Abstract
Background:
The relative importance of predictive factors for advanced non-small cell lung cancer (NSCLC) patients on epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor (TKI) treatment remains unclear.
Materials and methods:
We retrospectively enrolled advanced NSCLC patients with single first-generation EGFR-TKI treatment for ⩾5 years (Y) in Taiwan. Clinical data was collected and compared with those of another cohort with single first-line EGFR-TKI treatment for <5 Y. Plasma cell-free DNA (cfDNA) samples were collected from patient subsets, pre- and post-TKI, in the >5 Y group.
Results:
Overall, 128 and 278 patients were enrolled in the ⩾5 Y and <5 Y groups, respectively. Significant factors in the multivariate analysis of patients’ characteristics including Eastern Cooperative Oncology Group performance status 0–1, postoperative recurrence, without brain metastasis, oligometastasis (each score of 2), female sex, erlotinib use, and without bone metastasis (each score of 1), were incorporated into a risk scoring system. The area under the receiver operating characteristic curve was 0.82 [95% confidence interval (CI): 0.78–0.86]. Of the plasma cfDNA samples from 33 patients in the ⩾5 Y group, only 1 had a T790M in 25 patients without progressive disease. In 27 patients with single agent use for ⩾96 months, 22 (81.5%) received local treatment (surgery or radiotherapy) for the primary lung tumor before and during TKI treatment.
Conclusion:
For NSCLC patients with single first-generation EGFR-TKI use for ⩾5 Y, factors with different relative importance exist and the risk-scoring model is feasible with modest accuracy. The role of local treatment for primary tumors in patients with long-term TKI use requires further investigation.
Keywords: epidermal growth factor receptor, local treatment, more than five years, non-small cell lung cancer, scoring model, tyrosine kinase inhibitor
Introduction
The discovery of epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs) has resulted in a dramatic change in the treatment strategy of advanced EGFR-mutant non-small cell lung cancer (NSCLC) patients.1,2 Although most EGFR-mutant NSCLC patients initially respond to gefitinib, erlotinib, and afatinib, patients usually develop a progressive disease within 1 year after the initiation of treatment.3 However, a subgroup of patients experience a longer clinical benefit from EGFR-TKIs. There are few reports about this phenomenon. In a recent study, treatment with afatinib was reported to provide a significantly higher 24-month progression-free survival (PFS) rate than gefitinib in a first-line setting (17.6% versus 7.6%).4 Some case studies report that complete remission with EGFR-TKI treatment was maintained for more than 2 years.5–8 However, the real predictive factors for single EGFR-TKI long-term use in advanced EGFR-mutant NSCLC patients remains unclear. Therefore, we investigated the relative importance of predictive factors by studying the differences in the characteristics between patients with single EGFR-TKI use for ⩾5 years (⩾5 Y) and patients with <5 years (<5 Y) use.
Materials and methods
Patients
We retrospectively enrolled advanced NSCLC patients with single EGFR-TKI use for ⩾5 Y in nine medical centers in Taiwan under the ‘TKI Prolonged Single Agent Use over 5 Years’ (TIPS-5) study from September 2005 to September 2014. To be eligible for the study, patients were required to have cytological- or pathological-confirmed NSCLC, at inoperable advanced stage IIIB to stage IV, to have undergone single EGFR-TKI treatment for ⩾5 Y without progressive disease (PFS of EGFR-TKI ⩾5 Y), and a clear survival status during follow-up. Patients were excluded if they had undergone EGFR-TKI treatment for ⩾5 Y due to treatment beyond progression, another active malignancy, concomitant use of another anticancer medicine during the period of EGFR-TKI use, or if they had Eastern Cooperative Oncology Group performance status (ECOG PS) 4.
After forming the cohort of eligible patients with single EGFR-TKI use for ⩾5 Y, we enrolled another cohort with advanced NSCLC patients with first-line single EGFR-TKI use for <5 Y (PFS of EGFR-TKI < 5 Y) from January 2011 to June 2014 from the Taichung Veterans General Hospital. The development and validation of a prognostic risk score model was performed for the NSCLC patients with single EGFR-TKI use for ⩾5 Y. Another independent group of NSCLC patients who met the study criteria were enrolled as the validation cohort for the risk score model established for EGFR-TKI use for ⩾5 Y from February 2012 to August 2015 from the National Taiwan University Hospital. The study was conducted ethically in accordance with the World Medical Association Declaration of Helsinki, and was approved by the Institutional Review Board (IRB) of each hospital (Supplemental Table A.1). The informed consent documents for clinical data records and genetic testing were written by patients or were waived according to the requirement of each IRB.
Data records
The patients’ clinical characteristic and demographic data, including age, sex, family history, tumor stage, smoking status, ECOG PS, baseline EGFR mutation status, treatment history, metastatic site, and outcome variables were collected for analysis. We defined oligometastasis as no more than five metastatic lesions. The lung cancer tumor-node-metastasis staging was conducted according to the 8th edition of the American Joint Committee on Cancer staging system.9 Computed tomography of the chest was performed every 3 months for National Health Insurance reimbursement. The treatment response of EGFR-TKIs was confirmed using the Response Evaluation Criteria in Solid Tumors (Version 1.1).10
EGFR mutation test for tumor tissue
The methods of EGFR mutation-detection in tumor tissues of the patients in the single EGFR-TKI use for ⩾5 Y group were different in each hospital. The methods used for EGFR testing included direct sequencing, mutant type-specific sensitive methods [e.g., matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS)], the scorpion amplification refractory mutation system (ARMS), and Cobas EGFR Mutation Test. For the patients with single EGFR-TKI use for <5 Y, the EGFR mutation detection method was MALDI-TOF MS, performed as previously described.11 A rebiopsy of some patients in both groups after disease progression was conducted.
EGFR mutation detection of liquid biopsy in pre- and post- EGFR-TKI treatment plasma samples
A subset of patients in the single EGFR-TKI use for ⩾5 Y group had available pre- and post-EGFR-TKI treatment plasma samples. The post EGFR-TKI treatment plasma samples were collected, close to the latest follow-up dates in patients without progression or the dates of disease progression.
Plasma cell-free DNA (cfDNA) extraction was performed according to our previous study.12 EGFR mutation-detection including L858R, exon19 deletion, and T790M, was performed based on our previous studies with modifications.12–14 Briefly, the combination of peptide nucleic acid (PNA) and MALDI-TOF nucleotide mass spectrometry was utilized for experiments in the ISO15189-certified clinical center laboratory. PNA oligos used to specifically lock the wild-type allele were synthesized using PanaGene (Daejeon, Korea). All nucleotide MALDI-TOF MS assays were adapted from the MassARRAY System (Agena Bioscience, USA) according to the manufacturer’s instructions. Data were collected and analyzed using Typer 4 software (Agena Biosciences, USA). The tests were conducted at the National Center of Excellence for Clinical Trial and Research of the National Taiwan University Hospital.
Statistical methods
Univariate analysis using the Fisher’s exact test was performed to assess the differences in the characteristics between both groups. Logistic regression models were used to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) of the PFS of lung cancer for each variable. The variables showing significant associations with the 5-year PFS (p < 0.05) in the univariate analyses were included in a stepwise selection procedure to generate a final multiple model. The full model was used to develop a risk-scoring system for the prediction of the probability of PFS of lung cancer. The regression coefficients of the variables were converted into an integer risk score by rounding up the quotient of the regression coefficient by a single constant. The constant was the sex regression coefficient, defining the integer risk score for female sex to be one. Risk estimates for the 5-year PFS of lung cancer according to the sum of scores could be calculated.15 To evaluate the predictive accuracy of the risk score model, the receiver operating characteristic (ROC) curve was derived and the area under the ROC curve (AUROC) was calculated. All the statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). Two-tailed tests and p values < 0.05 for significance were used.
Results
Patients’ characteristics and demographic data
A total of 128 patients were enrolled in the ⩾5 Y use group, and 278 patients in the <5 Y use group. The baseline characteristics are shown in Table 1. A total of two hundred and one (49.5%) patients were ⩾65 years old, while two hundred and fifty-two patients were female (62.1%). A total of sixteen patients had stage IIIB to IIIC disease, and three hundred and eight patients had stage IV disease. The number of patients who had postoperative recurrence was eighty-two, while five patients had recurrence after concurrent chemoradiotherapy (CCRT). After the initial response to EGFR-TKI, radiotherapy for the primary tumor lesion only including stereotactic surgery was done for nine patients (three with oligometastasis and six with non-oligo-metastasis; eight in the ⩾5 Y use group). The characteristics of these nine patients are listed in Supplemental Table A.2. A total of one hundred and thirty-five patients had oligometastatic lesions (33.3%). The metastatic sites were the lungs (54.4%), brain (27.6%), bone (38.2%), liver (7.9%), and adrenal glands (3.0%).
Table 1.
Univariate analysis of factors associated with 5-year progression-free survival of lung cancer.
| Total | Progression-free survival | p-value | OR | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|
| Cases (n = 128) ⩾5 years, n (%) | Controls (n = 278) <5 years, n (%) | ||||||
| Age (years) | |||||||
| ⩾65 | 201 | 61 (47.66) | 140 (50.36) | 0.6127 | 1.00 | ||
| <65 | 205 | 67 (52.34) | 138 (49.64) | 1.114 | 0.733–1.694 | 0.6128 | |
| Sex | |||||||
| Male | 154 | 34 (26.56) | 120 (43.17) | 0.0014 | 1.00 | ||
| Female | 252 | 94 (73.44) | 158 (56.83) | 2.100 | 1.328–3.321 | 0.0015 | |
| Smoking | |||||||
| Current or former | 107 | 25 (19.53) | 82 (29.50) | 0.0342 | 1.00 | ||
| Never | 299 | 103 (80.47) | 196 (70.50) | 1.723 | 1.038–2.862 | 0.0355 | |
| Family history | |||||||
| No | 293 | 102 (87.93) | 191 (91.83) | 0.2531 | 1.00 | ||
| Yes | 31 | 14 (12.07) | 17 (8.17) | 1.542 | 0.731–3.255 | 0.2558 | |
| ECOG PS | |||||||
| 2–3 | 70 | 5 (3.91) | 65 (23.38) | <0.0001 | 1.00 | ||
| 0–1 | 336 | 123 (96.09) | 213 (76.62) | 7.507 | 2.943–19.146 | <0.0001 | |
| EGFR mutation | |||||||
| Exon 19 deletion | 170 | 43 (48.86) | 127 (46.86) | 0.7440 | 1.00 | ||
| Exon 21 L858R | 189 | 45 (51.14) | 144 (53.14) | 0.923 | 0.570–1.493 | 0.7441 | |
| EGFR-TKI | |||||||
| Gefitinib | 303 | 85 (66.41) | 218 (78.42) | 0.0098 | 1.00 | ||
| Erlotinib | 103 | 43 (33.59) | 60 (21.58) | 1.838 | 1.155–2.926 | 0.0103 | |
| Postoperative recurrence | |||||||
| No | 324 | 75 (58.59) | 249 (89.57) | <0.0001 | 1.00 | ||
| Yes | 82 | 53 (41.41) | 29 (10.43) | 6.068 | 3.603–10.218 | <0.0001 | |
| Oligo metastatic lesions or not (⩽5) | |||||||
| No | 271 | 53 (41.41) | 218 (78.42) | <0.0001 | 1.00 | ||
| Yes | 135 | 75 (58.59) | 60 (21.58) | 5.142 | 3.268–8.089 | <0.0001 | |
| Brain metastasis | |||||||
| Yes | 112 | 21 (16.41) | 91 (32.73) | 0.0006 | 1.00 | ||
| No | 294 | 107 (83.59) | 187 (67.27) | 2.479 | 1.459–4.215 | 0.0008 | |
| Lung metastasis | |||||||
| Yes | 225 | 74 (57.81) | 151 (54.32) | 0.5102 | 1.00 | ||
| No | 181 | 54 (42.19) | 127 (45.68) | 0.868 | 0.568–1.324 | 0.5104 | |
| Liver metastasis | |||||||
| Yes | 32 | 3 (2.34) | 29 (10.43) | 0.0050 | 1.00 | ||
| No | 374 | 125 (97.66) | 249 (89.57) | 4.853 | 1.450–16.240 | 0.0104 | |
| Bone metastasis | |||||||
| Yes | 155 | 27 (21.09) | 128 (46.04) | <0.0001 | 1.00 | ||
| No | 251 | 101 (78.91) | 105 (53.96) | 3.192 | 1.964–5.188 | <0.0001 | |
| Adrenal gland metastasis | |||||||
| Yes | 12 | 1 (0.78) | 11 (3.96) | 0.0792 | 1.00 | ||
| No | 394 | 127 (99.22) | 267 (96.04) | 5.232 | 0.668–40.969 | 0.1150 | |
ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor.
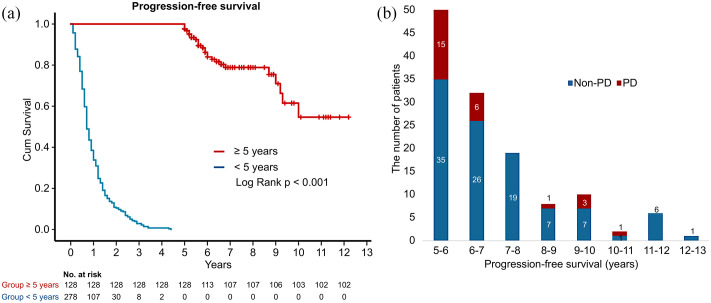
The Kaplan–Meier curves of the PFS of both groups are shown in Figure 1a. The PFS distribution in the ⩾5 Y use group is shown in Figure 1b. There were 27 patients with single-agent use for ⩾96 months, amongst whom, 22 (81.5%) were administered a local treatment for the primary lung tumor (16 with postoperative recurrence, two with CCRT recurrence, and four with radiotherapy) during treatment with TKIs.
Figure 1.
The PFS of the ⩾5 year and the <5 year use group. (a) The difference between the ⩾5 year and the <5 year use groups in Kaplan–Meier curve of PFS (b) The distribution of PFS in the ⩾5 year use group.
Cum survival, cumulative survival; PFS, progression-free survival; PD, progressive disease.
Univariate analysis of the clinical factors showed a significantly greater prevalence of the female sex, never-smokers, PS 0-1, erlotinib use, postoperative recurrence, and oligometastases (⩽5), but a lower prevalence of brain, liver, and bone metastases in the ⩾5 Y use group. There were no differences in age, family history, EGFR mutation subtypes, lung-to-lung, and adrenal metastases (Table 1).
In the multivariate analysis, female sex, ECOG PS 0-1, erlotinib use, postoperative recurrence, no brain metastasis, no bone metastasis, and with oligometastatic lesions (⩽5) showed a greater prevalence in the ⩾5 Y use group. There were no differences in never-smokers and liver metastasis (Table 2).
Table 2.
Multivariate analysis of factors associated with 5-year progression-free survival of lung cancer.
| Multivariate model (stepwise selection) | |||
|---|---|---|---|
| OR | 95% CI | p-value | |
| Sex | |||
| Male | 1.00 | ||
| Female | 2.223 | 1.294–3.817 | 0.0038 |
| ECOG PS | |||
| 2–3 | 1.00 | ||
| 0–1 | 5.201 | 1.914–14.131 | 0.0012 |
| EGFR-TKI | |||
| Gefitinib | 1.00 | ||
| Erlotinib | 1.989 | 1.136–3.484 | 0.0161 |
| Postoperative recurrence | |||
| No | 1.00 | ||
| Yes | 3.401 | 1.862–6.212 | <0.0001 |
| Oligo-metastatic lesions (⩽5) | |||
| No | 1.00 | ||
| Yes | 3.605 | 2.141–6.071 | <0.0001 |
| Brain metastasis | |||
| Yes | 1.00 | ||
| No | 3.400 | 1.794–6.446 | 0.0002 |
| Bone metastasis | |||
| Yes | 1.00 | ||
| No | 2.110 | 1.204–3.695 | 0.0090 |
ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor.
Risk score model of TKI use for more than 5 years
For the survey of the relative importance of each predictive factor, the risk scores of each factor for the 5-year EGFR-TKI single agent use of lung cancer are shown in Table 3. Predictors for EGFR-TKI use for ⩾5 Y in the derivation dataset were ECOG PS 0-1, postoperative recurrence, the absence of brain metastasis, and oligometastasis (each with a corresponding score of 2); female sex, erlotinib use, and without bone metastasis (each with a corresponding score of 1).
Table 3.
Risk scores of each factor for the 5-year progression-free survival of lung cancer.
| Multivariate model (stepwise selection) | |||
|---|---|---|---|
| Regression coefficient | p-value | Score | |
| Intercept | −1.3724 | <0.0001 | |
| Sex | |||
| Male | 0 | ||
| Female | 0.3994 | 0.0038 | 1 |
| ECOG PS | |||
| 2–3 | 0 | ||
| 0–1 | 0.8244 | 0.0012 | 2 |
| EGFR-TKI | |||
| Gefitinib | 0 | ||
| Erlotinib | 0.3439 | 0.0161 | 1 |
| Postoperative recurrence | |||
| No | 0 | ||
| Yes | 0.6120 | <0.0001 | 2 |
| Brain metastasis | |||
| Yes | 0 | ||
| No | 0.6120 | 0.0002 | 2 |
| Oligo-metastatic lesions or not (⩽5) | |||
| No | 0 | ||
| Yes | 0.6412 | <0.0001 | 2 |
| Bone metastasis | |||
| Yes | 0 | ||
| No | 0.3732 | 0.0090 | 1 |
ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor.
The nomogram of the multivariate model of the risk scores is shown in Figure 2. The chances of ⩾5 Y EGFR-TKI single-agent use in lung cancer patients ranged from 20% (score 0) to 95% (score 11). We used the ROC curve and the AUROCs to evaluate the predictive accuracy of the risk score model. The risk score model in the study using each factor had an AUROC of 0.82 (95% CI: 0.78–0.86, Supplemental Figure A.1A).
Figure 2.
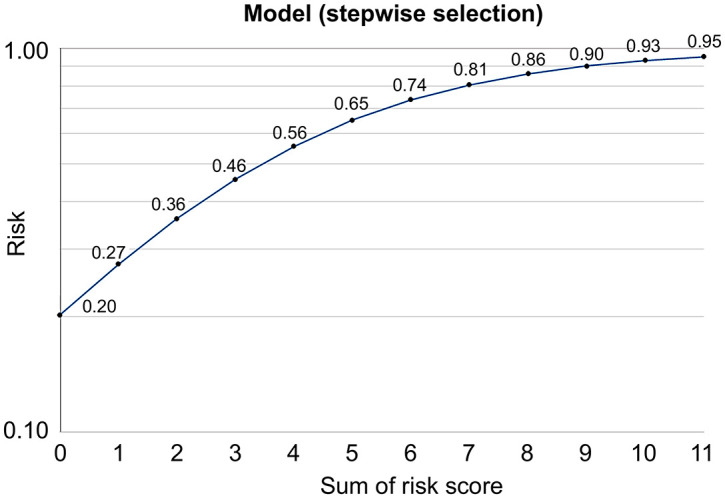
Nomogram of the multivariate model of the risk scores. The nomogram of the multivariate model of the risk scores were constructed to predict the chances of PFS ⩾5 years with EGFR-TKIs treatment in non-small cell lung cancer patients.
EGFR-TKI, epidermal growth factor receptor-tyrosine kinase inhibitors; PFS, progression-free survival.
The baseline characteristics of the validation cohort are shown in Supplemental Table A.3. A total of 177 patients were enrolled: 11 in the ⩾5 Y use group, and 166 in the <5 Y use group. A total of eighty-two (46.3%) patients were ⩾65 years old, while one hundred and twenty-three patients were female (69.5%). Six patients had stage IIIB to IIIFC disease, and one hundred and thirty-nine patients had stage IV disease. Thirty-two patients (18.1%) had postoperative recurrence. Fifty-six patients had oligometastatic lesions (31.6%). The proportions of the brain, lung, liver, bone, and adrenal gland metastases were 68.9%, 42.4%, 10.2%, 41.2%, and 8.5%, respectively. There were no patients with recurrence after CCRT or radiotherapy only for the primary tumor lesion after the response to EGFR-TKIs. When we applied the risk score model from the discovery to validation dataset, the predictive performance was AUROC 0.75 (95% CI: 0.64–0.86, Supplemental Figure A.1B).
EGFR mutation status
In the discovery dataset, the EGFR mutation test was not conducted in 30 patients in the ⩾5 Y use group, largely because the EGFR mutation test was previously not available in Taiwan. A total of one hundred and seventy patients harbored the exon 19 deletion mutation, 189 patients harbored the exon 21 L858R point mutation, and 15 patients had other mutations, including mixed mutations. There were two patients with undetected EGFR mutation.
In the ⩾5 Y use group, 26 patients had progressive disease after EGFR-TKIs treatment. Among them, eight patients underwent tissue rebiopsy, and T790M was detected in four patients. In the <5 Y use group, 72 patients underwent tissue rebiopsy, and 36 patients had T790M mutation (50%). The T790M mutation rates among patients with EGFR exon 19 deletion mutation, exon 21 L858R point mutation, and other mutations were 56.8%, 40.7%, and 0%, respectively (Supplemental Figure A.2).
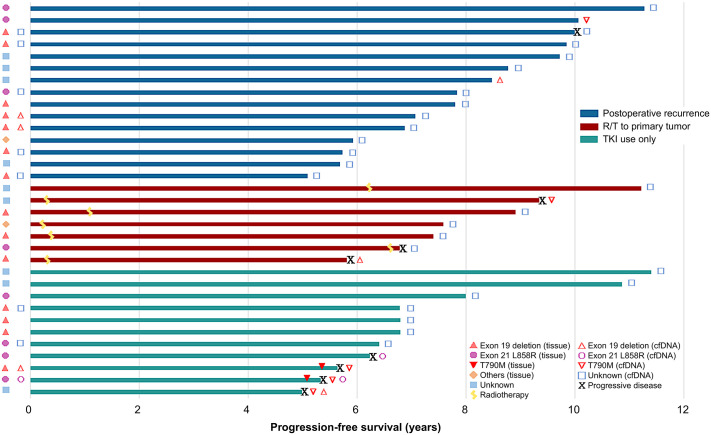
Regarding the cfDNA analysis in the group with ⩾5 Y single agent EGFR-TKI use, there were 33 patients with post-EGFR-TKI treatment plasma samples collected close to the dates of latest follow-up in patients without progression or the dates of disease progression (Figure 3). Among these 33 patients, 11 (33%) patients with pre-EGFR-TKI plasma samples were analyzed; seven had non-shedding tumors and four had shedding tumors (three with exon 19 deletion and one with L858R). Of these 33 patients with post-TKI plasma samples, 24 (72.7%) had a tumor tissue biopsy available for analysis before EGFR-TKI treatment; 14 had an exon 19 deletion, eight had L858R, and two had other mutations. There were nine patients who were not tested for EGFR mutation due to unavailability of the test.
Figure 3.
The swimmer plot for PFS in 33 patients of the ⩾5 year use group. The detailed data included treatment PFS, pre-treatment and post-treatment EGFR subtype of cfDNA and tissue biopsy, and the status of progressive disease.
cfDNA, circulating free DNA; EGFR, epidermal growth factor receptor; PFS, progression-free survival.
Of the 33 patients with post-EGFR-TKI treatment plasma samples for analysis, two were positive for EGFR mutation among 25 (75.8%) patients without progressive disease who were still under treatment with TKIs; one patient harbored T790M, and the other had EGFR exon 19 deletion. Eight (24.2%) patients had progressive disease under TKIs treatment, and T790M mutation was detected in four of them, including two with T790M alone and two with combined exon 19 deletion and L858R (Figure 3).
Discussion
Advanced NSCLC patients who were administered single first-generation EGFR-TKIs for ⩾5 Y without disease progression were uncommon. From this study, predictive factors with different relative importance exist; with ECOG PS 0-1, postoperative recurrence, without brain metastasis, and oligometastasis (each with a corresponding score of 2); and female sex, erlotinib use, and without bone metastasis (each with a corresponding score of 1). A risk-score model for EGFR-TKI ⩾5 Y use was established and verified in the validation dataset. In addition, local treatment of the primary tumor, including surgery, CCRT, and radiotherapy during EGFR-TKI use may provide further clinical benefit. From the cfDNA data, either shedding or non-shedding tumors can have a good outcome with long-term TKI use. For those patients with persistent disease control, most of them were without detectable EGFR mutation in plasma.
Several factors, such as female sex, good PS, oligometastasis, absence of brain metastasis, etc., are all well-known important prognostic factors for EGFR-mutant lung cancer patients treated with EGFR-TKIs. However, the relative importance of these factors has not been elucidated. As disease progression occurs about 1 year after the initiation of first-generation EGFR-TKIs in EGFR-mutant patients, an exaggerated emphasis on prolonged single EGFR-TKI use for ⩾5 Y could differentiate the relative importance of each factor clearer, especially through the risk score model.
The effect of local treatment of the primary lung tumor in advanced EGFR-mutant patients treated with EGFR-TKIs remains unclear. In a randomized phase III trial (ClinicalTrial.gov identifier: WJTOG 3405) that compared gefitinib with chemotherapy in patients with advanced EGFR-mutant NSCLC, postoperative recurrence was the only independent prognostic factor for overall survival (OS) analysis and was statistically better than de novo stage IIIB/IV disease. Using the Cox proportional hazards model, the hazard ratio (HR) was 0.459 (95% CI 0.312–0.673, p < 0.001).16 The OS of recurrent metastatic disease after treatment with curative intent may be better than that of de novo metastatic disease, as shown in several retrospective studies.17–20 In our study, besides postoperative recurrence, five patients had recurrence after CCRT for curative intent in the ⩾5 Y group.
In a study by Al-Halabi et al.,21 the initial progression of EGFR-TKI-treated cancers occurred in about half of the original disease sites, with-or-without distant metastasis. There were reports of survival improvement with radiotherapy for the primary and oligometastatic sites in EGFR-TKI-treated lung cancer patients.22,23 Furthermore, two reports found that primary tumor resection improved survival in stage IV NSCLC patients in the Surveillance, Epidemiology, and End Results database.24,25 Therefore, concerning the effect of the local treatment of primary tumors, consolidation stereotactic body radiation therapy (SBRT) and curative dose of radiotherapy only to the primary tumor in a subset of patients following TKI response seemed feasible. Here, there were nine patients who received radiotherapy, including SBRT for the primary tumor lesion after the initial response to EGFR-TKIs (eight patients in the ⩾5 Y use group and one patient in the <5 Y use group) even in the non-oligometastatic disease. Furthermore, 22 (81.5%) out of 27 patients with single agent use for ⩾96 months in the ⩾5 Y use group received a local treatment for the primary lung tumor. Regardless of the recurrence after operation or CCRT, or radiotherapy to the primary tumor during the response to EGFR-TKI use, a lower tumor burden could be one of the possible explanations for this difference in prognosis for local treatment to the primary lung tumor. Another hypothesis could be that after the primary tumor eradication, immune competence is restored, even if a metastatic tumor is present.26 More prospective studies are needed to investigate the effect of local treatment for primary tumors and its association with long-term EGFR-TKI use.
In the present study, there was no difference in the use of EGFR-TKIs for ⩾5 Y between patients with exon 19 deletion and those with L858R. However, the association between EGFR exon 19 deletions and prolonged survival in patients with advanced NSCLC treated with EGFR-TKIs has been reported in several studies.27–31 The phenomenon may be partly explained by more co-occurring mutations in patients with L858R mutation.32,33 The occurrence of concomitant mutations in patients with lung cancer using EGFR-TKIs for ⩾5 Y with sensitizing EGFR mutations needs to be investigated.
In this study, the risk score model using each factor had an AUROC of 0.82 (95% CI: 0.78–0.86) in the discovery dataset. It was a good tool for the prediction in patients with first-generation EGFR-TKI single agent use of more than 5 years. In the validation dataset, there were no patients with recurrence after CCRT or radiotherapy only for the primary tumor lesion after the response to EGFR-TKIs; this could reduce the predictive ability of our risk score model. However, even with this limitation, our risk score model still had a modest predictive ability (AUROC, 0.75). A larger sample size is needed in the future study.
According to the cfDNA data obtained in this study, EGFR mutation was detected in two out of 25 patients with persistent tumor control for ⩾5 Y. There is a need for further investigation as previous studies show that recurrences occur early in patients with detectable plasma EGFR mutation.34 In patients with disease progression, T790M was detected in 50% (4/8), compared with 36.9% in our previous study after treatment with first- and second-generation EGFR-TKIs.12
Our study had some limitations. Firstly, it was a retrospective study; therefore, it had more bias when compared with a prospective study. Secondly, all patients in this study were Taiwanese; therefore, our findings may not be generalizable to other ethnic populations. Thirdly, the cohort of the <5 Y TKI use group was from a single center, and the enrolment period was not the same as that of the ⩾5 Y TKI use group. Finally, in Taiwan, afatinib was reimbursed since May 2014, and osimertinib was reimbursed since April 2020. In the present study, we enrolled patients with single first-generation EGFR-TKI use for ⩾5 years. Therefore, few patients with afatinib or osimertinib as first-line treatment could be enrolled for analysis while we collected the data.
Conclusion
In conclusion, in advanced EGFR mutant NSCLC patients, predictive factors with different relative importance exist in single first-generation EGFR-TKI use for ⩾5 Y and were integrated into an established and verified risk-score model, which had a modest accuracy. According to the cfDNA data, both shedding and non-shedding tumors can have good outcomes with long-term TKI use. For patients with persistent disease control, most of them had an indetectable EGFR mutation in plasma. In addition, the role of local treatment for primary tumors in patients with long-term TKI use needs further investigation. Further prospective research is required to confirm these results.
Supplemental Material
Supplemental material, sj-pdf-1-tam-10.1177_17588359211018022 for The relative importance of predictive factors for single first-generation EGFR-TKI use for more than 5 years in patients with advanced non-small cell lung cancer: Taiwan multicenter TIPS-5 study by Yen-Hsiang Huang, Jen-Yu Hung, How-Wen Ko, Po-Lan Su, Chun-Liang Lai, Huang-Chih Chang, Te-Chun Hsia, Sheng-Hao Lin, Kuan-Li Wu, Cheng-Ta Yang, Wu-Chou Su, Yi-Chun Chu, Chin-Chou Wang, Wei-Yu Liao, Yen-Ting Lin, Ching-Hsiung Lin, Meng-Chih Lin, Kuo-Hsuan Hsu, Jeng-Sen Tseng, Tsung-Ying Yang, Kun-Chieh Chen, Mei-Hsuan Lee, Sung-Liang Yu, Chao-Chi Ho and Gee-Chen Chang in Therapeutic Advances in Medical Oncology
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Wei-Yu Liao  https://orcid.org/0000-0001-6383-3470
https://orcid.org/0000-0001-6383-3470
Kuo-Hsuan Hsu  https://orcid.org/0000-0002-8187-904X
https://orcid.org/0000-0002-8187-904X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Yen-Hsiang Huang, Division of Chest Medicine, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung; Institute of Biomedical Sciences, College of Life Sciences, National Chung Hsing University, Taichung.
Jen-Yu Hung, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung; Department of Internal Medicine, Kaohsiung Municipal Ta-Tung Hospital, Kaohsiung Medical University, Kaohsiung; Department of Internal Medicine, School of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung.
How-Wen Ko, Division of Lung Cancer and Interventional Bronchoscopy, Department of Thoracic Medicine, Linkou Chang Gung Memorial Hospital, Taoyuan.
Po-Lan Su, Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan.
Chun-Liang Lai, Division of Pulmonology and Critical Care, Department of Internal Medicine, Buddhist Dalin Tzu Chi Hospital, Chiayi; School of Medicine, Buddhist Tzu Chi University, Hualien; Institute of Molecular Biology Department of Life Science, National Chung Cheng University, Chiayi.
Huang-Chih Chang, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Kaohsiung; Graduate Institute of Clinical Medical Sciences, Chang Gung University, Taoyuan.
Te-Chun Hsia, Department of Respiratory Therapy, China Medical University, Taichung; Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, China Medical University Hospital, Taichung.
Sheng-Hao Lin, Division of Chest Medicine, Department of Internal Medicine, Changhua Christian Hospital, Changhua; Institute of Genomics and Bioinformatics, National Chung Hsing University, Taichung; Department of Recreation and Holistic Wellness, MingDao University, Changhua.
Kuan-Li Wu, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung; Department of Internal Medicine, Kaohsiung Municipal Ta-Tung Hospital, Kaohsiung Medical University, Kaohsiung; Department of Internal Medicine, School of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung; Graduate Institute of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung.
Cheng-Ta Yang, Division of Lung Cancer and Interventional Bronchoscopy, Department of Thoracic Medicine, Linkou Chang Gung Memorial Hospital, Taoyuan; Department of Respiratory Therapy, College of Medicine, Chang Gung University, Taoyuan.
Wu-Chou Su, Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan; Institute of Clinical Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan; Center of Applied Nanomedicine, National Cheng Kung University, Tainan.
Yi-Chun Chu, Division of Pulmonology and Critical Care, Department of Internal Medicine, Buddhist Dalin Tzu Chi Hospital, Chiayi.
Chin-Chou Wang, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Kaohsiung; Department of Respiratory Therapy, Kaohsiung Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Kaohsiung; Department of Respiratory Care, Chang Gung University of Science and Technology, Chiayi.
Wei-Yu Liao, Department of Internal Medicine, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei.
Yen-Ting Lin, Department of Internal Medicine, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei; Graduate Institute of Clinical Medicine, National Taiwan University College of Medicine, Taipei; Department of Medicine, National Taiwan University Cancer Center, Taipei.
Ching-Hsiung Lin, Division of Chest Medicine, Department of Internal Medicine, Changhua Christian Hospital, Changhua; Institute of Genomics and Bioinformatics, National Chung Hsing University, Taichung; Department of Recreation and Holistic Wellness, MingDao University, Changhua.
Meng-Chih Lin, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Kaohsiung; Department of Respiratory Therapy, Kaohsiung Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Kaohsiung.
Kuo-Hsuan Hsu, Division of Critical Care and Respiratory Therapy, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung.
Jeng-Sen Tseng, Division of Chest Medicine, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung; Institute of Biomedical Sciences, College of Life Sciences, National Chung Hsing University, Taichung; Faculty of Medicine, School of Medicine, National Yang-Ming University, Taipei.
Tsung-Ying Yang, Division of Chest Medicine, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung; Faculty of Medicine, School of Medicine, National Yang-Ming University, Taipei.
Kun-Chieh Chen, Division of Pulmonary Medicine, Department of Internal Medicine, Chung Shan Medical University Hospital, Taichung; Institute of Medicine, Chung Shan Medical University, Taichung; School of Medicine, Chung Shan Medical University, Taichung.
Mei-Hsuan Lee, Institute of Clinical Medicine, National Yang-Ming University, Taipei.
Sung-Liang Yu, Graduate Institute of Clinical Medicine, National Taiwan University College of Medicine, Taipei; Department of Clinical Laboratory Sciences and Medical Biotechnology, College of Medicine, National Taiwan University, Taipei; Centers of Genomic and Precision Medicine, National Taiwan University, Taipei; Department of Laboratory Medicine, National Taiwan University Hospital, Taipei; Institute of Medical Device and Imaging, College of Medicine, National Taiwan University, Taipei; Graduate Institute of Pathology, College of Medicine, National Taiwan University, Taipei.
Chao-Chi Ho, Department of Internal Medicine, National Taiwan University Hospital and National Taiwan University College of Medicine, No.7, Chung Shan South Road, Taipei, 100.
Gee-Chen Chang, Division of Pulmonary Medicine, Department of Internal Medicine, Chung Shan Medical University Hospital, No.110, Sec. 1, Jianguo N. Road, Taichung, 402; Institute of Biomedical Sciences, College of Life Sciences, National Chung Hsing University, Taichung; Faculty of Medicine, School of Medicine, National Yang-Ming University, Taipei; Institute of Medicine, Chung Shan Medical University, Taichung; School of Medicine, Chung Shan Medical University, Taichung.
References
- 1. Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004; 350: 2129–2139. [DOI] [PubMed] [Google Scholar]
- 2. Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004; 304: 1497–1500. [DOI] [PubMed] [Google Scholar]
- 3. Zhou C, Yao LD. Strategies to improve outcomes of patients with EGRF-mutant non-small cell lung cancer: review of the literature. J Thorac Oncol 2016; 11: 174–186. [DOI] [PubMed] [Google Scholar]
- 4. Park K, Tan EH, O’Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 2016; 17: 577–589. [DOI] [PubMed] [Google Scholar]
- 5. Gottschling S, Herpel E, Eberhardt WE, et al. The gefitinib long-term responder (LTR)-a cancer stem-like cell story? Insights from molecular analyses of German long-term responders treated in the IRESSA expanded access program (EAP). Lung Cancer 2012; 77: 183–191. [DOI] [PubMed] [Google Scholar]
- 6. Nakatomi K, Soda H, Kitazaki T, et al. Long-term survival in three patients with metastatic non-small cell lung cancer treated with gefitinib. Lung Cancer 2006; 52: 253–255. [DOI] [PubMed] [Google Scholar]
- 7. Jovanovic D, Stevic R, Velinovic M, et al. Durable complete remission of poor performance status metastatic lung adenocarcinoma patient treated with second-line erlotinib: a case report. Onco Targets Ther 2017; 10: 4347–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weber B, Sorensen BS, Knap MM, et al. Complete pathologic response in lung tumors in two patients with metastatic non-small cell lung cancer treated with erlotinib. J Thorac Oncol 2011; 6: 1946–1949. [DOI] [PubMed] [Google Scholar]
- 9. Amin MB, Edge SB, Greene FL, et al. (eds). AJCC cancer staging manual. 8th ed. New York, NY: Springer, 2017. [Google Scholar]
- 10. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 11. Hsu KH, Ho CC, Hsia TC, et al. Identification of five driver gene mutations in patients with treatment-naive lung adenocarcinoma in Taiwan. PLoS One 2015; 10: e0120852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Su KY, Tseng JS, Liao KM, et al. Mutational monitoring of EGFR T790M in cfDNA for clinical outcome prediction in EGFR-mutant lung adenocarcinoma. PLoS One 2018; 13: e0207001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Su KY, Kao JT, Ho BC, et al. Implementation and quality control of lung cancer EGFR genetic testing by MALDI-TOF mass spectrometry in Taiwan clinical practice. Sci Rep 2016; 6: 30944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Su KY, Chen HY, Li KC, et al. Pretreatment epidermal growth factor receptor (EGFR) T790M mutation predicts shorter EGFR tyrosine kinase inhibitor response duration in patients with non-small-cell lung cancer. J Clin Oncol 2012; 30: 433–440. [DOI] [PubMed] [Google Scholar]
- 15. Sullivan LM, Massaro JM, D’Agostino RB, Sr. Presentation of multivariate data for clinical use: the Framingham Study risk score functions. Stat Med 2004; 23: 1631–1660. [DOI] [PubMed] [Google Scholar]
- 16. Yoshioka H, Shimokawa M, Seto T, et al. Final overall survival results of WJTOG3405, a randomized phase III trial comparing gefitinib versus cisplatin with docetaxel as the first-line treatment for patients with stage IIIB/IV or postoperative recurrent EGFR mutation-positive non-small cell lung cancer. Ann Oncol 2019; 30: 1978–1984. [DOI] [PubMed] [Google Scholar]
- 17. Moore S, Leung B, Wu J, et al. Survival implications of de novo versus recurrent metastatic non-small cell lung cancer. Am J Clin Oncol 2019; 42: 292–297. [DOI] [PubMed] [Google Scholar]
- 18. Sekine I, Nokihara H, Yamamoto N, et al. Comparative chemotherapeutic efficacy in non-small cell lung cancer patients with postoperative recurrence and stage IV disease. J Thorac Oncol 2009; 4: 518–521. [DOI] [PubMed] [Google Scholar]
- 19. Yu HA, Sima CS, Hellmann MD, et al. Differences in the survival of patients with recurrent versus de novo metastatic KRAS-mutant and EGFR-mutant lung adenocarcinomas. Cancer 2015; 121: 2078–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tseng JS, Hsu KH, Zheng ZR, et al. Primary tumor resection is associated with a better outcome among advanced EGFR-mutant lung adenocarcinoma patients receiving EGFR-TKI treatment. Oncology. Epub ahead of print 7 September 2020. DOI: 10.1159/000509664. [DOI] [PubMed] [Google Scholar]
- 21. Al-Halabi H, Sayegh K, Digamurthy SR, et al. Pattern of failure analysis in metastatic EGFR-mutant lung cancer treated with tyrosine kinase inhibitors to identify candidates for consolidation stereotactic body radiation therapy. J Thorac Oncol 2015; 10: 1601–1607. [DOI] [PubMed] [Google Scholar]
- 22. Gong HY, Wang Y, Han G, et al. Radiotherapy for oligometastatic tumor improved the prognosis of patients with non-small cell lung cancer (NSCLC). Thorac Cancer 2019; 10: 1136–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hu F, Xu J, Zhang B, et al. Efficacy of local consolidative therapy for oligometastatic lung adenocarcinoma patients harboring epidermal growth factor receptor mutations. Clin Lung Cancer 2019; 20: e81–e90. [DOI] [PubMed] [Google Scholar]
- 24. Sun Z, Sui X, Yang F, et al. Effects of primary tumor resection on the survival of patients with stage IV extrathoracic metastatic non-small cell lung cancer: a population-based study. Lung Cancer 2019; 129: 98–106. [DOI] [PubMed] [Google Scholar]
- 25. Xu J, Fan L, Yu H, et al. Survival value of primary tumor resection for stage IV non-small-cell lung cancer: a population based study of 6466 patients. Clin Respir J. Epub ahead of print 16 April 2020. DOI: 10.1111/crj.13194. [DOI] [PubMed] [Google Scholar]
- 26. Danna EA, Sinha P, Gilbert M, et al. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res 2004; 64: 2205–2211. [DOI] [PubMed] [Google Scholar]
- 27. Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013; 31: 3327–3334. [DOI] [PubMed] [Google Scholar]
- 28. Jackman DM, Yeap BY, Sequist LV, et al. Exon 19 deletion mutations of epidermal growth factor receptor are associated with prolonged survival in non-small cell lung cancer patients treated with gefitinib or erlotinib. Clin Cancer Res 2006; 12: 3908–3914. [DOI] [PubMed] [Google Scholar]
- 29. Riely GJ, Pao W, Pham D, et al. Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res 2006; 12: 839–844. [DOI] [PubMed] [Google Scholar]
- 30. Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015; 16: 141–151. [DOI] [PubMed] [Google Scholar]
- 31. Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014; 15: 213–222. [DOI] [PubMed] [Google Scholar]
- 32. Offin M, Rizvi H, Tenet M, et al. Tumor mutation burden and efficacy of EGFR-tyrosine kinase inhibitors in patients with EGFR-mutant lung cancers. Clin Cancer Res 2019; 25: 1063–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hastings K, Yu HA, Wei W, et al. EGFR mutation subtypes and response to immune checkpoint blockade treatment in non-small-cell lung cancer. Ann Oncol 2019; 30: 1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tseng JS, Yang TY, Tsai CR, et al. Dynamic plasma EGFR mutation status as a predictor of EGFR-TKI efficacy in patients with EGFR-mutant lung adenocarcinoma. J Thorac Oncol 2015; 10: 603–610. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tam-10.1177_17588359211018022 for The relative importance of predictive factors for single first-generation EGFR-TKI use for more than 5 years in patients with advanced non-small cell lung cancer: Taiwan multicenter TIPS-5 study by Yen-Hsiang Huang, Jen-Yu Hung, How-Wen Ko, Po-Lan Su, Chun-Liang Lai, Huang-Chih Chang, Te-Chun Hsia, Sheng-Hao Lin, Kuan-Li Wu, Cheng-Ta Yang, Wu-Chou Su, Yi-Chun Chu, Chin-Chou Wang, Wei-Yu Liao, Yen-Ting Lin, Ching-Hsiung Lin, Meng-Chih Lin, Kuo-Hsuan Hsu, Jeng-Sen Tseng, Tsung-Ying Yang, Kun-Chieh Chen, Mei-Hsuan Lee, Sung-Liang Yu, Chao-Chi Ho and Gee-Chen Chang in Therapeutic Advances in Medical Oncology