Abstract
Prostate cancer is the second leading cause of cancer death in men. Its current treatment includes various physical and chemical approaches for the localized and advanced prostate cancer [e.g. metastatic castrate resistant prostate cancer (mCRPC)]. Although many new drugs are now available for prostate cancer, none is suitable for local treatment that can reduce adverse effects often associated with the current physical treatment. Of the drugs approved by FDA for mCRPC, the best mean improvement in overall survival is only about 4.8 months. Therefore, there is a need for improved treatment approaches for prostate cancer, especially drug-resistant cancer.
Ultrasound therapy represents a useful new physical approach for the drug-resistant cancer treatment by facilitating the entry of the related chemotherapy drug into the target cancer cells. There are two versions of ultrasound: High Intensity Focused Ultrasound (HIFU) and Low Intensity Pulsed Ultrasound (LIPUS). HIFU has been a promising treatment option for prostate cancer due to its noninvasiveness and various biological effects on cancer tissue. It has been approved for the treatment of cancer and in recent years there have been numerous findings suggesting HIFU can reduce cancer cell viability and possibly reverse the spread of cancerous tumors. LIPUS is currently being studied as an alternative treatment option for prostate cancer. Preliminary studies have found LIPUS to reduce cancer cell viability without the side effects seen in HIFU. Reversible cell membrane damage caused by LIPUS could allow increased uptake of anticancer drugs, enhancing cytotoxicity and death of cancer cells. In this way, a low dose of anticancer drug is more effective toward cancer cells while there is less damage to normal cells. The combination of LIPUS with certain chemotherapeutic agents can be an exciting physical-chemical combination therapy for prostate cancer. This review will focus on this topic as well as the clinical use of HIFU to provide an understanding of their current use and future potential role for prostate cancer therapy.
Keywords: LIPUS, HIFU, prostate cancer, treatment, ultrasound
Introduction
Since the discovery of prostate cancer by Dr. J. Adams in 1853 as “a very rare disease”, prostate cancer is now the most common diagnosed cancer in men, with over 174,000 new cases in 2019 and is the second leading cause of cancer death of men in the United States.1-3 Currently, there is a wide variety of treatment options for prostate cancer depending on its severity. For low or intermediate risk prostate cancer, patients can be treated with options such as active surveillance, minimally invasive ablative therapies, radiation therapy, or prostatectomy.4 For localized cancer the current recommended options are radiation therapy, brachytherapy or prostatectomy.1,2 As the disease progresses with metastases, chemotherapy is always a final option if it can be tolerated by the patient. However, recurrences from chemotherapy are common. Resistance to chemotherapy is the reason for 90% of drug failures in patients with metastatic cancer.5 Of the 6 drugs approved by FDA for the treatment of metastatic resistant prostate cancer (mCRPC), the best mean improvement in overall survival is only 4.8 months, most likely due to resistance development.6 In addition, chemotherapy often induces various severe side effects. Therefore, there is a need for new therapeutic approaches that can not only enhance efficacy but also reduce side effects.
Over the past decades, many new therapeutic approaches have been developed. These include chemical entities (currently there are 34 drugs approved by FDA for prostate cancer), physical entities (radiation, ultrasound) and their combinations. While there are many examples of chemical-chemical combinations1,2 physical-chemical combinations are less common. One such example is radiation in combination with androgen-ablative therapy. This combination is well known for improving time of relapse and survival.1 As of to date, there is no established ultrasound-drug combination to reach clinical trial stage, although the benefit of such combination can be considerable.
The present article will review the use of ultrasound for the treatment of prostate cancer, especially pertaining to its combination with anticancer drugs for potential treatment of prostate cancer. Since ultrasound can induce diverse effects to human cells (from harmless to lethal effect), it can offer a unique opportunity to optimize therapy when used in combination with chemotherapeutic agents.
General Properties of Ultrasound
Ultrasound waves refer to wave frequencies beyond the range the human ear can hear (>20 kHz).7 The ultrasound vibrations can cause a transfer of energy along a directed path and can propagate through the body, oscillating at varying pressures depending on the delivery of the ultrasound.8 There are usually three characteristics about ultrasound waves: frequency, wavelength, and velocity.9 Frequency is the number of times a particle experiencing a complete compression and rarefaction cycle in one second. Therapeutic ultrasound usually uses frequency ranges between 1 and 3 MHz and medical imaging applications use 1-20 MHz frequency ranges (Figure 1A). Ultra-high frequency ultrasonic transducers can also be used for research applications such as cellular stimulation and manipulation.10 Wavelength is the distance between two equivalent points on the waveform in a particular medium (Figure 1B). Depending on applications, appropriate pulse parameters are required. PRT (PRF) represents pulse repetition time. The duty factor is the fraction of time that an ultrasound pulse is actually being produced. The amplitude (Vp-p) of a sound wave is the maximum distance moved by a point on a wave measured from its equilibrium position and determines its intensity. Its intensity is an important parameter delivered from the ultrasound machine which is defined as the concentration of energy within the beam with units of W/cm2. The velocity of ultrasound is approximately 1500 m/sec in water and it can travel more rapidly in a denser medium.
Figure 1.
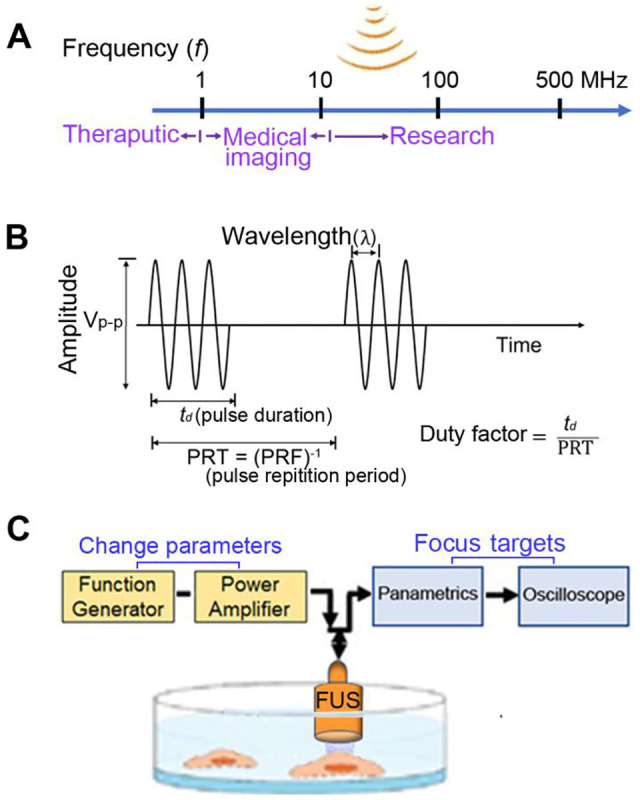
Sound waves and ultrasound parameters. (A) In ultrasound (US) field, different frequencies are used in water or different biological targets for different purposes. (B) Depending on applications, appropriate US pulse parameters are required. (C) In focused ultrasound (FUS), the stimulation parameters are easily adjusted by using a function generator and the focuses by an oscilloscope.
Focused ultrasound (FUS) produces a focused beam of acoustic energy that precisely and accurately reaches large targets in the body without damaging surrounding normal cells.11 The FUS stimulation parameters are easily adjusted by using a function generator (Figure 1C). The focal distance from the transducer to the surface of culture dish is aligned using a pulser-receiver (oscilloscope). FUS technology is approved for prostate cancer (see below) but also has been proposed for use in other cancer therapy.12,13
In this respect, there are two general types of ultrasound intensities used for therapeutic purposes: High Intensity Focused Ultrasound (HIFU) and Low Intensity Pulsed Ultrasound (LIPUS). High-intensity (>5 W/cm2) continuous FUS generates a systemic immune stimulatory effect resulting in tumor ablation.14 Pulsed FUS (i.e., non-continuous stimulus to minimize heat generation)15,16 may induce a more refined cellular/molecular immune response17 by initiating inflammatory responses which boost cancer immunotherapy.18 HIFU can lead to inertial cavitation of gas bubbles leading to cell death.19 This inertial cavitation can in turn lead to increased temperatures within cells.20 On the other hand, LIPUS causes stable cavitation without increasing temperature.
HIFU
HIFU was first approved by FDA in 2015. According to the recent NCCN Guidelines, ultrasound is recommended as a local therapeutic option for prostate cancer if radiation therapy fails.2 HIFU is usually defined as delivering ultrasound waves with an intensity of greater than 5 W/cm2.21 HIFU can generates tumor ablation through a systemic immune stimulatory effect.14 The main mechanisms of HIFU ablation involve mechanical and thermal effects.22 The thermal effect of HIFU is heat generation due to absorption of the acoustic energy with a rapid elevation of temperature in the local tissue. The local tissue temperature could be elevated to higher than 60°C by the thermal effect, causing tumor cell destruction via coagulation necrosis. The thermal effect is the major source from thermal ablation therapy using HIFU. In fact, HIFU with intensities in the range of 100 – 10,000 W/cm2 has been utilized clinically to destroy tumor cells.21 A spherical shaped transducer is thus used to focus the heat of the ultrasound on specific targeted cells. Many oncology centers use ultrasound with transducers between 0.8 and 3.5 MHz to not only treat prostate tumors, but also liver, kidney, or breast cancers because ultrasound therapy with frequencies between 0.8-3.5 MHz is more effective than standard diagnostic US for cancer treatment.23
Mechanical effects induced by HIFU are associated with acoustic pulses only at high intensities, including cavitation.22 Cavitation is defined as the physical forces of the sound waves on microenvironmental gases within fluid. As the sound waves propagate through the medium, the characteristic compression and rarefaction causes microscopic gas bubbles in the tissue fluid to contract and expand. It is generally thought that the rapid changes in pressure, both in and around the cell, may cause damage to the cell. There are two forms of cavitation: stable and inertial cavitation. Stable cavitation is the stable oscillation of the size of the bubble when exposed to a low-pressure acoustic field, and may play a role in US-enhanced drug and gene delivery. In contrast, inertial cavitation is violent oscillations of the bubble and rapid growth of the bubble during the rarefaction phase when they reach their size of resonance, eventually leading to the violent collapse and destruction of the bubble. The violent collapse will produce shock waves that produce free radicals and a cascade of molecular events and, in turn, damage the cancer cells. The mechanical effects can be explained by a concept of inertial cavitation. The heat generated by HIFU results in compression and rarefaction which then leads to formation of bubbles within the tissues. As the tissues are constantly heated and pressured by the HIFU, the bubbles are oscillating and expanding until reaching a size that would collapse asymmetrically, leading to tissue damage and cell death. However, it is less predictable and not as significant as the thermal effect, yet it can also play a role in damaging the targeted tissues.
Common devices used for HIFU are Sonablate and Ablatherm.24 Sonablate has a 4 MHz transducer with focal depth of 40 mm. Ablatherm has a 3 MHz transducer with adjustable focal length (19-26 mm). Both transducers can generate a focused ultrasound field that creates heat and cavitation which destroy the cancer tissues with a spindle-shaped elementary lesion of about 1.7 mm in diameter. The maximum depth that HIFU can penetrate prostatic tissues is about 30 mm.25 The therapeutic benefit of HIFU using Sonablate or Ablatherm in patients with prostate cancer is well established. This includes 61.2% to 95% 5-year disease-free survival rate for Sonablate and 69% to 84% 7-8-year disease-free survival rate for Ablatherm (See Table 1). However, HIFU can induce impotence (38.8% to 55.1%) and urinary incontinence (6.1% - 12.2%).28
Table 1.
HIFU for Prostate Cancer.
| Study design | Ultrasound settings | Efficacy | Side effects/limitation | References |
|---|---|---|---|---|
| Multi-institutional study of 111 patients | Transducer: 3.5MHz | Free survival rate at 2 years was 89% Mean PSA at 2 year was 2.3ng/ml |
UTI: 18/111 Transient dysuria: 17/111 Anejaculation: 16/111 |
26 |
| Retrospective study of 327 patients | Transducer: 3 MHz Focal length: 19-26 mm Max penetration depth: 30 mm |
Create spindle-shaped lession of 1.7 mm Biochemical failure free survival rate: 0.3% (P < 0.001) |
No side effect included in the study | 25 |
| Study of 163 patients who have prostatic cancer | Transducer: 3.5 MHz Max penetration HIFU: 24 mm |
86.4% has PSA nadir <1ng/ml 92.7% had negative biopsy after treatment No patient died due to prostate cancer within 4.8 +/- 1.2 years follow-up |
Most common: Obstruction due to necrosis or scarring (31% occurred with HIFU alone) | 27 |
| Cross sectional study of 223 patients who have prostatic cancer. | Transducer: 3 MHz Created lesion has: 1.7 mm diameter and 19-24 mm in length |
49% patients needed a second HIFU session due to positive biopsies after first HIFU treatment. The average time between first and second treatment is 7 months | Impotence: 49.8% Infravesical obstruction: 19.7% Stress incontinence: 7.6% |
28 |
| Study of 58 patients who have prostatic cancer | Transducer: 3 MHz Created lesion: 1.7 mm diameter and 19-24 mm in length. 5 seconds duration and 5 seconds delay between each shot |
78% of patients has PSA<0.5 ng/ml Successful rate of low-, intermediate-, and high-risk group are 85%, 77%, and 47% respectively |
No mention about side effects. Limitation: the sample size is so small to have significant impact |
29 |
| Study of 1002 patients who have prostate cancer | Transducer: 3 MHz 6 seconds treatment pulse and 4 seconds shot interval |
After an average of 7.9 weeks, median nadir PSA is 0.14 ng/ml 10-years survival rate was 80% |
Bladder outlet obstruction: 16.6% Urinary incontinence: 23.7% Acute urinary retention: 7.6% |
30 |
| Study of 227 patients who have prostate cancer | Ablatherm HIFU device (EDAP SA, Lyon, France) Transducer: 3 MHz Contiguous shots (5 seconds on, 5 seconds off) |
86% of patients treated with HIFU had negative control biopsies. Median nadir PSA was 0.1ng/ml 5-years disease free survival rate was 66% |
Side effect: 13% incontinence, 12% stenosis, 9% sloughing | 31 |
The benefit of combination of HIFU and anticancer drugs for prostate cancer is unknown. A previous study showed that the anticancer activity of anticancer drugs can be decreased by HIFU exposure when tested in human ovarian cancer cells.32 Since HIFU, when combined with anticancer drugs is known for inducing a lethal effect rather than a therapeutic effect to a defined area, its main use is for local ablation therapy and not for metastatic cancer.
LIPUS
LIPUS is defined as ultrasound applied at an intensity less than 3W/cm2 and the energy is released at a pulsed rate. The pulsed rate, commonly referred to as duty factor or duty cycle is usually set at 1:1 (50%) or 1:4 (20%) (the amount of time the energy is released versus being off in one second). In terms of penetration, 1 MHz frequency typically reaches tissue depths of 2.3-5 cm, while a 3 MHz frequency reaches depths of 0.8 -1.6 cm.33
Due to the low intensity from LIPUS, the thermal effect is minimal as opposed to HIFU. Most documented effects are mechanical (or nonthermal) cellular changes. Vibrations of cellular components due to ultrasound are known to cause cavitation within cells. LIPUS can cause stable cavitation resulting from the formation of gas bubbles which will take roughly 1000 cycles to reach their maximum size. Another mechanical effect is acoustic streaming that provides a driving force capable of displacing ions and small molecules. This mechanical pressure applied by the wave produces unidirectional movement of fluid along and around cell membranes which is known to affect diffusion rates and membrane permeability.34 Through cavitation, implosion of gas bubbles can create microjets of fluid, so possibly drugs can then enter targeted cells.35 Furthermore, non-collapsing bubbles near target cells may alter cellular membranes which can increase the influx of drugs into cells.36
LIPUS can be further utilized to combine with microbubbles to enhance the drug effect. Microbubbles are microspheres in the range of 1-8 µm size consisting a gas core stabilized by a surrounding shell made of phospholipids or other polymers. Drugs, antibodies or other proteins can be attached to the surface of shells, encased inside the shells, or embedded in the shell membrane.37– 39 Injection of such microbubbles and upon exposure to ultrasound can cause cavitation (shrinking and expanding to a size that can lead to rupture) which can generate shear stress and cell membrane permeability. Such effect can allow drugs or proteins to enter the cell and improve anticancer effect.
LIPUS has been investigated as a possible prostate cancer treatment option either alone or in combination with microbubbles [See Table 2]. Hou et al utilized LIPUS with microbubbles in order to induce microvessel damage in tumors leading to cell necrosis or apoptosis.40 Their results indicated that with LIPUS treatment four times per day, cell proliferation was inhibited, and apoptosis was promoted. However, when treated only once per day, they observed opposite effects indicating further research is needed to clearly define the ultrasound effects. Yang et al reported the effect of LIPUS plus microbubbles on microvessel disruption in prostate cancer.41,42 They concluded that the optimal ultrasound parameters are 20 kHz frequency, 1 W/cm2 intensity, 40% duty cycle and 3-minute duration to induce tumor ablation in mice implanted with PC3 cancer cells.
Table 2.
LIPUS Alone or in Combination With Drugs on Prostate Cancer.
| Cell line/xenograft | Parameters | Results | Reference |
|---|---|---|---|
| PC-3/xenograft | acoustic intensity (1 W/cm2), frequency (20 kHz), duty cycle (40%), and radiation duration (3 min) | cell proliferation was inhibited, and apoptosis was promoted | 40 |
| PC-3/xenograft | 0.50, 1.00 and 2.00 W/cm2, duty cycle of 20%, varying time | optimum conditions for microvessel disruption were found to be as follows: Sound intensity, 1.00 W/cm2; frequency, 20 Hz; duty cycle, 40%; microbubble volume, 0.20 ml; and irradiation time, 3 min. | 41 |
| PC-3/xenograft | frequency, 20 kHz; acoustic intensity, 1 W/cm2; duty cycle, 40% irradiation duration, 3 min. | exposure to microbubbles prior to radiofrequency ablation produced larger volumes of ablation, compared with treatment with RFA alone | 42 |
| PC-3/xenograft | frequency, 20 kHz; acoustic intensity, 1 W/cm2; duty cycle, 40% irradiation duration, 3 min. | USMB combined with docetaxel is more effective than USMB or docetaxel alone in inhibiting tumor growth via the enhancement of apoptosis, and the suppression of proliferation and angiogenesis | 43 |
| DU145 | intensity of 0.15, 0.30 and 0.45 W/cm2; irradiation time, 10, 20 and 30 sec | ultrasound with or without microbubbles in combination with simvastatin may additively or synergistically inhibit cell viability and induce apoptosis | 44 |
| DU145 | 21 kHz; power density, 0.113 W/cm2; exposure time, 2 min at a duty cycle of 70%, | microbubble/mitoxantrone combination significantly decreased cell viability (50.7%) when compared to drug alone and ultrasound alone | 45 |
| paclitaxel-resistant PC-3 cells | frequency of 1 MHz, intensity was set to 1.2 W/cm2 | ultrasound triggers ERs, which inhibit signal transduction pathway to downregulate the transcriptional activity of transporters and intracellular concentrations of paclitaxel are increased, promoting apoptosis and reversing drug resistance | 46 |
Abbreviations: USMB, ultrasound with microbubble; ER, endoplasmic reticulum; RFA, radiofrequency ablation.
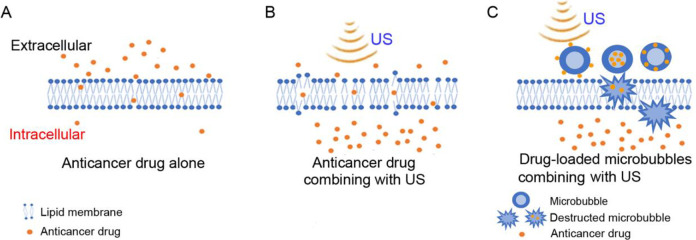
The use of LIPUS in combination with anticancer drugs has also been explored (Figure 2A, B). Anti-cancer drugs can inhibit various pathways known to regulate cancer proliferation and survival. Several classes of drugs/treatments have been developed to interfere with oncogenes/oncoproteins, androgen receptor signaling, bone metastasis, and immune response known to be involved in the progression of prostate cancer.47,48 There are now 36 drugs by FDA as of January 14, 2021.49 (Due to this extensive list of drugs, we like to refer those interested in therapeutic use of drugs for prostate cancer to the website and individual product information.) As is often the case with chemotherapy, drug resistance can occur with continued therapy,50 so improved methods to treat drug-resistant cancers are needed. The use of LIPUS in combination with anticancer drugs may offer a new treatment for drug resistant cancer (Table 2) (Figure 2A and B).
Figure 2.
Effect of membrane poration with ultrasound (US) on enhancement of anticancer drug delivery to cells. (A) The ability of the drug alone to cross the lipid membrane. (B) The proposed enhanced ability of the anticancer drug to cross the lipid membrane in combination with the use of US. (C) The anticancer drug-loaded microbubbles combining with US in the treatment of tumors. Anticancer drugs are attached to the surface of the microbubbles, encased inside the microbubbles, or embedded in the microbubble membrane.37-39 After ultrasound irradiation, microbubbles are destructed in tumor cells and drugs carried by microbubbles are released into tumor cells.
Yang et al studied the ultrasound combined with microbubbles (USMB) in further combination with docetaxel.43 They found that the mean tumor inhibition was 73.33%, 46.67% and 33.33% for ultrasound-docetaxel combination, docetaxel alone and ultrasound alone, respectively (Table 2). They believe the combination effect is due to inertial cavitation caused by USMB that generates jet streams. The jet streams in turn generate pores in vessel walls and cell membranes to enhance drug delivery (Figure 2C). Xu et al found a similar combination effect with ultrasound plus simvastatin.44 Their results indicated that USMB when combined with simvastatin significantly increased cellular apoptosis of DU-145 cells when compared to ultrasound and drug alone. This enhanced effect is proposed to be due to cavitation caused by ultrasound microbubbles and increased membrane permeability. Other prostate cancer studies also found the combination of USMB with anticancer drugs enhances chemotherapeutic effects through increased membrane permeability.45,46 Most of these reports related to the use of combination of USMB with anticancer drugs because microbubbles can amplify the impact of low-frequency ultrasound stimulation with the capability of long-distance penetration of cells physically coupled to microbubbles, as a result of a large difference in acoustic impedance between the surrounding media and the air inside the bubbles.51,52 However, ultrasound-anticancer drug combination without microbubble also has been demonstrated to induce significant change in prostate cancer cell viability.48
Discussion and Conclusion
While the use of HIFU for the treatment of localized prostate cancer is well accepted, the potential of LIPUS-anticancer drug combination can be an exciting area of research. LIPUS can penetrate a greater depth than HIFU and therefore is more versatile for application. Since LIPUS causes minimal damage to cells, it is also safer to normal tissues. In addition, if the effect of LIPUS causes reversible cell membrane damage that could allow anticancer drugs to enter cancer cells more readily, such combination with or without microbubbles may induce enhanced cytotoxicity to cancer cells. This can be especially useful if cancer cells are more sensitive to anticancer drugs than normal cells. Anticancer drugs like docetaxel and cabazitaxel can achieve a much lower IC50 for PC3 and DU145 prostate cancer cells compared to normal prostate cells, suggesting specificity of the drugs to cancer cells (our unpublished data). Future research on the application of LIPUS-anticancer drug combination can target both localized as well as certain metastatic sites. For local therapy such as the prostate, a lower dose of anticancer drug in combination with LIPUS may prove to be as effective but safer and more versatile than HIFU (Figure 2B). For metastatic prostate cancer, such combination may be also applied to common metastatic sites such as long bones, pelvis, lower spine, lymph nodes, liver, lungs or pleura in the thorax.53-55 Technical development of implanting a small transducer inducing LIPUS in combination with systemic anticancer drug therapy is needed. Potentially the development of intravascular catheter insertion with a small transducer without microbubbles could possibly enhance the versatility of LIPUS for metastatic prostate cancer in the future. In conclusion, LIPUS when used in combination with anticancer drug therapy could be an exciting example of a physical-chemical combination (Figure 2), resulting in potentially enhanced efficacy and reduced toxicity (in normal cells). Further research in this direction could be a worthwhile endeavor, especially for a patient with resistant prostate cancer in which there is no effective treatment.
Acknowledgments
The authors thank Ms. Yuna Kwon for her assistance on manuscript submission.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health grant GM126016 and USC BME Baum chair account (KKS).
ORCID iD: Mosses S. S. Chow, PharmD  https://orcid.org/0000-0001-7794-6214
https://orcid.org/0000-0001-7794-6214
References
- 1. Denmeade SR, Isaacs JT. A history of prostate cancer treatment. Nat Rev Cancer. 2002;2(5):389–396. doi:10.1038/nrc801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mohler JL, Antonarakis ES, Armstrong AJ, et al. Prostate cancer, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(5):479–505. doi:10.6004/jnccn.2019.0023 [DOI] [PubMed] [Google Scholar]
- 3. Rawla P. Epidemiology of prostate cancer. World J Oncol. 2019;10(2):63–89. doi:doi:10.14740/wjon1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Evans AJ. Treatment effects in prostate cancer. Mod Pathol. 2018;31(S1):S110–S121. doi:10.1038/modpathol.2017.158 [DOI] [PubMed] [Google Scholar]
- 5. Abdullah LN, Chow EK-H. Mechanisms of chemoresistance in cancer stem cells. Clin Transl Med. 2013;2:3. doi:10.1186/2001-1326-2-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. von Amsberg G, Merseburger AS. Treatment of metastatic, castration-resistant prostate cancer. Urologe A. 2020;59(6):673–679. doi:10.1007/s00120-020-01187-9 [DOI] [PubMed] [Google Scholar]
- 7. Lynn JG, Zwemer RL, Chick AJ, Miller AE. A new method for the generation and use of focused ultrasound in experimental biology. J Gen Physiol. 1942;26(2):179–193. doi:10.1085/jgp.26.2.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Seah BCQ, Teo BM. Recent advances in ultrasound-based transdermal drug delivery. Int J Nanomedicine. 2018;13:7749–7763. doi:10.2147/IJN.S174759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xin Z, Lin G, Lei H, Lue TF, Guo Y. Clinical applications of low-intensity pulsed ultrasound and its potential role in urology. Transl Androl Urol. 2016;5(2):255–266. doi:10.21037/tau.2016.02.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pan Y, Yoon S, Zhu L, Wang Y. Acoustic mechanogenetics. Curr Opin Biomed Eng. 2018;7:64–70. doi:10.1016/j.cobme.2018.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mittelstein DR, Ye J, Schibber EF, et al. Selective ablation of cancer cells with low intensity pulsed ultrasound. Appl Phys Lett. 2020;116:013701. doi:10.1063/1.5128627 [Google Scholar]
- 12. Lee NS, Yoon CW, Wang Q, et al. Focused ultrasound stimulates ER localized mechanosensitive PANNEXIN-1 to mediate intracellular calcium release in invasive cancer cells. Front Cell Dev Biol. 2020;8:504. doi:10.3389/fcell.2020.00504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weitz AC, Lee NS, Yoon CW, et al. Functional assay of cancer cell invasion potential based on mechanotransduction of focused ultrasound. Front Oncol. 2017;7:161. doi:10.3389/fonc.2017.00161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lu P, Zhu XQ, Xu ZL, Zhou Q, Zhang J, Wu F. Increased infiltration of activated tumor-infiltrating lymphocytes after high intensity focused ultrasound ablation of human breast cancer. Surgery. 2009;145(3):286–293. doi:10.1016/j.surg.2008.10.010 [DOI] [PubMed] [Google Scholar]
- 15. Hersh DS, Kim AJ, Winkles JA, Eisenberg HM, Woodworth GF, Frenkel V. Emerging applications of therapeutic ultrasound in neuro-oncology: moving beyond tumor ablation. Neurosurgery. 2016;79(5):643–654. doi:10.1227/NEU.0000000000001399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ziadloo A, Burks SR, Gold EM, et al. Enhanced homing permeability and retention of bone marrow stromal cells by noninvasive pulsed focused ultrasound. Stem Cells. 2012;30(6):1216–1227. doi:10.1002/stem.1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Curley CT, Sheybani ND, Bullock TN, Price RJ. Focused ultrasound immunotherapy for central nervous system pathologies: challenges and opportunities. Theranostics. 2017;7(15):3608–3623. doi:10.7150/thno.21225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. MAuri GA, Nicosia luc A, Xu Z, et al. Focused ultrasound: tumour ablation and its potential to enhance immunological therapy to cancer. Br J Radiol. 2018;91(1083):20170641. doi:10.1259/bjr.20170641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lentacker I, de Cock I, Deckers R, de Smedt SC, Moonen CTW. Understanding ultrasound induced sonoporation: definitions and underlying mechanisms. Adv Drug Deliv Rev. 2014;72:49–64. doi:10.1016/j.addr.2013.11.008 [DOI] [PubMed] [Google Scholar]
- 20. Phenix CP, Togtema M, Pichardo S, Zehbe I, Curiel L. High intensity focused ultrasound technology, its scope and applications in therapy and drug delivery. J Pharm Pharm Sci. 2014;17(1):136–153. doi:10.18433/j3zp5f [DOI] [PubMed] [Google Scholar]
- 21. Copelan A, Hartman J, Chehab M, Venkatesan AM. High-intensity focused ultrasound: current status for image-guided therapy. Semin Intervent Radiol. 2015;32(04):398–415. doi:10.1055/s-0035-1564793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhou Y-F. High intensity focused ultrasound in clinical tumor ablation. World J Clin Oncol. 2011;2(1):8–27. doi:10.5306/wjco.v2.i1.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sengupta S, Balla VK. A review on the use of magnetic fields and ultrasound for non-invasive cancer treatment. J Adv Res. 2018;14:97–111. doi:10.1016/j.jare.2018.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Colombel M, Gelet A. Principles and results of high-intensity focused ultrasound for localized prostate cancer. Prostate Cancer Prostatic Dis. 2004;7:289–294. doi:10.1038/sj.pcan.4500721 [DOI] [PubMed] [Google Scholar]
- 25. Pfeiffer D, Berger J, Gross A. Single application of high-intensity focused ultrasound as primary therapy of localized prostate cancer: treatment-related predictors of biochemical outcomes. Asian J Urol. 2015;2(1):46–52. doi:10.1016/j.ajur.2014.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rischmann P, Gelet A, Riche B, et al. Focal high intensity focused ultrasound of unilateral localized prostate cancer: a prospective multicentric hemiablation study of 111 patients. Eur Urol. 2017;71(2):267–273. doi:10.1016/j.eururo.2016.09.039 [DOI] [PubMed] [Google Scholar]
- 27. Blana A, Rogenhofer S, Ganzer R, et al. Eight years’ experience with high-intensity focused ultrasonography for treatment of localized prostate cancer. Urology. 2008;72(6):1329–1333. doi:10.1016/j.urology.2008.06.062 [DOI] [PubMed] [Google Scholar]
- 28. Blana A, Rogenhofer S, Ganzer R, Wild PJ, Wieland WF, Walter B. Morbidity associated with repeated transrectal high-intensity focused ultrasound treatment of localized prostate cancer. World J Urol. 2006;24(5):585–590. doi:10.1007/s00345-006-0107-x [DOI] [PubMed] [Google Scholar]
- 29. Lee HM, Hong JH, Choi HY. High-intensity focused ultrasound therapy for clinically localized prostate cancer. Prostate Cancer Prostatic Dis. 2006;9(4):439–443. doi:10.1038/sj.pcan.4500901 [DOI] [PubMed] [Google Scholar]
- 30. Crouzet S, Chapelon JY, Rouvière O, et al. Whole-gland ablation of localized prostate cancer with high-intensity focused ultrasound: oncologic outcomes and morbidity in 1002 patients. Eur Urol. 2014;65(5):907–914. doi:10.1016/j.eururo.2013.04.039 [DOI] [PubMed] [Google Scholar]
- 31. Poissonnier L, Chapelon JY, Rouvière O, et al. Control of prostate cancer by transrectal HIFU in 227 patients. Eur Urol. 2007;51(2):381–387. doi:10.1016/j.eururo.2006.04.012 [DOI] [PubMed] [Google Scholar]
- 32. Yu T, Zhang Y, He H, Zhou S, Liu Y, Huang P. Anticancer potency of cytotoxic drugs after exposure to high-intensity focused ultrasound in the presence of microbubbles and hematoporphyrin. Mol Pharm. 2011;8(4):1408–1415. doi:10.1021/mp2001846 [DOI] [PubMed] [Google Scholar]
- 33. Hayes BT, Merrick MA, Sandrey MA, Cordova ML. Three-MHz ultrasound heats deeper into the tissues than originally theorized. J Athl Train. 2004;39(3):230–234. http://www.ncbi.nlm.nih.gov/pubmed/15496991. Accessed July 29, 2020. [PMC free article] [PubMed] [Google Scholar]
- 34. Johns LD. Nonthermal effects of therapeutic ultrasound: the frequency resonance hypothesis. J Athl Train. 2002;37(3):293–299. PMID: 16558674; PMCID: PMC164359. [PMC free article] [PubMed] [Google Scholar]
- 35. Ohl CD, Arora M, Ikink R, et al. Sonoporation from jetting cavitation bubbles. Biophys J. 2006;91:4285–4295. doi:10.1529/biophysj.105.0753663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Feril LB, Jr, Kondo T. Biological effects of low intensity ultrasound: the mechanism involved, and its implications on therapy and on biosafety of ultrasound. J Radiat Res. 2004;45(4):479–489. doi:10.1269/jrr.45.479 [DOI] [PubMed] [Google Scholar]
- 37. Lee HJ, Yoon YI, Bae YJ. Theragnostic ultrasound using microbubbles in the treatment of prostate cancer. Ultrasonography. 2016;35(4):309–317. doi:10.14366/usg.16006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tu J, Zhang H, Yu J, Liufu C, Chen Z. Ultrasound-mediated microbubble destruction: a new method in cancer immunotherapy. Onco Targets Ther. 2018;11:5763–5775. doi:10.2147/OTT.S171019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gong Q, Gao X, Liu W, Hong T, Chen C. Drug-loaded microbubbles combined with ultrasound for thrombolysis and malignant tumor therapy. Biomed Res Int. 2019;2019. doi.org/10.1155/2019/6792465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hou R, Xu Y, Lu Q, Zhang Y, Hu B. Effect of low-frequency low-intensity ultrasound with microbubbles on prostate cancer hypoxia. Tumour Biol. 2017;39(10). doi:10.1177/1010428317719275 [DOI] [PubMed] [Google Scholar]
- 41. Yang Y, Bai W, Chen Y, Lin Y, Hu B. Optimization of low-frequency low-intensity ultrasound-mediated microvessel disruption on prostate cancer xenografts in nude mice using an orthogonal experimental design. Oncol Lett. 2015;10(5):2999–3007. doi:10.3892/ol.2015.3716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yang Y, Bai W, Chen Y, Zhang W, Wang M, Hu B. Low-frequency and low-intensity ultrasound-mediated microvessel disruption enhance the effects of radiofrequency ablation on prostate cancer xenografts in nude mice. Molecular Medicine Reports. 2015;12(5):7517–7525. doi:10.3892/mmr.2015.4375 [DOI] [PubMed] [Google Scholar]
- 43. Yang Y, Bai W, Chen Y, et al. Low-frequency ultrasound-mediated microvessel disruption combined with docetaxel to treat prostate carcinoma xenografts in nude mice: a novel type of chemoembolization. Oncol Lett. 2016;12(2):1011–1018. doi:10.3892/ol.2016.4703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xu WP, Shen E, Bai WK, Wang Y, Hu B. Enhanced antitumor effects of low-frequency ultrasound and microbubbles in combination with simvastatin by downregulating caveolin-1 in prostatic DU145 cells. Oncol Lett. 2014;7(6):2142–2148. doi:10.3892/ol.2014.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang Y, Bai WK, Shen E, Hu B. Sonoporation by low-frequency and low-power ultrasound enhances chemotherapeutic efficacy in prostate cancer cells in vitro. Oncol Lett. 2013;6(2):495–498. doi:10.3892/ol.2013.1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wu Y, Liu X, Qin Z, Hu L, Wang X. Low-frequency ultrasound enhances chemotherapy sensitivity and induces autophagy in PTX-resistant PC-3 cells via the endoplasmic reticulum stress-mediated PI3K/Akt/mTOR signaling pathway. Onco Targets Ther. 2018;11:5621–5630. doi:10.2147/OTT.S176744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Soekmadji C, Nelson CC. The emerging role of extracellular vesicle-mediated drug resistance in cancers: implications in advanced prostate cancer. Biomed Res Int. 2015;2015:454837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nevedomskaya E, Baumgart SJ, Haendler B. Recent advances in prostate cancer treatment and drug discovery. Int J Mol Sci. 2018;19(5):1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Drugs Approved for Prostate Cancer [Internet]. National cancer institute. January 14, 2021. https://www.cancer.gov/about-cancer/treatment/drugs/prostate.
- 50. Galletti G, Leach BI, Lam L, Tagawa ST. Mechanisms of resistance to systemic therapy in metastatic castration-resistant prostate cancer. Cancer Treat Rev. 2017;57:16–27. [DOI] [PubMed] [Google Scholar]
- 51. Chen D, Sun Y, Gudur MSR, et al. Two-bubble acoustic tweezing cytometry for biomechanical probing and stimulation of cells. Biophys J. 2015;108(1):32–42. doi:10.1016/j.bpj.2014.11.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fan Z, Sun Y, di Chen, et al. Acoustic tweezing cytometry for live-cell subcellular modulation of intracellular cytoskeleton contractility. Sci Rep. 2013;3. doi:10.1038/srep02176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gandaglia G, Abdollah F, Schiffmann J, et al. Distribution of metastatic sites in patients with prostate cancer: a population-based analysis. Prostate. 2014;74(2):210–216. doi:10.1002/pros.22742 [DOI] [PubMed] [Google Scholar]
- 54. Fornetti J, Welm AL, Stewart SA. Understanding the bone in cancer metastasis. J Bone Miner Res. 2018;33(12):2099–2113. doi:10.1002/jbmr.3618 [DOI] [PubMed] [Google Scholar]
- 55. Bubendorf L, Schöpfer A, Wagner U, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31(5):578–583. doi:10.1053/hp.2000.6698 [DOI] [PubMed] [Google Scholar]