Abstract
Long-term potentiation (LTP) is an important molecular mechanism for chronic pain in the anterior cingulate cortex (ACC), a key cortical region for pain perception and emotional regulation. Inhibiting ACC LTP via various manipulations or pharmacological treatments blocks chronic pain. Long-term depression (LTD) is another form of synaptic plasticity in the ACC, which is also proved to be involved in the mechanisms of chronic pain. However, less is known about the interactive relationship between LTP and LTD in the ACC. Whether the synaptic depression could be induced after synaptic LTP in the ACC is not clear. In the present study, we used multi-channel field potential recording systems to study synaptic depression after LTP in the ACC of adult mice. We found that low frequency stimulus (LFS: 1 Hz, 15 min) inhibited theta burst stimulation (TBS)-induced LTP at 30 min after the induction of LTP. However, LFS failed to induce depression at 90 min after the induction of LTP. Furthermore, NMDA receptor antagonist AP-5 blocked the induction of synaptic depression after potentiation. The GluN2B-selective antagonist Ro25-6981 also inhibited the phenomenon in the ACC, while the GluN2A-selective antagonist NVP-AAM077 and the GluN2C/D-selective antagonist PPDA and UBP145 had no any significant effect. These results suggest that synaptic LTP can be depressed by LTD in a time dependent manner, and GluN2B-containing NMDA receptors play important roles in this form of synaptic depression.
Keywords: Synaptic plasticity, synaptic depression, ACC, NMDA receptors, LTP, LTD, LFS
Introduction
Long-term potentiation (LTP) is an important molecular mechanism for generation and maintenance of chronic pain and pain-related emotions within the anterior cingulate cortex (ACC), a key cortical region for pain perception and emotional regulation.1,2 Synaptic potentiation in the ACC induced by the injury contributes to behavioral allodynia, hyperalgesia and spontaneous pain.1,3 Behavioral, genetic and pharmacological studies show that inhibiting or reducing LTP can produce analgesic effects in animal models of chronic pain. Depotentiation at nociceptive C-fibers functions to erase a memory trace of pain or relieve pain.4 Except for LTP, long-term depression (LTD) is another form of synaptic plasticity, which is also proved to be involved in mechanisms of chronic pain.5,6 Recently, Wang et al. reported that overexpression of Casp3 restores LTD within the ACC after nerve injury and reduces peripheral hypersensitivity.7 Few studies have shown that low-frequency stimulation (LFS)-induced LTD can also reverse LTP, occurring the synaptic depotentiation.8 However, the interactive relationship between LTP and LTD are not clear, especially for pain manipulation. Most studies about LTD only focus on the normal synapse condition, and do not explore the underlying mechanisms and functions under potentiated condition, for example after LTP.
Previous studies have shown that low-frequency stimulation (LFS) is a classical protocol to induce synaptic depression.9,10 In the hippocampus, the persistence of LTP could be disrupted by LFS and this synaptic depression was time-dependent.11,12 With the prolonged time intervals, it is more difficult to observe synaptic depression, indicating that synaptic depression after LTP is closely related to protein synthesis.12,13 Some studies also showed that LFS-induced depotentiation could be blocked by NMDA receptor antagonist AP-5.8,14,15 Direct application of NMDA revealed a time-dependent reversal of LTP in the CA1 region of hippocampal slices,8 indicating that LFS-induced synaptic depression after LTP in hippocampus requires the involvement of NMDA receptors.
Many studies have reported that LTP and LTD both existed in the ACC. However, no direct evidences showed whether synaptic depression exists after the induction of LTP in the ACC. In the present study, we used a 64-channel multi-electrode dish (MED64) recording system to examine LFS (1 Hz, 15 min)-induced synaptic depression after theta burst stimulation (TBS)-induced LTP in the ACC of adult mice. Our results showed that synaptic depression might be observed only within a short time window after potentiation, which provided an important mechanism for pain relief or forgetting. And this phenomenon is dependent on NMDA receptors, especially GluN2B-containing NMDA receptors.
Materials and methods
Animals
Adult male C57BL/6 mice (aged 6-8 weeks) were purchased from Experimental Animal Center of Xi’an Jiaotong University. All experimental animals were randomly housed in plastic cage with enough food and water under a 12-h light/dark cycle. Mice were adapted the environment at least one week before carrying out experiments. All experimental procedures were in accordance with the guidelines of the Ethics Committee of Xi’an Jiaotong University.
Brain slice preparation
Coronal ACC brain slices from C57BL/6 mice were prepared as described previously.16,17 Mice were anesthetized with 1% to 2% gaseous isoflurane within a short time and quickly decapitated. The whole brain was rapidly removed into ice-cold oxygenated (95% O2 and 5% CO2) cutting solution (in mM: 252 sucrose, 2.5 KCl, 6 MgSO4, 0.5 CaCl2, 25 NaHCO3, 1.2 NaH2PO4, and 10 glucose, pH 7.3 to 7.4) for a short time. Appropriate parts of the brain were then trimmed and the remaining brain block was glued onto the ice-cold platform of a vibrating tissue slicer (Leica VT1200S). Then coronal ACC brain slices (300 µm) were cut and transferred to an incubation chamber with oxygenated artificial cerebrospinal fluid (ACSF) (in mM: 124 NaCl, 2.5 KCl, 1 NaH2PO4, 1 MgSO4, 2 CaCl2, 25 NaHCO3 and 10 glucose, pH 7.3 to 7.4) at room temperature for at least 1-h incubation before conducting experiments.
The 64 multi-electrode array
MED64, a 64-channel recording system (Alpha-Med Sciences, Japan) was generally used throughout all the experiments for multi-channel field potential recordings. The MED64 P515A probe is an 8 × 8 array of 64 planar microelectrodes with a 150-µm interpolar distance. Before use, the surface of the MED64 P515A probe needs to be pre-treated with 0.1% polyethyleneimine (Sigma Aldrich) in 25 mM borate buffer (pH 8.4) overnight at room temperature. The probe surface was rinsed three times with sterile distilled water. After incubation for 1 h, one brain slice was transferred into the probe, and a fine mesh anchor were carefully positioned onto the slices to ensure slice stability during recordings. The different layers of ACC are placed to cover the surface of 64 microelectrodes. The slice was continuously perfused with oxygenated ACSF at the rate of 2-3 mL/min.
Field potential recordings
Multi-channel field potential recordings were based on our previous studies.10,18 After a minimum 1-hour recovery period of slices in the probe, one of the 64 channels was chosen as the optimum stimulation site, by which the best synaptic responses could be induced in surrounding channels. Biphasic constant current pulse test stimulation (0.2 ms) was applied once per minute for the stimulation channel to evoke the field excitatory postsynaptic potentials (fEPSPs). The optimum stimulation intensity was decided by which 40%-60% of the maximum number of channels could be induced. For LTP recordings, basal synaptic responses were stably recorded at least 30 min as a baseline, then a TBS (five trains of burst with four pulses at 100 Hz, at 200 ms interval; repeated five times at intervals of 10 s, with the same stimulation intensity and stimulation site with baseline) was given to induce stable LTP. LTP in a channel was defined if the potentiated synaptic response increased at least 20% of baseline during the entire recording period.
For LTD recordings, stable basal synaptic responses were recorded for 30 min, and then a classical LFS protocol (1 Hz, 15 min, with the same stimulation intensity and stimulation site with baseline) was given to induce LTD as described previously. The fEPSP responses were continuously recorded for 30 min after LFS on stimulation site. To test if synaptic depression would be produced after TBS-induced potentiation, LFS was given with different time windows after LTP induction. Furthermore, various antagonists were perfused to detect the characteristics of synaptic depression after LTP induction.
Drugs
Selective competitive NMDA receptor antagonist AP-5 (Cat. No. HB0225) and GluN2D-selective NMDA receptor antagonist UBP145 (Cat. No. HB4717) were purchased from HelloBio (Princeton, NJ, USA). GluN2A-selective NMDA receptors antagonist NVP-AAM077 (also called PEAQX tetrasodium hydrate; Cat. No. P1999) was from Sigma Aldrich (St Louis, MO, USA). GluN2B-selective NMDA receptors antagonist Ro25-6981 maleate (Cat. No. 1594) and GluN2C/D-selective NMDA receptor antagonist PPDA (Cat. No. 2530) were obtained from Tocris Cookson (Bristol, UK). Drugs were prepared as stock solutions for frozen aliquots at −20°C. All these drugs were diluted from the stock solution to the final concentration in the ACSF before immediate using.
Statistical analysis
Mobius software paired with the MED64 system, was used for all data acquisition and analysis. To quantify the data, the initial slope of fEPSP was measured separately for each channel. The percentage of the fEPSP slope of every minute was normalized by baseline average. Some activated channels with baseline response variation >5% were discarded. The 2-minute normalized fEPSP slopes were averaged as a data point for plotting figures (OriginPro 8.0). All data was presented as mean ± SEM, and SPSS 22.0 software was used for data analysis. Two-tail paired or unpaired t-test were used for statistical comparisons. In all cases, p < 0.05 was considered to be the criterion for statistical significance.
Results
LFS-induced synaptic depression in LTP-potentiated synapses of the ACC
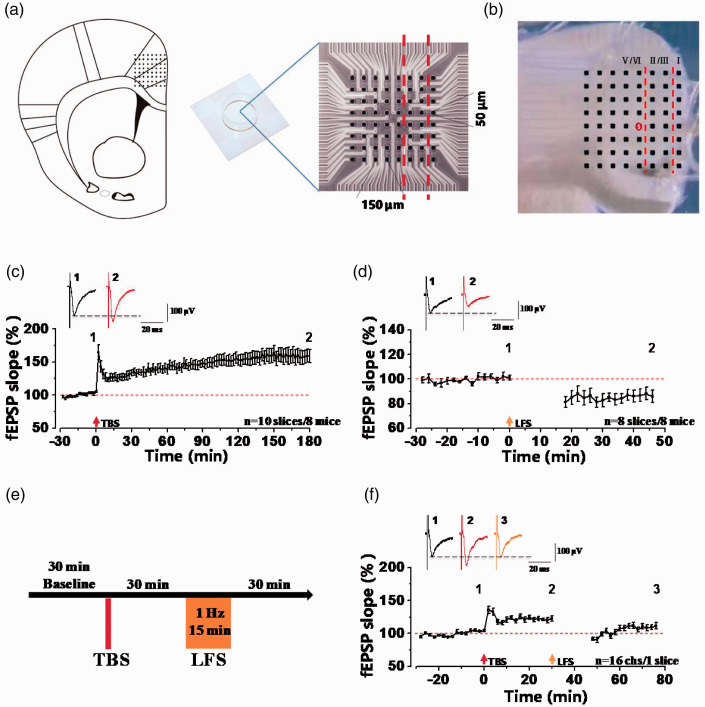
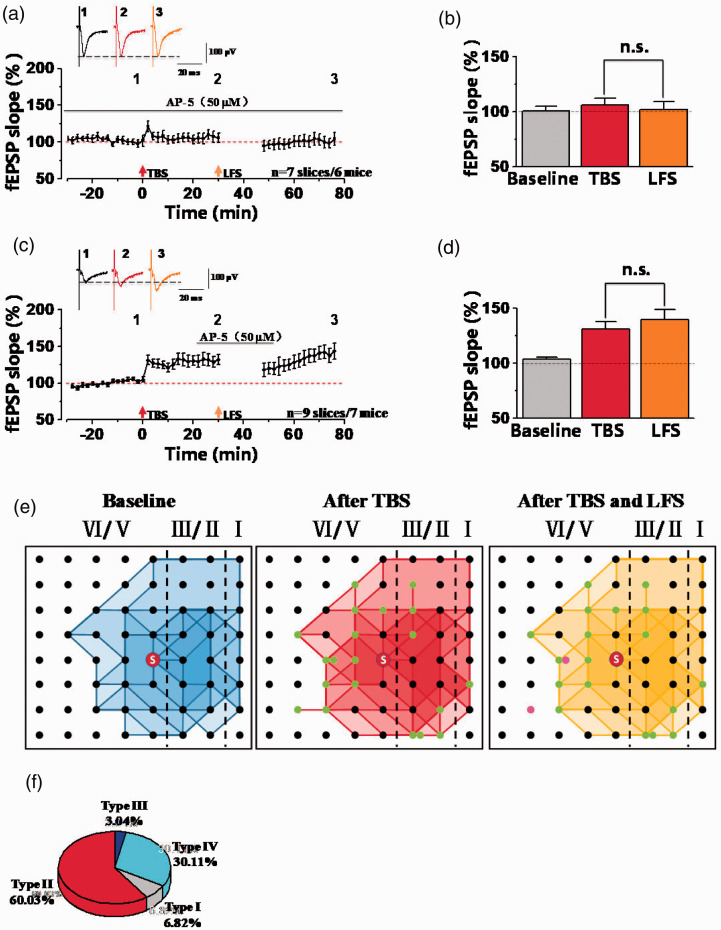
Previous reports showed that inhibition of LTP could relieve neuropathic pain.19,20 In our recent study, we used the extracellular field potential recording system MED64 to test whether LFS (1 Hz, 15 min) could reverse TBS-induced LTP in the ACC at synapse level. The different layers of ACC were covered onto the surface of 8 × 8 array MED64 P515A probe microelectrodes, and one channel of them in the deep layer (layer V) was chosen as the stimulation site which could evoke the best synaptic responses and the other 63 channels were used to record the evoked synaptic responses (Figure 1(a) and (b)). The fEPSP slope of all activated channels were recorded from different layers of the ACC around the stimulation site. At first, we recorded the TBS-induced LTP and LFS-induced LTD in the ACC independently. Consistent with previous reports, we also observed TBS-induced potentiated synaptic responses LTP in the ACC of male mice (157.99 ± 10.02% of the baseline, Figure 1(c)). Meanwhile, LFS-induced synaptic depression was also shown in the Figure 1(d) (86.78 ± 4.57% of the baseline). These results told us that synaptic potentiation and synaptic depression can be stably induced in the ACC of mice.
Figure 1.
LFS can induce synaptic depression after TBS-induced LTP at 30-min interval in the ACC of male mice. (a) Schematic diagram showed the scale of the location of MED-64 probe on the ACC slice (left), MED-64 P515A probe (right, 8 × 8 array of 64 planar microelectrodes with a 150-µm interpolar distance and an electrode size 50 × 50 µm). (b) One example microscopy photograph of the location of ACC slice and MED-64 probe. The red circle indicated the stimulation site. Vertical dashed lines indicated the layers of ACC slices. (c) Time-varying fEPSP slope of all recorded slices were summarized in male mice. LTP induced by TBS could sustain at least 3 h (n = 10 slices/8 mice; red arrow: TBS). Top: sample traces at 0 min (number 1), 180 min (number 2). (d) LTD in response to LFS (1 Hz, 15 min) was recorded from 8 slices of 8 mice (orange arrow: LFS). (e) Schematic diagram of an induction protocol about synaptic depression in potentiated synapses. (f) Synaptic depression induced by LFS was recorded from all 16 activated-channels of one slice within 30-min interval after TBS (red arrow: TBS; orange arrow: LFS). The dashed line indicated the mean basal synaptic responses.
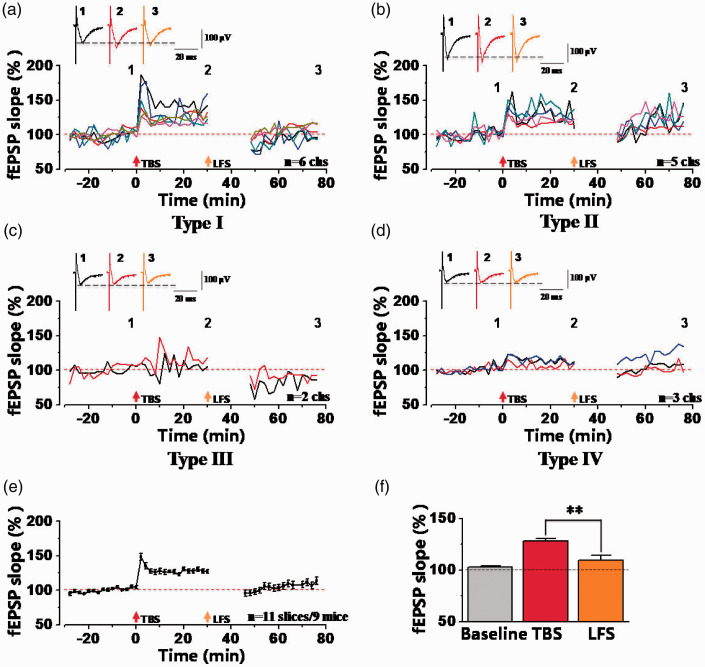
Next, we tested synaptic depression in TBS-induced potentiated synapses of the ACC. After recording stable baseline for at least 30 min, TBS was given to induce LTP. And then after 30 min, LFS (1 Hz, 15 min) was input to induce synaptic depression (Figure 1(e)). As shown in Figure 1(f), we found that TBS-induced LTP was reversed by LFS stimulation in one typical sample slice. By specific analysis of the type of synaptic responses, there were four types synaptic plasticity in the ACC after TBS and LFS stimulation. In one typical sample slice, there were 6 channels showed Type I (LTP + Depression) (Figure 2(a)), 5 channels showed Type II (LTP + No-Depression) (Figure 2(b)), 2 channels showed Type III (No-LTP + Depression) (Figure 2(c)), while 3 channels showed Type IV (No-LTP + No-Depression) (Figure 2(d)). In a total of 11 slices from 9 mice, the final fEPSP slope was potentiated to 127.98 ± 2.41% of the baseline at 30 min after TBS, and then the potentiated synaptic response was decreased to 109.42 ± 4.81% of the baseline at 30 min after LFS, which was significantly decreased relative to after TBS stimulation (**p<0.01, paired t-test; Figure 2(e) and (f)). These results suggest that LFS induced synaptic depression in potentiated synapses of the ACC.
Figure 2.
Four types of synaptic responses were observed in the ACC after TBS and LFS stimulation. (a–d) The sample temporal changes of fEPSP slopes with four types of plasticity from one the ACC slice. All recorded 16 channels were divided into four different types: 6 channels with type I (LTP + Depression) (a), 5 channels with type II (LTP + No-Depression) (b), 2 channels with type III (No-LTP + Depression) (c) and 3 channels with type IV (No-LTP + No-Depression) (d). Top: sample traces at 0 min (number 1), 30 min (number 2) and 75 min (number 3). (e) The pooled fEPSP slopes plot indicated the final averaged fEPSP slopes of all recorded channels, showing that LFS could induce synaptic depression after LTP induction within 30-min interval (n = 11 slices/9 mice). (f) Statistical results showed the last 10-min averaged fEPSP slopes from total activated channels at different time points (**p < 0.01, paired t-test). The dashed line indicated the mean basal synaptic responses.
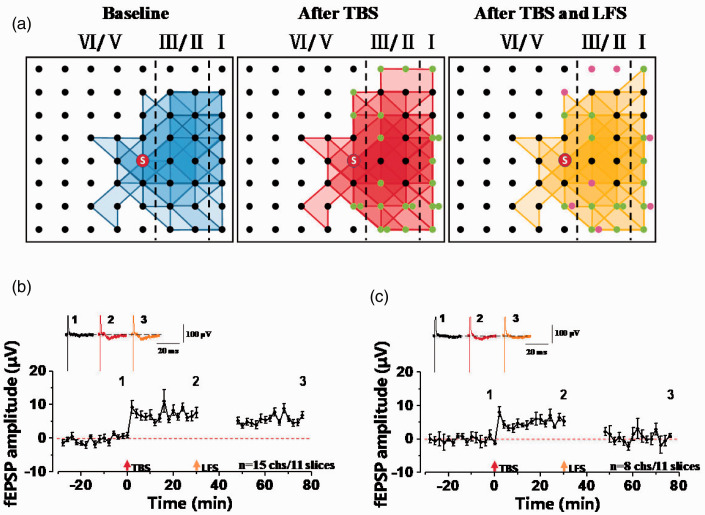
LFS reduced the recruited channels induced by TBS
Previous studies showed that some recruited synaptic responses can be observed using multi-channel recording system, which are originally inactive but can be recruited after LTP induction.18,21 In our study, we want to know whether LFS can affect the recruited synaptic responses induced by TBS. We found that there were 23 channels (2.09 ± 0.70 channels per slice) recruited after TBS from 11 slices of 10 mice. Among them, 15 channels still existed after LFS (1.36 ± 0.53 channels per slice), and time-varying fEPSP amplitudes were increased to about 6 µV over time which was about 0 µV during baseline (Figure 3(a) and (b)). However, there were 8 channels silenced (0.73 ± 0.25 channels per slice) after LFS, and the fEPSP amplitudes were increased over time after applying TBS and were decreased to baseline after LFS (Figure 3(a) and (c)). These results showed that TBS-induced recruited synaptic responses can be silenced by LFS.
Figure 3.
The network propagation of synaptic responses after TBS and LFS stimulation in the ACC. (a) Basal activated areas (blue), recruited areas (red) induced by TBS, and silent areas (yellow) induced by LFS in male mice, respectively. The recruited channels are shown as green dots and the silent channels are shown as purple dots. (b) Time-varying fEPSP amplitude from 15 recruited channels. These channels (green dots) are recruited by TBS and still exist after LFS. (c) Time-varying fEPSP amplitude from 8 silent channels. These channels (purple dots) are recruited by TBS and are silenced after LFS.
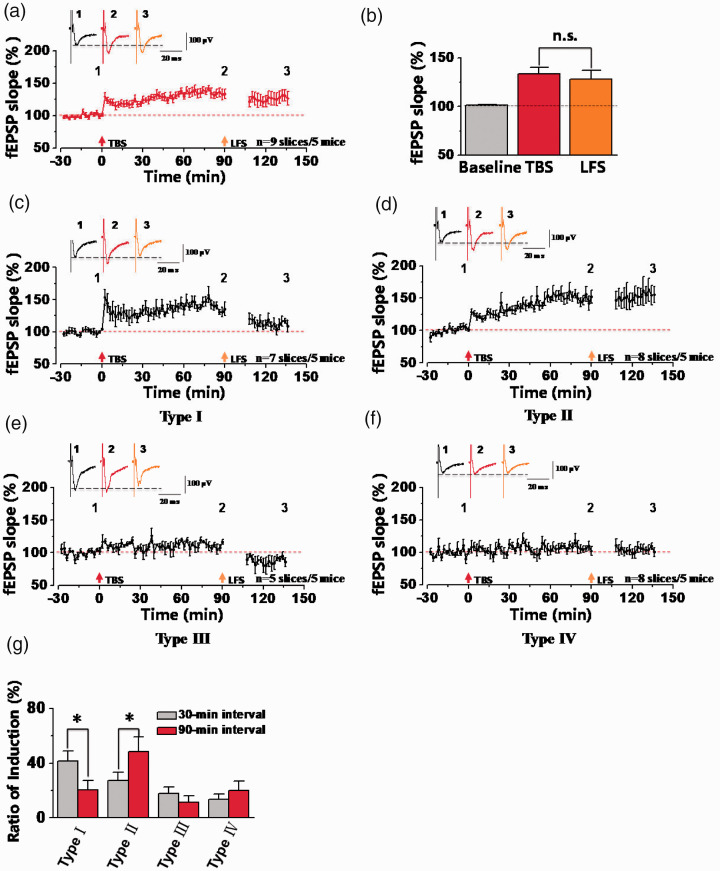
LFS-induced synaptic depression in potentiated synapses of the ACC was time-dependent
Next, we checked whether synaptic depression in potentiated synapses was dependent on the stimulus interval between TBS and LFS in the ACC. In our experiments, 90-min interval was performed between TBS and LFS in the ACC. As shown in the Figure 4, in a total of 9 slices from 5 mice, the final fEPSP slope was potentiated to 133.59 ± 6.60% of the baseline after TBS, and then the LTP was not significantly changed by LFS (128.04 ± 9.09% of the baseline at 30 min after LFS, Figure 4(a) and (b)). These results suggested that LFS failed to induce synaptic depression in potentiated synapses with long-term interval after TBS. However, by specific analysis of the type of synaptic responses, we also observed LFS-induced synaptic depression in potentiated synapses (Type I) from 38 channels out of 165 activated channels (n = 7 slices/5 mice) and their averaged fEPSP slope reached 111.47 ± 6.22% of the baseline after LFS (TBS: 135.56 ± 5.31% of the baseline; Figure 4(c)). In other activated channels, 86 channels showed LTP and no depression (Type II: n = 8 slices/5 mice; Figure 4(d)), 17 channels showed synaptic depression and no LTP (Type III: n = 5 slices/5 mice; Figure 4(e)), and 24 channels showed no any changes of synaptic plasticity (Type IV: n = 8 slices/5 mice; Figure 4(f)). In addition, we compared the difference of induction rate from four different synaptic responses within 30-min and 90-min intervals between TBS and LFS. In all activated channels, the induction rate of channels from type I was 41.67 ± 7.37% at 30-min interval and 20.55 ± 6.83% at 90-min interval (*p < 0.05, unpaired t-test). However, the induction rate of channels from type II at 30-min interval was lower than 90-min interval (*p < 0.05, unpaired t-test) and that no significant differences were observed in other two different types (Figure 4(g)). In summary, these results showed that LFS-induced synaptic depression was weakened at 90-min interval after LTP induction.
Figure 4.
Loss of synaptic depression in the ACC at 90-min interval between TBS and LFS stimulation. (a) The summarized plot showed the final averaged fEPSP slopes of all recorded channels at 90-min interval between TBS and LFS (n = 9 slices/5 mice). (b) Statistical results showed that no significant difference was observed between TBS and LFS. (c-f) The summarized plots of fEPSP slopes from four different types of synaptic responses. All recorded 165 channels were divided into four different types of synaptic responses from 5 mice, including type I (38 chs/7 slices), type II (86 chs/8 slices), type III (17 chs/5 slices), type IV (24 chs/8 slices). (g) The comparisons of the percentage of activated channels between 30-min and 90-min intervals for four different types of synaptic responses (*p < 0.05, unpaired t-test, compared with 30-min interval). n.s. means no significant difference, error bars indicated SEM.
NMDA receptors are required for synaptic depression in potentiated synapses of the ACC
NMDA receptors play important roles in synaptic potentiation and depression.6,20,22,23 We examined the effects of NMDA receptors on the synaptic depression in potentiated synapses in the ACC. At first, we applied NMDA receptors antagonist AP-5 (50 µM) in the whole recording time and found that AP-5 could block both TBS- and LFS-induced synaptic plasticity in the ACC (Figure 5(a) and (b); TBS: 106.16 ± 6.30% of the baseline; LFS: 101.97 ± 7.54% of the baseline). These results are consistent with our previous observation that NMDA receptors play crucial roles in LTP and LTD in the ACC. Next, we applied AP-5 for 30 min (started at 10 min before LFS and washed out at 5 min after LFS) after LTP successfully induced by TBS (Figure 5(c) to (e)). We found that LFS could not induce synaptic depression after LTP in the presence of AP-5 (TBS: 131.07 ± 7.22% of the baseline; LFS: 140.16 ± 9.24% of the baseline; n = 9 slices of 7 mice; Figure 5(d)). Meanwhile, we also analyzed some recruited synaptic responses in the presence of AP-5. Totally, there were 20 channels (2.22 ± 0.61 channels per slice) recruited after TBS from 9 slices of 7 mice. Among them, 18 recruited channels still existed after LFS (2.00 ± 0.68 channels per slice), and the remained 2 channels were depressed by LFS (Figure 5(e)). In the Figure 5(f), we also compared the induction rate of four different synaptic responses, found that the induction rates of channels from type I and type III were significantly decreased. Overall, these results show that synaptic depression in potentiated synapses was dependent on NMDA receptors in the ACC of adult male mice.
Figure 5.
NMDA receptors were required for synaptic depression after synaptic potentiation in the ACC. (a) The summarized fEPSP slope plot showed that bath applied AP-5 (NMDA receptor antagonist, 50 µM) throughout the whole recordings blocked the induction of LTP and LTD (n = 7 slices/6 mice). (b) Statistical results showed that no difference of fEPSP slopes after TBS and LFS in the presence of AP-5. (c) The summarized fEPSP slope plot showed that AP-5 inhibited LFS-induced synaptic depression after TBS-induced LTP (n = 9 slices/7 mice). (d) Statistical results showed that the fEPSP slope was not decreased by LFS in the presence of AP-5 after TBS-induced LTP. (e) The network propagation of synaptic responses after AP-5 applied. Basal activated areas (blue), recruited areas (red) induced by TBS, and silent areas (yellow) induced by LFS in male mice, respectively. The recruited channels are shown as green dots and the silent channels are shown as purple dots. n.s. means no significant difference, error bars indicated SEM. (f) The percentage of activated channels for four different types of synaptic responses after AP-5 applied.
GluN2B-containing NMDA receptors-dependent synaptic depression in potentiated synapses
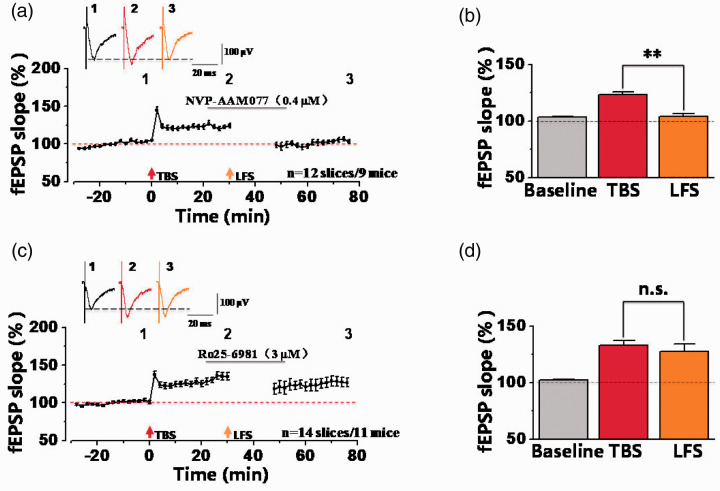
GluN2(A-D) subunits play crucial roles for functions of NMDA receptors.24 We wonder that which subtype of GluN2 subunits was involved in synaptic depression in potentiated synapses. Here, we tested the role of GluN2A and 2B-containing NMDA receptors in the ACC. Firstly, GluN2A-containing NMDA receptors antagonist NVP-AAM077 (0.4 µM) was applied for 30 min after TBS-induced LTP.25 As shown in the Figure 6(a) and (b), LFS-induced synaptic depression was still induced in the presence of NVP-AAM077. The fEPSP slope was potentiated to 123.48 ± 2.51% of the baseline after TBS. Then the potentiated synaptic response was decreased to 104.01 ± 2.28% of the baseline after LFS, which was significantly decreased more than that of TBS (**p < 0.01, paired t-test; n = 12 slices/9 mice). These results indicate that GluN2A doesn’t contribute to synaptic depression after potentiation in the ACC. Next, we tested the involvement of GluN2B-containing NMDA receptors by applying selective antagonist Ro25-6981 (3 µM) for 30 min.22 And we found that Ro25-6981 prevented LFS-induced synaptic depression after potentiation in the ACC. As illustrated in Figure 6(c) and (d), the synaptic response after LFS (127.67 ± 6.36% of the baseline) was not different from the potentiated synaptic response after TBS (132.96 ± 4.19% of the baseline) (n = 14 slices/11 mice). These data indicate that in the ACC of male mice, synaptic depression in potentiated synapses was dependent on GluN2B-containing NMDA receptors.
Figure 6.
GluN2B-containing NMDA receptors were involved in synaptic depression after synaptic potentiation. (a) The final fEPSP slope plot showed that bath-applied NVP-AAM077 (GluN2A-selective NMDA receptors antagonist, 0.4 µM) had no effect on synaptic depression after TBS-induced potentiation in the ACC (n = 12 slices/9 mice). (b) Statistical results showed that significant difference was observed between TBS and LFS by comparisons of the last 10-min fEPSP slopes in presence of NVP-AAM077 (TBS: 123.48 ± 2.51% of the baseline; LFS: 104.01 ± 2.28% of the baseline). **p<0.01, paired t-test, compared with TBS. (c) The normalized fEPSP slope plot showed that Ro25-6981 (GluN2B-selective NMDA receptors antagonist, 3 µM) inhibited the induction of synaptic depression after potentiation in the ACC (n = 14 slices/11 mice). (d) Statistical results showed that no any difference was observed between TBS and LFS in presence of Ro25-6981. n.s. means no significant difference, error bars indicated SEM.
The role of GluN2C/D-containing NMDA receptors in synaptic depression after potentiation
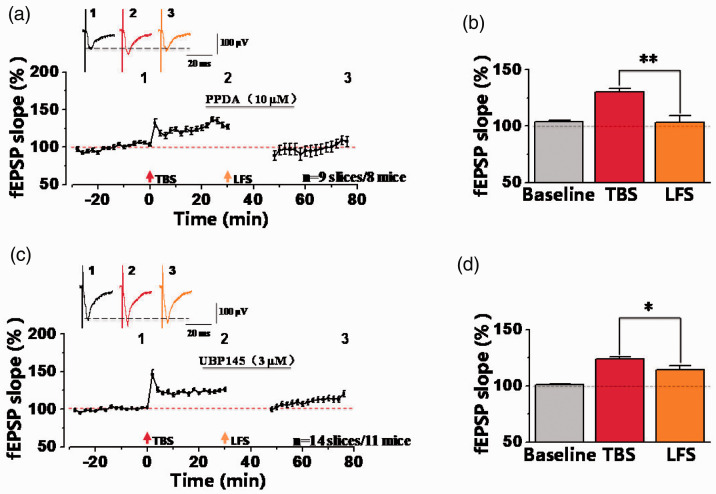
Except for GluN2A/B, increasing studies reported that GluN2C/D subunits also play important roles in functions of NMDA receptor,26–29 and are involved in synaptic plasticity.30–33 Here, we used GluN2C/D-selective antagonist PPDA (10 µM)29,34,35 and GluN2D-selective antagonist UBP145 (3 µM)33 to detect their effects on synaptic depression in potentiated synapses of the ACC. As shown in the Figure 7(a), PPDA (10 µM) was perfused for 30 min, and we found that PPDA failed to block the induction of synaptic depression induced by LFS after LTP-induction. The potentiated synaptic response (130.48 ± 2.80% of the baseline) was still decreased to the level of the baseline after LFS (102.98 ± 6.17% of the baseline) (**p < 0.01, paired t-test; Figure 7(a) and (b); n = 9 slices/8 mice), indicating that PPDA had no effect on the induction of synaptic depression in potentiated synapses. In addition, we used UBP145 (higher selectivity toward GluN2D) to observe whether GluN2D was involved (Figure 7(c) and (d)). These results showed that the averaged fEPSP slope after TBS (123.94 ± 1.77% of the baseline) was decreased to 114.43 ± 3.56% of the baseline by LFS in 14 slices from 11 mice (*p < 0.05, paired t-test; compared with TBS). Therefore, these results show that GluN2C/D-containing NMDA receptors may not involve in synaptic depression after potentiation in the ACC of male mice.
Figure 7.
GluN2C/D-containing NMDA receptors were not involved in synaptic depression after synaptic potentiation. (a) The summarized fEPSP slope plot about the effect of PPDA (GluN2C/D-selective NMDA receptors antagonist, 10 µM) on synaptic depression after potentiation (n = 9 slices/8 mice). (b) Statistical results showed that significant difference was observed between TBS and LFS by comparisons of the last 10 min fEPSP slopes in presence of PPDA (TBS: 130.48 ± 2.80% of the baseline; LFS: 102.98 ± 6.17%). **p<0.01, paired t-test, compared with TBS. (c) The averaged fEPSP slope plot about the effect of UBP145 (GluN2D-selective NMDA receptors antagonist, 3 µM) on synaptic depression after potentiation (n = 14 slices/11 mice). (d) Statistical results showed that the averaged fEPSP slope after TBS was higher than that of LFS (TBS: 123.94 ± 1.77%; LFS: 114.43 ± 3.56% of the baseline; *p<0.05, paired t-test, compared with TBS). Error bars indicated SEM.
Discussion
The ACC is suggested to be involved in chronic pain and pain-related emotions.20,36,37 LTP and LTD, two important synaptic plasticity in the ACC, correlate with each other for pain and pain relief.6,38 However, less is known about the interactive relationship between LTP and LTD in the ACC. In the present study, we found that LFS (1 Hz, 15 min) could induce synaptic depression after LTP induction within a short time window in the ACC. LFS can silence TBS-induced recruited synaptic responses in the ACC. Finally, we found that synaptic depression in potentiated synapses requires the involvement of GluN2B-containing NMDA receptors. This is the first time that we used multi-channel recordings to map LFS-induced network synaptic responses in potentiated synapses of the ACC, raising the possibility that LFS-induced synaptic depression after potentiation may be beneficial for relieving pain or erasing a memory trace.
In current study, we found that synaptic depression can be induced using LFS protocol after synaptic potentiation. This phenomenon is also thought as the process of plasticity of synaptic plasticity.39 We compared four different synaptic responses at different recording sites and observed higher induction rate from Type II and lower induction rate from Type I at 90-min interval than 30-min interval in the ACC, indicating that LTP has a lower sensitivity toward LFS with prolonged time window.40 This is consistent with previous reports in the hippocampus, showing that with the prolonged time intervals, LTP is more resistant to depotentiation due to gene transcription and local protein synthesis. Moreover, post-LTP was divided into at least two mechanistically distinct temporal phases: protein synthesis-independent E-LTP (early-phase LTP) and protein synthesis-dependent L-LTP (late-phase LTP). It is possible that E-LTP can be depotentiated by LFS and L-LTP is insensitive to LFS.12,13
Previous studies showed that some synapses which contained only NMDA receptors and no or very few AMPA receptors were silent under basal synaptic conditions. These silent synapses were functionally recruited or waken up because of the insertion of more AMPA receptors to postsynaptic membranes after LTP induction protocol applied.18,38,41,42 Conversely, some functional synapses can be transformed into silent synapses by endocytosis and inactivation of postsynaptic AMPA receptor after synaptic depression.10,38 Consistent with previous reports, we observed two different synaptic responses from inactivated channels under basal conditions and found that a part of recruited channels by TBS can be silenced after LFS, which may correlate with the insertion or removal of AMPA receptors. The insertion or removal of AMPA receptors was involved in the phosphorylation level of serine sites from C-terminal domains (CTDs).43
NMDA receptors play key roles in LTP and LTD.20 In our studies, we found that LFS-induced synaptic depression in potentiated synapses were significantly blocked by NMDA receptors antagonists AP-5, indicating that was dependent on NMDA receptors. Previous reports also showed that LFS-induced synaptic depression was partially dependent on NMDA receptors in the ACC,44 and that synaptic depotentiation was dependent on NMDA receptors in the hippocampus and spinal cord dorsal horn.8,14,15 The possible molecular and cellular mechanism was that NMDA receptors lead to influx of Ca2+, to activate downstream protein phosphatase (PP) cascades. And then the activation of PP cascades dephosphorylates GluA1 subunit of AMPARs, that leads to the reversal of LTP.14,45 However, some studies showed that the reversal of LTP was NMDA receptors-independent.46
GluN2A and GluN2B-containing NMDA receptors play crucial roles for synaptic plasticity and physiological functions. The expression of GluN2B was increased after peripheral inflammation, and pharmacological blockade of GluN2B selectively relieved inflammatory pain in the ACC.47 GluN2B receptors were also involved in morphine-induced analgesic tolerance in the ACC48,49 and contextual fear memory in the CA1 of hippocampus.50 In the ACC, GluN2A and GluN2B-containing NMDA receptors have been reported play different roles in different forms of NMDA receptor-dependent synaptic plasticity, such as LTP and LTD.6,20,51,52 Previous reports also showed that GluN2B-containing NMDA receptors were involved in novelty exploration-induced and PP-LFS-induced synaptic depotentiation.53,54 Consistent with previous reports, our present studies also found that GluN2B-containing NMDA receptors are critical for LFS-induced synaptic depression after potentiation in the ACC, but not GluN2A or GluN2C/D-containing NMDA receptors. However, some studies also find that synaptic depotentiation requires the involvement of GluN2A-containing NMDA receptors in the perirhinal cortex52 or hippocampus.51,55 Our results suggest that synaptic depotentiation or depression may employ different mechanisms at different brain regions,8 and we guessed that influx of Ca2+ via GluN2B-containing NMDA receptors could activate downstream PP1 cascades for removal of AMPA receptors to induce synaptic depotentiation in the ACC, but specific mechanisms are needed to be further studied.
In conclusion, it is important to understand molecular mechanism for synaptic depression after ACC LTP. From translational point of view, the restoration of LTD or the induction of synaptic depression after chronic pain will be beneficial for relieving chronic pain.
Footnotes
Authors' Contributions: M.X., X.H.L., and M.Z. designed the experiments. M.X., S.B.Z., and R.H.L. performed electrophysiological experiments and analyzed data. M.X., Q.Y.C., X.H.L., and M.Z. drafted the manuscript and finished the final version of the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: M.Z. is in part supported by grants from the Canadian Institute for Health Research (CIHR) project grants (PJT-148648 and 419286). X.H.L. is supported by grants from China Postdoctoral Science Foundation (2019M663669) and Basic Research Program of Natural Science in Shaanxi Province (2020JQ-085).
ORCID iDs: Qi-Yu Chen https://orcid.org/0000-0002-5707-6220
Xu-Hui Li https://orcid.org/0000-0003-4376-6252
References
- 1.Li XY, Ko HG, Chen T, Descalzi G, Koga K, Wang H, Kim SS, Shang Y, Kwak C, Park SW, Shim J, Lee K, Collingridge GL, Kaang BK, Zhuo M. Alleviating neuropathic pain hypersensitivity by inhibiting PKMzeta in the anterior cingulate cortex. Science 2010; 330: 1400–1404. [DOI] [PubMed] [Google Scholar]
- 2.Liu MG, Song Q, Zhuo M. Loss of synaptic tagging in the anterior cingulate cortex after tail amputation in adult mice. J Neurosci 2018; 38: 8060–8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhuo M. Cortical excitation and chronic pain. Trends Neurosci 2008; 31: 199–207. [DOI] [PubMed] [Google Scholar]
- 4.Drdla-Schutting R, Benrath J, Wunderbaldinger G, Sandkuhler J. Erasure of a spinal memory trace of pain by a brief, high-dose opioid administration. Science 2012; 335: 235–238. [DOI] [PubMed] [Google Scholar]
- 5.Collingridge GL, Peineau S, Howland JG, Wang YT. Long-term depression in the CNS. Nat Rev Neurosci 2010; 11: 459–473. [DOI] [PubMed] [Google Scholar]
- 6.Toyoda H, Zhao MG, Zhuo M. NMDA receptor-dependent long-term depression in the anterior cingulate cortex. Rev Neurosci 2006; 17: 403–413. [DOI] [PubMed] [Google Scholar]
- 7.Wang YJ, Liu MG, Wang JH, Cao W, Wu C, Wang ZY, Liu L, Yang F, Feng ZH, Sun L, Zhang F, Shen Y, Zhou YD, Zhuo M, Luo JH, Xu TL, Li XY. Restoration of cingulate long-term depression by enhancing non-apoptotic caspase 3 alleviates peripheral pain hypersensitivity. Cell Rep 2020; 33: 108369. [DOI] [PubMed] [Google Scholar]
- 8.Huang CC, Liang YC, Hsu KS. Characterization of the mechanism underlying the reversal of long term potentiation by low frequency stimulation at hippocampal CA1 synapses. J Biol Chem 2001; 276: 48108–48117. [DOI] [PubMed] [Google Scholar]
- 9.Wei F, Li P, Zhuo M. Loss of synaptic depression in mammalian anterior cingulate cortex after amputation. J Neurosci 1999; 19: 9346–9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu RH, Xue M, Li XH, Zhuo M. Sex difference in synaptic plasticity in the anterior cingulate cortex of adult mice. Mol Brain 2020; 13: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Staubli U, Scafidi J. Time-dependent reversal of long-term potentiation in area CA1 of the freely moving rat induced by theta pulse stimulation. J Neurosci 1999; 19: 8712–8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park P, Sanderson TM, Bortolotto ZA, Georgiou J, Zhuo M, Kaang BK, Collingridge GL. Differential sensitivity of three forms of hippocampal synaptic potentiation to depotentiation. Mol Brain 2019; 12: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woo NH, Nguyen PV. Protein synthesis is required for synaptic immunity to depotentiation. J Neurosci 2003; 23: 1125–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Dell TJ, Kandel ER. Low-frequency stimulation erases LTP through an NMDA receptor-mediated activation of protein phosphatases. Learn Mem 1994; 1: 129–139. [PubMed] [Google Scholar]
- 15.Fujii S, Saito K, Miyakawa H, Ito K-i, Kato H. Reversal of long-term potentiation (depotentiation) induced by tetanus stimulation of the input to CA1 neurons of guinea pig hippocampal slices. Brain Res 1991; 555: 112–122. [DOI] [PubMed] [Google Scholar]
- 16.Chen T, Lu JS, Song Q, Liu MG, Koga K, Descalzi G, Li YQ, Zhuo M. Pharmacological rescue of cortical synaptic and network potentiation in a mouse model for fragile X syndrome. Neuropsychopharmacology 2014; 39: 1955–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li XH, Matsuura T, Liu RH, Xue M, Zhuo M. Calcitonin gene-related peptide potentiated the excitatory transmission and network propagation in the anterior cingulate cortex of adult mice. Mol Pain 2019; 15: 1744806919832718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song Q, Zheng HW, Li XH, Huganir RL, Kuner T, Zhuo M, Chen T. Selective phosphorylation of AMPA receptor contributes to the network of long-term potentiation in the anterior cingulate cortex. J Neurosci 2017; 37: 8534–8548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li XH, Chen QY, Zhuo M. Neuronal adenylyl cyclase targeting central plasticity for the treatment of chronic pain. Neurotherapeutics 2020; 17: 861–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bliss TV, Collingridge GL, Kaang BK, Zhuo M. Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat Rev Neurosci 2016; 17: 485–496. [DOI] [PubMed] [Google Scholar]
- 21.Chen T, O'Den G, Song Q, Koga K, Zhang MM, Zhuo M. Adenylyl cyclase subtype 1 is essential for late-phase long term potentiation and spatial propagation of synaptic responses in the anterior cingulate cortex of adult mice. Mol Pain 2014; 10: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao MG, Toyoda H, Lee YS, Wu LJ, Ko SW, Zhang XH, Jia Y, Shum F, Xu H, Li BM, Kaang BK, Zhuo M. Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron 2005; 47: 859–872. [DOI] [PubMed] [Google Scholar]
- 23.Toyoda H, Zhao MG, Zhuo M. Roles of NMDA receptor NR2A and NR2B subtypes for long-term depression in the anterior cingulate cortex. The European Journal of Neuroscience 2005; 22: 485–494. [DOI] [PubMed] [Google Scholar]
- 24.Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci 2007; 8: 413–426. [DOI] [PubMed] [Google Scholar]
- 25.Wu LJ, Xu H, Ren M, Cao X, Zhuo M. Pharmacological isolation of postsynaptic currents mediated by NR2A- and NR2B-containing NMDA receptors in the anterior cingulate cortex. Mol Pain 2007; 3: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogden KK, Khatri A, Traynelis SF, Heldt SA. Potentiation of GluN2C/D NMDA receptor subtypes in the amygdala facilitates the retention of fear and extinction learning in mice. Neuropsychopharmacology 2014; 39: 625–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swanger SA, Vance KM, Pare JF, Sotty F, Fog K, Smith Y, Traynelis SF. NMDA receptors containing the GluN2D subunit control neuronal function in the subthalamic nucleus. J Neurosci 2015; 35: 15971–15983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ingram R, Kang H, Lightman S, Jane DE, Bortolotto ZA, Collingridge GL, Lodge D, Volianskis A. Some distorted thoughts about ketamine as a psychedelic and a novel hypothesis based on NMDA receptor-mediated synaptic plasticity. Neuropharmacology 2018; 142: 30–40. [DOI] [PubMed] [Google Scholar]
- 29.Prius-Mengual J, Perez-Rodriguez M, Andrade-Talavera Y, Rodriguez-Moreno A. NMDA receptors containing GluN2B/2C/2D subunits mediate an increase in glutamate release at hippocampal CA3-CA1 synapses. Mol Neurobiol 2019; 56: 1694–1706. [DOI] [PubMed] [Google Scholar]
- 30.Harney SC, Jane DE, Anwyl R. Extrasynaptic NR2D-containing NMDARs are recruited to the synapse during LTP of NMDAR-EPSCs. J Neurosci 2008; 28: 11685–11694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hrabetova S, Serrano P, Blace N, Tse HW, Skifter DA, Jane DE, Monaghan DT, Sacktor TC. Distinct NMDA receptor subpopulations contribute to long-term potentiation and long-term depression induction. J Neurosci 2000; 20: RC81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mirante O, Brandalise F, Bohacek J, Mansuy IM. Distinct molecular components for thalamic- and cortical-dependent plasticity in the lateral amygdala. Front Mol Neurosci 2014; 7: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen QY, Li XH, Lu JS, Liu Y, Lee JA, Chen YX, Shi W, Fan K, Zhuo M. NMDA GluN2C/2D receptors contribute to synaptic regulation and plasticity in the anterior cingulate cortex of adult mice. Mol Brain 2021; 14: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Costa BM, Feng B, Tsintsadze TS, Morley RM, Irvine MW, Tsintsadze V, Lozovaya NA, Jane DE, Monaghan DT. N-methyl-D-aspartate (NMDA) receptor NR2 subunit selectivity of a series of novel piperazine-2,3-dicarboxylate derivatives: preferential blockade of extrasynaptic NMDA receptors in the rat hippocampal CA3-CA1 synapse. J Pharmacol Exp Ther 2009; 331: 618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li YH, Li Y, Zheng L, Wang J. Postsynaptic GluN2B-containing NMDA receptors contribute to long-term depression induction in medial vestibular nucleus neurons of juvenile rats. Neurosci Lett 2020; 715: 134674. [DOI] [PubMed] [Google Scholar]
- 36.Koga K, Descalzi G, Chen T, Ko HG, Lu J, Li S, Son J, Kim T, Kwak C, Huganir RL, Zhao MG, Kaang BK, Collingridge GL, Zhuo M. Coexistence of two forms of LTP in ACC provides a synaptic mechanism for the interactions between anxiety and chronic pain. Neuron 2015; 85: 377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhuo M. Neural mechanisms underlying anxiety-chronic pain interactions. Trends Neurosci 2016; 39: 136–145. [DOI] [PubMed] [Google Scholar]
- 38.Luscher C, Malenka RC. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb Perspect Biol 2012; 4:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci 2008; 9: 387. [DOI] [PubMed] [Google Scholar]
- 40.Chen YL, Huang CC, Hsu KS. Time-dependent reversal of long-term potentiation by low-frequency stimulation at the hippocampal mossy fiber-CA3 synapses. J Neurosci 2001; 21: 3705–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liao D, Hessler NA, Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature 1995; 375: 400–404. [DOI] [PubMed] [Google Scholar]
- 42.Isaac JT, Nicoll RA, Malenka RC. Evidence for silent synapses: implications for the expression of LTP. Neuron 1995; 15: 427–434. [DOI] [PubMed] [Google Scholar]
- 43.Zhou Z, Liu A, Xia S, Leung C, Qi J, Meng Y, Xie W, Park P, Collingridge GL, Jia Z. The C-terminal tails of endogenous GluA1 and GluA2 differentially contribute to hippocampal synaptic plasticity and learning. Nat Neurosci 2018; 21: 50–62. [DOI] [PubMed] [Google Scholar]
- 44.Kang SJ, Liu MG, Chen T, Ko HG, Baek GC, Lee HR, Lee K, Collingridge GL, Kaang BK, Zhuo M. Plasticity of metabotropic glutamate receptor-dependent long-term depression in the anterior cingulate cortex after amputation. J Neurosci 2012; 32: 11318–11329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang CC, Liang YC, Hsu KS. A role for extracellular adenosine in time-dependent reversal of long-term potentiation by low-frequency stimulation at hippocampal CA1 synapses. J Neurosci 1999; 19: 9728–9738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bashir ZI, Collingridge GL. An investigation of depotentiation of long-term potentiation in the CA1 region of the hippocampus. Exp Brain Res 1994; 100: 437–443. [DOI] [PubMed] [Google Scholar]
- 47.Wu LJ, Toyoda H, Zhao MG, Lee YS, Tang J, Ko SW, Jia YH, Shum FW, Zerbinatti CV, Bu G, Wei F, Xu TL, Muglia LJ, Chen ZF, Auberson YP, Kaang BK, Zhuo M. Upregulation of forebrain NMDA NR2B receptors contributes to behavioral sensitization after inflammation. J Neurosci 2005; 25: 11107–11116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ko SW, Wu LJ, Shum F, Quan J, Zhuo M. Cingulate NMDA NR2B receptors contribute to morphine-induced analgesic tolerance. Mol Brain 2008; 1: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu LJ, Zhuo M. Targeting the NMDA receptor subunit NR2B for the treatment of neuropathic pain. Neurotherapeutics 2009; 6: 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang XH, Wu LJ, Gong B, Ren M, Li BM, Zhuo M. Induction- and conditioning-protocol dependent involvement of NR2B-containing NMDA receptors in synaptic potentiation and contextual fear memory in the hippocampal CA1 region of rats. Mol Brain 2008; 1: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science 2004; 304: 1021–1024. [DOI] [PubMed] [Google Scholar]
- 52.Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci 2004; 24: 7821–7828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qi Y, Hu NW, Rowan MJ. Switching off LTP: mGlu and NMDA receptor-dependent novelty exploration-induced depotentiation in the rat hippocampus. Cereb Cortex 2013; 23: 932–939. [DOI] [PubMed] [Google Scholar]
- 54.Park S, Lee S, Kim J, Choi S. Ex vivo depotentiation of conditioning-induced potentiation at thalamic input synapses onto the lateral amygdala requires GluN2B-containing NMDA receptors. Neurosci Lett 2012; 530: 121–126. [DOI] [PubMed] [Google Scholar]
- 55.Zhang L, Zhang P, Wang G, Zhang H, Zhang Y, Yu Y, Zhang M, Xiao J, Crespo P, Hell JW, Lin L, Huganir RL, Zhu JJ. Ras and rap signal bidirectional synaptic plasticity via distinct subcellular microdomains. Neuron 2018; 98: 783–800. [DOI] [PMC free article] [PubMed] [Google Scholar]