Abstract
The coronavirus disease (COVID-19) arises from the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) which is an enveloped RNA virus. COVID-19 has rapidly spread throughout the world by infecting more than 143 million people and causing 3.04 million deaths worldwide by 22 April 2021, confirmed by the World Health Organization. It caused great concern and pandemic all over the world, therewithal there has not been found any specific and efficient treatment yet. In the current review, we aimed to define the biophysical and biochemical aspects of SARS-CoV-2, including renin-angiotensin-system, cytokine storms, receptor binding, protein structural and functional features, molecular interactions, and conformational changes that take place during viral attachment and entering into human cells. It was also aimed to highlight the general hallmarks of COVID-19, including treatment strategies, diagnosis and even prevention. Thus, this review will serve as an updated comprehensive body of information and discussion on COVID-19 and will help the molecular scientists, biophysicists, clinicians, as well as medical engineers. Thereby, further understanding of COVID-19 will provide novel insights and advances in development of therapeutic potentials and vaccine alternatives as well as in detection of specific targets for diagnosis.
Keywords: COVID-19, Coronavirus, SARS-CoV-2, ACE2, Spike protein, Conformation
Abbreviations: SARS-CoV, severe acute respiratory syndrome coronavirus; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; COVID-19, coronavirus disease 2019; MERS-CoV, Middle Eastern Respiratory Syndrome Coronavirus; WHO, World Health Organization; CDC, Centre for Disease Control and Prevention; ACE2, angiotensin converting enzyme 2; RAS, renin-angiotensin system; ARB, angiotensin receptor blocker; ACEI, angiotensin-converting enzyme inhibitor; Ang I, angiotensin I; Ang II, angiotensin II; Ang (1–7), angiotensin (1–7); ATR1, type I angiotensin receptor; ACE, angiotensin converting enzyme; CRS, cytokine release syndrome; HMGB1, high-mobility group box 1 protein; DAMPs, damage-associated molecular patterns; PRRs, pathogen recognition receptors; TLRs, toll-like receptors; RBD, Receptor Binding Domain; RBM, Receptor Binding Motif; PD, peptidase domain; TM, transmembrane; CLD, collectrin-like domain; ORFs, open reading frames; NSPs, nonstructural proteins; PLpro, papain-like protease; 3CLpro, chymotrypsin-like protease; RdRp, RNA-dependent RNA polymerase; Hel, helicase; FP, fusion peptide; HR, heptad repeats; CRP, C-reactive protein; PCT, Procalcitonin
1. Introduction
According to WHO, in 2002, a virus which reminds of influenza started to infect the people in the Guangdong province of southern China. Shortly afterwards, in 2003, this virus was identified as SARS-CoV (World Health Organization, 2003a). The nature of the disease included influenza-like symptoms such as high body temperature (>38 °C), cough, shortness of breath and difficulty while breathing along with other minor symptoms as well (World Health Organization, 2003b). SARS epidemic has infected 8096 people primarily in China, Canada and Singapore followed by 774 deaths confirmed by WHO with an approximately 10% fatality rate (Organization, 2004). Moving on to 5 July 2003, WHO informed that the SARS-CoV was over as the chain of transmission between human-to-human had been broken (World Health Organization, 2003c). SARS-CoV still remains its popularity with its mechanism.
A secondary zoonotic coronavirus case happened 10 years later; in September 2012, a virus called MERS-CoV was first reported in Saudi Arabia. According to WHO, humans were able to get the infection once they contacted camels. As the original reservoir was investigated for a long time, it is believed that it may have spread to camels, thus caused an infection in humans (O'Keefe, 2016). The world has seen three important, communicable, and vital public health issues in these 20 years; one of them is still a big major problem in 2020 as known as Coronavirus disease 2019 (COVID-19).
COVID-19, caused by the novel SARS-CoV-2, started spreading on December 2019 with an unknown ethology. According to WHO there were some cases reported (with similar symptoms to pneumonia) by the Wuhan Municipal Health Commission (“Timeline of WHO's response to COVID-19,” n. d.). Towards the end of December, a descriptive study collected the samples from five patients with complaints of a serious lung condition as known as the complications of acute respiratory distress syndrome. The samples collected by the researchers confirmed the presence of a new beta coronavirus (Ren et al., 2020). At the beginning of January 2020, approximately 41 patients were identified with the symptoms of this novel coronavirus; furthermore, some of them had underlying diseases such as kidney disease, cardiovascular disease or diabetes (Huang et al., 2020). On 11 January, China found out that the outbreak might have originated from a seafood market in Wuhan City. Japan, the Republic of Korea, and Thailand were the first countries affected by COVID-19. Right after the outbreak, China started taking the necessary precautions against the virus by increasing the disinfection and sterilization processes but also providing infrared thermometer for the airports and stations (World Health Organization (WHO), 2020a). On February 11, 2020, this novel virus reached a name as ‘‘COVID-19″ by the WHO Director-General, this new name is an acronym for “coronavirus disease 2019". Furthermore, by this time China had exceeded 1000 deaths because of COVID-19 (“WHO Director-General's remarks at the media briefing on 2019-nCoV on 11 February 2020,” n. d.). One month later, on March 2020, WHO reported that the novel coronavirus had become a ‘‘pandemic’’ with its dynamic spread all over the world (World Health Organization (WHO), 2020b). COVID-19 is still continuing its spread across the world as a rapidly evolving global pandemic and crisis while researchers are still trying to develop effective treatments and vaccine.
The COVID-19 pandemic still concerns the whole world; thus, updated resources are required to develop strategies against this issue. Article reviewing is a fundamental and significant part of the scientific world. Currently, there are a large number of research articles about COVID-19 in the literature but the review articles are limited. Already published reviews about COVID-19 contains the points such as general information about COVID-19, including its emergence and spread, SARS-CoV-2 proteins, the interaction of SARS-CoV-2 with its receptor, clinical features of the disease and pathogenesis as well as some diagnosis and treatment methods (Bchetnia et al., 2020; Hu et al., 2021; Kilic et al., 2020; Naqvi et al., 2020; Yoshimoto, 2020). When compared to already published reviews, our current review mainly includes the biophysical and biochemical aspects of SARS-CoV-2, together with general characteristics and updated status on COVID-19. To our knowledge, for the first time, our current review includes detailed information in the biophysical part, which distinguishes it from previous review articles. In this review, we gathered structural and dynamical information that has been recently developed and clarified by using biophysical techniques since such comprehensive information is required to understand the functional properties of the virus, to develop strategies and finally to combat COVID-19. Although our current review focuses more on structural and functional characteristics of SARS-CoV-2, it also gives detailed information on other points such as diagnosis, treatment and prevention, serving as a comprehensive update on the rapidly evolving pandemic COVID-19.
With this current review article, we aimed to provide the latest investigations and highlight the general aspects of the novel coronavirus to the reader, getting detailed knowledge particularly about the biophysical and biochemical characteristics. Furthermore, this review supplies a literature review about the functional, structural properties of the SARS-CoV-2, its targeted therapies, and the vaccines regarding the new developments. It is believed that this review can be a reference to future studies by presenting various aspects and information about the SARS-CoV-2.
2. Viral properties and pathogenesis
Coronaviruses belong to the Nidovirales order, which includes families such as Coronaviridae. Provided by the genomic information, Coronaviruses have infectious, non-segmented and a single positive-sense RNA molecule. Coronaviruses and Toroviruses are within the virus family, Coronavidiae. While coronaviruses have pathogenic capacity on both animals and humans, Toroviruses are one of the main reasons for animal diarrhea (Peiris, 2012). Coronaviruses can be divided into 4 groups known as alpha, beta, gamma and delta, and people commonly get infected by alpha and beta coronaviruses as known as; 229 E, NL63, OC43, and HKU1 (CDC, 2020). They are well known for their positive single-stranded RNA genomes of approximately 30 kb, enveloped with nucleocapsid (Perlman and Netland, 2009). Recent studies have also shown that current coronavirus threatening public health belongs to betacoronavirus (Huang et al., 2020). It is really important to understand the nature of a virus to come up with the right treatment, thus, the studies regarding its pathogenesis carry a valid role. To find a relation between SARS-CoV-2 and other coronaviruses, a study calculated the sequence identity by collecting samples from different types. It was revealed that samples from COVID-19 infected patients presented a close relationship with two bat-derived coronaviruses: bat-SL-CoVZC45 and bat-SL-CoVZXC21. In fact, SARS-CoV-2 showed 88% identity with the two bat-derived coronaviruses. In comparison to these viruses, the associations between SARS-CoV-2 and SARS-CoV as well as MERS-CoV were more distant. While SARS-CoV showed ∼79% identity with SARS-CoV-2, MERS-CoV showed only ∼50% identity with SARS-CoV-2 (Lu et al., 2020). It was also reported that SARS-CoV-2 is 96% identical at the whole-genome level to another bat coronavirus (BatCoV-RaTG13) (Zhou et al., 2020). This close relationship may provide an evidence for SARS-CoV-2 to be originated in bats.
With the spread of the virus, new variants and mutations have started to become a problem in some regions. In 2020 December, a new variant appeared in the UK; it was revealed that this new variant was associated with the spike protein of the virus. In late December, it caused increased number of cases in the southeast of England. Authorities stated that the new mutation would not cause a major problem within the vaccine, as the vaccine affects many regions in the spike protein (Wise, 2020). A matched cohort study revealed the mortality rate to be slightly higher in the new strain (Challen et al., 2021). Another variant named 501Y.V2 was found in South Africa on December 18, 2020. It was discovered that this new variant had spread to various provinces. Furthermore, this variant was found to have 3 mutations in the spike protein (Tang et al., 2021; “WHO | SARS-CoV-2 Variants,” n. d.). Although the research is still ongoing, according to a study, South African strain may be more transmissible (Tegally et al., 2020).
Coronavirus has one of the best studied genome structures so far, and exhibits a high mutation rate (Cui et al., 2019). Mutations in this novel virus significantly affected the chain of transmission. Although SARS-CoV-2 contains amino acid mutations in its receptor-binding domain (Lu et al., 2020), both SARS-CoV and SARS-CoV-2 use their spike proteins to recognize the angiotensin converting enzyme 2 (ACE2) as a functional receptor on the target cell for entry (Hoffmann et al., 2020; Lan et al., 2020; Letko et al., 2020; Shang et al., 2020; Walls et al., 2020; Wang et al., 2020b; Yan et al., 2020). Recently, biophysical, and biochemical properties of SARS-CoV-2 have been studied in detail, which will be addressed in the following Sections 3, 4.
3. Structural and functional features of SARS-CoV-2
Phylogenetic analyses revealed that SARS-CoV-2 belongs to the betacoronavirus category of the Coronaviridae family. The coronavirus disease 2019 (COVID-19), rapidly spreading worldwide, is caused by this novel RNA virus SARS-CoV-2. To fight this global crisis, effective treatment and vaccine alternatives against COVID-19 are urgently required. Obviously, understanding the structural and functional features, dynamics and receptor recognition mechanism of SARS-CoV-2 is the first crucial step to develop the effective antiviral drug agents, novel targeted treatments, small molecular inhibitors, blocking antibodies, other therapeutics as well as to design an effective preventive vaccine against COVID-19. Knowing the structures and interactions on the atomic level will guide to identify potential targets by applying, for instance, in silico structure-assisted drug design or molecular docking procedures to find new drugs or to repurpose already approved drugs (Estrada, 2020; Hoffmann et al., 2020; Jin et al., 2020b). Hence, so far structural and functional relationship of SARS-CoV-2 proteins have been extensively studied by using biophysical techniques (e.g., cryogenic electron microscopy (Cryo-EM), X-ray crystallography), along with biochemical and biological assays (Lan et al., 2020; Shang et al., 2020; Walls et al., 2020; Wang et al., 2020b; Wrapp et al., 2020; Yan et al., 2020). Further studies on SARS-CoV-2 are still ongoing with increasing tendency to have integrated knowledge about molecular basis of recognition, molecular and atomic interactions as well as conformational changes occurring during viral attachment and entering into human cells.
Recently, biophysical and biochemical techniques have been applied for characterization of SARS-CoV-2 in the COVID-19 studies. Importantly, Cryo-EM (using deflection of electrons from frozen protein samples at liquid nitrogen temperatures) and X-ray crystallography (using scattering of X-rays from well-ordered protein crystals) have been used to determine the 3D structure of SARS-CoV-2 proteins at atomic resolution in a few Angstrom (Å) range. Later on, the atomic coordinates and maps of the protein 3D structures have been deposited in the Protein Data Bank (PDB), freely available to global community. In this regard, Lan et al. determined the crystal structure of the receptor-binding domain (RBD) of SARS-CoV-2 spike protein bound to the cell receptor ACE2 at 2.45 Å resolution (PDB ID: 6M0J) by using X-ray diffraction data (Lan et al., 2020). They also identified the amino acid residues of the SARS-CoV-2 RBD, essential for the ACE2 binding. Walls et al. determined the cryo-EM structures of the SARS-CoV-2 S ectodomain trimer in the closed (2.8 Å resolution, PDB ID: 6VXX) and partially opened (3.2 Å resolution, PDB ID: 6VYB) conformations (Walls et al., 2020). In another work, Wrapp et al. determined the cryo-EM structure of the SARS-CoV-2 spike protein in the prefusion conformation at 3.46 Å resolution and identified that one of the three receptor-binding domains is ‘up’ in a receptor-accessible conformation (PDB ID: 6VSB) (Wrapp et al., 2020). They also showed that SARS-CoV-2 S protein binds to human ACE2 with high affinity by utilizing the surface plasmon resonance, which is an optical technique used to determine the molecular interactions and binding kinetics. Obtaining a large amount of SARS-CoV-2 proteins (e.g. the spike protein) at high-quality is significant for development of vaccine alternatives or for research purposes. In this respect, Herrera et al. reported that ExpiCHO-S cell lines provided enhanced yields of spike proteins (Herrera et al., 2020). To validate the protein quality, stability and antigenicity, they analyzed the expressed and purified spike proteins by using the biochemical, biophysical and structural techniques and assays such as cryo-EM (3D structure, protein conformation), enzyme-linked immunosorbent assay (ELISA; antigenicity), protein microarray (antigenicity), flow cytometry (binding, specificity), analytical ultracentrifugation (oligomerization), SDS-page (protein size, molecular mass), light scattering (molecular mass, aggregation), and differential scanning fluorimetry (thermal stability, melting temperature). In another study associated with the COVID-19 studies, biochemical and biophysical characterization of the main protease, 3-chymotrypsin-like protease (3CLpro) from SARS-CoV-2 has been recently reported (Ferreira and Rabeh, 2020). Since 3CLpro of SARS-CoV-2 might be a promising antiviral drug target, its thermodynamic and kinetic stability at various pH and salt buffering conditions were studied by using differential scanning calorimetry, differential scanning fluorimetry and circular dichroism (CD) spectroscopy. The far UV-CD spectroscopic data in the 200–280 nm range was used for tracking of secondary structural elements (e.g. α-helix, β-sheets) and thus protein structural integrity at various pH and salt buffering conditions of 3CLpro. They reported that the protease has relatively high thermodynamic stabilities over a wide pH range but is less stable in the presence of salts. It is obvious that biophysical, biochemical, structural, and dynamical characterization of SARS-CoV-2 proteins helps to understand the viral features further, and thus, providing enormous information to discover strategies to combat COVID-19.
3.1. Structure of SARS-CoV-2
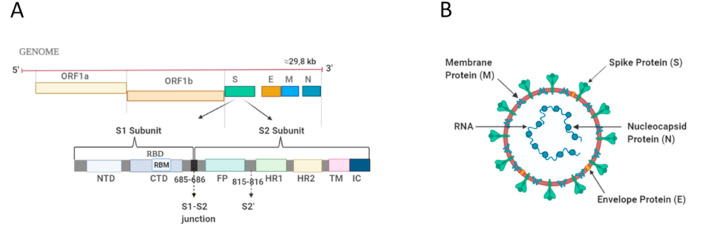
An RNA virus SARS-CoV-2 exhibits a spherical shape with a diameter of 60–140 nm and has many spikes (9–12 nm long) on its surface, and thus, it appears like a solar corona revealed by the electron microscopic data (Zhu et al., 2020). SARS-CoV-2 contains a positive-sense, single-stranded RNA genome. Its genome comprises ∼29.8 kilobases-long RNA and has 11 open reading frames (ORFs), including ORF1ab, ORF2, ORF3a, ORF4, ORF5, ORF6, ORF7a, ORF7b, ORF8, ORF9 and ORF10 genes (Yoshimoto, 2020) (Fig. 1 A). Accordingly, ORF1ab gene expresses a large polyprotein which is further cleaved by proteases into 16 nonstructural proteins (NSPs), including also major enzymes like NSP3 (papain-like proteases, PLpro), NSP5 (chymotrypsin-like protease, 3CLpro), NSP12 (RNA-dependent RNA polymerase, RdRp) and NSP13 (helicase, Hel) (Luk et al., 2019; Yoshimoto, 2020). SARS-CoV-2 is made up of four main structural proteins, responsible for replication processes and cell entering. These proteins are the spike (S) protein encoded by ORF2, the envelope (E) protein encoded by ORF4, the membrane (M) protein encoded by ORF5, and the nucleocapsid (N) protein encoded by ORF9. Besides, the gene fragments distributed among the structural genes encodes the accessory/helper proteins (Lu et al., 2020; Schoeman and Fielding, 2019; Wu et al., 2020; Yoshimoto, 2020). The enveloped structure of SARS-CoV-2 is made up of a bilayer lipids and proteins (S glycoprotein, E, M). A ribonucleoprotein core, which consists of the N protein bound to RNA genome, is located inside this viral envelope (Fig. 1B) (Naqvi et al., 2020; Yoshimoto, 2020).
Fig. 1.
Schematic representations of SARS-CoV-2 genome and structure. (A) Single-stranded RNA genome with a length of ∼29.8 kb, showing the spike protein in detail. (B) Structure of SARS-CoV-2, demonstrating its main structural proteins which are spike protein, membrane protein, envelope protein, and nucleocapsid protein. (Created with BioRender.com).
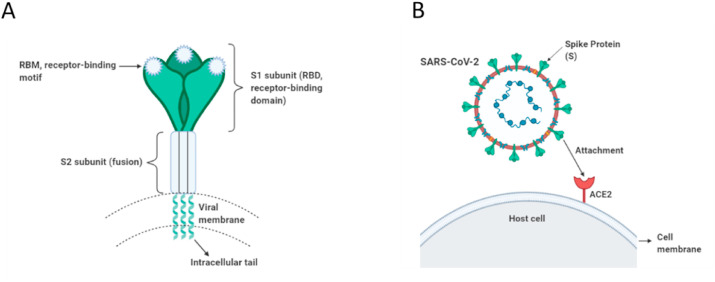
The smallest structural protein of SARS-CoV-2 is the E protein which is an integral membrane protein found in the viral membrane. It plays a number of key roles in the viral replication such as envelope formation, viral assembly, and it functions as ion channels and interacts with host cell proteins (Li et al., 2020a; Naqvi et al., 2020; Yoshimoto, 2020). The most abundant structural protein is the M protein which is an integral membrane protein that spans the viral membrane. It interacts with other main viral proteins and has significant role in the RNA packaging and viral assembly, shaping the viral envelope (Naqvi et al., 2020; Tang et al., 2020; Yoshimoto, 2020). The N protein is a ∼54 kDa protein that binds to viral RNA structurally and assembles into a helical ribonucleocapsid. A recent publication reported that its N-terminal is the RNA-binding domain while its C-terminal domain is responsible for homodimerization (PDB ID: 6WZO), very similar to that of SARS-CoV and MERS-CoV, and it forms large oligomers (tetramers) likely through nucleic acid-protein and protein-protein interactions (Ye et al., 2020). This nucleocapsid phosphoprotein is involved in the transcription, replication and packaging of the viral RNA (Naqvi et al., 2020; Schoeman and Fielding, 2019; Ye et al., 2020; Yoshimoto, 2020). The spike (S) proteins coated with polysaccharides are glycoproteins that a large number of S proteins protrude like a pedal-shaped spike on the surface of the virus envelope (Fig. 2 A). The S protein has 2 vital subunits for binding (S1 subunit) and fusion (S2 subunit) with the host cell. Briefly, the S protein facilities the entry into the human cells by attaching to a specific receptor (ACE2) located on the surface of the host cell (Fig. 2B). Those main structural proteins addressed above have collateral effects on the viral infection, and thus, they can be considered as potential drug targets as well. In the following sections of biophysical parts, the characteristics of spike protein can be comprehended in a more detailed way, which can help with the understanding of the connection of the virus with the human cells.
Fig. 2.
Schematic representations of SARS-CoV-2 spike protein and its attachment to host cell. (A) Trimeric spike protein of SARS-CoV-2, showing its functional subunits S1 (receptor binding domain) and S2 (membrane-fusion domain). (B) SARS-CoV-2 spike protein, representing its attachment to specific receptor angiotensin converting enzyme 2 (ACE2) located on the surface of the host cell for cell entry. (Created with BioRender.com).
3.2. Structure of spike (S) protein
Currently, it is well known that the COVID-19 infection initiates with the interaction of SARS-CoV-2 spike (S) protein (termed SARS-CoV-2 S) with human cells as a first step (Fig. 2B). The S proteins of both SARS-CoV-2 and SARS-CoV bind to human ACE2 receptor at high affinities in the nanomolar range to enter and invade the target human cells (Walls et al., 2020; Wang et al., 2020b; Wrapp et al., 2020). Thus, SARS-CoV-2 S protein is an essential target for therapeutic, diagnostic and biological research.
SARS-CoV-2 S protein has a high amino acid sequence identity with the S glycoproteins of SARS-CoV (76%) and BatCoV-RaTG13 (97%) (Walls et al., 2020; Yoshimoto, 2020; Zhou et al., 2020). SARS-CoV-2 has a longer spike protein with a length of 1273 amino acids in comparison to both SARS-CoV and MERS-CoV (Lu et al., 2020). A recent cryo-EM structural data revealed that SARS-CoV-2 S protein has a total structural weight of ∼438 kDa (Pdb ID: 6VXX) (Walls et al., 2020). The S protein, a heavily-glycosylated type I transmembrane protein, mediates attachment and entry into target cells. This large homotrimeric S protein, decorated with 22 N-linked glycans per protomer (Walls et al., 2020; Watanabe et al., 2020), is known for making protrusions like a crown on the viral surface. The S protein comprises of three parts: (i) an ectodomain having a triangular cross-section with a length of 160 Å (ii) a single-pass transmembrane anchor, and (iii) an intracellular tail (Li, 2016; Walls et al., 2020) (Fig. 2A). The ectodomain segment of homotrimeric S protein has S1 and S2 functional subunits in each monomer, which are associated with the receptor recognition and membrane fusion, respectively (Fig. 3 A and B). Accordingly, the S1 subunit exhibiting a V-shaped structure comprises a receptor binding domain (RBD) that binds to the receptor protein ACE2 located on the host cell surface, providing viral attachment. This is followed by the host cell membrane fusion through the S2 subunit so that the viral genomes can enter into target cells (Cascella et al., 2020; Li, 2016; Mathewson et al., 2008; Paul S. and Stanley, 2007; Walls et al., 2020). The S2 subunit harbours mainly α-helical secondary structures (Fig. 3B). It contains a fusion peptide (FP) region, heptad repeats (HR1 and HR2), a solely 40% identity with other SARS-CoVs (Cascella et al., 2020; Walls et al., 2020). Thus, the S2 subunit and the conserved motif in the S1 subunit can be thought as potential targets for neutralizing transmembrane domain (TM) and cytoplasmic domain, which is conserved in SARS-CoV-2 sharing 88% sequence identity, whereas the amino acid sequence of the receptor-binding domain in S1 shows antibodies against COVID-19.
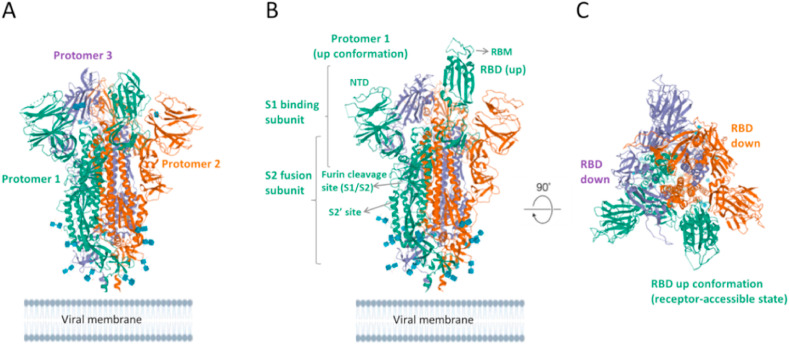
Fig. 3.
A 3D structure of homotrimeric SARS-CoV-2 S ectodomain. (A) The RBD on each protomer of trimeric S protein exhibits down conformation (closed) in the prefusion state (PDB ID: 6VXX). (B) The RBD of protomer 1 (green) of homotrimeric S protein is in the up conformation (open state) (PDB: 6VSB). (C) Top view of homotrimeric S glycoprotein, showing the RBDs in the up and down conformations (PDB ID: 6VSB). Attached carbohydrates are shown in blue-cubic shapes. Figures were reproduced from protein data bank (PDB). RBD, receptor binding domain; RBM: receptor binding motif; NTD, N-terminal domain.
Recent cryo-EM data (Carrique et al., 2020; Toelzer et al., 2020) have revealed bound unsaturated fatty acids on the RBD of the trimeric S protein. It was reported that the RBDs of trimeric SARS-CoV-2 S protein tightly and specifically binds to the essential free fatty acid linoleic acid in three composite binding pockets (Toelzer et al., 2020). Again recently reported, the acyl chains of unsaturated fatty acids bind to a hydrophobic pocket in one RBD of the trimeric S protein while the polar headgroups attach to an adjacent RBD, showing that lipid-like molecules modulate the S protein stability. Thereby, it was proposed in both studies that these binding pockets could be a promising target for development of small molecule-inhibitors or other therapeutics against SARS-CoV-2 (Carrique et al., 2020; Toelzer et al., 2020).
3.3. The S1/S2 junction and S2’ cleavage site
A cascade of events occurring during receptor attachment and proteolytic processing of trimeric S protein is required for cellular entry of SARS-CoV-2. The S protein is cleaved into S1 and S2 subunits by proteases on the host cell membrane. Significantly, a furin cleavage site was determined at the boundary between the S1/S2 subunits of the SARS-CoV-2 S protein, cleaved during S biogenesis (Walls et al., 2020). This S1/S2 junction is located in a disordered, solvent-exposed loop structure of the S protein (Wrapp et al., 2020) (Fig. 3B). This polybasic furin cleavage site (amino acid sequence: QTQTNSPRRARSVASQSIIA) is absent in the S protein of SARS-CoV (Yoshimoto, 2020). Additionally, the S2 subunit involves a protease cleavage site (called S2′ site) at the upstream of the hydrophobic fusion peptide. The S2’ cleavage site of SARS-CoV-2 S protein was found similar to that of SARS-CoV (Hoffmann et al., 2020). Recently determined that the S protein is cleaved at the S1/S2 and S2’ sites by host cell proteases such as furin and TMPRSS2 to activate the spike protein for a tight binding to the ACE2 receptor and for membrane fusion through multiple conformational alterations. This is crucial for expanding of SARS-CoV-2 cell and modulation of tropism, transmissibility and pathogenicity (Hoffmann et al., 2020; Walls et al., 2020). It was also explicitly showed that SARS-CoV-2 requires both host cell ACE2 receptor and serine protease TMPRSS2 for S protein priming to enter into the host cell, and thus, it was suggested that SARS-CoV-2 can be blocked by using TMPRSS2 inhibitors against COVID-19 (Hoffmann et al., 2020).
3.4. Conformational changes of S protein (open/closed or up/down states)
The trimeric S protein exhibits concerted conformational changes crucial for receptor binding and membrane fusion. Recently, open and closed conformations of SARS-CoV-2 spike glycoprotein has been detected (Walls et al., 2020). Accordingly, in the closed state of S trimers, the recognition motifs (RBMs) in the RBD of the S1 subunit are buried at the interface between protomers (Fig. 3A). However, in the open conformation of S1 at the trimer apex, the RBMs are exposed (Fig. 3B) which is required for ACE2 interaction followed by S2 conformational alterations in a concerted manner for protease cleavage at the S2’ site, membrane fusion and entry into target cells. Hence, Walls et al. (2020) proposed that trimeric S proteins of highly pathogenic human coronaviruses should undergo partially opened states, but human coronaviruses with common colds should have largely closed trimeric S proteins (Walls et al., 2020). In line with this study, the receptor-accessible (open or up conformation) and receptor-inaccessible (closed or down conformation) states of S trimers have been structurally determined (Wrapp et al., 2020) (Fig. 3C). In the prefusion conformation of S trimer, a single receptor-binding domain (RBD) of S1 in one protomer rotates up (receptor-accessible state) and becomes exposed to receptor binding (Fig. 3A). This renders the S trimer less stable, and results in a highly stable postfusion conformation of the S2 subunit and membrane fusion. In the closed or down conformation, RBD of S1 is angled through the central trimeric cavity (Wrapp et al., 2020). Recently, a stabilized prefusion SARS-CoV-2 spike ectodomain has been designed to enhance the yield and stability so that it can be a promising candidate for vaccine and diagnostic developments (Hsieh et al., 2020). Additionally, enhanced yields of high quality SARS-CoV-2 S proteins (recombinant S proteins) that have appropriate biochemical and biophysical properties were generated for investigations in both clinical and basic science (Herrera et al., 2020).
3.5. ACE2 receptor recognition by spike protein
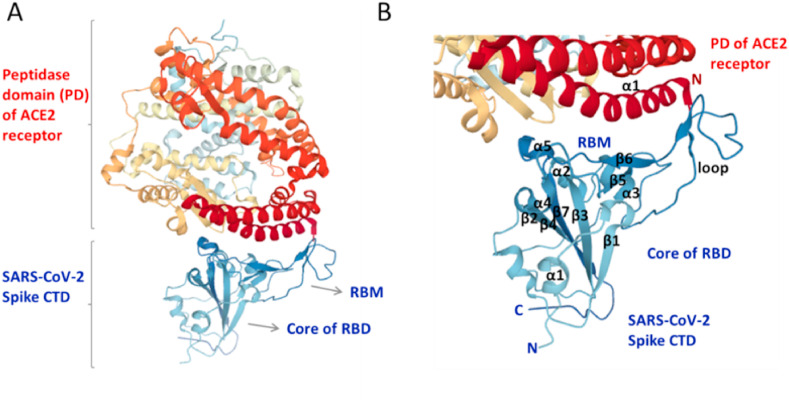
SARS-CoV-2 S protein binds to human cell receptor ACE2 with a high affinity of 14.7 nM analyzed by using surface plasmon resonance, which is much higher than that of SARS-CoV (Wrapp et al., 2020). ACE2 is a type I transmembrane α-helical protein. It comprises the N-terminal peptidase domain (PD) and C-terminal collectrin-like domain (CLD), involving a small extracellular domain, a long linker as well as a single TM helix. Recently reported that two S protein trimers can simultaneously bind to a human ACE2 homodimer, derived from the cryo-EM data of RBD-ACE2-B0AT1 ternary complex, existing as a dimer of heterodimers (Yan et al., 2020). Accordingly, mainly the neck domain (residues from 616 to 726) and also the peptidase domain (PD, residues from Ser19 to Asp615) of ACE2 contribute to this stable dimerization (without amino acid transporter B0AT1 contribution), through many polar interactions. Existence of the weak interaction at the PD dimer interface reveals its ability to transition to an open conformation, leading to a ∼25 Å-separation; thus, the up conformation (open) of RDB in S1 is necessary to bind to ACE2 (Yan et al., 2020), which is in line with the results of other recent biophysical studies (Walls et al., 2020; Wrapp et al., 2020). For recognition, the interaction occurs mainly between an extended loop in the receptor-binding motif (RBM) in the RBD of SARS-CoV-2 S protein (Gln498, Thr500, Asn501, Lys417, Tyr453, Gln474) and the N-terminal α1 helix of the extracellular PD of ACE2 (Tyr41, Gln42, Lys353, Arg357, Asp30, His34, Gln24) mainly through polar residues that undergo H-bonding (Yan et al., 2020) (Fig. 4 A and B). In line with this result, recently also revealed that the RBD of SARS-CoV-2 comprises the secondary structures of five antiparallel β-sheets, two short α-helices and loops (Lan et al., 2020). In the RBD of S1 subunit, short β5, β6, α4, α5 and loops constitute the RBM. This RBM is an extended region which includes most of the ACE2-interacting residues. Based on this X-ray crystallographic structure of the SARS-CoV-2 RBD–ACE2 complex, 17 residues in the SARS-CoV-2 RBD have networks with 20 residues of the ACE2 receptor through mainly hydrophilic interactions such as H-bonding and salt bridges. Existence of high structural similarity in the RBD–ACE2 interfaces strongly shows convergent evolutionary relationship between SARS-CoV-2 and SARS-CoV for strong ACE2 binding (Lan et al., 2020). In agreement with these results, recent studies have reported very similar and complimentary data, including the structural features and hotspots at the RBD–ACE2 interface although usage of different methodologies and sampling procedures (Shang et al., 2020; Wang et al., 2020b). Accordingly, the binding interface between the SARS-CoV-2 RBM and ACE2 forms a larger buried surface, includes a series of hydrophilic residues that makes strong polar contacts, and contains van der Waals contacts and aromatic interactions (Shang et al., 2020; Wang et al., 2020b). It is obviously clear from the biophysical sight that SARS-CoV-2 has important interconnections with ACE2, which will be addressed in the biochemical part through considering its function in Renin-Angiotensin-System.
Fig. 4.
Recognition of ACE2 receptor by SARS-Cov-2 spike protein. (A) A 3D structure of SARS-CoV-2 spike RBD in complex with ACE2 receptor (PDB ID: 6LZG). (B) A closer view to the RBM–ACE2 interface, showing the secondary structural elements of RBM and core subdomain of RBD (blue) and of N-terminal α1 helix of the extracellular PD of ACE2 (dark red). Figures were reproduced from protein data bank (PDB). ACE2, angiotensin-converting enzyme 2; PD, peptidase domain of ACE2; CTD, C-terminal domain of SARS-CoV-2 spike protein; RBD, receptor-binding domain; RBM, receptor-binding motif; C, C-terminal; N, N-terminal; α, α-helix; β, β-sheet secondary structures.
4. Entry of SARS-CoV-2 into the cell and the cellular events
4.1. Renin-angiotensin-system (RAS); angiotensin receptor blockers (ARBs); and angiotensin-converting enzyme inhibitors (ACEIs)
The receptor for SARS-CoV-2, known as ACE2 (angiotensin-converting enzyme 2), is located in host cell surface and it is a membrane-bound homologue of ACE. Additionally, ACE2 has uncovered the interaction between COVID-19 and RAS as it has crucial roles on viral mechanism (Gurwitz, 2020). ACE2 is known as a type I membrane protein, and the sites of its expression are mainly the lungs, heart and kidneys. Since the virus uses an enzyme (ACE2) that is part of the RAS, the medications which are commonly used to block the RAS are thought to have a positive effect in the treatment of COVID-19. ACEIs and ARBs are the agents which are related with Renin-Angiotensin System, and they are mainly prescribed for hypertension, congestive heart failure, chronic kidney disease as well as diabetes (Malha et al., 2020).
Even though ACE and ACE2 share 42% of amino acid identity, they have differences when it comes to enzymatic selectivity and function. These enzymes have significant effects in the Renin-Angiotensin System. Renin enzyme functions in the first step of RAS by catalysing the formation of Ang I (Angiotensin I) from angiotensinogen. The formed Ang I is converted into different molecules that have opposite hemodynamic effects. These molecules are known as vasoconstricting Ang II, and vasodilating Ang (1–7). Ang I is converted into Ang II by ACE, while the formed Ang II is converted into Ang (1–7) by ACE2. Ang II and Ang (1–7) have reverse vascular effects through their distinct receptors. Ang (1–7) is an antagonist of Ang II-mediated vasoconstriction, and interestingly, its antifibrotic effect was also studied, and thus, Ang (1–7) is thought to protect the lungs from injury due to lung diseases (Malha et al., 2020; Meng et al., 2014).
The primary route of entry for SARS-CoV-2 happens to be the respiratory system. Even though the kidney contains excessive amounts of ACE2, according to the results of a study, acute kidney injury incidence in COVID-19 was indicated as about 29%, and this incidence can be considered as low when compared to the 71% prevalence of lung injury. Problems regarding to kidneys, which can cause various types of injuries, seen in this disease are thought to be resulting from sepsis, chronic diseases, or an underlying kidney pathology that a patient might have. Other than this, there can be protective effects observed in kidneys that are based on ACE2 (Malha et al., 2020; Yang et al., 2020). To enter the cells, SARS-CoV-2 has a specific protein called “Spike” as it binds to ACE2. This event is thought to be the cause of lung injury in COVID-19. Internalization of the virus into the cell causes a reduction in the ACE2, and thus, it is possible to see an increase in Ang II levels. As a result, increased Ang II could cause lung injury by binding to its receptor ATR1 (Gurwitz, 2020; Kuba et al., 2005). Since COVID-19 became a pandemic, the urgent need for effective therapeutic interventions has led to consider RAS blockage due to its effect on viral entry and lung damage. A hypothesis has derived from the connection between ARBs and ACE2, and it has been stated that ARBs can be beneficial in treating COVID-19 by increasing ACE2 levels (Gurwitz, 2020). This hypothesis is supported by the consideration of the preventive role of ACE2 in lung damage. The main mechanism behind using ACEIs and ARBs is to prevent the formation of angiotensin 2, and to prevent the angiotensin 2 from functioning by binding its receptor and potentiate lung injury, respectively. Nevertheless, enhancement of the ACE2 may have a different effect on the virus as it can promote viruses to invade the cells (Malha et al., 2020).
Renin inhibitors and beta-blockers are molecules that can also act on the RAS by decreasing the generation of Ang I, Ang II and Ang (1–7). As it was also stated (Malha et al., 2020), these agents have not been focused in the discussion regarding the impact of RAS agents on SARS-CoV-2. Also, it would not be accurate to make conclusive statements on how the alterations on ACE2 in response to ACEIs and ARBs might affect COVID-19, due to limitations in the understanding of the drug action mechanisms on ACE2, and on viral-mediated lung injury (Malha et al., 2020). Ang II upregulates ACE and downregulates ACE2 expression (Koka et al., 2008). Therefore, it is expected to see upregulation in ACE2 levels by using ARBs and ACEIs due to their effects on Ang II. However, some studies have shown no increase in ACE2 levels after using ACEIs or ARBs (Burrell et al., 2005; Walters et al., 2017).
4.2. Interaction between ACE2 and Ang II
A study was done to understand the effect of Ang II in ACE2 expression in both in vivo and in vitro studies (Deshotels et al., 2014). According to the study, it has been stated that the absence of Ang II provides the interaction of type I angiotensin receptor (ATR1, the target of ARBs) with ACE2 on the cell membrane. It has been also stated that Ang II reduces the physical interaction between ACE2 and ATR1. On the other hand, Ang II induces ubiquitination and internalization/lysosomal degradation of ACE2 (Deshotels et al., 2014). Therefore, since ACE2 is the key enzyme used by SARS-CoV-2 for its entry into the cell, the additional results of further studies are needed to understand the main mechanism of the interaction between ACE2 and Ang II, and its effect on viral entry.
4.3. Role of oxidative stress on SARS-CoV-2 infection
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are produced by several cellular processes in the body (Adams et al., 2015; Finkel, 2011). These reactive oxygen/nitrogen species (RONS) such as hydroxyl radical (•OH), superoxide anion (O⁻₂), nitric oxide (NO), and peroxynitrite (ONOO⁻) are known as highly reactive molecules due to their unpaired valence electrons (Ntyonga-Pono, 2020). Normally, RONS are important biological molecules having roles in cell signaling and regulation of immune responses (Adams et al., 2015; Schieber and Chandel, 2014). Under normal physiological conditions, there is a balance between RONS and antioxidants. However, when this balance is disrupted due to the uncontrolled production of oxidants and dysregulation of antioxidant mechanisms, these powerful oxidants (free radicals) impair cellular functions and cause harmful effects on the body systems (Delgado-Roche and Mesta, 2020). This disruption of the balance between free radicals and antioxidants is called ‘oxidative stress' (Yoshikawa and Naito, 2002). Additionally, recent studies suggest that oxidative stress has an important role in the severity of SARS-CoV-2 infection, being linked with tissue damage (Laforge et al., 2020) and contributing to the induction of the cytokine storm (Cecchini and Cecchini, 2020).
RAS pathway contributes to the connection between oxidative stress and SARS-CoV-2 infection (Suhail et al., 2020). Ang II, the important molecule of the RAS pathway, produces reactive oxygen/nitrogen species by stimulating membrane-bound NADPH oxidase (Touyz, 2004; Wen, 2012). Additionally, ACE2 is another important molecule that degrades Ang II to Ang 1–7. This degradation results in the lowering of oxidative stress by inhibiting NADPH oxidase, and also preventing the production of RONS induced by Ang II (Lovren et al., 2008). This information is crucial in understanding the effect of this mechanism on the severity of COVID-19 symptoms. The binding of SARS-CoV-2 Spike protein to ACE2 increases the cellular concentration of Ang II, leading to an increased presence of RONS. This situation results in the occurrence of a cycle of oxidative stress and therefore increases the risk of severe illness from COVID-19 (Suhail et al., 2020). Additionally, a study has revealed that the reduction of the disulfide bonds of both ACE2 and SARS-CoV-2 Spike protein to thiol groups decreases the affinity of SARS-CoV-2 Spike protein for the ACE2 receptor. However, under oxidative stress, oxidation of thiols to disulfides increases the affinity of SARS-CoV-2 Spike protein for the ACE2 receptor, and therefore, may increase the severity of SARS-CoV-2 infection (Hati and Bhattacharyya, 2020).
Glutathione is an important tripeptide known for its ‘antioxidant effect’. It is involved in several processes such as detoxification, immune response, cellular division, and apoptosis (Forman et al., 2009). Glutathione exists in two states, reduced (GSH) and oxidized (GSSG). To protect the cells from oxidative stress, GSH reduces ROS and itself turns into its oxidized (GSSG) state (Silvagno et al., 2020). It is found that GSH levels and the patient's ability to recover from COVID-19 are connected, and having a high ROS/GSH ratio worsens COVID-19 symptoms (Polonikov, 2020). Vitamin D is another important molecule known for its antioxidant properties as well as its role in regulating immune function. A low level of vitamin D is also associated with increased severity of COVID-19 symptoms (Razdan et al., 2020). Therefore, it seems that maintaining an adequate glutathione and vitamin D level is crucial for fighting against COVID-19. There are many factors that can exacerbate or cause severe health issues due to SARS-CoV-2; the following section contains important immunological reactions, which is a response from body to SARS-CoV-2 infection. The molecules that were mentioned in the previous parts can be seen from a different perspective in the following section by understanding their connections and their functions within immunological reactions.
4.4. Cytokine Release Syndrome (CRS)
Cytokine Release Syndrome is a significant immunological event considering the SARS-CoV-2 that can affect multiple systems and may even cause death when untreated. It can also be defined as a systemic inflammatory response of human body caused by many factors such as infection and some drugs (Shimabukuro-Vornhagen et al., 2018). Patients with CRS are at a high risk of infection, and respiratory tract is known as the main site involved in infections. This consideration is derived from the fact that patients are treated with immunosuppressive agents which is a way of lowering their bodies’ immune response. Additionally, other facts are defined as immune dysregulation that associated with CRS and having tissue damages mainly at mucosal barriers. In response to damage, tissues release some molecules such as HMGB1 protein, ATP, uric acid, and DNA, which are the part of damage-associated molecular patterns (DAMPs) and function in supporting inflammatory responses. Pathogen recognition receptors (PRRs) are innate immunity receptors, and they can recognize DAMPs. Moreover, PRRs include toll-like receptors (TLRs) and the combining of these two receptors results in the activation of NF-ĸB and the release of cytokines such as IL-1, IL-6, TNFα and also other agents that play role in inflammation (Diamanti et al., 2020). Cytokine evaluation along with other molecules carries a significant role regarding COVID-19 patients. To assess the cytokine storm in patients, a study examined immunological features on different patients, including moderate and severe ones. Inflammatory factors were found to be higher in most severe patients. According to results, TNF-α, IL-6 and IL-10 levels were higher in many of the severe patients. In conclusion, these data may suggest a possible relation between cytokine syndrome and SARS-CoV-2, especially patients with severe symptoms should be evaluated very carefully and treated with these in mind (Chen et al., 2020a). Furthermore, SARS-CoV-2 induced lung injury may occur in patients, therefore treatment strategies should comprise many aspects of the virus. It was shown that alveolar epithelial cells happen to be the target cells of SARS-CoV-2. Along with ACE2, its main receptor, it can release fibrotic factors, which can induce lung fibrosis. Its mechanism can change gene expression, thus may start lung fibrosis, which can be very dangerous (Xu et al., 2020a). The diagnostic techniques for these issues will be addressed below.
5. Diagnosis
Clinical features of COVID-19 are known as: dry cough, sore throat, fever, weakness, fatigue, shortness of breath, or difficulty breathing, aches and pains, also including diarrhea, nausea or a runny nose. For those who have mild indications or asymptomatic patients must isolate themselves and consult to a medical provider (Organization, 2020). Imaging techniques, such as computed tomography (CT), are great opportunities for monitoring the effect of coronavirus on the lungs. Studies reported that the majority of patients have bilateral, multifocal lung lesions with ground glass opacities (Xu et al., 2020b). Even in the ultra-early stage without any clinical symptoms, SARS-CoV-2 infected patients can exhibit ground-glass opacities and patchy consolidations (Jin et al., 2020a). With the isolation of coronavirus, scientists have come a long way through laboratory techniques. Every country uses several mechanisms to distinguish the virus, but the goal is the same; detecting it so that precautions, treatments are made as soon as possible. As recently reported, a number of molecular and immunological diagnostic tests are currently available and can be improved further for detection of SARS-CoV-2 or the immune responses induced by this novel virus. Within laboratory testing, reverse-transcription PCR, and blood examinations are involved (Kilic et al., 2020). High leukocyte numbers, increased levels of plasma pro-inflammatory cytokines and abnormal respiratory findings are some important features that found in COVID-19 patients. The laboratory results of one of the COVID-19 patients revealed leukopenia with a leukocyte count of 2.91 × 109 cells/L and it was mostly composed of neutrophils (70%). A high level of 16.16 mg/L of blood C-reactive protein was noted that quite exceeds its normal range (Lei et al., 2020; Rothan and Byrareddy, 2020). The clinical features of the patient have been reported as cough, abnormal sounds from both lungs and a high body temperature (Lei et al., 2020; Rothan and Byrareddy, 2020). The high values of pro-inflammatory cytokines were noticed in severe COVID-19 patients, and these pro-inflammatory cytokines are reasoned to promote disease severity (Huang et al., 2020). It should also be questioned and given importance if the total lymphocyte is less than 0.8 × 109/L or if there is a decrease in CD4, CD8 T lymphocytes. Another diagnostic test includes RT-PCR technique with samples from respiratory tracts. For example, nose or throat swabs are tested within their RNA's (Chu et al., 2020; Jin et al., 2020a). Though both of the nose and throat swabs are used, some studies have detected the viral loads to be higher in the nose in comparison to the throat (Zou et al., 2020). This may be an interesting information regarding SARS-CoV-2 as pooled throat and nasal swab specimens are one of the most used diagnostic procedures to detect the virus (Chan et al., 2004). Besides laboratory, RT-PCR tests and imaging techniques, there is also blood gas analysis which can be useful for those people who are at high risk for severe illness as they can show increased muscle or liver enzymes, but also analyzing C-reactive protein (CRP) and Procalcitonin (PCT) can indicate an infection that takes place in the lungs (Jin et al., 2020a).
By understanding the virus and isolating it, scientists have discovered different ways to detect the viral load. Nonetheless, as mentioned before the RT-PCR test seems to be the gold standard to diagnose the novel coronavirus (Liu et al., 2020). The molecular logic of this test depends on taking the nasopharyngeal/oropharyngeal swabs and then the extraction of the viral RNA. With reverse transcription of the RNA to the complementary DNA, it gets amplificated (Sethuraman et al., 2020). There are different genes, of which different regions are targeted by manufacturers, such as virus envelope, virus nucleocapsid, RNA-dependent RNA polymerase gene (RdRp) or ORF1 gene. Cycle threshold, measuring the viral RNA, follows the procedure. It shows how many cycles of replications are needed in order to create a signal. Cycle threshold decreases inversely with increasing RNA loads. There are even some cases, where the PCR test may remain positive even after 3 weeks of the onset of severe illness, in comparison to mild ones, that are not positive after 3 weeks (Oliveira et al., 2020; Sethuraman et al., 2020; Zheng et al., 2020). Despite the fact that the RT-PCR test is considered as a gold standard diagnostic test, a study estimated the sensitivity to be 0.777 and specificity 0.988 (Padhye and Padhye, 2020). In comparison to PCR testing, CT scanning has some promising results for detection of the coronavirus and according to a study made in Wuhan, the sensitivity of the CT scans was around 97%, while the sensitivity of RT-PCR was 75% involving approximately a thousand of patients (Ai et al., 2020).
Besides the conventional methods, nanoparticles also started to acquire an important place when it comes to the detection of the virus. In a comprehensive research, low-cost nanoparticles were synthesized and it was discovered that magnetic nanoparticles could extract viral RNA easily and could be used for 50.000 COVID-19 tests (Chacón-Torres et al., 2020).
Saliva tests have also become popular with their ability to facilitate taking samples from patients. A study actually used saliva from 25 COVID-19 patients, analyzed by RT-PCR, and all of the samples were tested to be positive, which provides a reliable source for the diagnostic criteria (Azzi et al., 2020). Diagnosis is an important step to come up with effective therapeutic interventions, which can be understood better in the following section.
6. Treatment strategies against COVID-19
Currently, there is a global effort and many ongoing researches to find an effective way to treat this viral disease, these efforts include finding a functional vaccine and finding the right therapeutics. Some of these scientific strategies are now in the development of preclinical and clinical trials. As previously described, one of the most important steps considered as crucial, is the entrance of virus into human cells. There are countless studies searching for the prevention of this viral path. Thus, an urgent development of both effective vaccine alternatives and specific antiviral drugs against SARS-CoV-2 is globally needed. In this regard, broad-spectrum antiviral agents, which were formerly recommended and some of them were used against SARS-CoV and MERS-CoV, are currently considered to be effective against SARS-CoV-2. Hence, the mechanisms of action of these antiviral agents against SARS-CoV-2 are researched continuously.
6.1. Nonspecific antiviral drugs
The only option is the usage of broad-spectrum antiviral drugs such as HIV-protease inhibitors and Nucleoside analogues that could decrease virus infection till specific antiviral agents are found against COVID-19 (Lu, 2020). At present, nonspecific antiviral and antiflu drugs, including favipiravir, remdesivir (GS-5734), oseltamivir, lopinavir, ritonavir and chloroquine have been clinically used on COVID-19 patients, although there are still some arguments. The broad-spectrum antiviral remdesivir, chloroquine, and hydroxychloroquine were recently shown to be highly effective against COVID-19 (Wang et al., 2020a). However, it was also stated that the randomized controlled trials with hydroxychloroquine have not shown an effective result for prolonged survival in COVID-19 (Khuroo, 2020). As there has been significant debate and controversial data on chloroquine and hydroxychloroquine, a separate section on these drugs has been added (Please see section 6.2). Favipiravir (a guanosinenucleotid analog) acts as an inhibitor of RNA-dependent RNA polymerase (RdRp inhibitor) against RNA viruses, and thus, it is considered as a potential agent against SARS-CoV-2, although in vitro and preclinical studies are lacking (Kamps and Hoffmann, 2020; Şimşek Yavuz and Ünal, 2020). Former studies showed that remdesivir (a nucleotide analog) is another broad antiviral RdRp inhibitor against RNA viruses by increasing the RNA synthesis arrest, including Ebola virus, SARS-CoV and MERS-CoV (Cascella et al., 2020; Gordon et al., 2020; Kamps and Hoffmann, 2020; Sheahan et al, 2017, 2020; Şimşek Yavuz and Ünal, 2020, Williamson et al., 2020), and it was proposed to be an effective compound against coronaviruses (Gordon et al., 2020). Particularly, the remdesivir treatment in the early stage of COVID-19 was suggested to inhibit the development of pneumonia (Williamson et al., 2020). In addition, one of the treatments that have been attempted on 75 patients included twice a day orally 75 mg oseltamivir, 500 mg lopinavir (HIV-1 protease inhibitor), 500 mg ritonavir, and intravenously 0.25 g ganciclovir for 3–14 days, which are the existing antiviral drugs (Chen et al., 2020c). Moreover, the antiparasitic drug ivermectin has been researched over and over for a little while. It was shown to have inhibitory effects for nuclear protein import, but also proven that it can be a potential agent for HIV-1 and DENV (Wagstaff et al., 2012). The process of analyzing the safety of using high-dose ivermectin showed no difference in the intensity of experiencing undesirable results, in comparison to using standard-dose ivermectin (up to 400 μg/kg) (Navarro et al., 2020). Another study has observed the exposure to ivermectin during pregnancy, and it appears to show no neonatal deaths, preterm labours, or low birth weight but leaves a question, whether ivermectin can cause spontaneous abortions; therefore no proof has been found for the safety of ivermectin during pregnancy (Nicolas et al., 2020). To test the efficacy of ivermectin against novel coronavirus, one study has focused on in vitro experiments by infecting specific cells with SARS-CoV-2 isolate respectively followed by treatment with ivermectin. In one day, it was seen that ivermectin was able to decrease 93% of viral RNA in the supernatant and 99.8% in cell-associated viral RNA. By two days cells treated with ivermectin were observed even more successfully with a ∼5000-fold reduction of viral RNA resulting in a great reduction in all viral RNA, additionally, no differences were detected at 72 h. Moreover, at different times there were no toxic effects regarding the ivermectin. Based on these results it was hypothesized that a possible mechanism for ivermectin can be through inhibiting of IMPα/β1-mediated nuclear import of viral proteins (Caly et al., 2020).
6.2. Chloroquine and hydroxychloroquine
Hydroxychloroquine (HCQ) and chloroquine (CQ) are the known drugs for some specific diseases, in means of chloroquine has been prescribed for the treatment of malaria and also for preventing infection (chemoprevention), whereas hydroxychloroquine has been used for blood disorders (e.g. porphyria) and also for some autoimmune diseases which are related with joint inflammation (rheumatoid arthritis) and tissue damage (systemic lupus erythematosus) (Saqrane and El Mhammedi, 2020). In previous studies, HCQ and CQ were used to target SARS-CoV and other coronaviruses (e.g. Feline coronavirus was targeted by both HCQ and CQ, HCoV-229 E and HCoV-OC43 were targeted by CQ), and the results of the studies demonstrated the effective in vitro activity of these drugs on targeted coronaviruses (Biot et al., 2006; Keyaerts et al., 2009; Kono et al., 2008). As the use of HCQ and CQ was thought to be a potential treatment for COVID-19, these drugs were also used on SARS-CoV-2 infected cells, and this in vitro study resulted in finding HCQ to have higher potency in inhibiting SARS-CoV-2 when compared to CQ (Yao et al., 2020). Another study also demonstrated the effects of CQ on SARS-CoV-2, by using Vero E6 cells (Wang et al., 2020a). The known properties of chloroquine against SARS-CoV infection which are referred as increasing endosomal pH and interfering with the glycosylation of cellular receptor (ACE2) (Vincent et al., 2005), supported the effects of CQ against cell entry stages of SARS-CoV-2, with the EC90 value of 6.90 μM (Wang et al., 2020a). Additionally, according to the results of more than 100 patients, chloroquine phosphate was found to prevent the worsening of pneumonia and shorten the duration of the disease (Gao et al., 2020).
As previously described, the spike protein of coronavirus uses ACE2 receptor for a viral path, thus, new strategies against this protein have become popular to prevent it from cell entry. It has been shown that spike protein also uses sialic acids, which are connected to gangliosides on the cell surface. In other words, SARS-CoV-2 uses different molecules such as sialic acid, gangliosides to ensure its entry to the host cells. Sialic acids in the respiratory tract can be found through gangliosides or respectively glycoproteins. That is why scientists became interested towards this connection and a possible solution to break it down. According to the study, chloroquine, and hydroxychloroquine (more active form of chloroquine), may be a potential treatment against this novel virus. It was observed that CQ can bind to a specific sialic acid with its cationic group. As well as CQ, HCQ was also able to form a similar connection between sialic acid. Furthermore, it was seen that the OH group of hydroxychloroquine enhanced this binding. Therefore, in the presence of CQ or HCQ, the bond between spike protein and the ganglioside can be prevented, when these drugs bind to the same ganglioside. This may be a new approach of treatment towards patients with COVID-19 (Fantini et al., 2020).
Although it was formerly proved that these drugs may have antiviral properties, there are still some conflicts around these medications. The mechanism of CQ and HCQ remains different on every individual, especially it can vary between the 3 stages of coronavirus carriers as known as asymptomatic, mild, and severe. Immune system carries a valid role regarding to SARS-CoV-2 but CQ and HCQ can affect immune cells negatively, therefore it can be a major problem when it comes to the treatment of COVID-19 (Li et al., 2020b; Van Den Borne et al., 1997; Wallace et al., 1994). In conclusion, the effects, and mechanisms of these drugs on COVID-19 need further investigations both clinically and scientifically.
6.3. Plasma therapy as possible therapeutic agent
Since the investigations about the treatment of COVID-19 moves further, the importance of convalescent plasma therapy also raised by considering it as a potential treatment way. This technique is first based on collecting the blood of a person, who has been infected by the virus and then convalesced. The second process is separating the serum which contains increased antibodies in response to antigens. To combat the virus antigen of the newly infected person, the separated serum is injected into the patient. The production source of antibodies is the cells of the immune system which are called as “B cells”, and they can bind to specific molecules on the pathogen which can activate the immune response and called ‘‘antigen’’ (Ankcorn et al., 2019; Chen et al., 2020b). According to the former studies and reports, it has been confirmed that using convalescent plasma therapy in treating SARS-CoV has positive results (Cheng et al., 2005; Soo et al., 2004), and these results supported the use of convalescent plasma therapy on SARS-CoV-2 infected patients as well (Shen et al., 2020).
Recently, convalescent plasma therapy was administrated to 5 patients who were infected severely with SARS-CoV-2, and this administration resulted in an improvement in the clinical status of these patients. However, due to the small number of patients and also the study process, it is thought to be not accurate to make certain statements about this treatment's effectiveness on all COVID-19 patients (Shen et al., 2020). Researchers also underlined the risks of serum administration, which can be defined as serum sickness (known risk) and antibody-dependent enhancement of infection (theoretical risk). The known risk, serum sickness, is related to the transmission of infectious agents and triggering immunological reactions. However, antibody-dependent enhancement of infection is not considered as a known concern, and it is related to the enhancement of the infection to a different viral strain by antibodies to one form of coronavirus (Casadevall and Pirofski, 2020). Therefore, this concern raises the importance of determining the human monoclonal antibody for its role of neutralizing SARS-CoV-2.
In the section of “Structural and functional features of SARS-CoV-2" (above), it has been described in detail that the RBD of the S protein is the most variable part in SARS-CoV-2 with having crucial amino acids for binding to ACE2 receptor (Andersen et al., 2020; Wu et al., 2020; Zhou et al., 2020). This spike protein contains two significant subunits known as S1 and S2 subunits. The known role of S1 subunit is receptor binding, whereas S2 subunit is responsible for membrane-fusion. Thus, the epitopes of the spike protein are considered as specific targets for design of vaccine, neutralizing antibodies, or drug agents. A study was conducted on the effect of the anti-S1 human monoclonal antibody 80 R on SARS-CoV, and it resulted in the antibody binding to the conformational epitope on the S1 fragment and neutralizing SARS-CoV. The interaction between S1 subunit and ACE2 can also be prevented by this way (Sui et al., 2004). It is known that SARS-CoV-2 and SARS-CoV have different receptor binding domains (RBDs). According to another study, it has been shown that m396 and CR3014, which are the specific neutralizing antibodies for SARS-CoV spike protein, do not have ability to bind the spike protein of SARS-CoV-2. However, it was reported that at a concentration of 23.5 μg/ml, the antibody CR3022 can neutralize both SARS-CoV and SARS-CoV-2. Therefore, usage of CR3022 alone or by combining it with other neutralizing antibodies as potential therapeutics against COVID-19 was suggested (Tian et al., 2020).
By discovering the virus in a more detailed aspect, scientists have come through a lot of new treatment methods as mentioned before. Besides antiviral agents and plasma therapy, there are various methods that are being tried currently as well. Corticosteroid therapy has been newly searched. According to a study made with dexamethasone, it was found that it can be a potential method against acute respiratory distress syndrome (ARDS). It was revealed that moderate to severe ARDS patients receiving dexamethasone had more ventilator-free days than the control group (Villar et al., 2020). Another prospective study called the recovery trial in the UK found out 35% of reduction in the mortality rate, when the patients with mechanical ventilation received 6 mg of dexamethasone for 10 days. With that being said, there wasn't such benefit on mild to moderate cases (Singh et al., 2020).
Another study made in the USA searched for the effects of prophylactic anticoagulation against SARS-CoV-2 related deaths. It is known that this novel coronavirus can cause venous thromboembolism, which can lead to death (Bikdeli et al., 2020). The cohort study in the USA investigated the effects of prophylactic anticoagulation on patients admitted to the hospital with SARS-CoV-2, and they found that patients, who receive anticoagulation, had less risk of mortality. Furthermore, they also found that prophylactic anticoagulation had no association with severe bleeding (Rentsch et al., 2021).
Cenicriviroc is also another agent which is newly being searched for. It is a chemokine receptor antagonist additionally having anti-viral and anti-inflammatory characteristics. In an in vitro research, they found that cenicriviroc happened to have inhibitory properties against SARS-CoV-2 (Okamoto et al., 2020). An additional study dwelled on the agonist of the stimulator of interferon genes (STING) and its potential inhibitory mechanism against SARS-CoV-2. STING has many features including the control of host defence mechanism and immune pathway (Barber, 2015). It was shown that an agonist of STING, dimeric amidobenzimidazole (diABZI) was efficient against SARS-CoV-2 but also common cold coronavirus known as HCoV-229 E. They discovered that even in low dose of diABZI treatment, it was possible to prevent epithelial damage of the respiratory system (Zhu et al., 2021).
7. Vaccines
To stop the COVID-19 globally, effective vaccine alternatives are required as protective measures. Thus, it is obvious that clarifying the structural and functional properties of SARS-CoV-2 with biophysical and biochemical methods is an important fundamental study, and such studies can be transformed into practice. Since the beginning of the pandemic, scientists and research institutes have come a long way and made inroads into treatment strategies. With that being said, there are currently various types of vaccines available for the COVID-19 by using special types of techniques and efficacies. There are various factors which should be considered about vaccination and its prospects.
A study was performed to find out the percentage of vaccine protection that can be efficient to prevent an epidemic. It was revealed that at least 70% of protection by vaccination of 75% of the population is required to prevent an epidemic, and, with further confirmation, they found that in order to annihilate an ongoing epidemic the efficacy level of the vaccine should increase up to 80%. It can be seen that going back to normal life would require much efficacy and high coverage of the population. Nevertheless, these data do not mean that vaccines will be inadequate if they are less effective. The same study found that even in the case of 40% of protection, millions of people can be saved from ventilator and hospital bed days can be reduced. In short terms, a vaccination can save millions of lives (Bartsch et al., 2020). Besides the protection level, vaccines also vary according to their types such as mRNA vaccines, adenovirus vaccines or conventional inactivated vaccines.
Funded by BioNTech and Pfizer; BNT162b2 mRNA vaccine was one of the first ones released for usage. The logic for mRNA vaccine comes from injecting synthetic mRNA, which encodes viral antigens and thus causes an immune response. It stimulates the human cells to produce spike protein, to which, in return, the human body develops antibodies. Once the protein is produced, mRNA is removed by the cell. In comparison to conventional methods, they can be improved and produced very rapidly (Cao and Gao, 2021; Pardi et al., 2018). Clinical trials for BNT162b2 vaccine included more than forty thousand people, who received 2 doses of vaccine or placebo with 3 weeks of break. According to participants, local reactions were mostly due to pain, lower percentage of some participants also reported swelling or redness at the injection region. Overall, the complications were mild to moderate. Moreover, the percentage of severe systemic reactions was less than 0.9%, and most of the participants complained about a mild headache and fatigue. The efficacy of vaccine turned out to be quite decent with being almost 95%. However, the trial left some remained questions by not including pregnant women, immunocompromised patients, and children (Polack et al., 2020).
Besides mRNA vaccines, there are adenovirus vaccines such as ChAdOx1 nCoV-19 vaccine, known as AZD1222, which was developed at the Oxford University. It has an adenoviral vector that includes the spike protein. The study, which comprised different continents with 4 trials, found that ChAdOx1 nCoV-19 has around 70% protection without any severe cases of health concerns (Voysey et al., 2021). Another adenovirus vaccine was developed in the United States named Johnson and Johnson; it was further revealed that J&J vaccine was able to prevent moderate to severe cases at 66% with one additional shot (Livingston et al., 2021).
Vaccines with inactivated virus also seem to have successful results, the SARS-CoV-2 vaccine; BBIBP-CorV, which is being developed currently, turns out to be quite tolerable and successful without any severe adverse effects on phase 1 (Xia et al., 2021). CoronaVAC by the Sinovac Life Sciences has completed phase 1 and 2 on elderly people aged more than 60 without any safety concerns (Wu et al., 2021). The Sinovac's efficiency has been still investigated with the ongoing trials. There happen to be different aspects regarding to its efficacy from different countries. It was reported by the Brazilian researchers that the vaccine efficacy was between approximately 50.7 to 62.3%, while Turkey stated the efficacy to be around 83.5% (Costa, 2021; Dyer, 2021, Reuters, 2021). Another research from the University of Chile informed the efficacy two weeks after the 2nd dose as around 56.5% (Chik and Baptista, 2021).
8. Prevention
Candidate vaccine alternatives against COVID-19 have been still investigated; however, preventive measures can be taken with ease against a high-risk of contamination such as washing hands with soap or alcohol-based rub, social distancing at least 1 m, avoiding touching face, mouth, nose, and eyes which are portals of entry for the virus. To protect people from possible droplets, covering mouth or nose with the bent elbow has a valid significance, with that being in mind, seeking for medical attention in case of a fever or such as difficult breathing is also very important. For people being in another city or country, 14 days of isolation at home is a vital necessity (WHO, 2020). Mask use is an important step to protect other people from probable droplets that might come from symptomatic or asymptomatic person. It was also emphasized by WHO that people should avoid from the ‘Three Cs’ (closed spaces, crowded places, and close-contact settings) during the COVID-19 pandemic to prevent the human-to-human transmission.
According to WHO, it is also recommended not to meet with people in indoor settings; people should prefer outdoor gatherings instead of crowded places. In case of a meeting in a closed environment, they should take precautions such as wearing a mask, opening a window, supplying fresh air. When quarantined with a sick household, isolation must be fundamental, surfaces should be disinfected regularly, additionally sick person should be taken care and stay hydrated. When visiting a family member in a facility, the protective mask should be worn by both sides, hands should be washed, and social distance must be ensured. When shopping for groceries, one should not prefer peak hours, and shopping time should be kept as short as possible. If possible, people should check for online or telemedicine consultation, if not, patients should keep their social distance in facilities by wearing a mask and using a sanitizer. No matter the situation, it should be always remembered to keep the social distance, not touching the face, wearing protective equipment, and ensuring sanitizer (WHO, 2020).
9. Conclusion
In conclusion, novel coronavirus SARS-CoV-2 that causes COVID-19 has rapidly affected all countries in the world, becoming a global problem. Effective treatments and vaccine against COVID-19 are required urgently. Thus, intense investigations on the development of critical therapeutics and vaccine against SARS-CoV-2 have already started in the beginning of the first wave of COVID-19. Currently, both drug and vaccine studies, including clinical trials and hard laboratory works, are rapidly proceeding with great devotion. Thus, further understanding of the structural-functional features of SARS-CoV-2 and the mechanism of ACE2 recognition at the molecular level will help identify potential targets. Particularly, constitutes of the SARS-CoV-2 spike protein are very promising targets (such as epitope features on the RBD) for specific neutralizing antibodies, effective vaccine candidates and small-molecule inhibitors against COVID-19 pandemic as well as for diagnosis. This may also help to be ready for possible outbreaks which can emerge in the future due to similar coronaviruses.
In another respect, COVID-19 taught the humanity how a contagious disease can spread so easily and threaten many lives. It is also seen how self-preventive measures can be effective if applied properly. On the other hand, it showed the humanity how perspectives regarding the life can change drastically. Wearing a mask has become a part of humanity nowadays. Moreover, when it comes to the scientific world, it can be seen that this novel virus has led to many researches and projects. With the light of the scientists, myriad of important scientific data has been elicited and will be further revealed in the future as well. Over time, the effects and consequences of this novel coronavirus SARS-CoV-2 will become even more evident.
Declaration of competing interest
The authors declare that they have no conflict of interests.
References
- Adams L., Franco M.C., Estevez A.G. Reactive nitrogen species in cellular signaling. Exp. Biol. Med. 2015;240:711–717. doi: 10.1177/1535370215581314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W., Tao Q., Sun Z., Xia L. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296:E32–E40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020 doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankcorn M., Gallacher J., Ijaz S., Taha Y., Harvala H., Maclennan S., Thomson E.C., Davis C., Singer J.B., da Silva Filipe A., Smollett K., Niebel M., Semple M.G., Tedder R.S., McPherson S. Convalescent plasma therapy for persistent hepatitis E virus infection. J. Hepatol. 2019 doi: 10.1016/j.jhep.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzi L., Carcano G., Gianfagna F., Grossi P., Gasperina D.D., Genoni A., Fasano M., Sessa F., Tettamanti L., Carinci F., Maurino V., Rossi A., Tagliabue A., Baj A. Saliva is a reliable tool to detect SARS-CoV-2. J. Infect. 2020;81:e45–e50. doi: 10.1016/j.jinf.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber G.N. STING: infection, inflammation and cancer. Nat. Rev. Immunol. 2015 doi: 10.1038/nri3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch S.M., O'Shea K.J., Ferguson M.C., Bottazzi M.E., Wedlock P.T., Strych U., McKinnell J.A., Siegmund S.S., Cox S.N., Hotez P.J., Lee B.Y. Vaccine efficacy needed for a COVID-19 coronavirus vaccine to prevent or stop an epidemic as the sole intervention. Am. J. Prev. Med. 2020;59:493–503. doi: 10.1016/j.amepre.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bchetnia M., Girard C., Duchaine C., Laprise C. The outbreak of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): a review of the current global status. J. Infect. Public Health. 2020 doi: 10.1016/j.jiph.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E., Nigoghossian C. Der, Ageno W., Madjid M., Guo Y., Tang L.V., Hu Y., Giri J., Cushman M., Quéré I., Dimakakos E.P., Gibson C.M., Lippi G., Favaloro E.J., Fareed J., Caprini J.A., Tafur A.J., Burton J.R., Francese D.P., Wang E.Y., Falanga A., McLintock C., Hunt B.J., Spyropoulos A.C., Barnes G.D., Eikelboom J.W., Weinberg I., Schulman S., Carrier M., Piazza G., Beckman J.A., Steg P.G., Stone G.W., Rosenkranz S., Goldhaber S.Z., Parikh S.A., Monreal M., Krumholz H.M., Konstantinides S.V., Weitz J.I., Lip G.Y.H. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020 doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biot C., Daher W., Chavain N., Fandeur T., Khalife J., Dive D., De Clercq E. Design and synthesis of hydroxyferroquine derivatives with antimalarial and antiviral activities. J. Med. Chem. 2006;49:2845–2849. doi: 10.1021/jm0601856. [DOI] [PubMed] [Google Scholar]
- Burrell L.M., Risvanis J., Kubota E., Dean R.G., MacDonald P.S., Lu S., Tikellis C., Grant S.L., Lew R.A., Smith A.I., Cooper M.E., Johnston C.I. Myocardial infarction increases ACE2 expression in rat and humans. Eur. Heart J. 2005 doi: 10.1093/eurheartj/ehi114. [DOI] [PubMed] [Google Scholar]
- Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020 doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Gao G.F. EClinicalMedicine; 2021. mRNA vaccines: a matter of delivery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrique L., Duyvesteyn H.M., Malinauskas T., Zhao Y., Ren J., Zhou D., Walter T.S., Radecke J., Huo J., Ruza R.R., Shah P.N., Fry E.E., Stuart D.I. The SARS-CoV-2 Spike harbours a lipid binding pocket which modulates stability of the prefusion trimer. bioRxiv. 2020 doi: 10.1101/2020.08.13.249177. 2020.08.13.249177. [DOI] [Google Scholar]
- Casadevall A., Pirofski L.A. The convalescent sera option for containing COVID-19. J. Clin. Invest. 2020 doi: 10.1172/JCI138003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascella M., Rajnik M., Cuomo A., Dulebohn S.C., Di Napoli R. 2020. Features, Evaluation and Treatment Coronavirus (COVID-19) (StatPearls) [PubMed] [Google Scholar]
- CDC Human coronavirus types [WWW document] Cent. Dis. Control Prev. 2020 [Google Scholar]
- Cecchini R., Cecchini A.L. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med. Hypotheses. 2020;143 doi: 10.1016/j.mehy.2020.110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacón-Torres J.C., Reinoso C., Navas-León D.G., Briceño S., González G. Optimized and scalable synthesis of magnetic nanoparticles for RNA extraction in response to developing countries' needs in the detection and control of SARS-CoV-2. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-75798-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challen R., Brooks-Pollock E., Read J.M., Dyson L., Tsaneva-Atanasova K., Danon L. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: matched cohort study. BMJ. 2021;372 doi: 10.1136/bmj.n579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P.K.S., To W.K., Ng K.C., Lam R.K.Y., Ng T.K., Chan R.C.W., Wu A., Yu W.C., Lee N., Hui D.S.C., Lai S.T., Hon E.K.L., Li C.K., Sung J.J.Y., Tam J.S. Laboratory diagnosis of SARS. Emerg. Infect. Dis. 2004 doi: 10.3201/eid1005.030682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang Xiaoyun, Chen H., Yu H., Zhang Xiaoping, Zhang M., Wu S., Song J., Chen T., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Xiong J., Bao L., Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Wong R., Soo Y.O.Y., Wong W.S., Lee C.K., Ng M.H.L., Chan P., Wong K.C., Leung C.B., Cheng G. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur. J. Clin. Microbiol. Infect. Dis. 2005;24:44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chik H., Baptista E. South China Morning Post; 2021. Chile Covid-19 vaccination drive adds to Sinovac efficacy data [WWW Document]http://www.scmp.com/news/china/science/article/3128886/chile-covid-19-vaccination-drive-adds-sinovac-efficacy-data URL. (accessed 4.9.21) [Google Scholar]
- Chu D.K.W., Pan Y., Cheng S.M.S., Hui K.P.Y., Krishnan P., Liu Y., Ng D.Y.M., Wan C.K.C., Yang P., Wang Q., Peiris M., Poon L.L.M. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin. Chem. 2020 doi: 10.1093/clinchem/hvaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A.G. CNN Bras; 2021. Clinical study proving greater effectiveness of Coronavac is sent to Lancet [WWW Document]http://www.cnnbrasil.com.br/saude/2021/04/11/estudo-clinico-que-comprova-maior-eficacia-da-coronavac-e-enviado-para-lancet URL. (accessed 4.11.21) [Google Scholar]
- Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019 doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Roche L., Mesta F. Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection. Arch. Med. Res. 2020 doi: 10.1016/j.arcmed.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshotels M.R., Xia H., Sriramula S., Lazartigues E., Filipeanu C.M. Angiotensin II mediates angiotensin converting enzyme type 2 internalization and degradation through an Angiotensin II type I receptor-dependent mechanism. Hypertension. 2014 doi: 10.1161/HYPERTENSIONAHA.114.03743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti A.P., Rosado M.M., Pioli C., Sesti G., Laganà B. Cytokine release syndrome in COVID-19 patients, a new scenario for an old concern: the fragile balance between infections and autoimmunity. Int. J. Mol. Sci. 2020 doi: 10.3390/ijms21093330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer O. Covid-19: Chinese vaccines may need changes to improve efficacy, admits official. BMJ. 2021;373:n969. doi: 10.1136/bmj.n969. [DOI] [PubMed] [Google Scholar]
- Estrada E. COVID-19 and SARS-CoV-2. Modeling the present, looking at the future. Phys. Rep. 2020;192:109231. doi: 10.1016/j.physrep.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantini J., Di Scala C., Chahinian H., Yahi N. Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira J.C., Rabeh W.M. Biochemical and biophysical characterization of the main protease, 3-chymotrypsin-like protease (3CLpro) from the novel coronavirus SARS-CoV 2. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-79357-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011 doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman H.J., Zhang H., Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol. Aspect. Med. 2009 doi: 10.1016/j.mam.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020 doi: 10.5582/BST.2020.01047. [DOI] [PubMed] [Google Scholar]
- Gordon C.J., Tchesnokov E.P., Feng J.Y., Porter D.P., Götte M. The antiviral compound remdesivir potently inhibits RNAdependent RNA polymerase from Middle East respiratory syndrome coronavirus. J. Biol. Chem. 2020 doi: 10.1074/jbc.AC120.013056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev. Res. 2020 doi: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hati S., Bhattacharyya S. Impact of thiol-disulfide balance on the binding of covid-19 spike protein with angiotensin-converting enzyme 2 receptor. ACS Omega. 2020;5:16292–16298. doi: 10.1021/acsomega.0c02125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera N.G., Morano N.C., Celikgil A., Georgiev G.I., Malonis R.J., Lee J.H., Tong K., Vergnolle O., Massimi A.B., Yen L.Y., Noble A.J., Kopylov M., Bonanno J.B., Garrett-Thomson S.C., Hayes D.B., Bortz R.H., Wirchnianski A.S., Florez C., Laudermilch E., Haslwanter D., Fels J.M., Dieterle M.E., Jangra R.K., Barnhill J., Mengotto A., Kimmel D., Daily J.P., Pirofski L.-A., Chandran K., Brenowitz M., Garforth S.J., Eng E.T., Lai J.R., Almo S.C. Characterization of the SARS-CoV-2 S protein: biophysical, biochemical, structural, and antigenic analysis. bioRxiv Prepr. Serv. Biol. 2020 doi: 10.1101/2020.06.14.150607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C.-L., Goldsmith J.A., Schaub J.M., DiVenere A.M., Kuo H.-C., Javanmardi K., Le K.C., Wrapp D., Lee A.G., Liu Y., Chou C.-W., Byrne P.O., Hjorth C.K., Johnson N.V., Ludes-Meyers J., Nguyen A.W., Park J., Wang N., Amengor D., Lavinder J.J., Ippolito G.C., Maynard J.A., Finkelstein I.J., McLellan J.S. Structure-based design of prefusion-stabilized SARS-CoV-2 spikes. Science eabd0826. 2020 doi: 10.1126/science.abd0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B., Guo H., Zhou P., Shi Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021 doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y.H., Cai L., Cheng Z.S., Cheng H., Deng T., Fan Y.P., Fang C., Huang D., Huang L.Q., Huang Q., Han Y., Hu B., Hu F., Li B.H., Li Y.R., Liang K., Lin L.K., Luo L.S., Ma J., Ma L.L., Peng Z.Y., Pan Y.B., Pan Z.Y., Ren X.Q., Sun H.M., Wang Y., Wang Yun Yun, Weng H., Wei C.J., Wu D.F., Xia J., Xiong Y., Xu H.B., Yao X.M., Yuan Y.F., Ye T.S., Zhang X.C., Zhang Y.W., Zhang Y.G., Zhang H.M., Zhao Y., Zhao M.J., Zi H., Zeng X.T., Wang Yong Yan, Wang X.H. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil. Med. Res. 2020 doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z., Du X., Xu Y., Deng Y., Liu M., Zhao Y., Zhang B., Li X., Zhang L., Peng C., Duan Y., Yu J., Wang L., Yang K., Liu F., Jiang R., Yang Xinglou, You T., Liu Xiaoce, Yang Xiuna, Bai F., Liu H., Liu Xiang, Guddat L.W., Xu W., Xiao G., Qin C., Shi Z., Jiang H., Rao Z., Yang H. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582:289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- Kamps B., Hoffmann C. fourth ed. SteinHauser Verlag; 2020. COVID Reference. [Google Scholar]
- Keyaerts E., Li S., Vijgen L., Rysman E., Verbeeck J., Van Ranst M., Maes P. Antiviral activity of chloroquine against human coronavirus OC43 infection in newborn mice. Antimicrob. Agents Chemother. 2009;53:3416–3421. doi: 10.1128/AAC.01509-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khuroo M.S. Chloroquine and hydroxychloroquine in coronavirus disease 2019 (COVID-19). Facts, fiction and the hype: a critical appraisal. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.106101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic T., Weissleder R., Lee H. Molecular and immunological diagnostic tests of COVID-19 – current status and challenges. iScience. 2020;23:101406. doi: 10.1016/j.isci.2020.101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koka V., Xiao R.H., Chung A.C.K., Wang W., Truong L.D., Lan H.Y. Angiotensin II up-regulates Angiotensin I-Converting Enzyme (ACE), but down-regulates ACE2 via the AT1-ERK/p38 MAP kinase pathway. Am. J. Pathol. 2008 doi: 10.2353/ajpath.2008.070762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono M., Tatsumi K., Imai A.M., Saito K., Kuriyama T., Shirasawa H. Inhibition of human coronavirus 229E infection in human epithelial lung cells (L132) by chloroquine: involvement of p38 MAPK and ERK. Antivir. Res. 2008;77:150–152. doi: 10.1016/j.antiviral.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005 doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laforge M., Elbim C., Frère C., Hémadi M., Massaad C., Nuss P., Benoliel J.J., Becker C. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat. Rev. Immunol. 2020 doi: 10.1038/s41577-020-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., Zhang Q., Shi X., Wang Q., Zhang L., Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- Lei J., Li J., Li X., Qi X. CT imaging of the 2019 novel coronavirus (2019-NCoV) pneumonia. Radiology. 2020 doi: 10.1148/radiol.2020200236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F. Structure, function, and evolution of coronavirus spike proteins. Annu. Rev. Virol. 2016;3:237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Yuan L., Dai G., Chen R.A., Liu D.X., Fung T.S. Regulation of the ER stress response by the ion channel activity of the infectious bronchitis coronavirus envelope protein modulates virion release, apoptosis, viral fitness, and pathogenesis. Front. Microbiol. 2020;10:3022. doi: 10.3389/fmicb.2019.03022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Wang Y., Agostinis P., Rabson A., Melino G., Carafoli E., Shi Y., Sun E. Is hydroxychloroquine beneficial for COVID-19 patients? Cell Death Dis. 2020;11:1–6. doi: 10.1038/s41419-020-2721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R., Han H., Liu F., Lv Z., Wu K., Liu Y., Feng Y., Zhu C. Positive rate of RT-PCR detection of SARS-CoV-2 infection in 4880 cases from one hospital in Wuhan, China, from Jan to Feb 2020. Clin. Chim. Acta. 2020;505:172–175. doi: 10.1016/j.cca.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston E.H., Malani P.N., Creech C.B. The Johnson & Johnson vaccine for COVID-19. JAMA, J. Am. Med. Assoc. 2021 doi: 10.1001/jama.2021.2927. [DOI] [PubMed] [Google Scholar]
- Lovren F., Pan Y., Quan A., Teoh H., Wang G., Shukla P.C., Levitt K.S., Oudit G.Y., Al-Omran M., Stewart D.J., Slutsky A.S., Peterson M.D., Backx P.H., Penninger J.M., Verma S. Angiotensin converting enzyme-2 confers endothelial protection and attenuates atherosclerosis. Am. J. Physiol. Heart Circ. Physiol. 2008;295 doi: 10.1152/ajpheart.00331.2008. [DOI] [PubMed] [Google Scholar]
- Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci. Trends. 2020 doi: 10.5582/BST.2020.01020. [DOI] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk H.K.H., Li X., Fung J., Lau S.K.P., Woo P.C.Y. Molecular epidemiology, evolution and phylogeny of SARS coronavirus. Infect. Genet. Evol. 2019 doi: 10.1016/j.meegid.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malha L., Mueller F.B., Pecker M.S., Mann S.J., August P., Feig P.U. COVID-19 and the renin-angiotensin system. Kidney Int. Reports. 2020 doi: 10.1016/j.ekir.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewson A.C., Bishop A., Yao Y., Kemp F., Ren J., Chen H., Xu X., Berkhout B., van der Hoek L., Jones I.M. Interaction of severe acute respiratory syndrome-coronavirus and NL63 coronavirus spike proteins with angiotensin converting enzyme-2. J. Gen. Virol. 2008 doi: 10.1099/vir.0.2008/003962-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y., Yu C.H., Li W., Li T., Luo W., Huang S., Wu P.S., Cai S.X., Li X. Angiotensin-converting enzyme 2/angiotensin-(1-7)/mas axis protects against lung fibrosis by inhibiting the MAPK/NF-κB pathway. Am. J. Respir. Cell Mol. Biol. 2014 doi: 10.1165/rcmb.2012-0451OC. [DOI] [PubMed] [Google Scholar]
- Naqvi A.A.T., Fatima K., Mohammad T., Fatima U., Singh I.K., Singh A., Atif S.M., Hariprasad G., Hasan G.M., Hassan M.I. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: structural genomics approach. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2020 doi: 10.1016/j.bbadis.2020.165878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M., Camprubí D., Requena-Méndez A., Buonfrate D., Giorli G., Kamgno J., Gardon J., Boussinesq M., Munoz J., Krolewiecki A. Safety of high-dose ivermectin: a systematic review and meta-analysis. J. Antimicrob. Chemother. 2020 doi: 10.1093/jac/dkz524. [DOI] [PubMed] [Google Scholar]
- Nicolas P., Maia M.F., Bassat Q., Kobylinski K.C., Monteiro W., Rabinovich N.R., Menéndez C., Bardají A., Chaccour C. Safety of oral ivermectin during pregnancy: a systematic review and meta-analysis. Lancet Glob. Heal. 2020 doi: 10.1016/S2214-109X(19)30453-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntyonga-Pono M.P. COVID-19 infection and oxidative stress: an under-explored approach for prevention and treatment? Pan Afr. Med. J. 2020;35:12. doi: 10.11604/pamj.2020.35.2.22877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe L.C. Middle east respiratory syndrome coronavirus [WWW Document] Work. Heal. Saf. 2016 doi: 10.1177/2165079915607497. [DOI] [PubMed] [Google Scholar]
- Okamoto M., Toyama M., Baba M. The chemokine receptor antagonist cenicriviroc inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020;182 doi: 10.1016/j.antiviral.2020.104902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira B.A., de Oliveira L.C., Sabino E.C., Okay T.S. SARS-CoV-2 and the COVID-19 disease: a mini review on diagnostic methods. Rev. Inst. Med. Trop. Sao Paulo. 2020 doi: 10.1590/S1678-9946202062044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization W.H. World Heal; Organ: 2020. Coronavirus (Symptoms) [WWW Document] [Google Scholar]
- Organization W.H. World Heal. Organ; 2004. Summary of Probable SARS Cases with Onset of Illness from 1 November 2002 to 31 July 2003 (Based on Data as of the 31 December 2003. [WWW Document] [Google Scholar]
- Padhye N.S., Padhye N. vol. 4. 2020. p. 20078949. (Reconstructed Diagnostic Sensitivity and Specificity of the RT-PCR Test for COVID-19). medRxiv 2020. [DOI] [Google Scholar]
- Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines-a new era in vaccinology. Nat. Rev. Drug Discov. 2018 doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S.M., Stanley P. Fields Virology. sixth ed. 2007. Coronaviridae. [Google Scholar]
- Peiris J.S.M. Medical Microbiology: Eighteenth Edition. 2012. Coronaviruses. [DOI] [Google Scholar]
- Perlman S., Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat. Rev. Microbiol. 2009 doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/nejmoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonikov A. Endogenous deficiency of glutathione as the most likely cause of serious manifestations and death in COVID-19 patients. ACS Infect. Dis. 2020 doi: 10.1021/acsinfecdis.0c00288. [DOI] [PubMed] [Google Scholar]
- Razdan K., Singh K., Singh D. Vitamin D levels and COVID-19 susceptibility: is there any correlation? Med. Drug Discov. 2020;7:100051. doi: 10.1016/j.medidd.2020.100051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren L.L., Wang Y.M., Wu Z.Q., Xiang Z.C., Guo L., Xu T., Jiang Y.Z., Xiong Y., Li Y.J., Li X.W., Li H., Fan G.H., Gu X.Y., Xiao Y., Gao H., Xu J.Y., Yang F., Wang X.M., Wu C., Chen L., Liu Y.W., Liu B., Yang J., Wang X.R., Dong J., Li L., Huang C.L., Zhao J.P., Hu Y., Cheng Z.S., Liu L.L., Qian Z.H., Qin C., Jin Q., Cao B., Wang J.W. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin. Med. J. (Engl) 2020 doi: 10.1097/CM9.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentsch C.T., Beckman J.A., Tomlinson L., Gellad W.F., Alcorn C., Kidwai-Khan F., Skanderson M., Brittain E., King J.T., Ho Y.L., Eden S., Kundu S., Lann M.F., Greevy R.A., Ho P.M., Heidenreich P.A., Jacobson D.A., Douglas I.J., Tate J.P., Evans S.J.W., Atkins D., Justice A.C., Freiberg M.S. Early initiation of prophylactic anticoagulation for prevention of coronavirus disease 2019 mortality in patients admitted to hospital in the United States: cohort study. BMJ. 2021;372 doi: 10.1136/bmj.n311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuters . Reuters; 2021. Turkish study revises down Sinovac COVID-19 vaccine efficacy to 83.5% | Reuters [WWW Document]http://www.reuters.com/article/health-coronavirus-turkey-sinovac-int-idUSKBN2AV18P URL. (accessed 3.3.21) [Google Scholar]
- Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saqrane S., El Mhammedi M.A. Review on the global epidemiological situation and the efficacy of chloroquine and hydroxychloroquine for the treatment of COVID-19. New Microbes New Infect. 2020 doi: 10.1016/j.nmni.2020.100680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber M., Chandel N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014 doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol. J. 2019 doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethuraman N., Jeremiah S.S., Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA, J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.8259. [DOI] [PubMed] [Google Scholar]
- Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., Geng Q., Auerbach A., Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B., Leist S.R., Pyrc K., Feng J.Y., Trantcheva I., Bannister R., Park Y., Babusis D., Clarke M.O., MacKman R.L., Spahn J.E., Palmiotti C.A., Siegel D., Ray A.S., Cihlar T., Jordan R., Denison M.R., Baric R.S. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T.P., Sims A.C., Leist S.R., Schäfer A., Won J., Brown A.J., Montgomery S.A., Hogg A., Babusis D., Clarke M.O., Spahn J.E., Bauer L., Sellers S., Porter D., Feng J.Y., Cihlar T., Jordan R., Denison M.R., Baric R.S. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020;11:1–14. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C., Wang Z., Zhao F., Yang Yang, Li J., Yuan J., Wang F., Li D., Yang M., Xing L., Wei J., Xiao H., Yang Yan, Qu J., Qing L., Chen L., Xu Z., Peng L., Li Y., Zheng H., Chen F., Huang K., Jiang Y., Liu D., Zhang Z., Liu Y., Liu L. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA, J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimabukuro-Vornhagen A., Gödel P., Subklewe M., Stemmler H.J., Schlößer H.A., Schlaak M., Kochanek M., Böll B., von Bergwelt-Baildon M.S. Cytokine release syndrome. J. Immunother. Cancer. 2018 doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvagno F., Vernone A., Pescarmona G.P. 2020. The Role of Glutathione in Protecting against the Severe Inflammatory Response Triggered by Covid-19. Antioxidants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Şimşek Yavuz S., Ünal S. Antiviral treatment of covid-19. Turk. J. Med. Sci. 2020 doi: 10.3906/sag-2004-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.K., Majumdar S., Singh R., Misra A. Role of corticosteroid in the management of COVID-19: a systemic review and a Clinician's perspective. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14:971–978. doi: 10.1016/j.dsx.2020.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soo Y.O.Y., Cheng Y., Wong R., Hui D.S., Lee C.K., Tsang K.K.S., Ng M.H.L., Chan P., Cheng G., Sung J.J.Y. Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients. Clin. Microbiol. Infect. 2004;10:676–678. doi: 10.1111/j.1469-0691.2004.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhail S., Zajac J., Fossum C., Lowater H., McCracken C., Severson N., Laatsch B., Narkiewicz-Jodko A., Johnson B., Liebau J., Bhattacharyya S., Hati S. Role of oxidative stress on SARS-CoV (SARS) and SARS-CoV-2 (COVID-19) infection: a review. Protein J. 2020 doi: 10.1007/s10930-020-09935-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J., Li W., Murakami A., Tamin A., Matthews L.J., Wong S.K., Moore M.J., Tallarico A.S.C., Olurinde M., Choe H., Anderson L.J., Bellini W.J., Farzan M., Marasco W.A. Potent neutralization of severe acute respiratory syndrome (SARS) coronavirus by a human mAb to S1 protein that blocks receptor association. Proc. Natl. Acad. Sci. U. S. A. 2004 doi: 10.1073/pnas.0307140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J.W., Toovey O.T.R., Harvey K.N., Hui D.D.S. Introduction of the South African SARS-CoV-2 variant 501Y.V2 into the UK. J. Infect. 2021 doi: 10.1016/j.jinf.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T., Bidon M., Jaimes J.A., Whittaker G.R., Daniel S. Coronavirus membrane fusion mechanism offers a potential target for antiviral development. Antivir. Res. 2020 doi: 10.1016/j.antiviral.2020.104792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegally H., Wilkinson E., Giovanetti M., Iranzadeh A., Fonseca V., Giandhari J., Doolabh D., Pillay S., San E.J., Msomi N., Mlisana K., von Gottberg A., Walaza S., Allam M., Ismail A., Mohale T., Glass A.J., Engelbrecht S., van Zyl G., Preiser W., Petruccione F., Sigal A., Hardie D., Marais G., Hsiao M., Korsman S., Davies M.A., Tyers L., Mudau I., York D., Maslo C., Goedhals D., Abrahams S., Laguda-Akingba O., Alisoltani-Dehkordi A., Godzik A., Wibmer C.K., Sewell B.T., Lourenço J., Alcantara L.C.J., Kosakovsky Pond S.L., Weaver S., Martin D., Lessells R.J., Bhiman J.N., Williamson C., de Oliveira T. 2020. Emergence and Rapid Spread of a New Severe Acute Respiratory Syndrome-Related Coronavirus 2 (SARS-CoV-2) Lineage with Multiple Spike Mutations in South Africa. medRxiv. [DOI] [Google Scholar]
- Tian X., Li C., Huang A., Xia S., Lu S., Shi Z., Lu L., Jiang S., Yang Z., Wu Y., Ying T. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg. Microb. Infect. 2020 doi: 10.1080/22221751.2020.1729069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toelzer C., Gupta K., Yadav S.K.N., Borucu U., Garzoni F., Staufer O., Capin J., Spatz J., Fitzgerald D., Berger I., Schaffitzel C. Unexpected free fatty acid binding pocket in the cryo-EM structure of SARS-CoV-2 spike protein. bioRxiv. 2020 doi: 10.1101/2020.06.18.158584. 2020.06.18.158584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touyz R.M. Reactive oxygen species and angiotensin II signaling in vascular cells - implications in cardiovascular disease. Braz. J. Med. Biol. Res. 2004 doi: 10.1590/S0100-879X2004000800018. [DOI] [PubMed] [Google Scholar]
- Van Den Borne B.E.E.M., Dijkmans B.A.C., De Rooij H.H., Le Cessie S., Verweij C.L. Chloroquine and hydroxychloroquine equally affect tumor necrosis factor-α, interleukin 6, and interferon-γ production by peripheral blood mononuclear cells. J. Rheumatol. 1997;24:55–60. [PubMed] [Google Scholar]
- Villar J., Ferrando C., Martínez D., Ambrós A., Muñoz T., Soler J.A., Aguilar G., Alba F., González-Higueras E., Conesa L.A., Martín-Rodríguez C., Díaz-Domínguez F.J., Serna-Grande P., Rivas R., Ferreres J., Belda J., Capilla L., Tallet A., Añón J.M., Fernández R.L., González-Martín J.M., Álvarez J., Añón J.M., Asensio M.J., Blanco J., Blasco M., Cachafeiro L., del Campo R., Carbonell J.A., Carbonell N., Cariñena A., Carriedo D., Chico M., Conesa L.A., Corpas R., Cuervo J., Díaz-Domínguez F.J., Domínguez-Antelo C., Fernández L., Fernández R.L., Gamboa E., González-Luengo R.I., González-Martín J.M., Ortiz Díaz-Miguel R., Pérez-González R., Prieto A.M., Prieto I., Rojas-Viguera L., Romera M.A., Sánchez-Ballesteros J., Segura J.M., Serrano A., Solano R., Soler J.A., Soro M. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir. Med. 2020;8:267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G., Seidah N.G., Nichol S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., Bibi S., Briner C., Cicconi P., Collins A.M., Colin-Jones R., Cutland C.L., Darton T.C., Dheda K., Duncan C.J.A., Emary K.R.W., Ewer K.J., Fairlie L., Faust S.N., Feng S., Ferreira D.M., Finn A., Goodman A.L., Green C.M., Green C.A., Heath P.T., Hill C., Hill H., Hirsch I., Hodgson S.H.C., Izu A., Jackson S., Jenkin D., Joe C.C.D., Kerridge S., Koen A., Kwatra G., Lazarus R., Lawrie A.M., Lelliott A., Libri V., Lillie P.J., Mallory R., Mendes A.V.A., Milan E.P., Minassian A.M., McGregor A., Morrison H., Mujadidi Y.F., Nana A., O’Reilly P.J., Padayachee S.D., Pittella A., Plested E., Pollock K.M., Ramasamy M.N., Rhead S., Schwarzbold A.V., Singh N., Smith A., Song R., Snape M.D., Sprinz E., Sutherland R.K., Tarrant R., Thomson E.C., Török M.E., Toshner M., Turner D.P.J., Vekemans J., Villafana T.L., Watson M.E.E., Williams C.J., Douglas A.D., Hill A.V.S., Lambe T., Gilbert S.C., Pollard A.J., Aban M., Abayomi F., Abeyskera K., Aboagye J., Adam M., Adams K., Adamson J., Adelaja Y.A., Adewetan G., Adlou S., Ahmed K., Akhalwaya Y., Akhalwaya S., Alcock A., Ali A., Allen E.R., Allen L., Almeida T.C.D.S.C., Alves M.P.S., Amorim F., Andritsou F., Anslow R., Appleby M., Arbe-Barnes E.H., Ariaans M.P., Arns B., Arruda L., Azi P., Azi L., Babbage G., Bailey C., Baker K.F., Baker M., Baker N., Baker P., Baldwin L., Baleanu I., Bandeira D., Bara A., Barbosa M.A.S., Barker D., Barlow G.D., Barnes E., Barr A.S., Barrett J.R., Barrett J., Bates L., Batten A., Beadon K., Beales E., Beckley R., Belij-Rammerstorfer S., Bell J., Bellamy D., Bellei N., Belton S., Berg A., Bermejo L., Berrie E., Berry L., Berzenyi D., Beveridge A., Bewley K.R., Bexhell H., Bhikha S., Bhorat A.E., Bhorat Z.E., Bijker E., Birch G., Birch S., Bird A., Bird O., Bisnauthsing K., Bittaye M., Blackstone K., Blackwell L., Bletchly H., Blundell C.L., Blundell S.R., Bodalia P., Boettger B.C., Bolam E., Boland E., Bormans D., Borthwick N., Bowring F., Boyd A., Bradley P., Brenner T., Brown P., Brown C., Brown-O’Sullivan C., Bruce S., Brunt E., Buchan R., Budd W., Bulbulia Y.A., Bull M., Burbage J., Burhan H., Burn A., Buttigieg K.R., Byard N., Cabera Puig I., Calderon G., Calvert A., Camara S., Cao M., Cappuccini F., Cardoso J.R., Carr M., Carroll M.W., Carson-Stevens A., Carvalho Y. de M., Carvalho J.A.M., Casey H.R., Cashen P., Castro T., Castro L.C., Cathie K., Cavey A., Cerbino-Neto J., Chadwick J., Chapman D., Charlton S., Chelysheva I., Chester O., Chita S., Cho J.S., Cifuentes L., Clark E., Clark M., Clarke A., Clutterbuck E.A., Collins S.L.K., Conlon C.P., Connarty S., Coombes N., Cooper C., Cooper R., Cornelissen L., Corrah T., Cosgrove C., Cox T., Crocker W.E.M., Crosbie S., Cullen L., Cullen D., Cunha D.R.M.F., Cunningham C., Cuthbertson F.C., Da Guarda S.N.F., da Silva L.P., Damratoski B.E., Danos Z., Dantas M.T.D.C., Darroch P., Datoo M.S., Datta C., Davids M., Davies S.L., Davies H., Davis E., Davis, Judith, Davis, John, De Nobrega M.M.D., De Oliveira Kalid L.M., Dearlove D., Demissie T., Desai A., Di Marco S., Di Maso C., Dinelli M.I.S., Dinesh T., Docksey C., Dold C., Dong T., Donnellan F.R., Dos Santos T., dos Santos T.G., Dos Santos E.P., Douglas N., Downing C., Drake J., Drake-Brockman R., Driver K., Drury R., Dunachie S.J., Durham B.S., Dutra L., Easom N.J.W., van Eck S., Edwards M., Edwards N.J., El Muhanna O.M., Elias S.C., Elmore M., English M., Esmail A., Essack Y.M., Farmer E., Farooq M., Farrar M., Farrugia L., Faulkner B., Fedosyuk S., Felle S., Ferreira Da Silva C., Field S., Fisher R., Flaxman A., Fletcher J., Fofie H., Fok H., Ford K.J., Fowler J., Fraiman P.H.A., Francis E., Franco M.M., Frater J., Freire M.S.M., Fry S.H., Fudge S., Furze J., Fuskova M., Galian-Rubio P., Galiza E., Garlant H., Gavrila M., Geddes A., Gibbons K.A., Gilbride C., Gill H., Glynn S., Godwin K., Gokani K., Goldoni U.C., Goncalves M., Gonzalez I.G.S., Goodwin J., Goondiwala A., Gordon-Quayle K., Gorini G., Grab J., Gracie L., Greenland M., Greenwood N., Greffrath J., Groenewald M.M., Grossi L., Gupta G., Hackett M., Hallis B., Hamaluba M., Hamilton E., Hamlyn J., Hammersley D., Hanrath A.T., Hanumunthadu B., Harris S.A., Harris C., Harris T., Harrison T.D., Harrison D., Hart T.C., Hartnell B., Hassan S., Haughney J., Hawkins S., Hay J., Head I., Henry J., Hermosin Herrera M., Hettle D.B., Hill J., Hodges G., Horne E., Hou M.M., Houlihan C., Howe E., Howell N., Humphreys J., Humphries H.E., Hurley K., Huson C., Hyder-Wright A., Hyams C., Ikram S., Ishwarbhai A., Ivan M., Iveson P., Iyer V., Jackson F., De Jager J., Jaumdally S., Jeffers H., Jesudason N., Jones B., Jones K., Jones E., Jones C., Jorge M.R., Jose A., Joshi A., Júnior E.A.M.S., Kadziola J., Kailath R., Kana F., Karampatsas K., Kasanyinga M., Keen J., Kelly E.J., Kelly D.M., Kelly D., Kelly S., Kerr D., Kfouri R., de Á., Khan L., Khozoee B., Kidd S., Killen A., Kinch J., Kinch P., King L.D.W., King T.B., Kingham L., Klenerman P., Knapper F., Knight J.C., Knott D., Koleva S., Lang M., Lang G., Larkworthy C.W., Larwood J.P.J., Law R., Lazarus E.M., Leach A., Lees E.A., Lemm N.M., Lessa A., Leung S., Li Y., Lias A.M., Liatsikos K., Linder A., Lipworth S., Liu S., Liu X., Lloyd A., Lloyd S., Loew L., Lopez Ramon R., Lora L., Lowthorpe V., Luz K., MacDonald J.C., MacGregor G., Madhavan M., Mainwaring D.O., Makambwa E., Makinson R., Malahleha M., Malamatsho R., Mallett G., Mansatta K., Maoko T., Mapetla K., Marchevsky N.G., Marinou S., Marlow E., Marques G.N., Marriott P., Marshall R.P., Marshall J.L., Martins F.J., Masenya M., Masilela M., Masters S.K., Mathew M., Matlebjane H., Matshidiso K., Mazur O., Mazzella A., McCaughan H., McEwan J., McGlashan J., McInroy L., McIntyre Z., McLenaghan D., McRobert N., McSwiggan S., Megson C., Mehdipour S., Meijs W., Mendonça R.N.Á., Mentzer A.J., Mirtorabi N., Mitton C., Mnyakeni S., Moghaddas F., Molapo K., Moloi M., Moore M., Moraes-Pinto M.I., Moran M., Morey E., Morgans R., Morris, Susan, Morris, Sheila, Morris H.C., Morselli F., Morshead G., Morter R., Mottal L., Moultrie A., Moya N., Mpelembue M., Msomi S., Mugodi Y., Mukhopadhyay E., Muller J., Munro A., Munro C., Murphy S., Mweu P., Myasaki C.H., Naik G., Naker K., Nastouli E., Nazir A., Ndlovu B., Neffa F., Njenga C., Noal H., Noé A., Novaes G., Nugent F.L., Nunes G., O’Brien K., O’Connor D., Odam M., Oelofse S., Oguti B., Olchawski V., Oldfield N.J., Oliveira M.G., Oliveira C., Oosthuizen A., O’Reilly P., Osborne P., Owen D.R.J., Owen L., Owens D., Owino N., Pacurar M., Paiva B.V.B., Palhares E.M.F., Palmer S., Parkinson S., Parracho H.M.R.T., Parsons K., Patel D., Patel B., Patel F., Patel K., Patrick-Smith M., Payne R.O., Peng Y., Penn E.J., Pennington A., Peralta Alvarez M.P., Perring J., Perry N., Perumal R., Petkar S., Philip T., Phillips D.J., Phillips J., Phohu M.K., Pickup L., Pieterse S., Piper J., Pipini D., Plank M., Du Plessis J., Pollard S., Pooley J., Pooran A., Poulton I., Powers C., Presa F.B., Price D.A., Price V., Primeira M., Proud P.C., Provstgaard-Morys S., Pueschel S., Pulido D., Quaid S., Rabara R., Radford A., Radia K., Rajapaska D., Rajeswaran T., Ramos A.S.F., Ramos Lopez F., Rampling T., Rand J., Ratcliffe H., Rawlinson T., Rea D., Rees B., Reiné J., Resuello-Dauti M., Reyes Pabon E., Ribiero C.M., Ricamara M., Richter A., Ritchie N., Ritchie A.J., Robbins A.J., Roberts H., Robinson R.E., Robinson H., Rocchetti T.T., Rocha B.P., Roche S., Rollier C., Rose L., Ross Russell A.L., Rossouw L., Royal S., Rudiansyah I., Ruiz S., Saich S., Sala C., Sale J., Salman A.M., Salvador N., Salvador S., Sampaio M., Samson A.D., Sanchez-Gonzalez A., Sanders H., Sanders K., Santos E., Santos Guerra M.F.S., Satti I., Saunders J.E., Saunders C., Sayed A., Schim van der Loeff I., Schmid A.B., Schofield E., Screaton G., Seddiqi S., Segireddy R.R., Senger R., Serrano S., Shah R., Shaik I., Sharpe H.E., Sharrocks K., Shaw R., Shea A., Shepherd A., Shepherd J.G., Shiham F., Sidhom E., Silk S.E., da Silva Moraes A.C., Silva-Junior G., Silva-Reyes L., Silveira A.D., Silveira M.B.V., Sinha J., Skelly D.T., Smith D.C., Smith N., Smith H.E., Smith D.J., Smith C.C., Soares A., Soares T., Solórzano C., Sorio G.L., Sorley K., Sosa-Rodriguez T., Souza C.M.C.D.L., Souza B.S.D.F., Souza A.R., Spencer A.J., Spina F., Spoors L., Stafford L., Stamford I., Starinskij I., Stein R., Steven J., Stockdale L., Stockwell L.V., Strickland L.H., Stuart A.C., Sturdy A., Sutton N., Szigeti A., Tahiri-Alaoui A., Tanner R., Taoushanis C., Tarr A.W., Taylor K., Taylor U., Taylor I.J., Taylor J., te Water Naude R., Themistocleous Y., Themistocleous A., Thomas M., Thomas K., Thomas T.M., Thombrayil A., Thompson F., Thompson, Amber, Thompson K., Thompson, Ameeka, Thomson J., Thornton-Jones V., Tighe P.J., Tinoco L.A., Tiongson G., Tladinyane B., Tomasicchio M., Tomic A., Tonks S., Towner J., Tran N., Tree J., Trillana G., Trinham C., Trivett R., Truby A., Tsheko B.L., Turabi A., Turner R., Turner C., Ulaszewska M., Underwood B.R., Varughese R., Verbart D., Verheul M., Vichos I., Vieira T., Waddington C.S., Walker L., Wallis E., Wand M., Warbick D., Wardell T., Warimwe G., Warren S.C., Watkins B., Watson E., Webb S., Webb-Bridges A., Webster A., Welch J., Wells J., West A., White C., White R., Williams P., Williams R.L., Winslow R., Woodyer M., Worth A.T., Wright D., Wroblewska M., Yao A., Zimmer R., Zizi D., Zuidewind P. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagstaff K.M., Sivakumaran H., Heaton S.M., Harrich D., Jans D.A. Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem. J. 2012 doi: 10.1042/BJ20120150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace D.J., Linker-Israeli M., Hyun S., Klinenberg J.R., Stecher V. The effect of hydroxychloroquine therapy on serum levels of immunoregulatory molecules in patients with systemic lupus erythematosus - PubMed. J. Rheumatol. 1994:375–376. [PubMed] [Google Scholar]
- Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters T.E., Kalman J.M., Patel S.K., Mearns M., Velkoska E., Burrell L.M. Angiotensin converting enzyme 2 activity and human atrial fibrillation: increased plasma angiotensin converting enzyme 2 activity is associated with atrial fibrillation and more advanced left atrial structural remodelling. Europace. 2017 doi: 10.1093/europace/euw246. [DOI] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020 doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Zhang Y., Wu L., Niu S., Song C., Zhang Z., Lu G., Qiao C., Hu Y., Yuen K.Y., Wang Qisheng, Zhou H., Yan J., Qi J. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181:894–904. doi: 10.1016/j.cell.2020.03.045. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Allen J.D., Wrapp D., McLellan J.S., Crispin M. Site-specific glycan analysis of the SARS-CoV-2 spike. Science 84. 2020;369:330–333. doi: 10.1126/science.abb9983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H. Oxidative stress-mediated effects of angiotensin II in the cardiovascular system. World J. Hypertens. 2012;2:34. doi: 10.5494/wjh.v2.i4.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Coronavirus disease (COVID-19) advice for the public. Coronavirus Dis. 2020 2019. [Google Scholar]
- Williamson B.N., Feldmann F., Schwarz B., Meade-White K., Porter D.P., Schulz J., de Wit E. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature. 2020 doi: 10.1038/s41586-020-2423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise J. Covid-19: new coronavirus variant is identified in UK. BMJ. 2020;371:m4857. doi: 10.1136/bmj.m4857. [DOI] [PubMed] [Google Scholar]
- World Health Organization . World Heal; Organ: 2003. International travel and health SARS (severe acute respiratory syndrome. [WWW Document] [Google Scholar]
- World Health Organization . Who; 2003. WHO | World Health Organization issues emergency travel advisory [WWW Document] [Google Scholar]
- World Health Organization . World Heal; Organ: 2003. SARS: Breaking the Chains of Transmission. [WWW Document] [Google Scholar]
- World Health Organization (WHO) WHO Bull; 2020. Novel Coronavirus ( 2019-nCoV ) Situation Report - 1 21 January 2020. [WWW Document] [Google Scholar]
- World Health Organization (WHO) 2020. Coronavirus Disease 2019 (COVID-19) Situation Report – 51. [WWW Document]. World Heal. Organ. [DOI] [Google Scholar]
- Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.-L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 84. 2020;367:1260–1263. doi: 10.1101/2020.02.11.944462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., Hu Y., Tao Z.W., Tian J.H., Pei Y.Y., Yuan M.L., Zhang Y.L., Dai F.H., Liu Y., Wang Q.M., Zheng J.J., Xu L., Holmes E.C., Zhang Y.Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Hu Y., Xu M., Chen Z., Yang W., Jiang Z., Li M., Jin H., Cui G., Chen P., Wang L., Zhao G., Ding Y., Zhao Y., Yin W. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021 doi: 10.1016/S1473-3099(20)30987-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S., Zhang Y., Wang Y., Wang Hui, Yang Yunkai, Gao G.F., Tan W., Wu G., Xu M., Lou Z., Huang W., Xu W., Huang B., Wang Huijuan, Wang Wei, Zhang W., Li N., Xie Z., Ding L., You W., Zhao Y., Yang Xuqin, Liu Y., Wang Q., Huang L., Yang Yongli, Xu G., Luo B., Wang Wenling, Liu P., Guo W., Yang Xiaoming. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021;21:39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Xu X., Jiang L., Dua K., Hansbro P.M., Liu G. SARS-CoV-2 induces transcriptional signatures in human lung epithelial cells that promote lung fibrosis. Respir. Res. 2020;21 doi: 10.1186/s12931-020-01445-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Yu C., Qu J., Zhang L., Jiang S., Huang D., Chen B., Zhang Z., Guan W., Ling Z., Jiang R., Hu T., Ding Y., Lin L., Gan Q., Luo L., Tang X., Liu J. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur. J. Nucl. Med. Mol. Imag. 2020 doi: 10.1007/s00259-020-04735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 84. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., Liu X., Zhao L., Dong E., Song C., Zhan S., Lu R., Li H., Tan W., Liu D. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q., West A.M.V., Silletti S., Corbett K.D. Architecture and self-assembly of the SARS-CoV-2 nucleocapsid protein. bioRxiv Prepr. Serv. Biol. 2020 doi: 10.1101/2020.05.17.100685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa T., Naito Y. JMAJ; 2002. What is oxidative stress? [Google Scholar]
- Yoshimoto F.K. 2020. The Proteins of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS CoV-2 or N-COV19), the Cause of COVID-19. Protein J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S., Fan J., Yu F., Feng B., Lou B., Zou Q., Xie G., Lin S., Wang R., Yang X., Chen W., Wang Q., Zhang D., Liu Y., Gong R., Ma Z., Lu S., Xiao Y., Gu Y., Zhang J., Yao H., Xu K., Lu X., Wei G., Zhou J., Fang Q., Cai H., Qiu Y., Sheng J., Chen Y., Liang T. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X., Lou Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R., Di Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q., Zhang Y., Wang L., Yao X., Wu D., Cheng J., Pan X., Liu H., Yan Z., Gao L. Inhibition of coronavirus infection by a synthetic STING agonist in primary human airway system. Antivir. Res. 2021;187 doi: 10.1016/j.antiviral.2021.105015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., Yu J., Kang M., Song Y., Xia J., Guo Q., Song T., He J., Yen H.L., Peiris M., Wu J. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]