Abstract
Chronic orchialgia can be the result of pathological processes of the scrotal contents or stem from non-intrascrotal structures. Successful pain management depends on identifying the source of localized or referred pain. This is a case report of a 39-year-old male sports coach who presented with low back pain, right orchialgia, and sciatica refractory to conservative management. Magnetic resonance (MR) imaging revealed disc protrusion at L3/L4 and L4/L5 levels. Positive outcomes in relieving back and testicular pain were obtained after a total of 30 chiropractic sessions over a 9-week period. The evidence of the subjective improvement was corroborated by regression of the herniated discs documented on the repeat MR imaging. While chronic orchialgia is not an uncommon problem for men of all ages, it has seldom been described in association with lumbar discogenic disease. The current study provided preliminary support for a link between orchialgia and lumbar disc herniation. Chiropractic manipulation had provided a mechanistic alleviation of noxious lumbar stimuli, leading to symptomatic and functional improvements.
Keywords: chiropractic, discogenic disease, lumbar disc herniation, orchialgia, testicle
About 2.5%–4.8% of urologic clinic visits are related to orchialgia (testicular pain) (Sigalos & Pastuszak, 2017). Pain in the genitalia may be perceived from any of the scrotal contents, including the testicle, epididymis, vas deferens, spermatic cord, or para-testicular structures (Levine & Abdelsayed, 2018). Direct pathological processes of orchialgia vary from trauma, infection, torsion, pathological lesions, and scarring or functional changes following surgery (Levine & Abdelsayed, 2018). Extrascrotal origins such as pelvic floor dysfunction, ureterolithiasis, inguinal hernia, aortic aneurysms, vascular flow compromise, and entrapment neuropathy can also refer pain to the testes (Levine & Abdelsayed, 2018). Up to 50% of chronic orchialgia cases is of unknown etiology (Levine & Abdelsayed, 2018; Sigalos & Pastuszak, 2017), due to a poor understanding of the pathophysiology of orchialgia (Patel, 2017; Tan & Levine, 2017). Lumbosacral problems may be a commonly overlooked cause of orchialgia in men. Chronic orchialgia secondary to lumbar discogenic disease has only been described sporadically in the literature (Chu, 2020; Neff et al., 2017; Peng et al., 2014; Wouda et al., 2005). No accepted algorithm exists to guide clinicians in the evaluation and management of chronic orchialgia (Smith & Costabile, 2017). The current case study may shed some light on the association between orchialgia that is discogenic in nature, and a potential treatment option for discogenic-related chronic orchialgia. A literature review on this issue is also presented.
Case Presentation
A 39-year-old sports coach complained of constant low back pain with associated episodic right testicular pain for nearly a decade. He denied any prior trauma or systemic diseases. As a professional fencing competitor, the patient engaged in weightlifting, swimming, and hiking in the past 14 years. He started to experience a worsening back pain with radiating right testicular pain after a backpacking trip 7 years prior to seeking chiropractic treatment. The pain could be elicited by squat lifting, butterfly stroke swimming or coughing. A scrotal Doppler ultrasonography performed at initial urological consultation excluded trauma and direct testicular pathologies. Magnetic resonance imaging (MRI) performed for the orthopedic workup 2 years prior to chiropractic treatment revealed degenerative spondylitis of the lumbar spine. Oral medications (ibuprofen and 10 days of oral doxycycline), sports physiotherapy, and acupuncture did not provide any effective or long-lasting symptomatic improvement. His symptoms significantly worsened in the last 2 months, accompanied by radiating pain down the right leg, which he had not experienced previously. The painful symptoms had a considerable impact on his physical, emotional, sexual, and social functions, and he was unable to continue coaching fencing.
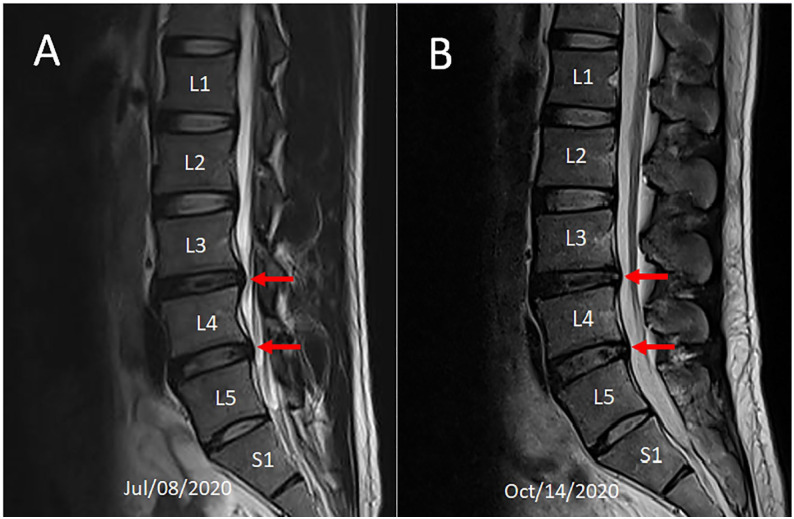
Physical examination of the testicles revealed that they were descended bilaterally and normal in size and consistency, with unrestricted movement within the scrotal sac. Active range of motion of the spine was normal. Low back soreness could be induced by passive lumbar extension and right lateral lumbar flexion. Palpation revealed tenderness and stiffness at L4/5 and L5/S1 segments. Spinal percussion with a reflex hammer over the L3 and L4 spinous processes elicited a Lhermitte’s sign (tingling sensation). Pinch-roll test of the skin revealed local hyperesthesia and tenderness over the right lower lumbar paraspinal region. MRI of the lumbar spine (Figure 1A) to assess the cause of the patient’s low back pain revealed decreased height of the L3/4, L4/5 and L5/S1 discs, and desiccation (reduced T2 weighted signal intensity) of the L3/4 disc. Disc protrusion at the L3/L4 and L4/L5 levels (red arrows) causing encroachment onto the left L3/4 intervertebral foramen and bilateral L4/L5 intervertebral foramina with more marked changes on the right than the left, and an impingement of right L4 nerve root were visualized. Chronic testicular pain caused by lumbar disc herniation was diagnosed on the basis of imaging findings and clinical history.
Figure 1.
Comparison of two MR scans over 3-month period. (A) Sagittal T2-weighted image before initiation of treatment showed decreased height of the L3/4, L4/5 and L5/S1 discs and reduced T2 weighted signal intensity (desiccation) of the L3/4 disc. Disc protrusion was seen at the L3/L4 and L4/L5 levels with indentation of the thecal sac (red arrows). (B) Follow-up image demonstrating regression of the thecal sac displacement (red arrows).
Following 6 consecutive days of lumbar manipulation to release intersegmental restriction combined with therapeutic ultrasound to provide deep heating to soft tissues, the patient’s pain complaints were substantially reduced. Subsequent treatment sessions consisted of intermittent motorized lumbar traction (Spine Decompression Device, WIZ Medical, Korea), therapeutic ultrasound, and spinal manipulation of the lower lumbar spine. The spinal decompression device was aimed specifically at L3/L4 and L4/L5 levels to restore his neurological dysfunction. Treatment frequency was reduced to three times weekly for a period of 8 weeks. Low back pain, testicular pain, and radiating leg pain were fully resolved by the end of treatment. Oswestry Disability Index (ODI) score was significantly reduced (50% at intake, 0% at 9 weeks, where 0 means no limitation and 100 means total disability). The World Health Organization Quality of Life (WHOQOL) score improved from 70% to 98% (100% being the highest possible well-being). Repeat MRI (Figure 1B) at 3-month follow-up documented the regression of disc extrusion. At the time of performing the repeat MRI, the patient had returned to sports coaching and had resumed participation in high-intensity workout.
Discussion
Weight training exerts force onto the spine and can pose a risk of spinal injury caused by shearing and compressive forces on the vertebral column. The cumulative effects of strenuous activities may influence disc health (Sørensen et al., 2011). Findings based on the Copenhagen Male Study (Sørensen et al., 2011) analyzing 5245 men without history of back disease at baseline demonstrated that frequent exposure to strenuous physical activity at work was a strong risk factor of the ensuing development of lumbar disc herniation. Chronic orchialgia has been sporadically reported as a manifestation of spinal problems in men (Chu, 2020; Neff et al., 2017; Peng et al., 2014; Wouda et al., 2005), but the precise pathophysiology link between lumbar pathology and orchialgia is poorly understood.
The somatic sensory innervation of the scrotum and testicles arises from the lumbar plexus through the iliohypogastric (T12, L1), ilioinguinal (L1), genitofemoral (L1, L2), and pudendal nerves (S2-S4) (Patel, 2017). Any organ or tissue sharing a common neural pathway (L1-L2 and S2-S4) with the scrotum or testicles can refer pain to the genital area (Leslie et al., 2020; Tan & Levine, 2017). Spinal disorders may be related to chronic orchialgia and are often overlooked in the medical workup. In cases of lumbar discogenic disease, chronic orchialgia can be attributed various mechanisms such as direct radicular irritation, entrapment neuropathy, pain referral, neurologic hypersensitivity, or Wallerian neurologic degeneration.
Radicular Irritation
The primary sensory function of the iliohypogastric, ilioinguinal, and genitofemoral nerves arise from the L1 and L2 spinal nerve roots of the upper lumbar plexus. Compressed or inflammatory irritation of the thoracolumbar nerve roots is a frequently overlooked cause of chronic orchialgia (Chu, 2020; Leslie et al., 2020). Intervertebral discs are in direct contact with the dorsal roots; discal bulging or degenerative disc changes can impinge the respective spinal nerve roots contributing to orchialgia (Cramer, 2014). Leakage of inflammatory cytokines from annular disc tears into the epidural space can injure adjacent nerves, leading to radicular pain in the absence of disc herniation (Shayota et al, 2019). Radicular pain is a symptom radiating in the distribution of a spinal nerve caused by an irritation of the sensory root or dorsal root ganglia (DRG) of the nerve. Depending on the number of nerve root(s) affected, pain is predictably distributed to areas corresponding to dermatomal or myotomal mappings (Alexander & Varacallo, 2019). Radiculopathy can be defined as the whole complex of symptoms arising from nerve root pathology.
Entrapment Neuropathy
Psoas muscle dysfunction or spasms can play a role in some cases of orchialgia (Chu, 2020). The psoas muscle and male genitalia share similar sources of lumbar innervations. The psoas major is innervated by the lumbar plexus via branches from L1-L3 nerves. The genitofemoral nerve also stems from L1 and L2 roots of the plexus. The psoas major inserts onto the lumbar discs, the vertebral bodies, and the transverse processes of the T12 to L5 levels. Lumbar discitis, through the action of pro-inflammatory cytokines, can agitate the psoas muscle and lead to iliopsoas tendinitis. Because the iliohypogastric, ilioinguinal, and hypogastric nerves travel through the psoas muscle, these nerves can be entrapped as they pass behind or through a hypertonic psoas muscle, contributing to groin and scrotal pain (Chu, 2020). Patients with iliopsoas tendinitis may exhibit a flexed antalgic lean to the painful side.
Pain Referral
Referred pain is pain felt at a location distant from the injured organ or painful stimulus/origin (Arendt-Nielsen & Svensson, 2001). Primary somatic sensory innervation of the testicle is from the ilioinguinal nerve and the genital branch of the genitofemoral nerve. Any organ that shares the same neural pathway with the scrotal contents (mostly L1, L2, and S2–4) can refer pain to the genital area (Leslie et al., 2020; Patel, 2017; Tan & Levine, 2017). The exact pathways transmitting discogenic stimulus from the lower back (L3- L5) to the testicles remain speculative.
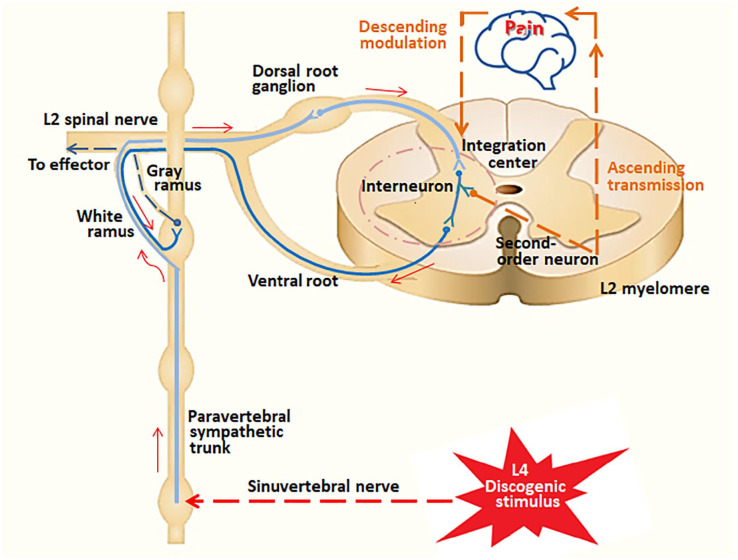
Lumbar discs are innervated by the sinuvertebral nerves (also known as Luschka nerve), that is formed from fibers coming from both gray rami communicants and ventral rami of the spinal nerve root (Das & Roy, 2018; Shayota et al, 2019). As shown in Figure 2, pain impulses flow through the sinuvertebral nerve (segmental) and travel along the paravertebral sympathetic trunk in a non-segmental fashion, via the white rami communicantes to the L2 (or L1) sensory nerve roots. The nociceptive signals then return to the spinal cord dorsal horn (Das & Roy, 2018; Murata et al., 2009), where the afferent information can be modulated or prioritized before sending to the brain (Smith et al., 2019). The pain impulses are carried predominately by the general visceral afferent (GVA) fibers to the spinal cord (Shayota et al, 2019). When the GVA fibers reach the dorsal horn of the cord, they terminate on second-order neurons (Sanvictores & Tadi, 2020). These neurons then send ascending signals to the brain for further processing. Normally, visceral afferent activity does not reach the level of consciousness. If the visceral afferent activity is pain related, it can reach the level of consciousness. It is generally agreed that two different afferent impulses synapse upon the same neuron, leading to a perception of misregistration in the brain (Arendt-Nielsen & Svensson, 2001; Jinkins, 2004); this is known as referred pain (Sanvictores & Tadi, 2020) (Figure 2).
Figure 2.
Proposed pathways for discogenic pain and referred orchialgia, using L4 discogenic pain as an example. Intervertebral discs are innervated by the sinuvertebral nerve (Shayota et al, 2019), which carries pain impulses from the injured disc to the sympathetic ganglion at the same level (L4). Any afferents from the L3 to S1 lumbosacral regions must detour up the paravertebral sympathetic trunk before re-entering the dorsal horn at upper lumbar (L1 or L2) level where rami communicantes are found (Das & Roy, 2018). Afferent impulses eventually ascend to the brain via the spinal cord. Different afferent fibers from the same spatial entry contribute to the mental picture of referred pain (Jinkins, 2004). The final stage of pain pathway involves integrating the ascending information into pain perception that elicits fight or flight behaviors (Patel, 2017).
Neurological Hypersensitivity
Sensitization of the primary afferent neurons to repetitive stimulation may lead to enhanced visceral perception (Chaban, 2010), accounting for a dramatically lower nociceptive threshold, and resulting in spontaneous nerve firing for all stimuli (Chaban, 2010; Leslie et al., 2020). Sensitization can also occur in response to inflammation. In a study of peripheral sensitization in nociceptive transmission on rat (Chaban, 2010), Chaban demonstrated that inflammation sensitized the adenosine triphosphate (ATP) response and enhanced the expression of ATP receptors with increasing neuronal hypersensitivity. Cross-sensitization can occur in non-inflamed viscera that are innervated by the same DRG in response to repeated stimulation of nociceptive transmitters such as ATP or substance P (Chaban, 2010). In cases of lumbar disc herniation, herniated disc materials may irritate the nerve root and the sinuvertebral nerve endings. The affected nerves become sensitized to mechanical stimuli by topical exposure to pro-inflammatory mediators. Inflammatory granulation with extensive innervation present in annular tears is also responsible for painful stimulation (Edgar, 2007). Brain-derived neurotrophic factor (BDNF) is a well-known mediator of pain plasticity that can cause the induction and maintenance of long-term potentiation in the brain and spinal cord (Sikandar & Sommer, 2019). It has been speculated that BDNF is implicated in the hypersensitization experienced in discogenic pain (Shayota et al, 2019). Axon disintegration in the distal nerve (Wallerian degeneration) instigates subsequent immune response and also contributes to neural hypersensitivity (Dubový, 2011).
Wallerian Degeneration
Wallerian degeneration is the self-destruction of the damaged axons initiated by the glial and immune cells (Patel, 2017). It is a cascade of inflammation in reaction to peripheral nerve injury, which simultaneously contributes to axonal regeneration and neuropathic pain induction (Dubový, 2011). These changes are termed neural plasticity and manifest as allodynia or hyperalgesia (Patel, 2017). Parekattil et al. (2020) have observed that a significant number of patients with chronic orchialgia have Wallerian degeneration in their spermatic cords when compared to the general male population. The ligation, ablation, or neuro-modulation of the affected nerve fibers may be considered as relevant approaches for pain relief in chronic orchialgia (Parekattil et al, 2020).
Back pain, originating in the lumbar region may cause referred unilateral testicular or scrotal pain by affecting the genitofemoral and inguinal nerves (Chu, 2020; Neff et al., 2017). Men engaged in lifting activities may be at risk for lumbar disc herniation. In the absence of symptoms or signs suggestive of L1 or L2 nerve root entrapment, pain referral or neuronal cross-sensitization should be considered as possible mechanisms of orchialgia, as in this case. The correlation between lesion regression illustrated on MRI and improvement of patient’s symptoms support the hypothesis of disc protrusion as the cause of his complaints. Sporadic case reports have described clinical benefit from chiropractic adjustment (Chu, 2020; Neff et al., 2017; Rowell & Rylander, 2012), manual therapy (Doubleday et al., 2003; Leone & Middleton, 2016), and back surgery (Asadian et al., 2016; Peng et al., 2014; Wouda et al., 2005) for patients with back and testicular pain. Biomechanical effects of chiropractic manipulation on symptom relief include relaxation of hypertonic musculature, release of entrapped tissues, disruption of periarticular adhesions, and restoration of spinal alignment (Chu, 2020). Treatment for chronic orchialgia suffers from lack of evidence-based guidance. A successful treatment outcome depends heavily on recognizing the origin of orchialgia. A multidisciplinary team approach may be considered to provide comprehensive medical care for these patients. Chiropractic care may be one of the conservative treatment options before surgery.
The current finding is limited by the fact that the pathways of testicular pain and mechanism of symptom relief remain speculative. A single sample without a control group prevents definitive conclusions for clinical results. The current case study suggests an often overlooked cause of chronic orchialgia and provides one plausible approach for the diagnosis and treatment of patients with chronic orchialgia. Further investigation is warranted before definitive conclusions can be drawn.
Conclusions
Chronic orchialgia may be attributed to a complex interplay between peripheral and central nociceptive mechanisms. Recognizing the factors contributing to testicular pain and dysfunction in patients can be challenging. Treatment strategies for chronic orchialgia have begun to shift toward musculoskeletal dysfunction, a possible overlooked contributor to pain and testicular symptoms. The chiropractic manipulation may have the potential to alleviate this issue by releasing compression of the original nociceptive stimulus. More research to better clarify the potential role of chiropractic manipulation are warranted.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Consent for Publication: Written informed consent for publication were obtained from the patient.
ORCID iD: Eric Chun Pu Chu  https://orcid.org/0000-0002-0893-556X
https://orcid.org/0000-0002-0893-556X
References
- Alexander C. E., Varacallo M. (2020). Lumbosacral radiculopathy. In: StatPearls, StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK430837/ [PubMed] [Google Scholar]
- Arendt-Nielsen L., Svensson P. (2001). Referred muscle pain: Basic and clinical findings. Clinical Journal of Pain, 17(1), 11–19. doi: 10.1097/00002508-200103000-00003. [DOI] [PubMed] [Google Scholar]
- Asadian L., Haddadi K., Zare A. (2016). Upper lumbar disk herniation presenting as chronic abdominal and scrotal pain: A case report. Neurosurgery Quarterly, 26(2), 177–179. doi: 10.1097/WNQ.0000000000000162 [DOI] [Google Scholar]
- Chaban V. V. (2010). Peripheral sensitization of sensory neurons. Ethnicity & Disease, 20(1 Suppl 1), S1-3-6. PMID:20521376 [PMC free article] [PubMed] [Google Scholar]
- Chu E. C. P. (2020). Taming of the testicular pain complicating lumbar disc herniation with spinal manipulation. American Journal of Men’s Health, 14(4), 1557988320949358. doi: 10.1177/1557988320949358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer G. D. (2014). The lumbar region. In: Cramer G. D., Darby S. A, Clinical Anatomy of the Spine, Spinal Cord, and ANS (3rd ed., pp. 246–311). Elsevier. 10.1016/B978-0-323-07954-9.00007-4 [DOI] [Google Scholar]
- Das G., Roy C. (2018). Rami communicans fibers in discogenic low back pain: The controversies. Indian Journal of Pain, 32(2), 60-62. doi: 10.4103/ijpn.ijpn_48_18 [DOI] [Google Scholar]
- Doubleday K. L., Kulig K., Landel R. (2003). Treatment of testicular pain using conservative management of the thoracolumbar spine: A case report. Archives of Physical Medicine Rehabilitation, 84(12), 1903–1905. doi: 10.1016/s0003-9993(03)00283-1 [DOI] [PubMed] [Google Scholar]
- Dubový P. (2011). Wallerian degeneration and peripheral nerve conditions for both axonal regeneration and neuropathic pain induction. Annals of Anatomy, 193(4), 267-275. doi: 10.1016/j.aanat.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Edgar M. A. (2007). The nerve supply of the lumbar intervertebral disc. Journal of Bone Joint Surgery, 89B(9), 1135-9. doi: 10.1302/0301-620X.89B9.18939 [DOI] [PubMed] [Google Scholar]
- Jinkins J. R. (2004). The anatomic and physiologic basis of local, referred and radiating lumbosacral pain syndromes related to disease of the spine. Journal of Neuroradiology, 31(3), 163-180. doi: 10.1016/s0150-9861(04)96988-x [DOI] [PubMed] [Google Scholar]
- Leone J. E., Middleton S. (2016). Nontraumatic testicular pain due to sacroiliac-joint dysfunction: A case report. Journal of Athletic Training, 51(8), 651-657. doi: 10.4085/1062-6050-51.10.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie S. W., Sajjad H., Siref L. E. (2020). Chronic testicular pain, orchialgia. In: StatPearls, StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK482481/ [PubMed] [Google Scholar]
- Levine L. A., Abdelsayed G. A. (2018). Chronic scrotal content pain: A diagnostic and treatment dilemma. Journal of Sexual Medicine, 15(9), 1212–1215. doi: 10.1016/j.jsexm.2018.07.008 [DOI] [PubMed] [Google Scholar]
- Murata Y., Kato Y., Miyamoto K., Takahashi T. (2009). Clinical study of low back pain and radicular pain pathways by using L2 spinal nerve root infiltration. A randomized, controlled, clinical trial. Spine, 34(19), 2008–2013. doi: 10.1097/BRS.0b013e3181b1fb96 [DOI] [PubMed] [Google Scholar]
- Neff S. M., Warnecke R. (2017). Chiropractic management of low back pain and testicle pain: A case report. Journal of the Academy of Chiropractic Orthopedists, 14(3), 36–41. [Google Scholar]
- Parekattil S. J., Ergun O., Gudeloglu A. (2020). Management of chronic orchialgia: Challenges and solutions– The current standard of care. Research and Reports in Urology, 2020, 199–210. doi: 10.2147/RRU.S198785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A. P. (2017). Anatomy and physiology of chronic scrotal pain. Translational Andrology and Urology, 6(Suppl 1), S51–S56. doi: 10.21037/tau.2017.05.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng B., Li D., Pang X. (2014). Degenerative lumbar spondylolisthesis with testicular pain. Pain Medicine, 15(1), 169–170. doi: 10.1111/pme.12246. [DOI] [PubMed] [Google Scholar]
- Rowell R. M., Rylander S. J. (2012). Low-back pain, leg pain, and chronic idiopathic testicular pain treated with chiropractic care. Journal of Alternative and Complementary Medicine, 18(4), 420–422. doi: 10.1089/acm.2010.0698 [DOI] [PubMed] [Google Scholar]
- Sanvictores T., Tadi P. (2020). Neuroanatomy, autonomic nervous system visceral afferent fibers and pain. In: StatPearls. StatPearls Publishing. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560843/. [PubMed] [Google Scholar]
- Shayota B., Wong T.L., Fru D., David G., Iwanaga J., Loukas M., Tubbs R. S. (2019). A comprehensive review of the sinuvertebral nerve with clinical applications. Anatomy & Cell Biology, 52(2), 128-133. doi: 10.5115/acb.2019.52.2.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigalos J. T., Pastuszak A.W. (2017). Chronic orchialgia: Epidemiology, diagnosis and evaluation. Translational Androloy and Urology, 6(Suppl 1), S37-S43. doi: 10.21037/tau.2017.05.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikandar S., Sommer C. (2019). Neurotrophins, cytokines, and pain. In: Wood J. N., (ed.), The Oxford Handbook of the Neurobiology of Pain. Oxford University Press. doi: 10.1093/oxfordhb/9780190860509.013.25 [DOI] [Google Scholar]
- Smith K. M., Browne T. J., Davis O. C., Coyle A., Boyle K. A., Watanabe M., Graham B. A. (2019). Calretinin positive neuron from an excitatory amplifier network in the spinal cord dorsal horn. eLife, 8, e49190. doi: 10.7554/eLife.49190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. P., Costabile R. A. (2017). Chronic orchialgia. Translational Androloy and Urology, 6(Suppl 1), S1. doi: 10.21037/tau.2017.05.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen I. G., Jacobsen P., Gyntelberg F., Suadicani P. (2011). Occupational and other predictors of herniated lumbar disc disease- A 33-year follow-up in the Copenhagen male study. Spine, 36(19), 1541–1546. doi: 10.1097/BRS.0b013e3181f9b8d4 [DOI] [PubMed] [Google Scholar]
- Tan W. P., Levine L. A. (2017). What can we do for chronic scrotal content pain? World Journal of Men’s Health, 35(3), 146–155. 10.5534/wjmh.17047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouda E. J., Leenstra S., Vanneste J. A. (2005). Scrotal pain as the presenting symptom of lumbar disc herniation: A report of 2 cases. Spine, 30(2), E47–E49. doi: 10.1097/01.brs.0000150633.36777.c8 [DOI] [PubMed] [Google Scholar]