Abstract
Background:
Oral candidiasis (OC) is an indirect indicator of cell-mediated immunodeficiency with a high predictive value of disseminated candidiasis. Here, we report the prevalence and factors associated with laboratory-confirmed OC in human immunodeficiency virus (HIV)-uninfected children with clinical OC attending the outpatient clinic or admitted in pediatric wards of the Bugando Medical Center (BMC).
Methods:
A cross-sectional study was conducted between January and June 2017. Social demographic and clinical data were collected using a pre-tested data collection tool. Oral swabs were collected using a sterile cotton swab and mycological culture was done to detect Candida spp. followed by susceptibility testing as per European Committee on Antimicrobial Testing (EUCAST) guidelines. Data were analyzed using STATA version 13 following study objectives.
Results:
A total of 325 non-repetitive oral swabs from HIV-uninfected children aged between 2 and 156 months were collected. Candida spp. were detected in 123 (37.8%) children. One (1.8%) C. albicans isolate was resistant to fluconazole, voriconazole, and posaconazole with minimum inhibitory concentrations (MIC) of 256 μg/ml, 32 μg/ml, and 0.31 μg/ml, respectively. Upon multivariate logistic regression analysis, being a male child (OR 2, 95% CI 1.2–3.2, p = 0.008) and having a history of antibiotic use (OR 1.8, 95% CI 1.1–2.8, p = 0.017) independently predicted laboratory-confirmed OC among HIV-uninfected children.
Conclusion:
Only a third of children with clinical OC were laboratory confirmed, and this was more likely in male children with a history of antibiotic use. Most of the isolates were highly susceptible to commonly used antifungal agents like fluconazole. Treatment of children at risk should be prioritized to reduce associated morbidity.
Keywords: oral candidiasis, Antibiotic use, Limited fungi diagnosis, C. albicans
Background
Oral candidiasis (OC) is an indirect indicator of cell-mediated immunodeficiency, with over 90% positive predictive value of oesophageal candidiasis.1 In human immunodeficiency virus (HIV)-infected individuals, Oesophageal candidiasis has been used as the major acquired immune deficiency syndrome (AIDS) defining illness.2 Oral Candida spp. colonization is one of the major risk factors for OC.3 In Tanzania, the prevalence of non-albicans Candida spp. (NAC spp.) colonization is higher among HIV-infected individuals than among uninfected individuals.4 However, data on patterns of Candida spp. causing OC in the HIV-uninfected pediatric population is still limited. The prevalence of OC is reported to range between 15% and 40% in HIV-infected children, with limited data in HIV-uninfected children.5 The lack of this data in HIV-uninfected children can lead to delayed diagnosis and delayed management of these children.
Due to the high burden of HIV in sub-Saharan Africa (SSA), the majority of studies from this region have focused on HIV-infected populations, with few studies in HIV-uninfected children. This affects the allocation of resources in the management of HIV-uninfected children at risk of getting OC. Candida albicans has long been established to be the leading cause of OC6,7; however, in recent decades NAC spp. have been documented to colonize the oral cavity of immunocompromised patients and subsequently cause infections.4
In HIV-uninfected children, the use of broad-spectrum antimicrobials, non-communicable diseases (such as diabetic mellitus, cancer), malnutrition, and prolonged hospitalizations are among the factors that can increase the risk of OC.8–11 In areas with limited fungal diagnosis and increased numbers of children at risk for OC, such as malnourished children and children with other comorbidities like sickle cell anemia and diabetic mellitus,12–14 understanding the distribution of Candida spp. causing OC and their susceptibility patterns is important for proper empirical management of these children. Additionally, increasing use of antifungals over-the-counter with no surveillance system to monitor the trend of resistance might increase the problem of antifungal resistance.8 Identifying children at risk of getting OC is crucial to reduce associated morbidity. Herein, we report the prevalence and factors associated with OC among HIV-uninfected children from Mwanza, Tanzania – data that are important in managing children with OC.
Methodology
Study design and settings
A cross-sectional hospital-based study was conducted among children with the clinical diagnosis of OC attending the outpatient clinic or admitted in pediatric wards of the Bugando Medical Center (BMC) between January and June 2017. BMC which is located in the city of Mwanza is a tertiary and teaching hospital of the Catholic University of Health and Allied Sciences located. BMC has a bed capacity of 1000, serving over 15 million people from Lake Zone regions. The pediatric department has a bed capacity of 120, while 45 children are attended daily as outpatients.
In this study, clinical diagnosis of OC was made by clinicians by observing the presence of white patches on the surface of the oral mucosal or tongue, or painful localized erythematous lesions in the buccal cavity, or erythematous or ulcerated fissures, typically affecting unilaterally or bilaterally commissures of the lip.15
Sample size, sampling, and study variables
The sample size was calculated using the Kish Leslie formula, a prevalence of 28.3% from a study conducted in Uganda was used.16 All children with a clinical diagnosis of OC were included in the study, these children were serially enrolled as they visited the hospital until the sample size was reached. All children were tested for HIV using the Tanzania HIV testing algorithm to exclude HIV-infected children.17,18 The main outcome of this study was laboratory-confirmed OC while independent variables were age, sex, hospital status, duration of hospitalization, and history of antimicrobial use.
Data collection and processing
Social demographic and clinical patient data were collected using a pre-tested questionnaire. Oral swabs were collected using a sterile cotton swab and transported to the microbiology laboratory in Stuart transport media (Delta lab, Barcelona, Spain) for culture and identification within 2 hours of sample collection.19
Isolation and identification of Candida spp
Samples were inoculated on Sabouraud’s Dextrose Agar supplemented with 50 mg/ml gentamicin and 50 mg/ml chloramphenicol (SDA) (Oxoid, Basingstoke, UK). Plates were incubated aerobically at 35°C for 24–48 hours. Culture positive was defined as SDA plate with more than made 10 colonies after 48 hours of incubation. Preliminary identification was done on CHROMagar (Oxoid) as previously described,4,20 followed by species confirmation at the Institute of Medical Microbiology, Gottingen, Germany, using the MALDI-ToF Mass Spectrometry (V4.0, database V6, Bruker Daltonics, Bremen, Germany).21
In vitro susceptibility assays
Antifungal susceptibility testing was done for fluconazole, voriconazole, posaconazole (Discovery Fine Chemicals, Bournemouth, UK), micafungin (Roth, Germany), caspofungin (Merck, Kenilworth, NJ, USA), and 5-fluorocytosine (Sigma Aldrich, St, Louis, MO, USA) following the guidelines laid down by the European Committee on Antimicrobial Testing (EUCAST).22 For fluconazole (C. albicans, C. glabrata, and C. tropicalis), voriconazole, posaconazole, and micafungin (C. albicans and C. tropicalis), the minimum inhibitory concentration (MIC) breakpoint interpretations were according to EUCAST guidelines.23 Pfaller et al. MIC breakpoints were used to interpret voriconazole, caspofungin, and 5-fluorocytosine susceptibility results for C. glabrata because these breakpoints are not indicated in EUCAST guidelines.24–26
Data analysis
Data were entered on an Excel spreadsheet for consistent check and cleaning then transferred to STATA version 13 for analysis. Categorical data were summarized using proportions while continuous data were summarized using the median and interquartile range (IQR).
Ethical considerations
Ethical approval to conduct this study was granted by the Joint Catholic University of Health and Allied Sciences/Bugando Medical Centre (CUHAS/BMC) research ethics and review committee with certificate number CREC/280/2017. Permission to conduct the study was sought from BMC. Parents/guardians of the children were requested to sign the written informed consent form before recruitment and their information was kept confidential. Culture results were communicated in a timely manner to the managing clinicians for patient care.
Results
A total of 325 (none repeated) children aged between 2 and 156 months with the clinical diagnosis of OC were recruited from January to June 2017. The median age (IQR) was 40 (16–84) months. A slight majority of children were male 212 (65.2%). Regarding hospitalization status, around one-third of the children (111; 34.2%) were inpatients. The median duration of hospitalization was 7 (IQR 5–10) days (Table 1).
Table 1.
Social demographic and clinical characteristics of 325 studied children.
| Variable | Frequency | % |
|---|---|---|
| Media age (IQR) months | 325 | 40 (16–84) |
| Sex | ||
| Female | 113 | 34.8 |
| Male | 212 | 65.2 |
| Hospital status | ||
| Outpatient | 214 | 65.8 |
| Inpatient | 111 | 34.2 |
| Median durations of hospitalization (IQR) daysa | 111 | 7 (5–10) |
| Antibiotic use | ||
| No | 154 | 47.4 |
| Yes | 171 | 52.6 |
| Antifungal use | ||
| No | 319 | 98.2 |
| Yes | 6 | 1.8 |
The median duration of hospitalization was only calculated for 111 admitted patients.
IQR, interquartile range.
Clinical presentation of the 325 studied children with clinical diagnosis of OC
Of 325 HIV-uninfected children involved in the study, a total of 236 (72.6%) presented with pseudomembranous candidiasis and 68 (20.9%) presented with angular cheilitis (Figure 1).
Figure 1.
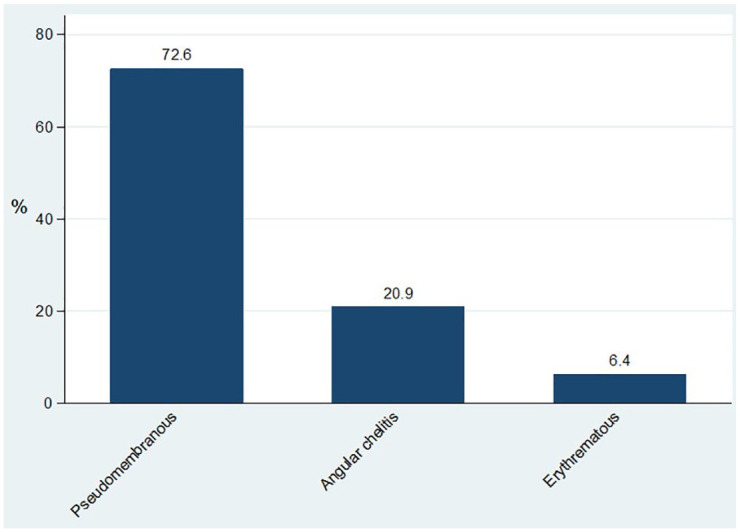
Clinical presentation of the 325 HIV-uninfected children.
Culture results and susceptibility
Of 325 HIV-uninfected children enrolled, 123 (37.8%) had culture-positive results indicating laboratory-confirmed OC. There was no statistical difference (p = 0.429) in the proportion of culture-positive results between patients with different clinical presentation. A total of 86 (n = 236, 36.4%), 27 (n = 68, 39.7%), and 10 (n = 21, 47.6%), had culture-positive results among patients presented with pseudomembranous candidiasis, angular cheilitis, and erythematous candidiasis, respectively.
NAC spp. were detected in only seven (5.7%) HIV-uninfected children using ChromoAgar; five presented with pseudomembranous, and two with erythematous candidiasis. Of them, C. tropicalis was detected in four patients and C. glabrata in three patients. Simple random selection was made to obtain 53 isolates for matrix assisted laser desorption ionization-time of flight mass spectrometry (MALDI TOF MS) confirmations of Candida spp., and antifungal susceptibility testing. On MALDI TOF MS, 10/53 (18.9%) NAC spp. were detected; including three C. glabrata, three C. tropicalis, three C. kefyr, and one K. ohmeri. All C. kefyr were detected as C. albicans on CHROMagar.
Of the 53 yeasts tested for antifungal susceptibility, only 1 (1.8%) C. albicans isolate was resistant to fluconazole, voriconazole, and posaconazole, with MICs of over 256 μg/ml, 32 μg/ml, and 0.31 μg/ml, respectively. The resistant strain had MICs for caspofungin, micafungin, and 5-flucytosine of 0.004 μg/ml, 0.125 μg/ml, and 0.063 μg/ml, respectively. Of 46 C. albicans tested for fluconazole susceptibility, the majority (43; 93.5%) had MIC of 0.25 μg/ml. In addition, the most frequently detected micafungin MIC among C. albicans was 0.063 μg/ml (Table 2).
Table 2.
Antifungal MIC distributions for the 53 Candida spp. tested.
| Candida spp. | Number tested | Agents | MIC50 (μg/ml) | |||
|---|---|---|---|---|---|---|
| Range tested | Range detected | Mode | % ⩽mode | |||
| C. albicans | 46 | Fluconazole | 0.250–256 | 0.250–256 | 0.250 | 93.4 |
| Voriconazole | 0.031–32 | 0.031–32 | 0.25 | 93.4 | ||
| Posaconazole | 0.004–4 | 0.016–0.31 | 0.023 | 67.4 | ||
| Caspofungin | 0.004–4 | 0.004–2 | 0.25 | 97.8 | ||
| Micafungin | 0.004–4 | 0.031–1.125 | 0.063 | 97.8 | ||
| 5-Fluorocytosine | 0.031–32 | 0.031–0.25 | 0.063 | 84.8 | ||
| Other yeast | 7 | Fluconazole | 0.250–256 | 0.25–4 | 0.5 | 71.4 |
| Voriconazole | 0.031–32 | 0.031–0.125 | 0.031 | 71.4 | ||
| Posaconazole | 0.004–4 | 0.016–0.25 | 0.25 | 100 | ||
| Caspofungin | 0.004–4 | 0.063–0.31 | 0.031 | 42.9 | ||
| Micafungin | 0.004–4 | 0.031–1.125 | 0.063 | 71.4 | ||
| 5-Fluorocytosine | 0.31–32 | 0.031–0.063 | 0.031 | 57.1 | ||
MIC, minimum inhibitory concentration.
Kodamaea (Pichia) ohmeri was detected in one patient, with MICs of 4 μg/ml, 0.031 μg/ml, 0.031 μg/ml, 2 μg/ml, 1.125 μg/ml, and 0.031 for fluconazole, voriconazole, posaconazole, Caspofungin, micafungin, and 5-flucytosine, respectively.
Upon univariate logistic regression analysis, being male [odds ratio (OR) 2, 95% confidence interval (CI) 1.2–3.1, p = 0.01], having a history of using antibiotics 2 weeks prior to the study (OR 1.8, 95% CI 1.2–2.9, p = 0.01) and increase in age (OR 0.994, 95% CI 0.988–0.999, p = 0.044) were significantly associated with laboratory-confirmed OC. In the multivariate logistic regression analysis, being a male child (OR 2, 95% CI 1.2–3.2, p = 0.008) and having a history of antibiotic use (OR 1.8, 95% CI 1.1–2.8, p = 0.017) independently predicted laboratory-confirmed OC among HIV-uninfected children (Table 3).
Table 3.
Factors associated with culture-positive oral swab among HIV-uninfected children.
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| Culture positive | OR (95%; CI) | p value | OR (95%; CI) | p value | |
| Age (months) | 108*, IQR (84–144) | 0.994 (0.988–0.999) | 0.044 | 0.993 (0.987–0.997) | 0.041 |
| Sex | |||||
| Female (113) | 34 (28.32%) | 1 | |||
| Male (112) | 91 (42.92%) | 1.90 (1.16–3.11) | 0.010 | 1.96 (1.19–3.25) | 0.008 |
| Antibiotic use | |||||
| No (154) | 47 (30.52%) | 1 | |||
| Yes (171) | 76 (44.44%) | 1.82 (1.15–2.87) | 0.010 | 1.762 (1.10–2.80) | 0.017 |
| Antifungal use | |||||
| No (319) | 120 (37.62%) | 1 | |||
| Yes (6) | 3 (50.00%) | 1.66 (0.32–8.34) | 0.540 | ||
| Duration of hospitalization (days) | 7*, IQR (5–10) | 1.01 (0.975–1.04) | 0.637 | ||
CI, confidence interval; HIV, human immunodeficiency virus; OR, odds ratio, IQR, interquartile range, *Median.
Discussion
OC is the commonest complaint among immunocompromised children. Much has been studied regarding OC among HIV-infected individuals, making OC the commonest oral fungal disease reported and an important progressing marker of HIV infection in children. In the current study, the prevalence of laboratory-confirmed OC was found to be 37.8%, which is similar to what has previously been reported among HIV-infected children (prevalence range of 15–40%).5 The high culture-negative results in children with clinical diagnosis of OC candidiasis may be due to the quantification cut-off point used in this study or the wrong clinical diagnosis.
As previously reported among HIV-uninfected children,27,28 the current study has proven increased age to be a protective factor for children from having OC. Besides, as in a previous study,27 the use of antibiotics was also found to independently predict culture-positive OC among HIV-uninfected children in this study. The history of antibiotic use has been reported to be a leading risk factor in candidiasis.28,29 This can be explained by the antimicrobial effect of antibiotics to oral bacteria microbiota, which leads to candida overgrowth within the oral cavity.
Looking at the distribution of Candida spp., C. albicans was the predominant yeast detected in this study. These findings are similar to previous studies in South Africa30–32 and other parts of the world33–35 among HIV-infected patients. Furthermore, these results are comparable with those of the previous study, which was done in Tanzania 10 years prior among individuals with primary and recurrent OC.36 The predominance of C. albicans as the species causing OC is partly contributed by their ability to produce virulence factors like phospholipases and proteases.37 These virulence factors are highly associated with Candida spp. causing OC.38–41 Furthermore, in our previous study,4 we documented that C. albicans was the predominant species colonizing both HIV-infected and uninfected populations, making it the predominant causative agents of endogenous oral candidiasis.
The current study has found a low resistance of Candida spp. to fluconazole. This might have been contributed by the low use of fluconazole and other antifungals in children, this is supported by the fact that only 6 children had hisotry of antifungal use in this study. The first line in treatment of OC in children is oral nystatin and was not commonly used by these children prior visit to the hopsital.42–44 The increased use of fluconazole has been reported previously to influence the development of fluconazole resistant C. albicans.7,45 The low resistance rate of Candida spp. to fluconazole has also been reported in study settings.7,36
In the current study, fluconazole resistance was documented to only one C. albicans; this isolate was also resistant to voriconazole and posaconazole. The cross-resistance observed in this isolate could be due to overexpression of the efflux pump – the common mechanism of azole resistance that confers resistance to several azoles.46,47
Study limitation
Due to limitations of resources, not all isolates were species confirmed by MALDI TOF and tested for susceptibility patterns. This could lead to underestimation of the prevalence of resistant isolates, especially in NAC spp and Candida spp. variaties.
Conclusion and recommendations
Only a third of children with clinical OC were laboratory confirmed, and this was more likely in male children with a history of antibiotic use. Most isolates were highly susceptible to commonly used antifungal agents like fluconazole. Continuous surveillance to monitor susceptibility trends in order to generate local data for empirical management of children with OC is highly recommended.
Acknowledgments
The authors would like to acknowledge the support provided by the Institute of Medical Microbiology, University Medical Center Goettingen, Germany, and the technical support of Mr. Vitus Silago from the department of Microbiology and Immunology, the Catholic University of Health and Allied Sciences, Mwanza, Tanzania and that of Agnieszka Goretzki and Yvonne Laukat from Institute of Medical Microbiology, University Medical Center Goettingen.
Footnotes
Author contributions: MFM, NL, and SEM designed the work. MFM and NL performed laboratory investigations. MFM and SEM analyzed and interpreted the data. MFM wrote the first draft of the manuscript, which was critically reviewed by SEM. All authors read and approved the final version of the manuscript.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Availability of data and material: The data is available upon request and the request should be made to the Director of Research and Innovation, Catholic University of Health and Allied Sciences.
ORCID iD: Martha F. Mushi  https://orcid.org/0000-0003-3511-2523
https://orcid.org/0000-0003-3511-2523
Contributor Information
Martha F. Mushi, Department of Microbiology/Immunology, Weill Bugando School of Medicine, The Catholic University of Health and Allied Sciences (CUHAS), PO BOX 1464, Mwanza, Tanzania.
Neema Loi, Instutite of Allied Sciences, Catholic University of Health and Allied Sciences, Mwanza, Tanzania.
Stephen E. Mshana, Department of Microbiology and Immunology, Weill Bugando School of Medicine, Catholic University of Health and Allied Sciences, Mwanza, Tanzania
References
- 1. Wilcox CM, Straub RF, Clark WS. Prospective evaluation of oropharyngeal findings in human immunodeficiency virus-infected patients with esophageal ulceration. Am J Gastroenterol 1995; 90: 1938–1941. [PubMed] [Google Scholar]
- 2. Thanyasrisung P, Kesakomol P, Pipattanagovit P, et al. Oral Candida carriage and immune status in Thai human immunodeficiency virus-infected individuals. J Med Microbiol 2014; 63: 753–759. [DOI] [PubMed] [Google Scholar]
- 3. Fong IW, Manuel L, Burford-Mason A. Asymptomatic oral carriage of Candida albicans in patients with HIV infection. Clin Invest Med 1997; 20: 85–93. [PubMed] [Google Scholar]
- 4. Mushi MF, Mtemisika CI, Bader O, et al. High oral carriage of non-albicans Candida spp. among HIV-infected individuals. Int J Infect Dis 2016; 49: 185–188. [DOI] [PubMed] [Google Scholar]
- 5. Walsh T, Butler K. Fungal infections complicating pediatric AIDS. In: Wilfert C, Pizzo PA. (eds) Pediatric AIDS: the challenge of HIV infection in infants, children, and adolescents. Baltimore, MD: The Williams & Wilkins Co, 1991, pp.225–244. [Google Scholar]
- 6. Staib P, Lermann U, Blass-Warmuth J, et al. Tetracycline-inducible expression of individual secreted aspartic proteases in Candida albicans allows isoenzyme-specific inhibitor screening. Antimicrob Agents Chemother 2008; 52: 146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mushi MF, Bader O, Taverne-Ghadwal L, et al. Oral candidiasis among African human immunodeficiency virus-infected individuals: 10 years of systematic review and meta-analysis from sub-Saharan Africa. J Oral Microbiol 2017; 9: 1317579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mushi MF, Masewa B, Jande M, et al. Prevalence and factor associated with over-the-counter use of antifungal agents’, in Mwanza City, Tanzania. Tanzania J Health Res 2017; 19: 1–8. [Google Scholar]
- 9. Taha TE, Graham SM, Kumwenda NI, et al. Morbidity among human immunodeficiency virus-1-infected and-uninfected African children. Pediatrics 2000; 106: e77. [DOI] [PubMed] [Google Scholar]
- 10. Petersen PE, Bourgeois D, Ogawa H, et al. The global burden of oral diseases and risks to oral health. Bull World Health Organ 2005; 83: 661–669. [PMC free article] [PubMed] [Google Scholar]
- 11. Belazi M, Velegraki A, Koussidou-Eremondi T, et al. Oral Candida isolates in patients undergoing radiotherapy for head and neck cancer: prevalence, azole susceptibility profiles and response to antifungal treatment. Oral Microbiol Immunol 2004; 19: 347–351. [DOI] [PubMed] [Google Scholar]
- 12. Ambrose EE, Makani J, Chami N, et al. High birth prevalence of sickle cell disease in Northwestern Tanzania. Pediatr Blood Cancer 2018; 65: e26735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ahmed MM, Hokororo A, Kidenya BR, et al. Prevalence of undernutrition and risk factors of severe undernutrition among children admitted to Bugando medical centre in Mwanza, Tanzania. BMC Nutrition 2016; 2: 49. [Google Scholar]
- 14. Mashuda F, Zuechner A, Chalya PL, et al. Pattern and factors associated with congenital anomalies among young infants admitted at Bugando medical centre, Mwanza, Tanzania. BMC Res Notes 2014; 7: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patil S, Rao RS, Majumdar B, et al. Clinical appearance of oral Candida infection and therapeutic strategies. Front Microbiol 2015; 6: 1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rwenyonyi CM, Kutesa A, Muwazi L, et al. Oral manifestations in HIV/AIDS-infected children. Eur J Dent 2011; 5: 291–298. [PMC free article] [PubMed] [Google Scholar]
- 17. Lyamuya EF, Aboud S, Urassa WK, et al. Evaluation of simple rapid HIV assays and development of national rapid HIV test algorithms in Dar es Salaam, Tanzania. BMC Infect Dis 2009; 9: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. National AIDS Control Programme. National guidelines for the management of HIV and AIDS. New Delhi: Ministry of Health and Social Welfare, 2015. [Google Scholar]
- 19. Williams DW, Lewis MAO. Oral Microbiology: isolation and identification of Candida from the oral cavity. Oral Dis 2000; 6: 3–11. [DOI] [PubMed] [Google Scholar]
- 20. Pfaller MA, Houston A, Coffmann S. Application of CHROMagar Candida for rapid screening of clinical specimens for Candida albicans, Candida tropicalis, Candida krusei, and Candida (Torulopsis) glabrata. J Clin Microbiol 1996; 34: 58–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bernhard M, Weig M, Zautner AE, et al. Yeast On-Target Lysis (YOTL), a procedure for making auxiliary mass spectrum data sets for clinical routine identification of yeasts. J Clin Microbiol 2014; 52: 4163–4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arendrup MC, Cuenca-Estrella M, Lass-Flörl C, et al. EUCAST technical note on the EUCAST definitive document EDef 7.2: method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts. Clin Microbiol Infect 2012; 18: E246–E247. [DOI] [PubMed] [Google Scholar]
- 23. EUCAST. The European Committee on Antimicrobial Susceptibility Testing, http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_6.0_Breakpoint_table.pdf (accessed 1 January 2016).
- 24. Pfaller M, Diekema D, Rex J, et al. Correlation of MIC with outcome for Candida species tested against voriconazole: analysis and proposal for interpretive breakpoints. J Clin Microbiol 2006; 44: 819–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pfaller M, Boyken L, Hollis R, et al. In vitro susceptibility of invasive isolates of Candida spp. to anidulafungin, caspofungin, and micafungin: six years of global surveillance. J Clin Microbiol 2008; 46: 150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pfaller M, Messer S, Boyken L, et al. In vitro activities of 5-fluorocytosine against 8,803 clinical isolates of Candida spp.: global assessment of primary resistance using National Committee for Clinical Laboratory Standards susceptibility testing methods. Antimicrob Agents Chemother 2002; 46: 3518–3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Akpan A, Morgan R. Oral candidiasis. Postgrad Med J 2002; 78: 455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mushi MF, Ngeta N, Mirambo MM, et al. Predictors of esophageal candidiasis among patients attending endoscopy unit in a tertiary hospital, Tanzania: a retrospective cross-sectional study. Afr Health Sci 2018; 18: 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oksala E. Factors predisposing to oral yeast infections. Acta Odontol Scand 1990; 48: 71–74. [DOI] [PubMed] [Google Scholar]
- 30. Blignaut E, Messer S, Hollis R, et al. Antifungal susceptibility of South African oral yeast isolates from HIV/AIDS patients and healthy individuals. Diagn Microbiol Infect Dis 2002; 44: 169–174. [DOI] [PubMed] [Google Scholar]
- 31. Blignaut E, Botes ME, Nieman HL. The treatment of oral candidiasis in a cohort of South African HIV/AIDS patients. SADJ 1999; 54: 605–608. [PubMed] [Google Scholar]
- 32. Blignaut E. Oral candidiasis and oral yeast carriage among institutionalised South African paediatric HIV/AIDS patients. Mycopathologia 2007; 163: 67–73. [DOI] [PubMed] [Google Scholar]
- 33. Cartledge JD, Midgley J, Gazzard BG. Non-albicans oral candidosis in HIV-positive patients. J Antimicrob Chemother 1999; 43: 419–422. [DOI] [PubMed] [Google Scholar]
- 34. Barchiesi F, Arzeni D, Del Prete M, et al. Fluconazole susceptibility and strain variation of Candida albicans isolates from HIV-infected patients with oropharyngeal candidosis. J Antimicrob Chemother 1998; 41: 541–548. [DOI] [PubMed] [Google Scholar]
- 35. Lattif AA, Banerjee U, Prasad R, et al. Susceptibility pattern and molecular type of species-specific Candida in oropharyngeal lesions of Indian human immunodeficiency virus-positive patients. J Clin Microbiol 2004; 42: 1260–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hamza OJ, Matee MI, Moshi MJ, et al. Species distribution and in vitro antifungal susceptibility of oral yeast isolates from Tanzanian HIV-infected patients with primary and recurrent oropharyngeal candidiasis. BMC Microbiol 2008; 8: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mushi MF, Bader O, Bii C, et al. Virulence and susceptibility patterns of clinical Candida spp. isolates from a tertiary hospital, Tanzania. Med Mycol. Epub ahead of print 31 October 2018. DOI: 10.1093/mmy/myy107. [DOI] [PubMed] [Google Scholar]
- 38. Tsang C, Chu F, Leung W, et al. Phospholipase, proteinase and haemolytic activities of Candida albicans isolated from oral cavities of patients with type 2 diabetes mellitus. J Med Microbiol 2007; 56: 1393–1398. [DOI] [PubMed] [Google Scholar]
- 39. Kumar CG, Kumar SSJ, Menon T. Phospholipase and proteinase activities of clinical isolates of Candida from immunocompromised patients. Mycopathologia 2006; 161: 213–218. [DOI] [PubMed] [Google Scholar]
- 40. Gokce G, Cerikcioglu N, Yagci A. Acid proteinase, phospholipase, and biofilm production of Candida spp. isolated from blood cultures. Mycopathologia 2007; 164: 265. [DOI] [PubMed] [Google Scholar]
- 41. Naglik JR, Rodgers CA, Shirlaw PJ, et al. Differential expression of Candida albicans secreted aspartyl proteinase and phospholipase B genes in humans correlates with active oral and vaginal infections. J Infect Dis 2003; 188: 469–479. [DOI] [PubMed] [Google Scholar]
- 42. Schäfer-Korting M, Blechschmidt J, Korting H. Clinical use of oral nystatin in the prevention of systemic candidosis in patients at particular risk. Mycoses 1996; 39: 329–339. [DOI] [PubMed] [Google Scholar]
- 43. Ozturk MA, Gunes T, Koklu E, et al. Oral nystatin prophylaxis to prevent invasive candidiasis in neonatal intensive care unit. Mycoses 2006; 49: 484–492. [DOI] [PubMed] [Google Scholar]
- 44. Howell A, Isaacs D, Halliday R, et al. Oral nystatin prophylaxis and neonatal fungal infections. Arch Dis Child Fetal Neonatal Ed 2009; 94: F429–F433. [DOI] [PubMed] [Google Scholar]
- 45. Enwuru CA, Ogunledun A, Idika N, et al. Fluconazole resistant opportunistic oro-pharyngeal candida and non-Candida yeast-like isolates from HIV infected patients attending ARV clinics in Lagos, Nigeria. Afr Health Sci 2008; 8: 142–148. [PMC free article] [PubMed] [Google Scholar]
- 46. Albertson GD, Niimi M, Cannon RD, et al. Multiple efflux mechanisms are involved in Candida albicans fluconazole resistance. Antimicrob Agents Chemother 1996; 40: 2835–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Niimi M, Firth NA, Cannon RD. Antifungal drug resistance of oral fungi. Odontology 2010; 98: 15–25. [DOI] [PubMed] [Google Scholar]


