Abstract
Background:
The patient global assessment of disease activity (PGA) is the major limiting factor to Boolean remission in patients with established rheumatoid arthritis (RA). Here, we investigated the limiting variables to disease remission in patients with early RA treated with conventional synthetic disease modifying anti-rheumatic drugs, also in relation to autoantibody status.
Methods:
Data were retrieved from 535 early RA patients (<12 months of symptoms) with an observation period of 6–12 months upon initiation of therapy with methotrexate aimed at the achievement of low disease activity based on the 28-joints disease activity score. Near-remission was defined as any of the four core items of Boolean remission >1 with the remaining three all ⩽1. Reasons for missing Boolean remission and predictors of near-remission subcategories were analyzed in relation to baseline disease variables.
Results:
After 6 and 12 months, near-remission was two-times more frequent than Boolean remission (25.6% and 26.9% at the two time-points). A 28-swollen joint count (SJC28) >1 was responsible for the majority of near-remission (56.2% and 57.6% at 6 and 12 months, respectively), and PGA > 1 accounted for approximatively 35% of the cases. Autoantibody-positivity independently predicted the risk of missing remission because of SJC28 > 1 [adjusted odds ratio (OR) 95% confidence interval (CI) 2.81 (1.59–4.9) at 6 months and 1.73 (1.01–3.01) at 12 months], whilst autoantibody-negativity was an independent predictor of PGA near-remission [adjusted OR (95% CI) 2.45 (1.25–4.80) at 6 months and 5.71 (2.47–13.2) at 12 months].
Conclusion:
In early RA, Boolean remission is more frequently missed because of persistent swollen joints. However, barriers to full-remission vary in relation to the autoantibody status. Autoantibody-positive patients more commonly experience residual swollen joints, whilst PGA more frequently impairs remission in autoantibody-negative patients. Efforts to target full-remission in early RA may thus require different strategies according to autoantibody profile.
Keywords: anti-citrullinated protein autoantibodies, early rheumatoid arthritis, near-remission, patient global assessment, patient reported outcomes, remission, rheumatoid factor
Background
In patients with rheumatoid arthritis (RA), abrogation of inflammation results in delay of joint damage progression, preservation of physical function and quality of life, and prevention of comorbidities.1 Furthermore, obtaining strict control of disease activity increases the chances of successful tapering of medications.2 The pursuit of disease remission is therefore the most important therapeutic goal in patients with RA according to modern treat-to-target (T2T) recommendations.3
In both trials and clinical practice, remission has been defined mostly according to the disease activity score on 28-joints (DAS28). However, at both the conventional cut-off of <2.6 and at lower thresholds, DAS28 remission rather reflects a state of low disease activity associated with sub-optimal outcomes in a large proportion of patients.4–6 As such, the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) jointly developed more stringent remission criteria in 2011.7 According to the Boolean-based definition, a patient is considered in remission when the tender joint count (TJC), the swollen joint count (SJC), the patient global assessment (PGA) of disease activity, and C-reactive protein (CRP) each do not exceed a score of one.7 Although the criteria were intended for use in research, both the original publication7 and subsequent treatment guidelines3 suggest their adoption as the new standard also in patient care.
Despite the widespread endorsement of the American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) criteria, the stringent Boolean-based definition of remission is, in fact, very hard to achieve in clinical practice.8–10 Far more commonly, patients miss remission solely because one of the four core items scoring >1 – a condition called near-remission.11 Depending on the missed core variable, the outcomes of near-remission may vary. Whilst residual swollen joints indeed indicate patients at risk of radiographic progression,12,13 PGA may be affected by several factors unrelated to inflammatory disease activity,14 and appears less strictly associated with joint damage and disability.15,16 Independent studies have recently shown that, in course of RA, PGA is the major limiting factor to full-remission.17–22 Based on this evidence, it has been suggested that the proportion of patients attaining adequate control of disease activity might actually be broader than that captured by the ACR/EULAR criteria, and that failure to achieve remission because of high PGA should be left out of T2T strategies.23
Caution is required, however, before introducing definitive changes in the current conception and management of remission in RA, as more thorough understanding of its frequency and limiting factors in different clinical settings is still needed.24,25 Studies addressing near-remission and its components have indeed focused largely on patient populations with established disease under variable treatment regimens.17–21 In this context, Boolean-remission may be particularly hard to achieve, and a number of factors also related to disease chronicity and disability may profoundly impact on patient-reported outcome (PRO) measures.14,26,27 Whether the state of near-remission is common also in early RA patients receiving T2T management is, in contrast, poorly defined,22 and no data indicate that the limiting variables to full-remission inevitably overlap in frequency and clinical significance those observed in established disease. Furthermore, early RA populations include significantly more autoantibody-negative patients,28,29 but the potential impact of the autoantibody status on the different components of disease remission is unknown.
The aim of this study was to analyze the frequency and the limiting factors for fulfilling Boolean remission in real-life in a yet unexplored early phase of RA, also in relation to disease-specific characteristics such as autoantibody status.
Patients and methods
Patients and follow up
Consecutive RA patients attending the Early Arthritis Clinic of the University Hospital of Pavia were included.30,31 Before October 2010, RA patients had to fulfill the ACR 1987 criteria at inclusion.32 After October 2010, patients were classified according to the ACR/EULAR 2010 criteria.33 All patients had symptoms duration <12 months and were glucocorticoid and disease modifying anti-rheumatic drug (DMARD)-naïve at their first assessment. After inclusion, patients were seen every 2 months in the first semester and every 3 months afterwards. Patients classified as RA according to the 1987 criteria were treated with methotrexate (MTX) from 10 mg/week up to 20 mg/week to achieve low disease activity (LDA, DAS28 <3.2). Low-dose oral prednisone (PDN) (12.5 mg/day for 2 weeks and 6.25 mg/day subsequently) was assigned randomly to about 50% of the patients.30 Patients classified as RA according to the 2010 criteria received MTX from 15 mg/week up to 25 mg/week to achieve a DAS28 <3.2. PDN (5 mg/day) was prescribed to all patients unless contraindicated.31 Alternative conventional synthetic (cs) DMARDs (leflunomide, sulfasalazine) were prescribed in patients with a contraindication (or early intolerance) to MTX; hydroxychloroquine was reserved for patients with very mild RA and/or severe comorbidities. If the target of LDA had not been reached with the first csDMARD, a combination with another csDMARD or with a biologic (b) or targeted synthetic (ts) DMARD was considered based on the presence of poor prognostic factors.
The study was conducted according to the declaration of Helsinki: all patients signed a written informed consent before inclusion, and the study protocol was approved by the local ethics committee.
Measurements
The data collection at baseline and follow up included demographic characteristics, symptom duration, tender and swollen joint count on 28 joints (TJC28, SJC28), th PGA, and physician’s assessment of disease activity (PhGA) on a 0–10 cm visual analogue scale (VAS), VAS for general health (GH) and pain (0–100 mm), the Health Assessment Questionnaire (HAQ), the erythrocyte sedimentation rate (ESR), and CRP. PGA was assessed systematically using the following formulation: “considering all the ways your arthritis has affected you, how do you feel your arthritis is today?”.14 Rheumatoid factor (RF) and anti-citrullinated protein autoantibodies (ACPA) were analyzed centrally in baseline sera by nephelometry and a second-generation ELiA (Phadia, Uppsala, Sweden) respectively, with cut-off values of 20 U/ml for RF and 10 U/ml for ACPA. Patients were classified as autoantibody-positive if RF and/or ACPA were above the reference cut-off values; autoantibody-negative in case of RF and ACPA both negative. At baseline, patients underwent ultrasonographic (US) examination of bilateral wrists and metacarpophalangeal joints using a Logiq 9 scanner (General Electrics Medical Systems, GE Healthcare, Chicago, IL, USA) with a multifrequency linear array transducer (8–15 MHz), according to the EULAR guidelines and their updates.34 Gray-scale (GS) and power Doppler (PD) signals were assigned to each joint in accordance with semi-quantitative 0–3 scales.35 An overall US score for GS and PD signal was calculated at each US assessment as the sum of either GS or PD signal scores obtained from each joint (range 0–36). All patients underwent postero–anterior radiographs of the hands, wrists, and feet at baseline. Erosive RA was defined based on the presence of an erosion score ⩾1 according to the Sharp/van der Heijde score.36
Definitions of remission
The achievement of disease remission was evaluated after 6 and 12 months of treatment. According to the ACR/EULAR Boolean-based definition,7 patients were classified in three remission states: (i) Boolean-based remission (TJC28, SJC28, CRP mg/dl, and PGA, all ⩽1); (ii) near-remission (just three of the four core items scoring ⩽1); (iii) non-remission (two or more criteria >1). Near-remission was further sub-classified based on the limiting variable.
Statistical analysis
Data were presented with means and standard deviations (SD), median and interquartile range (IQR), or relative frequencies, as appropriate. There was no imputation of missing data. Comparisons of disease characteristics between remission subgroups were made using independent samples t test, Mann–Whitney U test or χ2 test. The association between baseline demographic and clinical variables and near-remission (stratified according to the limiting variable) was investigated by means of univariable and multivariable logistic models including non-collinear variables with p < 0.2 at the univariable analysis. The independent associations of the autoantibody status were confirmed in multivariable models fitted to account for potential confounders irrespective of their p values at univariable analysis (age, gender, symptoms duration, baseline SJC28 and PGA, use of MTX and MTX starting dose, use of b/tsDMARDS, use of PDN, calendar year–quartiles). Results were presented as odds ratios (OR) and 95% confidence intervals (CI). All analyses were conducted using MedCalc® Version 12.7.0.0, and the level of significance was set at 0.05.
Results
Baseline characteristics of the study population
Out of a total population of 578 consecutive patients newly diagnosed with RA, 535 (92.6%) had 6 months follow-up data available after treatment start and were used for the current analyses. Of these, 23 patients were lost to follow up after the 6th month. Remission outcomes after 12 months were thus re-evaluated in 512 patients. Baseline characteristics of patients lost to follow up were not significantly different from the entire population (data not shown).
The demographic and baseline disease characteristics of the study population are shown in Table 1. Patients were predominantly female (72.5%), with a mean (SD) age of 59.3 (14.8) years and a median disease duration of 16 weeks (IQR 9–28). The mean (SD) DAS28 was 4.92 (1.18), the median (IQR) PGA was 6 (4–8) and the median (IQR) pain score was 54 (40–80) 48% of the patients was autoantibody-positive, and 38% presented with radiographic erosions already at baseline.
Table 1.
Baseline demographic and clinical characteristics of the study population.
| n = 535 patients | |
|---|---|
| Age, mean (SD) | 59.3 (14.8) |
| Female gender, n. (%) | 388 (72.5) |
| Symptoms duration, weeks, median (IQR) | 15.6 (9.4–28) |
| SJC28, median (IQR) | 7 (4–11) |
| TJC28, median (IQR) | 6 (3–11.3) |
| DAS28, mean (SD) | 4.92 (1.18) |
| SDAI, mean (SD) | 29.33 (13.50) |
| VAS pain, median (IQR) (0–100) | 54 (40–80) |
| PGA, median (IQR) (0–10) | 6 (4.1–8) |
| PhGA, median (IQR) (0–10) | 4.8 (3.5–6) |
| HAQ, median (IQR) (0–3) | 1.125 (0.625–1.75) |
| ESR, mm/1 h, median (IQR) | 24 (14–41) |
| CRP, mg/dl, median (IQR) | 0.88 (0.31–2.31) |
| RF positive, n (%) | 232 (43.4) |
| ACPA positive, n (%) | 179 (33.5) |
| RF and ACPA double-positive, n (%) | 149 (27.9) |
| RF and ACPA double-negative, n (%) | 271 (50.7) |
| Erosion SHS ⩾1, n (%) | 205 (38.3) |
| US-GS score, median (IQR) (0–36) | 7 (4–11) |
| US-PD score, median (IQR) (0–36) | 3 (0–8) |
ACPA, anti-citrullinated protein antibodies; CRP, C-reactive protein; DAS28, diseases activity score on 28 joints; ESR, erythrocyte sedimentation rate; GS, gray scale; HAQ, health assessment questionnaire; IQR, interquartile range; PD, power Doppler; PGA, patient global assessment; PhGA, physician global assessment; RF, rheumatoid factor; SD, standard deviation; SDAI, simplified disease activity index; SHS, Sharp van der Heijde score; SJC28, swollen joint count on 28 joints; TJC28, tender joint count on 28 joints; US, ultrasonography; VAS, visual analogue scale.
Frequency of near-remission and limiting variables to full-remission at follow up
Per study protocol, at the 6-month assessment all patients were still on therapy with csDMARDs (MTX in 89.7%), and the predefined target of LDA had been reached in 56.6% of cases, with 26.7% of the patients being in DAS28 remission. After the 6th month, patients continued to escalate MTX or received combination therapy with cs or b/ts DMARDs (84/512 patients, 16.4%) in case of failure to achieve the target of LDA despite maximum MTX dose. Collectively, at the 12 month assessment, 80% of the patients were in LDA, with 39.3% being in DAS28 remission.
Boolean-based remission was fulfilled in 69 patients (12.9%) at 6 months; of the remaining 466 patients, 329 (61.5%) were in non-remission, and 137 (25.6%) missed Boolean remission solely because one of the four items scoring >1 (near-remission). At 12 months, the proportion of Boolean remission slightly increased to 17.8%, whilst near-remission remained stable (25.8%). Less than half of the patients (45.5%) in full-remission at 6 months maintained stable remission also at the 12 months assessment, whilst 36.4% turned into a status of near-remission. Of the patients in near-remission at 6 months, 38.6% were still in near-remission, and 24.2% gained a status of full-remission.
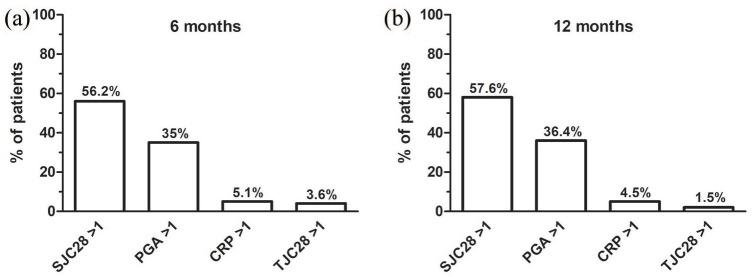
Figure 1 shows the distribution of the limiting factors for fulfilling Boolean remission after 6 and 12 months. SJC28 > 1 was responsible for the majority of near-remissions at both time points (56.2% and 57.6% at 6 and 12 months, respectively), whilst PGA > 1 accounted for 35% of the cases at 6 months and 36.4% at 12 months. TJC28 > 1 or CRP levels >1 mg/dl were found in less than 10% of near-remissions. In patients in near-remission at both the 6- and the 12-month follow up, the limiting variable remained mostly unchanged.
Figure 1.
Limiting variables to Boolean remission at 6 and 12 months. Histograms showing the proportion of patients failing to achieve full Boolean remission because of SJC28 >1, PGA >1, CRP levels >1 mg/dl, or TJC28 after 6 (a) and 12 months (b) from treatment start.
CRP, C-reactive protein; PGA, patient global assessment; SJC28, 28-swollen joint count; TJC28, 28-tender joint count.
Baseline characteristics associated with near-remission according to the limiting variable
As Boolean remission in our patients appeared precluded mainly by either persistent SJC28 or PGA > 1 at both earlier and later time points, we analyzed whether specific patient or disease characteristics at presentation could distinguish the different remission outcomes (Tables 2 and 3). None of the variables of disease activity or patient reported outcomes (PROs) at baseline could consistently discriminate between patients achieving SJC28 or PGA near-remission after 6 and 12 months from treatment start, except for slightly higher baseline SJC28 in SJC28 near-remission at 6 months and slightly worse baseline PROs in PGA near-remission at 12 months.
Table 2.
Baseline variables associated with near-remission at 6 months stratified for the missing item (PGA or SJC28).
| SJC28 near-remission n = 77 | PGA near-remission n = 48 | p | |
|---|---|---|---|
| Demographic | |||
| Age, mean (SD) | 59 (15.1) | 59.9 (15.3) | 0.75 |
| Female gender, n (%) | 56 (72.7) | 34 (70.8) | 0.98 |
| Disease characteristics | |||
| Duration, weeks, mean (SD) | 21.4 (21) | 20.2 (22.1) | 0.79 |
| RA 1987 criteria, n (%) | 62 (80.5) | 38 (79.2) | 0.96 |
| Disease activity | |||
| SJC28, mean (SD) | 8.3 (5) | 6 (3.8) | 0.01* |
| TJC28, mean (SD) | 4.7 (4.4) | 6 (5.2) | 0.14 |
| PhGA, mean (SD) | 4.6 (2) | 4.3 (2) | 0.53 |
| DAS28, mean (SD) | 4.67 (1.12) | 4.65 (1.29) | 0.92 |
| SDAI, mean (SD) | 24.89 (10.96) | 24.80 (10.43) | 0.97 |
| PROs | |||
| VAS pain, mean (SD) | 50.1 (28.3) | 53.3 (29.2) | 0.55 |
| PGA, mean (SD) | 5.2 (3) | 5.2 (2.8) | 0.99 |
| HAQ, mean (SD) | 1.02 (0.66) | 1.2 (0.97) | 0.21 |
| Laboratory | |||
| ESR, mean (SD) | 32.1 (21) | 28.2 (22.4) | 0.33 |
| CRP, mean (SD) | 1.69 (2.02) | 1.19 (1.44) | 0.14 |
| RF positive, n (%) | 47 (61) | 11 (22.9) | <0.001* |
| ACPA positive, n (%) | 38 (49.4) | 11 (22.9) | 0.005* |
| RF and/or ACPA positive, n (%) | 56 (72.7) | 15 (31.2) | <0.001* |
| Imaging | |||
| Erosion SHS score ⩾1, n (%) | 34 (44.2) | 17 (35.4) | 0.43 |
| US-GS score, mean (SD) | 8.2 (6.4) | 7.3 (5.1) | 0.44 |
| US-PD score, mean (SD) | 5.9 (6.6) | 5.7 (5.2) | 0.89 |
Significant at p < 0.05.
ACPA, anti-citrullinated protein antibodies; CRP, C-reactive protein; DAS28, disease activity score on 28 joints; ESR, erythrocyte sedimentation rate; GS, gray scale; HAQ, health assessment questionnaire; IQR, interquartile range; PD, power Doppler; PGA, patient global assessment; PhGA, physician global assessment; PROs, patient reported outcomes; RF, rheumatoid factor; SD, standard deviation; SDAI, simplified disease activity index; SHS, Sharp van der Heijde score; SJC28, swollen joint count on 28 joints; TJC28, tender joint count on 28 joints; US, ultrasonography; VAS, visual analogue scale.
Table 3.
Baseline variables associated with near-remission at 12 months stratified for the missing item (PGA or SJC28).
| SJC28 near-remission n = 76 | PGA near-remission n = 48 | p | |
|---|---|---|---|
| Demographic | |||
| Age, mean (SD) | 61.8 (13.4) | 59.1 (16) | 0.33 |
| Female gender, n (%) | 54 (71.1) | 30 (62.5) | 0.42 |
| Disease characteristics | |||
| Duration, weeks, mean (SD) | 19.1 (19.1) | 25.1 (24.7) | 0.19 |
| RA 1987 criteria, n (%) | 64 (84.2) | 39 (81.3) | 0.86 |
| Disease activity | |||
| SJC28, mean (SD) | 8.5 (5.2) | 7.6 (4.6) | 0.29 |
| TJC28, mean (SD) | 5.7 (5.6) | 7.2 (6.2) | 0.15 |
| PhGA, mean (SD) | 4.7 (2) | 4.5 (2.1) | 0.57 |
| DAS28, mean (SD) | 4.69 (1.19) | 4.77 (1.24) | 0.73 |
| SDAI, mean (SD) | 25.37 (13) | 24.45 (12.94) | 0.44 |
| PROs | |||
| VAS pain, mean (SD) | 48.4 (25.7) | 59.9 (28) | 0.02* |
| PGA, mean (SD) | 4.9 (2.7) | 6 (2.9) | 0.04* |
| HAQ, mean (SD) | 1.01 (0.72) | 1.25 (0.80) | 0.09 |
| Laboratory | |||
| ESR, mean (SD) | 34.8 (23.4) | 27.3 (18) | 0.07 |
| CRP, mean (SD) | 2.21 (2.81) | 1.44 (1.49) | 0.09 |
| RF positive, n (%) | 45 (59.2) | 6 (12.5) | <0.001* |
| ACPA positive, n (%) | 33 (43.4) | 6 (12.5) | <0.001* |
| RF and/or ACPA positive, n (%) | 50 (65.8) | 9 (18.8) | <0.001* |
| Imaging | |||
| Erosion SHS score ⩾1, n (%) | 35 (46.1) | 21 (43.8) | 0.95 |
| US-GS score, mean (SD) | 8.8 (5.2) | 7.6 (5.3) | 0.27 |
| US-PD score, mean (SD) | 6.1 (5.5) | 5.4 (5.6) | 0.52 |
Significant at p < 0.05.
ACPA, anti-citrullinated protein antibodies; CRP, C-reactive protein; DAS28, disease activity score on 28 joints; ESR, erythrocyte sedimentation rate; GS, gray scale; HAQ, health assessment questionnaire; IQR, interquartile range; PD, power Doppler; PGA, patient global assessment; PhGA, physician global assessment; PROs, patient reported outcomes; RF, rheumatoid factor; SD, standard deviation; SDAI, simplified disease activity index; SHS, Sharp van der Heijde score; SJC28, swollen joint count on 28 joints; TJC28, tender joint count on 28 joints; US, ultrasonography; VAS, visual analogue scale.
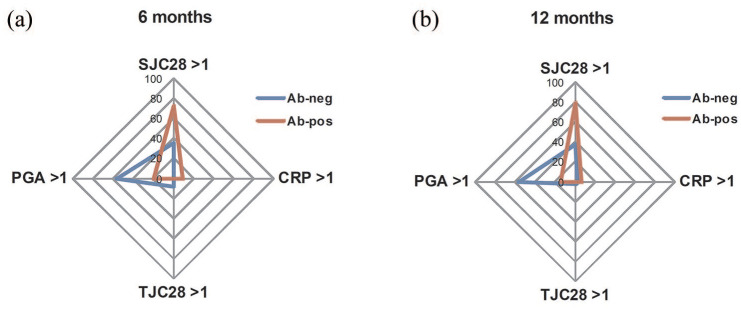
In contrast, significant differences were observed in autoantibody status. Indeed, patients in near-remission due to SJC28 > 1 were more frequently autoantibody-positive compared with patients in near-remission due to PGA > 1 at both the 6- and the 12-month time-points (72.7% versus 31.3% and 65.8% versus 18.8%, p < 0.001). As a result, the limiting variables to Boolean remission clearly differed between autoantibody-positive and -negative patients (Figure 2). In autoantibody-positive patients, SJC28 > 1 was responsible for 71.8% of near-remissions at 6 months and 79.4% at 12 months, compared with only 19.2% and 14.3% of cases attributable to PGA > 1 (p < 0.001 for both time points). In contrast, autoantibody-negative patients more frequently missed remission because of the PGA (55.9% and 56.5% at 6 and 12 months, respectively) rather than for persistent swollen joints (35.6% and 37.7%, p = 0.04 for both time points). After adjusting for confounders, autoantibody-positive patients were at increased risk of missing remission because of SJC28 >1 with an adjusted OR (95% CI) of 2.81 (1.59–4.96) at 6 months and of 1.73 (1.01–3.01) at 12 months, whilst autoantibody-negative patients missed remission because of PGA > 1 with an adjusted OR (95% CI) of 2.45 (1.25–4.80) at 6 months and 5.71 (2.47–13.2) at 12 months. Other independent predictors of SJC28 and PGA near-miss remission are listed in Table 4.
Figure 2.
Limiting variables to Boolean remission at 6 and 12 months stratified for the autoantibody status. Spider diagrams showing the frequency of the limiting variables to Boolean remission after 6 (a) and 12 months (b) from treatment start in autoantibody-positive (red) and -negative (blue) patients.
CRP, C-reactive protein; PGA, patient global assessment; SJC28, 28-swollen joint count; TJC28, 28-tender joint count.
Table 4.
Baseline predictors of near-remission stratified for the missing item (PGA or SJC28). Multivariable analysis.
| 6 months |
12 months |
|||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| SJC28 near-remission | ||||
| Female gender | − | − | − | − |
| RF and/or ACPA positive | 2.81 (1.59–4.96) | <0.001 | 1.73 (1.01–3.01) | 0.03 |
| SJC28 | 1.09 (1.02–1.16) | 0.006 | 1.09 (1.03–1.15) | 0.005 |
| CRP | − | − | − | − |
| TJC28 | 0.86 (0.81–0.92) | <0.001 | 0.90 (0.85–0.96) | 0.001 |
| PGA | − | − | 0.99 (0.97–0.99) | 0.01 |
| PGA near remission | ||||
| Female gender | − | − | 1.64 (0.82–3.28) | 0.16 |
| RF and/or ACPA positive | 0.41 (0.21–0.80) | 0.009 | 0.18 (0.08–0.41) | <0.001 |
| SJC28 | 0.94 (0.87–1.01) | 0.09 | − | − |
| CRP | 0.87 (0.73–1.03) | 0.11 | 0.91 (0.80–1.04) | 0.18 |
| TJC28 | − | − | − | − |
| PGA | 1.01 (0.99–1.08) | 0.10 | − | − |
ACPA, anti-citrullinated protein antibodies; CI, confidence interval; CRP, C-reactive protein; OR, odds ratio; PGA, patient global assessment; RF, rheumatoid factor; SJC28, swollen joint count on 28 joints; TJC28, tender joint count on 28 joints.
Characteristics of near-miss remission according to the limiting variable
We then investigated the specific disease characteristics of patients in near-remission (stratified for the limiting variable) in comparison with full-remission and non-remission (Table 5). Irrespective of the autoantibody status, SJC28 near-remission at 6 months presented a mean (SD) number of residual swollen joints of 3.2 (1.5), and nearly 15% of these patients had ⩾6 active joints. Data were similar in SJC28 near-remission at 12 months. In patients in PGA near-remission, as expected, VAS scores for pain and PGA were significantly higher compared with both SJC28 near-remission and full-remission, and were comparable with those of patients in non-remission. However, and unexpectedly, measures of objective disease activity were also slightly increased compared with full-remission, with higher CRP levels and SJC28.
Table 5.
Characteristics of patients in different remission states at 6 and 12 months.
| SJC28 near-remission | PGA near-remission | Remission | Non-remission | |
|---|---|---|---|---|
| 6 months | n = 77 | n = 48 | n = 69 | n = 329 |
| Disease activity | ||||
| SJC28, mean (SD) | 3.2 (1.5)* | 0.5 (0.5)*§ | 0.5 (0.6)*# | 4.3 (3.1)*§ |
| TJC28, mean (SD) | 0.2 (0.4)* | 0.3 (0.6)§ | 0.1 (0.4)§# | 4 (4.9)*§# |
| PROs | ||||
| VAS pain, mean (SD) (0–100) | 6.9 (13.7)* | 29.6 (23.6)*§ | 3.9 (7.7)*§# | 35.9 (25.4)*§# |
| PGA, mean (SD) (0–10) | 0.3 (0.4)* | 3.4 (2.3)*§ | 0.2 (0.3)§# | 3.5 (2.)*# |
| Laboratory | ||||
| ESR, mm/1 h, mean (SD) | 15.8 (9.9)* | 16.6 (11.7)§ | 13.5 (9.9)*§# | 21.6 (15.9)*§# |
| CRP, mg/dl, mean (SD) | 0.27 (0.23)* | 0.35 (0.25)*# | 0.25 (0.23)*# | 0.78 (1.79)*# |
| 12 months | n = 76 | n = 48 | n = 91 | n = 289 |
| Disease activity | ||||
| SJC28, mean (SD) | 2.9 (1.5)* | 0.6 (0.5)*§ | 0.4 (0.5)*§# | 3.7 (2.5)*§# |
| TJC28, mean (SD) | 0.2 (0.4)* | 0.3 (0.4)§ | 0.1 (0.3)*§# | 3.8 (5)*§# |
| PROs | ||||
| VAS pain, mean (SD) (0–100) | 5.8 (8.8)* | 35.2 (24.7)*§ | 6 (10.3)§# | 38.3 (26.5)*§# |
| PGA, mean (SD) (0–10) | 0.2 (0.3)* | 3.9 (2.5)*§ | 0.3 (0.4)§# | 3.5 (2.4)*# |
| Laboratory | ||||
| ESR, mm/1 h, mean (SD) | 17.9 (12.7)* | 18.7 (17.1)§ | 15.6 (11.2)*§# | 21.6 (17)*§# |
| CRP, mg/dl, mean (SD) | 0.31 (0.50)* | 0.36 (0.26)*§ | 0.26 (0.22)*§# | 0.82 (2.03)*§# |
p < 0.05 based on ANOVA analysis of variance with Student–Newman–Keuls test for all pairwise comparisons.
SJC28 near-remission versus PGA near-remission, remission and non-remission.
PGA near-remission versus remission and non-remission.
Remission versus non-remission.
CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; PGA, patient global assessment; PhGA, physician global assessment of disease activity; PROs, patient reported outcomes; SD, standard deviation; SJC28, swollen joint count on 28 joints; TJC28, tender joint count on 28 joints; VAS, visual analogue scale.
Discussion
In this study, we demonstrate that, in patients with RA, despite early diagnosis and T2T, stringent remission according to the ACR/EULAR criteria is hard to achieve. Far more commonly, patients miss Boolean remission solely because one of the four core variables scoring >1. Compared with established RA, in which the barrier to Boolean remission is often represented by a PGA > 1, in patients with early RA the achievement of stringent remission is more often precluded by the persistence of swollen joints. Relevantly, the limiting factor to full-remission varies according to autoantibody status. Autoantibody-positive patients largely miss remission because of SJC28, whilst the PGA is the limiting variable to full-remission more often in autoantibody-negative patients.
The achievement of stringent remission in early RA soon after treatment start conveys most benefits in terms of physical function, halt of joint damage progression and quality of life.6 As such, disease remission should be better assessed by means of the ACR/EULAR criteria, which are more restrictive compared with the DAS28.3 In our cohort, symptom duration before treatment start was, on average, within the window of opportunity of 15–19 weeks, MTX was used as the anchor drug and increased to the target, and glucocorticoid co-medication was introduced systematically in more recent years. This strategy is recognized as largely non-inferior compared with immediate start of biological DMARDs in the achievement of disease remission in early RA.37 Nevertheless, Boolean remission remains hard to achieve, being observed in less than 20% of the patients in this study and in similar early arthritis cohorts.10 Although we cannot exclude that adoption of more stringent therapeutic targets might in principle improve the outcomes, neither earlier treatment initiation within the phase of undifferentiated arthritis and early combination with biological DMARDs, as in the IMPROVED study,38 nor treatment to the target of imaging remission, such as in the ARCTIC trial,39 are apparently associated with higher rates of ACR/EULAR remission.
Far more commonly, RA patients fulfill three of the four required Boolean criteria – a condition defined near-remission.11 Studies in different clinical practice cohorts of established RA have consistently shown that PGA is the limiting variable to full-remission in more than 70% of the cases,11,21 and omitting the PGA nearly doubles the rate of patients achieving remission of inflammation as assessed objectively.16–22 Whilst the condition of near-miss remission was nearly as twice more common than full-remission also in patients with early RA in our study, remission was missed more frequently because of persistent swollen joints, and this was observed at both early and later time points. Apart from inter-studies differences related to the lack of standardized administration of the PGA,14,40,41 our results indicate that the current management of early RA in daily life is still insufficient at rapidly controlling synovitis. Although the adoption of more stringent therapeutic targets does not apparently modify the outcomes of remission,38,39 we cannot exclude that more targeted therapies could impact differently on core variables of disease activity. Accordingly, early combination with bDMARDs in the U-Act-Early trial has been shown to be associated with faster suppression of synovial inflammation compared with MTX monotherapy.42 The low proportion of patients initiating a b/tsDMARD in our cohort precluded testing of whether more targeted therapies would reduce the rate of remission missed because of SJC8. However, our results indicate that, in the early phases after treatment start with csDMARDs, patients with RA should be monitored carefully for disease progression/relapse even when most of the parameters of disease activity are apparently well-controlled. Indeed, persistent swollen joints are the major drivers of radiographic progression also in patients in remission.12,13,43,44
Yet, a smaller though significant proportion of patients presented with poor self-assessment of the disease despite good control of objective inflammation also in our cohort. The reasons for the lower rates of PGA near-remission in our cohort of early RA compared with established disease remain to be demonstrated,17–22 but may reflect possible differences in patients’ perception of the disease in course of different phases, with higher rates of satisfaction as soon as treatment is started and the first benefits are being experienced. Of particular relevance for clinical practice, no demographic or clinical variables at presentation could effectively discriminate between SJC28 and PGA near-remission apart from autoantibody status. Indeed, autoantibody-positive patients nearly exclusively missed remission because of a SJC28 > 1. In these patients, treatment intensification should be strongly considered in order to preserve long-term joint integrity, as RA-associated autoantibodies are recognized as an additional and inflammation-independent risk factor for bone and cartilage destruction.45,46 In contrast, the PGA was the limiting variable to full-remission predominantly in autoantibody-negative patients. This finding is not surprising as several studies have shown that, among patients with RA, disproportionate pain is observed more frequently within the autoantibody-negative subgroup.47,48
In established RA, the PGA score reflects chronic pain, fatigue, anxiety, and loss of function, whilst correlation with joint involvement and acute phase reactants is low.14,16,17,19,20 As the condition of remission solely missed because of the PGA has been shown to largely overlap full-remission in clinical characteristics and outcomes,15,20 a dual strategy separately targeting biologic inflammation and patient symptoms is currently been proposed.23 However, in our cohort of early RA patients, PGA near-remission unexpectedly also presented with slightly higher levels of objective inflammation as compared with patients in Boolean remission. Although specific studies on the major drivers of the PGA in the early phases of RA are lacking, it is possible that the PGA may collect partly different information in the different phases of the disease.49 Soon after disease onset, the consequences of disease chronicity on pain sensitization and bone destruction may not yet have severely impacted on patients’ self-assessments,50 and the PGA may more specifically reflect disease activity. Collectively, our results thus suggest caution in the pursuit of a dual target strategy in early RA missing remission solely because of the PGA, as some of these patients may present persistent clinical or subclinical inflammation potentially susceptible of immunosuppressive therapy.
Our study has limitations. The lack of standardization of administration of the PGA,14,40,41 as well as the imprecise reproducibility of joint counts,51 may affect comparisons with previous studies. However, the PGA was assessed systematically using the same formulation, and joint evaluations were performed by experienced rheumatologists working at a single center, thus making the proportion of PGA and SJC28 near-remission observed in this study reliable. Still, our data refer mostly to treatment with csDMARDs, and we cannot exclude that the use of b/tsDMARDs may alter the distribution of the limiting variables to Boolean remission also in patients with early RA.24 Compared with studies addressing near-remission in established RA, the proportion of autoantibody-negative patients in our cohort and in similar early RA populations is expected to be higher.28,29 Although the application of the 2010 criteria might have led to RA over-diagnosis, especially in autoantibody-negative subjects,52 differential diagnoses were carefully excluded, and the vast majority of our patients also fulfilled the 1987 criteria for RA. This allowed us to highlight statistically and clinically significant differences between serological disease subgroups. Still, the low number of single RF- and ACPA-positive patients hampers further definition on the possible independent associations of the two antibody systems with remission outcomes. Although the rate of SJC28 near-remission remained high also after 12 months from treatment start, a longer follow up is needed to establish at which time point the PGA becomes the major limiting factor to Boolean remission.
In conclusion, our results indicate that incomplete suppression of synovitis represents a major obstacle to the achievement of stringent disease remission in patients with early RA in the context of conventional treatment strategies with first-line csDMARDs. Autoantibody-positive patients more often present with persistent joint swelling even when other parameters of disease activity and self-reported assessments appear well-controlled, and should be monitored carefully for disease progression. In contrast, PROs limit full-remission predominantly in autoantibody-negative patients. However, in early RA, near-remission due to the PGA exceeding the cut-off of 1 maintains higher levels of objective inflammation as compared with Boolean remission, suggesting cautious evaluation of disease activity also in this condition.
Footnotes
Author contributions: SB contributed to the conception of the work, to the analysis and interpretation of data and to the drafting of the work. LDS contributed to the conception of the work, to the acquisition and interpretation of data and to the drafting of the work. AM contributed to the interpretation of data and revised the manuscript critically for important intellectual content. GS contributed to the acquisition and interpretation of data and revised the manuscript critically. BX contributed to the acquisition and interpretation of data and revised the manuscript critically. CM contributed to the conception of the work and revised the manuscript critically for important intellectual content. All the authors provided final approval of the version to be published.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this study was supported in part by funding from the IRCCS Policlinico San Matteo Foundation, Pavia, Italy.
Ethics approval information and consent to participate: IRCCS Policlinico San Matteo Foundation Ethics Committee n.20070001302.
All patients signed a written informed consent before the inclusion and the study protocol.
Consent for publication: Not applicable.
ORCID iD: Serena Bugatti  https://orcid.org/0000-0002-5396-7077
https://orcid.org/0000-0002-5396-7077
Availability of data and materials: Data relevant to the study are included in the article. Deidentified participant rough data are available from the corresponding author (serena.bugatti@unipv.it) upon reasonable request. The Authors declare that parts of the article have been published on the preprint server Research Square. DOI: 10.21203/rs.3.rs-22550/v1. https://www.researchsquare.com/article/rs-22550/v1
Contributor Information
Serena Bugatti, Division of Rheumatology, IRCCS Policlinico San Matteo Foundation University Hospital, Viale Golgi 19, Pavia, 27100, Italy; Department of Internal Medicine and Therapeutics, University of Pavia, Pavia, Italy.
Ludovico De Stefano, Division of Rheumatology, IRCCS Policlinico San Matteo Foundation, Pavia, Italy; Department of Internal Medicine and Therapeutics, University of Pavia, Pavia, Italy.
Antonio Manzo, Division of Rheumatology, IRCCS Policlinico San Matteo Foundation, Pavia, Italy; Department of Internal Medicine and Therapeutics, University of Pavia, Pavia, Italy.
Garifallia Sakellariou, Istituti Clinici Scientifici Maugeri, Pavia, Italy; Department of Internal Medicine and Therapeutics, University of Pavia, Pavia, Italy; Istituti Clinici Scientifici Maugeri, Pavia, Italy.
Blerina Xoxi, Division of Rheumatology, IRCCS Policlinico San Matteo Foundation, Pavia, Italy.
Carlomaurizio Montecucco, Division of Rheumatology, IRCCS Policlinico San Matteo Foundation, Pavia, Italy; Department of Internal Medicine and Therapeutics, University of Pavia, Pavia, Italy.
References
- 1. Smolen JS, Breedveld FC, Burmester GR, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis 2015; 75: 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schett G, Emery P, Tanaka Y, et al. Tapering biologic and conventional DMARD therapy in rheumatoid arthritis: current evidence and future directions. Ann Rheum Dis 2016; 75: 1428–1437. [DOI] [PubMed] [Google Scholar]
- 3. Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020; 79: 685–699. [DOI] [PubMed] [Google Scholar]
- 4. Mäkinen H, Kautiainen H, Hannonen P, et al. Is DAS28 an appropriate tool to assess remission in rheumatoid arthritis? Ann Rheum Dis 2005; 64: 1410–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Felson D. Defining remission in rheumatoid arthritis. Ann Rheum Dis 2012; 71(Suppl. 2): i86–i88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aletaha D, Smolen JS. Remission in rheumatoid arthritis: missing objectives by using inadequate DAS28 targets. Nat Rev Rheumatol 2019; 15: 633–634. [DOI] [PubMed] [Google Scholar]
- 7. Felson DT, Smolen JS, Wells G, et al. American College of Rheumatology/European League against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Arthritis Rheum 2011; 63: 573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shahouri SH, Michaud K, Mikuls TR, et al. Remission of rheumatoid arthritis in clinical practice: application of the American College of Rheumatology/European League against Rheumatism 2011 remission criteria. Arthritis Rheum 2011; 63: 3204–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thiele K, Huscher D, Bischoff S, et al. Performance of the 2011 ACR/EULAR preliminary remission criteria compared with DAS28 remission in unselected patients with rheumatoid arthritis. Ann Rheum Dis 2013; 72: 1194–1199. [DOI] [PubMed] [Google Scholar]
- 10. Britsemmer K, van Schaardenburg D, Boers M, et al. Prevalence and validity of ACR/EULAR remission in four European early rheumatoid arthritis cohorts. Clin Exp Rheumatol 2018; 36: 362–370. [PubMed] [Google Scholar]
- 11. Studenic P, Smolen JS, Aletaha D. Near misses of ACR/EULAR criteria for remission: effects of patient global assessment in Boolean and index-based definitions. Ann Rheum Dis 2012; 71: 1702–1705. [DOI] [PubMed] [Google Scholar]
- 12. Aletaha D, Alasti F, Smolen JS. Rheumatoid arthritis near remission: clinical rather than laboratory inflammation is associated with radiographic progression. Ann Rheum Dis 2011; 70: 1975–1980. [DOI] [PubMed] [Google Scholar]
- 13. Aletaha D, Smolen JS. Joint damage in rheumatoid arthritis progresses in remission according to the disease activity score in 28 joints and is driven by residual swollen joints. Arthritis Rheum 2011; 63: 3702–3711. [DOI] [PubMed] [Google Scholar]
- 14. Nikiphorou E, Radner H, Chatzidionysiou K, et al. Patient global assessment in measuring disease activity in rheumatoid arthritis: a review of the literature. Arthritis Res Ther 2016; 18: 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferreira RJO, Welsing PMJ, Jacobs JWG, et al. Revisiting the use of remission criteria for rheumatoid arthritis by excluding patient global assessment: an individual meta-analysis of 5792 patients. Ann Rheum Dis. Epub ahead of print 6 October 2020. DOI: 10.1136/annrheumdis-2020-217171. [DOI] [PubMed] [Google Scholar]
- 16. Studenic P, Felson D, de Wit M, et al. Testing different thresholds for patient global assessment in defining remission for rheumatoid arthritis: are the current ACR/EULAR Boolean criteria optimal? Ann Rheum Dis 2020; 79: 445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vermeer M, Kuper HH, van der Bijl AE, et al. The provisional ACR/EULAR definition of remission in RA: a comment on the patient global assessment criterion. Rheumatology (Oxford) 2012; 51: 1076–1080. [DOI] [PubMed] [Google Scholar]
- 18. Balogh E, Dias JM, Orr C, et al. Comparison of remission criteria in a tumour necrosis factor inhibitor treated rheumatoid arthritis longitudinal cohort: patient global health is a confounder. Arthritis Res Ther 2013; 15: R221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ferreira RJO, Dougados M, Kirwan J, et al. Drivers of patient global assessment in patients with rheumatoid arthritis who are close to remission: an analysis of 1588 patients. Rheumatology (Oxford) 2017; 56: 1573–1578. [DOI] [PubMed] [Google Scholar]
- 20. Ferreira RJO, Duarte C, Ndosi M, et al. Suppressing inflammation in rheumatoid arthritis: does patient global assessment blur the target? A practice-based call for a paradigm change. Arthritis Care Res 2018; 70: 369–378. [DOI] [PubMed] [Google Scholar]
- 21. Ferreira RJO, Carvalho PD, Ndosi M, et al. Impact of patient’s global assessment on achieving remission in patients with rheumatoid arthritis: a multinational study using the METEOR database. Arthritis Care Res 2019; 71: 1317–1325. [DOI] [PubMed] [Google Scholar]
- 22. Gossec L, Kirwan JR, de Wit M, et al. Phrasing of the patient global assessment in the rheumatoid arthritis ACR/EULAR remission criteria: an analysis of 967 patients from two databases of early and established rheumatoid arthritis patients. Clin Rheumatol 2018; 37: 1503–1510. [DOI] [PubMed] [Google Scholar]
- 23. Ferreira RJO, Ndosi M, de Wit M, et al. Dual target strategy: a proposal to mitigate the risk of overtreatment and enhance patient satisfaction in rheumatoid arthritis. Ann Rheum Dis 2019; 78: e109. [DOI] [PubMed] [Google Scholar]
- 24. Aletaha D, Wang X, Zhong S, et al. Differences in disease activity measures in patients with rheumatoid arthritis who achieved DAS, SDAI, or CDAI remission but not Boolean remission. Semin Arthritis Rheum 2020; 50: 643–644. [DOI] [PubMed] [Google Scholar]
- 25. Boers M. Patient global assessment to define remission in rheumatoid arthritis:quo vadis? Ann Rheum Dis. Epub ahead of print 6 November 2020. DOI: 10.1136/annrheumdis-2020-218802. [DOI] [PubMed] [Google Scholar]
- 26. Sokka T, Kankainen A, Hannonen P. Scores for functional disability in patients with rheumatoid arthritis are correlated at higher levels with pain scores than with radiographic scores. Arthritis Rheum 2000; 43: 386–389. [DOI] [PubMed] [Google Scholar]
- 27. Radner H, Yoshida K, Tedeschi S, et al. Different rating of global rheumatoid arthritis disease activity in rheumatoid arthritis patients with multiple morbidities. Arthritis Rheumatol 2017; 69: 720–727. [DOI] [PubMed] [Google Scholar]
- 28. Gwinnutt JM, Symmons DPM, MacGregor AJ, et al. Have the 10-year outcomes of patients with early inflammatory arthritis improved in the new millennium compared with the decade before? Results from the Norfolk Arthritis Register. Ann Rheum Dis 2018; 77: 848–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lukas C, Mary J, Debandt M, et al. Predictors of good response to conventional synthetic DMARDs in early seronegative rheumatoid arthritis: data from the ESPOIR cohort. Arthritis Res Ther 2019; 21: 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Montecucco C, Todoerti M, Sakellariou G, et al. Low-dose oral prednisone improves clinical and ultrasonographic remission rates in early rheumatoid arthritis: results of a 12-month open-label randomised study. Arthritis Res Ther 2012; 14: R112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Balduzzi S, Scirè CA, Sakellariou G, et al. In early inflammatory polyarthritis more intensive management according to the 2010 ACR/EULAR criteria leads to higher rates of clinical remission: comparison of two cohorts treated according to different treat-to-target protocols. Clin Exp Rheumatol 2017; 35: 401–405. [PubMed] [Google Scholar]
- 32. Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988; 31: 315–324. [DOI] [PubMed] [Google Scholar]
- 33. Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Ann Rheum Dis 2010; 69: 1580–1588. [DOI] [PubMed] [Google Scholar]
- 34. Backhaus M, Burmester GR, Gerber T, et al. Guidelines for musculoskeletal ultrasound in rheumatology. Ann Rheum Dis 2001; 60: 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bugatti S, Manzo A, Benaglio F, et al. Serum levels of CXCL13 are associated with ultrasonographic synovitis and predict power Doppler persistence in early rheumatoid arthritis treated with non-biological disease-modifying anti-rheumatic drugs. Arthritis Res Ther 2012; 14: R34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol 2000; 27: 261–263. [PubMed] [Google Scholar]
- 37. Verhoeven MMA, Welsing PMJ, Bijlsma JWJ, et al. Effectiveness of remission induction strategies for early rheumatoid arthritis: a systematic literature review. Curr Rheumatol Rep 2019; 21: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Akdemir G, Heimans L, Bergstra SA, et al. Clinical and radiological outcomes of 5-year drug-free remission-steered treatment in patients with early arthritis: IMPROVED study. Ann Rheum Dis 2018; 77: 111–118. [DOI] [PubMed] [Google Scholar]
- 39. Paulshus Sundlisæter N, Olsen IC, Aga A-B, et al. Predictors of sustained remission in patients with early rheumatoid arthritis treated according to an aggressive treat-to-target protocol. Rheumatology (Oxford) 2018; 57: 2022–2031. [DOI] [PubMed] [Google Scholar]
- 40. Khan NA, Spencer HJ, Abda EA, et al. Patient’s global assessment of disease activity and patient’s assessment of general health for rheumatoid arthritis activity assessment: are they equivalent? Ann Rheum Dis 2012; 71: 1942–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ferreira RJO, de Wit M, Henriques M, et al. ‘It can’t be zero!’ Difficulties in completing patient global assessment in rheumatoid arthritis: a mixed methods study. Rheumatology (Oxford) 2020; 59: kez467. [DOI] [PubMed] [Google Scholar]
- 42. Bijlsma JWJ, Welsing PMJ, Woodworth TG, et al. Early rheumatoid arthritis treated with tocilizumab, methotrexate, or their combination (U-Act-Early): a multicentre, randomised, double-blind, double-dummy, strategy trial. Lancet 2016; 388: 343–355. [DOI] [PubMed] [Google Scholar]
- 43. Bugatti S, Manzo A, Caporali R, et al. Assessment of synovitis to predict bone erosions in rheumatoid arthritis. Ther Adv Musculoskelet Dis 2012; 4: 235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bugatti S, Sakellariou G, Luvaro T, et al. Clinical, imaging, and pathological suppression of synovitis in rheumatoid arthritis: is the disease curable? Front Med 2018; 5: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bugatti S, Manzo A, Montecucco C, et al. The clinical value of autoantibodies in rheumatoid arthritis. Front Med 2018; 5: 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Steffen U, Schett G, Bozec A. How autoantibodies regulate osteoclast induced bone loss in rheumatoid arthritis. Front Immunol 2019; 10: 1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Doss J, Mo H, Carroll RJ, et al. Phenome-wide association study of rheumatoid arthritis subgroups identifies association between seronegative disease and fibromyalgia. Arthritis Rheumatol 2017; 69: 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Challa DN, Kvrgic Z, Cheville AL, et al. Patient-provider discordance between global assessments of disease activity in rheumatoid arthritis: a comprehensive clinical evaluation. Arthritis Res Ther 2017; 19: 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bugatti S, De Stefano L, Favalli EG, et al. Increasing the threshold for patient global assessment in defining remission may have a different impact in patients with early and established rheumatoid arthritis. Ann Rheum Dis. Epub ahead of print 20 April 2020. DOI: 10.1136/annrheumdis-2020-217488. [DOI] [PubMed] [Google Scholar]
- 50. Walsh DA, McWilliams DF. Mechanisms, impact and management of pain in rheumatoid arthritis. Nat Rev Rheumatol 2014; 10: 581–592. [DOI] [PubMed] [Google Scholar]
- 51. Cheung PP, Gossec L, Mak A, et al. Reliability of joint count assessment in rheumatoid arthritis: a systematic literature review. Semin Arthritis Rheum 2014; 43: 721–729. [DOI] [PubMed] [Google Scholar]
- 52. van der Linden MP, Knevel R, Huizinga TW, et al. Classification of rheumatoid arthritis: comparison of the 1987 American College of Rheumatology criteria and the 2010 American College of Rheumatology/European League against Rheumatism criteria. Arthritis Rheum 2011; 63: 37–42. [DOI] [PubMed] [Google Scholar]