correlated with the severity of HA (σ = 0.46, p = 0.002) and the presence of DR and MH (σ = 0.57, p <0.001, and σ = 0.56, p <0.001, respectively). Laboratory findings at the time of liver sampling (Table 3) correlated with histological features. In fact, serum bilirubin and INR directly correlated with BDL (σ = 0.34, p = 0.03, and σ = 0.55, p = 0.002, respectively), DR (σ = 0.36, p = 0.02, and σ = 0.52, p = 0.004, respectively), the extent of fibrosis (σ = 0.38, p = 0.01, and σ = 0.72, p <0.001, respectively), and the stage of the disease (σ = 0.37, p = 0.02, and σ = 0.68, p <0.001, respectively). Serum albumin levels inversely correlated with HA (σ = −0.45, p = 0.004), BDL (σ = −0.60, p <0.001), MH (σ = −0.32, p = 0.05), DR (σ = −0.36, p = 0.03), fibrosis (σ = −0.68, p <0.001), and stage of the disease (σ = −0.73, p <0.001). Finally, platelet count inversely correlated with DR (σ = −0.32, p = 0.04), fibrosis (σ = −0.61, p <0.001), and the stage of the disease (σ = −0.48, p = 0.002).
Table 2.
Histological features in 44 liver samples of 35 patients with PSC.
| Histological feature | PSC liver samples∗ (N = 44) |
|---|---|
| Disease duration at the time of sampling (years)† | 1 (0–9) |
| Biopsy length (mm)‡ | 16 (10–25) |
| Cholangitis activity | |
| Absent | 30 (68.2) |
| Mild | 7 (15.9) |
| Moderate | 6 (13.6) |
| Marked | 1 (2.3) |
| Hepatitis activity | |
| Absent | 19 (43.1) |
| Mild | 8 (18.2) |
| Moderate | 8 (18.2) |
| Marked | 9 (20.5) |
| Bile duct loss | |
| Score 0 | 12 (27.3) |
| Score 1 | 10 (22.7) |
| Score 2 | 12 (27.3) |
| Score 3 | 10 (22.7) |
| Fibrosis | |
| Score 0 | 4 (9.1) |
| Score 1 | 14 (31.8) |
| Score 2 | 15 (34.1) |
| Score 3 | 11 (25.0) |
| Stage | |
| Stage 1 | 3 (6.8) |
| Stage 2 | 14 (31.8) |
| Stage 3 | 15 (34.1) |
| Stage 4 | 12 (27.3) |
| Ductular reaction | |
| Score 0 | 0 (0) |
| Score 1 | 10 (22.7) |
| Score 2 | 16 (36.4) |
| Score 3 | 18 (40.9) |
| Metaplastic hepatocytes | |
| Score 0 | 10 (22.7) |
| Score 1 | 11 (25.0) |
| Score 2 | 21 (47.7) |
| Not available | 2 (4.6) |
Categorical variables are described as frequency (%).
Disease duration is reported as median (IQR).
Biopsy length is referred only to liver biopsies (N = 35) and is reported as median (IQR). PSC, primary sclerosing cholangitis.
Table 3.
Biochemical findings at the time of liver sampling.
| Biochemical variable | Value |
|---|---|
| Total bilirubin (μmol/L) | 19.0 (9.6–52.9) 3 (6.8) |
| ALT × ULN | 1.7 (1.2–3.2) 5 (11.4) |
| γGT × ULN | 3.6 (1.4–7.2) 5 (11.4) |
| ALP × ULN | 2.4 (1.4–3.2) 5 (11.4) |
| Serum albumin (g/L) | 38.3 (30.4–41.2) 3 (6.8) |
| Platelets count (× 109/mm3) | 221 (158–41) 5 (11.4) |
| INR | 1.1 (1.0–1.2) 15 (34.1) |
Variables are reported as median (IQR). Missing data are reported in italic and are expressed as frequency (%).
γGT, gamma-glutamyltranspeptidase; ALP, alkaline phosphatase; ALT, alanine aminotransferase; INR international normalised ratio; ULN, upper limit of normal.
Expression of CS markers (p16 and p21) and correlations with histological and biochemical findings
The expression of CS markers, p16 and p21, in NBD and DR is summarised in Table 4. Overall, the expression of CS markers in NBD and in DR was observed in the majority of cases. No differences in the expression of CS markers in NBD and DR were found according to different types of disease, i.e. large duct, small duct, and PSC-autoimmune hepatitis (AIH) variant (data not shown). The extent of p16 expression in NBD directly correlated with HA (σ = 0.34, p = 0.026), fibrosis (σ = 0.49, p = 0.001), and disease stage (σ = 0.41, p = 0.006), whereas p16 expression in DR directly correlated with BDL (σ = 0.42, p = 0.005), fibrosis (σ = 0.71, p <0.001), disease stage (σ = 0.60, p <0.001), and the presence of MH (σ = 0.54, p <0.001). p21 expression in NBD and DR directly correlated with HA (σ = 0.46, p = 0.004, and σ = 0.31, p = 0.043, respectively), BDL (σ = 0.50, p = 0.002, and σ = 0.32, p = 0.03, respectively), fibrosis (σ = 0.44, p = 0.006, and σ = 0.53, p <0.001, respectively), stage (σ = 0.45, p = 0.006, and σ = 0.48, p = 0.001, respectively), MH (σ = 0.52, p = 0.002, and σ = 0.57, p <0.001, respectively), and DR (σ = 0.47, p = 0.004, and σ = 0.59, p <0.001, respectively). Results of univariate analysis are reported in Table 5 and graphically summarised in Figs. S3 and S4.
Table 4.
Expression of cellular senescence markers in native bile duct and ductular reaction.
| Senescence marker | PSC liver samples∗ (n = 44) |
|---|---|
| p16 in native bile duct | |
| Score 0 | 12 (27.3) |
| Score 1 | 15 (34.1) |
| Score 2 | 17 (38.6) |
| p16 in ductular reaction | |
| Score 0 | 8 (18.2) |
| Score 1 | 13 (29.5) |
| Score 2 | 23 (52.3) |
| p21 in native bile duct | |
| Score 0 | 8 (18.2) |
| Score 1 | 19 (43.2) |
| Score 2 | 10 (22.7) |
| Not available | 7 (15.9) |
| p21 in ductular reaction | |
| Score 0 | 14 (31.8) |
| Score 1 | 20 (45.5) |
| Score 2 | 10 (22.7) |
p16, p16INK4A; PSC, primary sclerosing cholangitis.
Categorical variables are described as frequency (%).
Table 5.
Histological features associated with CS cholangiocyte marker expression (univariate analysis).
| Regression coefficient | 95% CI | p value | |
|---|---|---|---|
| p16 in NBD | |||
| HA | 0.39 | 0.07 to 0.93 | 0.025 |
| BDL | 0.25 | −0.08 to 0.76 | 0.107 |
| Fibrosis | 0.49 | 0.26 to 0.89 | 0.001 |
| Stage | 0.43 | 0.17 to 0.81 | 0.003 |
| MH | 0.26 | −0.05 to 0.59 | 0.093 |
| DR | 0.22 | −0.08 to 0.51 | 0.148 |
| p16 in DR | |||
| HA | 0.27 | −0.05 to 0.87 | 0.082 |
| BDL | 0.34 | 0.13 to 0.97 | 0.012 |
| Fibrosis | 0.69 | 0.57 to 1.11 | <0.001 |
| Stage | 0.58 | 0.38 to 0.99 | <0.001 |
| MH | 0.51 | 0.25 to 0.84 | 0.001 |
| DR | 0.54 | 0.29 to 0.82 | <0.001 |
| p21 in NBD | |||
| HA | 0.46 | 0.26 to 1.26 | 0.004 |
| BDL | 0.50 | 0.33 to 1.28 | 0.001 |
| Fibrosis | 0.29 | 0.18 to 0.99 | 0.006 |
| Stage | 0.45 | 0.18 to 0.99 | 0.006 |
| MH | 0.54 | 0.26 to 0.92 | 0.001 |
| DR | 0.46 | 0.18 to 0.90 | 0.004 |
| p21 in DR | |||
| HA | 0.28 | −0.03 to 0.93 | 0.068 |
| BDL | 0.33 | 0.05 to 0.95 | 0.030 |
| Fibrosis | 0.53 | 0.34 to 1.01 | <0.001 |
| Stage | 0.49 | 0.27 to 0.94 | 0.001 |
| MH | 0.57 | 0.35 to 0.95 | <0.001 |
| DR | 0.59 | 0.36 to 0.89 | <0.001 |
BDL, bile duct loss; CS, cellular senescence; DR, ductular reaction; HA, hepatitis activity; MH, metaplastic hepatocytes; NBD, native bile duct; p16, p16INK4A; p21, p21WAF1/Cip1. In Bold p values < 0.05.
By multivariate logistic regression analysis, p16 expression in DR was independently associated with the stage of the disease (HR 13.62, 95% CI 2.86–64.92, p = 0.001), fibrosis (HR 16.02, 95% CI 2.99–85.55, p = 0.001), BDL (HR 19.19, 95% CI 1.96–187.68, p = 0.011), and MH (HR 8.75, 95% CI 1.61–47.69, p = 0.012). Moreover, p21 expression in NBD was independently associated with HA (HR 5.49, 95% CI 1.46–20.73, p = 0.012), BDL (HR 10.39, 95% CI 1.11–97.08, p = 0.04), and DR (HR 13.44, 95% CI 1.69–106.99, p = 0.014).
As expected, all CS markers were significantly and reciprocally correlated: p16 expression in NBD was related to p16 expression in DR (σ = 0.496, p = 0.001), and p21 expression in NBD was related to p21 expression in DR (σ = 0.557, p = 0.002). Moreover, p16 expression in NBD and in DR was associated with p21 expression in NBD (σ = 0.461, p = 0.012, and σ = 0.610, p <0.0001, respectively) and p21 expression in DR (σ = 0.354, p = 0.034, and σ = 0.640, p <0.0001, respectively), confirming their role in the same cell death process. We also found a direct correlation between p16 expression, in both NBD and DR, and the amount of macrophages (σ = 0.416, p = 0.0002, and σ = 0.532, p = 0.006, respectively) and myofibroblasts in the PTs (σ = 0.31, p = 0.0006, and σ = 0.544, p = 0.02, respectively). The portal number of macrophages and myofibroblasts was also directly related to p21 expression, in both NBD and DR (σ = 0.569, p = 0.0001, and σ = 0.532, p <0.0001, for macrophage infiltration, respectively; σ = 0.527, p = 0.0006, and σ = 0.57, p <0.0001, for myofibroblast infiltration, respectively). However, portal macrophages were directly correlated with the amount of portal myofibroblasts, fibrosis, BDL, and the stage of the disease (σ = 0.73, p <0.0001; σ = 0.53, p <0.0001; σ = 0.507, p = 0.001; and σ = 0.555, p <0.0001, respectively). Finally, portal myofibroblasts were associated with BDL and the stage of the disease (σ = 0.55, p <0.0001, and σ = 0.649, p <0.0001, respectively). Furthermore, we investigated the correlations between CS markers and laboratory findings, and we observed that both p16 and p21 expression in DR positively correlated with INR (σ = 0.55, p = 0.002, and σ = 0.43, p = 0.02, respectively) and inversely correlated with serum albumin (σ = −0.36, p = 0.024, and σ = −0.35, p = 0.027, respectively) and platelet count (σ = −0.60, p <0.001, and σ = −0.40, p = 0.010). Moreover, p21 expression in DR directly correlated with total bilirubin (σ = 0.34, p = 0.031), and p16 expression in NBD directly correlated with INR (σ = 0.37, p = 0.046) and inversely correlated with albumin (σ = −0.40, p = 0.02) and platelet count (σ = −0.41, p = 0.008). Finally, p21 expression in NBD inversely correlated with albumin (σ = −0.38, p = 0.029) and platelet count (σ = −0.47, p = 0.02). By multivariate analysis, p16 expression in DR was independently associated with serum albumin (HR −5.27, 95% CI −25.9 to −2.07, p = 0.04).
Prognostic value of histological findings and senescence markers
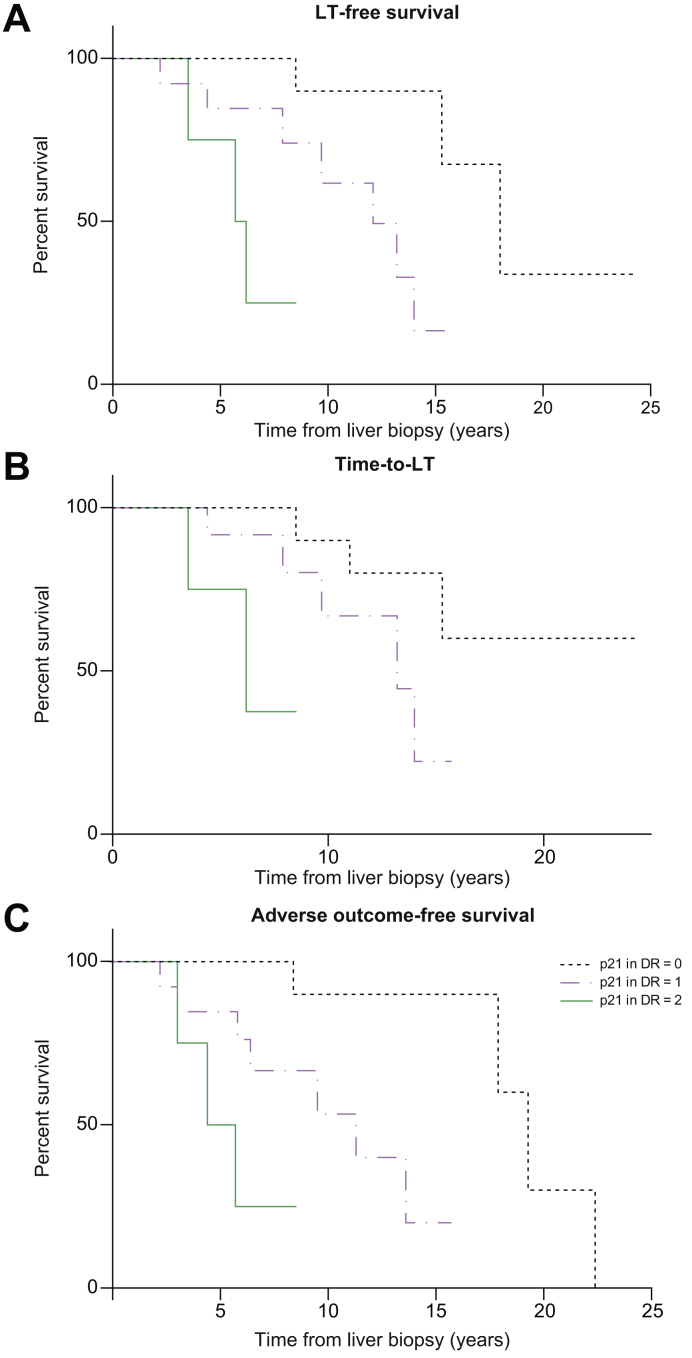
As already reported, for all the prognostic analyses, we only considered 30 patients with PSC at the time of the first liver biopsy. The overall median LT-free survival was 14 (11.3–16.7) years. During this follow-up, 10 LT have been recorded, all for end-stage liver disease, as well as 3 cases of CCA (2 intrahepatic and 1 extrahepatic CCA), 1 GBCA, 1 HCC, 2 colorectal cancers, and 5 PSC-related deaths. The evaluation of the prognostic impact of each histological finding by univariate analysis showed that fibrosis was significantly associated with LT-free survival and adverse outcome-free survival (p = 0.015 and p = 0.014, respectively). When exploring the relationship between CS marker expression in NBD and DR and clinical outcomes, we observed a higher expression of p21 in DR in patients who then developed bacterial cholangitis (odds ratio [OR] 13.93, p = 0.001). Furthermore, multivariate analysis showed that p21 expression in DR was independently associated with LT-free survival, time to LT, and adverse outcome-free survival (HR 4.86, 95% CI 1.84–12.82, p = 0.001; HR 3.37, 95% CI 1.26–10.10, p = 0.017; and HR 5.09, 95% CI 1.92–13.52, p = 0.001, respectively) (Fig. 3).
Fig. 3.
Survival figures according to p21 expression in ductular reaction.
Kaplan–Meier curves showing (A) LT-free survival (log-rank test, HR 4.86, 95% CI 1.84–12.82, p = 0.001), (B) time to LT (log-rank test, HR 3.37, 95% CI 1.26–10.10, p = 0.017), and (C) adverse outcome-free survival (log-rank test, HR 5.09, 95% CI 1.92–13.52, p = 0.001). DR, ductular reaction; HR, hazard ratio; LT, liver transplantation; p21, p21WAF1/Cip1.
Discussion
In this study, we demonstrated that CS in cholangiocytes is present in patients with PSC in all disease stages, and the extent of senescence correlates not only with histological and clinical severity, but also with patients’ outcomes. We documented that, in patients with PSC, CS is present in cholangiocytes of both NBD and DR in about 70% and 80% of cases, respectively. Expression of senescence-associated markers has been previously reported in PSC, but only a small number of patients with advanced disease stage were analysed. Indeed, 8 cases of PSC in stages 3 and 4 were studied by Sasaki et al.,9 whereas 9 explanted livers from patients with PSC and liver cirrhosis were evaluated in the study by Tabibian et al.12 The present case series is representative of all different stages of the disease, even if only a minority of patients were in a very early stage. Novel information provided by this study is that cholangiocyte senescence is an early event in PSC, which suggests that it can be involved in the disease pathogenesis. Tabibian et al. reported that, differently from PBC, senescence in PSC cholangiocytes was independent of telomere shortening, suggesting an insult-induced (and non-replicative) senescence.12 We here reported some clinical elements that may be in favour of an insult-induced senescence in PSC: the presence of CS also in early stages, the association between the extent of CS and the frequency of bacterial cholangitis, and the lack of an association between CS and patient’s age. As proposed in PBC,9 we here suggest that senescent cholangiocytes may be prone to further injury also in PSC, and this likely determines BDL. Coherently, by multivariate analysis, we demonstrated that p21 expression in the NBDs was independently associated with BDL. Moreover, senescence in DR can be a mechanism further contributing to BDL, histological progression, and an eventual clinical worsening. Indeed, by multivariate analysis, we demonstrated that p16 expression in DR was independently associated with Nakanuma’s stage and its components, i.e. BDL and fibrosis, consistently with previous observations in patients with PBC.11 Earlier studies also showed that senescent cells lose their function,20 thereby becoming unable to replace damaged cholangiocytes, and this may justify the association between DR senescence and BDL. However, CS seems to be directly involved in fibrogenesis. Indeed, Ferreira-Gonzalez et al.14 recently reported that in a mouse model of biliary disease, senescent cholangiocytes were able to induce profound alterations in the hepatic microenvironment, by recruiting myofibroblasts and macrophages, causing collagen deposition, transforming growth factor beta (TGFβ) production, and induction of senescence in the surrounding cholangiocytes and hepatocytes. Moreover, they reported that inhibition of TGFβ signalling disrupts the transmission of senescence and restores liver function.14 In our series, we found a significant correlation between the expression of CS markers by cholangiocytes of NBD and DR and the amount of macrophages and myofibroblasts recruited in the PTs, and between the presence of these cells in the PTs and the amount of fibrosis, BDL, and the stage of the disease, supporting the theory proposed by these authors. Kyritsi et al.15 demonstrated that p16 immunoreactivity, biliary senescence, and SASP levels are increased in the MDR2−/− mouse model of PSC compared with wild-type mice, and decreased, together with fibrosis, by the downregulation of p16 by using p16 Vivo-Morpholino. All these observations suggest that cholangiocyte CS may represent a promising therapeutic target in PSC. The association between senescence and disease severity is confirmed by the significant correlation we observed between CS and liver function tests. In fact, at the univariate analysis, both p16 and p21 expression in DR and p16 in NBD were associated with an increased INR and a decreased serum albumin level and platelet count. By multivariate analysis, p16 in DR remained independently and negatively associated with serum albumin. The link between senescence in DR and a reduced synthetic function is not easy to explain. We may suggest that senescent cholangiocytes in DR can no longer support hepatocyte regeneration, thus contributing to the reduction of the volume of functioning parenchyma as long as fibrosis progresses. Interestingly, we demonstrated for the first time that the expression of senescence markers in the NBD and in DR is independently associated with HA in PSC. Senescent cells, although not proliferating, are metabolically active and capable of affecting their microenvironment. They are regarded as pro-inflammatory and immunogenic21 and secrete many pro-inflammatory cytokines, chemokines, growth factors, and proteases, which cause chronic inflammation. Therefore, it could be hypothesised that the inflammatory activity in PSC is a senescence-related phenomenon and not always a histological feature of AIH-PSC overlap, as traditionally considered. This would further support the idea that the term ‘overlap’ should be abandoned, as HA in PSC may actually represent a PSC subphenotype rather than a separate clinical entity.22 The relation between cholangiocyte senescence and patients’ outcome is noteworthy. Indeed, we observed that p21 expression in DR was independently associated with all the clinical endpoints, namely, LT-free survival, time to LT, and adverse outcome-free survival, with the latter also including cirrhosis decompensation. This has never been described before in chronic biliary diseases, although a single study reported a role for hepatocyte senescence in predicting patients’ outcome in alcoholic and non-alcoholic fatty liver disease.23 p21 expression is easily assessed by immunohistochemistry; therefore, adding cholangiocytes senescence assessment to standard histological examination may improve the value of liver biopsy for risk stratification in patients with PSC and as a surrogate endpoint in clinical trials.24 The population included in this study showed some slight differences compared with epidemiological data, i.e. lower age at diagnosis, different sex prevalence, and a lower association with IBD. However, we consider our population representative of PSC, since we found significant similarities in the development of clinical outcomes and in the overall survival, which were both comparable with those observed in multicentric cohorts of patients followed up in reference and liver transplant centres.2 Moreover, our cohort of patients was prospectively followed up by the same clinician in the same centre for the entire period, and this allowed a systematic and precise report of each clinical event. It is understood that our findings, although interesting, are limited by the restricted number of included patients and the retrospective design of the study. However, a proper prospective design would not have been feasible, as liver biopsy is no longer required for PSC diagnosis, except for variant syndrome with AIH or small-duct PSC or in the setting of clinical trials. Although this latter situation may permit the assessment of cholangiocyte CS marker expression in patients with PSC at different time points, it could also potentially select special groups of patients, which we tried to avoid.
In conclusion, this study demonstrated that cholangiocyte senescence is detectable in all stages of PSC and is associated with the histological and clinical severity of the disease. Furthermore, we observed a strong relationship between the proportion of senescent cells in DR and the development of adverse outcomes, thus suggesting that cholangiocyte senescence may represent a promising therapeutic target. Finally, our findings may support a brand-new prognostic role for liver biopsy when performed in clinical practice and in the setting of clinical trials.
Financial support
FPR was funded by the Department of Surgery, Oncology and Gastroenterology, University of Padova, Padova (DOR 2020).
Authors’ contributions
Conceptualisation, investigation, and methodology: NC, SS, FPR, MG, AF. Data curation: NC, SS, AF, GC, MG, SDM, FPR, VG. Formal analysis: NC, SS. Writing – original draft: NC, SS. Writing – review and editing: FPR, MG, AF, SDM. Funding acquisition: FRP. Validation: NC, SS, FPR, AR, MG, SDM, GC, VG
Data availability statement
Owing to the confidential nature of the data in the study, the data collected in the study will not be available to access.
Conflicts of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2021.100286.
Contributor Information
Nora Cazzagon, Email: nora.cazzagon@gmail.com.
Francesco Paolo Russo, Email: francescopaolo.russo@unipd.it.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Karlsen T.H., Folseraas T., Thorburn D., Vesterhus M. Primary sclerosing cholangitis – a comprehensive review. J Hepatol. 2017;67:1298–1323. doi: 10.1016/j.jhep.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 2.Weismüller T.J., Trivedi P.J., Bergquist A., Imam M., Lenzen H., Ponsioen C.Y. Patient age, sex, and inflammatory bowel disease phenotype associate with course of primary sclerosing cholangitis. Gastroenterology. 2017;152:1975–1984. doi: 10.1053/j.gastro.2017.02.038. e8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergquist A., Ekbom A., Olsson R., Kornfeldt D., Lööf L., Danielsson A. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol. 2002;36:321–327. doi: 10.1016/s0168-8278(01)00288-4. [DOI] [PubMed] [Google Scholar]
- 4.Guicciardi M.E., Trussoni C.E., LaRusso N.F., Gores G.J. The spectrum of reactive cholangiocytes in primary sclerosing cholangitis. Hepatology. 2020;71:741–748. doi: 10.1002/hep.31067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakanuma Y., Sasaki M., Harada K. Autophagy and senescence in fibrosing cholangiopathies. J Hepatol. 2015;62:934–945. doi: 10.1016/j.jhep.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 6.Gorgoulis V., Adams P.D., Alimonti A., Bennett D.C., Bischof O., Bishop C. Cellular senescence: defining a path forward. Cell. 2019;179:813–827. doi: 10.1016/j.cell.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 7.He S., Sharpless N.E. Senescence in health and disease. Cell. 2017;169:1000–1011. doi: 10.1016/j.cell.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aravinthan A.D., Alexander G.J.M. Senescence in chronic liver disease: is the future in aging? J Hepatol. 2016;65:825–834. doi: 10.1016/j.jhep.2016.05.030. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki M., Ikeda H., Haga H., Manabe T., Nakanuma Y. Frequent cellular senescence in small bile ducts in primary biliary cirrhosis: a possible role in bile duct loss. J Pathol. 2005;205:451–459. doi: 10.1002/path.1729. [DOI] [PubMed] [Google Scholar]
- 10.Sasaki M., Ikeda H., Yamaguchi J., Nakada S., Nakanuma Y. Telomere shortening in the damaged small bile ducts in primary biliary cirrhosis reflects ongoing cellular senescence. Hepatology. 2008;48:186–195. doi: 10.1002/hep.22348. [DOI] [PubMed] [Google Scholar]
- 11.Sasaki M., Ikeda H., Yamaguchi J., Miyakoshi M., Sato Y., Nakanuma Y. Bile ductular cells undergoing cellular senescence increase in chronic liver diseases along with fibrous progression. Am J Clin Pathol. 2010;133:212–223. doi: 10.1309/AJCPWMX47TREYWZG. [DOI] [PubMed] [Google Scholar]
- 12.Tabibian J.H., O’Hara S.P., Splinter P.L., Trussoni C.E., LaRusso N.F. Cholangiocyte senescence by way of N-ras activation is a characteristic of primary sclerosing cholangitis. Hepatology. 2014;59:2263–2275. doi: 10.1002/hep.26993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tabibian J.H., Trussoni C.E., O’Hara S.P., Splinter P.L., Heimbach J.K., LaRusso N.F. Characterization of cultured cholangiocytes isolated from livers of patients with primary sclerosing cholangitis. Lab Invest. 2014;94:1126–1133. doi: 10.1038/labinvest.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferreira-Gonzalez S., Lu W.-Y., Raven A., Dwyer B., Man T.Y., O’Duibhir E. Paracrine cellular senescence exacerbates biliary injury and impairs regeneration. Nat Commun. 2018;9:1020. doi: 10.1038/s41467-018-03299-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kyritsi K., Francis H., Zhou T., Ceci L., Wu N., Yhang Z. Downregulation of p16 decreases biliary damage and liver fibrosis in the Mdr2−/− mouse model of primary sclerosing cholangitis. Gene Expr. 2020;20:89–103. doi: 10.3727/105221620X15889714507961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.European Association for the Study of the Liver EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237–267. doi: 10.1016/j.jhep.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Harada K., Hsu M., Ikeda H., Zeniya M., Nakanuma Y. Application and validation of a new histologic staging and grading system for primary biliary cirrhosis. J Clin Gastroenterol. 2013;47:174–181. doi: 10.1097/MCG.0b013e31827234e4. [DOI] [PubMed] [Google Scholar]
- 18.de Vries E.M.G., Verheij J., Hubscher S.G., Leeflang M.M.G., Boonstra K., Beuers U. Applicability and prognostic value of histologic scoring systems in primary sclerosing cholangitis. J Hepatol. 2015;63:1212–1219. doi: 10.1016/j.jhep.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 19.de Vries E.M.G., de Krijger M., Färkkilä M., Arola J., Schirmacher P., Gotthardt D. Validation of the prognostic value of histologic scoring systems in primary sclerosing cholangitis: an international cohort study. Hepatology. 2017;65:907–919. doi: 10.1002/hep.28963. [DOI] [PubMed] [Google Scholar]
- 20.Campisi J. The role of cellular senescence in skin aging. J Investig Dermatol Symp Proc. 1998;3:1–5. [PubMed] [Google Scholar]
- 21.Soto-Gamez A., Quax W.J., Demaria M. Regulation of survival networks in senescent cells: from mechanisms to interventions. J Mol Biol. 2019;431:2629–2643. doi: 10.1016/j.jmb.2019.05.036. [DOI] [PubMed] [Google Scholar]
- 22.Boberg K.M., Chapman R.W., Hirschfield G.M., Lohse A.W., Manns M.P., Schrumpf E. Overlap syndromes: the International Autoimmune Hepatitis Group (IAIHG) position statement on a controversial issue. J Hepatol. 2011;54:374–385. doi: 10.1016/j.jhep.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Aravinthan A., Pietrosi G., Hoare M., Jupp J., Marshall A., Verrill C. Hepatocyte expression of the senescence marker p21 is linked to fibrosis and an adverse liver-related outcome in alcohol-related liver disease. PLoS One. 2013;8 doi: 10.1371/journal.pone.0072904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ponsioen C.Y., Chapman R.W., Chazouillères O., Hirschfield G.M., Karlsen T.H., Lohse A.W. Surrogate endpoints for clinical trials in primary sclerosing cholangitis: review and results from an International PSC Study Group consensus process. Hepatology. 2016;63:1357–1367. doi: 10.1002/hep.28256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Owing to the confidential nature of the data in the study, the data collected in the study will not be available to access.