Abstract
Background:
The prediction of the individual’s response to disease modifying antirheumatic drugs (DMARDs) in rheumatoid arthritis (RA) is challenging and often limited. Here we evaluated the influence of patients’ expectations towards a change in treatment with DMARD on clinical outcome in RA.
Methods:
One hundred patients (74 female) with RA (2010 ACR/EULAR classification criteria) and an upcoming change in DMARD treatment due to non-response or adverse effects were included. Patients’ treatment beliefs, health-related quality of life and treatment expectations were measured using the Beliefs about Medicines Questionnaire (BMQ), the Short Form 36, and self-designed questions about expectations before treatment initiation (T0), and DAS28-CRP was calculated at T0 and after 4 months (T4). Associations between patients’ beliefs and expectations and changes in DAS28-CRP (T0 to T4, ΔDAS28-CRP) were explored by regression analyses after multiple imputation.
Results:
A total of 99 patients were included, of whom 84 completed all questionnaires. Thirty-six percent of all variability in treatment response (ΔDAS28-CRP) was explained by expectations assessed with the questionnaires and the C-reactive protein (CRP)-value at T0. Among these, the expected improvement rate, with 10.5%, as well as the CRP-value at T0, with 10.6%, had the greatest positive effect whereas the fear of adverse effects, with 11.4%, and the BMQ.concern scale, with 9.0%, had the greatest negative impact on ΔDAS28.
Conclusion:
Patients’ expectations towards newly induced DMARD therapies influence clinical response and may serve as possible explanatory factors for treatment response affecting subjective and objective outcome parameters.
Clinical trial registration number:
DRKS00017005
Keywords: behavior, biomarkers, DMARDs, patient attitude to health, quality of life, rheumatoid arthritis
Introduction
In rheumatoid arthritis (RA), the concept of early treatment and “treat-to-target” have led to increased disease remission rates.1 Still, about 20% of our patients do not achieve the goal of remission despite high therapy standards.2,3 In the past years, several baseline parameters predictive for treatment response have been identified and extensively evaluated: among these, female gender, current smoking as well as longer disease duration, several genetic markers and previous failure of disease-modifying antirheumatic drugs (DMARDs) are associated with lower treatment response.4–7 Even combined, these parameters do not reliably predict treatment response. Furthermore, most of these predictors are not modifiable and hence do not serve as targets for therapeutic interventions. Additional parameters with an impact on treatment response need to be identified for individualized targeted interventions, also beyond the direct pathophysiological inflammatory focus.
The psychosocial dimensions of RA have received considerable attention in the past years. It is generally accepted that the psychosocial state impacts parameters such as disease activity and health-related quality of life (HrQoL) at baseline, but also longitudinally.8–10 As such, an association of anxiety, coping with pain and fatigue at baseline with disease activity after 3–12 months was reported.11 Other analyses focused on illness perceptions in patients with RA and found a significant impact of illness perceptions on physical and mental HrQoL at baseline.12 However, the association of initial illness perception on outcomes such as HrQoL and disease activity has not yet been investigated. Patients’ expectations, as central components of illness beliefs,13 and their association on clinical outcome in RA, has also been assessed in only few studies.9,10 Dasgupta et al.9 reported a strong correlation of high expectations and a decrease in the subjective Disease Activity Score 28 (DAS28) components tender and swollen joint count (TJC and SJC) after switching the treatment to a TNFα-inhibitor.
The first study to relate patients’ attitudes towards a specific medication to patient-reported outcome was the analysis of participants enrolled in the BeSt trial.14,15 The retrospective survey proved a positive impact of fulfilled therapy preferences on the subjective general health and thus underlined the importance of assessing and considering patients’ treatment expectations for their health improvement.
In the current study it was our aim to investigate the longitudinal effect of psychological status. We sought to assess whether expectations of RA patients towards newly initiated therapies affect clinical outcome in terms of DAS28-CRP response after 4 months and whether expectations might thus serve as a new prognostic marker and a potential therapeutic target.
Methods
Patients and measures
The prospective study was approved by the ethics committee of the Medical Faculty at Heinrich-Heine-University Duesseldorf (#3933) in October 2012. Inclusion criteria were a diagnosed RA (2010 American College of Rheumatology/European League Against Rheumatism (EULAR) classification criteria) with an upcoming DMARD initiation or change of DMARD treatment due to non-response or drug intolerance or side effects according to their treating physician. A total of 100 patients were recruited between October 2012 and July 2014 at the Sana Hospital Duisburg, Germany (70 patients) and at the St. Josef Hospital Wuppertal, Germany (30 patients). Exclusion criteria were insufficient language skills, lack of a written informed consent and conditions that require cessation of immunosuppressive therapies (e.g. malignancies, major infections). One patient was excluded due to diagnosis of lung cancer and discontinuation of DMARD therapy. All patients gave their full written informed consent. Collected data of the patients at baseline included diagnosis, demographics including education, antibody-status, C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) value, comorbidities, detailed medication, DAS28-CRP, the Short Form 36 (SF-36) as measure of HrQoL, the Beliefs about Medicines Questionnaire (BMQ) and six questions regarding treatment and general expectations. DAS28-CRP was again calculated after 4 months (T1). The timeframe of 3 months to reevaluate treatment efficacy as recommended by EULAR was extended by 1 month (T1) to make sure a treatment related effect was visible at follow-up.16 The treating physicians and patients were free in their treatment choice as long as decisions were in accordance with the then current treatment guidelines for RA,17 since all drugs approved for the treatment of RA are also reimbursed by health insurance companies. Off label uses, which require a special request, have not been noted in this study.
Assessment of expectations
Six questions were developed to assess patients’ expectations towards a newly administered medical treatment. Patients were asked for (a) the general expected [patient expectation (PE)] improvement with new therapy (PE_general improvement), (b) an expected improvement rate (PE_improvement rate), (c) the fear of adverse effects (AEs) (PE_fear of AE), (d) concerns about the route of administration (PE_route of administration), (e) the feeling of being well informed about the therapy (PE_information) and (f) the general attitude towards an accompanying glucocorticosteroid treatment (PE_steroid therapy). All items were answered using a five-point Likert scale from 1 to 5. Items a, b, d and f were inspired by the questionnaire about patient preferences for treatment strategies from the BeSt trial.15 The questions were tested for content-related consistency and comprehensibility in 20 patients. All items were evaluated separately and frequency of each score among all patients was reported. All questions and their possible answers are provided in the Supplemental material Table S1 online.
BMQ
The BMQ is a validated questionnaire comprising two sections with the German version consisting of a total of six scales and 28 items.18,19 The first section assesses general beliefs about pharmaceuticals as a class of treatment; the second part evaluates beliefs about medication prescribed for a specific illness. In the German version, the BMQ-general comprises the scales overuse, harm and benefit of medicines and the patients’ sensitivity towards medication, meanwhile the BMQ-specific is composed of the necessity scale and the concern scale. All items are answered using a five-point Likert scale which varies from “strongly disagree” to “strongly agree”. Scores for each scale are summed up and evaluated on a continuous scale. The ranges for each scale are as follows: BMQ.overuse, BMQ.harm and BMQ.necessity 4–20, BMQ.sensitivity 5–20, BMQ.concern 6–30 and BMQ.necessity 5–20. Higher scores indicate stronger beliefs about the assessed aspects concerning medicines. The German version of the BMQ was used after signed consent of Prof. Robert Horne and Dr. Yvonne Nestoriuc.
DAS28
To assess disease activity, the CRP-based DAS28 was used. The DAS28-CRP is a composite score comprising the acute phase parameters CRP, the patient reported disease activity in terms of the patient global assessment (PGA) as well as the TJC and the SJC.20 The change in DAS28 and its components between T0 and T4 was presented as ΔDAS28, calculated by as DAS28T0 – DAS28T4. A DAS28 of <2.6 indicates clinical remission, ⩾2.6 to <3.2 low disease activity, 3.2 to <5.1 moderate disease activity and a DAS28 of ⩾5.1 resembles high disease activity.
SF-36
The SF-36 is a generic survey measuring HrQoL.21 It has been validated for the use in general22 as well as clinical populations especially suffering chronic diseases.23 The 36-item questionnaire comprises the eight health domains physical functioning, role limitations due to physical problems, bodily pain, general health perception, energy/vitality, social functioning, role limitations due to emotional problems, and mental health. The domains can be summarized as the physical component summary (PCS) and the mental component summary (MCS). A summary scale >50 means a value above the mean value of the standard sample, a value <50 lies below the mean standard value. We used the German version validated in 1995.24
Statistical analysis
Data management and analyses were performed using R Version 3.2.1 (The R Foundation for Statistical Computing) with a significance level of α = 0.05. Descriptive data are presented as absolute and relative values in percent or as median and first quartile to third quartile [interquartile range (IQR) = Q1 to Q3] and a Welch two sample t-test was applied for statistical analyses.
Evaluation of all questionnaires was carried out according to their specific evaluation protocols. The PE was analysed by counting the given answers of each category within the Likert scale.
A linear mixed model was developed according to the Aikaike Information Criterion (AIC) based on complete case analysis. The impact of questionnaires and demographic data including the baseline glucocorticosteroid dosage on changes in the DAS28 components CRP, patient global assessment (PGA), TJC and SJC between T0 and T1 (ΔCRP, ΔPGA, ΔTJC, ΔSJC) were assessed for each DAS28-CRP component separately. After identification of the relevant factors associated with a significant decrease in PGA, TJC, SJC and CRP, their effect sizes on ΔDAS28 were calculated. To adjust for potential impact of missing data, multiple imputation by the chained equation method was carried out prior to further analyses.25 Multiple Imputation using Chained Equations employs the observed data to estimate values for missing data. We used the R package “mice”.26 Following the recommendation, the number of imputations is chosen greater than maximum (5, 100 × f), where f refers to the proportion of participants having any variables missing.25 The imputed data sets were used for further linear mixed regression with ΔDAS28 as dependent variable, disease duration, CRP at T0, ESR at T0, PE_improvement rate, PE_route of administration, PE_fear of AE, BMQ.concern, BMQ.sensitivity, BMQ.benefit, glucocorticosteroid dosage at T0, SF-36 physical role functioning (SF-36.rolph), SF-36 emotional role functioning (SF-36.rolem), SF-36 mental health (SF-36.mhi), SF-36 general health perception (SF-36.ghp), SF-36 social role functioning (SF-36.social) and SF-36 bodily pain (SF-36.pain) as covariates and gender and education as independent variables in order to calculate effect sizes of previously identified parameters. All scales were normalized and the model adjusted for the possible confounders gender, education and age.
Results
Clinical and demographic data
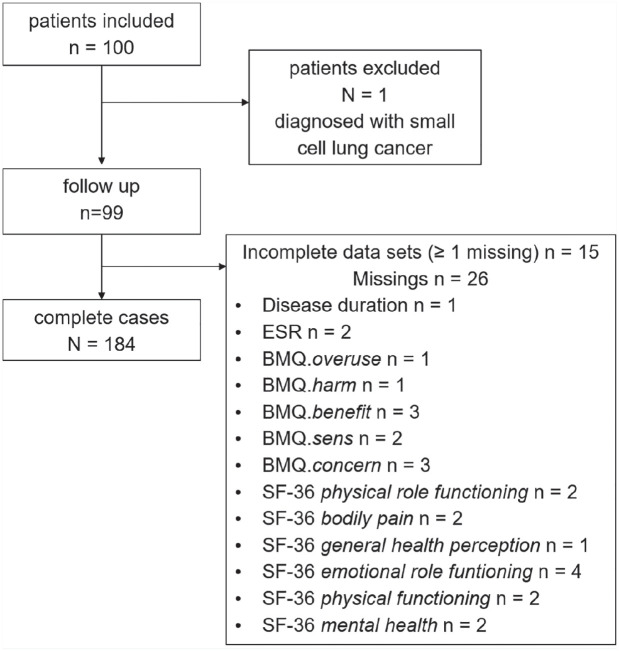
Data of 99 patients (74 female) with a median age of 62 years (IQR 53.5–70.0) were analysed. Eighty-four of the 99 (64 female) individuals completed all questionnaires (Figure 1). By decision of the managing physicians, 53 individuals were started on a biological DMARD (bDMARD), whereas 46 received a new conventional synthetic DMARD (csDMARD) at T0. bDMARD and csDMARD receivers did not differ in demographic features or disease activity (Table 1). Ninety-nine percent had failed DMARDs before. Regarding previous therapies, 62.6% had received methotrexate, 31.3% another csDMARD and 5.1% had been treated with a bDMARD either alone or in combination with a csDMARD. In one case RA was newly diagnosed.
Figure 1.
Flow chart of our prospective cohort study.
BMQ, Beliefs About Medicines Questionnaire; ESR, erythrocyte sedimentation rate; SF-36, Short Form 36.
Table 1.
Clinical and demographic features.
| Baseline | Total N = 99 |
bDMARDs n = 53 |
csDMARDs n = 46 |
|---|---|---|---|
| Female, n (%) | 74 (74.7) | 40 (75.5) | 34 (73.9) |
| Age in years, median (IQR) | 62.0 (53.5–70.0) | 62 (50–70) | 61.5 (56.25–70) |
| Disease duration in years, median (IQR) | 5.5 (2–13) | 6 (2–15) | 4 (2–9) |
| RF-positivity, n (%) | 72 (72.7) | 41 (77.4) | 31 (67.4) |
| ACPA-positivity, n (%) | 75 (75.8) | 41 (77.4) | 34 (73.9) |
| DAS28-CRP at T0, median (IQR) | 3.8 (3.4–4.3) | 4.0 (3.4–4.4) | 3.8 (3.4–4.2) |
| CRP in mg/dl, median (IQR) | 1.0 (0.4–3.25) | 0.8 (0.4–3.2) | 1.6 (0.7–3.2) |
| PGA in mm, median (IQR) | 62.0 (50.0–80.0) | 62.0 (50.0–78.0) | 63.0 (45.75–80.0) |
| TJC, median (IQR) | 4 (3–6) | 5 (3–7) | 4 (3–6) |
| SJC, median (IQR) | 4 (2–6) | 4 (2–6) | 4 (2–5) |
| Erosions, n (%) | 76 (76.8) | 44 (83) | 32 (69.6) |
| Prednisone equivalent dosage in mg/day, median (IQR) at T0 | 5 (0–7.5) | 5 (0–7.5) | 7.5 (5–9.4) |
ACPA, anti-citrullinated protein antibodies; bDMARD, biologic disease-modifying anti-rheumatic drug; CRP, C-reactive protein; csDMARD, synthetic disease-modifying anti-rheumatic drug; DAS28, Disease Activity Score 28; IQR, interquartile range; PGA, patient global assessment; RF, rheumatoid factor; SJC, swollen joint count; TJC, tender joint count.
Items on patient expectation
The questions on expectations revealed that 80.8% surely or rather surely expected a health improvement and about half (50.2%) anticipated a fast response. The number who were slightly frightened represented 39.4%, whereas 12.1% were very frightened. Most patients (69.7%) felt well or very well informed about the newly initiated medication, 3% did not feel well informed. Of all patients 61.6% were reluctant to very reluctant regarding an accompanying prednisolone treatment, whereas 9.1% did not have any concerns about prednisone. There was no difference in expectations regarding the therapy in general and glucocorticosteroid treatment between bDMARD and csDMARD receivers.
BMQ
Patients believed in the necessity of their medication for maintaining health [median 21 (19–23)]. Patients considered medications highly beneficial [median 16 (14–18)] and mildly harmful [10 (8–12)]. For detailed BMQ results see Supplemental Table S2.
SF-36
The median PCS was 40.9 (IQR 33.6–54.3) at T0. The median MCS amounted to 29.5 (24.5–35.5). For detailed SF-36 results see Supplemental Table S3.
Change in DAS28-CRP between T0 and T1
Mean ΔDAS28-CRP (DAS28-T0 – DAS28-T1) was 1.3 (IQR 0.6–2.2; −34%). Mean ΔCRP amounted to 0.3 (0–2.3; −40%), mean ΔPGA was 23 (6.5–44.5; −41%), mean ΔTJC amounted to 2 (1–5; −48%) and mean ΔSJC was 2 (1–4; −41%) (Figure 2).
Figure 2.
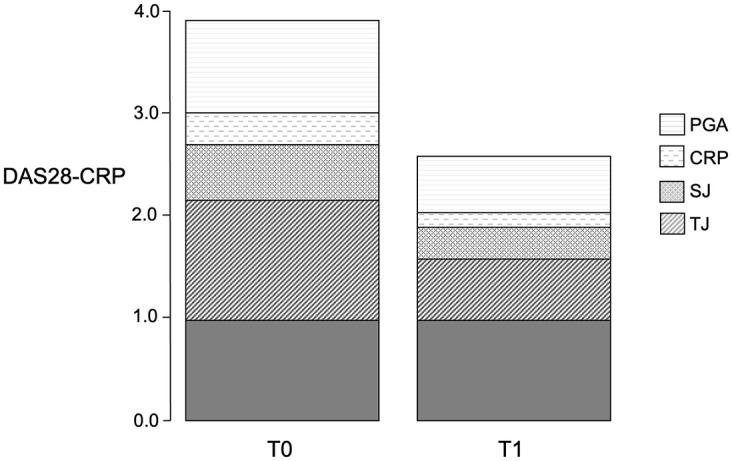
Changes in DAS28-CRP and its components from T0 to T1 after 4 months.
CRP, C-reactive protein; DAS28-CRP, Disease Activity Score 28-CRP; PGA, patient global assessment; SJC, swollen joint count; TJC, tender joint count.
Explanation of ΔDAS28-CRP subdomains
Regression models using the AIC allowed for estimation of the assessed parameters’ impact on ΔDAS28-CRP and its components. A negative estimate indicates a decrease in the DAS28-CRP subdomain over time and hence has a positive impact, meanwhile a positive estimate shows an increase in the subdomain value between T0 and T1. All parameters identified as associated with DAS28-CRP subdomains are listed in Table 2.
Table 2.
Explanation of changes in the DAS28-CRP subdomains between T0 and T1 according to the Aikaike Information Criterion.
| Subdomains | Explanatory factors | Estimate | Confidence interval |
|---|---|---|---|
| PGA | Female gender | 13.17 | −1.53 to 27.86 |
| BMQ.concern | 4.31 | 2.32 to 6.30 | |
| CRP T0 | 3.88 | −0.39 to 8.15 | |
| SF-36.vital | 0.37 | −0.03 to 0.76 | |
| PE_improvement rate | −6.61 | −12.33 to −0.88 | |
| BMQ.sensitivity | −2.26 | −3.81 to −0.71 | |
| Glucocorticosteroid dosage T0 | −1.10 | −2.27 to 0.08 | |
| Disease duration | −0.52 | −1.28 to 0.24 | |
| SF-36.social | −0.17 | −0.42 to 0.07 | |
| TJC | BMQ.overuse | 0.36 | 0.12 to 0.60 |
| BMQ.sensitivity | 0.18 | 0.02 to 0.34 | |
| Glucocorticosteroid dosage T0 | 0.09 | −0.03 to 0.22 | |
| ESR T0 | 0.05 | 0.02 to 0.09 | |
| Education | −0.93 | −1.70 to −0.15 | |
| CRP T0 | −0.67 | −1.03 to −0.32 | |
| BMQ.harm | −0.48 | −0.74 to −0.21 | |
| Disease duration | −0.26 | −0.35 to −0.17 | |
| SF-36.rolph | −0.04 | −0.07 to −0.02 | |
| SJC | PE_route of administration | 0.68 | 0.19 to 1.17 |
| BMQ.overuse | 0.27 | 0.04 to 0.51 | |
| ESR T0 | 0.02 | −0.01 to 0.06 | |
| Education | −0.92 | −1.68 to −0.16 | |
| BMQ.harm | −0.33 | −0.57 to −0.10 | |
| Disease duration | −0.24 | −0.32 to −0.15 | |
| SF-36.rolph | −0.03 | −0.06 to −0.002 | |
| CRP | BMQ.benefit | 0.15 | 0.02 to 0.28 |
| BMQ.concern | 0.09 | −0.01 to 0.19 | |
| SF-36.ghp | 0.02 | 0.003 to 0.05 | |
| ESR T0 | 0.01 | −0.005 to 0.03 | |
| SF-36.rolem | 0.01 | −0.002 to 0.01 | |
| CRP T0 | −1.01 | −1.20 to −0.83 | |
| PE_fear of AEs | −0.33 | −0.62 to −0.03 | |
| SF-36.mhi | −0.02 | −0.04 to 0.001 |
A negative estimate indicates a decrease in the subdomain between T0 and T1, a positive estimate shows an increase, hence worsening of the subdomain.
AE, adverse effect; BMQ, Beliefs about Medicines Questionnaire; CRP, C-reactive protein; DAS28, Disease Activity Score 28; ESR, erythrocyte sedimentation rate; PE, questionnaire about patient expectation; PGA, patient global assessment; SF-36, Short Form 36; SF-36.ghp, SF-36 general health perceptions; SF-36.mhi, SF-36 mental health; SF-36.rolph, SF-36 physical role functioning; SF-36.rolem, SF-36 emotional role functioning; SF-36.vital, ; SJC, swollen joint count; TJC, tender joint count.
Explanation of total ΔDAS28
After analyzing the individual components of the DAS28-CRP (CRP, PGA, TJC, SJC), we combined all their possible explanatory factors as independent variables to a regression model with the dependent variable ΔDAS28-CRP. A total of 15 persons had missing values in the independent variables (Figure 1). Thus, the proportion of subjects with missing values was f = 15/99 = 15.2% and 20 imputations were performed.
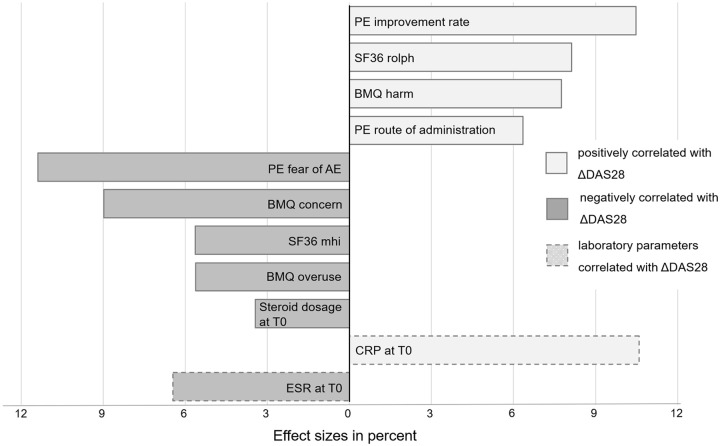
The regression model revealed that 36% of the variability in ΔDAS28-CRP could be explained by the factors previously identified as associated with a change in DAS28-CRP subdomains: the normalized model adjusted for gender and education identified the patients’ fear of AE with 11.4%, the expected improvement rate with 10.5% and the CRP-value at T0 with 10.6% as the main explanatory factors of ΔDAS28-CRP, where the fear of AE was negatively correlated with ΔDAS28-CRP (a higher fear led to less clinical improvement), and the expected improvement rate and the CRP value were positively correlated with ΔDAS28-CRP. The BMQ.concern contributed an amount of 9.0% to the variability in ΔDAS28-CRP (negative correlation). Other, positively correlated and highly influential factors, were the SF-36 physical role function with 8.1%, the BMQ.harm (7.8%) and the concerns about the route of administration (6.4%). An only minor effect was seen for the glucocorticosteroid dosage at baseline (3.4%) (positive correlation). In addition to the SF-36 physical role function, the SF-36 subscales mental health, physical functioning and emotional role functioning accounted for 14.3% of ΔDAS28 variability (5.6%, 5.3% and 3.4% respectively) (Figure 3).
Figure 3.
Predictive variables and relative effect sizes for ΔDAS28-CRP.
AE, adverse effect; BMQ, Beliefs About Medicines Questionnaire, CRP, C-reactive protein; DAS28-CRP, Disease Activity Score 28-CRP; ESR, erythrocyte sedimentation rate; PE, patient expectation; SF-36.mhi, Short Form-36 mental health; SF-36.rolph, Short Form-36 physical role functioning.
Discussion
Psychological factors play a substantial role in patients with RA, affecting both physical and mental wellbeing as well as their disease course.8 So far, characterization of psychological factors and determination of their predictive value for treatment response has not been performed in depth. Particularly, expectations have so far been analysed only as a whole and specific aspects of expectations have not yet been assessed separately.
In the present study, it was our aim to further investigate this very individual aspect. We focused on expectations and treatment preferences in patients with RA and set out to assess the effect sizes of expectations towards new treatments and beliefs about medicines on treatment response.
Among these, the expected improvement rate had the greatest positive effect, whereas the fear of AE and the BMQ.concern showed the greatest negative impact on ΔDAS28 in our patient population.
As a high expected improvement rate implicates both the general belief in a positive effect of the treatment and a fast effect, this item contains two important aspects of treatment outcome expectations.27 Accordingly, the fear of AE and BMQ.concern indicate specific (fear of AE) and general (BMQ.concern) feared harm of the treatment.
These findings could at least partly be classified as placebo response as expectations have been recognized as key factors for the formation of placebo and nocebo effects.28,29 Pre-existing optimistic expectations about therapeutic outcomes can thus amplify the effect, while pessimistic expectations might induce negative treatment outcomes or lead to the absence of effects.30 This not only holds true in clinical trials but plays a role in day-to-day clinical settings, too.31 In order to develop future interventions, it is important to understand and differentiate patient expectations and analyze their impact on treatment response independent from the complex construct of placebo response.
The ability to explain a total of 36% of variety in DAS28-CRP response by ‘psychological factors’ such as expectations and beliefs together with the CRP-value at baseline, provides a great opportunity to estimate therapy response with simple (patient-reported) measures, adjust treatment and/or patients’ expectations and consequently improve clinical outcome. A previous study evaluating best predictive parameters for treatment response was able to explain a maximum variance of 29% in treatment response according to the EULAR response criteria in RA patients by the parameters DAS28 at baseline and ACPA-positivity, synovial TNF expression and synovial lymphocyte aggregates.32 Unfortunately, different statistical analyses were applied in this study; hence, direct comparison is not possible.
Interestingly, in the current study, expectations not only affected the total ΔDAS28-CRP response and its (semi-)subjective subdomains PGA, TJC and SJC, but seemed to be associated with the objective CRP value as well. Previous studies could not show this connection: Cordingley et al.10 revealed an association of several psychological factors such as illness consequences and illness identity with the total DAS28-CRP score at baseline. Furthermore, the group evaluated the impact of psychological factors on single DAS28 subdomains and stated a strong correlation of depression and the PGA. In contrast, no belief or mood measures were associated with the SJC while the CRP-level among others correlated with the BMQ.concern and depression. The GO-MORE study reported a positive influence of expectations regarding golimumab treatment in csDMARD non-responders on the outcome measured as ΔDAS28-ESR score.9 While PGA and TJC were significantly affected by patients’ expectations, no association with CRP, ESR or SJC was found.
An explanation for these contrasting findings might be the different study design. While we investigated the effect size for each item without grouping answers or patients, Dasgupta et al.9 formed different expectation groups with the risk of losing power and missing borderline significant results.
Furthermore, there is evidence that optimization of a patient’s expectations before heart surgery leads to smaller increases of inflammatory markers after surgery, showing the influence of psychological measures on objective parameters.33
With the CRP value at baseline as a major explanatory factor of treatment response, our findings are in consistency with previously published results.34,35 Other studies, however, presented different results on CRP value and therapy response, leaving the literature rather inconclusive.36,37
In the present investigation, patients were treated with a bDMARD or csDMARD according to guidelines and physicians’ choice. In contrast to the randomized studies above, all patients were involved in the process of treatment decision-making. Consequently, preferences were not assessed separately, as opposed to the BeSt trial.15 Nonetheless, we did not find evidence for any difference between csDMARD- and bDMARD-receivers regarding treatment beliefs and expectations, indicating no substantial differences in patients’ preferences between the two groups. Interestingly, there was no significant effect of the type of intervention (csDMARD versus bDMARD) on our outcome ΔDAS28-CRP and its subdomains. To the best of our knowledge, studies comparing response to csDMARDs and bDMARDs in patients after previous therapy failure have not yet been published. This important observation should be evaluated in future randomized trials.
Conforming with previous studies, higher glucocorticosteroid dosages at baseline were associated with less DAS28-CRP response, explainable by the reductive effect of higher doses on disease activity prior to T0.38 Since the use of glucocorticosteroids is often disliked by patients and might influence adherence and treatment response, we assessed the willingness to take concomitant glucocorticosteroids.15 However, we found no association of patients’ attitude towards glucocorticosteroids and treatment response.
SF-36 subscales had slightly smaller effects on DAS28-CRP response compared with expectations and treatment beliefs and only little influence on the DAS28-CRP subdomains. A maximum effect was seen for SF-36 physical role functioning with 8.1%. As previously reported, lower functional status is associated with higher disease activity in RA patients.39 Nevertheless, associations between HrQoL at baseline and treatment response have, as far as we know, not yet been established. We assume that HrQoL subdomains form an additional dimension to consider before treatment initiation.
The direct influence of individual parameters on treatment response can be determined properly only with the knowledge of the patients’ adherence. This is (negatively) influenced by, among other things, unmet therapy preferences, low expectations and a negative belief about medicines.40,41 Therefore, expectations can also have an indirect influence on therapy response via adherence. Given the rather high treatment necessity beliefs value (BMQ.necessity) of 21 that serves as an indicator for high adherence,42 an indirect impact of low expectations on treatment response seems unlikely.
With patients’ expectations and more precisely factors like the fear of AE, the expected improvement rate and the BMQ.concern, influencing outcome parameters, this study identified promising targets for non-immunomodulating therapies. According to the expectation model developed by Rief and Petrie30 three factors contribute to the formation of individuals’ expectations. These are (a) prior experiences with the health care system and treatments, (b) social influences about health issues established through peers or social media and (c) the patient’s own cognitive constructions and personality structure. All of these aspects are promising targets for effective therapeutic approaches other than the conventional molecular targets.
The present study had a few limitations: first, data acquisition with self-reported questionnaires is critical due to the lacking possibility to verify data. We tried to counteract this concern by reducing the number of questions to a minimum. Still a selection bias due to the non-randomized and voluntary patient enrolment cannot be ruled out and further confirmatory analyses will be necessary to allow generalisability of our data. Second, common questionnaires on treatment expectations focus predominantly on surgical interventions and are suboptimal for the chronically ill RA patients. Hence, a general tool to assess treatment expectations in RA needs to be developed to reproduce the results and further investigate the association of expectations and treatment response. Furthermore, many of these findings are exploratory and have a potential impact on treatment strategies so that our data need validation in independent confirmatory studies to reach a higher level of evidence. Also, our results include new findings with potential impact on treatment strategies so that our data need validation in future trials. Although adjustments for the potential confounders age, education, disease duration and gender were carried out, we cannot rule out residual confounders we did not assess in our analyses with a potential influence on both expectations and treatment outcome.
Conclusion
The present study showed a high impact of patients’ expectations towards new medication on treatment response affecting both subjective and objective parameters. The integration of patients’ preferences and their expectations in treatment decisions could significantly increase clinical outcome. Hence, a more detailed analysis of prevailing expectations and factors that influence these expectations could identify valuable leverage points for non-pharmaceutical options to improve outcome.
Supplemental Material
Supplemental material, sj-pdf-1-tab-10.1177_1759720X211015829 for Treatment expectations as a possible prognostic factor for DMARD response in rheumatoid arthritis: a prospective cohort study by Johanna Mucke, Ralph Brinks, Argyri Dimitriou, Jutta G. Richter and Matthias Schneider in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-pdf-2-tab-10.1177_1759720X211015829 for Treatment expectations as a possible prognostic factor for DMARD response in rheumatoid arthritis: a prospective cohort study by Johanna Mucke, Ralph Brinks, Argyri Dimitriou, Jutta G. Richter and Matthias Schneider in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-pdf-3-tab-10.1177_1759720X211015829 for Treatment expectations as a possible prognostic factor for DMARD response in rheumatoid arthritis: a prospective cohort study by Johanna Mucke, Ralph Brinks, Argyri Dimitriou, Jutta G. Richter and Matthias Schneider in Therapeutic Advances in Musculoskeletal Disease
Acknowledgments
The authors thank Dr. Astrid Thiele, Dr. Christian Bergerhausen and their teams for realization of the study and for helping with patient recruitment.
Footnotes
Authors’ Note: Ralph Brinks is also affiliated with Medical Biometry and Epidemiology, Witten/Herdecke University, Faculty of Health/School of Medicine, Witten, Germany.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: This research was supported by the Hiller Research Foundation.
Data availability statement: The data underlying this article will be shared on reasonable request to the corresponding author.
Ethics statement and informed consent: This study complies with the Declaration of Helsinki. The study was approved by the ethics committee of the Medical Faculty of Heinrich-Heine University and all patients gave their full informed written consent.
ORCID iD: Johanna Mucke  https://orcid.org/0000-0001-8915-7837
https://orcid.org/0000-0001-8915-7837
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Johanna Mucke, Policlinic and Hiller Research Unit for Rheumatology, Heinrich-Heine-University, Moorenstrasse 5, Duesseldorf, 40225, Germany.
Ralph Brinks, Policlinic and Hiller Research Unit for Rheumatology, Heinrich-Heine-University Duesseldorf, Germany.
Argyri Dimitriou, Policlinic and Hiller Research Unit for Rheumatology, Heinrich-Heine-University Duesseldorf, Germany; Department for Internal Medicine, Zollikerberg Hospital, Zurich, Switzerland.
Jutta G. Richter, Policlinic and Hiller Research Unit for Rheumatology, Heinrich-Heine-University Duesseldorf, Germany
Matthias Schneider, Policlinic and Hiller Research Unit for Rheumatology, Heinrich-Heine-University Duesseldorf, Germany.
References
- 1. Stoffer MA, Schoels MM, Smolen JS, et al. Evidence for treating rheumatoid arthritis to target: results of a systematic literature search update. Ann Rheum Dis 2016; 75: 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Drosos AA, Pelechas E, Voulgari PV. Rheumatoid arthritis treatment. A back to the drawing board project or high expectations for low unmet needs? J Clin Med 2019; 8: 1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaltsonoudis E, Pelechas E, Voulgari PV, et al. Unmet needs in the treatment of rheumatoid arthritis. An observational study and a real-life experience from a single university center. Semin Arthritis Rheum 2019; 48: 597–602. [DOI] [PubMed] [Google Scholar]
- 4. Anderson JJ, Wells G, Verhoeven AC, et al. Factors predicting response to treatment in rheumatoid arthritis: the importance of disease duration. Arthritis Rheum 2000; 43: 22–29. [DOI] [PubMed] [Google Scholar]
- 5. Hyrich KL, Watson KD, Silman AJ, et al. Predictors of response to anti-TNF-alpha therapy among patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Rheumatology (Oxford) 2006; 45: 1558–1565. [DOI] [PubMed] [Google Scholar]
- 6. Nair N, Wilson AG, Barton A. DNA methylation as a marker of response in rheumatoid arthritis. Pharmacogenomics 2017; 18: 1323–1332. [DOI] [PubMed] [Google Scholar]
- 7. Kleinert S, Tony H-P, Krause A, et al. Impact of patient and disease characteristics on therapeutic success during adalimumab treatment of patients with rheumatoid arthritis: data from a German noninterventional observational study. Rheumatol Int 2012; 32: 2759–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Santiago T, Geenen R, Jacobs JWG, et al. Psychological factors associated with response to treatment in rheumatoid arthritis. Curr Pharm Des 2015; 21: 257–269. [DOI] [PubMed] [Google Scholar]
- 9. Dasgupta B, Combe B, Louw I, et al. Patient and physician expectations of add-on treatment with golimumab for rheumatoid arthritis: relationships between expectations and clinical and quality of life outcomes. Arthritis Care Res (Hoboken) 2014; 66: 1799–1807. [DOI] [PubMed] [Google Scholar]
- 10. Cordingley L, Prajapati R, Plant D, et al. Impact of psychological factors on subjective disease activity assessments in patients with severe rheumatoid arthritis. Arthritis Care Res (Hoboken) 2014; 66: 861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kuijper TM, Luime JJ, Xiong H, et al. Effects of psychosocial factors on monitoring treatment effect in newly diagnosed rheumatoid arthritis patients over time: response data from the tREACH study. Scand J Rheumatol 2018; 47: 178–184. [DOI] [PubMed] [Google Scholar]
- 12. Berner C, Erlacher L, Fenzl KH, et al. A cross-sectional study on self-reported physical and mental health-related quality of life in rheumatoid arthritis and the role of illness perception. Health Qual Life Outcomes 2018; 16: 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Petrie KJ, Weinman J. Why illness perceptions matter. Clin Med (Lond) 2006; 6: 536–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goekoop-Ruiterman YPM, de Vries-Bouwstra JK, Allaart CF, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum 2005; 52: 3381–3390. [DOI] [PubMed] [Google Scholar]
- 15. Goekoop-Ruiterman YPM, de Vries-Bouwstra JK, Allaart CF, et al. Patient preferences for treatment: report from a randomised comparison of treatment strategies in early rheumatoid arthritis (BeSt trial). Ann Rheum Dis 2007; 66: 1227–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smolen JS, Landewé R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017; 76: 960–977. [DOI] [PubMed] [Google Scholar]
- 17. Smolen JS, Landewé R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 2014; 73: 492–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health 1999; 14: 1–24. [Google Scholar]
- 19. Mahler C, Hermann K, Horne R, et al. Patients’ beliefs about medicines in a primary care setting in Germany. J Eval Clin Pract 2012; 18: 409–413. [DOI] [PubMed] [Google Scholar]
- 20. Prevoo ML, van ’t Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995; 38: 44–48. [DOI] [PubMed] [Google Scholar]
- 21. McHorney CA, Ware JE, Raczek AE. The MOS 36-item Short-Form health survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 1993; 31: 247–263. [DOI] [PubMed] [Google Scholar]
- 22. Jenkinson C, Layte R, Coulter A, et al. Evidence for the sensitivity of the SF-36 health status measure to inequalities in health: results from the Oxford healthy lifestyles survey. J Epidemiol Community Health 1996; 50: 377–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hermann BP, Vickrey B, Hays RD, et al. A comparison of health-related quality of life in patients with epilepsy, diabetes and multiple sclerosis. Epilepsy Res 1996; 25: 113–118. [DOI] [PubMed] [Google Scholar]
- 24. Bullinger M. German translation and psychometric testing of the SF-36 health survey: preliminary results from the IQOLA project. International quality of life assessment. Soc Sci Med 1995; 41: 1359–1366. [DOI] [PubMed] [Google Scholar]
- 25. Harrell FE. Regression modeling strategies. 2nd ed. Cham: Springer International Publishing, 2015. [Google Scholar]
- 26. van Buuren S, Groothuis-Oudshoorn K. MICE: Multivariate Imputation by Chained Equations in R. J Stat Soft 2011; 45: 1–67. [Google Scholar]
- 27. Laferton JAC, Kube T, Salzmann S, et al. Patients’ expectations regarding medical treatment: a critical review of concepts and their assessment. Front Psychol 2017; 8: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stewart-Williams S, Podd J. The placebo effect: dissolving the expectancy versus conditioning debate. Psychol Bull 2004; 130: 324–340. [DOI] [PubMed] [Google Scholar]
- 29. Younger J, Gandhi V, Hubbard E, et al. Development of the Stanford Expectations of Treatment Scale (SETS): a tool for measuring patient outcome expectancy in clinical trials. Clin Trials 2012; 9: 767–776. [DOI] [PubMed] [Google Scholar]
- 30. Rief W, Petrie KJ. Can psychological expectation models be adapted for placebo research? Front Psychol 2016; 7: 1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hegerl U, Mergl R. The clinical significance of antidepressant treatment effects cannot be derived from placebo-verum response differences. J Psychopharmacol 2010; 24: 445–448. [DOI] [PubMed] [Google Scholar]
- 32. Klaasen R, Thurlings RM, Wijbrandts CA, et al. The relationship between synovial lymphocyte aggregates and the clinical response to infliximab in rheumatoid arthritis: a prospective study. Arthritis Rheum 2009; 60: 3217–3224. [DOI] [PubMed] [Google Scholar]
- 33. Rief W, Shedden-Mora MC, Laferton JAC, et al. Preoperative optimization of patient expectations improves long-term outcome in heart surgery patients: results of the randomized controlled PSY-HEART trial. BMC Med 2017; 15: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Narváez J, Magallares B, Díaz Torné C, et al. Predictive factors for induction of remission in patients with active rheumatoid arthritis treated with tocilizumab in clinical practice. Semin Arthritis Rheum 2016; 45: 386–390. [DOI] [PubMed] [Google Scholar]
- 35. Pers Y-M, Fortunet C, Constant E, et al. Predictors of response and remission in a large cohort of rheumatoid arthritis patients treated with tocilizumab in clinical practice. Rheumatology (Oxford) 2014; 53: 76–84. [DOI] [PubMed] [Google Scholar]
- 36. van den Borne BE, Landewé RB, Rietveld JH, et al. Chloroquine therapy in patients with recent-onset rheumatoid arthritis: the clinical response can be predicted by the low level of acute-phase reaction at baseline. Clin Rheumatol 1999; 18: 369–372. [DOI] [PubMed] [Google Scholar]
- 37. Mancarella L, Bobbio-Pallavicini F, Ceccarelli F, et al. Good clinical response, remission, and predictors of remission in rheumatoid arthritis patients treated with tumor necrosis factor-alpha blockers: the GISEA study. J Rheumatol 2007; 34: 1670–1673. [PubMed] [Google Scholar]
- 38. Miwa Y, Takahashi R, Ikari Y, et al. Clinical characteristics of rheumatoid arthritis patients achieving functional remission with six months of biological DMARDs treatment. Intern Med 2017; 56: 903–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Donisan T, Bojincă VC, Dobrin MA, et al. The relationship between disease activity, quality of life, and personality types in rheumatoid arthritis and ankylosing spondylitis patients. Clin Rheumatol 2017; 36: 1511–1519. [DOI] [PubMed] [Google Scholar]
- 40. van den Bemt BJF, van Lankveld WGJM. How can we improve adherence to therapy by patients with rheumatoid arthritis? Nat Clin Pract Rheumatol 2007; 3: 681. [DOI] [PubMed] [Google Scholar]
- 41. Scheiman-Elazary A, Duan L, Shourt C, et al. The rate of adherence to antiarthritis medications and associated factors among patients with rheumatoid arthritis: a systematic literature review and metaanalysis. J Rheumatol 2016; 43: 512–523. [DOI] [PubMed] [Google Scholar]
- 42. Horne R, Chapman SCE, Parham R, et al. Understanding patients’ adherence-related beliefs about medicines prescribed for long-term conditions: a meta-analytic review of the necessity-concerns framework. PLoS One 2013; 8: e80633. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tab-10.1177_1759720X211015829 for Treatment expectations as a possible prognostic factor for DMARD response in rheumatoid arthritis: a prospective cohort study by Johanna Mucke, Ralph Brinks, Argyri Dimitriou, Jutta G. Richter and Matthias Schneider in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-pdf-2-tab-10.1177_1759720X211015829 for Treatment expectations as a possible prognostic factor for DMARD response in rheumatoid arthritis: a prospective cohort study by Johanna Mucke, Ralph Brinks, Argyri Dimitriou, Jutta G. Richter and Matthias Schneider in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-pdf-3-tab-10.1177_1759720X211015829 for Treatment expectations as a possible prognostic factor for DMARD response in rheumatoid arthritis: a prospective cohort study by Johanna Mucke, Ralph Brinks, Argyri Dimitriou, Jutta G. Richter and Matthias Schneider in Therapeutic Advances in Musculoskeletal Disease