Abstract
The objective of this study is to investigate the association between periodontitis (PD) and erectile dysfunction (ED).
A systematic review and meta-analysis on data was extracted and conducted according to PRISMA. Relevant articles were selected from a literature search using MEDLINE, EMBASE, Scopus, Web of Science and CENTRAL from inception until August 2, 2020. Both randomized and nonrandomized controlled studies were included. Case reports, case series, nonsystematic reviews and trials published as abstract were excluded. Odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were used to estimate the association between PD and the risk of ED. The meta-analysis was conducted with RevMan 5.3. Methodological quality assessment was carried out using the Newcastle-Ottawa Quality Assessment Scale and the quality of evidence was assessed using the GRADE approach.
Six articles (215008 subjects) were included for analysis. Of the participants, 38,675 cases were compared to 1,76,333 healthy controls. Based on the random effects model, periodontitis was associated with an increased risk of ED (OR = 2.56, 95% CI: 1.70–3.85) as compared with the non-periodontitis individuals. The findings were statistically significant with a p < .0001. The statistical heterogeneity was high across all studies (I2 = 98%, p < .00001). Estimates of total effects were generally consistent with the sensitivity and subgroup analyses.
Within the limits of the available evidence, our review and meta-analysis showed that a significant association exists between the PD and ED. The results should be interpreted with caution due to high degree of inconsistency across all the studies.
Keywords: periodontitis, periodontal disease, gingivitis, periodontal, erectile dysfunction, sexual, dysfunction, impotence
Periodontitis (PD), a multifactorial and complex inflammatory disease in tooth-supporting tissues, is categorized by loss of periodontal tissue support (Papapanou et al., 2018). Though the microbial plaque biofilm initiates the process, progression is largely due to an exaggerated host immune-inflammatory response. It is a major public health problem with a significant impact on an individual’s quality of life (QoL) (Nazir et al., 2020). A high prevalence, ranging from 20% to 50%, has been reported globally (Nazir et al., 2020).
Erectile dysfunction (ED) is an impairment in the arousal phase of the sexual response and the inability to attain and maintain an erection required for satisfactory sexual performance (Burnett et al., 2018). Though etiological and risk factors are related to ED, the principal risk factor is age. Risk factors are classified in organic and psychogenic components (Nguyen et al., 2017). The prevalence of ED is difficult to estimate due to the variability in the definitions used (Kessler et al., 2019). ED represents an increasing health concern with a significant impact on the QoL of men globally (Kessler et al., 2019).
Evidence suggests shared risk factors between PD and ED, including diabetes, smoking, hypertension, and coronary heart disease (Oğuz et al., 2013; Shariff et al., 2016; Sharma et al., 2011; Singh et al., 2017). Literature supports a pathophysiological link between ED and PD, such as systemic inflammation, oxidative stress, and endothelial dysfunction. Periodontal treatment may result in an improvement of endothelial function by reducing the level of systemic biomarkers (Changal et al., 2019; Shariff et al., 2016; Singh et al., 2017). Animal studies indicated a decrease in the expression of endothelial nitric oxide synthase (eNOS) and NOS activity in penile cavernous tissue, caused by a mild systemic inflammatory status in periodontitis as a possible mechanism for the effect of periodontitis on penile erection (Zuo et al., 2011). The link between chronic periodontitis (CP) and ED has been investigated, but there is no evidence yet that CP is a risk factor for the development of ED. Many of the studies were relatively small, resulting in effect estimates with wide confidence intervals (CIs).
So far, three meta-analyses have been conducted about the association between ED and PD (Liu et al., 2017; Wang et al., 2016; Zhou et al., 2019). Though the meta-analyses indicated a positive association between CP and ED, the heterogeneity between the studies was significant. The purpose of this meta-analysis is to update the available evidence regarding this association by the addition of new studies and a quality assessment using GRADE PRO. We used the trial sequential analysis (TSA) to determine if the existing literature base is sufficient to draw firm conclusions about the association between PD and ED.
Methods
This review paper was conducted according to the `Preferred Reporting Items for Systematic Review and Meta-analysis' (PRISMA) statement. The review protocol was registered on OSF at https://osf.io/nmzu6.
The research question was formulated using the Participant (adult males), Intervention (patients with periodontal disease), Comparison (patients without periodontal disease) and Outcomes (erectile dysfunction) (PICO) model. The research question was whether PD is associated with ED.
MEDLINE, EMBASE, Web of Science and CENTRAL and SCOPUS were systematically searched from its inception until June 22, 2020, as well as two trial registers (ClinicalTrials.gov and World Health Organisation International Clinical Trials Registry Platform). A re-search was carried out on the August 2 to avoid missing of any articles. We searched all studies that examined the association between PD and ED, regardless of the measured outcomes. Case reports, case series, non-systematic reviews, and trials published as abstracts were excluded. The search strategy used is outlined in Table 1.
Table 1.
Search Strategy.
| Search terms | Total | Database |
|---|---|---|
| ALL FIELDS: (periodontitis and impotence) OR ALL FIELDS: (periodontitis and sexual dysfunction) OR ALL FIELDS: (periodontitis and erectile dysfunction) OR ALL FIELDS: (periodontal disease and impotence) OR ALL FIELDS: (periodontal disease and sexual dysfunction) OR ALL FIELDS: (periodontal disease and erectile dysfunction) OR ALL FIELDS: (gingivitis and impotence) OR ALL FIELDS: (gingivitis and sexual dysfunction) OR ALL FIELDS: (gingivitis and erectile dysfunction) | 47 | WES |
|
#1 (impotence) OR ("sexual dysfunction") OR ("erectile dysfunction") OR ("sexual function") (Word variations have been searched)
#2 (periodontitis) OR ("periodontal disease") OR ("periodontal infection") OR (gingivitis) OR ("chronic periodontitis") (Word variations have been searched) #3 #1 AND #2 |
10 | Cochrane |
| ((((((((“erectile dysfunction”[MeSH Terms] OR (“erectile”[All Fields] AND “dysfunction”[All Fields])) OR “erectile dysfunction”[All Fields]) OR “impotence”[All Fields]) OR “impotent”[All Fields]) OR “impotency”[All Fields]) OR “erectile dysfunction”[All Fields]) OR “sexual function”[All Fields]) OR “sexual dysfunction”[All Fields]) AND ((((((((((((“periodontal”[All Fields] OR “periodontally”[All Fields]) OR “periodontically”[All Fields]) OR “periodontics”[MeSH Terms]) OR “periodontics”[All Fields]) OR “periodontic”[All Fields]) OR “periodontitis”[MeSH Terms]) OR “periodontitis”[All Fields]) OR “periodontitides”[All Fields]) OR ((((((((“periodontal”[All Fields] OR “periodontally”[All Fields]) OR “periodontically”[All Fields]) OR “periodontics”[MeSH Terms]) OR “periodontics”[All Fields]) OR “periodontic”[All Fields]) OR “periodontitis”[MeSH Terms]) OR “periodontitis”[All Fields]) OR “periodontitides”[All Fields])) OR (((((((“gingiva”[MeSH Terms] OR “gingiva”[All Fields]) OR “gingival”[All Fields]) OR “gingivally”[All Fields]) OR “gingivals”[All Fields]) OR “gingivitis”[MeSH Terms]) OR “gingivitis”[All Fields]) OR “gingivitides”[All Fields])) OR “periodontal infection”[All Fields]) OR “chronic periodontitis”[All Fields]) | 46 | MEDLINE |
|
#3. #1 AND #2
#2. 'impotence'/exp OR impotence OR 'sexual dysfunction' OR 'sexual function' OR 'erectile dysfunction' #1. 'periodontitis'/exp OR periodontitis OR 'periodontal infection' OR gingivitis OR 'periodontal disease' OR 'chronic periodontitis'/exp OR 'chronic periodontitis' |
146 | Embase |
| ( ALL ( periodontitis AND impotence ) OR ALL ( periodontitis AND sexual AND dysfunction ) OR ALL ( periodontitis AND erectile AND dysfunction ) OR ALL ( periodontal AND disease AND impotence ) OR ALL ( periodontal AND disease AND sexual AND dysfunction ) OR ALL ( periodontal AND disease AND erectile AND dysfunction ) OR ALL ( gingivitis AND impotence ) OR ALL ( gingivitis AND sexual AND dysfunction ) OR ALL ( gingivitis AND erectile AND dysfunction ) ) | 1182 | SCOPUS |
Articles not written in English were included if the journal provided an English-translated version. All the bibliographies of the chosen studies were hand-searched for additional studies. The authors were contacted at least twice if any data were missing.
The inclusion criteria were any observational or interventional study with adult males, >18 years, investigating the association between PD and ED, based on clear diagnostic criteria for PD and ED. The primary outcome was ED. The diagnosis of PD was based on accepted classifications, including the International Classification of Disease, 9th edition and the Clinical Modification (ICD-9-CM), including clinical periodontal measurements such as bleeding on probing, plaque index, probing pocket depth, clinical attachment loss, and radiographic bone loss. The diagnosis of ED was standardized based on accepted classifications such as the Clinical modification coding (ICD-9-CM), International Index of Erectile Function (IIEF) questionnaire or the sexual Health Inventory for Men (SHIM) questionnaire and the International Classification of Disease, 9th edition.
The titles and abstracts were independently screened in terms of the eligibility criteria by two authors (AA and MN). If both authors were confident that a study was unsuitable, the study was excluded. Any disagreements at this stage were resolved by the third author (FF). The same two reviewers (AA and MN) independently screened the full texts of qualifying papers. Any discrepancies at this stage were discussed with the third author (FF) for a final decision.
All the studies were assessed for a risk of bias, using the Newcastle-Ottawa (NCO) Scale. The GRADE assessments of the quality of evidence and summary of findings were independently performed by two authors (AA and MN). Any disagreements were resolved by the third author (FF). Using the Cochrane Handbook, the quality of evidence was assessed based on five criteria (risk of bias, inconsistency, indirectness, imprecision and publication bias).
Statistical analyses were done using the RevMan Review Manager, Version 5.3. Analysis of funnel plots was not performed, as there were less than 10 studies in each of the measured outcomes. The I2 test was used to assess the heterogeneity of studies. The values of <40%, 40%–60% and >60% were used to determine low, moderate and substantial heterogeneity, respectively. A two-sided p-value of <.05 was considered to denote statistical significance of heterogeneity. All findings were reported as odds ratios (OR) with 95% (CI). A random-effect model analysis (Mantel-Haenszel method) was used to pool the estimates. To investigate the presence of small study effects on the measured outcomes, a sensitivity analysis was done to explain the diversity of the results in different studies using the following factors as stratifying variables: type of study, location, and confounder adjustment to detect any changes in the magnitude and direction of statistical findings. In addition, sensitivity analyses were performed by sequentially removing each study (from the most recent trials) and re-analyzing the remaining dataset of the outcomes with substantial heterogeneity.
Using the trial sequential analysis software version 0.9.5.5 Beta, the TSA was performed using a diversity adjusted information size to prevent the risk of random type I errors and the multiplicity phenomenon due to repeated significance testing in meta-analyses. The required meta-analysis information size (RIS) and adjusted significance thresholds were derived based on a 5% risk of type 1 error, power of 90% and a relative risk reduction of 10%.
Results
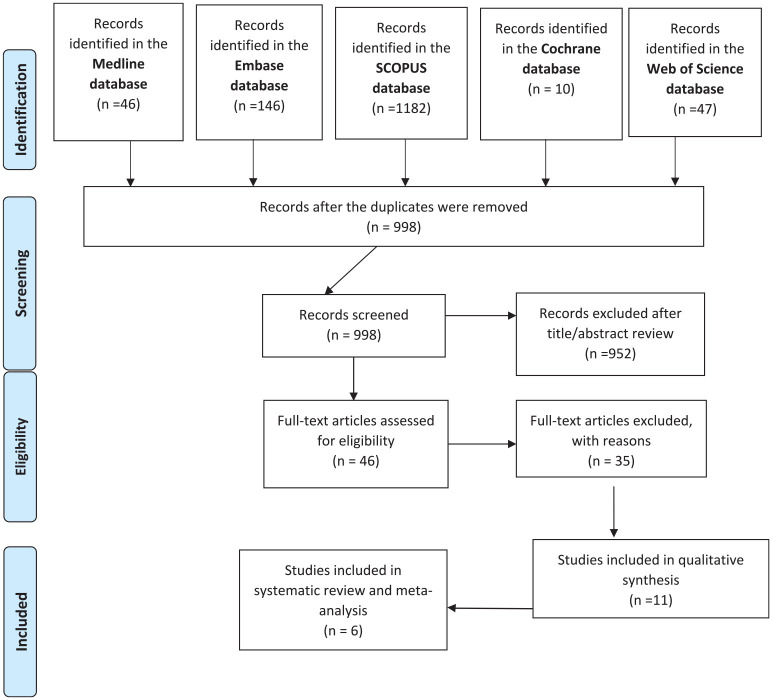
The search from PubMed, Embase, Cochrane, Web of Science and SCOPUS yielded 1431 citations. After removal of the duplicates, 998 citations were screened in terms of the title and abstract and 46 articles were retrieved for full text screening. Six articles (38675 subjects) were included in the final review after the full text screening (Figure 1). The PRISMA flowchart is presented in Figure 1.
Figure 1.
PRISMA flowchart demonstrating the reports identified, screened, and included in the review.
The characteristics of the studies are summarized in Table 2. All studies were observational and single-center in nature. No potential conflict of interest was declared in the six observational studies. The characteristics of the excluded articles are illustrated in Table 3.
Table 2.
Clinical Characteristics of the Included Studies.
| Study (year) | Location | Design | Study population | Age | N | Confounders assessed | Diagnosis of PD | Diagnosis of ED | Clinical outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Zaadik (2009) | Israel | Cross- sectional study R |
Males ≥ 25 years of age | 39.5 ± 6.7 |
‡305 Mild ED = 51, Moderate ED = 18; Severe ED = 1 Control = 235 |
Education, ED severity, Smoking | Alveolar bone loss | SHIM questionnaire scores ≤ 21 | Males with ED (mild and moderate) compared to males without ED had significantly more prevalent CP |
| Keller (2012) | China | Case-control study R |
Males ≥ 18 years of age | 49.3 ± 12.5; 49.4 for cases 49.2 for controls | 1,97,136 | Age, urbanization, income, geographic region, hypertension, diabetes, hyperlipidemia, coronary heart disease, obesity, alcohol | ICD-9-CMcode523.4 periodontal examination and probing of the sulcus and radiographs |
ICD-9-CMcode 607.84 IIEF-5 (International Index of ED) |
ED cases were more likely to have been previously diagnosed with CP than controls |
| Oguz (2013) | Turkey | Cross- sectional study R |
30y to 40y males | 162 | Age, incomes, education level, BMI, CP severity DM, heart disease, hypertension, smoking | Mild: PPD ≥ 4 mm BOP (<15 tooth sites) Severe PD:PPD ≥ 15 mm BOP (≥ 15 tooth sites) | IIEF Questionnaire | Patients with ED had more CP compared to the non-ED group | |
| Tsao (2014) | China | Case-control study | 20y to 80y males | 48.3 ± 12.5 | 15315 Cases = 5,105, Control = 10,210 |
age, hypertension, ischemic heart disease, DM, hyperlipidemia, obesity | ICD-9-CMcode523.4, periodontal examination and radiographs | ICD-9-CM codes23.0 | ED cases were more likely to have been previously diagnosed with CP than controls |
| Martin (2018) | Spain | Case-control study P |
18y to 70y males ≥11 teeth |
Controls 53 ± 8 Cases 53 ± 9 |
158 | Age, cardiovascular diseases, DM, triglycerides | BOP ≥ 1s ite PPD ≥ 4 mm AL ≥ 3 mm | IIEF ≥ 25 | Patients with CP were more likely to have ED compared to periodontally healthy men |
| Chou (2014) | Taiwan | Cross- sectional Study R | 18–28 years males | 21.65 ± 2.609 years | 1932 | Age, BMI, Waist, smoking | Comprehensive dental examination | IIEF‑5 questionnaire | Incidence of ED in young males with CPD was higher than those without CPD. |
Note. ‡Authors when contacted clarified the number of periodontitis subjects among the ED and non ED groups.
Table 3.
Characteristics of the Excluded Studies.
| Study (year) | Location | Design | Study population | Age | N | Confounders assessed | Diagnosis of PD | Diagnosis of ED | Reason for exclusion |
|---|---|---|---|---|---|---|---|---|---|
| Eltas (2013) | Turkey | Randomized controlled clinical trial P |
Moderate or severe ED and periodontitis 30 to 40 years ≥20 teeth + male Exclusion-systemic diseases ( DM, HT, Heart disease) + Periodontal therapy last 12 months + systemic antibiotic last 6 months |
ED + NSPT: 38.1 ± 6.1 ED: 36.6 ± 6.9 |
120 ED + NSPT = 60, ED = 60 |
CVD; DM HBP Periodontal therapy Smoking Systemic antibiotics | Oral examination AAP criteria (PI, BOP, PD, CAL) PL ≥ 4 mm AL ≥ 3 mm |
IIEF ≥ 25 | Selection bias, no control group |
| Matsumoto (2013) | Japan | Cross-sectional study R |
88 male patients, Aged 20–85 Adjustment made for age alone |
50.9 ± 16.6 | 88 | None | CPD self-check sheet | IIEF ≥ 25 | Confusing scoring system of periodontitis (self-check sheet for diagnosis of chronic periodontitis) |
| Uppal (2014) | India | Cross-sectional study | 53 males, aged 25–40 ≥ 20 permanent teeth | 53 | Systemic diseases, Alcohol Smoking | SHIM questionnaire scores ≤ 21 | Selection bias (included subjects were diagnosed with ED)/ No control group | ||
| Lee (2017) | Korea | Cohort study | Males ≥ 20 years | 2,68,296 | Age, income insurance residence area DM cerebral infraction myocardial infarction smoking angina pectoris health status | ICD-10codeK05.3 | Periodontal flap surgery | ||
| Sharma (2011) | India | Cross-sectional | Males, 25–40 years ≥ 20 permanent teeth |
103 | Periodontal examination and radiographs | SHIM questionnaire scores ≤ 21 | Selection bias, no control group |
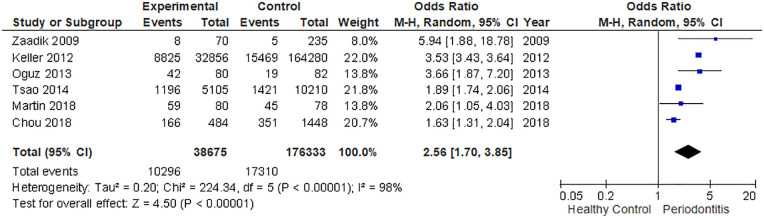
The current meta-analysis included 38,675 cases and 1,76,333 healthy controls. Based on the data of the studies, the pooled summary OR was 2.56 (95% CI: 1.70–3.85) in the random-effect model for the group with PD compared to the non-PD group (Figure 2). In other words, the periodontitis group were 2.56-fold more likely to be diagnosed with ED. The findings were statistically significant with a p < .0001. However, the statistical heterogeneity was high across all studies (I2 = 98%, p < .00001).
Figure 2.
Forest plot of the association between chronic periodontitis and ED.
Figure 3 in the Supplement, presents the TSA of the association of CP and ED. The cumulative number of patients included in the meta-analysis is represented in the X-axis. The Y-axis represents the cumulative Z score. The required meta-analysis sample size was 2,64,239 patients. The cumulative Z curve (blue line) crossed the trial sequential significance boundary (red) for statistical significance after the first study was included showing that the first study found a strong association, the last studies did not change the estimate very much.
Sensitivity analyses were performed for the year of publication, study design, adjusted confounders, influence of individual study on overall risk of ED and the estimated effects remained unchanged (Supplement: Figs 4, 5, and 6). By sequentially removing the most recent trials and re-analyzing the remaining dataset, similar OR and 95% CI were obtained after the exclusion (Table 4). This indicated a high degree of stability of the results.
Table 4.
Sensitivity Analysis After Each Study Was Excluded by Turns.
| Study omitted | OR (95%) for remainders | p | Heterogeneity |
|---|---|---|---|
| I2 (%) | |||
| Zaadik et al | 2.38 (1.55, 3.64) | <.0001 | 98 |
| Tsao et al | 2.80 (1.71, 4.59) | <.0001 | 92 |
| Oguz et al | 2.42 (1.55, 3.76) | <.0001 | 98 |
| Martin et al | 2.65 (1.70, 4.12) | <.00001 | 98 |
| Keller et al | 2.02 (1.60, 2.56) | <.00001 | 57 |
| Chou et al | 2.87 (1.83, 4.49) | <.00001 | 98 |
In terms of the risk of biased assessment, all studies were graded as low risk (Table 5). The summary of the findings with the quality of the evidence (GRADE) assessment is demonstrated in Supplement Table 1. The findings revealed a positive association between chronic PD and ED.
Table 5.
Risk of Bias Assessment: Newcastle-Ottawa Quality Assessment Scale.
| NO | Reference | Case definition |
Representativeness of cases |
Selection of controls | Definition of controls | Comparability by design and analysis | Ascertainment of exposure | Same method of ascertainment for cases and controls | Nonresponse rate | Score |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Zadik (2008) | * | * | X | X | * | * | * | * | 7 |
| 2 | Keller (2012) | * | * | * | * | ** | * | * | * | 9 |
| 3 | Oguz (2012) | * | * | * | * | ** | * | * | * | 7 |
| 4 | Tsao (2014) | * | * | * | * | ** | * | * | * | 9 |
| 5 | Martin (2018) | * | * | * | * | ** | * | * | * | 9 |
| 6 | Chou (2018) | * | * | * | * | *X | * | * | * | 8 |
Note. *indicates that the feature is present; x, that the feature is absent. But for comparability by design this checklist awards maximum of two stars (**), one (*) or none if the feature is completely absent.
Discussion
The current meta-analysis is an update of the existing evidence regarding the association between PD and ED. As of now, three meta-analyses investigated this issue (Liu et al., 2017; Wang et al., 2016; Zhou et al., 2019). However, this was the first meta-analysis to use TSA to determine if the existing literature is sufficient to draw firm conclusions about the association. Major updates of this meta-analysis are the inclusion of a new study (Chou et al., 2018), the performance of the TSA and the quality assessment using GRADE PRO.
Our meta-analysis revealed a statistically significant association between PD and ED, based on the summarized results of six observational studies (Chou et al., 2018; Keller et al., 2012; Martín et al., 2018; Oğuz et al., 2013; Tsao et al., 2015; Zadik et al., 2009), with 38675 cases and 1,76,333 controls. Sensitivity and subgroup analyses were performed due to the statistically significant heterogeneity (I2 = 98%) but could not rule out the explanation of the heterogeneity. A previous meta-analysis performed a subgroup analysis in terms of age which also could not reduce the increased heterogeneity (Wang et al., 2016).
Although, our TSA findings demonstrated firm evidence in support of the association between PD and ED, the high degree of statistical heterogeneity warrants further studies to address the issue of increased heterogeneity.
We excluded the study of Matsumoto et al. because the diagnosis was based on the Chronic Periodontal Disease Self-Check Sheet (Matsumoto et al., 2014). Several studies assessed this topic using various designs, and almost all the studies confirmed the relationship between ED and PD. The only exception was the study reported by Sharma, which did not show any statistical significance in the association (Sharma et al., 2011). Two studies reported an intervention with treatment (Eltas et al., 2013; Lee et al., 2017). The reasons why studies were not included in the current are available in Table 3.
PD and ED share risk factors such as age, obesity, smoking, diabetes mellitus, metabolic syndrome, hypertension, cardiovascular diseases, and excessive drinking (Machado et al., 2020; Zuo et al., 2011). Both, also share common pathophysiological pathways, including endothelial dysfunction and inflammation (Kulshrestha et al., 2020). The evidence is strengthened by the findings that smoking cessation, reduction of obesity, and avoiding other common risk factors are safe and effective means to reduce the incidence of both diseases. Evidence support the statement that providing periodontal treatment can reduce the risk of ED (Eltas et al., 2013). Endothelial dysfunction is a possible explanation for the association between PD and ED. It has been confirmed in experimental and clinical studies that PD could impair vascular endothelial dysfunction (Amar et al., 2003; Blum et al., 2007) by increasing the expression of proinflammatory cytokines and adhesion molecules. In addition, PD induce a mild systematic inflammatory status and decrease the activity of endothelial nitric oxide synthase and nitric oxide synthase in penile cavernous tissues of rats (Kulshrestha et al., 2020; Pedrotti et al.; Zuo et al., 2011). Periodontal therapy may improve damaged vascular endothelial function and results in significant improvement in the function (Gurav, 2014; Scannapieco et al., 2010). Another possible suggestion for the link between PD and ED could be via the level of testosterone (Corona et al., 2008; Corona & Maggi, 2010; Singh et al., 2011; Steffens et al., 2020).
The strengths of this meta-analysis is the use of cumulative meta-analysis and the TSA to explore the results. We conducted a comprehensive literature search in several databases PubMed, Embase and Cochrane library, Web of Science and Scopus to obtain a summary measure of the association between CP and ED, reducing the risk of missing studies that may lead to the risk of selection bias. The inclusion of an increased number of studies and a larger number of participants from six studies is another strength of the current study. The confirmation of the stability of the results through the sensitivity analysis and the high quality of the studies included (NCO) is another strength of the study. The elimination of possible disparities due to confounders, study design, and location, sub group analysis was performed.
The limitations of the current study include a lack of standardized criteria for the diagnosis of ED. Almost all of the studies confirmed ED based on the IIEF or SHIM questionnaires, without using an ultrasound of the penis. These methods, however have their own limitations (Levinson et al., 2010). The lack of consistency in the assessment of PD is another limitation. The lack of an uniform accurate definition may have contributed to bias in our review. The studies included were observational, with their own inherent limitations of potential bias. Although the sensitivity analysis confirmed that the statistical results were stable, the substantial heterogeneity present could not be explained due to the potential biases involved and detracted from the strength of the evidence. However, the quality of the evidence from the GRADE assessment for all the measured outcomes were low due to the high degree of inconsistency. The results of this meta-analysis should be interpreted with caution due to the high degree of inconsistency.
The results of the current meta-analysis provide evidence to further support the positive association between PD and ED. The high degree of inconsistency from the GRADE assessment warrants the need of well-designed controlled clinical trials and prospective studies. Patients identified with PD should be screened for ED and treated appropriately.
Conclusion
Within the limits of the available evidence, our review and meta-analysis indicated that a significant association exists between CP and ED. Nevertheless, the results should be interpreted with caution due to the high degree of inconsistency.
Supplemental Material
Supplemental material, sj-pdf-1-jmh-10.1177_15579883211007277 for The Association Between Periodontitis and Erectile Dysfunction: A Systematic Review and Meta-Analysis by Fathima Farook, Azzam Al Meshrafi, Nuzaim Mohamed Nizam and Abdulsalam Al Shammari in American Journal of Men’s Health
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Self-funded
ORCID iD: Fathima Farook  https://orcid.org/0000-0002-2257-2662
https://orcid.org/0000-0002-2257-2662
Supplemental Material: Supplemental material for this article is available online.
References
- Amar S., Gokce N., Morgan S., Loukideli M., Van Dyke T. E., Vita J. A. (2003). Periodontal disease is associated with brachial artery endothelial dysfunction and systemic inflammation. Arteriosclerosis, Thrombosis, and Vascular Biology, 23(7), 1245–1249. [DOI] [PubMed] [Google Scholar]
- Blum A., Kryuger K., Eizenberg M. M., Tatour S., Vigder F., Laster Z., Front E. (2007). Periodontal care may improve endothelial function. European Journal of Internal Medicine, 18(4), 295–298. [DOI] [PubMed] [Google Scholar]
- Burnett A. L., Nehra A., Breau R. H., Culkin D. J., Faraday M. M., Hakim L. S., Heidelbaugh J., Khera M., McVary K. T., Miner M. M., Nelson C. J. (2018). Erectile dysfunction: AUA guideline. The Journal of Urology, 200(3), 633–641. [DOI] [PubMed] [Google Scholar]
- Changal K., Bashir R., Sheikh M. A. (2019). Periodontal therapy improves serum soluble E-selectin levels and endothelial function; A meta-analysis. Arteriosclerosis, Thrombosis, and Vascular Biology, 39(Suppl_1), A222–A222. [Google Scholar]
- Chou M.-H., Liu C.-Y., Yang M.-H., Chou Y.-C., Wu S.-T., Cha T.-L., Tsao C.-W. (2018). Chronic periodontal disease correlated with sezual function in young males. Formosan Journal of Surgery, 51(5), 175. [Google Scholar]
- Corona G., Forti G., Maggi M. (2008). Why can patients with erectile dysfunction be considered lucky? The association with testosterone deficiency and metabolic syndrome. The Aging Male, 11(4), 193–199. [DOI] [PubMed] [Google Scholar]
- Corona G., Maggi M. (2010). The role of testosterone in erectile dysfunction. Nature Reviews Urology, 7(1), 46. [DOI] [PubMed] [Google Scholar]
- Eltas A., Oguz F., Uslu M. O., Akdemir E. (2013). The effect of periodontal treatment in improving erectile dysfunction: A randomized controlled trial. Journal of Clinical Periodontology, 40(2), 148–154. [DOI] [PubMed] [Google Scholar]
- Gurav A. N. (2014). The implication of periodontitis in vascular endothelial dysfunction. European Journal of Clinical Investigation, 44(10), 1000–1009. [DOI] [PubMed] [Google Scholar]
- Keller J. J., Chung S. D., Lin H. C. (2012). A nationwide population-based study on the association between chronic periodontitis and erectile dysfunction. Journal of Clinical Periodontology, 39(6), 507–512. [DOI] [PubMed] [Google Scholar]
- Kessler A., Sollie S., Challacombe B., Briggs K., Van Hemelrijck M. (2019). The global prevalence of erectile dysfunction: A review. BJU International, 124(4), 587–599. [DOI] [PubMed] [Google Scholar]
- Kulshrestha R., Chaudhuri G. R., Bhattacharya K., Dutta S., Sengupta P. (2020). Periodontitis as an independent factor in pathogenesis of erectile dysfunction. Biomedical and Pharmacology Journal, 13(1), 01–04. [Google Scholar]
- Lee J.-H., Choi J.-K., Kim S.-H., Cho K.-H., Kim Y.-T., Choi S.-H., Jung U.-W. (2017). Association between periodontal flap surgery for periodontitis and vasculogenic erectile dysfunction in Koreans. Journal of Periodontal & Implant Science, 47(2), 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson A. W., Ward N. T., Sanda M. G., Mettee L. Z., Wei J. T., Su L.-M., Litwin M.S., Pavlovich C. P. (2010). Comparison of validated instruments measuring sexual function in men. Urology, 76(2), 380–386. [DOI] [PubMed] [Google Scholar]
- Liu L., Li E., Zhong S., Li Y., Yang Z., Kang R., Zhao S. K., Li F. T., Wan S. P., Zhao Z. (2017). Chronic periodontitis and the risk of erectile dysfunction: A systematic review and meta-analysis. International Journal of Impotence Research, 29(1), 43–48. [DOI] [PubMed] [Google Scholar]
- Machado V., Lopes J., Patrão M., Botelho J., Proença L., Mendes J. J. (2020). Validity of the association between periodontitis and female infertility conditions: a concise review. Reproduction, 160(3), R41–R54. [DOI] [PubMed] [Google Scholar]
- Martín A., Bravo M., Arrabal M., Magán-Fernández A., Mesa F. (2018). Chronic periodontitis is associated with erectile dysfunction. A case–control study in european population. Journal of Clinical Periodontology, 45(7), 791–798. [DOI] [PubMed] [Google Scholar]
- Matsumoto S., Matsuda M., Takekawa M., Okada M., Hashizume K., Wada N., Hori J., Tamaki G., Kita M., Iwata T., Kakizaki H. (2014). Association of ED with chronic periodontal disease. International Journal of Impotence Research, 26(1), 13–15. [DOI] [PubMed] [Google Scholar]
- Nazir M., Al-Ansari A., Al-Khalifa K., Alhareky M., Gaffar B., Almas K. (2020). Global prevalence of periodontal disease and lack of its surveillance. The Scientific World Journal, 2020(8). 10.1155/2020/2146160.2146160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H. M. T., Gabrielson A. T., Hellstrom W. J. (2017). Erectile dysfunction in young men—a review of the prevalence and risk factors. Sexual Medicine Reviews, 5(4), 508–520. [DOI] [PubMed] [Google Scholar]
- Oğuz F., Eltas A., Beytur A., Akdemir E., Uslu M. Ö., Güneş A. (2013). Is there a relationship between chronic periodontitis and erectile dysfunction? The Journal of Sexual Medicine, 10(3), 838–843. [DOI] [PubMed] [Google Scholar]
- Papapanou P. N., Sanz M., Buduneli N., Dietrich T., Feres M., Fine D. H., Flemmig T.F., Garcia R., Giannobile W.V., Graziani F., Greenwell H. (2018). Periodontitis: Consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. Journal of Periodontology, 89, S173–S182. [DOI] [PubMed] [Google Scholar]
- Pedrotti S., Pontillo V., Nassar P. O., da Costa K. F., Beu C. C. L., Nassar C. A. Influence of Experimental Periodontitis on Periodontal Tissues and Penis of Rats. [Google Scholar]
- Scannapieco F. A., Dasanayake A. P., Chhun N. (2010). Does periodontal therapy reduce the risk for systemic diseases? Dental Clinics, 54(1), 163–181. [DOI] [PubMed] [Google Scholar]
- Shariff J. A., Ingleshwar A., Lee K. C., Zavras A. I. (2016). Relationship between chronic periodontitis and erectile dysfunction: A narrative review. Journal of Oral Diseases, 2016. [Google Scholar]
- Sharma A., Pradeep A., Raju P, A. (2011). Association between chronic periodontitis and vasculogenic erectile dysfunction. Journal of Periodontology, 82(12), 1665–1669. [DOI] [PubMed] [Google Scholar]
- Singh B. P., Makker A., Tripathi A., Singh M. M., Gupta V. (2011). Association of testosterone and bone mineral density with tooth loss in men with chronic periodontitis. Journal of Oral Science, 53(3), 333–339. [DOI] [PubMed] [Google Scholar]
- Singh V. P., Nettemu S. K., Nettem S., Hosadurga R., Nayak S. U. (2017). Oral health and erectile dysfunction. Journal of Human Reproductive Sciences, 10(3), 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens J. P., Valenga H. M., Santana L. C. L., Albaricci M. C. d. C., Kantarci A., Spolidorio L. C. (2020). Role of testosterone and androgen receptor in periodontal disease progression in female rats. Journal of Periodontology, 91(4), 545–553. [DOI] [PubMed] [Google Scholar]
- Tsao C. W., Liu C. Y., Cha T. L., Wu S. T., Chen S. C., Hsu C. Y. (2015). Exploration of the association between chronic periodontal disease and erectile dysfunction from a population-based view point. Andrologia, 47(5), 513–518. [DOI] [PubMed] [Google Scholar]
- Wang Q., Kang J., Cai X., Wu Y., Zhao L. (2016). The association between chronic periodontitis and vasculogenic erectile dysfunction: A systematic review and meta-analysis. Journal of Clinical Periodontology, 43(3), 206–215. [DOI] [PubMed] [Google Scholar]
- Zadik Y., Bechor R., Galor S., Justo D., Heruti R. J. (2009). Erectile dysfunction might be associated with chronic periodontal disease: Two ends of the cardiovascular spectrum. The Journal of Sexual Medicine, 6(4), 1111–1116. [DOI] [PubMed] [Google Scholar]
- Zhou X., Cao F., Lin Z., Wu D. (2019). Updated evidence of association between periodontal disease and incident erectile dysfunction. The Journal of Sexual Medicine, 16(1), 61–69. [DOI] [PubMed] [Google Scholar]
- Zuo Z., Jiang J., Jiang R., Chen F., Liu J., Yang H., Cheng Y. (2011). Effect of periodontitis on erectile function and its possible mechanism. The Journal of Sexual Medicine, 8(9), 2598–2605. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jmh-10.1177_15579883211007277 for The Association Between Periodontitis and Erectile Dysfunction: A Systematic Review and Meta-Analysis by Fathima Farook, Azzam Al Meshrafi, Nuzaim Mohamed Nizam and Abdulsalam Al Shammari in American Journal of Men’s Health