Abstract
Circular RNAs (circRNAs) are a large class of noncoding RNAs that are emerging as critical regulators of various cellular processes that are involved in the physiopathological mechanism of many human diseases, such as cardiovascular disease, atherosclerosis, diabetes mellitus, and carcinogenesis. Autophagy is a conserved and catabolic cellular process that degrades unfolded, misfolded, or damaged protein aggregates or organelles to maintain cellular homeostasis under physiological and pathological conditions. Increasing evidence has shown a link between circRNAs and autophagy that is closely related to the occurrence and development of human diseases, including cancer. In this review, we highlight recent advances in understanding the functions and mechanisms of circRNAs in the regulation of autophagy in cancer. These autophagy-related circRNAs contribute to cancer development and progression in various types of human cancer by activating or inhibiting autophagy. Cumulative research on the relationship between circRNAs and autophagy regulation provides critical insight into the essential role that circRNAs play in carcinogenesis and suggests new targets for tumor therapy.
Keywords: circRNA, autophagy, oncogene, tumor, regulator
Graphical abstract
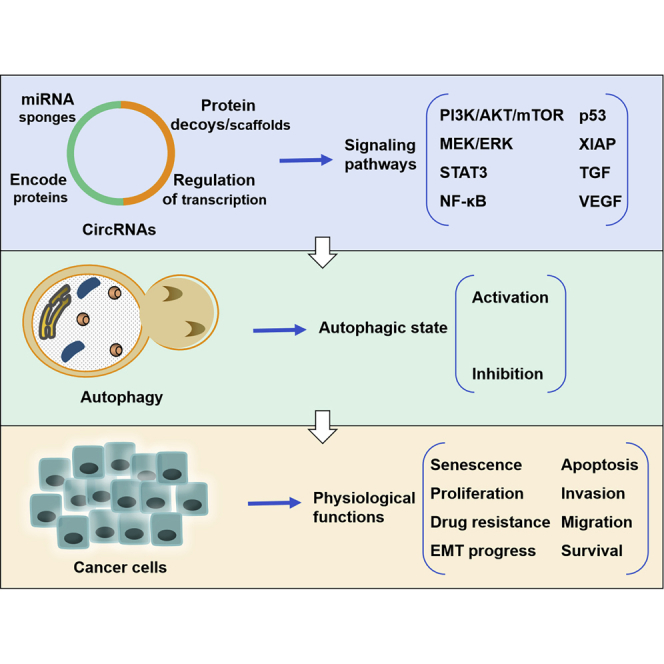
circRNAs serve as oncogenes in the regulation of many biological processes of cancer cells by activating or inhibiting autophagy. In turn, autophagy may be regulated by circRNAs in cancer. The relationship between circRNAs and autophagy provides insight into the role of circRNAs in carcinogenesis, suggesting new targets for anti-cancer therapy.
Introduction
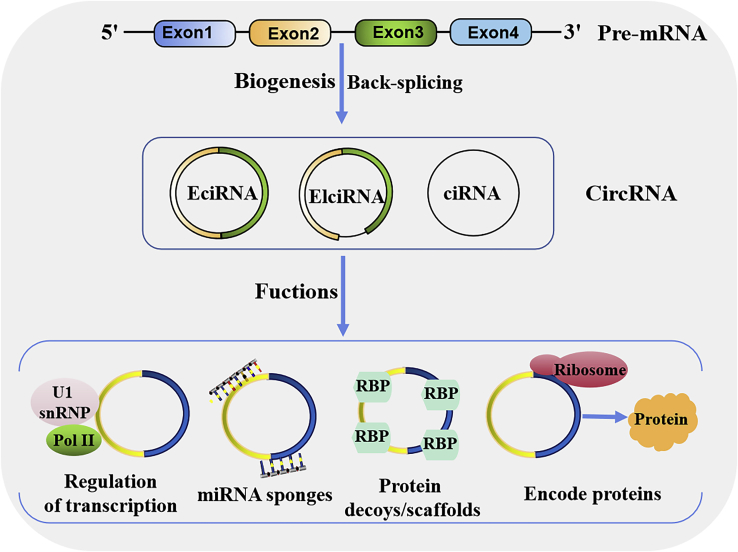
Circular RNAs (circRNAs) are a large class of noncoding RNAs (ncRNAs) that are approximately 100 nt in length and have a covalently closed-loop structure without any 5′-3′ polarity or a polyadenylated tail.1,2 Unlike canonical splicing of precursor mRNA (pre-mRNA), circRNAs are produced by back-splicing of pre-mRNA, a noncanonical splicing process. Although the detailed mechanisms of circRNA biogenesis are still continuously studied and confirmed, most circRNAs are formed by exon cyclization, and some circRNAs are lasso structures formed by intron cyclization. According to their different compositions, circRNAs are classified into three categories: exonic circRNAs (EcircRNAs) formed by exon sequences only, intronic circRNAs (CiRNAs) formed by introns, and exon-intron circRNAs (EIciRNAs) formed by exon and intron sequences (Figure 1).3 However, at present, an understanding of the complexity and functionality of circRNAs remains elusive and requires further investigation.
Figure 1.
circRNA biogenesis and function
circRNAs are mainly produced from gene transcripts by back-splicing. Based on their exonic and/or intronic source sequences, circRNA can be grouped into three major categories: exonic circRNAs (EciRNAs), exon-intron circRNAs (EIciRNAs), and intron-derived circRNAs (CiRNAs). circRNAs can interact with U1 small nuclear ribonucleoproteins (snRNPs) and polymerase II (Pol II) to promote gene transcription. circRNAs can reduce miRNA function by sponging miRNAs. circRNAs can regulate the function of proteins by working as protein decoys or scaffolds. circRNAs can be templates for translation to encode peptides, then produce proteins.
In eukaryotic cells, circRNAs are widely expressed in various tissues and organs and have highly stable and conservative properties, and circRNAs have emerged as crucial mediators of the occurrence and development of a wide variety of diseases, including cancers.3, 4, 5, 6 Accumulating evidence has revealed that circRNAs play important roles in gene expression at the transcriptional and posttranscriptional levels by acting as microRNA (miRNA) sponges and protein scaffolds and by interacting with RNA-binding proteins (RBPs) (Figure 1),4 which may potentially function as biomarkers for diagnosis and therapeutic targets in human cancer.
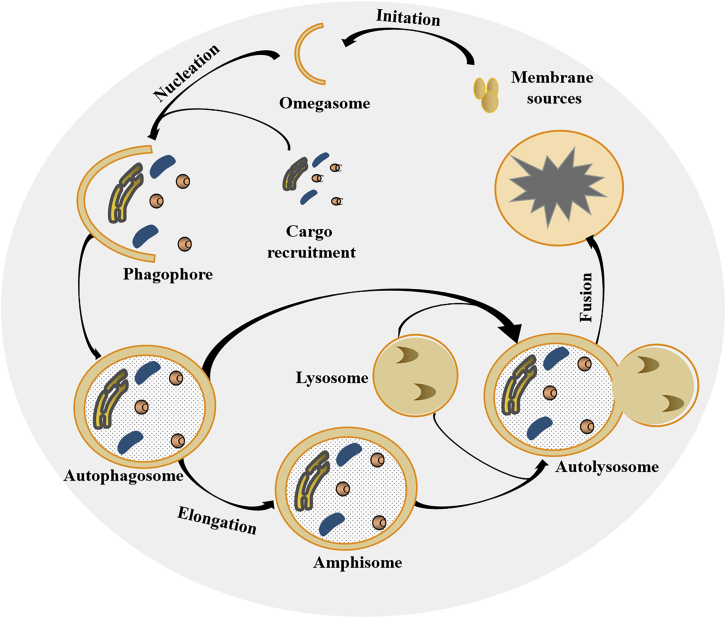
In recent years, increasing evidence has shown that there is a significant association between circRNAs and autophagy. Autophagy is a conserved and catabolic cellular process that delivers damaged or useless proteins or other cytoplasmic components to double-membrane vesicles (autophagosomes), and then to lysosomes or vacuoles for degradation.7 The process involves various steps, including initiation, nucleation, phagophore formation and elongation, and autolysosome fusion (Figure 2).8 Each step is tightly regulated by a core set of autophagy (ATG)-related proteins and transcription factors, such as unc-51-like autophagy-activating kinases (ULKs), target of rapamycin (mTOR in mammals), forkhead box O (FOXO) transcription factors, cAMP response element-binding proteins (CREBs), and cyclic AMP (cAMP)-dependent transcription factors (ATFs).9,10 Thus, mTOR inhibitors targeting these regulators are often used to stimulate or inhibit autophagy; for example, mTOR inhibitors of rapamycin act as autophagy inducers, and inhibitors of ULK1 and ULK2S BI-0206965 act as autophagy inhibitors.11
Figure 2.
The general process of autophagy
Upon various stresses, a portion of the cytoplasm is engulfed by an isolation membrane to form the omegasome. Cargo recruitment is tethered to the omegasome to form the phagophore, resulting in the generation of an autophagosome. Then, the autophagosome either fuses directly with lysosomes to form an autolysosome, or it first fuses with late endosomes to generate an amphisome, which then fuses with lysosomes to produce an autolysosome. In the autolysosome, the cargo is degraded and recycled to provide cellular energy.
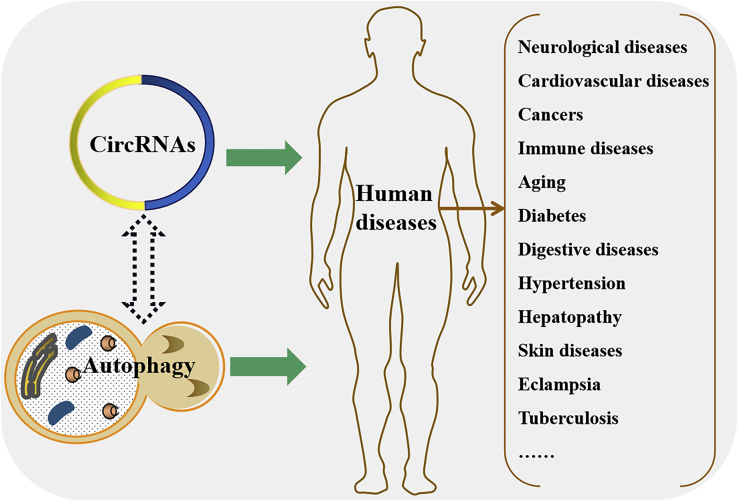
The activation markers of autophagy are microtubule-associated protein 1A/1B-light chain 3 (LC3) and selective autophagy adaptor sequestosome 1 (SQSTM1, also known as p62). There are two forms of LC3: cytosolic LC3-I and membrane-bound LC3-II, which form from LC3-I. LC3-II can bind to ubiquitin-p62 to form the LC3-II-p62 complex, leading to a decrease in p62 and the occurrence of autophagy.12 Autophagy has been defined as an autodigestive pathway that is essential for maintaining cellular homeostasis in response to various cellular stresses, including nutrient deprivation, hypoxia, oxidative stress, chemical and physical damage, and pathogen invasion.13,14 An increasing number of studies have revealed that autophagy plays a crucial role in regulating many pathological and physiological processes that are also regulated by circRNAs (Figure 3), such as cancers, autoimmune disorders, cardiovascular diseases, and neurodegenerative diseases.15, 16, 17, 18 That is, there may be crosstalk between circRNAs and autophagy in the development and progression of these human diseases.
Figure 3.
There may be crosstalk between circRNAs and autophagy in multiple human diseases
Both circRNAs and autophagy are associated with the development and progression of human diseases, in which the same types of diseases are listed in the right bracket. As circRNAs can activate or inhibit autophagy to control gene expression in these diseases, we speculate that the functions of autophagy in human physiology and pathology may also be mediated by regulating the expression of circRNAs.
In cancer, autophagy is considered a double-edged sword, depending on the nature and cellular context, such as tumor type, stage, grade, and genetic relationships.19,20 On the one hand, autophagy acts as a cell death and tumor suppressor mechanism by maintaining genomic stability.16,21 On the other hand, autophagy contributes to tumorigenesis by assisting tumor cell survival and proliferation under stress, either from the tumor microenvironment or induced by tumor therapy.19,22 Thus, targeting the autophagy process by intervening with the regulators involved in the process, including circRNAs, has emerged as a promising novel approach for cancer treatment.23 In this review, we summarize the functions and underlying mechanisms of circRNAs in regulating autophagy in cancer, which may help us to understand the pathogenesis of this function and determine the feasibility of circRNA and autophagy-mediated diagnosis and therapy for cancer.
Autophagy-related circRNA in cancer
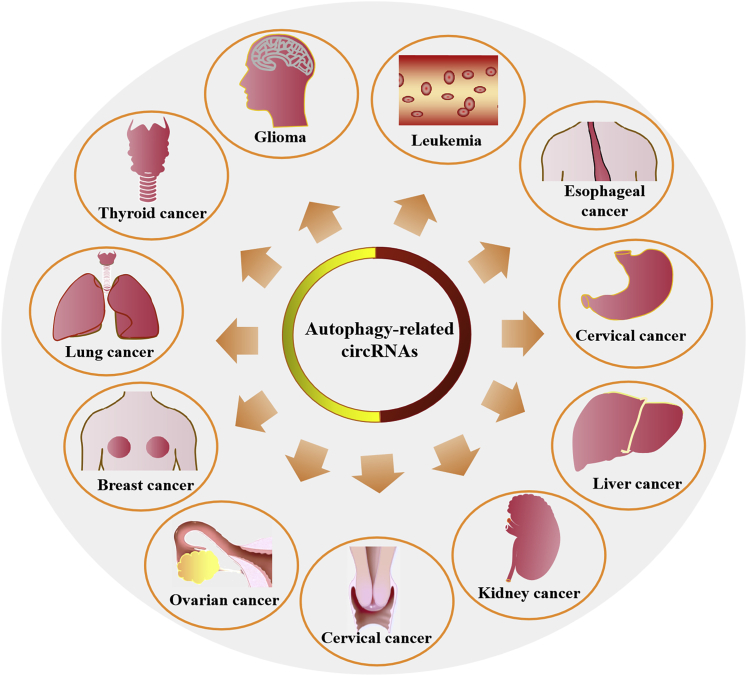
circRNAs can activate or inhibit autophagy by regulating autophagy-related proteins and pathways at any step in the autophagy process.24 Although the effects of autophagy on cancer are conflicting, almost all of the autophagy-related circRNAs that have been reported to date could further contribute to cancer development and progression in various types of human cancer, as shown in Figure 4. Autophagy-related circRNAs are positively correlated with the primary tumor size, the stages of the metastatic process, and the mortality rate of these cancer patients. These effects are mediated by the circRNAs activating or inhibiting autophagy.
Figure 4.
Cancer types related to autophagy-related circRNAs
circRNAs are associated with the development and progression of human cancers by activating or inhibiting autophagy. The types of cancers are listed in the figure.
The protumorigenic roles of circRNAs in regulating autophagy have been reported to manipulate various cellular processes, such as cell proliferation, apoptosis, migration, invasion, metastasis, and drug resistance, and these roles are associated with many signaling molecules, including ATGs, tumor protein p53 (p53), JNK kinase family, Ras, wingless-type MMTV integration site family (Wnt), human epidermal growth factor receptor 2 (HER2), and miRNAs.
circRNAs promote cancer by activating autophagy
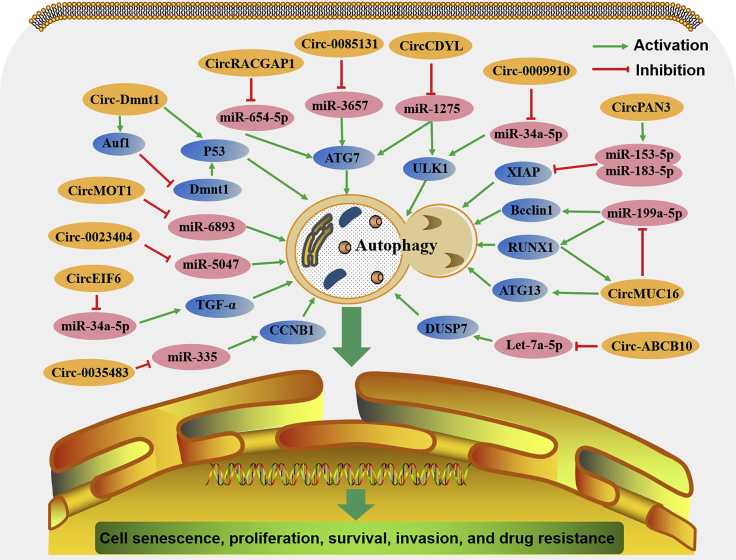
Several circRNAs are known to play important roles in development and progression by activating autophagy through multiple signaling pathways (Figure 5).
Figure 5.
The comprehensive mechanism of circRNAs in activating autophagy in cancer cells
circRNAs are involved in cancer cell senescence, proliferation, survival, invasion, and drug resistance through activating autophagy mediated by many signaling pathways.
Regulation of cell senescence, proliferation, survival, and invasion
circ-Dnmt1 (circRNA-102439, originating from mRNA RefSeq: NM_001130823.1) has been discovered to be expressed at high levels in breast cancer (BC) tissues and cell lines.25 Overexpression of circ-Dnmt1 inhibited cellular senescence and increased tumor cell proliferation and xenograft growth by stimulating cellular autophagy. Researchers have found that circ-Dnmt1 can interact with both p53 and AUF1 to promote their nuclear translocation. Then, p53 nuclear translocation could induce cellular autophagy, while AUF1 nuclear translocation reduces Dnmt1 mRNA instability, leading to the release of its inhibitory effect on p53 transcription, further enhancing autophagy.25 Similarly, circCDYL (circ-0008285, derived from the CDYL gene) is another newly discovered circRNA with high levels of expression in BC and it is positively related to a poor prognosis, shorter survival time, and a poor clinical response to therapy in BC patients.26 circCDYL overexpression increased the proliferation of BC cells via autophagy by acting as a miR-1275 sponge to significantly increase the expression of two essential autophagy regulators: ATG protein 7 (ATG7) and ULK1.26 These results indicate that circ-Dnmt1 and circCDYL act as oncogenes by activating autophagy in BC.
In one study of epithelial ovarian cancer (EOC), RNA sequencing analysis revealed that all circRNAs generated from the gene MUC16 were significantly elevated compared to normal ovarian tissue.27 circMUC16 (circ-0049116) is one of the circRNAs derived from MUC16 that is increased in EOC tissues, where high levels are positively linked to the tumor stage and grade of EOC. Further study found that circMUC16 was also increased in EOC cell lines and could enhance cell proliferation, invasion, and metastasis by promoting autophagy. Mechanistically, circMUC16 acted as a miR-199a-5p sponge to induce autophagy by regulating the apoptosis-related genes Beclin1 and RUNX1. In turn, RUNX1 can enhance circMUC16 transcription. Moreover, circMUC16 could directly bind to the autophagy-related 13 homolog (S. cerevisiae) (ATG13) and promote its expression, further contributing to cell autophagy.27 These data suggest that circMUC16 enhances tumor phenotypes by promoting autophagy through the miR-199a-5p/Beclin1/RUNX1 or ATG13 pathway.
Regulation of drug resistance
Autophagy has been regarded as a double-edged sword for tumor cell apoptosis, and impairment results in drug resistance and may depend on tumor characteristics and genomic context.20,28,29 Recent evidence has shown that circRNA-mediated autophagy can induce drug resistance in many types of cancer cells. Low levels of circMTO1 (circ-0007874) have been proven to play a role as a tumor suppressor in many cancers, including colorectal cancer,30 bladder cancer,31 BC,32 and hepatocellular carcinoma.33 Overexpression of circMTO1 could inhibit tumor cell proliferation, viability, invasion, metastasis, and epithelial-to-mesenchymal transition (EMT), as well as reverse drug resistance.30, 31, 32, 33 However, in cervical cancer, circMTO1 was shown to have high levels of expression in tumor tissues and cell lines, which contributed to tumor chemoresistance by activating autophagy.34 In the presence of cisplatin (DDP), the autophagy inhibitor 3-MA could significantly impair cell viability, which was mediated by circMTO1 overexpression.34,35 Mechanistically, the autophagy-mediated chemoresistance of circMTO1 has been associated with miR-6893.34 Similar to circMTO1, circ-0023404 was also shown to have high levels of expression and to play an oncogenic role in cervical cancer.35,36 circ-0023404 could activate autophagy and mediate tumor chemoresistance to DDP by sponging miRNA-5047.35 These results indicate that circMTO1 and circ-0023404 inhibit cell chemosensitivity to DDP by activating autophagy in cervical cancer.
A relationship between circRNA and autophagy has also been reported in recent studies of one of the two types of leukemia based on whether the leukemia is fast growing or slower growing. In chronic myeloid leukemia (CML), circ-0009910, a circRNA of 315 nt derived from the MFN2 gene, was proven to play a role in promoting cancer cell resistance to imatinib by activating autophagy.37 circ-0009910 was upregulated in imatinib-resistant CML serum samples and was positively associated with shorter survival in CML patients. As a miR-34a-5p sponge, circ-0009910 could mediate the imatinib resistance of CML via ULK1-induced autophagy.37 In acute myeloid leukemia (AML), circPAN3 (circ-0100181) was similarly found to promote drug resistance through autophagy.38 circPAN3, originating from the Pan3 gene transcript, was highly expressed in doxorubicin (ADM)-resistant AML cells and played an essential role in the acquired drug resistance of AML. Other studies found that circPAN3 could promote autophagy through the AMPK/mTOR pathway, resulting in the occurrence of AML drug resistance.38
Thyroid carcinoma is divided into papillary thyroid carcinoma (PTC), anaplastic thyroid carcinoma (ATC), and medullary thyroid carcinoma (MTC), depending on cell differentiation. circEIF6 (circ-0060060), a circRNA with 799 nt that originates from 5,226-bp genomic DNA, was found to be highly expressed in PTC and ATC tissues and cells but not in MTC.39 Overexpression of circEIF6 was proven to enhance the autophagy induced by DDP, resulting in apoptosis impairment and the enhancement of resistance to DDP in PTC and ATC cells. When circEIF6 was inhibited, miR-144-3p expression was increased, transforming growth factor (TGF)-α expression declined, and DDP resistance was weakened.39 These data suggest that the DDP resistance induced by EIF6-mediated autophagy in PTC and ATC cells is regulated by the miR-144-3p/TGF-α axis.
circ-0035483, a circRNA with 1,157 nt that originates from 12,284-bp genomic DNA, is overexpressed in renal clear cell carcinoma (KIRC) tissues and cells.40 In the presence of gemcitabine, circ-0035483 overexpression promoted autophagy in renal cancer cells, resulting in apoptosis inhibition and the promotion of tumor growth. While circ-0035483 silencing could inhibit autophagy and cell proliferation, it could also enhance the sensitivity of renal cancer cells to gemcitabine. An in-depth study showed that circ-0035483 could promote autophagy, resulting in the occurrence of AML drug resistance, working as a sponge to bind with miR-335, leading to the enhancement of cyclin B1 (CCNB1) expression and gemcitabine resistance.40
circRACGAP1 (circ-004582) is a circRNA originating from Rac GTPase-activating protein 1 (RACGAP1). It was reported to take part in apatinib-induced autophagy and apoptosis sensitivity in gastric cancer (GC) cells.41 Apatinib could increase circRACGAP1 expression and trigger autophagy by decreasing miR-3657 and increasing ATG7 expression in GC cells. When circRACGAP1 was silenced, autophagy was inhibited, and apatinib-induced apoptosis was improved.41 These data indicate that blockade of the circRACGAP1/miR-3657/ATG7 axis may be a potential therapeutic strategy to enhance GC cell sensitivity to apatinib.
circ-0085131 is a circRNA derived from the circularization of the PABPC1 genome, and it is highly expressed in non-small cell lung carcinoma (NSCLC).42 Clinically, a higher level of circ-0085131 was associated with higher recurrence rates and worse survival of NSCLC patients. Overexpression of circ-0085131 could promote cell proliferation and the resistance of NSCLC cells to DDP. Furthermore, it was proven that circ-0085131-mediated DDP resistance of NSCLC cells was induced by activating autophagy. circ-0085131 can work as a competing RNA of miR-654-5p to trigger ATG7 expression, thereby increasing cell autophagy and chemoresistance.42 These results suggest that silencing of the circ-0085131/miR-654-5p/ATG7 axis could inhibit cell proliferation and enhance cell chemosensitivity to DDP in NSCLC.
circ-ABCB10 (with 17 cricRNAD IDs, generated from ATP binding cassette subfamily B member 10) is a circRNA highly expressed in many cancers, including esophageal squamous cell carcinoma (ESCC),43 oral squamous cell carcinoma,44 lung cancer,45,46 thyroid cancer,47 BC,48 and gliomas.49 It acts as an oncogene by promoting cell proliferation, migration, and invasion while inhibiting cell apoptosis.43, 44, 45, 46, 47, 48, 49 circ-ABCB10 also plays an important role in the development of tumor drug resistance.45,50 The knockdown of circ-ABCB10 could restore the sensitivity of DDP-resistant lung cancer cells and increase DDP-induced apoptosis by the miR-556-3p/AK4 axis.45 In BC cells, circ-ABCB10 knockdown could also increase the sensitivity of paclitaxel (PTX)-resistant cells to PTX and inhibit cell invasion.50 In addition, circ-ABCB10 knockdown effectively inhibited tumor growth in vivo. However, the autophagy of PTX-resistant BC cells is also suppressed by circ-ABCB10 intervention, which suggests that circ-ABCB10 may contribute to the PTX resistance of BC. Mechanistically, the let-7a-5p/DUSP7 axis was proven to be involved in the effects of circ-ABCB10 in PTX-resistant BC cells.50
circRNAs promote cancer by inhibiting autophagy
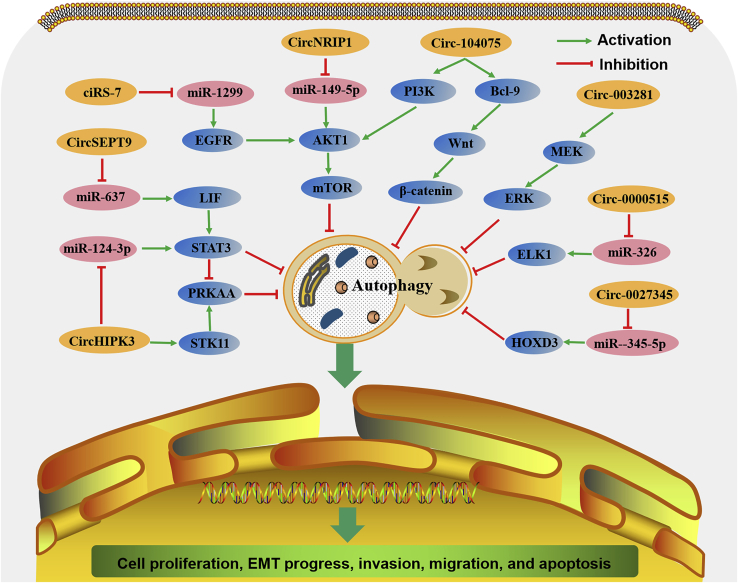
Unlike the abovementioned circRNAs that activate autophagy, some other circRNAs can inhibit autophagy, although they are also highly expressed in tumor tissues and play oncogenic roles (Figure 6). In the context of these circRNAs, autophagy is often considered to have antitumor activity by impairing tumor angiogenesis or inhibiting the malignant transformation of tumor cells.51,52
Figure 6.
The comprehensive mechanism of circRNAs in inhibiting autophagy in cancer cells
circRNAs are involved in cancer cell proliferation, EMT, invasion, migration, and apoptosis through inhibiting autophagy mediated by many signaling pathways.
Regulation of cell proliferation, EMT progression, invasion, and migration
Both circ-003281 and circNRIP1 (circ-0004771) are significantly upregulated in human GC tumors and cells.53,54 circ-003281 is a circRNA derived from the CEP128 gene 53, while circNRIP1 arises from the NRIP gene.54 Clinically, higher levels of circ-003281 or circNRIP1 are associated with tumor size, more advanced tumor stages, more metastatic lymphoid nodes, and worse survival rates of GC patients.53,54 circ-0032821 or circNRIP1 silencing significantly decreased cell proliferation, EMT, migration, and invasion but increased autophagy in GC cells.53,54 circ-0032821 overexpression had an opposite effect on GC cells, where the suppressive effect on autophagy could be counteracted by administering the autophagy enhancer rapamycin.53 circNRIP1, whose transcription can be promoted by quaking, could promote energy production activities and inhibit catabolic activities, including autophagy, leading to GC tumor growth and metastasis.54 Mechanistically, the tumor promotor role of circ-0032821 is mediated by the mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK) signaling pathway,53 while that of circNRIP1 is mediated by sponging miR-149-5p to activate the AKT1/mTOR signaling pathway.54
circHIPK3 is a circRNA originating from HIPK3 that has been considered an oncogene because it regulates cell growth in many cancer tissues and cells.55,56 In NSCLC cells, there is also a positive relationship between circHIPK3 and tumor cell biology.57 circHIPK3 silencing significantly inhibited cell proliferation, migration, and invasion. However, the regulation of cell autophagy mediated by circHIPK3 silencing is STK11-dependent.57 STK11 plays essential roles in the phosphorylation of PRKAA in the STK11-PRKAA pathway.58 In STK11 mutant NSCLC cells, circHIPK3 silencing markedly increased autophagy by interacting with miR-124-3p to activate the STAT3 PRKAA/AMPK signaling pathway, whereas in STK11 wild-type NSCLC cells, circHIPK3 silencing inhibited autophagy by decreasing the activity of STK11-pPRKAA.56 Moreover, there is an antagonistic relationship between circHIPK3 and linear HIPK3 (linHIPK3), with autophagy mediated by opposing STAT3 regulation. The ratio between circHIPK3 and linHIPK3 (C:L ratio) may reflect autophagy levels in NSCLC. A high C:L ratio (>0.49) represents a low autophagic flow, while a low C:L ratio (<0.49) represents a high autophagic flow. Clinically, in comparison with normal tissue, the C:L ratio was significantly higher in tumors with lower survival rates, especially in advanced-stage NSCLC, which indicates an anti-autophagic environment in NSCLC tissue.57 These results suggest that circHIPK3 functions as an oncogene and autophagy inhibitor in lung cancer.
circ-0000515 is a circRNA arising from ribonuclease P RNA component H1 (RPPH1) that is highly expressed in cervical cancer tissues and cells.59 Silencing of circ-0000515 significantly inhibits proliferation and invasion and promotes the apoptosis and autophagy of cervical cancer cells. In addition, circ-0000515 silencing inhibited tumor growth in nude mice in vivo. Further study found that circ-0000515 acted as a sponge of miR-326 to increase the expression of ELK1, resulting in the upregulation of PCNA and MMP-9 and the downregulation of LC3.59 These results demonstrate that circ-0000515 acts as an oncogene in cervical cancer via the miR-326/ELK1 axis.
circSEPT9 (circ-0005320), whose biogenesis is upregulated by E2F1 and EIF4A3, is a circRNA generated from the SEPT9 gene.60 In triple-negative BC (TNBC), circSEPT9 is upregulated in tumor tissues and cell lines. High levels of circSEPT9 were positively associated with advanced tumor stages and a poor prognosis. circSEPT9 silencing significantly suppressed cell proliferation, migration, and invasion, but induced cell cycle arrest, apoptosis, and autophagy in TNBC cells, whereas circSEPT9 overexpression exerted the opposite effects as circSEPT9 silencing. In addition, the knockdown of circSEPT9 inhibited tumor growth and metastasis in nude mice in vivo. Further research into the mechanism showed that circSEPT9 acts as a sponge of miR-637 to upregulate the expression of leukemia inhibitory factor (LIF), leading to activation of the STAT3 signaling pathway and the promotion of TNBC progression.60 These data demonstrated that circSEPT9 has pro-tumor functions in the development of TNBC via the circSEPT9/miR-637/LIF axis.
Regulation of the autophagy-inhibiting roles of starvation and rapamycin
ciRS-7 (circ-0001946, also called CDR1as) is a circRNA generated from the CDR1 gene. As a conserved sponge of miR-7, ciRS-7 acts as a promoter in many cancers.61, 62, 63, 64 Similar to other tumors, ciRS-7 is also highly expressed and acts as an oncogene in the progression of ESCC by improving tumor growth and metastasis.65 Moreover, a recent study proved that ciRS-7 was involved in the regulation of autophagy in ESCC cells.66 ciRS-7 overexpression markedly blocked starvation- and rapamycin-induced autophagy in ESCC cells, while ciRS-7 silencing obviously increased starvation- and rapamycin-induced cell autophagy, which indicates that ciRS-7 acts as an autophagy inhibitor. Mechanistically, further research found that ciRS-7 functions as a miR-1299 sponge to activate EGFR-AKT-mTOR signaling, resulting in the inhibition of autophagy in ESCC cells.66 These data indicate that ciRS-7 participates in the autophagy-inhibiting roles of starvation and rapamycin through the miR-1299/EGFR/AKT/mTOR axis in ESCC.
Regulation of the antitumor function of matrine
Matrine, an alkaloid extracted from the leguminous plant Sophora flavescens, has been reported to exert multiple pharmacological effects, including antitumor activity, in many types of cancers, such as melanoma,67 GC,68 CML,69 acute lymphoblastic leukemia (ALL),70 glioblastoma,71 and hepatocellular carcinoma (HCC).72 Many regulators and signaling pathways are involved in the antitumor effects of matrine, including miRNAs,68 ERK/MAPK,69 and phosphatidylinositol 3-kinase (PI3K)/AKT.71 Recently, studies have shown that circRNA acting as an inhibitor of autophagy was involved in the antitumor mechanism of matrine.73,74 In HCC cells, matrine could inhibit cell growth, migration, and invasion while increasing cell apoptosis and autophagy.74 Overexpression of circ-0027345, which was downregulated by matrine, reversed the effects of matrine on HCC cells.73 In addition, circ-0027345 can act as a miR-345-5p sponge to upregulate the levels of HOXD3, whose knockdown can reverse the tumor-promoting effects of matrine in HCC cells, which indicates that matrine regulates the viability, apoptosis, cell cycle, migration, invasion, and autophagy of HCC by inhibiting the circ-0027345/miR345-5p/HOXD3 axis.73 Similarly, matrine can repress cell viability and induce cell apoptosis and autophagy in the glioma cell line U251.74 Overexpression of circ-104075, which was downregulated by matrine, reversed the effects of matrine on U251 cells. Moreover, circ-104075 overexpression can also reactivate the Wnt/β-catenin and PI3K/AKT signaling pathways, both of which are suppressed by matrine.74 Bcl-9 is an essential coactivator of the Wnt/β-catenin pathway, which suggests that matrine enhances cell apoptosis and autophagy of glioma cells through inhibition of the circ-104075/Bcl-9/Wnt/β-catenin and circ-104075/PI3K/AKT pathways.
Other circRNAs associated with autophagy-related miRNAs
In addition to the autophagy-related circRNAs that were just discussed, other circRNAs may also be considered autophagy related in cancer,75,76 because the target miRNAs of these circRNAs are autophagy related.76,77 For example, circ-101280 was reported to promote cell proliferation in HCC cells by sponging miR-375.75 In another study, miR-375 inhibited autophagy in HCC cells under hypoxic conditions.77 This indicates that the antitumor effect of circ-101280 may be achieved by promoting autophagy, which certainly needs further study. Another typical example is miR-663a-5p and miR-154-3p, which were downregulated in pancreatic cancer (PC) cells treated with the autophagy inhibitor chloroquine.76 Competing endogenous RNA (ceRNA) microarray analysis showed that nine circRNAs (circ-0003176, circ-0048579, circ-0063706, circ-0071922, circ-0078989, circ-079319, circ-0083080, circ-0089643, and circ-0090372) whose expression was upregulated in PC cells were thought to have binding sites for miR-663a-5p, while five upregulated circRNAs (circ-0000156, circ-0004089, circ-0006461, circ-0015157, and circ-0038665) were thought to have binding sites for miR-154-3p.76 These prospective ceRNA networks indicate that these circRNAs are involved in the regulation of the autophagy inhibition of chloroquine as sponges of miR-663a-5p and miR-154-3p. Of course, these prospective ceRNA networks need further study to support these findings.
circRNAs may suppress cancer by activating or inhibiting autophagy
circRNAs not only play a critical role in promoting tumor angiogenesis and progression but also exert tumor-suppressive effects in many types of cancer, including HCC, bladder cancer, GC, BC, lung cancer, colorectal cancer, and oral squamous cell carcinoma.5,78 Similar to tumor-promoting circRNAs, tumor-suppressing circRNAs can regulate cell proliferation, migration, invasion, and death by acting as miRNA sponges and protein regulators or by regulating the transcription of linear RNAs.78 However, to date, a relationship between tumor-suppressing circRNAs and autophagy has not been reported. Further investigation is needed to determine whether there is a circRNA whose tumor-suppression effects are mediated by activating or inhibiting autophagy.
Emerging role in cancer diagnostics and therapeutics of circRNAs mediated by autophagy
With the development of biology and medical science, an increasing number of autophagy-related circRNAs will be discovered. It has become increasingly clear that they play important roles in cancer development, progression, drug resistance, recurrence, and metastasis, which indicates that circRNAs are potential diagnostic and prognostic biomarkers and promising molecular therapeutic targets in human cancer (Table 1).
Table 1.
circRNAs associated with autophagy regulation in human cancer
| circRNA | Origin of gene | RefSeq | Ensembl no. | Expression in cancer | Role in cancer | Function in autophagy | Related cellular process | Related gene | Tumor type | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| circ-Dnmt1 | DNMT1 | NM_001032355.1 | ENSG00000130816 | upregulated | oncogene | pro-autophagy | cellular senescence, cell proliferation | p53, AUF1 | breast cancer | 25 |
| circCDYL | CDYL | NM_004824.4 | ENSG00000153046 | upregulated | oncogene | pro-autophagy | cell proliferation | miR-1275, ATG7, ULK1 | breast cancer | 26 |
| circMUC16 | MUC16 | NM_024690.2 | ENSG00000181143 | upregulated | oncogene | pro-autophagy | cell proliferation, invasion, metastasis | miR-199a-5, Beclin1, RUNX1, ATG13 | epithelial ovarian cancer | 27 |
| circMTO1 | MTO1 | NM_133645.3 | ENSG00000135297 | upregulated | oncogene | pro-autophagy | drug resistance | miR-6893 | cervical cancer | 34 |
| circ-0023404 | RNF121 | NM_018320.5 | ENSG00000137522 | upregulated | oncogene | pro-autophagy | drug resistance | miRNA-5047 | cervical cancer | 35 |
| circ-0009910 | MFN2 | NM_014874.4 | ENSG00000116688 | upregulated | oncogene | pro-autophagy | drug resistance | miR-34a-5p, ULK1 | chronic myeloid leukemia | 37 |
| circPAN3 | PAN3 | NM_175854.8 | ENSG00000152520 | upregulated | oncogene | pro-autophagy | drug resistance | AMPK/mTOR pathway | acute myeloid leukemia | 38 |
| circEIF6 | EIF6 | NM_002212.4 | ENSG00000242372 | upregulated | oncogene | pro-autophagy | drug resistance | miR-144-3p, TGF-α | thyroid carcinoma | 39 |
| circ-0035483 | ND | ND | ND | upregulated | oncogene | pro-autophagy | drug resistance | AMPK/mTOR pathway, miR-335, CCNB1 | renal clear cell carcinoma | 40 |
| circRACGAP1 | RACGAP1 | NM_013277.5 | ENSG00000161800 | upregulated | oncogene | pro-autophagy | drug resistance | miR-3657, ATG7 | gastric cancer | 41 |
| circ-0085131 | PABPC1 | NM_002568.4 | ENSG00000070756 | upregulated | oncogene | pro-autophagy | drug resistance | miR-654-5p, ATG7 | non-small cell lung carcinoma | 42 |
| circ-ABCB10 | ABCB10 | NM_012089.3 | ENSG00000135776 | upregulated | oncogene | pro-autophagy | drug resistance | let-7a-5p, DUSP7 | breast cancer | 50 |
| Circ-003281 | CEP128 | NM_152446.5 | ENSG00000100629 | upregulated | oncogene | anti-autophagy | cell proliferation, EMT, migration, invasion | MEK/ERK pathway | gastric cancer | 53 |
| circHIPK3 | HIPK3 | NM_005734.5 | ENSG00000110422 | upregulated | oncogene | anti-autophagy | cell proliferation, migration, invasion | miR124-3p, STK11, PRKAA, AMPK | non-small cell lung carcinoma | 56 |
| circNRIP1 | NRIP1 | NM_003489.4 | ENSG00000180530 | upregulated | oncogene | anti-autophagy | cell proliferation, EMT, migration, invasion | miR-149-5p, AKT1, mTOR | gastric cancer | 58 |
| circ-0000515 | RPPH1 | NR_002312.1 | ENSG00000277209 | upregulated | oncogene | anti-autophagy | cell proliferation, invasion, apoptosis | miR-326, ELK1, PCNA, MMP-9 | cervical cancer | 59 |
| circSEPT9 | SEPT9 | NM_001113491.2 | ENSG00000184640 | upregulated | oncogene | anti-autophagy | cell proliferation, migration, invasion, cycle, apoptosis | miR-637, LIF, STAT3 | breast cancer | 60 |
| ciRS-7 | CDR1 | NM_004065.2 | ENSG00000288642 | upregulated | oncogene | anti-autophagy | function of starvation and rapamycin | EGFR/Akt/mTOR pathway | esophageal squamous cell carcinoma | 66 |
| circ-0027345 | ND | ND | ND | upregulated | oncogene | anti-autophagy | function of matrine | miR345-5p, HOXD3 | hepatocellular carcinoma | 73 |
| circ-104075 | ND | ND | ND | upregulated | oncogene | anti-autophagy | function of matrine | Bcl-9, PI3K, AKT, Wnt/β-catenin | glioma | 74 |
ND, not determined.
In addition, circRNAs are detectable in many human body fluids, such as blood, saliva, urine, breast milk, and especially serum exosomes. Exosomes are tiny vesicles secreted by cells (including tumor cells) that play an important role in regulating intercellular communication by carrying various molecular substances. Exosomes can carry circRNAs and protect them from degradation, so the expression of circRNAs in serum exosomes can better reflect their real level in the body. Therefore, taking advantage of their stability and high specificity, exosomal circRNAs may be stable tumor diagnosis and treatment markers that could easily be detected. Therefore, the existence, expression levels, functions, and molecular mechanisms of circRNAs mediated by autophagy in cancer serum exosomes need to be further elucidated.
circRNA in cancer may be regulated by autophagy
As sequencing technologies and bioinformatics rapidly develop, an increasing number of circRNAs that regulate autophagy in cancer will be identified. Thus, it would be interesting to investigate whether autophagy could, in turn, affect the expression of circRNAs (Figure 3). It has been reported that autophagy plays an essential role in the maintenance of cellular RNA homeostasis by degrading several types of RNAs, including miRNAs.79,80 Autophagy could regulate miRNA homeostasis by degradation via the selective autophagy receptor calcium binding and coiled-coil domain 2 (CALCOCO2), targeting the miRNA machinery factors dicer 1, ribonuclease type III (DICER1), and argonaute RNA-induced silencing complex (RISC) component 1/argonaute-1 (EIF2C1/AGO1).81 The inhibition of autophagy can decrease miRNA levels and lead to the depression of miRNA activity, which suggests that autophagy acts as a checkpoint for the maintenance of miRNA abundance and activity.81 Additionally, miRNAs and long noncoding RNAs (lncRNAs) can be regulated by autophagy, including plasmacytoma variant translocation 1 (PVT1).82,83 Although the mechanism is unknown, the elevation of PVT1 mediated by autophagy has an important function in the development and progression of diabetic nephropathy.82,84 As another important type of noncoding RNA, circRNA expression is also most likely regulated by autophagy, at least during its back-splicing generation process, which may result in the impairment of circRNA functions in human physiology and pathology, including cancer. Thus, extensive further investigations are desperately needed.
Conclusions
As both circRNAs and autophagy play crucial roles in the development and progression of cancer, the regulatory relationship between them is attracting increasing research attention. However, in the limited related research to date, only a few circRNAs have been found to be involved in the biology of cancer by activating or inhibiting autophagy, and there is limited knowledge of the molecular mechanisms underlying the relationship. The underlying mechanism in autophagy-related circRNAs in cancer is usually that they act as sponges of miRNAs to form a circRNA-miRNA-mRNA regulatory axis, leading to the enhancement of cancer development. In addition, autophagy-related circRNAs can also interact with cancer-related genes/proteins and signaling pathways to affect their biological functions or regulate the expression of linear RNAs. With the growing knowledge base about circRNAs and autophagy in cancer, more complicated functions and underlying mechanisms will be elucidated, perhaps involving epigenetic regulation, such as DNA methylation, histone modification, or chromatin remodeling. Nevertheless, considerable efforts and in-depth studies are expected to obtain more information about autophagy-related circRNAs and their utility as potential novel prognostic biomarkers and therapeutic targets in cancer treatment.
Acknowledgments
This study was supported by the Natural Science Foundation of Shandong Province of China (ZR2020MH250) and by the Qingdao Source Innovation Program of China (19-6-2-49-cg and 18-6-6-63-nsh).
Author contributions
Z.Z., Y.Z., and P.L. designed the review and contributed to manuscript preparation. Z.Z. and Y.Z. wrote the manuscript. J.G., X.H., and C.S. performed technical and administrative support. Z.Z. and J.L. prepared the figures. C.L. and Y.W. revised the manuscript. All authors read and approved the final manuscript.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Zhixia Zhou, Email: zhou_zhixia@qdu.edu.cn.
Peifeng Li, Email: peifli@qdu.edu.cn.
References
- 1.Wilusz J.E. A 360° view of circular RNAs: From biogenesis to functions. Wiley Interdiscip. Rev. RNA. 2018;9:e1478. doi: 10.1002/wrna.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hentze M.W., Preiss T. Circular RNAs: Splicing’s enigma variations. EMBO J. 2013;32:923–925. doi: 10.1038/emboj.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang Q., Hann S.S. Biological roles and mechanisms of circular RNA in human cancers. OncoTargets Ther. 2020;13:2067–2092. doi: 10.2147/OTT.S233672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han B., Chao J., Yao H. Circular RNA and its mechanisms in disease: From the bench to the clinic. Pharmacol. Ther. 2018;187:31–44. doi: 10.1016/j.pharmthera.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Meng S., Zhou H., Feng Z., Xu Z., Tang Y., Li P., Wu M. circRNA: Functions and properties of a novel potential biomarker for cancer. Mol. Cancer. 2017;16:94. doi: 10.1186/s12943-017-0663-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shan C., Zhang Y., Hao X., Gao J., Chen X., Wang K. Biogenesis, functions and clinical significance of circRNAs in gastric cancer. Mol. Cancer. 2019;18:136. doi: 10.1186/s12943-019-1069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janku F., McConkey D.J., Hong D.S., Kurzrock R. Autophagy as a target for anticancer therapy. Nat. Rev. Clin. Oncol. 2011;8:528–539. doi: 10.1038/nrclinonc.2011.71. [DOI] [PubMed] [Google Scholar]
- 8.Kume S., Koya D. Autophagy: A novel therapeutic target for diabetic nephropathy. Diabetes Metab. J. 2015;39:451–460. doi: 10.4093/dmj.2015.39.6.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Füllgrabe J., Klionsky D.J., Joseph B. The return of the nucleus: Transcriptional and epigenetic control of autophagy. Nat. Rev. Mol. Cell Biol. 2014;15:65–74. doi: 10.1038/nrm3716. [DOI] [PubMed] [Google Scholar]
- 10.Amaravadi R.K. Transcriptional regulation of autophagy in RAS-driven cancers. J. Clin. Invest. 2015;125:1393–1395. doi: 10.1172/JCI81504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Towers C.G., Thorburn A. Therapeutic targeting of autophagy. EBioMedicine. 2016;14:15–23. doi: 10.1016/j.ebiom.2016.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang P., Mizushima N. LC3- and p62-based biochemical methods for the analysis of autophagy progression in mammalian cells. Methods. 2015;75:13–18. doi: 10.1016/j.ymeth.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 13.Levine B., Kroemer G. Biological functions of autophagy genes: A disease perspective. Cell. 2019;176:11–42. doi: 10.1016/j.cell.2018.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glick D., Barth S., Macleod K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010;221:3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rebecca V.W., Amaravadi R.K. Emerging strategies to effectively target autophagy in cancer. Oncogene. 2016;35:1–11. doi: 10.1038/onc.2015.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuervo A.M. Autophagy: In sickness and in health. Trends Cell Biol. 2004;14:70–77. doi: 10.1016/j.tcb.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Shibutani S.T., Saitoh T., Nowag H., Münz C., Yoshimori T. Autophagy and autophagy-related proteins in the immune system. Nat. Immunol. 2015;16:1014–1024. doi: 10.1038/ni.3273. [DOI] [PubMed] [Google Scholar]
- 18.Reggiori F., Klionsky D.J. Autophagic processes in yeast: Mechanism, machinery and regulation. Genetics. 2013;194:341–361. doi: 10.1534/genetics.112.149013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White E. Deconvoluting the context-dependent role for autophagy in cancer. Nat. Rev. Cancer. 2012;12:401–410. doi: 10.1038/nrc3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kondo Y., Kanzawa T., Sawaya R., Kondo S. The role of autophagy in cancer development and response to therapy. Nat. Rev. Cancer. 2005;5:726–734. doi: 10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- 21.Sun T. Long noncoding RNAs act as regulators of autophagy in cancer. Pharmacol. Res. 2018;129:151–155. doi: 10.1016/j.phrs.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Moosavi M.A., Haghi A., Rahmati M., Taniguchi H., Mocan A., Echeverría J., Gupta V.K., Tzvetkov N.T., Atanasov A.G. Phytochemicals as potent modulators of autophagy for cancer therapy. Cancer Lett. 2018;424:46–69. doi: 10.1016/j.canlet.2018.02.030. [DOI] [PubMed] [Google Scholar]
- 23.Klionsky D.J., Abdelmohsen K., Abe A., Abedin M.J., Abeliovich H., Acevedo Arozena A., Adachi H., Adams C.M., Adams P.D., Adeli K. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) Autophagy. 2016;12:1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J., Wang P., Wan L., Xu S., Pang D. The emergence of noncoding RNAs as Heracles in autophagy. Autophagy. 2017;13:1004–1024. doi: 10.1080/15548627.2017.1312041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du W.W., Yang W., Li X., Awan F.M., Yang Z., Fang L., Lyu J., Li F., Peng C., Krylov S.N. A circular RNA circ-DNMT1 enhances breast cancer progression by activating autophagy. Oncogene. 2018;37:5829–5842. doi: 10.1038/s41388-018-0369-y. [DOI] [PubMed] [Google Scholar]
- 26.Liang G., Ling Y., Mehrpour M., Saw P.E., Liu Z., Tan W., Tian Z., Zhong W., Lin W., Luo Q. Autophagy-associated circRNA circCDYL augments autophagy and promotes breast cancer progression. Mol. Cancer. 2020;19:65. doi: 10.1186/s12943-020-01152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gan X., Zhu H., Jiang X., Obiegbusi S.C., Yong M., Long X., Hu J. circMUC16 promotes autophagy of epithelial ovarian cancer via interaction with ATG13 and miR-199a. Mol. Cancer. 2020;19:45. doi: 10.1186/s12943-020-01163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mariño G., Niso-Santano M., Baehrecke E.H., Kroemer G. Self-consumption: the interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2014;15:81–94. doi: 10.1038/nrm3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faccenda D., Campanella M. Molecular regulation of the mitochondrial F1Fo-ATPsynthase: Physiological and pathological significance of the inhibitory factor 1 (IF(1)) Int. J. Cell Biol. 2012;2012:367934. doi: 10.1155/2012/367934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ge Z., Li L.F., Wang C.Y., Wang Y., Ma W.L. circMTO1 inhibits cell proliferation and invasion by regulating Wnt/β-catenin signaling pathway in colorectal cancer. Eur. Rev. Med. Pharmacol. Sci. 2018;22:8203–8209. doi: 10.26355/eurrev_201812_16513. [DOI] [PubMed] [Google Scholar]
- 31.Li Y., Wan B., Liu L., Zhou L., Zeng Q. Circular RNA circMTO1 suppresses bladder cancer metastasis by sponging miR-221 and inhibiting epithelial-to-mesenchymal transition. Biochem. Biophys. Res. Commun. 2019;508:991–996. doi: 10.1016/j.bbrc.2018.12.046. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y., Dong Y., Zhao L., Su L., Luo J. Circular RNA-MTO1 suppresses breast cancer cell viability and reverses monastrol resistance through regulating the TRAF4/Eg5 axis. Int. J. Oncol. 2018;53:1752–1762. doi: 10.3892/ijo.2018.4485. [DOI] [PubMed] [Google Scholar]
- 33.Han D., Li J., Wang H., Su X., Hou J., Gu Y., Qian C., Lin Y., Liu X., Huang M. Circular RNA circMTO1 acts as the sponge of microRNA-9 to suppress hepatocellular carcinoma progression. Hepatology. 2017;66:1151–1164. doi: 10.1002/hep.29270. [DOI] [PubMed] [Google Scholar]
- 34.Chen M., Ai G., Zhou J., Mao W., Li H., Guo J. circMTO1 promotes tumorigenesis and chemoresistance of cervical cancer via regulating miR-6893. Biomed. Pharmacother. 2019;117:109064. doi: 10.1016/j.biopha.2019.109064. [DOI] [PubMed] [Google Scholar]
- 35.Guo J., Chen M., Ai G., Mao W., Li H., Zhou J. hsa_circ_0023404 enhances cervical cancer metastasis and chemoresistance through VEGFA and autophagy signaling by sponging miR-5047. Biomed. Pharmacother. 2019;115:108957. doi: 10.1016/j.biopha.2019.108957. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J., Zhao X., Zhang J., Zheng X., Li F. Circular RNA hsa_circ_0023404 exerts an oncogenic role in cervical cancer through regulating miR-136/TFCP2/YAP pathway. Biochem. Biophys. Res. Commun. 2018;501:428–433. doi: 10.1016/j.bbrc.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Cao H.X., Miao C.F., Sang L.N., Huang Y.M., Zhang R., Sun L., Jiang Z.X. circ_0009910 promotes imatinib resistance through ULK1-induced autophagy by sponging miR-34a-5p in chronic myeloid leukemia. Life Sci. 2020;243:117255. doi: 10.1016/j.lfs.2020.117255. [DOI] [PubMed] [Google Scholar]
- 38.Shang J., Chen W.M., Wang Z.H., Wei T.N., Chen Z.Z., Wu W.B. circPAN3 mediates drug resistance in acute myeloid leukemia through the miR-153-5p/miR-183-5p-XIAP axis. Exp. Hematol. 2019;70:42–54.e3. doi: 10.1016/j.exphem.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 39.Liu F., Zhang J., Qin L., Yang Z., Xiong J., Zhang Y., Li R., Li S., Wang H., Yu B. Circular RNA EIF6 (hsa_circ_0060060) sponges miR-144-3p to promote the cisplatin-resistance of human thyroid carcinoma cells by autophagy regulation. Aging (Albany NY) 2018;10:3806–3820. doi: 10.18632/aging.101674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yan L., Liu G., Cao H., Zhang H., Shao F. hsa_circ_0035483 sponges hsa-miR-335 to promote the gemcitabine-resistance of human renal cancer cells by autophagy regulation. Biochem. Biophys. Res. Commun. 2019;519:172–178. doi: 10.1016/j.bbrc.2019.08.093. [DOI] [PubMed] [Google Scholar]
- 41.Ma L., Wang Z., Xie M., Quan Y., Zhu W., Yang F., Zhao C., Fan Y., Fang N., Jiang H. Silencing of circRACGAP1 sensitizes gastric cancer cells to apatinib via modulating autophagy by targeting miR-3657 and ATG7. Cell Death Dis. 2020;11:169. doi: 10.1038/s41419-020-2352-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kong R. Circular RNA hsa_circ_0085131 is involved in cisplatin-resistance of non-small-cell lung cancer cells by regulating autophagy. Cell Biol. Int. 2020;44:1945–1956. doi: 10.1002/cbin.11401. [DOI] [PubMed] [Google Scholar]
- 43.Zhang W.Q., Liu K.Q., Pei Y.X., Tan J., Ma J.B., Zhao J. circ-ABCB10 promotes proliferation and invasion of esophageal squamous cell carcinoma cells by modulating microRNA-670-3p. Eur. Rev. Med. Pharmacol. Sci. 2020;24:6088–6096. doi: 10.26355/eurrev_202006_21504. [DOI] [PubMed] [Google Scholar]
- 44.Chen F., Li X.H., Liu C., Zhang Y., Wang R.J. circ-ABCB10 accelerates the malignant progression of oral squamous cell carcinoma by absorbing miRNA-145-5p. Eur. Rev. Med. Pharmacol. Sci. 2020;24:681–690. doi: 10.26355/eurrev_202001_20045. [DOI] [PubMed] [Google Scholar]
- 45.Wu Z., Gong Q., Yu Y., Zhu J., Li W. Knockdown of circ-ABCB10 promotes sensitivity of lung cancer cells to cisplatin via miR-556-3p/AK4 axis. BMC Pulm. Med. 2020;20:10. doi: 10.1186/s12890-019-1035-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng J.P., Dai Y.M., Chen Z., Chen Q., Zheng Y., Lin X., Cui T.J. Retraction. Eur. Rev. Med. Pharmacol. Sci. 2020;24:7200. doi: 10.26355/eurrev_202007_21850. [DOI] [PubMed] [Google Scholar]
- 47.Han X.T., Jiang J.Q., Li M.Z., Cong Q.M. Retraction. Eur. Rev. Med. Pharmacol. Sci. 2020;24:9774. doi: 10.26355/eurrev_202010_23170. [DOI] [PubMed] [Google Scholar]
- 48.Liang H.F., Zhang X.Z., Liu B.G., Jia G.T., Li W.L. Circular RNA circ-ABCB10 promotes breast cancer proliferation and progression through sponging miR-1271. Am. J. Cancer Res. 2017;7:1566–1576. [PMC free article] [PubMed] [Google Scholar]
- 49.Sun W.Y., Lu Y.F., Cai X.L., Li Z.Z., Lv J., Xiang Y.A., Chen J.J., Chen W.J., Liu X.M., Chen J.B. circ-ABCB10 acts as an oncogene in glioma cells via regulation of the miR-620/FABP5 axis. Eur. Rev. Med. Pharmacol. Sci. 2020;24:6848–6857. doi: 10.26355/eurrev_202006_21674. [DOI] [PubMed] [Google Scholar]
- 50.Yang W., Gong P., Yang Y., Yang C., Yang B., Ren L. circ-ABCB10 contributes to paclitaxel resistance in breast cancer through let-7a-5p/DUSP7 axis. Cancer Manag. Res. 2020;12:2327–2337. doi: 10.2147/CMAR.S238513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Galluzzi L., Pietrocola F., Bravo-San Pedro J.M., Amaravadi R.K., Baehrecke E.H., Cecconi F., Codogno P., Debnath J., Gewirtz D.A., Karantza V. Autophagy in malignant transformation and cancer progression. EMBO J. 2015;34:856–880. doi: 10.15252/embj.201490784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heras-Sandoval D., Pérez-Rojas J.M., Hernández-Damián J., Pedraza-Chaverri J. The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell. Signal. 2014;26:2694–2701. doi: 10.1016/j.cellsig.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 53.Jiang Y., Zhang Y., Chu F., Xu L., Wu H. circ_0032821 acts as an oncogene in cell proliferation, metastasis and autophagy in human gastric cancer cells in vitro and in vivo through activating MEK1/ERK1/2 signaling pathway. Cancer Cell Int. 2020;20:74. doi: 10.1186/s12935-020-1151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang X., Wang S., Wang H., Cao J., Huang X., Chen Z., Xu P., Sun G., Xu J., Lv J., Xu Z. Circular RNA circNRIP1 acts as a microRNA-149-5p sponge to promote gastric cancer progression via the AKT1/mTOR pathway. Mol. Cancer. 2019;18:20. doi: 10.1186/s12943-018-0935-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zheng Q., Bao C., Guo W., Li S., Chen J., Chen B., Luo Y., Lyu D., Li Y., Shi G. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat. Commun. 2016;7:11215. doi: 10.1038/ncomms11215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Y., Zheng F., Xiao X., Xie F., Tao D., Huang C., Liu D., Wang M., Wang L., Zeng F., Jiang G. circHIPK3 sponges miR-558 to suppress heparanase expression in bladder cancer cells. EMBO Rep. 2017;18:1646–1659. doi: 10.15252/embr.201643581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen X., Mao R., Su W., Yang X., Geng Q., Guo C., Wang Z., Wang J., Kresty L.A., Beer D.G. Circular RNA circHIPK3 modulates autophagy via MIR124-3p-STAT3-PRKAA/AMPKα signaling in STK11 mutant lung cancer. Autophagy. 2020;16:659–671. doi: 10.1080/15548627.2019.1634945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shackelford D.B., Shaw R.J. The LKB1-AMPK pathway: Metabolism and growth control in tumour suppression. Nat. Rev. Cancer. 2009;9:563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tang Q., Chen Z., Zhao L., Xu H. Circular RNA hsa_circ_0000515 acts as a miR-326 sponge to promote cervical cancer progression through up-regulation of ELK1. Aging (Albany NY) 2019;11:9982–9999. doi: 10.18632/aging.102356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zheng X., Huang M., Xing L., Yang R., Wang X., Jiang R., Zhang L., Chen J. The circRNA circSEPT9 mediated by E2F1 and EIF4A3 facilitates the carcinogenesis and development of triple-negative breast cancer. Mol. Cancer. 2020;19:73. doi: 10.1186/s12943-020-01183-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pan H., Li T., Jiang Y., Pan C., Ding Y., Huang Z., Yu H., Kong D. Overexpression of circular RNA ciRS-7 abrogates the tumor suppressive effect of miR-7 on gastric cancer via PTEN/PI3K/AKT signaling pathway. J. Cell. Biochem. 2018;119:440–446. doi: 10.1002/jcb.26201. [DOI] [PubMed] [Google Scholar]
- 62.Weng W., Wei Q., Toden S., Yoshida K., Nagasaka T., Fujiwara T., Cai S., Qin H., Ma Y., Goel A. Circular RNA ciRS-7-A promising prognostic biomarker and a potential therapeutic target in colorectal cancer. Clin. Cancer Res. 2017;23:3918–3928. doi: 10.1158/1078-0432.CCR-16-2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hansen T.B., Jensen T.I., Clausen B.H., Bramsen J.B., Finsen B., Damgaard C.K., Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 64.Sang M., Meng L., Liu S., Ding P., Chang S., Ju Y., Liu F., Gu L., Lian Y., Geng C. Circular RNA ciRS-7 maintains metastatic phenotypes as a ceRNA of miR-1299 to target MMPs. Mol. Cancer Res. 2018;16:1665–1675. doi: 10.1158/1541-7786.MCR-18-0284. [DOI] [PubMed] [Google Scholar]
- 65.Hansen T.B., Kjems J., Damgaard C.K. Circular RNA and miR-7 in cancer. Cancer Res. 2013;73:5609–5612. doi: 10.1158/0008-5472.CAN-13-1568. [DOI] [PubMed] [Google Scholar]
- 66.Meng L., Liu S., Ding P., Chang S., Sang M. Circular RNA ciRS-7 inhibits autophagy of ESCC cells by functioning as miR-1299 sponge to target EGFR signaling. J. Cell. Biochem. 2020;121:1039–1049. doi: 10.1002/jcb.29339. [DOI] [PubMed] [Google Scholar]
- 67.Wei Y.P., Wang X.H., Liu G., Zhang J.F., Yang Y.X., Zhang J., Song X.L., Li Z.D., Zhao L.D. Matrine exerts inhibitory effects in melanoma through the regulation of miR-19b-3p/PTEN. Int. J. Oncol. 2018;53:791–800. doi: 10.3892/ijo.2018.4414. [DOI] [PubMed] [Google Scholar]
- 68.Liu Z.M., Yang X.L., Jiang F., Pan Y.C., Zhang L. Matrine involves in the progression of gastric cancer through inhibiting miR-93-5p and upregulating the expression of target gene AHNAK. J. Cell. Biochem. 2020;121:2467–2477. doi: 10.1002/jcb.29469. [DOI] [PubMed] [Google Scholar]
- 69.Ma L., Xu Z., Wang J., Zhu Z., Lin G., Jiang L., Lu X., Zou C. Matrine inhibits BCR/ABL mediated ERK/MAPK pathway in human leukemia cells. Oncotarget. 2017;8:108880–108889. doi: 10.18632/oncotarget.22353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tetik Vardarlı A., Düzgün Z., Erdem C., Kaymaz B.T., Eroglu Z., Çetintas V.B. Matrine induced G0/G1 arrest and apoptosis in human acute T-cell lymphoblastic leukemia (T-ALL) cells. Bosn. J. Basic Med. Sci. 2018;18:141–149. doi: 10.17305/bjbms.2017.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Z., Wu Y., Wang Y., Jin Y., Ma X., Zhang Y., Ren H. Matrine inhibits the invasive properties of human glioma cells by regulating epithelial-to-mesenchymal transition. Mol. Med. Rep. 2015;11:3682–3686. doi: 10.3892/mmr.2015.3167. [DOI] [PubMed] [Google Scholar]
- 72.Wang Y., Zhang S., Liu J., Fang B., Yao J., Cheng B. Matrine inhibits the invasive and migratory properties of human hepatocellular carcinoma by regulating epithelial-mesenchymal transition. Mol. Med. Rep. 2018;18:911–919. doi: 10.3892/mmr.2018.9023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lin S., Zhuang J., Zhu L., Jiang Z. Matrine inhibits cell growth, migration, invasion and promotes autophagy in hepatocellular carcinoma by regulation of circ_0027345/miR-345-5p/HOXD3 axis. Cancer Cell Int. 2020;20:246. doi: 10.1186/s12935-020-01293-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chi G., Xu D., Zhang B., Yang F. Matrine induces apoptosis and autophagy of glioma cell line U251 by regulation of circRNA-104075/BCL-9. Chem. Biol. Interact. 2019;308:198–205. doi: 10.1016/j.cbi.2019.05.030. [DOI] [PubMed] [Google Scholar]
- 75.Cao S., Wang G., Wang J., Li C., Zhang L. hsa_circ_101280 promotes hepatocellular carcinoma by regulating miR-375/JAK2. Immunol. Cell Biol. 2019;97:218–228. doi: 10.1111/imcb.12213. [DOI] [PubMed] [Google Scholar]
- 76.Wei D.M., Jiang M.T., Lin P., Yang H., Dang Y.W., Yu Q., Liao D.Y., Luo D.Z., Chen G. Potential ceRNA networks involved in autophagy suppression of pancreatic cancer caused by chloroquine diphosphate: A study based on differentially-expressed circRNAs, lncRNAs, miRNAs and mRNAs. Int. J. Oncol. 2019;54:600–626. doi: 10.3892/ijo.2018.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chang Y., Yan W., He X., Zhang L., Li C., Huang H., Nace G., Geller D.A., Lin J., Tsung A. miR-375 inhibits autophagy and reduces viability of hepatocellular carcinoma cells under hypoxic conditions. Gastroenterology. 2012;143:177. doi: 10.1053/j.gastro.2012.04.009. 87.e8. [DOI] [PubMed] [Google Scholar]
- 78.Li Z., Ruan Y., Zhang H., Shen Y., Li T., Xiao B. Tumor-suppressive circular RNAs: Mechanisms underlying their suppression of tumor occurrence and use as therapeutic targets. Cancer Sci. 2019;110:3630–3638. doi: 10.1111/cas.14211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Frankel L.B., Lubas M., Lund A.H. Emerging connections between RNA and autophagy. Autophagy. 2017;13:3–23. doi: 10.1080/15548627.2016.1222992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shan C., Chen X., Cai H., Hao X., Li J., Zhang Y., Gao J., Zhou Z., Li X., Liu C. The emerging roles of autophagy-related microRNAs in cancer. Int. J. Biol. Sci. 2021;17:134–150. doi: 10.7150/ijbs.50773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gibbings D., Mostowy S., Voinnet O. Autophagy selectively regulates miRNA homeostasis. Autophagy. 2013;9:781–783. doi: 10.4161/auto.23694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li Z., Hao S., Yin H., Gao J., Yang Z. Autophagy ameliorates cognitive impairment through activation of PVT1 and apoptosis in diabetes mice. Behav. Brain Res. 2016;305:265–277. doi: 10.1016/j.bbr.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 83.Gao J., Chen X., Shan C., Wang Y., Li P., Shao K. Autophagy in cardiovascular diseases: Role of noncoding RNAs. Mol. Ther. Nucleic Acids. 2020;23:101–118. doi: 10.1016/j.omtn.2020.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Millis M.P., Bowen D., Kingsley C., Watanabe R.M., Wolford J.K. Variants in the plasmacytoma variant translocation gene (PVT1) are associated with end-stage renal disease attributed to type 1 diabetes. Diabetes. 2007;56:3027–3032. doi: 10.2337/db07-0675. [DOI] [PubMed] [Google Scholar]