Abstract
Childhood adversity has been associated with elevated risk for psychopathology. We investigated whether development of functional brain networks important for executive function (EF) could serve as potential mediators of this association. We analyzed data of 475 adolescents, a subsample of the multisite longitudinal NCANDA (National Consortium on Alcohol and Neurodevelopment in Adolescence) cohort with completed measures of childhood trauma, resting-state functional brain connectivity data, and symptoms of internalizing and externalizing psychopathology at baseline and follow-up years 1–4. Using parallel process latent growth models, we found that childhood adversity was associated with increased risk for externalizing/internalizing behaviors. We specifically investigated whether functional connectivity of the dorsal anterior cingulate cortex (dACC) to brain regions within the cingulo-opercular (CO) network, a well-known EF network that underlies control of attention and self-regulation, mediates the association between adversity and symptoms of psychopathology. We found that childhood adversity, specifically child neglect was negatively associated with functional connectivity of the dACC within the CO network, and that this connectivity mediated the association between neglect and externalizing behaviors. Our study advances a mechanistic understanding of how childhood adversity may impact the development of psychopathology, highlighting the relevance of dACC functional networks particularly for externalizing psychopathology.
Keywords: Childhood adversity, Functional brain connectivity, Dorsal anterior cingulate cortex, Developmental psychopathology, Externalizing
1. Introduction
Childhood adversity is associated with elevated risk for psychopathology, including all commonly occurring forms of internalizing and externalizing behaviors in youth (Heleniak et al., 2016; McLaughlin et al., 2012). Adolescence is a critical time period for the emergence of psychopathological syndromes, and attempts have been made to relate this phenomenon to simultaneous changes in neurobiological structure and function and its cognitive and affective correlates (Giedd et al., 2008; Gogtay et al., 2004). In this regard, a pivotal role is ascribed to the development of executive function (EF), an overarching human cognitive ability to flexibly select, maintain, and act upon goal-relevant information, while also suppressing irrelevant distractions (Badre, 2011; Lenartowicz et al., 2010; Luna et al., 2015). EF, thus, encompasses fundamental cognitive functions such as sustained attention, conflict processing, and self-regulation. Impairments in EF are a transdiagnostic factor determining mental disorders (McTeague et al., 2016). Impaired EF has also been suggested as a potential mechanism to explain the impact of childhood adversity on the development of psychopathology, which include maladaptive cognitive and behavioral responses to distress based on emotion regulation deficits (Heleniak et al., 2016).
EF starts to develop during childhood, yet it improves and refines markedly during adolescence (Best and Miller, 2010; Crone, 2009; Luna et al., 2015), highlighting the relevance of this period in transitioning to healthy vs. impaired neurocognition. The development of EF during adolescence critically depends on brain maturation, including synaptic pruning (Petanjek et al., 2011) and myelination (Simmonds et al., 2014), which enhance connectivity within and between brain systems (Luna and Sweeney, 2004). Particularly the dorsal anterior cingulate cortex (dACC) is an important hub region for these processes (Ordaz et al., 2013; Tucker et al., 2015). Functionally, the dACC frequently co-activates with the bilateral anterior insula, with which it forms the core of a cingulo-opercular (CO) network in adults (Dosenbach et al., 2006; Han et al., 2019; Power and Petersen, 2013). Functional networks are characterized by dense internal connectivity, and thus network organization of the brain is defined by the network affiliation of each region of the connectome (Newman, 2006). Indeed, brain network function that correlates with EF has been grouped into two networks, the CO and the fronto-parietal (FP) network (Dosenbach et al., 2006). In task related brain states, while the FP network has been ascribed to the initiation and adjustment of moment-to-moment task control, the CO network provides sustained set-maintenance over the entire task duration. The distinct groupings of these two networks have not only been found during the performance of cognitive tasks, but also during resting state (Power et al., 2011). Additionally, age-related neurodevelopmental changes have been observed in these functional network organizations (Dosenbach et al., 2010; Fair et al., 2009; Kelly et al., 2009; Ordaz et al., 2013). During childhood, the CO network is not functionally distinct, but is part of a larger set of regions. Adolescence marks the period during which the key dACC region segregates from its FP connections to form the CO network (Fair et al., 2007). Particularly, increased CO network integration during adolescence has also been shown to moderate the effect of age in the transition to adult-level EF (Marek et al., 2015). Moreover, in our recent, albeit small sample research in adolescents with childhood adversity, we have found that reduced strength of maturing functional connectivity of the dACC in the CO network is associated with greater child neglect severity (Mishra et al., 2020).
A critical distinction in developmental psychopathology is manifested in patterns of disordered self-control, which relate to internalizing or externalizing problems. These behavioral patterns may occur as early as childhood and pave a developmental pathway to negative social and behavioral outcomes (Hofstra et al., 2002; King et al., 2004; Reef et al., 2010). While internalizing behavior appears in the form of withdrawal, anxiety and depression, externalizing behaviors relate to hyperactivity, aggression and destructive behavior. A linkage between childhood adversity and various psychopathological syndromes is promoted by adaptive and enduring psychobiological responses to threat that differentially shape developmental learning processes (Baldwin, 2013). More consistently reported brain changes in the context of adversity, which have been linked to the development of psychopathology, include structural changes of the hippocampus and anterior cingulate cortex (ACC), amygdala volume and reactivity, as well as a decreased hemispheric integrity of the corpus callosum; however, changes were found to vary with regards to the type of maltreatment that was experienced (Teicher et al., 2016a; Teicher and Samson, 2016, 2013). Very few longitudinal studies have investigated a link between childhood adversities, functional brain connectivity and child/adolescent psychopathology (Barch et al., 2018; Herringa et al., 2016). These studies highlight the role of functional connectivity in predefined regions corresponding to impulse control and emotion processing, as distinctly relevant to internalizing and externalizing behaviors. However, while a meta-analysis in adults highlights the overall relevance of dACC / anterior insula based network integrity across various psychiatric diagnoses, which is consistent with transdiagnostic deficits in EF (Goodkind et al., 2015), no study to date has investigated dACC connectivity as a mediator of childhood adversity and developmental psychopathology.
On the one hand, impaired EF and associated structure and function of brain circuits have been argued as common mechanisms underlying mental health sequelae in individuals who have suffered childhood adversities. Yet, emerging evidence indicates that these alterations in EF development only occur following specific forms of childhood adversity (Lambert et al., 2017; Sheridan et al., 2017; Teicher et al., 2016b). A recent conceptual framework distinguishes adversities in two main dimensions, namely threat and deprivation, with unique emotional, cognitive, and neurobiological developmental pathways relevant to the emergence of psychopathology (Sheridan and McLaughlin, 2016). Child abuse is associated with experiences of violence or risk of violence, and is thus categorized under the dimension of threat. Child neglect involves a lack of parental care and constitutes a form of deprivation of social and emotional inputs. Associations of threat or deprivation with psychopathology are suggested to be mediated by alterations in pruning and potentiation of synapses during neurodevelopment (Sheridan and McLaughlin, 2016). Experiences of deprivation in particular have been found linked to impairments in EF, thereby increasing the risk for psychopathology (McLaughlin et al., 2014; Sheridan et al., 2017). A recent longitudinal study provides evidence that, while both early life deprivation and threat increase risk for internalizing and externalizing psychopathology in adolescents, only the link between childhood deprivation and developing externalizing behaviors is mediated by impaired cognitive abilities (Miller et al., 2018). In our recent cross-sectional research on a subsample of the current cohort of adolescents, we identified dACC functional connectivity among that of other regions, to mediate the association between childhood adversity and EF self-reports (Silveira et al., 2018). This study investigated the association between childhood adversity, in the forms of child abuse and child neglect, and developing functional brain connectivity underlying EF. At the same time, we investigated the link between the brain network connectivity associated with adversity and the development of internalizing and externalizing behaviors. Specifically, we investigated how child abuse and neglect associate with functional connectivity of the dACC in the CO network, and whether these neurofunctional correlates mediate the development of externalizing and internalizing psychopathology.
2. Materials and methods
2.1. Sample
We analyzed data of 475 adolescents (mean age 16.97, age range 12–22 years; 52.4 % female), a subsample of the 831 multisite NCANDA (National Consortium on Alcohol & Neurodevelopment in Adolescence) cohort. NCANDA uses an accelerated longitudinal design to sample subjects from a broad span of baseline ages (Duncan et al., 1996; Miyazaki and Raudenbush, 2000). This allows to address development across larger age ranges than traditional cohort designs. The subsample for the current research was selected according to existing reports on all measurements that are relevant to our hypotheses: (a) self-report on childhood adversity in one of the years of assessment, (b) resting-state functional magnetic resonance imaging (rs-fMRI) data in at least two years of assessment, (c) parent-report for adolescents under age 18 or self-report for those over age 18 on symptoms of psychopathology in at least two years of assessment. Psychopathological syndrome data was missing in 9 cases at follow-up year 1, in 14 cases at follow-up year 2, in 40 cases at follow-up year 3 and in 109 cases (23 %) at follow-up year 4. Rs-fMRI data was missing in 57 cases at study baseline, in 25 cases at follow-up year 1, in 36 cases at follow-up year 2, in 62 cases at follow-up year 3 and in 133 cases (28 %) at follow-up year 4.
The study was approved by the institutional review board of each NCANDA site. All subjects underwent informed assent or consent annually. When under age 18, the participants’ legal guardians provided written informed consent to participate and the youth provided written informed assent. Participants over age 18 provided written informed consent themselves.
2.2. Assessments
2.2.1. Childhood adversity
Childhood adversity was assessed using the 28-items brief screening version of the Childhood Trauma Questionnaire, short form (CTQ-SF; Bernstein et al., 2003). The CTQ-SF is a self-administered retrospective inventory consisting of five categories of child neglect and abuse. Each category entails five items that are rated on a 5-point Likert scale ranging from never true (= 1) to very often true (= 5). For descriptive purposes, subscale scores were summed and those with low to moderate adversity were identified using recommended cut-off scores of subscale sums (Bernstein & Fink, 1998; emotional abuse ≥ 9, physical abuse ≥ 8, sexual abuse ≥ 6, emotional neglect ≥ 10, physical neglect ≥ 8; Table 1). Subscale scores were averaged to inform composite scores for child neglect (i.e., emotional neglect, physical neglect; sample mean = 1.55 ± .60; range = 1–5) and abuse (i.e., emotional abuse, physical abuse, sexual abuse; sample mean = 1.21 ± .33; range = 1–5).
Table 1.
Samples descriptives (mean ± standard deviation) of time-invariant covariates at baseline and time-varying neurofunction and psychopathology at baseline and at follow-up after one (Y1), two (Y2), three (Y3) and four (Y4) years. SES = socioeconomic status, AUD = alcohol use disorder, dACC = dorsal anterior cingulate cortex, CO = cingulo-opercular network.
| Table 1. Study Sample | Baseline | |
|---|---|---|
| Age | 16.97 ± 2.31 | |
| Sex | Male | 47.6 % |
| Female | 52.4 % | |
| Ethnicity | Caucasian | 73.3 % |
| African American | 13.9 % | |
| Asian | 6.3 % | |
| Mixed or other | 6.5 % | |
| SES | 16.80 ± 2.35 | |
| High alcohol use | 17.1 % | |
| AUD family history | .18 ± .38 | |
| MRI scanner type | Siemens | 38.9 % |
| GE | 61.1 % | |
| Childhood adversity | Neglect | 1.55 ± .60 |
| Abuse | 1.21 ± .33 | |
| ≥ low to moderate: | ||
| Emotional abuse | 17.7 % | |
| Physical abuse | 16.2 % | |
| Sexual abuse | 5.1 % | |
| Emotional neglect | 34.7 % | |
| Physical neglect | 24.6 % | |
| Longitudinal Measures | Baseline | Y1 | Y2 | Y3 | Y4 |
|---|---|---|---|---|---|
| dACC-CO connectivity | .29 ± .13 (n = 418) |
.23 ± .13 (n = 450) |
.28 ± .14 (n = 439) |
.27 ± .14 (n = 413) |
.25 ± .13 (n = 342) |
| Externalizing psychopathology (T-scores) | 42.73 ± 8.51 (n = 475) |
43.87 ± 9.04 (n = 466) |
44.00 ± 9.31 (n = 461) |
44.78 ± 9.71 (n = 435) |
45.00 ± 9.68 (n = 366) |
| Internalizing psychopathology (T-scores) | 44.63 ± 9.01 (n = 475) |
46.02 ± 10.30 (n = 466) |
45.94 ± 10.91 (n = 461) |
46.68 ± 10.80 (n = 435) |
47.58 ± 11.24 (n = 366) |
2.2.2. Psychopathology
Internalizing and externalizing behaviors in adolescents under the age of 18 years were determined using parent-reports on the school age version of the Child Behavior Checklist (CBCL) and, if those were not available, self-reports on the Youth Self Report (YSR) of the Achenbach System of Empirically Based Assessments (ASEBA) were used (Achenbach and Rescorla, 2000). Subjects older than 18 years completed the ASEBA Adult Self Report (ASR). All three scales consist of 112 statements about the adolescent’s behaviors that are rated on a 3-point Likert scale from 0 (= not true) to 2 (= very true). Scores were summed up into composite scales for internalizing and externalizing behaviors, respectively. The internalizing score is composed of syndrome scales for anxious/depressed and withdrawn-depressed behaviors, and somatic complaints. The score for externalizing behaviors consists of rule-breaking and aggressive behavior syndrome scales. The CBCL provides sex and age normed T-scores. In the current study, we are using normative T-scores for the 12–18 years age range. Higher scores indicate greater degrees of behavioral and emotional problems. T-scores of ≥64 on internalizing and externalizing scales indicate deviant behavior that is clinically relevant.
2.2.3. Demographics
Demographic variables included age, gender, ethnicity (Caucasian, African American, Asian or mixed) and socio-economic status (SES). SES was determined by the highest level of education achieved by either parent from 1 (1 st grade) to 20 (4+ years of graduate/professional school) (Von Rueden et al., 2006). Parental income was not used as an indicator of SES due to substantial differences in salaries across the NCANDA study sites (Sullivan et al., 2016). As the NCANDA study was designed to inform associations between alcohol use and brain development, youth at risk for heavy drinking were oversampled. Control variables therefore included family history of AUDs and early alcohol use prior to the study. AUD family history density index was calculated for first- and second-degree relatives ([# Positive Parents] + [# Positive Grandparents * 0.5]; range of 0–4) (Stoltenberg et al., 1998). Early alcohol use was defined as a dichotomous variable indicating exceeding age-defined drinking thresholds at baseline, potentially indicating some level of alcohol-related risk (“high alcohol use”; n = 81) (Pfefferbaum et al., 2017). At baseline, higher frequencies of externalizing problems were seen in subjects with high alcohol use (7.9 %) than in other participants (5.8 %) (Brown et al., 2015).
2.3. Imaging acquisition
We used rs-fMRI data that was assessed at baseline and follow-up years 1–4 for all 475 participants to extract functional brain connectivity measures. Neuroimaging data were acquired on two different 3.0 T MRI scanner models in NCANDA. 38.9 % of participants were scanned with a Magnetom Trio Trim (Siemens Healthcare, Germany) and 61.1 % with a MR750 (GE Healthcare, USA). Scan protocols were consistent between sites and scanners. High-resolution anatomical T1 scans (1.2 mm slices thickness, repetition time [TR] =1900 ms, echo time [TE] =2.92 ms, 11° flip angle, 240 mm field of view [FoV]) and gradient echo-planar imaging (EPI) scans were collected (5 mm slice thickness, 32 slices, TR =2200 ms, TE =30 ms, 79° flip angle, FoV = 240, 240 × 240 matrix).
2.4. Data analysis
Statistical analyses were conducted using MATLAB (The Mathworks, Inc., Natick, MA) and SPSS 19.0 (IBM Corp., Armonk, NY). Rs-fMRI data were analyzed using the CONN functional connectivity toolbox (https://www.nitrc.org/projects/conn) (Whitfield-Gabrieli and Nieto-Castanon, 2012). The first five volumes of the EPI scans were discarded to allow for magnetic saturation effects. Data preprocessing included slice time correction, data realignment to the first functional volume, individual rigid T1-to-EPI co-registration, segmentation and normalization of the co-registered T1 and EPI volumes in the MNI stereotactic space. To minimize noise and residual inter-subject differences, spatial smoothing was applied using an isotropic Gaussian kernel with 8 mm full width at half maximum. Physiological artifacts and residual subject movements were removed from the data using regression of six rigid-body motion parameters, which were estimated in the realignment step, and regression of outlier scans, based on scrubbing of bad data points as identified by artifact detection. Scan acquisitions with framewise displacement >.3 mm were regarded as motion confounded. While temporal censoring techniques like scrubbing attenuate motion artifacts, this results in reduced time series and degrees of freedom. On average, 28.1 ± 6.3 % of volumes were discarded from the analyses. Subject-level analyses additionally included regression of white matter signals (WM) and cerebrospinal fluid (CSF). For both WM and CSF areas, five potential noise components were defined (Chai et al., 2012). The implemented anatomical component-based noise correction procedure (aCompCor) includes WM and CSF noise components (Behzadi et al., 2007), subject-motion parameters, and identified outlier scans (Power et al., 2014). The aCompCor approach of data preprocessing is similar to models that include global signal regression and was found to be relatively effective in mitigating the impact of motion (Ciric et al., 2017). None of the subjects were removed completely during the preprocessing procedure.
We analyzed functional connectivity between predefined regions of interest (ROI-to-ROI), which undergo substantial development during adolescence and are known to subserve executive functioning. We determined the relationship of this functional connectivity to childhood adversity and the extent to which this connectivity mediates a link between adversity and psychopathology. Regions within the CO network were manually created as 12 mm radius spheres with peak MNI coordinates from previous literature (Fair et al., 2007). The dACC (MNI coordinates: -2, 7, 50) was defined as a seed region based on prior evidence that it segregates from its FP connections to form the CO network during typical adolescence (Fair et al., 2007), as well as our prior results that dACC functional connectivity relates to childhood adversity (Mishra et al., 2020) and mediates the link between adversity and self-reported EF in a cross-sectional sample of adolescents (Silveira et al., 2018). Other regions of the CO include the bilateral anterior insula (left: -36, 17, 3; right: 38, 19, 0), anterior prefrontal cortex (left: -28, 53, 16; right: 28, 51, 25) and thalamus (left: -13, -14, 4; right: 11, -14, 6). Functional network connectivity between dACC and CO-ROIs was calculated by median connectivity weights of blood-oxygen level dependent (BOLD) time-series.
Mediation analyses were conducted using AMOS 26.0 (Arbuckle, 2019). We used parallel process latent growth modeling (LGM) as an application of structural equation models for longitudinal mediation analyses. Missing data on repeated measures was addressed using Bayesian multiple imputation (MI) as implemented in AMOS. To capture the uncertainty in predicting data given the observed information, MI generated 10 different data sets, each containing a set of maximum likelihood estimates for missing values. The conversion criterion was satisfied. The LGMs were then fit to each imputed data set, and model fit indices as well as standardized estimates were pooled across all datasets (van Ginkel, 2020; Wu and Jia, 2013). In two LGMs, the median connectivity measures extracted in the previous analyses were used to inform latent random intercept and random linear slope of neurofunctional development within the CO network. Similarly, scores on the ASEBA, were used to model intercept and slope of internalizing and externalizing behaviors respectively. Loadings for the slope factors were set equal to values of the time variable, defined with a value of 0 at study baseline and an incremental increase of 1 for each consecutive year of follow-up assessment. Loadings for the intercepts were set equal to 1. Intercept and slope factors represent functions of means and individual deviations from those means. Means of slopes indicate changes in functional connectivity or externalizing/internalizing behaviors over time. The variances of intercepts and slopes are residual variances. Equality constraints were included on uncorrelated variance residuals of time-varying indicators across measurement occasions (Grimm and Widman, 2010). Negative error variance estimates were obtained from rs-fMRI data, i.e., Heywood case (Rindskopf, 1984). In accordance with Van Driel’s method (Van Driel, 1978), residual slope variance of σ2 = 0.00, 95 % CI [-0.00, .00] and a critical ratio of -.56 indicates that the improper estimate can be seen as a result of sampling fluctuations rather than specification errors (Chen et al., 2001). Therefore, residual slope variance of rs-fMRI data was constrained to 0 (Dillon et al., 1987). To investigate longitudinal mediation effects, we modeled parallel processes from CTQ-SF neglect and abuse scores to the intercept of dACC-CO connectivity (= baseline status of connectivity) and then to the slope of externalizing/internalizing behaviors (= change in psychopathology over time), as well as from neglect and abuse scores to the intercept of psychopathology and then to the slope of dACC-CO connectivity. This setup assumes that when modeling an intercept as putative mediator of latent growth, a temporal order between mediator and outcome can be presumed (von Soest and Hagtvet, 2011). Indirect effects between child neglect/abuse and slopes of connectivity or externalizing/internalizing behaviors were calculated by multiplying the paths that constitute the respective effects and served as indicators of the extent of mediation. We additionally tested whether child neglect and abuse associations with psychopathology, and mediating effects can be traced to functional connectivity between the core CO nodes, the dACC and bilateral anterior insula. Additionally, growth curves of internalizing and externalizing behaviors as well as resting-state functional connectivity were plotted (see supplement S1).
Age, sex, ethnicity, SES, high alcohol use, AUD family history, and MRI scanner type were included as covariates. Thereby, ethnicity as categorical variable was dummy coded into several dichotomous variables, i.e., Caucasian, African American, Asian, mixed or other group membership. Even though age was assessed at each data collection time point, it was treated as a time-invariant covariate at study baseline. There was missing data on SES scores in 8.8 % of cases. These missing values were imputed by using the group mean (Cheung, 2007). All covariates except for scanner type were modeled with paths on latent variables (i.e., intercepts and slopes) of both functional connectivity measures and internalizing/externalizing scores. Covariance between sample demographics, abuse and neglect scores were included in the model. Due to non-normality of CTQ-SF and CBCL scores, we performed bootstrapping of maximum likelihood estimation, using 1000 bootstrap samples and a Boot factor of 1. We report bias-corrected confidence intervals of 95 %. χ2 statistics, Comparative Fit Index (CFI) and Root Mean Square Error of Approximation (RMSEA) were used as indicators of SEM fit.
3. Results
3.1. Covariance of childhood adversity and sample demographics
We found significant positive covariance for child abuse and neglect scores (β = .07, 95 % CI [.05, .11], p = .001). Further, both childhood adversity scales, i.e., neglect and abuse, significantly covaried with age, indicating that the reported severity of adverse childhood experiences is higher in older adolescents (Table 2). Besides, SES negatively covaried with both neglect and abuse scores. Covariates related to alcohol consumption in relatives and adolescents themselves showed a consistent positive association with exposure to childhood adversity. AUD family history was thereby related to both child neglect and abuse, and high alcohol use was related to child abuse.
Table 2.
Significant covariances and paths of parallel process latent growth models to link sample demographics to childhood adversity, functional network connectivity and (1) externalizing and (2) internalizing behavior.
| 95 % CI |
|||
|---|---|---|---|
| β,p | lower CI | upper CI | |
| Model 1&2: Covariances | |||
| Age ↔ Abuse | .10 * | .03 | .17 |
| Age ↔ Neglect | .14* | .01 | .26 |
| SES ↔ Abuse | −.11* | −.19 | −.04 |
| SES ↔ Neglect | −.19* | −.32 | −.07 |
| AUD family history ↔ Abuse | .03* | .01 | .05 |
| AUD family history ↔ Neglect | .03* | .01 | .05 |
| High alcohol use ↔ Abuse | .02* | .00 | .03 |
| Model 1: Externalizing behavior | |||
| High alcohol use → E Intercept | .19* | .07 | .30 |
| SES → E Intercept | −.10* | −.20 | −.01 |
| AUD family history → E Intercept | .16* | .06 | .27 |
| Scanner → FC Intercept | −.14* | −.25 | −.05 |
| Age → FC Intercept | 14* | .02 | .26 |
| AUD family history → FC Intercept | −.13* | −.25 | −.01 |
| AUD family history → FC Slope | −.12* | −.21 | −.02 |
| Model 2: Internalizing behavior | |||
| AUD family history → I Intercept | .12* | .00 | .23 |
| Age → I Intercept | .12* | .00 | .23 |
| Age → I Slope | −.18* | −.30 | −.06 |
| Scanner → FC Intercept | −.14* | −.25 | −.04 |
| Age → FC Intercept | .14* | .02 | .24 |
| AUD family history → FC Intercept | −.13* | −.25 | −.01 |
| AUD family history → FC Slope | −.11* | −.21 | −.02 |
Note. SES = socioeconomic status, AUD = alcohol use disorder, FC = functional connectivity from dorsal anterior cingulate cortex to regions of the cingulo-opercular network, E = externalizing, I = internalizing, β = regression weight, CI = confidence interval, p(*) = significant paths.
3.2. Externalizing behavior
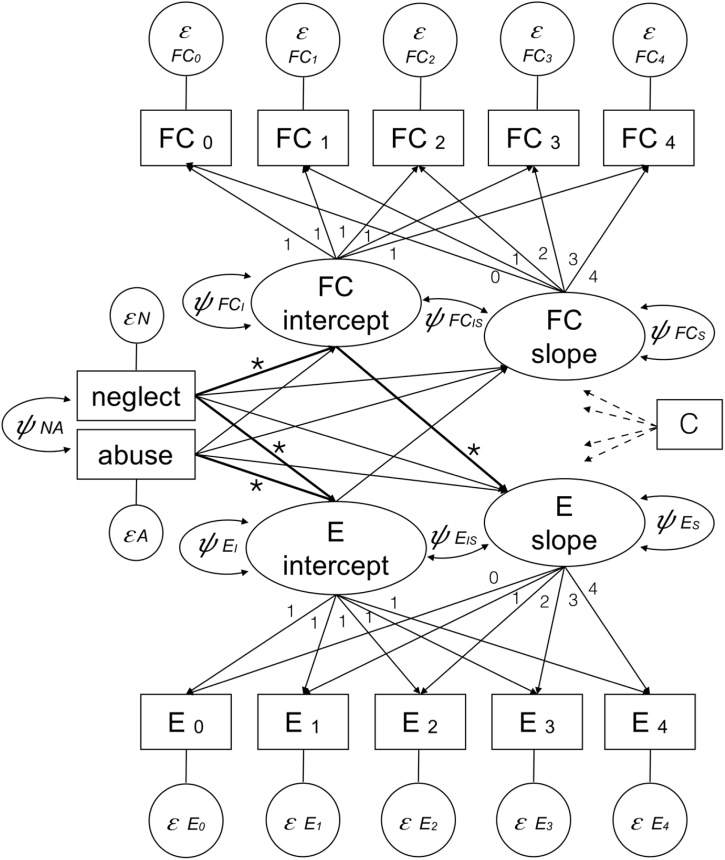
The LGM for externalizing behavior had a good overall fit pooled across all 10 multiple imputed datasets with χ2 = 343.38, p < .000, CFI = .86 and RMSEA = .07, 95 % CI [.06, .08]. Externalizing behavior increased significantly over the 5 years of assessment with a mean slope of μ = .41, 95 % CI [.34, .50]. Functional connectivity between the dACC node and other regions of the CO network increased slightly on average (μ = .01, 95 % CI [.01, .01]). Both child neglect and abuse were associated with higher scores on the latent intercept of externalizing scores (Table 3). Besides, we found a negative association between neglect scores and median dACC-CO network connectivity. There was no direct association of neglect or abuse severity and slopes, i.e., a change in functional connectivity or externalizing symptoms over time. Yet, we found that lower functional dACC-CO network connectivity was associated with an increased slope of externalizing behaviors; thereby, mediating the link between child neglect and the development of externalizing behavior as indicated by a significant indirect effect (Table 3). Notably, the reverse pathway of adversity-associated externalizing symptoms mediating the development of functional connectivity was not significant (Fig. 1).
Table 3.
Paths and indirect effects of parallel process latent growth models to link childhood adversity to functional network connectivity and (1) externalizing and (2) internalizing behavior.
| 95 % CI |
|||
|---|---|---|---|
| β,p | lower CI | upper CI | |
| Model 1: Externalizing behavior | |||
| Neglect → FC Intercept | −.15* | −.26 | −.03 |
| Neglect → FC Slope | .10 | −.00 | .20 |
| Neglect → E Intercept | .11* | .00 | .24 |
| Neglect → E Slope | .06 | −.09 | .22 |
| Abuse → FC Intercept | .02 | −.10 | .15 |
| Abuse → FC Slope | −.06 | −.16 | .05 |
| Abuse → E Intercept | .15* | .03 | .29 |
| Abuse → E Slope | .01 | −.13 | .15 |
| FC Intercept → E Slope | −.18* | −.33 | −.03 |
| E Intercept → FC Slope | −.03 | −.06 | .12 |
| Neglect → E Intercept → FC Slope | .00 | −.01 | .02 |
| Neglect → FC Intercept → E Slope | .03* | .00 | .07 |
| Abuse → E Intercept → FC Slope | .01 | −.01 | .03 |
| Abuse → FC Intercept → E Slope | −.00 | −.03 | .02 |
| Model 2: Internalizing behavior | |||
| Neglect → FC Intercept | −.15* | −.26 | −.04 |
| Neglect → FC Slope | .10 | −.00 | .19 |
| Neglect → I Intercept | .13* | .00 | .24 |
| Neglect → I Slope | .11 | −.03 | .26 |
| Abuse → FC Intercept | .02 | −.10 | .15 |
| Abuse → FC Slope | −.04 | −.15 | .06 |
| Abuse → I Intercept | .17* | .05 | .32 |
| Abuse → I Slope | −.00 | −.16 | .15 |
| FC Intercept → I Slope | −.05 | −.22 | .13 |
| I Intercept → FC Slope | .03 | −.07 | .12 |
| Neglect → I Intercept → FC Slope | .00 | −.01 | .02 |
| Neglect → FC Intercept → I Slope | .01 | −.02 | .04 |
| Abuse → I Intercept → FC Slope | .00 | −.01 | .02 |
| Abuse → FC Intercept → I Slope | −.00 | −.02 | .01 |
Note. FC = functional connectivity from dorsal anterior cingulate cortex to regions of the cingulo-opercular network, E = externalizing, I = internalizing, β = regression weight, CI = confidence interval, p(*) = significant paths and indirect effects.
Fig. 1.
Parallel process latent growth model. ε = error term, ψ = variance and covariance parameters of latent variables, FC = functional connectivity, E = externalizing behavior, C = covariates (i.e., age, gender, ethnicity, socio-economic status, high alcohol use, family history of alcohol use disorder), * = significant paths.
With regard to the demographic variables, there was a significant association of high alcohol use as well as SES with the latent intercept of externalizing behaviors (Table 2). Scanner type and age were also significantly associated with the intercept of functional connectivity. AUD family history was found to be associated with latent intercepts of externalizing scores and dACC-CO functional connectivity, and with the latent slope of this functional connectivity. Results were not exclusively driven by dACC-anterior insula functional connectivity (see supplement S2).
3.3. Internalizing behavior
The internalizing behavior model had an overall acceptable model fit with χ2 = 391.50, p < .001, CFI = .89 and RMSEA = .07, 95 % CI [.06, .07]. Internalizing behavior increased significantly over time with a mean slope of μ = .77, 95 % CI [.63, .94]. Again, there was a significant negative association of neglect scores with the latent intercept of dACC-CO network connectivity and a positive association of both neglect and abuse with the latent intercept of internalizing behaviors (Table 3). There was no direct or indirect link between child neglect or abuse severity and dACC-CO network neurodevelopment or the development of internalizing behaviors over time.
With regard to the demographic variables, the association of scanner type and age with the latent intercept of functional network connectivity, and of AUD family history with the latent intercept and slope of functional connectivity and with the latent intercept of internalizing behaviors, were found to be analogous to the externalizing behavior model (Table 2). We additionally found an association of age with both latent intercept and slope of internalizing symptoms.
4. Discussion
We used the accelerated longitudinal NCANDA dataset to investigate developmental trajectories linking childhood adversity, functional brain connectivity, and psychopathology in adolescence. Regarding the prevalence of adverse childhood experiences, our sample is comparable to previously reported lifetime prevalence metrics in the general population (Kim et al., 2017). The occurrence of child neglect, particularly emotional neglect, is thereby much higher than the occurrence of child abuse. We found a significant association of child neglect with functional connectivity from the dACC to other regions of the CO network. The dACC plays a crucial role in the development of EF (Luna et al., 2015; Ordaz et al., 2013), particularly as a core region of the CO network that is undergoing segregation and maturation during adolescence (Dosenbach et al., 2010, 2006; Fair et al., 2009, 2007; Kelly et al., 2009; Marek et al., 2015). While there was no direct association of childhood adversity with developmental growth in brain connectivity or psychopathology, we found that the link between adversity and the development of externalizing behaviors is mediated by functional dACC network connectivity. Thus, our findings highlight that this mediating effect of dACC-CO connectivity is specific to child neglect. While childhood adversity is generally associated with higher risk of psychopathology, different developmental pathways including alterations in structural and functional neurodevelopment have been suggested to relate to the specific types of adversity experiences (Sheridan and McLaughlin, 2016; Teicher et al., 2016b). Our result is in accordance with previous findings, which indicate that particularly neglect as a form of deprivation, is linked to psychopathogenesis via impaired neural functions underlying EF (McLaughlin et al., 2019; Miller et al., 2018; Spratt et al., 2012).
We further found positive main effects of child neglect and abuse severity on both externalizing and internalizing behaviors in youth. This is in line with the notion that childhood adversity is a risk factor for psychopathology and its onset in adolescence (Heleniak et al., 2016; McLaughlin et al., 2012). However, no mediation by dACC-CO connectivity was found for the development of internalizing behaviors. Previous research suggests that dysregulated self−control can predict both internalizing and externalizing behaviors in children and adolescents (Hughes and Ensor, 2011) and mediate the association of childhood adversity with both internalizing and externalizing behaviors (Heleniak et al., 2016). Studies in adults further highlight that impaired structural as well as functional dACC / anterior insula based network integrity is a consistent and transdiagnostic factor in psychopathology (Goodkind et al., 2015). Yet, in this large sample, we found dACC-CO network connectivity to specifically mediate the link between child neglect and the development of externalizing behavior. This result also dovetails with our prior research showing that interventions that strengthen dACC-CO functional connectivity in neglected adolescents, primarily resolve hyperactivity that is core to externalizing behaviors (Mishra et al., 2020).
Interestingly, recent longitudinal studies highlight a similar link between childhood adversities and EF or EF related functional connectivity that can be related to externalizing, but not internalizing psychopathology (Barch et al., 2018; Miller et al., 2018). The distinction in developmental trajectories of externalizing and internalizing behaviors may be due to the nature of impaired self-control. While self-regulation of internalizing behavior is contextualized in the presence of aversive stimuli, self-regulation of externalizing behavior is relevant in the context of appetitive stimuli (Tucker et al., 2015). Our results indicate a possible mechanistic pathway for how EF impairments following child neglect particularly affect the phenotypic expressions of externalizing behavior characterized by a lack of inhibitory control towards environmental stimuli. The CO network shows considerable overlap with the salience network, particularly in the core nodes of dACC and bilateral anterior insula (Power and Petersen, 2013; Seeley et al., 2007). Functional coupling between these core regions has been associated with the presentation of salient events as well as with transient attention (Han et al., 2019). Thus, impacted functional connectivity from the CO/salience network core nodes may lead to heightened risk for externalizing symptoms due to a lack of self-control in the presence of salient environmental stimuli. However, when testing for a mediating effect specifically of dACC connectivity with the bilateral insula, the results could not be traced to connectivity between these CO core nodes; our results, thus, appear to be a product of overall dACC-CO within-network connectivity.
Additionally, using parallel process latent growth models, we simultaneously tested the reverse relationship between internalizing or externalizing behaviors and the development of functional brain connectivity. Neither internalizing nor externalizing phenotypes of adolescent psychopathology could be linked to developmental trajectories of dACC-CO network connectivity. These results support the notion that functional connectivity patterns can be a useful biomarker relevant to individual differences in behavior, but not vice versa, with implications for neurally-targeted personalized medicine (Finn and Constable, 2016). While the majority of functional connectivity based prediction studies have focused on classifying disease status at the time of the fMRI scan, this study contributes to a research gap on individual variations and longitudinal associations with dimensional behavior-based clinical phenotypes (Rosenberg et al., 2018; Silveira et al., 2020).
NCANDA oversamples youth at higher risk for risky alcohol use patterns. We therefore included AUD risk related covariates in our analyses, i.e., drinking patterns that exceed age-related thresholds and an index for AUD family history. We found high covariance between both these risk factors and childhood adversity, indicating that adolescents who experienced childhood adversity are more likely to have parents or grandparents with AUDs, and that those who experienced child abuse are more likely to drink more alcohol than others of the same age. High alcohol use was further associated with higher levels of externalizing behaviors. AUD family history was related to higher levels of both externalizing and internalizing behaviors as well as with lower baseline status and decelerated development of dACC-CO functional network connectivity. This indicates that alcohol use related factors within the family may be important precursors of both baseline status and development of psychopathology in youth. Previous literature provides evidence that particularly children of alcoholics show elevated levels of internalizing and externalizing symptoms in childhood and adolescence (Hussong et al., 2007, 2008).
Due to several sample characteristics, the generalizability of study results is limited. Specifically, the sample is characterized by a high socioeconomic status, since mean parental education level indicates at least one year of graduate/professional school. Besides, the sample consists of mainly Caucasian adolescents. Prevalence of reported childhood adversity is similar to other mainly Caucasian adolescent samples, apart from less emotional abuse in this NCANDA sample (Paivio and Cramer, 2004). However, overall severity of child abuse and neglect was low, particularly as compared to adolescent psychiatric populations (Bernstein et al., 1997). Besides, levels of internalizing and externalizing psychopathology are below the normative age-expected levels. Another limitation of the current research is a sole focus on dACC resting state functional connections. Due to this specific scope, the current study is not exhaustive in explaining alternative neurofunctional pathways that may be relevant to the development of externalizing or internalizing psychopathology. The focus on dACC connectivity in particular is based on a body of research that suggests its formation during adolescence, and its association with EF (Marek et al., 2015; Ordaz et al., 2013) and childhood adversity (Teicher et al., 2016a; Teicher and Samson, 2016). A further limitation of this study is that no EF task is included in the analyses. Interpretation of results are thus based on previous findings that link dACC and CO network connectivity to EF and its development during adolescence. Therefore, no explicit conclusions can be drawn about EF in the current sample. Future studies are needed to investigate a direct link between dACC-CO network connectivity, the development of EF performance and psychopathology.
Taken together, our study advances a mechanistic understanding of how childhood adversity may be linked to the development of psychopathology during adolescence. Notably, it supports the notion of different developmental trajectories dependent on the type of experienced adversity, neglect vs. abuse. Further, it highlights the relevance of dACC based functional networks in mediating a link between child neglect and externalizing behaviors. Results from this study may inform the body of research directed towards neurotherapeutic approaches by identifying specific functional brain connectivity as possible intervention and prevention targets.
Data statement
Data from the National Consortium on Alcohol and Neurodevelopment in Adolescence (NCANDA) is available upon request. For further information, please visit https://www.niaaa.nih.gov/ncanda-data-distribution-agreement
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
This research was supported by University of California San Diego (UCSD) lab start-up funds (Mishra) and the German National Academy of Sciences Leopoldina fellowship (Silveira). Reported data was obtained from the multisite National Consortium on Alcohol & Neurodevelopment in Adolescence (NCANDA) study. This work was made possible with support by NIH Grants AA021697, AA021695, AA021692, AA021696, AA021681, AA021690, and AA021691. Data used here are from the data releases Pohl, K.M., Podhajsky, S., Sullivan, E.V., Pfefferbaum, A.: The NCANDA_PUBLIC_4Y_REDCAP_V02 Data Release of the National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA), Sage Bionetworks Synapse. https://dx.doi.org/10.7303/syn24226662 and Pohl, K.M., Podhajsky S., Sullivan, E.V., Pfefferbaum, A.: The NCANDA_PUBLIC_4Y_RESTINGSTATE_V01 Data Release of the National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA), Sage Bionetworks Synapse. https://dx.doi.org/10.7303/syn25671177 distributed to the public according to the NCANDA Data Distribution agreement: www.niaaa.nih.gov/research/major-initiatives/national-consortium-alcohol-and-neurodevelopment-adolescence/ncanda-data.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2021.100962.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Achenbach T.M., Rescorla L. University of Vermont Research Center for Children, Youth, & Families; Burlington, VT: 2000. Manual for the ASEBA Preschool Forms and Profiles. [Google Scholar]
- Arbuckle J.L. 2019. Amos 26.0 User’s Guide. [Google Scholar]
- Badre D. Defining an ontology of cognitive control requires attention to component interactions. Top. Cogn. Sci. 2011;3:217–221. doi: 10.1111/j.1756-8765.2011.01141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin D.V. Primitive mechanisms of trauma response: an evolutionary perspective on trauma-related disorders. Neurosci. Biobehav. Rev. 2013;37:1549–1566. doi: 10.1016/j.neubiorev.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Barch D.M., Belden A.C., Tillman R., Whalen D., Luby J.L. Early childhood adverse experiences, inferior frontal gyrus connectivity, and the trajectory of externalizing psychopathology. J. Am. Acad. Child Adolesc. Psychiatry. 2018;57:183–190. doi: 10.1016/j.jaac.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y., Restom K., Liau J., Liu T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein D.P., Ahluvalia T., Pogge D., Handelsman L. Validity of the childhood trauma questionnaire in an adolescent psychiatric population. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:340–348. doi: 10.1097/00004583-199703000-00012. [DOI] [PubMed] [Google Scholar]
- Bernstein D.P., Stein J.A., Newcomb M.D., Walker E., Pogge D., Ahluvalia T., Stokes J., Handelsman L., Medrano M., Desmond D., Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abus. Negl. 2003;27:169–190. doi: 10.1016/S0145-2134(02)00541-0. PMID: 12615092. [DOI] [PubMed] [Google Scholar]
- Best J.R., Miller P.H. A developmental perspective on executive function. Child Dev. 2010;81:1641–1660. doi: 10.1111/j.1467-8624.2010.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S.A., Brumback T.Y., Tomlinson K., Cummins K., Thompson W.K., Nagel B.J., De Bellis M.D., Hooper S.R., Clark D.B., Chung T., Hasler B.P., Colrain I.M., Baker F.C., Prouty D., Pfefferbaum A., Sullivan E.V., Pohl K.M., Rohlfing T., Nichols B.N., Chu W., Tapert S.F. The National Consortium on Alcohol and Neuro-Development in Adolescence (NCANDA): a multisite study of adolescent development and substance use. J. Stud. Alcohol Drugs. 2015;213:895–908. doi: 10.15288/jsad.2015.76.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai X.J., Castañán A.N., Öngür D., Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. Neuroimage. 2012;59:1420–1428. doi: 10.1016/j.neuroimage.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Bollen K.A., Paxton P., Curran P.J., Kirby J.B. Improper solutions in structural equation models: causes, consequences, and strategies. Sociol. Methods Res. 2001;29:468–508. [Google Scholar]
- Cheung M.W. Missing time-invariant covariates in latent growth models under the assumption of missing completely at random. Organ. Res. Methods. 2007;10:609–634. [Google Scholar]
- Ciric R., Wolf D.H., Power J.D., Roalf D.R., Baum G.L., Ruparel K., Shinohara R.T., Elliott M.A., Eickhoff S.B., Davatzikos C., Gur R.C., Gur R.E., Bassett D.S., Satterthwaite T.D. Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. Neuroimage. 2017;154:174–187. doi: 10.1016/j.neuroimage.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone E.A. Executive functions in adolescence: inferences from brain and behavior. Dev. Sci. 2009;12:825–830. doi: 10.1111/j.1467-7687.2009.00918.x. [DOI] [PubMed] [Google Scholar]
- Dillon W.R., Kumar A., Mulani N. Offending estimates in covariance structure analysis: comments on the causes of and solutions to heywood cases. Psychol. Bull. 1987;101:126–135. [Google Scholar]
- Dosenbach N.U.F., Visscher K.M., Palmer E.D., Miezin F.M., Wenger K.K., Kang H.C., Burgund E.D., Grimes A.L., Schlaggar B.L., Petersen S.E. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. PMCID: PMC3621133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U.F., Nardos B., Cohen A.L., Fair D.A., Power J.D., Church J.a, Nelson S.M., Wig G.S., Vogel A.C., Coalson R.S., Pruett J.R., Barch D.M., Petersen S.E., Schlaggar B.L. Prediction of individual brain maturity using fMRI. Science (80-.) 2010;329:1358–1361. doi: 10.1016/j.jrurstud.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan S.C., Duncan T.E., Hops H. Analysis of longitudinal data within accelerated longitudinal designs. Psychol. Methods. 1996;1:236–248. [Google Scholar]
- Fair D.A., Dosenbach N.U.F., Church J.A., Cohen A.L., Brahmbhatt S., Miezin F.M., Barch D.M., Raichle M.E., Petersen S.E., Schlaggar B.L. Development of distinct control networks through segregation and integration. Proc. Natl. Acad. Sci. 2007;104:13507–13512. doi: 10.1111/iwj.12198. PMCID: PMC1940033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Cohen A.L., Power J.D., Dosenbach N.U.F., Church J.A., Miezin F.M., Schlaggar B.L., Petersen S.E. Functional brain networks develop from a ‘“Local to Distributed”’ organization. PLoS Comput. Biol. 2009;5:14–23. doi: 10.1371/journal.pcbi.1000381. PMCID: PMC2671306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn E.S., Constable R.T. Individual variations in functional brain connectivity: implications for personalized approaches to psychiatric illness. Dialogues Clin. Neurosci. 2016;18:277–287. doi: 10.31887/DCNS.2016.18.3/efinn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J.N., Keshavan M., Paus T. Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513.Why. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C., Nugent T.F., Herman D.H., Clasen L.S., Toga A.W., Rapoport J.L., Thompson P.M. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkind M., Eickhoff S.B., Oathes D.J., Jiang Y., Chang A., Jones-Hagata L.B., Ortega B.N., Zaiko Y., Roach E., Korgaonkar M., Grieve S., Galatzer-Levy I., Fox P.T., Etkin A. Identification of a comon neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72:305–315. doi: 10.1001/jamapsychiatry.2014.2206.Identification. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm K.J., Widman K.F. Residual structures in latent growth curve modeling. Struct. Equ. Model. 2010;17:424–442. [Google Scholar]
- Han S.W., Eaton H.P., Marois R. Functional fractionation of the cingulo-opercular network: alerting insula and updating cingulate. Cereb. Cortex. 2019;29:2624–2638. doi: 10.1093/cercor/bhy130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heleniak C., Jeness J.L., Vander Stoep A., McCauley E., McLaughlin K.A. Childhood maltreatment exposure and disruptions in emotion regulation: a transdiagnostic pathway to adolescent internalizing and externalizing psychopathology. Cognit. Ther. Res. 2016;40:394–415. doi: 10.1007/s10608-015-9735-z.Childhood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa R.J., Burghy C.A., Stodola D.E., Fox M.E., Davidson R.J., Essex M.J. Enhanced prefrontal-amygdala connectivity following childhood adversity as a protective mechanism against internalizing in adolescence. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2016;1:326–334. doi: 10.1016/j.bpsc.2016.03.003.Enhanced. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstra M.B., Van Der Ende J., Verhulst F.C. Child and adolescent problems predict DSM-IV disorders in adulthood: a 14-year follow-up of a Dutch epidemiological sample. J. Am. Acad. Child Adolesc. Psychiatry. 2002;41:182–189. doi: 10.1097/00004583-200202000-00012. [DOI] [PubMed] [Google Scholar]
- Hughes C., Ensor R. Individual differences in growth in executive function across the transition to school predict externalizing and internalizing behaviors and self-perceived academic success at 6 years of age. J. Exp. Child Psychol. 2011;108:663–676. doi: 10.1016/j.jecp.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Hussong A.M., Wirth R.J., Edwards M.C., Curran P.J., Chassin L.A., Zucker R.A. Externalizing symptoms among children of alcoholic parents: Entry points for an antisocial pathway to alcoholism. J. Abnorm. Child Psychol. 2007;116:529–542. doi: 10.1037/0021-843X.116.3.529.Externalizing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussong A.M., Cai L., Curran P.J., Flora D.B., Chassin L., Zucker R.A. Disaggregating the distal, proximal, and time-varying effects of parent alcoholism on children’s internalizing symptoms. J. Abnorm. Child Psychol. 2008;36:335–346. doi: 10.1007/s10802-007-9181-9.Disaggregating. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A.M.C., Di Martino A., Uddin L.Q., Shehzad Z., Gee D.G., Reiss P.T., Margulies D.S., Castellanos F.X., Milham M.P. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb. Cortex. 2009;19:640–657. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- Kim H., Wildeman C., Jonson-Reid M., Drake B. Lifetime prevalence of investigating child maltreatment among US children. Am. J. Public Health. 2017;107:274–280. doi: 10.2105/AJPH.2016.303545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King S.M., Iacono W.G., Mcgue M. Childhood externalizing and internalizing psychopathology in the prediction of early substance use. Addiction. 2004;99:1548–1559. doi: 10.1111/j.1360-0443.2004.00893.x. [DOI] [PubMed] [Google Scholar]
- Lambert H.K., King K.M., Monahan K.C., McLaughlin K.A. Differential associations of threat and deprivation with emotion regulation and cognitive control in adolescence. Dev. Psychopathol. 2017;29:929–940. doi: 10.1017/S0954579416000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenartowicz A., Kalar D.J., Congdon E., Poldrack A. Towards an ontology of cognitive control. Top. Cogn. Sci. 2010;2:678–692. doi: 10.1111/j.1756-8765.2010.01100.x. [DOI] [PubMed] [Google Scholar]
- Luna B., Sweeney J.A. The emergence of collaborative brain function: fMRI studies of the development of response inhibition. Ann. N. Y. Acad. Sci. 2004;1021:296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- Luna B., Marek S., Larsen B., Tervo-Clemmens B., Chahal R. An integrative model of the maturation of cognitive control. Annu. Rev. Neurosci. 2015;38:151–170. doi: 10.1126/science.1249098.Sleep. PMCID: PMC5661874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek S., Hwang K., Foran W., Hallquist M.N., Luna B. The contribution of network organization and integration to the development of cognitive control. PLoS Biol. 2015;13:1–25. doi: 10.1371/journal.pbio.1002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Green J.G., Gruber M.J., Sampson N.A., Zaslavsky A.M., Kessler R.C. Childhood adversities and first onset of psychiatric disorders in a national sample of US adolescents. Arch. Gen. Psychiatry. 2012;69:1151–1160. doi: 10.1001/archgenpsychiatry.2011.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Sheridan M.A., Lambert H.K. Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neurosci. Biobehav. Rev. 2014 doi: 10.1016/j.neubiorev.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Weissman D., Bitrán D. Childhood adversity and neural development: a systematic review. Annu. Rev. Dev. Psychol. 2019:1. doi: 10.1146/annurev-devpsych-121318-084950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague L.M., Goodkind M.S., Etkin A. Transdiagnostic impairment of cognitive control in mental illness. J. Psychiatr. Res. 2016;83:37–46. doi: 10.1016/j.jpsychires.2016.08.001.Transdiagnostic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.B., Sheridan M.A., Hanson J.L., McLaughlin K.A., Bates J.E., Lansford J.E., Pettit G.S., Dodge K.A. Dimensions of deprivation and threat, psychopathology, and potential mediators: a multi-year longitudinal analysis. J. Abnorm. Psychol. 2018;127:160–170. doi: 10.1037/abn0000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra J., Sagar R., Parveen S., Kumaran S., Modi K., Maric V., Ziegler D.A., Gazzaley A. Closed-loop digital meditation for neuro-cognitive & behavioral development in adolescents with childhood neglect. Nat. Transl. Psychiatry. 2020;10:1–13. doi: 10.1038/s41398-020-0820-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki Y., Raudenbush S.W. Tests for linkage of multiple cohorts in an accelerated longitudinal design. Psychol. Methods. 2000;5:44. doi: 10.1037/1082-989x.5.1.44. [DOI] [PubMed] [Google Scholar]
- Newman M.E.J. Modularity and community structure in networks. Proc. Natl. Acad. Sci. 2006;103:8577–8582. doi: 10.1073/pnas.0601602103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordaz S.J., Foran W., Velanova K., Luna B. Longitudinal growth curves of brain function underlying inhibitory control through adolescence. J. Neurosci. 2013;33:18109–18124. doi: 10.1523/JNEUROSCI.1741-13.2013. PMCID: PMC3828464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paivio S.C., Cramer K.M. Factor structure and reliability of the childhood trauma questionnaire in a Canadian undergraduate student sample. Child Abus. Negl. 2004;28:889–904. doi: 10.1016/j.chiabu.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Petanjek Z., Judaš M., Šimic G., Rašin M.R., Uylings H.B.M., Rakic P., Kostovic I. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc. Natl. Acad. Sci. 2011;108:13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A., Kwon D., Brumback T., Thompson W.K., Cummins K., Tapert S.F., Brown S.A., Colrain I.M., Baker F.C., Prouty D., De Bellis M.D., Clark D.B., Nagel B.J., Chu W., Park S.H., Pohl K.M., Sullivan E.V. Altered brain developmental trajectories in adolescents after initiating drinking. Am. J. Psychiatry. 2017;175:370–380. doi: 10.1176/appi.ajp.2017.17040469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Petersen S.E. Control-related systems in the human brain. Curr. Opin. Neurobiol. 2013;23:223–228. doi: 10.1016/j.conb.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Cohen A.L., Nelson S.M., Wig G.S., Barnes K.A., Church J.A., Vogel A.C., Laumann T.O., Miezin F.M., Schlaggar B.L., Petersen S.E. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. PMCID: PMC3222858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Mitra A., Laumann T.O., Snyder A.Z., Schlaggar B.L., Petersen S.E. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reef J., Diamantopoulou S., Meurs I., Verhulst F., Ende J. Predicting adult emotional and behavioral problems from externalizing problem trajectories in a 24-year longitudinal study. Eur. Child Adolesc. Psychiatry. 2010;19:577–585. doi: 10.1007/s00787-010-0088-6. [DOI] [PubMed] [Google Scholar]
- Rindskopf D. Structural equation models: empirical identification, Heywood cases, and related problems. Sociol. Methods Res. 1984;13:109–119. [Google Scholar]
- Rosenberg M.D., Casey B.J., Holmes A.J. Prediction complements explanation in understanding the developing brain. Nat. Commun. 2018;9:1–13. doi: 10.1038/s41467-018-02887-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan M.A., McLaughlin K.A. Neurobiological models of the impact of adversity on education. Curr. Opin. Behav. Sci. 2016 doi: 10.1016/j.cobeha.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan M.A., Peverill M., Finn A.S., McLaughlin K.A. Dimensions of childhood adversity have distinct associations with neural systems underlying executive functioning. Dev. Psychopathol. 2017;29:1777–1794. doi: 10.1017/S0954579417001390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira S., Nooner K.B., Nagel B.J., Tapert S.F., De Bellis M.D., Mishra J. Cognitive control networks and related vulnerabilities for alcohol abuse in adolescents with childhood trauma. Program No. 645.04. 2018 Neuroscience Meeting Planner. Society for Neuroscience; San Diego, CA; 2018. [Google Scholar]
- Silveira S., Shah R., Nooner K.B., Nagel B.J., Tapert S.F., de Bellis M.D., Mishra J. Impact of childhood trauma on executive function in adolescence—mediating functional brain networks and prediction of high-risk drinking. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2020;5:499–509. doi: 10.1016/j.bpsc.2020.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds D., Hallquist M.N., Asato M., Luna B. Developmental stages and sex differences of white matter and behavioral development through adolescence: a longitudinal diffusion tensor imaging (DTI) study. Neuroimage. 2014;15:356–368. doi: 10.1016/j.neuroimage.2013.12.044.Developmental. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt E.G., Friedenberg S.L., Swenson C.C., LaRosa A., De Bellis M.D., Macias M.M., Summer A.P., Hulsey T.C., Des Runyon K., Brady K.T. The effects of early neglect on cognitive, language, and behavioral functioning in childhood. Psychology (Irvine) 2012;3:175. doi: 10.4236/psych.2012.32026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltenberg S.F., Mudd S., Blow F.C., Hill E.M. Evaluating measures of family history of alcoholism: density versus dichotomy. Addiction. 1998;93:1511–1520. doi: 10.1046/j.1360-0443.1998.931015117.x. [DOI] [PubMed] [Google Scholar]
- Sullivan E.V., Brumback T., Tapert S.F., Fama R., Prouty D., Brown S.A., Cummins K., Thompson W.K., Colrain I.M., Fiona C., De Bellis M.D., Hooper S.R., Clark D.B., Chung T., Nagel B.J., Nichols B.N., Rohlfing T., Chu W., Pohl K.M., Pfefferbaum A. Cognitive, emotion control, and motor performance of adolescents in the NCANDA study: contributions from alcohol consumption, age, sex, ethnicity, and family history of addiction. Neuropsychology. 2016;30:449–473. doi: 10.1037/neu0000259.Cognitive. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher M.H., Samson J.A. Childhood maltreatment and psychopathology: a case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am. J. Psychiatry. 2013;170:1114–1133. doi: 10.1176/appi.ajp.2013.12070957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher M.H., Samson J.A. Annual Research Review: enduring neurobiological effects of childhood abuse and neglect. J. Child Psychol. Psychiatry Allied Discip. 2016;57:241–266. doi: 10.1111/jcpp.12507. PMCID: PMC4760853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher M.H., Samson J.A., Anderson C.M., Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat. Rev. Neurosci. 2016;17:652–666. doi: 10.1016/j.micron.2017.11.003. PMID: 27640984. [DOI] [PubMed] [Google Scholar]
- Teicher M.H., Samson J.A., Anderson C.M., Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat. Rev. Neurosci. 2016;17:652–666. doi: 10.1016/j.micron.2017.11.003. [DOI] [PubMed] [Google Scholar]
- Tucker D.M., Poulsen C., Luu P. Critical periods for the neurodevelopmental processes of externalizing and internalizing. Dev. Psychopathol. 2015;27:321–346. doi: 10.1017/S0954579415000024. [DOI] [PubMed] [Google Scholar]
- Van Driel O. On various causes of improper solutions in maximum likelihood factor analysis. Psychometrika. 1978;43:225–243. [Google Scholar]
- van Ginkel J.R. Standardized regression coefficients and newly proposed estimators for R2 in multiply imputed data. Psychometrika. 2020;85:185–205. doi: 10.1007/s11336-020-09696-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Rueden U., Gosch A., Rajmil L., Bisegger C., Ravens-Sieberer U. Socioeconomic determinants of health related quality of life in childhood and adolescence: results from a European study. J. Epidemiol. Community Health. 2006;60:130–135. doi: 10.1136/jech.2005.039792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Soest T., Hagtvet K.A. Mediation analysis in a latent growth curve modeling framework. Struct. Equ. Model. 2011;18:289–314. doi: 10.1080/10705511.2011.557344. [DOI] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Wu W., Jia F. A new procedure to test mediation with missing data through nonparametric bootstrapping and multiple imputation. Multivariate Behav. Res. 2013;48:663–691. doi: 10.1080/00273171.2013.816235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.