Abstract
The research field of ferroptosis has been enjoying exponential growth over the past few years, since the term was coined in 2012. This unique modality of cell death, driven by iron-dependent phospholipid peroxidation, is regulated by multiple cellular metabolic events, including redox homeostasis, iron handling, mitochondrial activity, and metabolism of amino acids, lipids and sugars, in addition to numerous signaling pathways relevant to disease. Intriguingly, therapy-resistant cancer cells, particularly those of the mesenchymal state and prone to metastasis, are exquisitely vulnerable to ferroptosis. Further, numerous organ injuries and degenerative pathologies are driven by ferroptosis. As such, pharmacological modulation of ferroptosis, via both its induction and inhibition, holds great potential for the treatment of drug-resistant cancers, ischemic organ injuries, and other degenerative diseases linked to overwhelming lipid peroxidation. In this Review, we seek to provide an extensive and critical analysis of the current understanding of the molecular mechanisms and regulatory networks of ferroptosis, the potential physiological functions of ferroptosis in tumor suppression and immune surveillance, and its pathological roles and potential for therapeutics. Importantly, as in all rapidly evolving new research areas, issues and confusions exist due to misconceptions and inappropriate use of experimental tools – this Review also tries to address these issues and to provide practical guidelines. Finally, we discuss important concepts and pressing questions that should be a focus of future ferroptosis research.
Cells represent the fundamental organizing unit of life. Cell proliferation, differentiation, functional characteristics, and ultimately cell death, are therefore of critical importance in the myriad manifestations of life. The fate and functions of cells are impacted by both environmental and genetic cues. One of the most critical axes for cell fate determination is how cells respond to oxidative stress, since most living organisms rely on oxygen as the ultimate electron acceptor in reductive/oxidative (redox)-based metabolic processes. Among the conditions and oxygen-derived species that cause oxidative stress in cells, the oxidative modification of lipids in membrane bilayers (e.g., lipid peroxidation) has emerged as a nexus for integrating a series of environmental and genetic inputs, including heat and radiation exposure, metabolism, redox homeostasis, immune surveillance, and intercellular contacts, as well as oncogenic and tumor suppressive signaling. In fact, these seemingly disparate processes are tightly integrated to instruct cells’ decisions to undergo ferroptosis, a distinct cell death paradigm resulting from unrestrained lipid peroxidation (Fig. 1). Ferroptosis is now appreciated as likely one of the most widespread and ancient forms of cell death, as although originally studied in mammalian systems1, ferroptosis-like cell death has also been observed in evolutionarily remote species, such as those belonging to the kingdoms of plants, protozoa, and fungi2–4.
Figure 1. An overview of ferroptosis.
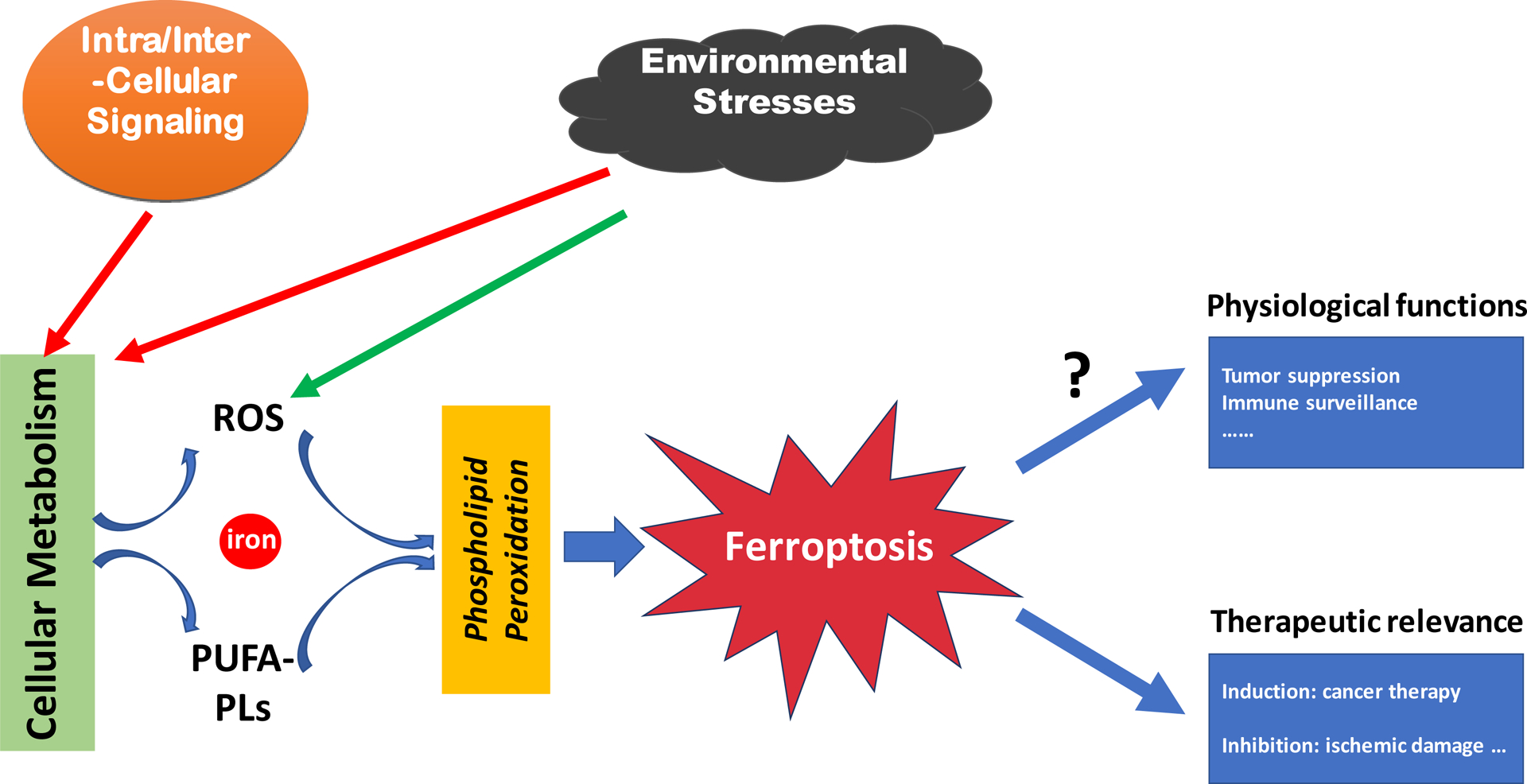
A schematic chart showing that ferroptosis is executed by phospholipid peroxidation, a process relying on metabolic products reactive oxygen species (ROS), phospholipid containing polyunsaturated fatty acid chain(s) (PUFA-PL), and transition metal iron, and that intra- and intercellular signaling events and environmental stresses can impact ferroptosis by regulating cellular metabolism and ROS level. The figure also shows the role of ferroptosis in disease and its potential physiological functions.
Historically, cell death was initially considered to be passive and unregulated, until apoptosis was discovered in the 1970s as the prototypical form of programmed cell death (PCD), as it is executed by a developmentally programmed pathway5. The broader category of regulated cell death (RCD) refers to death programs that are molecularly regulated, but not necessarily developmentally programmed6. Ferroptosis meets this RCD criterion: it is driven by lethal lipid peroxidation, a consequence of cellular metabolism and imbalanced redox homeostasis, and it can be suppressed by blocking lipid peroxidation directly or through depleting iron, via pharmacological or genetic means. Importantly, mounting evidence suggests potential physiological roles of ferroptosis in tumor suppression and immunity (Fig. 1). More recently, ferroptosis has also been implicated in the development of certain fungal species4 and in developmental aging of nematodes7. Furthermore, the pathophysiological relevance of ferroptosis, especially as a therapeutic modality in cancer and ischemic organ damage, has been convincingly established (Fig. 1).
Although the term “ferroptosis” was coined fairly recently in 2012, ferroptosis-like cell death was observed long before, for instance, as a type of oxidative-stress-induced cell death termed “oxytosis”8. Pioneering work by Harry Eagle in the 1950s and 1960s had shown that deprivation of the amino acid cyst(e)ine can cause cell death9, and that the endogenous synthesis of cysteine using methionine and glucose, i.e., transsulfuration, makes cells resistant to such cell death10,11. Cysteine, either taken up in its oxidized form as cystine by the system xc− cystine/glutamate antiporter (a transmembrane protein complex containing subunits SLC7A11 and SLC3A2)12,13 and sodium-dependent neutral amino acid transporter B(0)AT1 (SLC6A19), or in its reduced form cysteine by the neutral amino acid transporter, or generated by endogenous production via transsulfuration, is the rate-limiting substrate for the biosynthesis of reduced glutathione (GSH)14. GSH, the most abundant reductant in mammalian cells, is important for iron-sulfur cluster biogenesis and is a cofactor for multiple enzymes, including glutathione peroxidases (GPX) and glutathione-S-transferases. It has been demonstrated genetically that GSH synthesis, system xc−, and glutathione peroxidase 4 (GPX4) can all protect cells from death triggered by diverse oxidative stress conditions, particularly those causing thiol deprivation, including inhibition of system xc− activity15–19. As the roles of GSH, system xc−, and GPX4 become established in ferroptosis, we can now put all these early studies in the context of ferroptosis.
The field of ferroptosis is nonetheless nascent in many ways, having only coalesced from the adjacent fields of amino acid and lipid metabolism, iron homeostasis, redox and selenium biology, and cell death in the last few years. There has been an exponential growth in the number of published studies on ferroptosis, necessitating a thorough and critical look at recent advances to crystalize for diverse communities of researchers the key findings, questions, and challenges surrounding this topic. Herein, we provide an in-depth analysis of the mechanisms and regulation of ferroptosis, its potential physiological functions, and role in disease and therapy. We discuss emerging questions and challenges that are conceptually important for the field. We also provide practical and experimental suggestions to guide ferroptosis research.
Mechanisms governing ferroptosis
Recent years have witnessed rapid progress in the mechanistic understanding of ferroptosis. Through the initial discovery of the role of the cystine-import-GSH-GPX4 machinery in suppressing ferroptosis, the role of phospholipid hydroperoxides (PLOOHs) as the executioners of ferroptosis is now established. More recently, GPX4-independent ferroptosis surveillance pathways have been identified. Furthermore, the mechanisms of PLOOH synthesis, particularly the synthesis and activation of polyunsaturated fatty acids (PUFAs), the precursor of PLOOHs, have been extensively investigated in the context of ferroptosis. Importantly, all these studies converge on cellular metabolism and have revealed an intimate relationship between ferroptosis and metabolic pathways.
The canonical GPX4-regulated ferroptosis pathway.
In a campaign for the discovery of novel small molecule anti-cancer therapies, the Stockwell Lab conducted a high-throughput screen, beginning in 2001, leading to the discovery of a series of compounds that induce a unique form of non-apoptotic, non-necroptotic cell death reported in 200320. Counter screening revealed that multiple iron chelators and lipophilic radical-trapping antioxidants (RTAs) inhibited this type of cell death21. The iron requirement inspired coining the term “ferroptosis”1. Mechanistic investigations identified two cellular components, inhibition of which induces ferroptotic death: system xc− cystine/glutamate antiporter and GPX4, inhibited by the compounds erastin and RSL3, respectively1,22,23.
GPX4, a selenoprotein originally discovered by Ursini and colleagues through biochemical purification, is the major enzyme catalyzing the reduction of PLOOHs in mammalian cells24,25. Reduction of phospholipid and cholesterol hydroperoxides to their corresponding alcohols by GPX4 requires the catalytic selenocysteine residue of GPX4 and two electrons provided mainly by GSH, but also by other low molecular thiols or even protein thiols26. The thorough study of the first conditional Gpx4 knockout mouse model by the Conrad group provided early evidence that loss of Gpx4 causes lipid-peroxidation-dependent, non-apoptotic cell death in murine embryonic fibroblasts, and neurodegeneration in hippocampus and cortical regions of the brain19. This and several other mouse models have helped to delineate a more detailed picture of the in vivo relevance of ferroptosis as discussed further later.
As GPX4 is the major PLOOH-neutralizing enzyme, a general mechanism underlying erastin/RSL3-induced ferroptosis emerged: both compounds inactivate GPX4 – RSL3 does so directly, and erastin does so indirectly by inhibiting cystine import, thus depriving cells of cysteine, an essential cellular antioxidant and a building block of GSH. Consequently, PLOOHs accumulate, possibly causing rapid and unrepairable damage of plasma membrane, leading to cell death (Fig. 2A). Conceptually, these findings establish ferroptosis as a cell death modality with mechanisms distinct from other known death processes. The pharmacological and genetic tools developed herein enable, and have become indispensable for, ferroptosis research.
Figure 2. Ferroptosis-suppressing pathways.
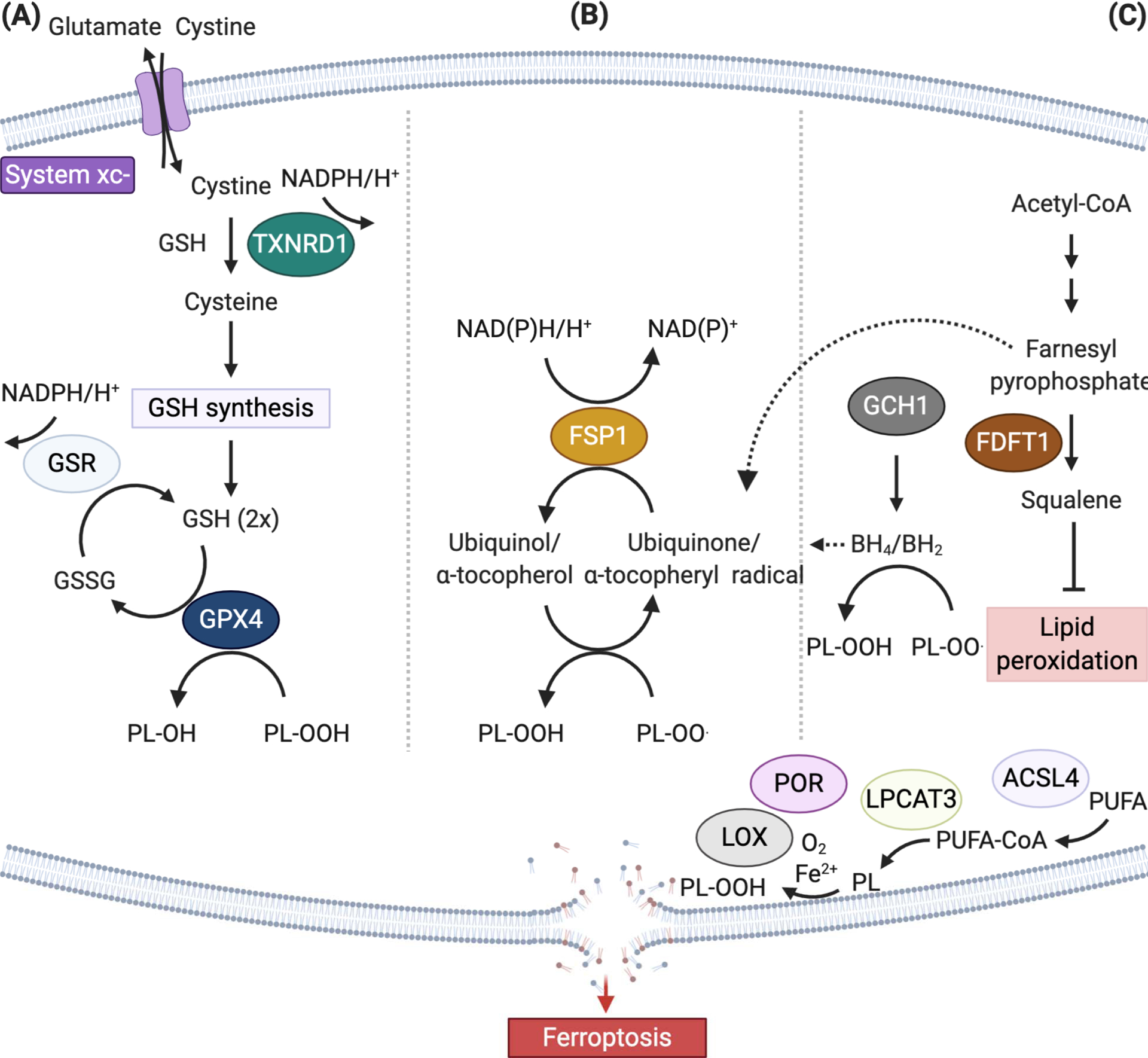
(A) The canonical ferroptosis controlling axis entails uptake of cystine via the cystine-glutamate antiporter, designated system xc−, glutathione (GSH)- and/or thioredoxin reductase 1 (TXNRD1)-dependent reduction of cystine to cysteine, GSH biosynthesis, and glutathione peroxidase 4 (GPX4)-mediated reduction of phospholipid hydroperoxides (PL-OOH) yielding the corresponding alcohols (P-LOH). Recycling of oxidized glutathione (GSSG) is achieved via glutathione-disulfide reductase (GSR) using electrons provided by NADPH/H+. (B) In two independent genetics screens the FSP1/ubiquinone (CoQ10) system has been recently identified that completely protects against ferroptosis induced by pharmacological inhibition or genetic deletion of GPX4. FSP1 prevents lipid peroxidation and associated ferroptosis via reduction of ubiquinol/α-tocopherol on the level of lipid radicals unlike GPX4/GSH. (C) Alternate ferroptosis-suppressive mechanisms include squalene- and di-/tetrahydrobiopterin (BH2/BH4)-mediated inhibition of lipid peroxidation, although the chemical mechanisms how this is achieved remains to be shown (Abbreviations: ACSL4, acyl-CoA synthetase long chain family member 4; FDFT1, Farnesyl-diphosphate farnesyltransferase 1; GCH1, GTP cyclohydrolase 1, LOX, lipoxygenase; LPCAT3 lysophosphatidylcholine acyltransferase 3, POR, cytochrome P450 oxidoreductase, PUFA, polyunsaturated fatty acid).
Phospholipid peroxidation.
Unrestrained lipid peroxidation is the hallmark of ferroptosis. Early research in the 1950s pointed towards a nexus for the suppression of lipid peroxidation by the trace element selenium, as well as vitamin E and cysteine27,28. Initiation of lipid peroxidation requires the abstraction of a bisallylic hydrogen atom, in between two carbon-carbon double bonds, from polyunsaturated fatty acyl moieties present on phospholipids (PUFA-PLs) incorporated in lipid bilayers, forming a carbon-centered radical, and subsequent reaction with molecular oxygen to yield a peroxyl radical29,30 (Fig. 3). If not converted to a lipid hydroperoxide and reduced to the corresponding alcohol, propagation of free-radical-mediated reactions lead to formation of a myriad of secondary products, breakdown of membrane integrity and ultimately rupturing of organelle and cell membranes. Thus, membranes containing a high PUFA-PL content, as evident in neurons, are particularly vulnerable to peroxidation. In line with this, genome-wide haploid and CRISPR/Cas9-based screens unveiled two membrane-remodeling enzymes, acyl-CoA synthetase long chain family member 4 (ACSL4) and lysophosphatidylcholine acyltransferase 3 (LPCAT3)31–33, as important drivers of ferroptosis. The role of ACSL4 in the ferroptotic process is based on its ability to ligate preferably long chain PUFAs, including arachidonic acid (20:4) and adrenic acid (22:4), with coenzyme A, whereupon they can be re-esterified in phospholipids by various LPCAT enzymes. Genetic loss of ACSL4 causes a dramatic shift from long chain PUFA tails to short chain and monounsaturated fatty acyl tails (MUFAs) in phospholipids31,34, in a manner similar to what is seen in wild-type cells treated with pharmacological inhibition of ACSL435. Such a dramatic change in the phospholipidome of ACSL4-null cells enables proliferation of Gpx4 knockout cells for months, a genetic rescue of Gpx4 that was not observed before31. Along the same line, exogenous supplementation of MUFAs, stearoyl-CoA desaturase (SCD1)-mediated cellular MUFA production, and an ACSL3-dependent enrichment of membranes with MUFAs was reported to lower the cells’ propensity to succumb to ferroptosis36–38. Notably, expression of ACSL4 in a subset of triple negative breast cancer cell lines correlates with their sensitivity towards ferroptosis inducers31, a correlation that seems to be shared with therapy-resistant, mesenchymal cancer cells39 and clear-cell renal carcinoma33. Suppression of ACSL4 expression thus may be a principal mechanism in desensitizing cells to ferroptosis, regulated by diverse signaling pathways40,41. Conversely, increased expression/activity of ACSL4 might promote ferroptosis under various pathophysiological contexts42,43.
Figure 3. Mechanisms of phospholipid peroxidation.

Lipid peroxidation, the hallmark of ferroptosis, occurs in both non-enzymatic and enzymatic ways (marked by a dashed box). For the latter, lipoxygenases (LOXs) and/or cytochrome P450 oxidoreductase (POR) have been implied in initiating the process of lipid peroxidation by dioxygenation of lipids, although definitive genetic evidence for an involvement of lipoxygenases in the ferroptotic process is lacking. Lipid peroxidation can be divided in three phases, i.e. initiation, propagation and termination, as indicated with the differently colored arrows. The lipid peroxidation-inhibiting systems – involving enzymes and small molecules – act on the different levels of the lipid peroxidation cascade (Abbreviations: ETC, electron transport chain; FSP1, ferroptosis suppressor protein 1; Fe2+, ferrous iron, Fe3+, ferric iron, GPX4, glutathione peroxidase; LOX, lipoxygenase; POR, cytochrome P450 oxidoreductase; L•, lipid radical; L-H, lipid; L-O•, alkoxyl radical; L-OO•, peroxyl radical; L-OH, lipid alcohol; L=O, lipid carbonyl, NOX, NADPH oxidase; OH− hydroxide ion; O2·−, superoxide anion; RTA, radical trapping antioxidant).
While there is no doubt that the degree of unsaturation of lipid bilayers is key in determining the sensitivity of cells to ferroptosis, there are many uncertainties and debates on how lipid peroxidation is initiated. A bis-allylic carbon adjacent to a conjugated diene has among the weakest of C–H bonds known and an increase in the number of these structures augments the rate of autoxidation of lipids, as is evident in the spoiling of PUFA-containing foods under ambient oxygen tension. Conceivably, non-enzymatic initiation of lipid peroxidation can be sparked by alkoxyl radicals in lipids or hydroxyl radicals, in a Fenton-type radical chemistry using iron as the catalyst44 (Fig. 3) (detailed later).
Certain lipoxygenases (LOXs), which are non-heme iron-dependent dioxygenases, can directly oxygenate PUFAs of biological membranes45, raising the prospect that LOXs may mediate ferroptosis. This possibility was supported by the observations that some pharmacological inhibitors of LOX can inhibit ferroptosis19,46, and that 12/15-LOX knockout or LOX inhibitor baicalein protected mice against ischemic brain injury and edema formation47,48. Nonetheless, genetic removal of 12/15-LOX on the Gpx4 knockout background repeatedly failed to prevent ferroptosis in mouse fibroblasts, acute kidney injury and associated lethality in vivo49, nor did it restore CD8+ T cells in T-cell specific Gpx4−/− mice50. These data suggest that alternative mechanisms compensate for Alox15 loss and/or that the frequently used “LOX-specific” inhibitors exert non-specific RTA activity51. Indeed, a recent study confirmed that most frequently used LOX inhibitors harbor RTA activity52, thus challenging a substantial role of LOXs in ferroptosis. Further, combined downregulation of all human LOX isoenzymes failed to prevent RSL3-induced ferroptosis, although it provided substantial rescue against erastin-induced ferroptosis, likely due to the fact that erastin treatment activates lipoxygenases37.
Can individual LOXs play a more prominent role in ferroptosis, at least under certain specific contexts? Deletion of Alox15 or Alox12 showed protective roles in specific mouse models of neurodegeneration or cancer suppression47,53, respectively. However, it should be stressed that these enzymes play important physiological roles in the immune system by directly modulating (neuro)inflammatory processes and the tumor microenvironment via generating pro- and anti-inflammatory molecules54,55, thus not necessarily directly related to ferroptosis. Equally relevant to this notion are previous findings that GPX4 controls the activities of lipoxygenases and cyclooxygenases by the so-called cellular peroxide tone, as both types of eicosanoid-metabolizing enzymes require oxidation of their iron by lipid hydroperoxide to capture and incorporate molecular oxygen into PUFAs54. Notably, it has been reported that ALOX12 is essential for p53-dependent ferroptosis triggered by peroxides, and this form of ferroptosis is rather unique as it appears to be independent of ACSL453; PE-binding protein-1 (PEBP1) has been reported to complex with certain LOXs, altering their substrate specificity towards PUFA-PLs56.
Besides the controversial role of LOXs in ferroptosis, recent findings suggest that the ubiquitously expressed cytochrome P450 oxidoreductase (POR) plays a role in initiating lipid peroxidation57. After accepting electrons from POR by using NADPH as electron donor, downstream electron acceptors, such as cytochrome P450 and CYB5A, are reduced, which could subsequently either directly or indirectly trigger lipid peroxidation by abstracting methylene hydrogen from PUFAs or by reducing ferric iron57,58.
Despite the widespread lack of PUFAs in bacterial membranes, Pseudomonas aeruginosa expresses a secreted lipoxygenase (PA-LOX)59. This enzyme was shown to induce oxidation of membrane lipids of human red blood cells60, and was able to induce ferroptosis in human bronchial epithelial cells61. Accordingly, patients suffering from cystic fibrosis presented increased levels of oxidized arachidonic acid in phosphatidylethanolamine61, which was reported to be one of the main phospholipid species detected in cells and tissues dying by ferroptosis34. Such a ferroptosis-inducing mechanism across organisms is intriguing and warrants further investigations to determine whether this mechanism is exploited by other lower organisms.
Cell metabolism and ferroptosis.
It is obvious that multiple metabolic reactions can lead to the cellular production of PLOOHs, and mounting evidence has demonstrated that metabolism plays a central role in ferroptosis. Studies by Jiang and colleagues, seeking to determine how metabolism contributes to cell fate determination unraveled the intricate relationship of ferroptosis with metabolism62–64. They started by chasing the elusive cell-death-promoting function of autophagy. As a crucial survival mechanism in response to various stresses, whether and how autophagy can also promote cell death (i.e., “autophagic cell death”) has been debated for decades65. Unexpectedly, they found that autophagy promotes a rapid non-apoptotic, non-necroptotic form of cell death, upon amino acid starvation (a condition that triggers potent autophagy), but only in the presence of full serum. They subsequently found that the iron-carrier transferrin and the amino acid glutamine in serum were required for this form of cell death and that deprivation of the amino acid cystine from the cell culture medium was sufficient to trigger the death. Hence, this type of cell death is ferroptosis induced by cystine starvation (mimicking erastin) and dependent on iron-transferrin62. The role of autophagy in cystine-deprivation-induced ferroptosis is via autophagic degradation of the iron-storage protein ferritin (aka ferritinophagy), which results in an increase of cellular labile iron content, and thus sensitization to ferroptosis63,66 (Fig. 4). The requirement of glutamine metabolism, or glutaminolysis, for cysteine-deprivation-induced ferroptosis62 directly links ferroptosis to cell metabolism. The anaplerotic function of glutaminolysis in generating α-ketoglutarate to fuel the mitochondrial TCA cycle suggests the involvement of normal metabolic function of mitochondria in ferroptosis, which was subsequently validated through a variety of pharmacological, cellular, and genetic analyses64 (Fig. 4). Notably, mitochondria have been shown earlier to be active participants in oxytosis8,67.
Figure 4. Metabolism and cell signaling in ferroptosis.
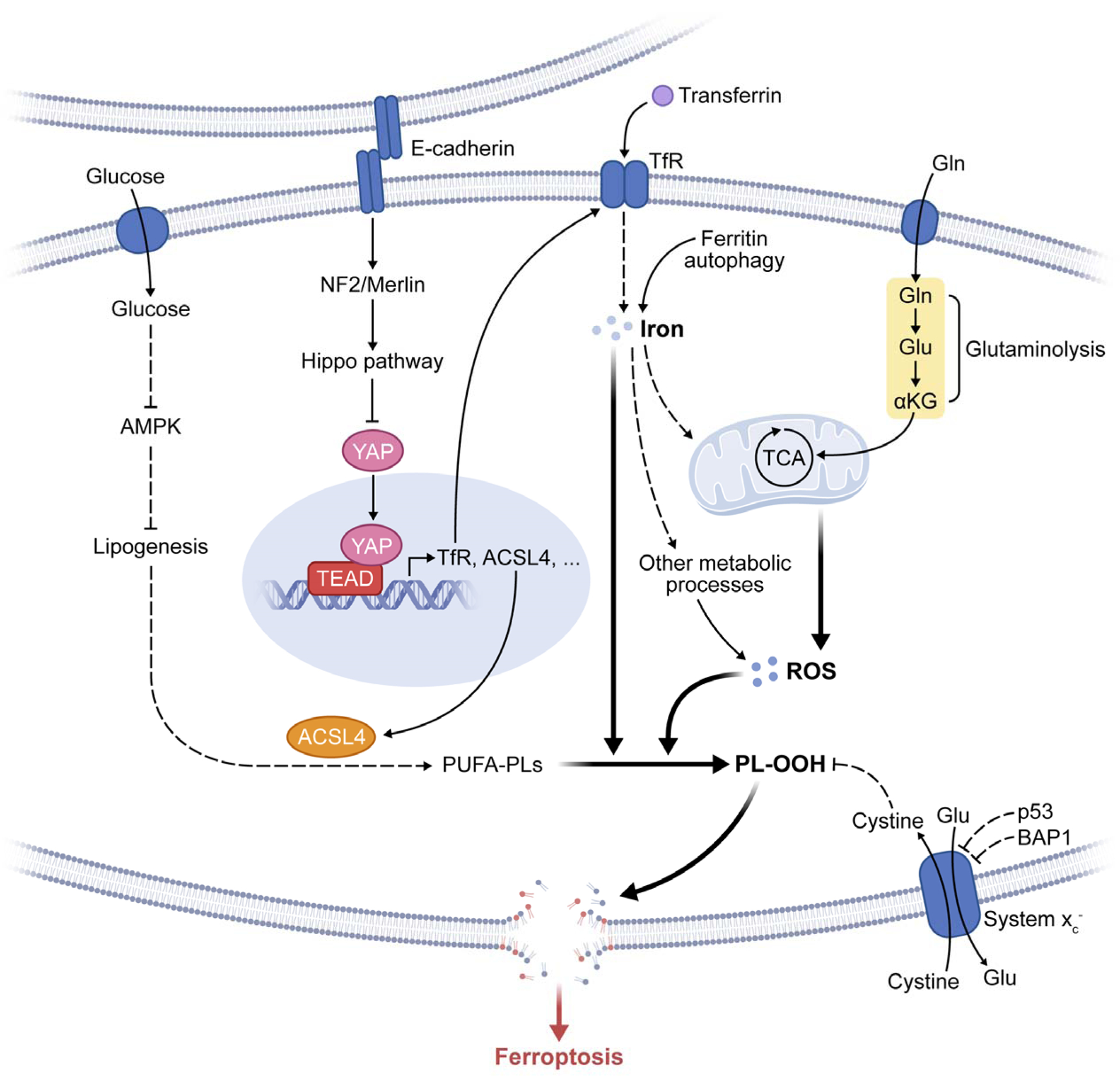
The figure depicts the regulation of ferroptosis by multiple metabolic events (such as lipogenesis, autophagy, and mitochondrial TCA cycle) and signaling pathways (such as E-cadherin-NF2-Hippo-YAP pathway, glucose-regulated AMPK signaling, and p53 and BAP1 tumor suppressor function). See text for detail. Abbreviations: TfR, transferrin receptor; PL-OOH, phospholipid hydroperoxide; PUFA-PL, phospholipid with polyunsaturated fatty acid chain; ROS, reactive oxygen species; TCA, mitochondrial TCA cycle; Gln, glutamine; Glu, glutamate; αKG, α-ketoglutarate.
The involvement of mitochondria in ferroptosis underscores its metabolic nature (Fig. 4), and based on these findings, one can also raise a plethora of hypotheses and questions. For example, would glucose, a major fuel of mitochondrial TCA cycle, regulate ferroptosis? Indeed, glucose starvation has been recently shown to suppress ferroptosis68,69. However, mechanistically this appears to be mainly due to AMPK signaling, instead of TCA cycle engagement (detailed later). And on a related note, if the ferroptotic function of glutamine is solely through its anaplerotic role in the TCA cycle, then how can glutamine be essential for cysteine-deprivation-induced ferroptosis even when cells are cultured in the presence of abundant glucose62? Thus, a detailed study of both TCA-cycle-dependent and independent roles of glutaminolysis in ferroptosis is warranted. Furthermore, and fundamental to ferroptosis, does mitochondrial metabolism promote ferroptosis by generating specific lipid precursors for PLOOH synthesis (intermediates of the TCA cycle can participate in lipogenesis), or via the generation of reactive oxygen species (ROS; natural byproducts of oxidative metabolic reactions)? The observation that both the mitochondrial activity and glutaminolysis are crucial for cysteine-deprivation-induced ferroptosis, but are dispensable for that induced by GPX4 inhibition64, lend more support to the latter mechanism.
Besides mitochondria, plant cells possess another unique organelle specialized in metabolism – the chloroplast – to conduct photosynthesis, which comprises a chain of oxidative-reductive anabolic reactions. As ferroptosis has been observed in plants2,70–72, an intriguing speculation is that chloroplasts may also play an important role in the regulation of ferroptosis in plants.
Iron in ferroptosis.
The essential function of cell metabolism, especially phospholipid peroxidation, in ferroptosis provides insights into why this death modality depends on iron. First, LOXs and POR, metabolic enzymes implicated in phospholipid peroxidation, require iron for catalysis; and iron is also essential for a plethora of metabolic enzymes involved in the generation of cellular ROS. Second, the non-enzymatic, iron-dependent Fenton chain reaction is likely essential for ferroptosis: when GPX4 is inhibited, PLOOHs can persist longer, initiating Fenton reaction to rapidly amplify PLOOHs, the hallmark of ferroptosis29 (Fig. 3). PLOOHs can react with both ferrous and ferric ions to generate free radicals PLO• and PLOO•, respectively, and these free radicals react with PUFA-PLs to further propagate PLOOH production.
Given the central role of iron in cell viability and death, it is not a surprise that cellular iron homeostasis is under exquisite control, mainly through a posttranscriptional network dictated by iron-regulatory proteins IRP1 and IRP273,74 to regulate intracellular iron storage/release and import/export. Conceivably, many cellular processes alter the sensitivity of cells toward ferroptosis by changing cellular labile iron contents. Transferrin and its receptor work together to promote ferroptosis by importing iron into the cell62. Conversely, mechanisms that enhance cellular iron export have been shown to render cells more resistant to ferroptosis75,76. As discussed, ferritin and its autophagic degradation can also regulate ferroptosis63,66. Further, liberating iron from degrading heme via hemeoxygenase-1 (HO-1)-mediated heme degradation has also been implicated in ferroptosis; yet a series of conflicting data suggest HO-1 to either promote or suppress ferroptosis77,78.
Recent in vivo mouse model studies further illustrated the role of iron regulation in ferroptosis. For example, genetic deletion of ferritin heavy chain promotes cardiomyopathy, likely via enhancing ferroptosis79. Intriguingly, hepatocyte-specific knockout of the transferrin gene in mice led to rather unexpected phenotype: feeding the knockout mice with high dietary diet increased iron loading in hepatocytes, cells normally responsible for transferrin synthesis in the body, and rendered the mice more susceptible to liver fibrosis, which could be ameliorated by lipophilic RTAs80. This study suggests that in the absence of transferrin, SLC39A14 is compensatorily upregulated in hepatocytes, leading to excessive import of iron.
GPX4-independent surveillance pathways.
Although the cyst(e)ine-GSH-GPX4 axis is considered the main ferroptosis-regulating system in mammals22,49, genome-wide screens have recently uncovered GPX4-independent mechanisms. Using either a genetic suppressor screen or a synthetic lethal CRISPR-Cas9 screen, the Conrad group and Olzmann group independently identified ferroptosis suppressor protein 1 (FSP1) as the second mainstay of ferroptosis81,82, acting differently from the thiol-dependent axis. FSP1 was previously called AIFM283,84, as it is homologous with AIFM1 (apoptosis inducing factor mitochondria associated 1)85. AIFM1, initially considered to be pro-apoptotic (like AIFM2), is nowadays considered to mediate transport and proper folding of mitochondrial intermembrane proteins instead86. Similarly, FSP1 lacks substantial pro-apoptotic function, but rather protects cells from ferroptosis induced by inhibition or genetic deletion of GPX481,82.
FSP1 is myristoylated and associates with several cell membrane structures including plasma membrane, Golgi apparatus, and perinuclear structures. Mutation of the myristoylation site abrogates its cell-protective function. Mechanistically, due to its NADH:ubiquinone oxidoreductase activity87, FSP1 suppresses lipid peroxidation and ferroptosis by either reducing ubiquinone (CoQ10)/semihydroquinone to yield ubiquinol, which in turn may directly reduce lipid radicals to terminate lipid autoxidation, or indirectly via regenerating oxidized α-tocopheryl radical (vitamin E)81,82, the most powerful natural chain breaking antioxidant in lipids (Fig. 2B). These studies thus also solved a long-standing mystery why there is so much extramitochondrial ubiquinone in some cells and tissues88, in contrast to its canonical role in the mitochondrial electron transport chain.
Using a CRISPR/Cas9-based activator screen, GTP cyclohydrolase-1 (GCH1) was recently reported to protect against ferroptosis via its metabolic products tetrahydrobiopterin (BH4) and dihydrobiopterin (BH2)89. BH4 was shown to prevent depletion of phospholipids containing two polyunsaturated fatty acyl tails, likely involving a dual mechanism by both acting as a direct RTA and being involved in CoQ10 synthesis89,90 (Fig. 2C). While the role of GCH1 in protecting tissues and organs from ferroptosis remains to be elucidated, knockout studies showed that loss of Gch1 in mice causes bradycardia and embryonic death during mid-gestation91. Besides these systems which either directly act on peroxides in lipid bilayers or on phospholipid radicals via naturally occurring RTAs, still other cell-intrinsic mechanisms may exist that protect against deleterious lipid peroxidation. In this context, accumulation of squalene, a metabolite of the cholesterol pathway, was reported to confer anti-ferroptotic activity in cholesterol-auxotrophic ALK (anaplastic lymphoma kinase)-positive anaplastic large cell lymphoma cell lines and primary tumors, albeit it remains to be shown whether this is a cancer subtype-specific effect or a general protective mechanism92 (Fig. 2C).
Regulation of ferroptosis
Conceivably, biological processes that modulate ferroptosis-promoting or surveillance molecules, redox and iron homeostasis, and cell metabolism could impact ferroptosis. As expected, the oxidative-stress-responsive transcription factor NRF2 can mitigate ferroptosis by stimulating the expression of its multiple canonical target genes (see93 for a comprehensive review). Additionally, mounting evidence has demonstrated that under specific biological contexts, multiple signaling pathways can dictate the susceptibility of cells to ferroptosis.
Regulation of GPX4 and FSP1.
Despite their prevailing importance for ferroptosis, little is known how GPX4 and FSP1 are regulated on the transcriptional, translational and, equally important, post-translational/activity level, under both physiological and pathological conditions. Nevertheless, it seems that both GPX4 and FSP1, at least to some extent, intersect with the mevalonate pathway: isopentenylation stabilizes the selenocysteine-specific tRNA (Trsp), which is required for the synthesis of selenoenzymes including GPX4, and as one of the final metabolites of the mevalonate pathway, ubiquinone (CoQ10), is a major substrate of FSP194.
As one of 25 dedicated selenoproteins in humans, GPX4 expression is tightly regulated94. For instance, selenium supplementation was shown to boost its expression through an SP1- and-TFAP2c-dependent manner in neurons95. However, it should be noted that GPX4 can be regarded a housekeeping protein being constitutively expressed in most tissues and organs, unlike those true selenium-responsive proteins such as selenoprotein P (SELENOP), GPX1, and GPX3. Other transcription factors that have been reported to regulate GPX4 expression include CEBP1 and C/EBPs in enterocytes96, and NF-Y in some cancer cells97. Post-transcriptionally, guanine-rich sequence-binding factor 1 (GRSF1) was reported to bind to the 5’UTR of the mitochondrial form of the Gpx4 mRNA, causing increased translation of mitochondrial GPX4 that plays an essential role during sperm development98,99. The relevance of these regulatory events to ferroptosis, however, remains undefined.
Accumulating evidence suggests that GPX4 is regulated on the activity and stability level. For instance, impaired GSH-dependent reduction of the active site selenocysteine, due to sustained oxidative stress and concomitant lack of GSH may cause irreversible inactivation of GPX4 by formation of a redox-dead dehydroalanine in a process known as ß-cleavage100. Moreover, formation of a selenylamide between the selenenic acid and a neighboring amino acid may protect the enzyme from irreversible inactivation. However, it needs to be further explored whether any of these mechanisms are at play during pathological conditions, such as ischemia/reperfusion injuries (IRI), albeit it has been reported that intestinal IRI is associated with much lower GPX4 levels42. In addition, several well-established ferroptosis inducers including RSL3 ultimately cause GPX4 depletion, either through covalent inhibition of the active site selenocysteine, impairment of the mevalonate metabolism or general iron-dependent oxidative stress101–103.
As another prominent ferroptosis suppressor, FSP1 was originally described as a p53-responsive gene, therefore initially called p53-responsive gene 3 (PRG3)104. FSP1 is a target of transcription factors NRF2105, CEBP106, and PPARα107. Interestingly, in T-cell lymphoblastic lymphoma cells, FSP1 expression was reported to be upregulated by the long non-coding RNA (lncRNAs) maternally expressed 3 (MEG3) gene and suppressed by miR-214108, two regulatory RNAs involved in tumor development. Beyond transcriptional regulation, almost nothing is known how the oxidoreductase activity of FSP1 is regulated and how its differing subcellular localization impacts on its role in different physiological and pathophysiological processes81–83,106. But the promiscuity of FSP1 toward both the reducing and oxidizing substrates (including NADH, NADPH, CoQ10 and α-tocopherol) suggests its complex regulation.
E-cadherin-NF2-Hippo-YAP signaling and ferroptosis.
The Hippo-YAP pathway is involved in a myriad of biological functions, including cell proliferation and organ size control109,110. The investigation of the role of this pathway in ferroptosis was initiated from an interesting observation that cells grown with high density are often more resistant to ferroptosis induced by both cysteine deprivation and GPX4 inhibition41. This observation is reminiscent of earlier observations; a directly relevant one is that cells were shown to survive Gpx4 knockout if they are cultured at extremely high density or as spheres19,111. Mechanistically, the cell density effect on ferroptosis in epithelial cells is mediated by E-cadherin-mediated cell-cell contacts, and such intercellular interaction activates intracellular Hippo signaling through NF2/merlin tumor suppressor, and thus inhibits the transcription co-regulatory activity of oncoprotein YAP; various target genes of YAP, including ACSL4, TfR1, and possibly others, are regulators of ferroptosis41 (Fig. 4). Consistent with this finding, TAZ, the close homolog of YAP, has also been shown to enhance ferroptosis in a cell-density-regulated manner in kidney cancer cells that predominantly express TAZ instead of YAP112. Further, a closer analysis of the target gene profile of YAP and TAZ indicates that YAP and TAZ transcriptionally modulate various processes relevant to ferroptosis, including iron homeostasis, oxidative response, and cellular metabolism113,114.
The role of the E-cadherin-NF2-Hippo-YAP pathway in dictating ferroptosis sensitivity has important implications. First, as multiple components of this pathway are frequently mutated in cancer, ferroptosis induction might be exploited as a potential therapeutic approach for the treatment of these specific cancers, a topic further discussed later. Second, as such cell density-dependent ferroptosis was also observed in non-epithelial cells that do not express E cadherin41, would other cadherins or cell adhesion molecules also mediate similar mechanisms for ferroptosis suppression? Thirdly, the Hippo-YAP pathway is important in development and interacts with various other signaling pathways, thus one might investigate the potential function of ferroptosis in normal biology through its link to the Hippo-YAP pathway. Speculatively, did cadherins evolve in multicellular organisms in such a way that they not only physically connect cells, mediate intercellular communication via transducing signals into intracellular machinery, but also function as an ancient mechanism to protect cells from oxidative stress challenges and its most devastating consequence, ferroptosis? All these events are essential for the being of a multicellular organism. Indeed, the expression of cadherin proteins can be traced back all the way to some ancestral metazoan species115.
AMPK signaling and ferroptosis.
Intuitively, energy and metabolic stress should result in the loss of energy and thus a cascading failure of systems needed to maintain homeostasis, such as energy-dependent ion gradients across cell membranes116, ultimately resulting in cell death. Moreover, metabolically stressing cells with glucose starvation increases ROS production117, suggesting that glucose starvation promotes ferroptosis. Surprisingly, glucose starvation instead blocks ferroptosis68,69. This protective effect was dependent on the activity of energy-sensing kinase, AMPK. Hence, when glucose is absent, AMPK is activated, turning on an energy stress-protective program against ferroptosis that involves impaired biosynthesis of PUFAs, which are essential for lipid peroxidation-driven ferroptosis34,37 (Fig. 4). This finding has practical applications, as activating this energy stress program was found to protect against renal IRI68. It may be that this protective mechanism exists as a first line of defense against organ injury, which can often involve energy failure along with the injury.
Hypoxia signaling and ferroptosis.
Given that ferroptosis is death by phospholipid peroxidation, a longstanding question has been whether it is dependent on oxygen concentrations. An early experiment indicated little loss of sensitivity to erastin-induced ferroptosis in 1% oxygen, suggesting that hypoxia does not suppress ferroptosis23. A recent study indicated that hypoxia sensing can in fact drive sensitivity to ferroptosis: clear cell carcinomas were found to be highly sensitive to ferroptosis induced by GPX4 inhibition, and this sensitivity was driven by HIF2α through PUFA lipid remodeling via hypoxia-inducible, lipid droplet-associated protein (HILPDA)33. Clear cell carcinomas have a characteristic clear cytoplasmic staining upon histology staining and are difficult to treat118, indicating clinical implication of this finding. Also, it is tempting to speculate that the HIF2α-HILPDA-driven sensitization to ferroptosis may be an ancient means to eliminate nascent hypoxic tumors.
Potential biological functions of ferroptosis
The mechanisms of ferroptosis suggest its potential evolutionary origin: ever since living organisms on Earth began to use oxygen to drive metabolism, essentially through a series of chemical redox reactions, the transition metal iron has become the prime factor to catalyze these reactions. An inevitable side product of such iron-dependent, redox-based metabolism are ROS, including PLOOHs. When the cellular level of PLOOHs exceeds a certain threshold, cells succumb to ferroptosis. Conceivably, multiple surveillance mechanisms like GPX4 and FSP1 have evolved to protect cells from ferroptosis. Thus speculatively, ferroptosis might be a most ancient form of regulated cell death.
But is there any beneficial, physiologically relevant role for ferroptosis? In theory, this is possible. A related example might be ROS and lipid hydroperoxides: first considered as mere toxic “byproducts” of metabolism and later found to confer essential physiological functions, for instance in cell signaling and immunity, they are even deliberately generated at several subcellular sites to regulate cellular physiological events119–121. Similarly, ferroptosis might also be adapted to accomplish roles beneficial for life. Mounting evidence, although indirect, suggests the physiological function of ferroptosis in tumor suppression and immune surveillance.
Ferroptosis in tumor suppression.
Multiple tumor suppressors have been shown to sensitize cells to ferroptosis. Therefore, a reasonable hypothesis is that ferroptosis contributes to the antitumor activity of these tumor suppressors, i.e., tumor suppression might be an innate physiological function of ferroptosis.
Among such tumor suppressors, the involvement of p53 in ferroptosis has been thoroughly investigated. Through a detailed analysis of specific lysine acetylation sites of p53, Gu and colleagues found that p53 can potentiate ferroptosis via suppressing the transcription of system xc– subunit SLC7A11, and this function may contribute to the tumor suppressive function of p53 in vitro and in vivo122,123. Furthermore, a cancer-prone single nucleotide polymorphism of p53, P47S, was found to be enriched in the African population and to make cancer cells more resistant to ferroptosis124. While these findings are consistent with a role of ferroptosis in p53-mediated tumor suppression, it is not clear whether the loss of ferroptosis-promoting activity of p53 is the only functional consequence caused these specific mutations. Notably, in contrast to these studies, p53 has also been reported to prevent ferroptosis via modulating its other transcriptional targets125,126. Given that p53 can regulate a large cohort of target genes that are involved in various biological processes, its precise role in ferroptosis can well be context-dependent.
Similar to p53, the tumor suppressor and epigenetic regulator BAP1 can also promote ferroptosis by downregulating SLC7A11 expression127. But unlike p53, whose ferroptosis-promoting activity alone has been suggested to be sufficient to suppress tumorigenesis in vivo123, it is not clear how significantly the ferroptosis-promoting activity of BAP1 contributes to its tumor-suppressive function.
Fumarase, an enzyme catalyzing the conversion of fumarate to malate in the TCA cycle, is a bona fide tumor suppressor in leiomyoma and papillary renal cell carcinoma128. It has been elusive how this metabolic enzyme can counter-intuitively suppress tumorigenesis, and how it does so only in a few specific cancer types. The involvement of fumarase in the ferroptosis-promoting function of the mitochondrial TCA cycle64 sheds light to these questions: during the development of certain types of cancer, tumor cells encounter substantial oxidative stress and are primed to undergoing ferroptosis. Under these conditions, loss of fumarase function, although impairing growth capability, renders cells more resistant to ferroptosis, thus enhancing their survival and potential of cancerous transformation.
Ferroptosis in immune surveillance.
Unlike other forms of cell death such as apoptosis, pyroptosis and necroptosis, which are clearly engaged by the immune system129, it remains largely obscure whether sensitizing or triggering ferroptosis by extrinsic or intrinsic mechanisms may fulfill a similar “physiological role”. Due to the multifaceted nature and the involvement of numerous metabolic pathways directly impinging on ferroptosis, it is highly likely that certain key nodes of the ferroptosis process are targeted by the immune system. Early findings by Sato’s laboratory suggested that system xc– activity is suppressed by interferon-γ (IFN-γ)130. Such a mechanism is employed by CD8+ T cells in sensitizing tumor cells toward ferroptosis131. Whether such a direct mechanism of immune cells on other ferroptosis nodes might be of physiological relevance remains elusive. Early studies indicated that interleukins 4 and 13 suppress GPX4 expression in certain cells coinciding with an increased expression of ALOX15, thus permitting a full-blown immune activation by metabolites of the arachidonate pathway132. Since GPX4 is known to suppress the activities of lipoxygenases and cyclooxygenases by lowering the lipid peroxide tone54, it is possible that impaired activity of GPX4 may have a profound effect on the secretion of pro- and anti-inflammatory lipid mediators, which in turn may alert the innate immune system for the presence of cells and tissues in a ferroptosis-sensitive state.
The potential immune function of ferroptosis is not limited to the mammalian system. A recent report suggests that ferroptosis promotes host immunity of rice against infection by fungus M. Oryzae4. A ferroptosis-like cell death of specific rice blast cells, associated with lipid peroxidation and inhibitable by iron chelators, prevents M. Oryzae infection. Intriguingly, ferroptosis-like death of specific cells of M. Oryzae is conversely required for its host invasion and development in the host. As such, this finding also implicates, for the first time, a potential developmental role of ferroptosis.
Role of ferroptosis in disease and therapeutic opportunities
Although the physiological function of ferroptosis remains obscure, its role in a plethora of human diseases has been extensively documented. Importantly, pharmacological modulation of ferroptosis has been demonstrated to be a promising therapeutic venue for the treatment of cancer and IRI in diverse preclinical animal models. While in this section we focus on the role and therapeutic implication of ferroptosis in cancer and IRI, ferroptosis has been implicated in the pathogenesis of many other diseases, as discussed in Box-1.
Box 1 – Diseases possibly related to ferroptosis.
In addition to cancer and ischemic organ injuries, ferroptosis has been implicated in the pathogenesis of a growing list of other diseases, such as neurodegeneration19,165–167,182, liver and lung fibrosis80,183, autoimmune diseases184,185, Mycobacterium-tuberculosis-induced tissue necrosis186, cigarette-smoking-associated chronic obstructive pulmonary disease187,188, and a rare genetic neurological disorder called Pelizaeus-Merzbacher Disease189. While this long list speaks to the clinical relevance and therapeutic potential of ferroptosis-modulating approaches, further investigation is required to determine if there is indeed a causative role of ferroptosis in these diseases. For example, in most cases the general observation is that non-apoptotic cell death was observed in the disease tissue, and that a ferroptosis inhibitor, often a lipophilic RTA, could mitigate the observed cell death and in some cases, alter the severity of the symptom. However, lipid peroxides can regulate immunity and inflammation, processes that play important roles in all the listed diseases. Therefore, caution is needed to distinguish whether the observed effect of lipophilic RTA is via modulation of inflammation or ferroptosis, or both. A detailed mechanistic interrogation of tissue cell death associated with the disease, including examining specific in vivo ferroptosis biomarkers (which the field sorely miss), will be crucial for this purpose.
Ferroptosis in cancer.
Ferroptosis has been linked to cancer since the very beginning of the field: the initial discovery of chemical inducers of ferroptosis is the result of a hunting for novel cancer therapeutic compounds20,21. Subsequent mechanistic studies have revealed that numerous cancer-relevant genes and signaling pathways regulate ferroptosis. The observations that mesenchymal and dedifferentiated cancer cells, which are often resistant to apoptosis and common therapeutics, as well as so called “therapy-persister” cancer cells, are highly susceptible to ferroptosis inducers39,133,134, further underscores the promise of ferroptosis induction as a novel cancer therapy.
Conceptually, since ferroptosis is an oxidative stress-induced, metabolic form of cell death, it seems logical to propose that cancer cells may have higher tendency to undergo ferroptosis, due to overall more active metabolism and higher ROS load. Adding to this notion, it has been shown that cancer cells often demand high iron contents135,136, which may further sensitize them to ferroptosis. However, cancer cells may also harness additional genetic or epigenetic alterations to counter these metabolic and oxidative burdens, such as increased expression of SLC7A11 or upregulation of the anti-oxidative transcription factor NRF2137. Therefore, whether a given cancer is more sensitive or resistant to ferroptosis induction is dictated by its specific genetic background. The genomics of cancer, as well as various other parameters as discussed below, should be considered for the development of ferroptosis induction-based cancer therapy.
Ferroptosis-related cancer biomarkers and genetic alterations
Multiple oncoproteins, tumor suppressors, and oncogenic signal transduction pathways can regulate ferroptosis. Therefore, their alterations in cancer can be used as biomarkers to predict the responsiveness of cancer cells to ferroptosis-inducing therapies.
Taking the E-cadherin-NF2-Hippo-YAP pathway as an example and based on the TCGA analysis, loss of function mutation of tumor suppressor E cadherin is a frequent event in breast lobular invasive carcinoma (~65%) and diffusive gastric adenocarcinoma (~25%), among other cancers; loss of function mutation of NF2 occurs in >30% of mesothelioma and in all of a group of benign diseases known as NF2 diseases; similarly, mutation of Hippo components LATS1/2 tumor suppressors also occurs in a variety of cancers. Although genetic mutation of YAP is rare, its overexpression and post-translational activation is frequently observed in cancer. Importantly, malignant mutations of these genes usually drive metastasis, protect cancer cells from apoptosis, and make them more resistant to common cancer therapies138,139. Therefore, the finding that these same mutations sensitize cancer cells to ferroptosis41 not only makes these mutations potential biomarkers for ferroptosis-inducing therapies, but also unveils an unusual “Achilles’ heel” of these malignant cells and suggests unique therapeutic opportunity of ferroptosis induction. This opportunity is particularly attractive for gastric cancer and mesothelioma, both of which currently lack effective treatment140,141.
Potential ferroptosis-inducing cancer therapies
The development of ferroptosis induction-based cancer therapies is being actively pursued. While several untargeted nanoparticle-based strategies to deliver iron, peroxides and other toxic cargoes to kill tumor cells have been tested, the presence of multiple enzymes that control ferroptosis enables the development of targeted approaches142,143. Perhaps the most obvious target is GPX4, as it is expressed in most cancer cell lines and is important for their survival22. Yet, GPX4 lacks a classical small molecule binding pocket, and the available GPX4 inhibitors covalently modify the selenocysteine residue of GPX4 as well as other selenoproteins144, raising the issue of specificity. These inhibitors are also highly reactive and thus unstable, but this may be overcome by developing masked prodrugs which can be metabolically converted into their active forms intracellularly145,146. Nonetheless, the main caveat remains that GPX4 is essential for various peripheral tissues, such as kidney tubular cells and certain neuronal subpopulations in mice29, thus targeting GPX4 will probably cause substantial side effects.
Unlike targeting GPX4, approaches to limit cellular cyst(e)ine availability by inhibiting system xc– emerge to be highly promising given the fact that Slc7a11 knockout in mice does not cause major pathologies147, and that the expression of SLC3A2/SLC7A11 negatively correlates with clinical outcome of melanoma and glioma patients131,148. Indeed, studies to restrain tumor growth and tumor metastasis in various tumor entities by inhibiting system xc– either pharmacologically149,150 or genetically151–157 in mouse models have provided highly promising results. The higher vulnerability of various tumor tissues to system xc− inhibition than that of normal tissues is likely due to the more active metabolism and other alterations in these tumor cells, making them subjective to sustained oxidative stress and thus more addictive to system xc− function. Obviously, in order to apply system xc− inhibition-based therapies, a careful stratification of patient tumor tissues is required to examine system xc− expression (e.g., SLC7A11 overexpression may indicate cancer cell addiction to cystine)82 and other biomarkers that determine the sensitivity of tumors to system xc− inhibition.
Similar to SLC7A11, knockout of FSP1 does not cause embryonic lethality or apparent pathologies158, suggesting a broad therapeutic window for targeting FSP1. Moreover, FSP1 is abundantly expressed in a large number of cancer cell lines and is the highest ranked gene correlating with the resistance to GPX4 inhibitors in a panel of 860 cancer cell lines82. Cancer cells deprived of GPX4 can be efficiently killed by FSP-specific inhibitor iFSP1, while in GPX4-proficient cancer cells iFSP1 synergizes with RSL382. Therefore, FSP1 inhibitors may find their way into the clinics, especially in therapy-resistant or dedifferentiating cancer entities.
Ferroptosis induction-based treatment might also be combined with other therapeutic approaches, with immune checkpoint blockade and radiotherapy as potential options. Combining immune checkpoint blockade with other therapies has been actively pursued. One rationale is that these other therapies may induce immunogenic cell death in tumor tissues to enhance the potency of immune checkpoint blockade. Intriguingly, Zou and colleagues discovered that anti-PDL1 therapy can potentiate ferroptosis-inducing therapy131. They found that anti-PDL1 antibodies stimulated CD8+ T cells to secrete IFNg, and that IFNg subsequently triggered downregulation of both subunits of system xc− in tumor cells, thus sensitizing cancer cells to ferroptosis. Therefore, immunotherapy in combination with ferroptosis induction represents a promising treatment in that the two therapeutic modalities mutually potentiate each other, leading to synergistic anticancer effect. As of radiation, recent evidence indicates that it can induce ferroptosis on its own, and can synergize with ferroptosis inducers and immunotherapy, providing potentially effective therapeutic combination regimens43,159,160. This sensitizing effect was observed at the level of cell death, lipid peroxidation, ferroptosis-linked gene expression changes, and lipidomic changes, as well as in cell-line and patient-derived xenograft cancer models and freshly isolated patient glioma slice cultures. Moreover, cytoplasmic but not nuclear radiation was found to synergize with ferroptosis inducers, suggesting the involvement of lipid-peroxidizing activity and glutathione-depleting activity of radiation in ferroptosis, but not its canonical DNA-damaging effects. On the other hand, the ferroptosis-inducing effects of radiation and immunotherapy have been shown to be synergistic, driven by radiation-induced ATM activation and immunotherapy-driven SLC7A11 downregulation160. Finally, radiation has been shown to upregulate ACSL4 in cancer43. Therefore, the ferroptosis effect of radiation is likely mediated by multiple mechanisms and may be context-dependent. Notably, ferroptosis is implicated as a contributor to some of the adverse events of radiation, such as lung fibrosis and killing of granulocyte-macrophage hematopoietic progenitor cells161,162.
Ferroptosis in IRI.
Ischemia followed by reperfusion can induce massive cell death and inflammatory response in the affected organs, resulting in devastating diseases including brain stroke, ischemic heart diseases, and injuries of liver and kidney. Remarkably, ischemic heart disease remains to be the disease that causes highest mortality worldwide163. Strong evidence, as detailed below, indicates that ferroptosis is a major contributor to IRI-associated cell death, due to, at least partially, oxidative stress induced by ischemia. These findings suggest that ferroptosis inhibition is a potential therapeutic approach for the treatment of IRI diseases.
Brain and heart
Multiple studies have established a role of ferroptosis in neurotoxicity and brain injuries, suggesting the therapeutic potential of ferroptosis inhibition. An ex vivo experiment using rat hippocampal slice culture showed that glutamate-induced neuronal excitotoxic death can be blocked by the ferroptosis inhibitor ferrostatin-11. As glutamate neurotoxicity is involved in stroke and various neurodegenerative diseases164, and high concentration of extracellular glutamate can induce ferroptosis through inhibiting system xc− function1, probably ferroptosis contributes to the pathogenesis of these brain diseases. Consistently, genetic studies confirmed that conditional Gpx4 deletion can cause symptoms mimicking neurodegeneration19,165–167. Further, iron chelators and lipophilic RTAs have been tested for stroke and neurodegeneration in diverse experimental systems75,166,168,169. Although the adverse effect of systemic use of iron chelators remains a serious concern, these experiments have nevertheless validated the significant contribution of ferroptosis in these diseases.
The role of ferroptosis in ischemic heart disease has also been extensively investigated. In an ex vivo system mimicking IRI of mouse heart, it has been shown that iron chelators and glutaminolysis inhibitors significantly mitigated cardiomyocyte cell death and damage of heart tissue and function, suggesting the potential therapeutic value of ferroptosis-targeting for the treatment of ischemic heart disease62. More recently, an in vivo mouse model study further confirmed this notion170.
To design feasible ferroptosis-targeted therapies to treat the fatal diseases of stroke and ischemic heart injury, many important factors need to be considered. For example, both diseases can be extremely acute and only allow a short time window for intervention; therefore, can ferroptosis-targeted therapies be applied timely thereby exerting their effects rapidly enough? Moreover, since there are multiple ferroptosis surveillance pathways, and ischemia/reperfusion may induce ferroptosis in different organs via selectively disrupting one of these surveillance pathways, can we develop specific ferroptosis inhibitors that can effectively treat a certain disease while causing minimal side effect to other organs?
IRI associated with organ transplantation
Besides the brain, perhaps the kidney is the most ferroptosis-sensitive organ identified in adult mammals; survival of the proximal kidney tubular cells depends on functional GPX449, and they are at high risk in response to an IRI scenario as occurring during kidney transplantation. Consistently, ferroptosis inhibitors have been shown to mitigate kidney tubular cell death and acute renal failure in models of warm IRI, in the genetic model of inducible whole body Gpx4 deletion, and in folic acid-induced acute kidney injury (AKI)49,171,172. These studies suggest that ferroptosis causes activation of the innate immune system via necroinflammation associated with specific molecular alterations172–174. Another frequently transplanted organ is the liver. While mice with hepatocyte-specific ablation of Gpx4 die neonatally, high dietary vitamin E can compensate for the lack of GPX4175. Moreover, liproxstatin-1 protects the liver parenchyma from IRI49. Lastly, ferroptosis has also been implicated in heart transplantation176. Collectively, ferroptosis inhibitors might be effective drugs to alleviate tissue detriment inflicted by oxygen deprivation/reoxygenation in different organs.
Potential therapeutic targets for IRI
There are several potential points of therapeutic intervention in the ferroptosis network, although each node of intervention carries its own risk/reward profile. Given that ferroptosis is driven by phospholipid peroxidation, one strategy is to introduce agents that prevent the peroxidation process, for example, by preventing the propagation of lipid peroxyl radicals through the administration of lipophilic RTAs, such as liproxstatin51. These agents are highly effective in cell models of ferroptosis and can also be effective in some in vivo contexts. Their pharmacological properties need to be further improved before human trials can be considered. A related approach is to administer PUFAs that are chemically resistant to peroxidation, such as by incorporation of deuterium at the bis-allylic carbon that is normally susceptible to peroxidation37,177. Such deuterated PUFAs have been shown to be effective inhibitors of ferroptosis and a variety of chronic ferroptosis-linked degenerative diseases, although with lower potency than ferrostatin and liproxstatin37,102. Similarly, monounsaturated fatty acids have been shown to suppress ferroptosis and could be exploited as a potential therapy36,37.
A second strategy for blocking lipid peroxidation is to either inhibit enzymes that drive lipid peroxidation or to deplete the labile iron pool, e.g., through using iron chelators. Iron chelators have been explored in numerous clinical indications but have selectivity and safety issues to content with. On the other hand, targeting enzymes responsible for generating PUFA-PLs and PLOOHs, such as ACSL4, LPCAT3, LOXs, and POR, might be feasible. As defined molecular targets, they are preferable from a target-centric drug discovery standpoint. However, they also have other functions in various tissues, complicating the potential therapeutic outcomes.
Conclusions and perspectives
Ferroptosis is a concept that integrates into a cohesive and satisfying whole previously disparate aspects involving the metabolism of iron, selenium, amino acids, lipids, and redox chemistry (Fig. 5). This increasingly vibrant tapestry intersects a broad array of biological processes, including both normal physiology and diverse pathophysiologies. From our current vantage point surveying the landscape that is encompassed by the field of ferroptosis, we can share some concerns, challenges, and a preview of potential discoveries to be made in the future.
Figure 5. Relationship of ferroptosis to other biological processes and diseases.
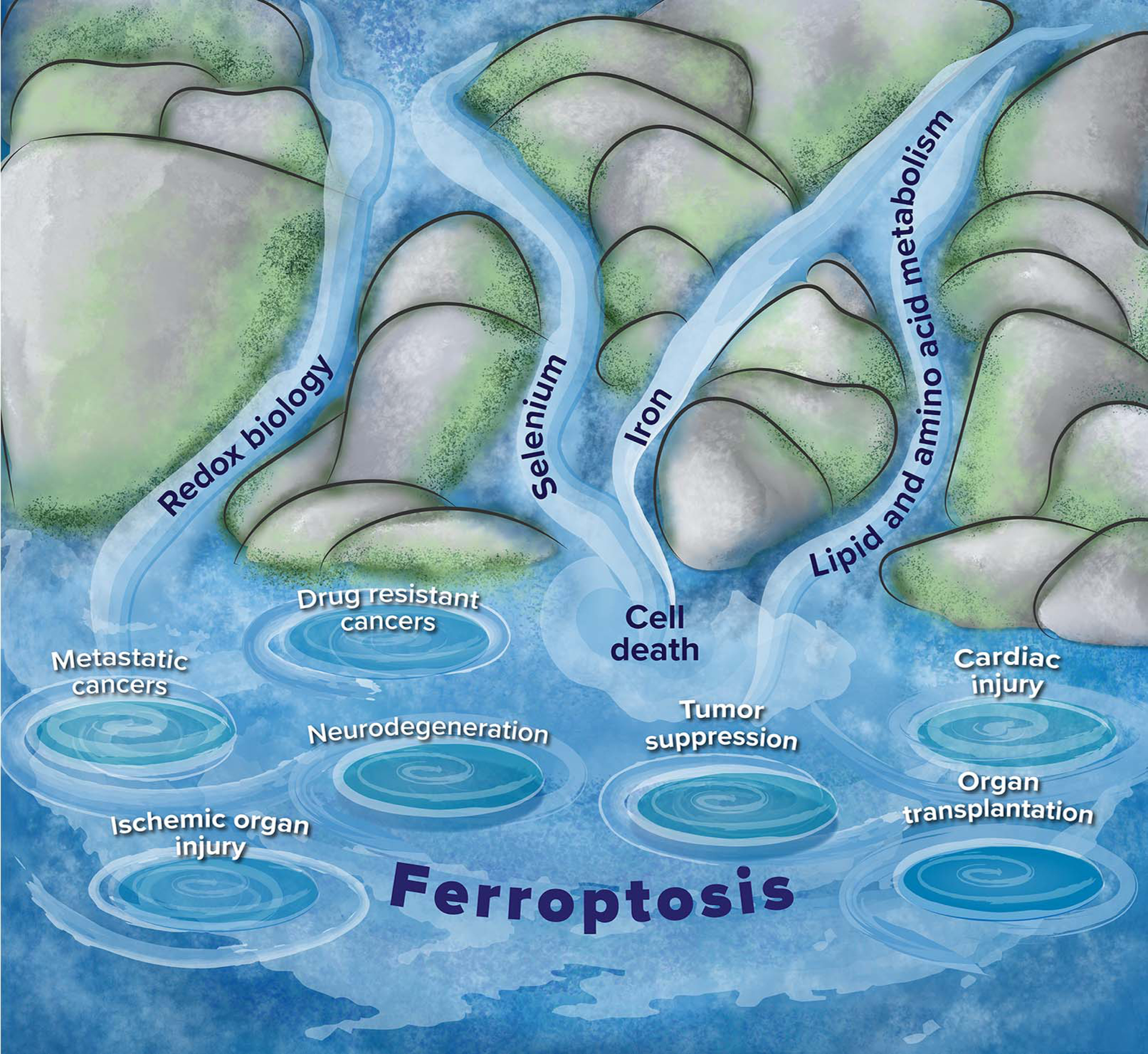
The figure depicts streams of related fields that feed into the interdisciplinary field of ferroptosis, as well as some diseases for which ferroptosis has been implicated. Redox biology, selenium, iron and lipid and amino acid metabolism (streams) are related biological processes and fields that control aspects of ferroptosis. The whirlpools represent diseases that have been linked to ferroptosis: metastatic cancers and drug-resistant cancers have sensitivity to ferroptosis inducers, tumor suppression occurs in some cases through inducing ferroptosis, and neurodegeneration, ischemic organ injury, cardiac injury and organ transplantation involve activation of ferroptosis as part of degenerative processes.
First, we share some concerns. While there is enthusiasm for the increasing explanatory power of the concept of ferroptosis and its interdigitation with diverse aspects of biology, care must be taken to rigorously test the role of ferroptosis in biological processes. Suppression of cell death by a single inhibitor of ferroptosis cannot be taken as sufficient evidence that ferroptosis is involved in a process of interest. The current operational definition of ferroptosis178 is that of a cell death process suppressed by both iron depletion and lipophilic RTAs, such as ferrostatin-1, liproxstatin-1, vitamin E, or CoQ10. Both requirements are critical, as there are iron-dependent lethal mechanisms distinct from ferroptosis179 that may involve lysosomal toxicity, for example, and oxidative stress mechanisms independent of iron1. We suggest adding another requirement: direct detection of lipid peroxidation (using LC-MS/MS, fluorescent dyes, or antibodies such as 1F83). The mobilization and upregulation of TfR1 is another potential marker recently reported180,181. Although its generality remains to be established, this new marker allows for the differentiation of contexts involving oxidative stress versus ferroptosis. Additional markers of ferroptosis would be highly valuable to the field. Another significant concern is various improper experimental approaches used in ferroptosis research, an inevitable problem in any newly emerging area. To address this issue, Box-2 and Box-3 present a practical guideline for ferroptosis investigation, in which important considerations for in vivo and in vitro study of ferroptosis are described. In particular, we list certain inappropriate tools and approaches that have frequently appeared in the literature but should be avoided in future research.
Box 2 – Potential caveats when using genetically engineered model systems to study ferroptosis.
Sensitivity to ferroptosis is inevitably linked to intra- and extra-cellular redox conditions. For instance, SLC7A11 is upregulated in many cell lines and promptly induced in ex vivo-cultured cells/organs due to the virtual absence of the reduced form of cysteine in culture media. While the knockout of Slc7a11 is well-tolerated in mice because cysteine is present in vivo in its reduced form that is taken up into cells by neutral amino acids transporters147, almost all cell lines with Slc7a11-knockout invariably die when cultured in vitro as all cysteine is present in its oxidized form in culture medium. These stark discrepancies in redox conditions between cell culture-based and in vivo studies call for a careful interpretation of findings solely based on in vitro studies. Equally important, when using system xc− inhibitors it needs to be assessed whether SLC7A11 is expressed at all in the cell line to be studied. Alarmingly, many currently available antibodies against SLC7A11 are unspecific and unsuitable for determining SLC7A11 levels.
When intervening with GPX4 activity, one needs to consider that GPX4 is promiscuous not only using glutathione as substrate, but also other thiol-containing compounds and even protein thiols26. This is of particular significance when using ferroptosis-inducing agents that act at the different steps of the cyst(e)ine-glutathione-GPX4 axis. Moreover, since cell density has a profound impact on the cells’ sensitivity toward ferroptosis19,41, it needs to be carefully controlled. Notably, since GPX4 requires selenium for its biosynthesis and sera contain varying amounts of selenium (and vitamin E), different cell culture sera may have a profound impact on the results. This is also of vital importance when using Gpx2−/− mice since chows can vary in their vitamin E contents (both tocopherols and tocotrienols), which have major impact on the phenotypes of knockout mice175,190,191. Therefore, this should also be considered in future studies when using pathological models or tumor xenografts. Consistently, supplementation with selenium and vitamin E has been shown to be cancer-promoting in some contexts192.
Box 3 – Experimental consideration when using pharmacological tools to probe ferroptosis.
Small molecule tools are valuable for probing ferroptosis. However, several issues involving specificity, biological relevance, pharmacokinetics and pharmacodynamics must be considered when using such tools. First, when using pharmacological inhibitors of a certain enzyme to assess whether the enzyme regulates ferroptosis, one needs to determine whether the purported inhibitor can act as lipophilic RTAs and thus inhibit ferroptosis independent of the enzyme under study, as occurred in some LOX-ferroptosis studies51,52,193. Second, it is important to verify in the experimental system whether a given small molecule probe indeed affects the intended target or pathway. For example, in using RSL3 to inhibit GPX4, or erastin to inhibit system xc−, it is important to test whether those targets are inhibited by each probe using a biochemical assay or a pharmacodynamic marker of target inhibition, such as mRNA expression changes. Moreover, when using small molecule in animal studies, it is important to first determine the appropriate formulation and route of administration to achieve and acceptable pharmacokinetic profile and pharmacodynamic response of the target tissue. Many small molecules that are potent and selective probes in cellular assays have no utility in animal models due to poor solubility, stability and/or pharmacokinetics. A few common approaches that are inappropriate and thus should be avoided are listed below:
RSL3 has low solubility and difficult to measure pharmacokinetics in mice, not suitable suited for in vivo animal studies, except in some cases by direct injection into tissues or tumors. Erastin has low solubility and low metabolic stability, unsuitable for use in animal studies (imidazole ketone erastin (IKE) has good solubility, high stability and favorable pharmacokinetics and pharmacodynamics in mice, and has been validated for in vivo studies). Similarly, ferrostatin-1 is not suitable for in vivo studies, whereas liproxstatin-1 and vitamin E can be used in vivo as ferroptosis inhibitors. In vivo data generated by using iron chelators should be treated with caution, as depleting iron may lead to a plethora of consequences in addition to ferroptosis inhibition, and it is unclear whether a specific outcome is caused by iron chelation or an off-target activity of the compounds. Inducing or potentiating ferroptosis in cell culture by adding iron (ferric or ferrous) into the culture medium is not appropriate for the study of ferroptosis mechanisms, as free iron ions catalyze Fenton chain reaction in the presence of oxygen and trace amount of lipid peroxides (generated by accidental cell death or ferroptosis triggered by other inducers), thus amplifying extracellular lipid peroxidation and killing cells rapidly.
Second, we note a major challenge. Despite significant progress in understanding the mechanisms regulating ferroptosis, we still do not know how cells ultimately die. Uncontrolled peroxidation of PUFA-PLs is the most downstream step identified; it may be that peroxidized phospholipids cause membrane damage or even pore formation, compromising membrane integrity. However, a recent report found that phospholipids containing two PUFA tails are particularly effective drivers of ferroptosis89, suggesting that crosslinking may be an aspect of the membrane damage upon ferroptosis. In this scenario, it may be that limiting fluidity of membrane components causes failure of some vital functions associated with membranes, resulting in cell death (analogous to vascular stiffening seen in vivo). However, other possibilities exist, such as that oxidized PUFA-PLs decompose into reactive electrophiles that then damage other macromolecules. In this model, reactive electrophiles serve the function in ferroptosis that caspases serve in apoptosis—to inactivate key structural and functional proteins within cells. We hope that over the next several years, the mechanism of ferroptosis execution will be unambiguously elucidated.
Third and finally, we can predict some potential future discoveries that lie ahead in the field of ferroptosis. Identifying precise biomarkers for in vivo ferroptosis, as cleaved caspase-3 for apoptosis in vivo and in tissue sections, will be fundamentally important for determining the physiological function and therapeutic role of this cell death modality. Identifying the enigmatic natural triggers for ferroptosis also remains a pressing challenge, but one that may see breakthroughs in the coming years. The following molecules are potentially, or proximate to, the natural ferroptosis triggers—glutamate, p53, iron, radiation, and PUFA-PLs. Glutamate is an abundant neurotransmitter, and excess extracellular glutamate is sufficient to inhibit system xc−, resulting in ferroptosis1. Nature may exploit this effect of extracellular glutamate to induce ferroptosis in developmental or other physiological contexts, such as the elimination of unnecessary neurons in the sculpting of neural circuits. The tumor suppressor p53 induces ferroptosis likely to suppress tumor development; induction of p53 in the presence of basal lipid peroxidative damage may be a natural means of eliminating certain stressed cells through ferroptosis. High levels of iron alone can trigger ferroptosis in diverse model systems, suggesting that in some contexts, releasing ferritin-stored iron or increasing iron import might be a means of eliminating cells through ferroptosis. Low to moderate doses of ionizing radiation have been shown to induce ferroptosis. Thus, contexts in which radiation is abundant, or radioprotective cellular mechanisms are compromised might be circumstances in which ferroptosis is naturally induced. For example, there is speculation that there might once have been life on Mars, which either has been lost or has moved underground due to the harsh radiation present on the planet surface. Thus, high radiation environments might make ferroptosis a default program that needs to be continuously counteracted for life to survive and evolve, by establishing a network of anti-ferroptotic mechanisms; and downregulating these protective mechanisms would then serve as a natural trigger for driving ferroptosis. Finally, PUFA uptake and PUFA-PL synthesis may be sufficient in some contexts to induce ferroptosis; in such cases, a cell could be induced to die by ferroptosis by release of PUFAs in the extracellular environment, or by driving expression of enzymes such as LPCAT3 and ACSL4 that stimulate PUFA-PL synthesis. Some of these mechanisms may be involved in development, tumor suppression and clearance of infected cells by the immune system, and regulation of tissue size.
In summary, there is a wealth of foreseeable opportunity to elucidate both the execution mechanisms of ferroptosis and the contexts in which this form of cell death is naturally harnessed. Such studies will illuminate how Nature chooses to co-opt ferroptosis to an array of purposes beyond disease and therapy.
Acknowledgements
X.J. is supported by NIH grants R01CA204232 and R01CA166413, as well as NCI cancer centre core grant P30 CA008748 to MSKCC. X.J. is an inventor on patents and patent applications involving programmed cell death and autophagy. B.R.S. is supported by NCI grants P01CA87497 and R35CA209896 and NINDS grant R61NS109407. B.R.S. is an inventor on patents and patent applications involving ferroptosis and co-founded and serves as a consultant to Inzen Therapeutics and Nevrox Limited. M.C. is supported by the Deutsche Forschungsgemeinschaft (DFG) CO 291/5-2 and CO 291/7-1, the German Federal Ministry of Education and Research (BMBF) VIP+ program NEUROPROTEKT (03VP04260), the Helmholtz Validation Fund (0042), the Ministry of Science and Higher Education of the Russian Federation (075-15-2019-1933), the Else Kröner-Fresenius-Stiftung, and the m4 Award provided by the Bavarian Ministry of Economic Affairs, Regional Development and Energy (StMWi). M.C. has further received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No. GA 884754). M.C. is an inventor on patents for some of the compounds described herein.
Glossary:
- Alkoxyl radicals
a reactive species consisting of a hydrocarbon chain attached to an oxygen with an unpaired electron
- Anaplerosis
replenishment of TCA cycle intermediates
- Bis-allylic carbon
a carbon that is in between and adjacent to two carbon-carbon double bonds
- Conjugated diene
two carbon-carbon double bonds separated by a carbon-carbon single bond, such that the pi systems of the two double bonds form a continuous delocalized pi system
- Dihydrobiopterin (BH2)
a natural biopterin metabolite in the partially oxidized form
- Immune checkpoint blockade
the process of activating immune cells by inhibiting a signaling pathway that normally suppresses their activity
- Ischemia/reperfusion injury
the organ injury resulting from limited blood flow and oxygen deprivation followed by reintroduction of blood flow and oxygenation
- Mevalonate pathway
a lipid biosynthesis pathway that produces terpenes-derived compounds such as cholesterol and coenzyme Q10
- Selenoproteins
proteins into which the element selenium is incorporated, mainly via the incorporation of selenocysteine
- Tetrahydrobiopterin (BH4)
a natural biopterin metabolite in the fully reduced form
- Transferrin
a protein that transports iron into cells through receptor-mediated endocytosis
References
- 1.Dixon SJ et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072, doi: 10.1016/j.cell.2012.03.042 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Distefano AM et al. Heat stress induces ferroptosis-like cell death in plants. Journal of Cell Biology 216, 463–476 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bogacz M & Krauth-Siegel RL Tryparedoxin peroxidase-deficiency commits trypanosomes to ferroptosis-type cell death. Elife 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen Q, Liang ML, Yang F, Deng YZ & Naqvi NI Ferroptosis contributes to developmental cell death in rice blast. New Phytol (2020). [DOI] [PubMed] [Google Scholar]
- 5.Kerr JF, Wyllie AH & Currie AR Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26, 239–257 (1972). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galluzzi L et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death and Differentiation 25, 486–541 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jenkins NL et al. Changes in ferrous iron and glutathione promote ferroptosis and frailty in aging Caenorhabditis elegans. Elife 9, doi: 10.7554/eLife.56580 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan S, Schubert D & Maher P Oxytosis: A novel form of programmed cell death. Current topics in medicinal chemistry 1, 497–506 (2001). [DOI] [PubMed] [Google Scholar]
- 9.Eagle H Nutrition needs of mammalian cells in tissue culture. Science 122, 501–514 (1955). [DOI] [PubMed] [Google Scholar]
- 10.Eagle H, Piez KA & Oyama VI The biosynthesis of cystine in human cell cultures. J Biol Chem 236, 1425–1428 (1961). [PubMed] [Google Scholar]
- 11.Coltorti M, De Ritis F & Giusti G [Enzymatic mechanisms of transsulfuration in biology and clinical practice]. G Clin Med 37, 285–323 (1956). [PubMed] [Google Scholar]
- 12.Bannai S & Kitamura E Transport interaction of L-cystine and L-glutamate in human diploid fibroblasts in culture. J Biol Chem 255, 2372–2376 (1980). [PubMed] [Google Scholar]
- 13.Sato H, Tamba M, Ishii T & Bannai S Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem 274, 11455–11458 (1999). [DOI] [PubMed] [Google Scholar]
- 14.Meister A Glutathione metabolism. Methods Enzymol 251, 3–7, doi: 10.1016/0076-6879(95)51106-7 (1995). [DOI] [PubMed] [Google Scholar]
- 15.Shi ZZ et al. Glutathione synthesis is essential for mouse development but not for cell growth in culture. Proc Natl Acad Sci U S A 97, 5101–5106 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yant LJ et al. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic Biol Med 34, 496–502. (2003). [DOI] [PubMed] [Google Scholar]
- 17.Banjac A et al. The cystine/cysteine cycle: a redox cycle regulating susceptibility versus resistance to cell death. Oncogene 27, 1618–1628 (2008). [DOI] [PubMed] [Google Scholar]
- 18.Mandal PK et al. System x(c)− and thioredoxin reductase 1 cooperatively rescue glutathione deficiency. J Biol Chem 285, 22244–22253, doi:M110.121327 [pii] 10.1074/jbc.M110.121327 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seiler A et al. Glutathione Peroxidase 4 Senses and Translates Oxidative Stress into 12/15-Lipoxygenase Dependent- and AIF-Mediated Cell Death. Cell Metab 8, 237–248 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Dolma S, Lessnick SL, Hahn WC & Stockwell BR Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 3, 285–296 (2003). [DOI] [PubMed] [Google Scholar]
- 21.Yagoda N et al. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature 447, 864–868 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang WS et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 156, 317–331, doi: 10.1016/j.cell.2013.12.010 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dixon SJ et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife 3 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ursini F, Maiorino M, Valente M, Ferri L & Gregolin C Purification from pig liver of a protein which protects liposomes and biomembranes from peroxidative degradation and exhibits glutathione peroxidase activity on phosphatidylcholine hydroperoxides. Biochim Biophys Acta 710, 197–211. (1982). [DOI] [PubMed] [Google Scholar]
- 25.Ursini F, Maiorino M & Gregolin C The selenoenzyme phospholipid hydroperoxide glutathione peroxidase. Biochim Biophys Acta 839, 62–70. (1985). [DOI] [PubMed] [Google Scholar]
- 26.Maiorino M, Conrad M & Ursini F GPx4, Lipid Peroxidation, and Cell Death: Discoveries, Rediscoveries, and Open Issues. Antioxid Redox Signal, doi: 10.1089/ars.2017.7115 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Schwarz K & Foltz CM SELENIUM AS AN INTEGRAL PART OF FACTOR 3 AGAINST DIETARY NECROTIC LIVER DEGENERATION. Journal of the American Chemical Society 79, 3292–3293, doi: 10.1021/ja01569a087 (1957). [DOI] [Google Scholar]
- 28.Bieri JG An Effect of Selenium and Cystine on Lipide Peroxidation in Tissues Deficient in Vitamin E. Nature 184, 1148–1149, doi: 10.1038/1841148a0 (1959). [DOI] [PubMed] [Google Scholar]
- 29.Conrad M & Pratt DA The chemical basis of ferroptosis. Nat Chem Biol 15, 1137–1147, doi: 10.1038/s41589-019-0408-1 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Bateman L Olefin oxidation. Quarterly Reviews, Chemical Society 8, 147–167 (1954). [Google Scholar]
- 31.Doll S et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol 13, 91–98, doi: 10.1038/nchembio.2239 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dixon SJ et al. Human Haploid Cell Genetics Reveals Roles for Lipid Metabolism Genes in Nonapoptotic Cell Death. ACS chemical biology 10, 1604–1609, doi: 10.1021/acschembio.5b00245 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zou Y et al. A GPX4-dependent cancer cell state underlies the clear-cell morphology and confers sensitivity to ferroptosis. Nat Commun 10, 1617, doi: 10.1038/s41467-019-09277-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kagan VE et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol 13, 81–90, doi: 10.1038/nchembio.2238 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim JH, Lewin TM & Coleman RA Expression and characterization of recombinant rat Acyl-CoA synthetases 1, 4, and 5. Selective inhibition by triacsin C and thiazolidinediones. J Biol Chem 276, 24667–24673, doi: 10.1074/jbc.M010793200 (2001). [DOI] [PubMed] [Google Scholar]
- 36.Magtanong L et al. Exogenous Monounsaturated Fatty Acids Promote a Ferroptosis-Resistant Cell State. Cell Chem Biol 26, 420–432 e429, doi: 10.1016/j.chembiol.2018.11.016 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang WS et al. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci U S A 113, E4966–4975, doi: 10.1073/pnas.1603244113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tesfay L et al. Stearoyl-CoA Desaturase 1 Protects Ovarian Cancer Cells from Ferroptotic Cell Death. Cancer Res 79, 5355–5366, doi: 10.1158/0008-5472.CAN-19-0369 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Viswanathan VS et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature, doi: 10.1038/nature23007 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown CW, Amante JJ, Goel HL & Mercurio AM The alpha6beta4 integrin promotes resistance to ferroptosis. J Cell Biol 216, 4287–4297, doi: 10.1083/jcb.201701136 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu J et al. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature 572, 402–406, doi: 10.1038/s41586-019-1426-6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y et al. Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ, doi: 10.1038/s41418-019-0299-4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lei G et al. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res 30, 146–162, doi: 10.1038/s41422-019-0263-3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Porter NA, Caldwell SE & Mills KA Mechanisms of free radical oxidation of unsaturated lipids. Lipids 30, 277–290, doi: 10.1007/bf02536034 (1995). [DOI] [PubMed] [Google Scholar]
- 45.Kuhn H, Banthiya S & van Leyen K Mammalian lipoxygenases and their biological relevance. Biochim Biophys Acta 1851, 308–330, doi: 10.1016/j.bbalip.2014.10.002 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, Maher P & Schubert D A role for 12-lipoxygenase in nerve cell death caused by glutathione depletion. Neuron 19, 453–463, doi:S0896–6273(00)80953–8 [pii] (1997). [DOI] [PubMed] [Google Scholar]
- 47.van Leyen K et al. Baicalein and 12/15-lipoxygenase in the ischemic brain. Stroke 37, 3014–3018, doi:01.STR.0000249004.25444.a5 [pii] 10.1161/01.STR.0000249004.25444.a5 (2006). [DOI] [PubMed] [Google Scholar]
- 48.Jin G et al. Protecting against cerebrovascular injury: contributions of 12/15-lipoxygenase to edema formation after transient focal ischemia. Stroke 39, 2538–2543, doi:STROKEAHA.108.514927 [pii] 10.1161/STROKEAHA.108.514927 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Friedmann Angeli JP et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol 16, 1180–1191, doi: 10.1038/ncb3064 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsushita M et al. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. The Journal of experimental medicine 212, 555–568, doi: 10.1084/jem.20140857 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zilka O et al. On the Mechanism of Cytoprotection by Ferrostatin-1 and Liproxstatin-1 and the Role of Lipid Peroxidation in Ferroptotic Cell Death. ACS Cent Sci 3, 232–243, doi: 10.1021/acscentsci.7b00028 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shah R, Shchepinov MS & Pratt DA Resolving the Role of Lipoxygenases in the Initiation and Execution of Ferroptosis. ACS Cent Sci 4, 387–396, doi: 10.1021/acscentsci.7b00589 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chu B et al. ALOX12 is required for p53-mediated tumour suppression through a distinct ferroptosis pathway. Nat Cell Biol 21, 579–591, doi: 10.1038/s41556-019-0305-6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Proneth B & Conrad M Ferroptosis and necroinflammation, a yet poorly explored link. Cell Death Differ 26, 14–24, doi: 10.1038/s41418-018-0173-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Friedmann Angeli JP, Krysko DV & Conrad M Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat Rev Cancer 19, 405–414, doi: 10.1038/s41568-019-0149-1 (2019). [DOI] [PubMed] [Google Scholar]
- 56.Wenzel SE et al. PEBP1 Wardens Ferroptosis by Enabling Lipoxygenase Generation of Lipid Death Signals. Cell 171, 628–641 e626, doi: 10.1016/j.cell.2017.09.044 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zou Y et al. Cytochrome P450 oxidoreductase contributes to phospholipid peroxidation in ferroptosis. Nat Chem Biol 16, 302–309, doi: 10.1038/s41589-020-0472-6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ghosh MK, Mukhopadhyay M & Chatterjee IB NADPH-initiated cytochrome P450-dependent free iron-independent microsomal lipid peroxidation: specific prevention by ascorbic acid. Mol Cell Biochem 166, 35–44, doi: 10.1023/a:1006841228483 (1997). [DOI] [PubMed] [Google Scholar]
- 59.Vance RE, Hong S, Gronert K, Serhan CN & Mekalanos JJ The opportunistic pathogen Pseudomonas aeruginosa carries a secretable arachidonate 15-lipoxygenase. Proc Natl Acad Sci U S A 101, 2135–2139, doi: 10.1073/pnas.0307308101 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aldrovandi M et al. Specific oxygenation of plasma membrane phospholipids by Pseudomonas aeruginosa lipoxygenase induces structural and functional alterations in mammalian cells. Biochim Biophys Acta Mol Cell Biol Lipids 1863, 152–164, doi: 10.1016/j.bbalip.2017.11.005 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dar HH et al. Pseudomonas aeruginosa utilizes host polyunsaturated phosphatidylethanolamines to trigger theft-ferroptosis in bronchial epithelium. J Clin Invest 128, 4639–4653, doi: 10.1172/JCI99490 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gao M, Monian P, Quadri N, Ramasamy R & Jiang X Glutaminolysis and Transferrin Regulate Ferroptosis. Mol Cell 59, 298–308, doi: 10.1016/j.molcel.2015.06.011 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gao M et al. Ferroptosis is an autophagic cell death process. Cell Res 26, 1021–1032, doi: 10.1038/cr.2016.95 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gao M et al. Role of Mitochondria in Ferroptosis. Mol Cell 73, 354–363 e353, doi: 10.1016/j.molcel.2018.10.042 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Doherty J & Baehrecke EH Life, death and autophagy. Nat Cell Biol 20, 1110–1117, doi: 10.1038/s41556-018-0201-5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hou W et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy 12, 1425–1428, doi: 10.1080/15548627.2016.1187366 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tan SL, Sagara Y, Lin YB, Maher P & Schubert D The regulation of reactive oxygen species production during programmed cell death. Journal of Cell Biology 141, 1423–1432 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee H et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nature Cell Biology 22 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li C et al. LKB1-AMPK axis negatively regulates ferroptosis by inhibiting fatty acid synthesis. Signal Transduct Target Ther 5, 187, doi: 10.1038/s41392-020-00297-2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gao Z et al. OsCUL3a-Associated Molecular Switches Have Functions in Cell Metabolism, Cell Death, and Disease Resistance. J Agric Food Chem 68, 5471–5482, doi: 10.1021/acs.jafc.9b07426 (2020). [DOI] [PubMed] [Google Scholar]
- 71.Hajdinak P, Czobor A & Szarka A The potential role of acrolein in plant ferroptosis-like cell death. PLoS One 14, e0227278, doi: 10.1371/journal.pone.0227278 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dangol S, Chen Y, Hwang BK & Jwa NS Iron- and Reactive Oxygen Species-Dependent Ferroptotic Cell Death in Rice-Magnaporthe oryzae Interactions. Plant Cell 31, 189–209, doi: 10.1105/tpc.18.00535 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang DL, Ghosh MC & Rouault TA The physiological functions of iron regulatory proteins in iron homeostasis - an update. Front Pharmacol 5, 124, doi: 10.3389/fphar.2014.00124 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Andrews NC & Schmidt PJ Iron homeostasis. Annu Rev Physiol 69, 69–85, doi: 10.1146/annurev.physiol.69.031905.164337 (2007). [DOI] [PubMed] [Google Scholar]
- 75.Tuo QZ et al. Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol Psychiatr 22, 1520–1530 (2017). [DOI] [PubMed] [Google Scholar]
- 76.Brown CW et al. Prominin2 Drives Ferroptosis Resistance by Stimulating Iron Export. Dev Cell 51, 575–586 e574, doi: 10.1016/j.devcel.2019.10.007 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kwon MY, Park E, Lee SJ & Chung SW Heme oxygenase-1 accelerates erastin-induced ferroptotic cell death. Oncotarget 6, 24393–24403, doi: 10.18632/oncotarget.5162 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sun X et al. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology 63, 173–184, doi: 10.1002/hep.28251 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fang X et al. Loss of Cardiac Ferritin H Facilitates Cardiomyopathy via Slc7a11-Mediated Ferroptosis. Circulation research 127, 486–501, doi: 10.1161/CIRCRESAHA.120.316509 (2020). [DOI] [PubMed] [Google Scholar]
- 80.Yu Y et al. Hepatic Transferrin Plays a Role in Systemic Iron Homeostasis and Liver Ferroptosis. Blood, doi: 10.1182/blood.2019002907 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bersuker K et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575, 688–692, doi: 10.1038/s41586-019-1705-2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Doll S et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 575, 693–698, doi: 10.1038/s41586-019-1707-0 (2019). [DOI] [PubMed] [Google Scholar]
- 83.Wu M, Xu LG, Li X, Zhai Z & Shu HB AMID, an apoptosis-inducing factor-homologous mitochondrion-associated protein, induces caspase-independent apoptosis. J Biol Chem 277, 25617–25623, doi: 10.1074/jbc.M202285200 (2002). [DOI] [PubMed] [Google Scholar]
- 84.Ohiro Y et al. A novel p53-inducible apoptogenic gene, PRG3, encodes a homologue of the apoptosis-inducing factor (AIF). FEBS Lett 524, 163–171, doi: 10.1016/s0014-5793(02)03049-1 (2002). [DOI] [PubMed] [Google Scholar]
- 85.Susin SA et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397, 441–446, doi: 10.1038/17135 (1999). [DOI] [PubMed] [Google Scholar]
- 86.Reinhardt C et al. AIF meets the CHCHD4/Mia40-dependent mitochondrial import pathway. Biochim Biophys Acta Mol Basis Dis 1866, 165746, doi: 10.1016/j.bbadis.2020.165746 (2020). [DOI] [PubMed] [Google Scholar]
- 87.Elguindy MM & Nakamaru-Ogiso E Apoptosis-inducing Factor (AIF) and Its Family Member Protein, AMID, Are Rotenone-sensitive NADH:Ubiquinone Oxidoreductases (NDH-2). J Biol Chem 290, 20815–20826, doi: 10.1074/jbc.M115.641498 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nyquist SE, Barr R & Morre DJ Ubiquinone from rat liver Golgi apparatus fractions. Biochim Biophys Acta 208, 532–534, doi: 10.1016/0304-4165(70)90228-x (1970). [DOI] [PubMed] [Google Scholar]
- 89.Kraft VAN et al. GTP Cyclohydrolase 1/Tetrahydrobiopterin Counteract Ferroptosis through Lipid Remodeling. ACS Cent Sci 6, 41–53, doi: 10.1021/acscentsci.9b01063 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Soula M et al. Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nat Chem Biol, doi: 10.1038/s41589-020-0613-y (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Douglas G et al. A requirement for Gch1 and tetrahydrobiopterin in embryonic development. Dev Biol 399, 129–138, doi: 10.1016/j.ydbio.2014.12.025 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Garcia-Bermudez J et al. Squalene accumulation in cholesterol auxotrophic lymphomas prevents oxidative cell death. Nature 567, 118–122, doi: 10.1038/s41586-019-0945-5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Anandhan A, Dodson M, Schmidlin CJ, Liu P & Zhang DD Breakdown of an Ironclad Defense System: The Critical Role of NRF2 in Mediating Ferroptosis. Cell Chem Biol 27, 436–447, doi: 10.1016/j.chembiol.2020.03.011 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Conrad M & Proneth B Selenium: Tracing Another Essential Element of Ferroptotic Cell Death. Cell Chem Biol 27, 409–419, doi: 10.1016/j.chembiol.2020.03.012 (2020). [DOI] [PubMed] [Google Scholar]
- 95.Alim I et al. Selenium Drives a Transcriptional Adaptive Program to Block Ferroptosis and Treat Stroke. Cell 177, 1262–1279 e1225, doi: 10.1016/j.cell.2019.03.032 (2019). [DOI] [PubMed] [Google Scholar]
- 96.Speckmann B et al. Induction of glutathione peroxidase 4 expression during enterocytic cell differentiation. J Biol Chem 286, 10764–10772, doi: 10.1074/jbc.M110.216028 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huang HS, Chen CJ & Chang WC The CCAAT-box binding factor NF-Y is required for the expression of phospholipid hydroperoxide glutathione peroxidase in human epidermoid carcinoma A431 cells. FEBS Lett 455, 111–116, doi: 10.1016/s0014-5793(99)00866-2 (1999). [DOI] [PubMed] [Google Scholar]
- 98.Ufer C et al. Translational regulation of glutathione peroxidase 4 expression through guanine-rich sequence-binding factor 1 is essential for embryonic brain development. Genes Dev 22, 1838–1850 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schneider M et al. Mitochondrial glutathione peroxidase 4 disruption causes male infertility. Faseb J 23, 3233–3242, doi:fj.09–132795 [pii] 10.1096/fj.09-132795 (2009). [DOI] [PubMed] [Google Scholar]
- 100.Orian L et al. Selenocysteine oxidation in glutathione peroxidase catalysis: an MS-supported quantum mechanics study. Free Radic Biol Med 87, 1–14, doi: 10.1016/j.freeradbiomed.2015.06.011 (2015). [DOI] [PubMed] [Google Scholar]
- 101.Shimada K et al. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat Chem Biol 12, 497–503, doi: 10.1038/nchembio.2079 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gaschler MM et al. FINO2 initiates ferroptosis through GPX4 inactivation and iron oxidation. Nat Chem Biol 14, 507–515, doi: 10.1038/s41589-018-0031-6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen GQ et al. Artemisinin compounds sensitize cancer cells to ferroptosis by regulating iron homeostasis. Cell Death Differ 27, 242–254, doi: 10.1038/s41418-019-0352-3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Horikoshi N, Cong J, Kley N & Shenk T Isolation of differentially expressed cDNAs from p53-dependent apoptotic cells: activation of the human homologue of the Drosophila peroxidasin gene. Biochem Biophys Res Commun 261, 864–869, doi: 10.1006/bbrc.1999.1123 (1999). [DOI] [PubMed] [Google Scholar]
- 105.Chorley BN et al. Identification of novel NRF2-regulated genes by ChIP-Seq: influence on retinoid X receptor alpha. Nucleic Acids Res 40, 7416–7429, doi: 10.1093/nar/gks409 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nguyen HP et al. Aifm2, a NADH Oxidase, Supports Robust Glycolysis and Is Required for Cold- and Diet-Induced Thermogenesis. Mol Cell 77, 600–617 e604, doi: 10.1016/j.molcel.2019.12.002 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Venkatesh D et al. MDM2 and MDMX promote ferroptosis by PPARalpha-mediated lipid remodeling. Genes Dev 34, 526–543, doi: 10.1101/gad.334219.119 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fan FY et al. The inhibitory effect of MEG3/miR-214/AIFM2 axis on the growth of T-cell lymphoblastic lymphoma. Int J Oncol 51, 316–326, doi: 10.3892/ijo.2017.4006 (2017). [DOI] [PubMed] [Google Scholar]
- 109.Pan D The hippo signaling pathway in development and cancer. Dev Cell 19, 491–505, doi: 10.1016/j.devcel.2010.09.011 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhao B, Lei QY & Guan KL The Hippo-YAP pathway: new connections between regulation of organ size and cancer. Curr Opin Cell Biol 20, 638–646, doi: 10.1016/j.ceb.2008.10.001 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schneider M et al. Absence of Glutathione Peroxidase 4 Affects Tumor Angiogenesis through Increased 12/15-Lipoxygenase Activity. Neoplasia 12, 254–263 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yang WH et al. The Hippo Pathway Effector TAZ Regulates Ferroptosis in Renal Cell Carcinoma. Cell Rep 28, 2501–2508 e2504, doi: 10.1016/j.celrep.2019.07.107 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zanconato F, Cordenonsi M & Piccolo S YAP/TAZ at the Roots of Cancer. Cancer Cell 29, 783–803, doi: 10.1016/j.ccell.2016.05.005 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zanconato F et al. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat Cell Biol 17, 1218–1227, doi: 10.1038/ncb3216 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gul IS, Hulpiau P, Saeys Y & van Roy F Evolution and diversity of cadherins and catenins. Exp Cell Res 358, 3–9, doi: 10.1016/j.yexcr.2017.03.001 (2017). [DOI] [PubMed] [Google Scholar]
- 116.Green DR, Galluzzi L & Kroemer G Metabolic control of cell death. Science 345, 1466-+ (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hay N Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy? Nature Reviews Cancer 16, 635–649 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sugiyama T et al. Clinical characteristics of clear cell carcinoma of the ovary - A distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer 88, 2584–2589 (2000). [PubMed] [Google Scholar]
- 119.Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q & Griendling KK Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circulation research 122, 877–902, doi: 10.1161/CIRCRESAHA.117.311401 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ray PD, Huang BW & Tsuji Y Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 24, 981–990, doi: 10.1016/j.cellsig.2012.01.008 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sies H & Jones DP Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Bio (2020). [DOI] [PubMed] [Google Scholar]
- 122.Jiang L et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 520, 57–62, doi: 10.1038/nature14344 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang SJ et al. Acetylation Is Crucial for p53-Mediated Ferroptosis and Tumor Suppression. Cell Rep 17, 366–373 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jennis M et al. An African-specific polymorphism in the TP53 gene impairs p53 tumor suppressor function in a mouse model. Genes Dev 30, 918–930, doi: 10.1101/gad.275891.115 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xie Y et al. The Tumor Suppressor p53 Limits Ferroptosis by Blocking DPP4 Activity. Cell Rep 20, 1692–1704, doi: 10.1016/j.celrep.2017.07.055 (2017). [DOI] [PubMed] [Google Scholar]
- 126.Tarangelo A et al. p53 Suppresses Metabolic Stress-Induced Ferroptosis in Cancer Cells. Cell Rep 22, 569–575, doi: 10.1016/j.celrep.2017.12.077 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang Y et al. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat Cell Biol 20, 1181–1192, doi: 10.1038/s41556-018-0178-0 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tomlinson IP et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet 30, 406–410, doi: 10.1038/ng849 (2002). [DOI] [PubMed] [Google Scholar]
- 129.Green DR The Coming Decade of Cell Death Research: Five Riddles. Cell 177, 1094–1107, doi: 10.1016/j.cell.2019.04.024 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sato H, Fujiwara K, Sagara J & Bannai S Induction of cystine transport activity in mouse peritoneal macrophages by bacterial lipopolysaccharide. Biochem J 310 (Pt 2), 547–551 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang W et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature, doi: 10.1038/s41586-019-1170-y (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schnurr K, Borchert A & Kuhn H Inverse regulation of lipid-peroxidizing and hydroperoxyl lipid-reducing enzymes by interleukins 4 and 13. FASEB J 13, 143–154, doi: 10.1096/fasebj.13.1.143 (1999). [DOI] [PubMed] [Google Scholar]
- 133.Hangauer MJ et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature 551, 247–250, doi: 10.1038/nature24297 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tsoi J et al. Multi-stage Differentiation Defines Melanoma Subtypes with Differential Vulnerability to Drug-Induced Iron-Dependent Oxidative Stress. Cancer Cell 33, 890–904 e895, doi: 10.1016/j.ccell.2018.03.017 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Manz DH, Blanchette NL, Paul BT, Torti FM & Torti SV Iron and cancer: recent insights. Ann Ny Acad Sci 1368, 149–161 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Torti SV & Torti FM Iron and cancer: more ore to be mined. Nature Reviews Cancer 13, 342–355 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.de la Vega MR, Chapman E & Zhang DD NRF2 and the Hallmarks of Cancer. Cancer Cell 34, 21–43 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hansen CG, Moroishi T & Guan KL YAP and TAZ: a nexus for Hippo signaling and beyond. Trends Cell Biol 25, 499–513, doi: 10.1016/j.tcb.2015.05.002 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Piccolo S, Dupont S & Cordenonsi M The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev 94, 1287–1312, doi: 10.1152/physrev.00005.2014 (2014). [DOI] [PubMed] [Google Scholar]
- 140.Johnston FM & Beckman M Updates on Management of Gastric Cancer. Curr Oncol Rep 21, 67, doi: 10.1007/s11912-019-0820-4 (2019). [DOI] [PubMed] [Google Scholar]
- 141.Bueno R et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat Genet 48, 407–416, doi: 10.1038/ng.3520 (2016). [DOI] [PubMed] [Google Scholar]
- 142.Hassannia B, Vandenabeele P & Vanden Berghe T Targeting Ferroptosis to Iron Out Cancer. Cancer Cell 35, 830–849, doi: 10.1016/j.ccell.2019.04.002 (2019). [DOI] [PubMed] [Google Scholar]
- 143.Hassannia B et al. Nano-targeted induction of dual ferroptotic mechanisms eradicates high-risk neuroblastoma. J Clin Invest 128, 3341–3355, doi: 10.1172/JCI99032 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen Y et al. Quantitative Profiling of Protein Carbonylations in Ferroptosis by an Aniline-Derived Probe. J Am Chem Soc 140, 4712–4720, doi: 10.1021/jacs.8b01462 (2018). [DOI] [PubMed] [Google Scholar]
- 145.Eaton JK, Ruberto RA, Kramm A, Viswanathan VS & Schreiber SL Diacylfuroxans Are Masked Nitrile Oxides That Inhibit GPX4 Covalently. J Am Chem Soc 141, 20407–20415, doi: 10.1021/jacs.9b10769 (2019). [DOI] [PubMed] [Google Scholar]
- 146.Eaton JK et al. Selective covalent targeting of GPX4 using masked nitrile-oxide electrophiles. Nat Chem Biol 16, 497–506, doi: 10.1038/s41589-020-0501-5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sato H et al. Redox imbalance in cystine/glutamate transporter-deficient mice. J Biol Chem 280, 37423–37429, doi:M506439200 [pii] 10.1074/jbc.M506439200 (2005). [DOI] [PubMed] [Google Scholar]
- 148.Robert SM et al. SLC7A11 expression is associated with seizures and predicts poor survival in patients with malignant glioma. Sci Transl Med 7, 289ra286, doi: 10.1126/scitranslmed.aaa8103 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chung WJ et al. Inhibition of cystine uptake disrupts the growth of primary brain tumors. J Neurosci 25, 7101–7110 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang Y et al. Imidazole Ketone Erastin Induces Ferroptosis and Slows Tumor Growth in a Mouse Lymphoma Model. Cell Chem Biol 26, 623–633 e629, doi: 10.1016/j.chembiol.2019.01.008 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen RS et al. Disruption of xCT inhibits cancer cell metastasis via the caveolin-1/beta-catenin pathway. Oncogene 28, 599–609, doi: 10.1038/onc.2008.414 (2009). [DOI] [PubMed] [Google Scholar]
- 152.Savaskan NE et al. Small interfering RNA-mediated xCT silencing in gliomas inhibits neurodegeneration and alleviates brain edema. Nat Med 14, 629–632 (2008). [DOI] [PubMed] [Google Scholar]
- 153.Arensman MD et al. Cystine-glutamate antiporter xCT deficiency suppresses tumor growth while preserving antitumor immunity. Proc Natl Acad Sci U S A 116, 9533–9542, doi: 10.1073/pnas.1814932116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Daher B et al. Genetic Ablation of the Cystine Transporter xCT in PDAC Cells Inhibits mTORC1, Growth, Survival, and Tumor Formation via Nutrient and Oxidative Stresses. Cancer Res 79, 3877–3890, doi: 10.1158/0008-5472.CAN-18-3855 (2019). [DOI] [PubMed] [Google Scholar]
- 155.Lim JKM et al. Cystine/glutamate antiporter xCT (SLC7A11) facilitates oncogenic RAS transformation by preserving intracellular redox balance. Proc Natl Acad Sci U S A 116, 9433–9442, doi: 10.1073/pnas.1821323116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Badgley MA et al. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science 368, 85–89, doi: 10.1126/science.aaw9872 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sato M et al. Loss of the cystine/glutamate antiporter in melanoma abrogates tumor metastasis and markedly increases survival rates of mice. Int J Cancer 147, 3224–3235, doi: 10.1002/ijc.33262 (2020). [DOI] [PubMed] [Google Scholar]
- 158.Mei J, Webb S, Zhang B & Shu HB The p53-inducible apoptotic protein AMID is not required for normal development and tumor suppression. Oncogene 25, 849–856, doi: 10.1038/sj.onc.1209121 (2006). [DOI] [PubMed] [Google Scholar]
- 159.Ye LF et al. Radiation-Induced Lipid Peroxidation Triggers Ferroptosis and Synergizes with Ferroptosis Inducers. ACS chemical biology 15, 469–484 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lang X et al. Radiotherapy and Immunotherapy Promote Tumoral Lipid Oxidation and Ferroptosis via Synergistic Repression of SLC7A11. Cancer Discov 9, 1673–1685, doi: 10.1158/2159-8290.CD-19-0338 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Li X et al. Ferroptosis inhibitor alleviates Radiation-induced lung fibrosis (RILF) via down-regulation of TGF-1. J Inflamm-Lond 16 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang XH et al. Ionizing radiation induces ferroptosis in granulocyte-macrophage hematopoietic progenitor cells of murine bone marrow. Int J Radiat Biol 96, 584–595 (2020). [DOI] [PubMed] [Google Scholar]
- 163.Benjamin EJ et al. Heart Disease and Stroke Statistics-2017 Update A Report From the American Heart Association. Circulation 135, E146–E603 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lau A & Tymianski M Glutamate receptors, neurotoxicity and neurodegeneration. Pflug Arch Eur J Phy 460, 525–542 (2010). [DOI] [PubMed] [Google Scholar]
- 165.Chen LJ, Hambright WS, Na R & Ran QT Ablation of the Ferroptosis Inhibitor Glutathione Peroxidase 4 in Neurons Results in Rapid Motor Neuron Degeneration and Paralysis. Journal of Biological Chemistry 290, 28097–28106 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hambright WS, Fonseca RS, Chen LJ, Na R & Ran QT Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biology 12, 8–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wirth EK et al. Neuronal selenoprotein expression is required for interneuron development and prevents seizures and neurodegeneration. Faseb Journal 24, 844–852 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Belaidi AA & Bush AI Iron neurochemistry in Alzheimer’s disease and Parkinson’s disease: targets for therapeutics. J Neurochem 139, 179–197 (2016). [DOI] [PubMed] [Google Scholar]
- 169.Masaldan S, Bush AI, Devos D, Rolland AS & Moreau C Striking while the iron is hot: Iron metabolism and ferroptosis in neurodegeneration. Free Radical Bio Med 133, 221–233 (2019). [DOI] [PubMed] [Google Scholar]
- 170.Fang XX et al. Ferroptosis as a target for protection against cardiomyopathy. P Natl Acad Sci USA 116, 2672–2680 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Linkermann A et al. Synchronized renal tubular cell death involves ferroptosis. Proc Natl Acad Sci U S A 111, 16836–16841, doi: 10.1073/pnas.1415518111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Martin-Sanchez D et al. Ferroptosis, but Not Necroptosis, Is Important in Nephrotoxic Folic Acid-Induced AKI. J Am Soc Nephrol 28, 218–229, doi: 10.1681/ASN.2015121376 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Belavgeni A, Meyer C, Stumpf J, Hugo C & Linkermann A Ferroptosis and Necroptosis in the Kidney. Cell Chem Biol 27, 448–462, doi: 10.1016/j.chembiol.2020.03.016 (2020). [DOI] [PubMed] [Google Scholar]
- 174.Wen Q, Liu J, Kang R, Zhou B & Tang D The release and activity of HMGB1 in ferroptosis. Biochem Biophys Res Commun 510, 278–283, doi: 10.1016/j.bbrc.2019.01.090 (2019). [DOI] [PubMed] [Google Scholar]
- 175.Carlson BA et al. Glutathione peroxidase 4 and vitamin E cooperatively prevent hepatocellular degeneration. Redox Biol 9, 22–31, doi: 10.1016/j.redox.2016.05.003 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Li W et al. Ferroptotic cell death and TLR4/Trif signaling initiate neutrophil recruitment after heart transplantation. J Clin Invest 129, 2293–2304, doi: 10.1172/JCI126428 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Shchepinov MS Polyunsaturated Fatty Acid Deuteration against Neurodegeneration. Trends in Pharmacological Sciences 41, 236–248, doi: 10.1016/j.tips.2020.01.010 (2020). [DOI] [PubMed] [Google Scholar]
- 178.Stockwell BR et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 171, 273–285, doi: 10.1016/j.cell.2017.09.021 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mai TT et al. Salinomycin kills cancer stem cells by sequestering iron in lysosomes. Nat Chem 9, 1025–1033 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Feng H et al. Transferrin Receptor Is a Specific Ferroptosis Marker. Cell Rep 30, 3411−+ (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Aron AT, Loehr MO, Bogena J & Chang CJ An Endoperoxide Reactivity-Based FRET Probe for Ratiometric Fluorescence Imaging of Labile Iron Pools in Living Cells. Journal of the American Chemical Society 138, 14338–14346 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Devos D et al. Targeting Chelatable Iron as a Therapeutic Modality in Parkinson’s Disease. Antioxid Redox Sign 21, 195–210 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhang Z et al. Activation of ferritinophagy is required for the RNA-binding protein ELAVL1/HuR to regulate ferroptosis in hepatic stellate cells. Autophagy 14, 2083–2103, doi: 10.1080/15548627.2018.1503146 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hu CL et al. Reduced expression of the ferroptosis inhibitor glutathione peroxidase-4 in multiple sclerosis and experimental autoimmune encephalomyelitis. J Neurochem 148, 426–439, doi: 10.1111/jnc.14604 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kim EH, Wong SW & Martinez J Programmed Necrosis and Disease:We interrupt your regular programming to bring you necroinflammation. Cell Death Differ 26, 25–40, doi: 10.1038/s41418-018-0179-3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Amaral EP et al. A major role for ferroptosis in Mycobacterium tuberculosis-induced cell death and tissue necrosis. The Journal of experimental medicine 216, 556–570, doi: 10.1084/jem.20181776 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Park EJ, Park YJ, Lee SJ, Lee K & Yoon C Whole cigarette smoke condensates induce ferroptosis in human bronchial epithelial cells. Toxicol Lett 303, 55–66, doi: 10.1016/j.toxlet.2018.12.007 (2019). [DOI] [PubMed] [Google Scholar]
- 188.Yoshida M et al. Involvement of cigarette smoke-induced epithelial cell ferroptosis in COPD pathogenesis. Nat Commun 10, 3145, doi: 10.1038/s41467-019-10991-7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Nobuta H et al. Oligodendrocyte Death in Pelizaeus-Merzbacher Disease Is Rescued by Iron Chelation. Cell Stem Cell 25, 531–541 e536, doi: 10.1016/j.stem.2019.09.003 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wortmann M et al. Combined deficiency in glutathione peroxidase 4 and vitamin e causes multiorgan thrombus formation and early death in mice. Circulation research 113, 408–417, doi: 10.1161/CIRCRESAHA.113.279984 (2013). [DOI] [PubMed] [Google Scholar]
- 191.Altamura S et al. Glutathione peroxidase 4 and vitamin E control reticulocyte maturation, stress erythropoiesis and iron homeostasis. Haematologica 105, 937–950, doi: 10.3324/haematol.2018.212977 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Klein EA et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 306, 1549–1556, doi: 10.1001/jama.2011.1437 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Shah R, Farmer LA, Zilka O, Van Kessel ATM & Pratt DA Beyond DPPH: Use of Fluorescence-Enabled Inhibited Autoxidation to Predict Oxidative Cell Death Rescue. Cell Chem Biol 26, 1594–1607 e1597, doi: 10.1016/j.chembiol.2019.09.007 (2019). [DOI] [PubMed] [Google Scholar]


