Abstract
Objectives:
Fellow involvement in patient care is important for education, but effect on patient care is unclear. Our aim was to compare patient outcomes in gynecologic oncology attending clinics versus a fellow training clinic at a large academic medical center.
Methods:
A retrospective review of consecutive gynecologic oncology patients from six attending clinics and one faculty-supervised fellow clinic was used to analyze differences based on patient demographics, cancer characteristics, and practice patterns. Primary outcome was overall survival (OS); secondary outcomes included recurrence-free survival (RFS), postoperative complications and chemotherapy within the last 30 days of life. Survival analyses were performed using Kaplan-Meier curves with log-rank tests.
Results:
Of 159 patients, 76 received care in the attending clinic and 83 in the fellow clinic. Patients in the fellow clinic were younger, less likely to be Caucasian, and more overweight, but cancer site and proportion of advanced stage disease were similar. Both clinics had similar rates of moderate to severe adverse events related to surgery (15% vs. 8%, p=0.76), chemotherapy (21% vs. 23%, p=0.40), and radiation (14% vs. 17%, p=0.73). There was no difference in median RFS in the fellow compared to attending clinic (38 vs. 47 months, p=0.78). OS on both univariate (49 months--fellow clinic, 60 months--attending clinic vs. p = 0.40) and multivariate analysis [hazard ratio 1.3 (0.57, 2.75), P = 0.58] was not significantly different between groups.
Conclusions:
A fellow-run gynecologic oncology clinic designed to provide learning opportunities does not compromise patient outcomes and is a safe and feasible option for fellow education.
Introduction
Gynecologic oncology fellowship programs are tasked with training physicians in the care of women with and at risk for gynecologic cancers. In addition to formal didactic education, faculty achieve this aim by providing graduated autonomy in patient management leading to independent practice. Though there are standardized educational milestones, fellowship program directors and health care administrators face the practical dilemma of creating an environment that fosters effective practice- and system-based learning while granting the ability to exercise independent thinking[1]. The process for and limits to providing graduated autonomy to trainees in the operating room have been explored[2, 3]. However, little is known about the safety or effectiveness of strategies for fellow training in the outpatient setting, despite this being an important component of fellowship education[1, 4, 5].
Recent studies have documented gaps in learning among gynecologic oncology fellows. Graduating fellows risk being unprepared for dealing with postoperative complications, developing management plans, and executing palliative care principles[6-8]. To address these gaps and better develop graduated autonomy in the outpatient arena, some training programs employ a fellow-run clinic. While well adapted for education, fellow-run clinics may lead to worse patient outcomes if inexperienced fellows make frequent errors. Fellow involvement in colorectal surgical care has been shown to lead to outcomes similar to those achieved by attending physicians alone[9]. Whether similar outcomes occur in fellow-run gynecologic oncology clinics is unknown.
Here we report our institutional experience with a fellow-run gynecologic oncology outpatient clinic with attending physician oversight that provides care to low-income, underserved women. Similar clinics that target this population have been shown to mitigate survival disparities when compared to their private clinic counterparts, but fellows were not the principal providers[10]. The objective of this study was to compare patient outcomes in a fellow training clinic to those achieved in attending clinics. Our goal was to investigate whether this model of fellowship training could provide practical learning opportunities to achieve graduated autonomy without impairing quality of care.
Methods
We performed a retrospective cohort study to evaluate all new patients who sought care for gynecologic malignancy by providers within the Division of Gynecologic Oncology at Washington University School of Medicine and Barnes-Jewish Hospital from May 2010 to May 2012. This period was chosen in order to achieve a minimum follow-up period of 5 years. This study was approved by Washington University's Human Research Protection Office.
Patients were included in the final analysis if they were older than 18 years of age with a histologically confirmed diagnosis of uterine, ovarian, or cervical cancer. Patients were excluded when diagnosed with synchronous primary malignancies or rare histologies, pregnancy, benign disease, or crossover between clinics at any stage in their care. Women were also excluded if an outside provider performed their surgeries, provided surveillance, or delivered chemotherapy. In an effort to capture patient outcomes that best reflected provider management strategies versus patient non-adherence, we also limited our sample population to those who had consistent follow-up in our clinics, defined as fewer than four consecutive missed appointments.
As part of its nonprofit mission, Barnes-Jewish Hospital in St. Louis has provided indigent care to the surrounding community for more than a century. Combining humanitarian and medical education objectives, these clinics are staffed by residents and fellows and provide care to uninsured and Medicaid-eligible patients. Throughout the study period, patients received discounted care and were not denied care for financial reasons. Management decisions in the fellow clinic were made by one of four clinical fellows per academic year at each visit under the in-person supervision of an attending gynecologic oncologist (LSM). Fellows were allotted graduated responsibility and were allowed to execute management plans with any clinical faculty to take advantage of individual faculty strengths and practice patterns. However, in development of management plans, fellows were asked to consider evidence based medicine including National Comprehensive Cancer Network Evidence Blocks, in addition to cost-effectiveness and potential for financial toxicity. The attending clinic patients were seen by the same attending gynecologic oncologist throughout their care. Residents were routinely integrated in the outpatient care of patients in the fellow clinic, but due to scheduling, they had minimal exposure to attending clinics. Though both clinics had access to social worker referrals, the fellow clinic has ongoing hospital support to maintain an on-site social worker who is readily available to assist patients with outpatient resources to reduce financial toxicity. Otherwise, both clinics have dedicated nurse coordinators, clinical trial opportunities, radiation oncologists, and infusion support. Patients were treated in the same operating rooms and postoperative care was delivered on the same hospital floor.
The primary outcome was overall survival (OS). Date of death was determined using electronic medical records and public death records. Secondary outcomes included recurrence-free survival (RFS) and key quality measures: postoperative complications within 30 days of surgery based on the Accordion severity grading[11], incidence of grade 3 or 4 chemotherapy- or radiation-related adverse events based on Common Terminology Criteria for Adverse Events version 5.0[12], and treatment given within the last 30 days of life. Power calculations for predetermined sample size quotas were not utilized given lack of universally accepted and verified differences in survival based on gynecologic oncology fellow training. Instead, we designed our study to provide a reasonable time frame of 2 years with 5 year follow-up. Nevertheless, based on a post-hoc calculation, a sample size of 75 in the attending clinic group and 80 in the fellow clinic group was needed to achieve 80% power to detect non-inferiority of OS in the fellow clinic group.
Patient and treatment characteristics were descriptively summarized and compared using Chi-squared or Fisher’s exact tests, Student’s t-tests, and Mann-Whitney U tests as appropriate. OS and RFS were compared using Kaplan-Meier curves with log-rank tests and a Cox-proportional hazard model, adjusting for potential confounders. All tests were two-sided with the significance level set at 0.05.
Results
We assessed a total of 191 records for patients who met initial selection criteria. Of those, 32 (27 from fellow clinic and 5 from attending clinics) were excluded for inconsistent follow-up, leaving 159 patients for final statistical analysis. The median follow-up was 51 months (range 2-109). Table 1 displays the patient characteristics. Women seen in the fellow clinic were on average nearly a decade younger, less likely to be Caucasian, more likely to have Medicaid coverage or be uninsured, and have a higher median body mass index. Patients were equivalent with respect to median Charlson Comorbidity Index score. Although cervical cancer was overrepresented in the fellow clinic (35% vs. 22%), the distribution of disease sites and grade were not significantly different in any of the cancer types. The fellow clinic group had a higher proportion of advanced (stage III/IV) disease at 39% vs. 22% (p = 0.04), although the rate of recurrence was similar (p = 0.76).
Table 1.
Patient Characteristics
| Attending Clinic N=76 (%) |
Fellow Clinic N=83 (%) |
P value | |
|---|---|---|---|
| Age in years (mean ± SD) | 66.2 ± 15.8 | 57.2 ± 10.7 | <0.001 |
| Race | <0.001 | ||
| Caucasian | 69 (91) | 48 (58) | |
| African American | 7 (9) | 29 (35) | |
| Other | 0 (0) | 6 (7) | |
| Insurance Coverage1 | <0.001 | ||
| Commercial / Medicare | 73 (96) | 25 (30) | |
| Medicaid / Uninsured | 6 (8) | 58 (70) | |
| Median BMI (IQR) kg/m2 | 28 (24-35) | 34 (28-43) | 0.002 |
| Median Charlson Comorbidity Index (IQR) | 4 (3-7) | 6 (3-8) | 0.17 |
| Disease site | 0.16 | ||
| Uterus | 41 (54) | 41 (49) | |
| Ovary | 18 (24) | 13 (16) | |
| Cervix | 17 (22) | 29 (35) | |
| Stage2 | 0.04 | ||
| I/II | 58 (76) | 49 (59) | |
| III/IV | 17 (22) | 32 (39) | |
| Unknown | 1 (1) | 2 (2) | |
| Incidence of recurrence | 23 (30) | 24 (29) | 0.76 |
Numbers are reported as column percentage with the denominator excluding missing values unless otherwise noted.
Insurance status was not mutually exclusive among individual patients.
Unknown data due to discrepancy in documentation.
SD = standard deviation. IQR = interquartile range.
Treatment characteristics are represented in Table 2. Timely treatment initiation (either surgical or medical) was similar between the two groups with the median time to therapy being 20 days in the fellow clinic compared to 18 days in the attending clinic (p = 0.56). Both groups had similar rates of laparoscopic versus open surgical approaches (p = 0.59). The proportion of patients who received surgery only was lower in the fellow clinic group than the attending clinic group, 24% vs. 43%, respectively p=0.001. Otherwise, treatment rates with regards to receiving chemotherapy only as well as surgery with adjuvant treatment of any kind was similar between the groups. The proportion of patients who received any number of chemotherapy cycles and radiation treatment was 28% in the fellow clinic vs. 7% in the attending clinic (p=0.001).
Table 2.
Types of treatment completed and route of hysterectomy, where applicable
| Attending Clinic N=76 (%) |
Fellow Clinic N=83 (%) |
|
|---|---|---|
| Treatment1 | ||
| Surgery only | 33 (43) | 20 (24) |
| Surgery + chemotherapy | 18 (24) | 13 (16) |
| Surgery + radiation | 8 (11) | 2 (2) |
| Surgery + chemotherapy + radiation | 10 (13) | 13 (16) |
| Chemotherapy + radiation only | 5 (7) | 23 (28) |
| Chemotherapy only | 2 (3) | 4 (5) |
| Radiation only | 0 (0) | 6 (7) |
| No treatment | 0 (0) | 2 (2) |
| Hysterectomy type2 | ||
| Open | 57 (83) | 36 (75) |
| Laparoscopic | 6 (9) | 7 (15) |
| Robotic | 6 (9) | 5 (10) |
All treatments are mutually exclusive.
Hysterectomy route percentages with denominator equal to total number of patients undergoing hysterectomy in each group (n = 69 in attending clinic and n = 48 in fellow clinic).
Postoperatively, there were no significant differences in 30-day complication rates between groups. Regarding adverse events related to non-surgical therapies, patients also did not differ in their rates of grade 3+ toxicities related to chemotherapy and/or radiation (Table 3). There were no immediate-postoperative or treatment-related deaths.
Table 3.
Rates of complications from treatment
| Attending Clinic N=76 (%) |
Fellow Clinic N=83 (%) |
P value | |
|---|---|---|---|
| Surgical Complication Severity1 | |||
| Mild | 13 (19) | 5 (10) | 0.50 |
| Moderate | 5 (7) | 6 (13) | |
| Severe | 1 (1) | 1 (2) | |
| No complications | 50 (72) | 37 (77) | |
| Type of Surgical Complication2 | |||
| Infectious3 | 9 (12) | 6 (7) | 0.32 |
| Hematologic | 2 (3) | 1 (1) | 0.61 |
| Cardiovascular | 1 (1) | 0 (0) | 0.48 |
| Gastrointestinal | 6 (8) | 4 (5) | 0.52 |
| Other | 3 (4) | 1 (1) | 0.35 |
| Grade 3 or 4 Chemotherapy Complications4 | 8 (23) | 11 (21) | 0.40 |
| Grade 3 or 4 Radiation Therapy Complications5 | 4 (17) | 6 (14) | 0.73 |
| 1st visit to treatment - median days (IQR) | 18 (10-30) | 20 (15-32) | 0.56 |
| Chemotherapy within last 30 days of life4 | 5 (14) | 1 (2) | 0.03 |
Denominator includes only patients who underwent surgery: n = 69 (attending clinic) and n = 48 (fellow clinic). No missing data. Complications are classified and graded according to the Accordion scale.
Not mutually exclusive
Includes both superficial and deep surgical site infections
Denominator includes only patients who received chemotherapy: n = 35 (attending clinic) and n = 53 (fellow clinic)
Denominator includes only patients who received radiation therapy: n = 23 (attending clinic) and n = 44 (fellow clinic)
IQR = interquartile range
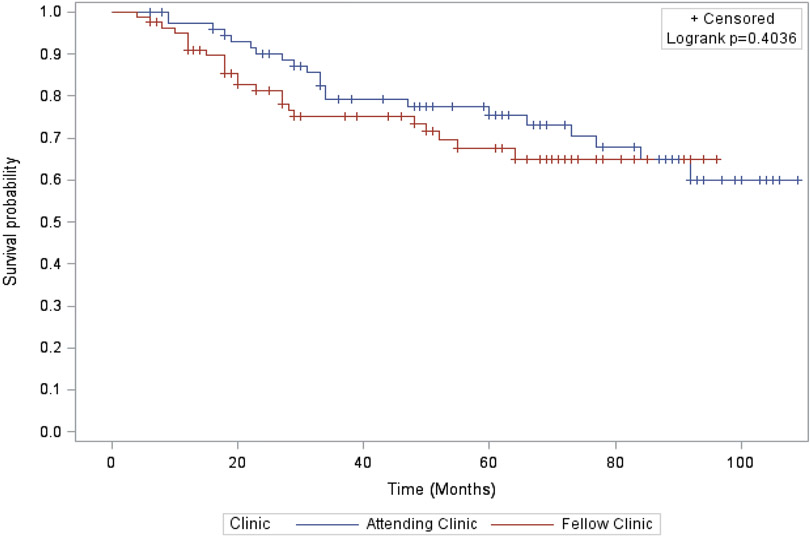
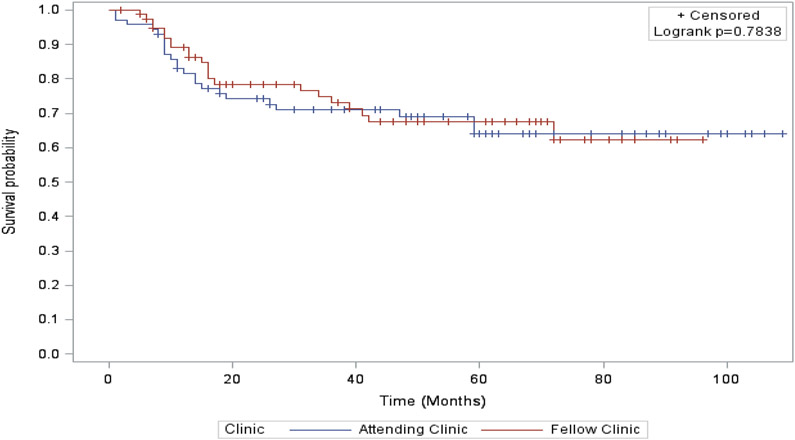
Survival outcomes were not significantly different in the two care settings. We did not identify a difference in our primary outcome, median OS before (60 months in the attending clinic vs. 49 months in the fellow clinic, p = 0.40; Figure 1) or after adjustment for age, race, BMI, cancer site, and cancer stage [hazard radio (HR) for survival for women cared for in the fellows’ clinic 1.3 (0.57, 2.75), P = 0.58]. RFS also was similar between the two care settings (47 vs. 38 months, p = 0.78; Figure 2). The adjusted HR for recurrence among patients in the fellow clinic was 0.59 [(0.24, 1.46), p = 0.25)].
Figure 1. Overall Survival.
Overall survival estimates by Kaplan-Meier assessment stratified by clinic groups. Differences compared statistically using the log-rank test.
Figure 2. Recurrence-free survival.
Recurrence-free survival estimates by Kaplan-Meier assessment stratified by clinic groups. Differences compared statistically using the log-rank test.
We also evaluated for differences in chemotherapy administration within 30 days of death, a key metric for assessing quality of cancer care. Among women who received chemotherapy, those in the fellow clinic received a median of 1 line of chemotherapy [interquartile range (IQR) 1-2] compared to 2 in the attending clinic (IQR 1-4) (p = 0.004). In addition, only one patient (1.9%) in the fellow clinic group received chemotherapy within the last 30 days of life compared to 5 (14.3%) in the attending clinic group (p = 0.03). There was no difference in patients hospitalized in the last 30 days of life with 6 of 83 (7.9%) in the fellow clinic vs. 6 of 76 (7.2%) in the faculty clinic (p = 0.87).
Discussion
A gynecologic oncology fellow-run clinic under faculty supervision can provide the graduated autonomy critical to fellows’ professional development without sacrificing quality of care. Using the attending clinic as a reference, after adjusting for age, race, BMI, cancer site, stage, and recurrence status, the aHR for OS was statistically similar in the fellow clinic at 1.3 (0.57, 2.75). Women cared for in the fellows’ clinic received treatment in a timely fashion, had similar rates of minimally invasive surgery, and comparable rates of surgical, chemotherapy, and radiation adverse events.
These data support the concept of a fellow-run clinic as a safe alternative to fellow education integrated into faculty clinics. Our clinic model most closely resembles the focused faculty model as described by Bodenheimer et al[13]. As compared to the dispersed or hybrid models which have multiple faculty who invite trainees to their own ambulatory setting, our model functions with a gynecologic oncology faculty who spends dedicated time in clinic to supervise, teach, and provide feedback to trainees. The investigators concluded that focused faculty care systems result in greater patient continuity, more effective use of clinic resources, and better demonstration to trainees how clinics should successfully function. This sentiment was also shared among both program directors and hospital administrators. With respect to the advantages of continuity, Fortuna et al has shown that greater continuity among trainee-run clinics leads to improved patient outcomes[14]. Whether a dispersed model with lack of attending continuity would lead to similar outcomes remains to be explored.
There is a paucity of data comparing clinic models specifically among gynecologic oncology fellowship programs, but our model comprises several components of previously successful experiences. Anderson et al. demonstrated that among trainees in obstetrics and gynecology, practical training in an ambulatory setting was the most valuable method of education they experienced[15]. Fellow involvement in a collaborative surgical setting in the colorectal subspecialty resulted in equivalent postoperative complication rates, surgical margin status, and readmission rates when compared to faculty-only cases[9]. In addition, Eskander et al. observed that higher quality experience with end of life care directly resulted in fellows feeling significantly more prepared to manage these patients after completion of training[8]. Our study elevates this body of literature by demonstrating a successful curricula that optimizes gynecologic oncology fellow leaning opportunities without compromising patient care[16].
This study has several limitations, including limited numbers that might obscure meaningfully worse survival among women seen in the fellows’ clinic. It is also difficult to differentiate the possible impact of fellows’ management on survival from the known negative prognostic impact of non-white race and poverty on cancer survival[17]. As a single-institution retrospective cohort study, we are inherently limited by the potential for missing or incorrectly documented data. In addition, we compare two very heterogeneous cohorts with respect to different grades, stages, and primary disease sites. Although it would be ideal to compare large numbers of each of these categories, the volume from a single institution does not allow for that. Despite these limitations, our comparison represents a realistic management experience that could potentially be applied to other academic training centers.
We also acknowledge that our fellow clinic model may be criticized because it segregates patients based on insurance status, which in the United States is typically associated with non-white ethnicity. As expected, we found that women managed through the fellows’ clinic were more likely to be non-white and were more likely to have cervical cancer, a preventable cancer associated with well-documented disparities[18]. Nonetheless, academic medical centers must develop strategies to meet both their community responsibility to deliver high-value care to under-resourced patients that balances cost and quality while fulfilling their social responsibility for medical education to ensure competent care for future patients. It is also the responsibility of both faculty and fellows to not only recognize, but address disparities in cancer care delivery, many of which are driven by race, socioeconomic status, and other social determinants of health. At our institution, although trainees provide care at reduced salary cost, we have shown that quality measures achieved through a fellow clinic appear to be similar to those achieved by faculty clinics. In fact, cost-conscious care may provide patient benefit by minimizing low-value and potentially toxic care, such as chemotherapy toward the end of life. By sharing our experience, it is our hope that other academic institutions will look at their medical education curricula and create meaningful changes to overcome health inequities while maintaining a strong commitment to training future leaders in medicine.
Methods for improvement in value-based measures of care, although not specifically addressed in our data set, should be further explored. As quality becomes the focus of reimbursement, value-based measures and assessments are required to improve costs while maximizing patient outcomes[19-21]. Specific to gynecologic oncology care, prior studies have demonstrated the feasibility of providing low-cost care to underserved women, though none included a fellow training clinic[22-24]. We suspect our clinic model may serve this benefit, but cost analysis would be necessary to confirm. Our study does however, demonstrate that our fellow-run clinic is not inferior in regards to outcomes when compared to attending clinics. It serves as a viable model to achieve both high levels of patient care while balancing the needs of education for fellows.
In summary, our findings support the concept that a dedicated fellows’ clinic allowing for graduated autonomy is safe. Whether it is effective in preparing fellows for independent practice should be a focus for further research.
Highlights:
A gynecologic oncology fellow-run clinic model allows for graduated autonomy without compromising patient care.
Fellow clinic patients achieved overall and recurrence-free survival that was comparable to patients managed by attendings.
Fellow clinic patients received timely care and had similar rates of surgical, chemotherapy, and radiation adverse events.
Gynecologic oncology fellows prescribed less chemotherapy within the last 30 days of life compared to attendings.
Footnotes
Conflict of Interest: Dr. Thaker reports personal fees from Celsion, personal fees from Stryker, grants and personal fees from Glaxo-Smith Kline, grants and personal fees from Merck, personal fees from Abbvie, and personal fees from Astra Zeneca, outside the submitted work. Dr. Powell reports personal fees from Merck, personal fees from Tesaro, personal fees from Clovis Oncology, personal fees from AstraZeneca, personal fees from Roche/Genentech, and personal fees from GOG Foundation, outside the submitted work. Dr. Kuroki reports a grant from Washington University Institute of Clinical and Translational Sciences (KL2) during the conduct of the study. All other authors declare no potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hoffman MS, Bodurka DC. Surgical education and training program development for gynecologic oncology: American perspective. Gynecologic oncology. 2009;114:S47–51. [DOI] [PubMed] [Google Scholar]
- [2].Soliman PT, Iglesias D, Munsell MF, Frumovitz M, Westin SN, Nick AM, et al. Successful incorporation of robotic surgery into gynecologic oncology fellowship training. Gynecologic oncology. 2013;131:730–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ring KL, Ramirez PT, Conrad LB, Burke W, Wendel Naumann R, Munsell MF, et al. Make New Friends But Keep the Old: Minimally Invasive Surgery Training in Gynecologic Oncology Fellowship Programs. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2015;25:1115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hansen HH, Bajorin DF, Muss HB, Purkalne G, Schrijvers D, Stahel R. Recommendations for a global core curriculum in medical oncology. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:4616–25. [DOI] [PubMed] [Google Scholar]
- [5].Scribner DR Jr., Baldwin J, Gold MA. Factors affecting fellowship satisfaction among gynecologic oncology fellows. Gynecologic oncology. 2001;80:74–8. [DOI] [PubMed] [Google Scholar]
- [6].Urban RR, Ramzan AA, Doo DW, Galan HL, Harper L, Omurtag K, et al. Fellow Perceptions of Residency Training in Obstetrics and Gynecology. Journal of surgical education. 2019;76:93–8. [DOI] [PubMed] [Google Scholar]
- [7].Urban RR, Ramzan AA, Doo DW, Sheeder J, Guntupalli SR. The perceptions of gynecologic oncology fellows on readiness for subspecialty training following OB/GYNRESIDENCY. Gynecol Oncol Rep. 2019;28:104–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Eskander RN, Osann K, Dickson E, Holman LL, Rauh-Hain JA, Spoozak L, et al. Assessment of palliative care training in gynecologic oncology: a gynecologic oncology fellow research network study. Gynecologic oncology. 2014;134:379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Collins D, Machairas N, Duchalais E, Landmann RG, Merchea A, Colibaseanu DT, et al. Participation of Colon and Rectal Fellows in Robotic Rectal Cancer Surgery: Effect on Surgical Outcomes. Journal of surgical education. 2017. [DOI] [PubMed] [Google Scholar]
- [10].Behbakht K, Abu-Rustum NR, Lee S, San Juan A, Massad LS. Characteristics and survival of cervical cancer patients managed at adjacent urban public and university medical centers. Gynecologic oncology. 2001;81:40–6. [DOI] [PubMed] [Google Scholar]
- [11].Strasberg SM, Linehan DC, Hawkins WG. The accordion severity grading system of surgical complications. Ann Surg. 2009;250:177–86. [DOI] [PubMed] [Google Scholar]
- [12].Institute NC. Common Terminology Criteria for Adverse Events (CTCAE). 2017. [Google Scholar]
- [13].Bodenheimer T, Knox M, Kong M. Models of Faculty Involvement in Primary Care Residency Teaching Clinics. Academic medicine : journal of the Association of American Medical Colleges. 2020;95:190–3. [DOI] [PubMed] [Google Scholar]
- [14].Fortuna RJ, Garfunkel L, Mendoza MD, Ditty M, West J, Nead K, et al. Factors Associated With Resident Continuity in Ambulatory Training Practices. J Grad Med Educ. 2016;8:532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Anderson ML, Ogunwale A, Clark BA, Kilpatrick CC, Mach CM. Preferences and Outcomes for Chemotherapy Teaching in a Postgraduate Obstetrics and Gynecology Training Program. Journal of surgical education. 2015;72:936–41. [DOI] [PubMed] [Google Scholar]
- [16].Buckheit C, Willis-Gray M, Dotters-Katz S. Trends in Fellow Education Research Among Obstetric and Gynecologic Subspecialties. Obstetrics and gynecology. 2018;132 Suppl 1:8s–13s. [DOI] [PubMed] [Google Scholar]
- [17].DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA: a cancer journal for clinicians. 2019;69:211–33. [DOI] [PubMed] [Google Scholar]
- [18].Musselwhite LW, Oliveira CM, Kwaramba T, de Paula Pantano N, Smith JS, Fregnani JH, et al. Racial/Ethnic Disparities in Cervical Cancer Screening and Outcomes. Acta cytologica. 2016;60:518–26. [DOI] [PubMed] [Google Scholar]
- [19].Porter ME. What is value in health care? The New England journal of medicine. 2010;363:2477–81. [DOI] [PubMed] [Google Scholar]
- [20].Havrilesky LJ, Fountain C. Can we maximize both value and quality in gynecologic cancer care? A work in progress. American Society of Clinical Oncology educational book American Society of Clinical Oncology Meeting. 2014:e268–75. [DOI] [PubMed] [Google Scholar]
- [21].Urban RR, He H, Alfonso R, Hardesty MM, Goff BA. The end of life costs for Medicare patients with advanced ovarian cancer. Gynecologic oncology. 2018;148:336–41. [DOI] [PubMed] [Google Scholar]
- [22].Seamon LG, Tarrant RL, Fleming ST, Vanderpool RC, Pachtman S, Podzielinski I, et al. Cervical cancer survival for patients referred to a tertiary care center in Kentucky. Gynecologic oncology. 2011;123:565–70. [DOI] [PubMed] [Google Scholar]
- [23].Doll KM, Puliaev R, Chor J, Roston A, Patel UA, Patel A. Detection of gynecologic cancers in indigent women in an urban inner-city hospital. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2012;22:1113–7. [DOI] [PubMed] [Google Scholar]
- [24].Perez JA Jr., Awar M, Nezamabadi A, Ogunti R, Puppala M, Colton L, et al. Comparison of Direct Patient Care Costs and Quality Outcomes of the Teaching and Nonteaching Hospitalist Services at a Large Academic Medical Center. Academic medicine : journal of the Association of American Medical Colleges. 2018;93:491–7. [DOI] [PubMed] [Google Scholar]