Abstract
Objective
Losartan and activation of the peroxisome proliferator-activated receptor-γ (PPARγ) have been previously reported to alleviate the progression of osteoarthritis (OA). However, the nature of the interaction between losartan and PPARγ in OA remains elusive. Therefore, we aimed to investigate the mechanism of the regulation of PPARγ by losartan in the context of OA.
Methods
Clinical samples of OA patients were collected and the chondrocytes were further isolated, and used to construct OA chondrocyte model via induction with IL-1β. An OA mouse model was developed by the surgical destabilization of the medial meniscus (DMM). OA chondrocytes were treated with losartan, PPARγ siRNA and the PPAR-γ agonist GW1929 alone or in combination. Furthermore, the OA mice were treated with varying doses of losartan to determine the best mode of administration and treatment dose. Subsequently, the DMM mice were treated with losartan and GW9662. Expression of PPARγ, key proteins of the transforming growth factor-beta1 (TGF-β1) signaling pathway and the markers of OA degeneration were evaluated by the Western blot analysis, while effects on OA inflammatory factors were determined by ELISA.
Results
The downregulation of PPARγ and the upregulation of TGF-β1 signaling pathway were detected in the OA cartilage tissues and chondrocytes. Losartan treatment or PPARγ activation contributes to reduced levels of IL-6, IL-1β, TNF-α, and COX-2, expression of TGF-β1, MMP-13, ADAMTS-4, ADAMTS-5, HtrA1, and iNOS, along with reduced Smad2 and Smad3 phosphorylation, but elevated PPARγ and Collagen II expression in vivo and in vitro. Additionally, the intraarticular injection of losartan into the knee joint proved to be the best mode of administration, and 10 mg/mL being the optimal treatment concentration.
Conclusion
Our results show that losartan could arrest the progression of OA by upregulating PPARγ expression and inactivating the TGF-β1 signaling pathway.
The translational potential of this article: Our results provide a biological rationale for the use of losartan as a potential candidate for OA treatment.
Keywords: Osteoarthritis, Losartan, PPARγ, TGF-β1 signaling pathway, IL-1β
1. Introduction
Osteoarthritis (OA), the most common chronic joint disease, is characterized by the failure in repairing damaged cartilage due to the biochemical and biomechanical changes in the joint, and which has an increasing incidence closely associated with the aging population and the obesity epidemic [[1], [2], [3]]. The pathologic changes in OA joints typically include the formation of osteophytes, the degeneration of ligaments, knee and menisci, the degradation of the articular cartilage, hypertrophy of the joint capsule, and synovial inflammation and thickening of the subchondral bone [4]. Moreover, there are various well-established risk factors of OA, including being overweight, obesity, knee injury, repetitive use of joints, joint laxity, female gender, muscle weakness, old age, and bone density [5,6]. Additionally, inflammation also contributes to the progression of OA, presenting as clinical features such as effusion or synovitis due to thickening of the synovium [7,8]. Furthermore, it has been proven that interleukin (IL)-1β induces inflammation in chondrocytes and thus promotes OA progression in a murine model [9]. Moreover, the angiotensin II receptor antagonist losartan has been revealed to attenuate the progression of OA in the synovial temporomandibular joint [10]. In addition, losartan also promotes joint repair by reducing the formation of fibrocartilage in a rabbit model with osteochondral defect [11]. More importantly, losartan has been previously reported to activate the peroxisome proliferator-activated receptor-γ (PPARγ) to inhibit inflammation in renal injury [12]. Therefore, we speculated that losartan may help to alleviate OA via effects on the PPARγ signaling pathway.
PPARγ belongs to the PPAR subfamily of nuclear hormone receptors, which includes PPAR- and PPAR-. PPARγ is a ligand-gated transcription factor, and has been associated with the differentiation of adipocytes, lipid metabolism, lipid storage, insulin sensitivity and glucose homeostasis [13]. The overexpression of PPARγ has been recently documented to repress the degree of inflammation in patients with metabolic syndrome [14]. Another prior study has demonstrated that the upregulation of PPARγ showed inhibitory effects on inflammation in IL-1β-treated chondrosarcoma cells [15]. Furthermore, PPARγ has also been established to suppress the release of transforming growth factor-β1 (TGF-β1) in airway smooth muscle cells (ASMCs), which may be relevant to asthma therapeutics [16]. TGF-β1 is a pleiotropic and multifunctional cytokine that derives from multiple types of cells, including endothelial cells, epithelial cells, smooth muscle cells, fibroblasts, and most immune cells [17]. Additionally, a prior study has demonstrated that repression of the TGF-β1/Smad signaling pathway resulted in reduced cartilage injury in a rat model of OA [18]. Another study has previously suggested that losartan inhibits the TGF-β1 signaling pathway and thereby decreases articular cartilage degeneration in OA mice [19]. Based on the abovementioned findings, we hypothesized that losartan could potentially inhibit the occurrence of OA via suppression of the PPARγ-mediated TGF-β1 signaling pathway, and that elucidating this pathway might provide new therapeutic target for the treatment of OA.
2. Materials and methods
2.1. Ethics statement
The current study was performed with the approval of the ethics committee of our hospital and in strict accordance with the Declaration of Helsinki. Informed signed consents were obtained from all the included participants and their families. The experiments involving animals were performed strictly according to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. Great efforts were made to minimize the number and suffering of the included animals.
2.2. Sample collection
The articular cartilage tissues from the knee and serum were obtained from 10 OA patients at our hospital who had previously received joint replacement surgery and 10 patients (normal control) who had previously undergone amputation due to trauma. Any specimens with infection or other diseases were excluded according to the medical history, preoperative X-ray film, and postoperative visual observation. All the OA patients were diagnosed according to the OA knee joint standards of the Orthopaedic Branch of the Chinese Medical Association (2007).
2.3. Detection of PPARγ, TGF-β1 signaling pathway-related proteins, OA-related inflammatory factors, and degeneration markers in cartilage tissues
The OA cartilage samples were obtained from severe abrasion sites in the load-bearing area of the tibial plateau of the knee joint of the included OA patients, and normal cartilage was obtained from corresponding regions in the normal samples. Subsequently, the OA cartilage and normal cartilage tissues were digested with type II collagenase, followed by measuring expression of PPARγ, TGF-β1 signaling pathway-related proteins (TGF-β1, Smad2, and Smad3), OA-related inflammatory factors (interleukin [IL]-6, IL-1β, tumor necrosis factor-α [TNF-α], and COX-2), and degeneration markers (matrix metalloproteinase [MMP]-13, a disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif [ADAMTS]-4, ADAMTS-5, HtrA1, inducible nitric oxide synthase [iNOS], and Collagen II) in the samples.
2.4. Chondrocyte culture
The normal cartilage tissues were rinsed with phosphate buffer saline (PBS) and the clean cartilage surface was harvested and cut into pieces, which were then placed into 15 mL centrifuge tubes. Each tube was added with 2–3 volumes of type II collagenase, mixed well, and further incubated at 37 °C with 5% CO2 for 12 h. Subsequently, the obtained ablated cartilage tissue mixture was filtered through a 60-mesh filter. The resultant filtrate was transferred into 15 mL centrifuge tubes and added with PBS to a final volume of 12 mL. This sample was centrifuged for 6 min at 800 r/min, and the lower layer of resultant filtrate containing chondrocytes was collected. Subsequently, 6–8 mL of 15% fetal bovine serum (FBS) and Dulbecco's modified Eagle's medium (DMEM)/F12 complete medium (containing 1% streptomycin and penicillin) were added to obtain the chondrocyte solution adjusted to a concentration of approximately 1 × 105 cells/mL. The chondrocyte solution was aliquoted into 25 mm2 culture bottles and placed in an incubator under standard conditions. The culture medium was changed every 3 days and the cells were subcultured when the cells reached approximately 80% confluence. The final chondrocyte preparation was identified by immunohistochemical staining with anti-type II collagen antibody.
2.5. IL-1β-treated chondrocyte culture, transfection and grouping
In order to elucidate the effect of losartan on OA, we first used IL-1β to treat chondrocytes to construct an OA cell model. The separated normal chondrocytes were cultured with 10 ng/mL IL-1β for 24 h. Then, the IL-1β-treated chondrocytes were assigned to the following 13 groups: blank (treatment with normal saline), losartan (treatment with 5 μM losartan), negative control small interfering RNA (NC siRNA) (transfection with NC siRNA sequence), PPARγ siRNA (transfection with PPARγ siRNA sequence), losartan + NC siRNA (treatment with 5 μM losartan and transfection with NC siRNA sequence), losartan + PPARγ siRNA (treatment with 5 μM losartan and transfection with PPARγ siRNA sequence), dimethyl sulfoxide (DMSO) (treatment with 1 μM DMSO), GW1929 (treatment with 1 μM GW1929, a PPAR-γ agonist), GW1929 + PBS (co-treatment with 1 μM GW1929 and 10 ng/mL PBS), GW1929 + TGF-β1 (co-treatment with 1 μM GW1929 and 10 ng/mL TGF-β1), losartan + PBS (co-treatment with 5 μM losartan and 10 ng/mL PBS), losartan + TGF-β1 (co-treatment with 5 μM losartan and 10 ng/mL TGF-β1), losartan + PPARγ siRNA + PBS (transfection with PPARγ siRNA sequence and treatment with 5 μM losartan and 10 ng/mL PBS), and losartan + PPARγ siRNA + TGF-β1 group (transfection with PPARγ siRNA sequence and treatment with 5 μM losartan and 10 ng/mL TGF-β1). Transfection was conducted in accordance to the manufacturer's instructions of Lipofectamin 2000 reagents (Invitrogen, Carlsbad, CA, USA). The PPARγ siRNA sequences were as follows: 5′-GACAUUCCAUUCACAAGAA-3' (sense) and 5′-UUCUUGUGAAUGGAAUGUCTT-3' (antisense).
2.6. Western blot analysis
Radio-immunoprecipitation assay cell lysis buffer (P0013C, Beyotime Institute of Biotechnology, Shanghai, China) containing phenylmethylsulfonyl fluoride was added into cells and tissues for total protein extraction. The culture was then placed on ice for 30 min, centrifuged at 8000 g for 10 min, and the resultant supernatant was collected. Additionally, a bicinchoninic acid kit was employed to estimate the protein concentration in the supernatant. Subsequently, 50 μg of protein was dissolved in 2 × sodium dodecyl sulfate (SDS) sample buffer and boiled for 5 min. The protein sample was then subjected to SDS-polyacrylamide gel electrophoresis. Furthermore, the separated protein was electroblotted onto a polyvinylidene fluoride membrane using the wet transfer method, which was then followed by 1-h blocking with 5% skimmed milk powder at room temperature. The membrane was subsequently probed overnight at the temperature of 4 °C with diluted primary rabbit antibodies against PPARγ (1 : 500, ab59256), TGF-β1 (1 : 1000, #3709, Cell Signaling Technologies, Beverly, MA, USA), Smad2 (1 : 2000, ab40855), Smad3 (1 : 1500, ab40854), phosphorylated (p)-Smad2 (1 : 500, ab53100), p-Smad3 (1 : 1000, ab193297), MMP-13 (1 : 3000, ab39012), ADAMTS-4 (1 : 2000, ab185722), ADAMTS-5 (1 : 250, ab41037), Collagen II (1 : 5000, ab34712), iNOS (1 : 500, ab3523), high-temperature requirement A serine peptidase 1 (HtrA1; 1 : 1000, ab38611), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; ab9485, 1:2500, internal reference). All the antibodies except TGF-β1 were purchased from Abcam Inc (Cambridge, UK). Subsequently, the membrane was re-probed with goat anti-rabbit immunoglobulin G H&L secondary antibody (1 : 2000, ab97051, Abcam Inc.) which was labeled by horseradish peroxidase for the duration of 1 h. Following this, the membrane was placed on a clean glass plate and developed using the enhanced chemiluminescence reagents (BB-3501, Amersham, Little Chalfont, UK). The image analysis system (Bio-Rad, Hercules, CA, USA) was employed to take photograph and the results were analyzed using the Quantity One v4.6.2 software. The protein levels were expressed as the ratio of the gray value of each protein to that of internal reference.
2.7. Enzyme-linked immunosorbent assay (ELISA)
Cell culture supernatant was harvested from 24-well plates following lipopolysaccharide (LPS) administration, after which the concentration of inflammatory cytokines was measured by ELISA according to the manufacturer's protocols (R&D Systems, Abingdon, UK) and normalized to cell protein concentration. The serum and cell solutions to be tested were harvested for the further evaluation of the levels of TNF-α (RAB0477), IL-6 (RAB0308), cyclooxygenase-2 (COX-2; RAB1034) and IL-1β (RAB0274) in accordance to the manufacturer's protocols of ELISA Kits (Sigma–Aldrich Chemical Company, St Louis, MO, USA).
2.8. Destabilization of medial meniscus (DMM)-induced OA mouse model establishment
A total of 160C57BL/6 mice (weighing 18–22 g, aged 8–10 weeks, Hunan SJA Laboratory Animal Co., Ltd., Hunan, China) were obtained for the current study. In brief, one knee joint of each mice was randomly selected, and the OA mouse model was established as previously reported [20]. Subsequent to the operation, the mice were returned to their home cages and were free to move about, and were treated with intraperitoneal injection of penicillin to prevent post-operative infection. Ten sham-operated mice were used as controls. In these mice, the medial meniscus tibial ligament of the contralateral knee was not cut off, but the exposure and suturing methods were otherwise similar to those for OA modeling. HE staining and safranin-O-fast green staining were performed post hoc to verify the successful establishment of the OA model. The OA model mice with were divided into control (treatment with normal saline) and IL-1β (intraarticular injection of 10 ng/mL IL-1β) groups. After 12 weeks post-surgery, the mice were euthanized, and expression of related genes in cartilage tissues was detected by Western blot analysis and ELISA.
2.9. Exploring of the best intervention method
Our initial aim was to determine the best mode of administration and concentration of losartan for treating OA mice. For these purposes, we assigned the successfully induced OA mice into oral treatment and knee joint intracavitary injection treatment groups. In the oral treatment OA mouse groups, treatments were placebo (controls), or oral losartan administered in drinking water at 0.1 mg/kg/day (low dose), 1 mg/kg/day (medium dose), 10 mg/kg/day (high dose), or 100 mg/kg/day (extremely high dose) beginning on the day after surgery and continuing until the day of euthanasia. The knee joint intracavitary injection treatment groups were treated with 10 μL volumes of saline control or containing losartan at concentrations of 0.1 mg/mL (low dose), 1 mg/mL (medium dose), 10 mg/mL (high dose), or 100 mg/mL (extremely high dose) subsequent to the operation, with treatment on weeks 2, 4, 6, 8, and 10 post-operation (n = 10 in each group). The losartan was purchased from the LKT Laboratories (St Paul, MN, USA). The mice were euthanized at the 12th week subsequent to the operation and the knees of the surgery sides were collected for histological analysis. Expression of relevant genes in articular cartilage of the tibial plateau was detected using Western blot analysis.
2.10. In vitro study of attenuation of OA by losartan
Base on initial studies in of the optimal losartan treatment doses in OA mice indicated, we administered intraarticular losartan at a dose of 10 mg/mL losartan +5 mg/kg DMSO, or 10 mg/mL losartan +5 mg/kg GW9662. The mice were then euthanized 12 weeks after the operation for further histological analysis. The expression of relevant genes in cartilage was detected by Western blot analysis.
The knee joints were dissected and fixed with 10% neutral buffered formalin (NBF) for 72 h. The fixed knee joint tissues were then decalcified in 10% ethylenediaminetetraacetic acid (EDTA) disodium (E9884-1 KG, Sigma–Aldrich, St. Louis, MO, USA) for 4 weeks, dehydrated in an ethanol series, cleared with xylene, and embedded in paraffin. Then, 5-μm sagittal sections were prepared on a microtome and collected on Superfrost glass slides (Thermo Fisher Scientific, Waltham, MA, USA) at levels where the medial tibial plateau and anterior and posterior horns of the medial meniscus were visible. These sections were subsequently deparaffinized using xylene and hydrated using an ethanol gradient and water for further histological analysis.
2.11. Histology
HE staining was performed to reveal the general morphology of the knee joint. Additionally, Safranin-O-fast green staining (IHC World; http://www.ihcworld.com/_protocols/special_stains/safranin_o.htm) was modified by extending the Safranin O step to30 min to detect the proteoglycan and glycosaminoglycan matrices [20].
2.12. Immunohistochemistry
OA and normal cartilage tissues were deparaffinized with xylene and rehydrated with gradient ethanol, followed by microwave antigen retrieval. The tissues were then immersed in 0.3% H2O2 to block the endogenous peroxidase and then immunostained with primary antibody against CCNO (1:1000, ab47682, Abcam) overnight. The following day, tissues were added with secondary antibody for 30 min of reaction. Finally, tissues were developed with 3,3-diaminobenzidine and counterstained with hematoxylin. Semiquantitative evaluation on the immunohistochemistry of CCNO was carried out using an immunoscore according to the percentage of stained cells and their staining intensity, as previously described. The intensity score was defined as follows: 0 = no evident staining; 1 = weak intensity; 2 = moderate intensity; 3 = strong intensity; 4 = very strong intensity. The fraction score was determined based on the proportion of positively stained cells (0–100%). The mean value of the immunoscores was recorded following observation under a microscope in ten randomly selected high-power fields. The histologic classification of the specimens was determined by two pathologists in an independent manner.
2.13. Histological analysis
Safranin-O-fast green staining was conducted to detect the OA cartilage damage and subchondral bone plate (SBP) thickness, whereas Safranin-O/hematoxylin was conducted for the purpose of synovitis scoring. Representative microscopic images were taken, which captured the entire articular cartilage of the tibial plateau in each section. Subsequently, the Osteoarthritis Research Society International (OARSI) scoring system (grade 0–6) was adopted to measure OA severity/cartilage damage [20].
2.14. Statistical analysis
All measurement data were shown as mean ± standard deviation and was analyzed using the SPSS 19.0 software (IBM Corp., Armonk, NY, USA), with a level of significance set as p < 0.05. The data between two groups were compared employing the unpaired t-test and comparisons amongst multiple groups were performed using the one-way analysis of variance (ANOVA), followed by the Tukey's post-hoc test.
3. Results
3.1. PPARγ was poorly expressed and TGF-β1 signaling pathway was activated in cartilage tissues and chondrocytes with OA
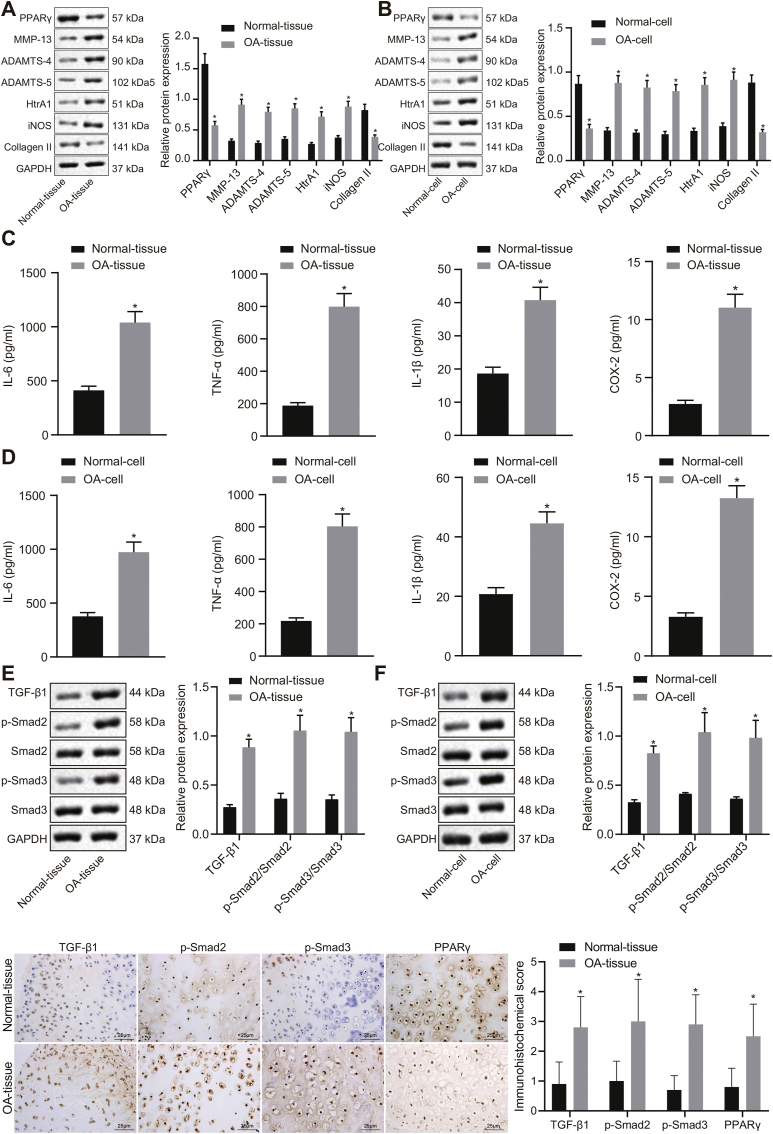
In comparison to the normal cartilage tissues and chondrocytes, expression of PPARγ and Collagen II was significantly reduced, while levels of MMP-13, ADAMTS-4, ADAMTS-5, HtrA1, and iNOS were significantly increased in OA cartilage tissues and chondrocytes (p < 0.05; Fig. 1A and B). Additionally, IL-6, IL-1β, TNF-α, and COX-2 levels were observed to be abundant in OA cartilage tissues and IL-1β-treated chondrocytes (Fig. 1C and D). Additionally, Western blot analysis and immunohistochemistry revealed high expression of TGF-β1, and increased extents of Smad2 and Smad3 phosphorylation in OA cartilage tissues and IL-1β-treated chondrocytes (Fig. 1E and F). These results suggested the occurrence of downregulated PPARγ expression, activated TGF-β1 signaling pathway, and increased expression of OA related inflammatory factors, as well as increased degeneration markers in OA cartilage tissues and IL-1β-treated chondrocytes.
Fig. 1.
Downregulation of PPARγ and activation of the TGF-β1 signaling pathway in OA model cartilage tissues and chondrocytes. A, Expression of PPARγ, MMP-13, ADAMTS-4, ADAMTS-5, HtrA1, iNOS, and Collagen II in cartilage tissues examined by Western blot analysis normalized to GAPDH (n = 10). B, Expression of PPARγ, MMP-13, ADAMTS-4, ADAMTS-5, HtrA1, iNOS, and Collagen II in chondrocytes examined by Western blot analysis normalized to GAPDH (n = 3). C, IL-6, IL-1β, TNF-α, and COX-2 levels in serum of clinical samples measured by ELISA (n = 10). D, IL-6, IL-1β, TNF-α, and COX-2 levels in chondrocytes measured by ELISA (n = 3). E, Expression of TGF-β1, Smad2, and Smad3, along with the extent of Smad2 and Smad3 phosphorylation in cartilage tissues examined by Western blot analysis normalized to GAPDH, and immunohistochemistry (n = 10). F, Expression of TGF-β1, Smad2, and Smad3, along with the extent of Smad2 and Smad3 phosphorylation in chondrocytes examined by Western blot analysis normalized to GAPDH, and immunohistochemistry (n = 3). ∗p < 0.05 vs. normal cartilage tissues and normal chondrocytes.
3.2. Losartan reduced expression of OA-related genes in IL-1β-treated chondrocytes by increasing PPARγ
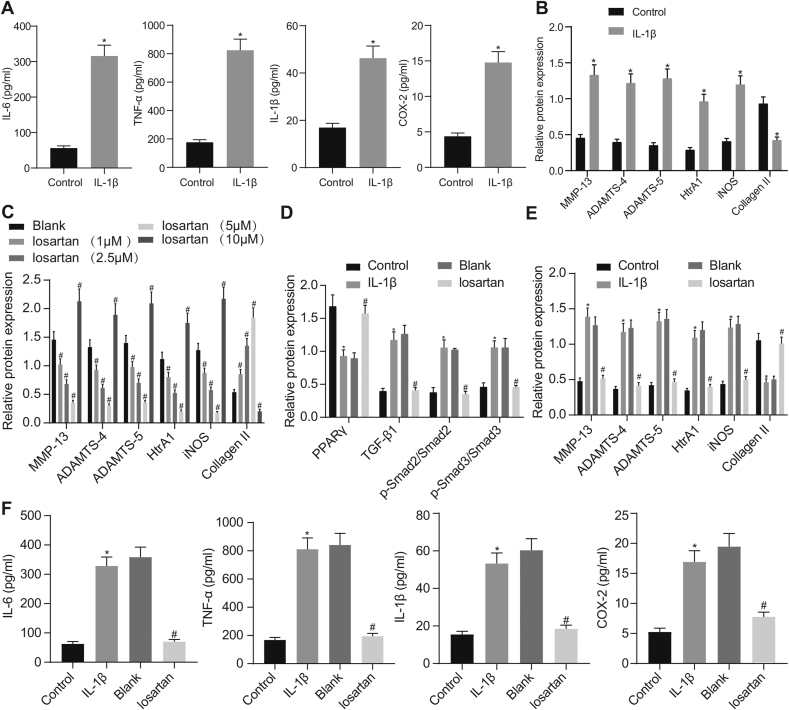
ELISA and Western blot analysis revealed that expression of IL-6, IL-1β, TNF-α, COX-2, MMP-13, ADAMTS-4, ADAMTS-5, HtrA1, and iNOS was strikingly elevated, while that of Collagen II was remarkably lower in OA chondrocytes in comparison to the normal chondrocytes (Fig. 2A and B). Treatment with 1 μM, 2.5 μM or 5 μM losartan resulted in reduced expression of MMP-13, ADAMTS-4, ADAMTS-5, HtrA1, and iNOS, but enhanced the expression of Collagen II in OA chondrocytes. However, treatment with 10 μM losartan resulted in opposite results in OA chondrocytes (Fig. 2C). Therefore, we chose a treatment concentration of 5 μM losartan for subsequent experiments. Then, expression of TGF-β1, MMP-13, ADAMTS-4, ADAMTS-5, HtrA1, and iNOS along with the extent of Smad2 and Smad3 phosphorylation was found to be significantly diminished, whereas expression of Collagen II and PPARγ was remarkably enhanced in OA chondrocytes (Fig. 2D and E). ELISA displayed that the levels of IL-6, IL-1β, TNF-α, and COX-2 were diminished in OA chondrocytes when treated with 5 μM losartan (Fig. 2F). Cumulatively, the aforementioned findings established that losartan resulted in increased PPARγ expression and inactivation of the TGF-β1 signaling pathway in chondrocytes.
Fig. 2.
Losartan downregulates expression of OA-related genes in OA chondrocytes. A, The levels of IL-6, IL-1β, TNF-α, and COX-2 in OA chondrocytes evaluated by ELISA. B, Expression of MMP-13, ADAMTS-4, ADAMTS-5, HtrA1, iNOS, and Collagen II in OA chondrocytes assessed by Western blot analysis normalized to GAPDH. C, Expression of MMP-13, ADAMTS-4, ADAMTS-5, HtrA1, iNOS, and Collagen II in OA chondrocytes after treatment with 1, 2.5, 5 or 10 μM losartan evaluated by Western blot analysis normalized to GAPDH. D, Expression of PPARγ, TGF-β1, Smad2, and Smad3, along with the extent of Smad2 and Smad3 phosphorylation in OA chondrocytes after treatment with 5 μM losartan evaluated by Western blot analysis normalized to GAPDH. E, Expression of MMP-13, ADAMTS-4, ADAMTS-5, HtrA1, iNOS, and Collagen II in OA chondrocytes after treatment with 5 μM losartan evaluated by Western blot analysis normalized to GAPDH. F, The levels of IL-6, IL-1β, TNF-α, and COX-2 in OA chondrocytes after treatment with 5 μM losartan evaluated by ELISA. The experiment was repeated three times. ∗p < 0.05 vs. control chondrocytes; #p < 0.05 vs. OA chondrocytes.
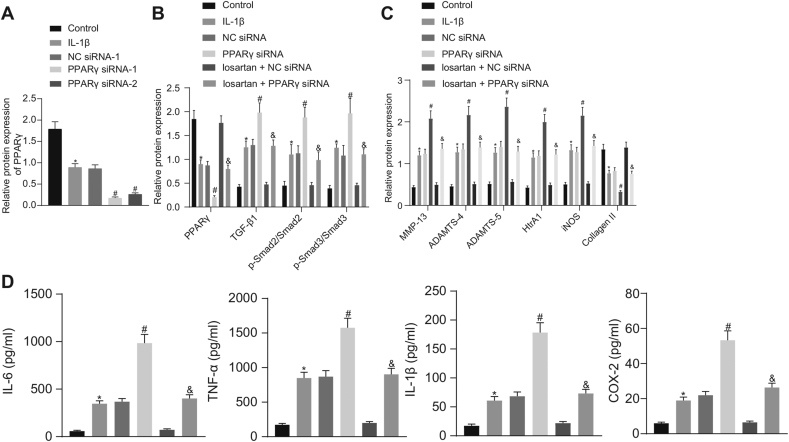
Western blot analysis (Fig. 3A) demonstrated that expression of PPARγ was significantly reduced subsequent to the PPARγ silencing. The PPARγ siRNA-1 showed the best silencing efficacy and was therefore selected for subsequent experiments. Furthermore, results of Western blot analysis established that PPARγ siRNA treatment led to a significant decrease of PPARγ and Collagen II expression, as well as the increased expression of TGF-β1, MMP-13, ADAMTS-4, ADAMTS-5, HtrA1 and iNOS, along with enhanced extent of Smad2 and Smad3 phosphorylation, all of which was reversed by losartan treatment (Fig. 3B and C). Additionally, the ELISA detection revealed elevated levels of IL-6, IL-1β, TNF-α, and COX-2 in OA chondrocytes subsequent to the silencing of PPARγ, and that these effects were reversed by treatment with losartan (Fig. 3D). The abovementioned results suggest that losartan could downregulate expression of OA-related genes in IL-1β-treated chondrocytes by upregulating PPARγ expression.
Fig. 3.
Losartan downregulates expression of OA-related genes by upregulating PPARγ expression in chondrocytes. A, The silencing efficacy of PPARγ in chondrocytes assessed by Western blot analysis normalized to GAPDH. Normal chondrocytes were used as controls, and OA chondrocytes treated with or without NC siRNA, PPARγ siRNA, losartan + NC siRNA or losartan + PPARγ siRNA. B, Expression of PPARγ, TGF-β1, Smad2, and Smad3, along with the extent of Smad2 and Smad3 phosphorylation in chondrocytes evaluated by Western blot analysis normalized to GAPDH. C, Expression of MMP-13, ADAMTS-4, ADAMTS-5, HtrA1, iNOS, and Collagen II in chondrocytes evaluated by Western blot analysis normalized to GAPDH. D, The levels of IL-6, IL-1β, TNF-α, and COX-2 in chondrocytes evaluated by ELISA. The experiment was repeated three times. ∗p < 0.05 vs. control chondrocytes; #p < 0.05 vs. OA chondrocytes treated with NC siRNA; & p < 0.05 vs. OA chondrocytes treated with losartan + NC siRNA.
3.3. PPARγ downregulated expression of OA-related genes in IL-1β-treated chondrocytes by inactivating the TGF-β1/smad2/3 signaling pathway
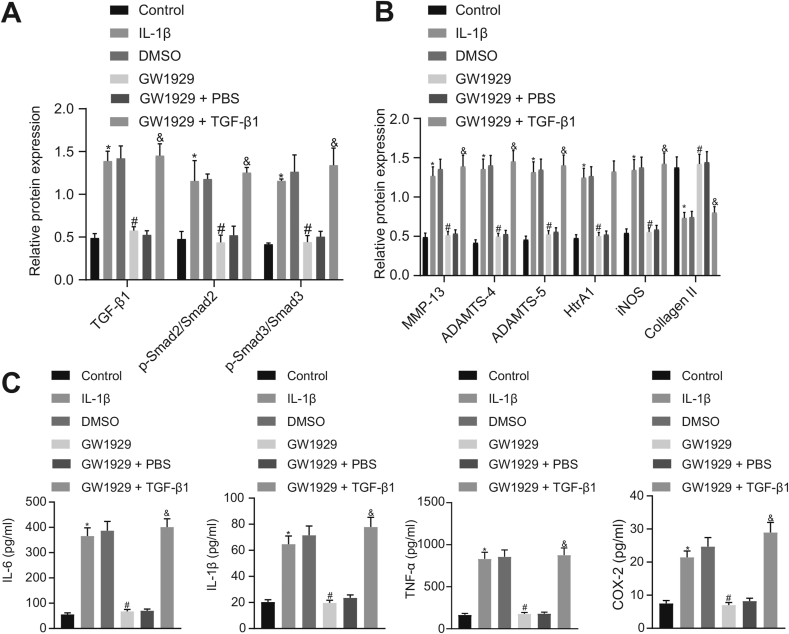
The results of the Western blot analysis showed that expression of TGF-β1, MMP-13, ADAMTS-4, ADAMTS-5, HtrA1, and iNOS along with the extent of Smad2 and Smad3 phosphorylation was significantly decreased, whereas expression of Collagen II was markedly increased in the OA chondrocytes in the presence of the PPARγ agonist GW1929, which was subsequently abrogated by TGF-β1 treatment (Fig. 4A and B). Furthermore, the ELISA results established that the levels of IL-6, IL-1β, TNF-α, and COX-2 were significantly reduced in OA chondrocytes treated with GW1929, which was also found to be reversed by TGF-β1 treatment (Fig. 4C). Based on these findings, we conclude PPARγ could repress expression of OA-related genes in chondrocytes via the inactivation of the TGF-β1/Smad2/3 signaling pathway.
Fig. 4.
PPARγ inactivates the TGF-β1/Smad2/3 signaling pathway and suppresses expression of OA-related genes in chondrocytes. Normal chondrocytes were used as controls and OA chondrocytes were treated with or without DMSO, GW1929, GW1929 + PBS or GW1929 + TGF-β1. A, Expression of TGF-β1, Smad2, and Smad3, along with the extent of Smad2 and Smad3 phosphorylation in chondrocytes evaluated by Western blot analysis normalized to GAPDH. B, Expression of MMP-13, ADAMTS-4, ADAMTS-5, HtrA1, iNOS, and Collagen II in chondrocytes evaluated by Western blot analysis normalized to GAPDH. C, The levels of IL-6, IL-1β, TNF-α, and COX-2 in chondrocytes detected by ELISA. The experiment was repeated three times. ∗p < 0.05 vs. control chondrocytes; #p < 0.05 vs. OA chondrocytes treated with DMSO; & p < 0.05 vs. OA chondrocytes treated with GW1929 + PBS.
3.4. Losartan downregulated expression of OA-related genes via PPARγ-mediated TGF-β1/smad2/3 signaling pathway inactivation in IL-1β-treated chondrocytes
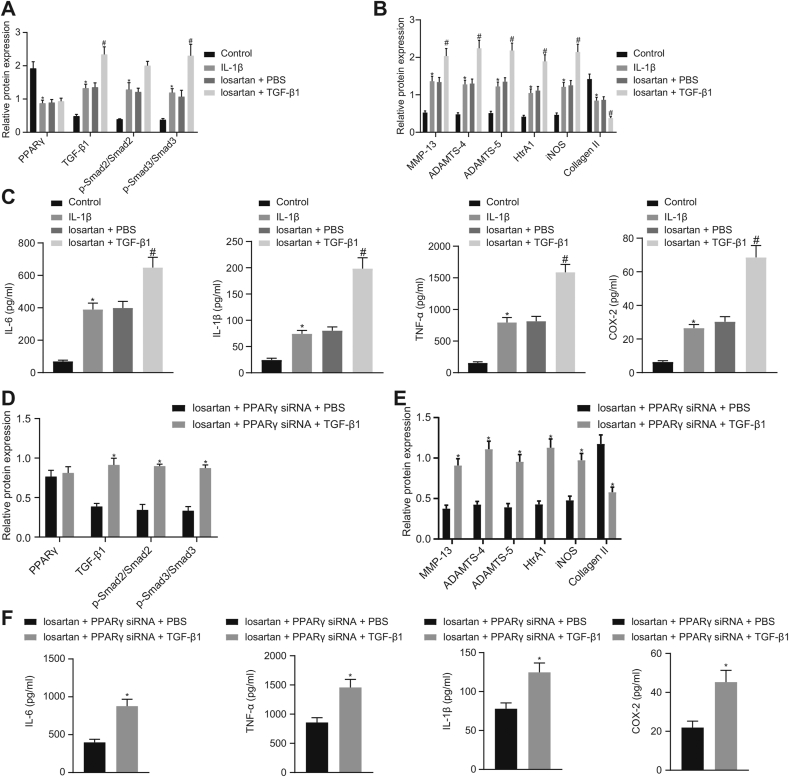
Concomitant treatment with losartan and TGF-β1 did not affect PPARγ expression, but resulted in much higher expression of TGF-β1, MMP-13, ADAMTS-4, ADAMTS-5, HtrA1, iNOS, greater extent of Smad2 and Smad3 phosphorylation, as well as lower expression of Collagen II in OA chondrocytes in comparison to losartan treatment alone (Fig. 5A and B). Additionally, the ELISA results demonstrated that the TGF-β1 treatment resulted in elevated levels of IL-6, IL-1β, TNF-α, and COX-2 in OA chondrocytes in the presence of losartan (Fig. 5C).
Fig. 5.
Losartan diminishes expression of OA-related genes through PPARγ-mediated TGF-β1/Smad2/3 signaling pathway inactivation in chondrocytes. Normal chondrocytes were used as controls, and OA chondrocytes were treated with or without losartan + PBS or losartan + TGF-β1. A, Expression of PPARγ, TGF-β1, Smad2, and Smad3, along with the extent of Smad2 and Smad3 phosphorylation in chondrocytes evaluated by Western blot analysis normalized to GAPDH. B, Expression of MMP-13, ADAMTS-4, ADAMTS-5, HtrA1, iNOS, and Collagen II in chondrocytes evaluated by Western blot analysis normalized to GAPDH. C, The levels of IL-6, IL-1β, TNF-α, and COX-2 in chondrocytes evaluated by ELISA. OA chondrocytes were treated with losartan + PPARγ siRNA + PBS or losartan + PPARγ siRNA + TGF-β1. D, Expression of PPARγ, TGF-β1, Smad2, and Smad3, along with the extent of Smad2 and Smad3 phosphorylation in chondrocytes evaluated by Western blot analysis normalized to GAPDH. E, Expression of MMP-13, ADAMTS-4, ADAMTS-5, HtrA1, iNOS, and Collagen II in chondrocytes evaluated by Western blot analysis normalized to GAPDH. F, The levels of IL-6, IL-1β, TNF-α, and COX-2 in chondrocytes evaluated by ELISA. The experiment was repeated three times. ∗p < 0.05 vs. control chondrocytes or OA chondrocytes treated with losartan + PPARγ siRNA + PBS; #p < 0.05 vs. OA chondrocytes treated with losartan + PBS.
Furthermore, the results of the Western blot analysis demonstrated no significant difference in expression of PPARγ, but an increase was established in expression of TGF-β1, MMP-13, ADAMTS-4, ADAMTS-5, HtrA1, and iNOS, along with greater extent of Smad2 and Smad3 phosphorylation as well as a decrease in the expression of Collagen II in OA chondrocytes after treatment with losartan + PPARγ siRNA + TGF-β1 as compared to the treatment with losartan + PPARγ siRNA + PBS (Fig. 5D and E). Additionally, the ELISA results suggested that treatment with losartan + PPARγ siRNA significantly increased the levels of IL-6, IL-1β, TNF-α, and COX-2 in OA chondrocytes after TGF-β1 treatment (Fig. 5F). Taken together, these results suggest that suggested that losartan could potentially elicit the PPARγ-mediated inactivation of the TGF-β1/Smad2/3 signaling pathway and thus decrease expression of OA-related genes in IL-1β-treated chondrocytes.
3.5. The best intervention method and concentration of losartan in the treatment of OA mice
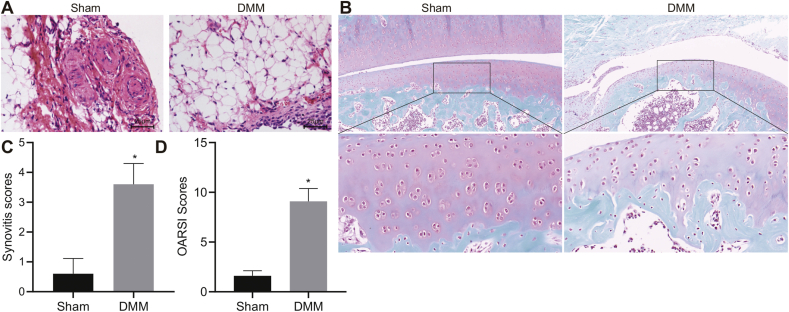
Analysis with HE staining of knee joint synovial tissues depicted normal synovium and an orderly arrangement of cells in the synovium and subintima in sham-operated mice. In contrast, DMM mice showed massive inflammatory cells infiltration into synovial tissues, fibrous tissue proliferation and synovium cell proliferation and derangement (Fig. 6A). Additionally, the Safranin-O-fast green staining revealed that, in comparison to the sham-operated mice, the DMM mice showed severe cartilage damage (Fig. 6B). Meanwhile, synovitis scores and OARSI scores were found to be higher in DMM mice in comparison to the sham-operated mice (Fig. 6C and D). The aforementioned results confirmed the successful establishment of OA mouse models.
Fig. 6.
Morphological characteristics of cartilage tissues of DMM-induced OA mice. A, Histopathological changes on the synovium of mouse knee joint observed by HE staining after OA model establishment (400 × ). B, Degree of cartilage damage of mouse knee joint assessed by safranin-O-fast green staining after OA model establishment (400 × ). C, Synovitis scores of mice after OA model establishment. D, OARSI scores of mice after OA model establishment. OA mice were orally administered with normal saline, 0.1, 1, 10, or 100 mg/kg losartan, or intraarticularly injected with normal saline, 0.1, 1, 10 or 100 mg/mL losartan. ∗p < 0.05 vs. sham-operated mice.
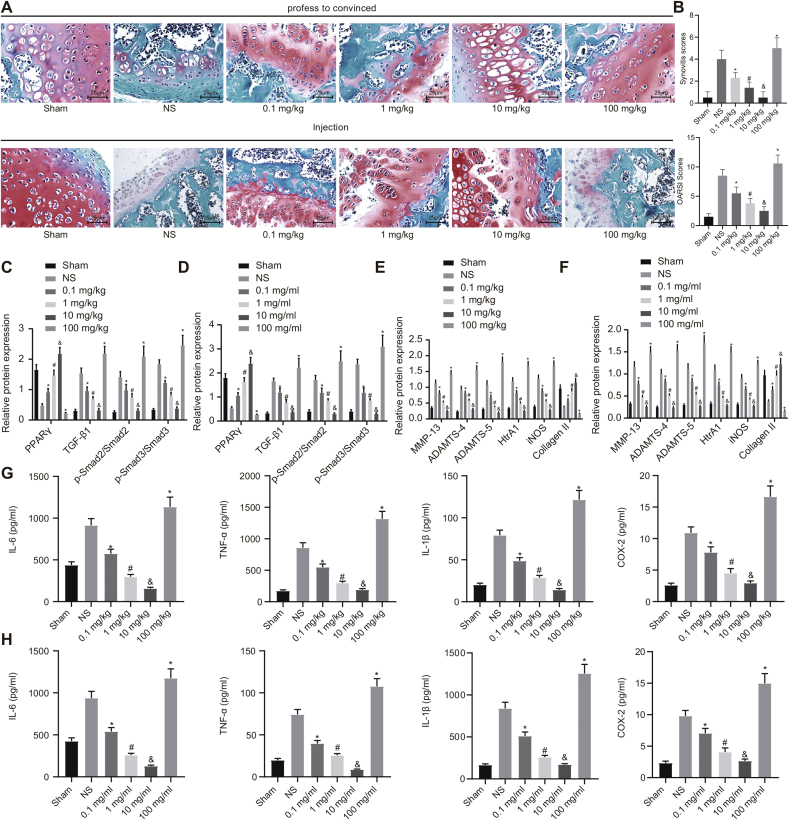
In order to explore the best method of administration and the concentration of losartan for the treatment of OA mice, the OA mice were orally administered (mg/kg) or intraarticularly injected (mg/mL) with varying concentrations of losartan. Safranin-O-fast green staining analysis indicated that losartan with low, medium, and high doses (0.1, 1, or 10 mg/kg) effectively promoted cartilage regeneration in OA mice, amongst which treatment with high dose losartan (10 mg/kg) showed the best therapeutic effect, while losartan with extremely high concentration (100 mg/kg) aggravated the cartilage destruction in OA mice. Furthermore, the therapeutic effect of intraarticular injection of losartan in the knee joint was significantly better in comparison to the oral treatment in OA mice (Supplementary Figs. 1A and B). Additionally, the results of Western blot analysis indicated that losartan of low, medium, and high doses (0.1, 1 or 10 mg/kg) resulted in lower expression of TGF-β1, MMP-13, ADAMTS-4, ADAMTS-5, HtrA1, and iNOS, along with the lesser extent of Smad2 and Smad3 phosphorylation but enhanced expression of Collagen II and PPARγ in cartilage tissues of OA mice. Moreover, when OA mice were treated with a high dose of losartan (10 mg/kg), expression of the above genes reached their highest or lowest levels. However, treatment with extremely high dose of losartan (100 mg/kg) resulted in the opposite effects in contrast to treatment with a high dose (10 mg/kg) (Supplementary Figs. 1C–F). Furthermore, the results of the ELISA revealed that the levels of IL-6, IL-1β, TNF-α, and COX-2 were diminished in the cartilage tissues of the OA mice subsequent to the treatment with low, medium, and high doses (0.1, 1 or 10 mg/kg) of losartan, amongst which the high concentration of losartan (10 mg/kg) exhibited the lowest levels of the related inflammatory factors. However, treatment with extremely high dose of losartan (100 mg/kg) resulted in enhanced levels of IL-6, IL-1β, TNF-α, and COX-2 in cartilage tissues of OA mice (Supplementary Figs. 1G and H). Taken together, intraarticular injection of losartan into the knee joint was the best mode of administration and the optimal treatment concentration was 10 mg/mL.
3.6. Losartan repressed OA in DMM mice via PPARγ-dependent inactivation of the TGF-β1/smad2/3 signaling pathway
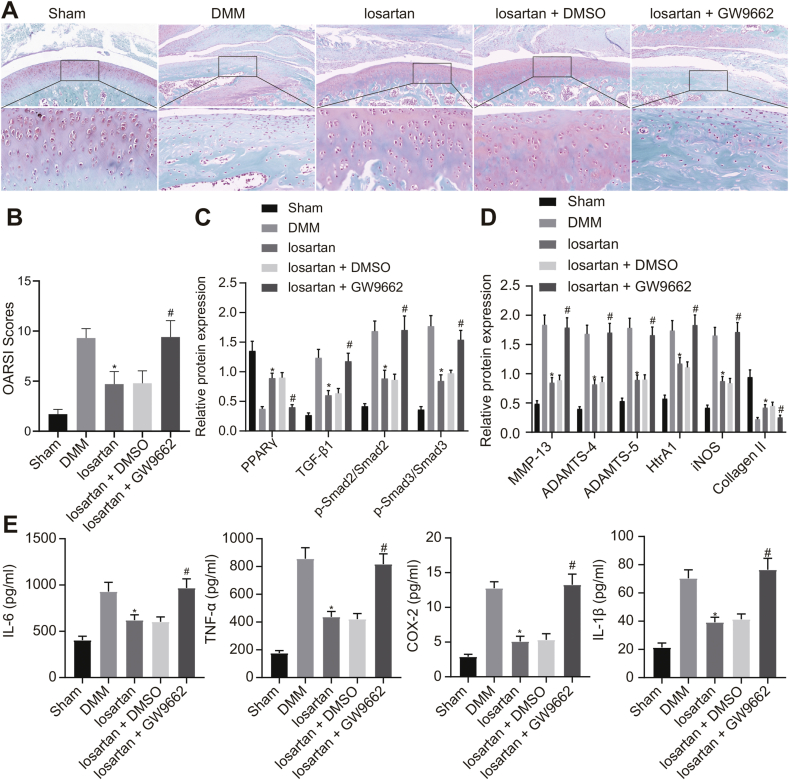
The results of the safranin-O-fast green assay revealed that the treatment with losartan resulted in decreased cartilage damage of the knee joint in DMM mice, which was neutralized by GW9662 treatment (Fig. 7A and B). Additionally, the Western blot analysis demonstrated that expression of TGF-β1, MMP-13, ADAMTS-4, ADAMTS-5, HtrA1, and iNOS along with the extent of Smad2 and Smad3 phosphorylation were significantly diminished, whereas expression of Collagen II and PPARγ was notably elevated in response to losartan treatment in DMM mice. This effect was reversed by additional treatment with GW9662, while the PPARγ expression remained unchanged (Fig. 7C and D).
Fig. 7.
Losartan alleviates OA in DMM mice via PPARγ-mediated inactivation of the TGF-β1/Smad2/3 signaling pathway. Sham-operated mice were used as controls, and DMM mice were treated with or without losartan, losartan + DMSO or losartan + GW9662. A, Degree of cartilage damage of knee joint in mice assessed by safranin-O-fast green staining (400 × ). B, OARSI scores of knee joint cartilage in mice. C, Expression of PPARγ, TGF-β1, Smad2, Smad3, along with the extent of Smad2 and Smad3 phosphorylation in cartilage tissues of OA mice evaluated by Western blot analysis normalized to GAPDH. D, Expression of MMP-13, ADAMTS-4, ADAMTS-5, HtrA1, iNOS, and Collagen II in cartilage tissues of mice evaluated by Western blot analysis normalized to GAPDH. E, The levels of IL-6, IL-1β, TNF-α, and COX-2 in cartilage tissues of mice evaluated by ELISA. ∗p < 0.05 vs. DMM mice; #p < 0.05 vs. DMM mice treated with losartan + DMSO.
Furthermore, based on the results of ELISA, the levels of IL-6, IL-1β, TNF-α, and COX-2 were found to be significantly reduced in the cartilage tissues of DMM mice subsequent to the treatment with losartan, which was counteracted by the additional treatment with GW9662 (Fig. 7E). Conclusively, losartan could upregulate the PPARγ expression to suppress the TGF-β1/Smad2/3 signaling pathway, therefore attenuating OA in DMM mice.
4. Discussion
OA is well recognized to be closely associated with inflammatory factors produced by the synovium and chondrocytes [21,22]. Joint replacement is an effective treatment of symptomatic end-stage OA although the long-term postoperative results of this surgery have been correlated with poor functional outcomes and limited lifespan of prostheses [23]. Therefore, it is urgent to explore more effective and safer alternative treatment approaches for OA. A prior study has reported that the type II angiotensin receptor antagonist losartan has protective properties against articular cartilage degeneration caused by OA in mice [19]. However, the molecular mechanism of losartan's effects on the progression of OA remains elusive. Therefore, the current study aimed to identify the specific molecular mechanism of losartan underlying the delay in OA progression via PPARγ mediation. Consequently, this dissertation clarified that the upregulation of PPARγ was involved in the alleviatory effects of OA by inactivating the TGF-β1 signaling pathway.
The results from the present study demonstrated that expression of IL-6, IL-1β, TNF-α, COX-2, MMP-13, ADAMTS-4, ADAMTS-5, HtrA1, and iNOS was strikingly reduced, while expression of Collagen II was remarkably increased in OA chondrocytes after losartan treatment. Losartan, as an antagonist of the Angiotensin II receptor, has been widely used in the treatment of hypertension [24]. Additionally, inflammatory cytokines like IL-1β and TNF-α have also been recognized to promote inflammatory mediator production and MMPs expression to modulate the pathogenesis of OA [25]. Meanwhile, COX2 is also a regulator of inflammation [26]. Furthermore, MMPs and the ADAMTS family of proteins have been documented to be closely correlated with cartilage degeneration in OA [27]. Moreover, a prior study has revealed a reduction of Collagen II expression in conjunction with cellular aging-induced OA [28]. A study conducted by Chen et al. showed that articular cartilage degeneration in OA mice was repressed by losartan treatment [19]. Additionally, another study has unraveled the ability of losartan to repress inflammation and expression of TNF-a and IL-6 in arthritically injured forepaws [29]. More importantly, losartan has also been found to alleviate OA progression in the temporomandibular synovial joint by suppressing the degeneration of articular cartilage of condylar [10], which suggested the potential protective effects of losartan against osteoarthritic-related injury. Therefore, in the current study we tested that prediction that treatment with losartan could alleviate OA progression.
In the subsequent experiments, we found PPARγ upregulation and TGF-β1 signaling pathway inactivation in cartilage tissues, chondrocytes and OA mice with losartan treatment. Meanwhile, losartan treatment resulted in repressed expression of inflammation- and degeneration-related genes in OA mice and chondrocytes by inactivating the TGF-β1 signaling pathway via PPARγ upregulation. These results are in agreement with a prior study where losartan protected against liver ischemia/reperfusion injury by activating PPARγ [30]. Besides, Yamamoto et al. also found that losartan could repress inflammation in renal injury by activating PPARγ [12]. PPARγ is an essential component of normal endochondral osteogenesis, as well as cartilage growth and development [31]. Accumulating evidence has uncovered that activation of PPARγ could impair IL-1β-induced inflammation in human OA chondrocytes [32,33]. Furthermore, a prior study exhibited that losartan inactivated the TGF-β1 signaling pathway to impair OA progression in the synovial temporomandibular and knee joints of a chondrodysplasia mouse model [10]. The PPARγ agonist, 15-deoxy-Δ-12, 14-prostaglandin J2, has also been reported to inhibit TGF-β1 expression, thereby decreasing synovial fibrosis in OA fibroblasts [34]. Moreover, another previous study has elucidated that TGF-β1 contributes to the activation of the Smad2/3 signaling pathway in OA [35]. More importantly, the inactivation of the TGF-β1/Smad signaling pathway has been shown to attenuate cartilage injury and OA in rats [18].
5. Conclusion
Taken together, results of the current study illustrate that PPARγ and the TGF-β1 signaling pathway were critically involved in response to OA after treatment with losartan. Specifically, losartan contributed to the repression of TGF-β1 signaling pathway by upregulating PPARγ, thus alleviating OA by decreasing the expression IL-6, IL-1β, TNF-α, COX-2, MMP-13, ADAMTS-4, ADAMTS-5, HtrA1, and iNOS, as well as increasing Collagen II expression. This discovery broadens our understanding of the complex mechanism underlying losartan/PPARγ/TGF-β1 signaling in the progression of OA and provides a potential new therapeutic target for the treatment of OA, which may eventually be translatable into the clinical setting.
Contributions
(I) Conception and design: Zhenhan Deng, Weimin Zhu (II) Administrative support: Wei Lu (III); Provision of study materials: Wei Jiang, Weimin Zhu (IV); Collection and assembly of data: Zhenhan Deng, Fei Chen (V) Data analysis and interpretation: Zhenhan Deng, Yuwei Liu, Jinping Wang; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors.
Funding
This study was supported by the National Natural Science Foundation of China (81902303, 81902233, 81902558, 81672234), Guangdong Basic and Applied Basic Research Foundation (2020A151501048), China Postdoctoral Science Foundation (2019M653080), Shenzhen Double Chain Project for Innovation and Development Industry supported by Bureau of Industry and Information Technology of Shenzhen (201806081524201510), Shenzhen Science and Technology Project (RCBS20200714114856299, JCYJ20190806164216661, GJHZ20180416164801042, JCYJ20180305124912336) and the Clinical Research Project of Shenzhen Second People's Hospital (20203357028, 20203357007, 20173357201814).
Declaration of competing interest
The authors have no conflicts of interest to declare.
Acknowledgments
We would like to acknowledge Pro. Shanshan Gao from University of Colorado Anschutz Medical Campus for helping in study design. We would like to give our sincere appreciation to the reviewers for their helpful comments on this article.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jot.2021.03.005.
Contributor Information
Zhenhan Deng, Email: dengzhenhan@email.szu.edu.cn.
Fei Chen, Email: fchen@sibs.ac.cn.
Yuwei Liu, Email: lyw699@csu.edu.cn.
Jinping Wang, Email: 787079848@qq.com.
Wei Lu, Email: weilu9309@gmail.com.
Wei Jiang, Email: docjw@sina.com.
Weimin Zhu, Email: szhzwm@email.szu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Supplementary Figure 1.
The best intervention method and concentration of losartan in the treatment of OA mice. A, Degree of cartilage damage of knee joint after treatment with different concentrations of losartan assessed by safranin-O-fast green staining (400 × ). B, OARSI scores of mice after treatment with different concentrations of losartan. C and D, Expression of PPARγ, TGF-β1, Smad2, and Smad3, along with the extent of Smad2 and Smad3 phosphorylation in OA mice after treatment with different concentrations of losartan evaluated by Western blot analysis normalized to GAPDH. E and F, Expression of MMP-13, ADAMTS-4, ADAMTS-5, HtrA1, iNOS, and Collagen II in mice after treatment with different concentrations of losartan evaluated by Western blot analysis normalized to GAPDH. G and H, The levels of IL-6, IL-1β, TNF-α, and COX-2 in mice after treatment with different concentrations of losartan evaluated by ELISA. ∗p < 0.05 vs. OA mice treated with normal saline; #p < 0.05 vs. OA mice treated with 0.1 mg/mL or 0.1 mg/kg losartan; & p < 0.05 vs. OA mice treated with 1 mg/mL or 1 mg/kg losartan.
References
- 1.Li Y., Xiao W., Wu P., Deng Z., Zeng C., Li H. The expression of SIRT1 in articular cartilage of patients with knee osteoarthritis and its correlation with disease severity. J Orthop Surg Res. 2016;11:144. doi: 10.1186/s13018-016-0477-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tu M., Li Y., Zeng C., Deng Z., Gao S., Xiao W. MicroRNA-127-5p regulates osteopontin expression and osteopontin-mediated proliferation of human chondrocytes. Sci Rep. 2016;6:25032. doi: 10.1038/srep25032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bijlsma J.W., Berenbaum F., Lafeber F.P. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377:2115–2126. doi: 10.1016/S0140-6736(11)60243-2. [DOI] [PubMed] [Google Scholar]
- 4.Loeser R.F., Goldring S.R., Scanzello C.R., Goldring M.B. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y., Jordan J.M. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26:355–369. doi: 10.1016/j.cger.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y., Xiao W., Luo W., Zeng C., Deng Z., Ren W. Alterations of amino acid metabolism in osteoarthritis: its implications for nutrition and health. Amino Acids. 2016;48:907–914. doi: 10.1007/s00726-015-2168-x. [DOI] [PubMed] [Google Scholar]
- 7.Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!) Osteoarthritis Cartilage. 2013;21:16–21. doi: 10.1016/j.joca.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Li Y.S., Luo W., Zhu S.A., Lei G.H. T cells in osteoarthritis: alterations and beyond. Front Immunol. 2017;8:356. doi: 10.3389/fimmu.2017.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yin W., Lei Y. Leonurine inhibits IL-1beta induced inflammation in murine chondrocytes and ameliorates murine osteoarthritis. Int Immunopharm. 2018;65:50–59. doi: 10.1016/j.intimp.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 10.Thomas M., Fronk Z., Gross A., Willmore D., Arango A., Higham C. Losartan attenuates progression of osteoarthritis in the synovial temporomandibular and knee joints of a chondrodysplasia mouse model through inhibition of TGF-beta1 signaling pathway. Osteoarthritis Cartilage. 2019;27:676–686. doi: 10.1016/j.joca.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 11.Utsunomiya H., Gao X., Deng Z., Cheng H., Nakama G., Scibetta A.C. Biologically regulated marrow stimulation by blocking TGF-beta1 with losartan oral administration results in hyaline-like cartilage repair: a rabbit osteochondral defect model. Am J Sports Med. 2020 doi: 10.1177/0363546519898681363546519898681. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto S., Zhong J., Yancey P.G., Zuo Y., Linton M.F., Fazio S. Atherosclerosis following renal injury is ameliorated by pioglitazone and losartan via macrophage phenotype. Atherosclerosis. 2015;242:56–64. doi: 10.1016/j.atherosclerosis.2015.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nobs S.P., Kopf M. PPAR-gamma in innate and adaptive lung immunity. J Leukoc Biol. 2018;104:737–741. doi: 10.1002/JLB.3MR0118-034R. [DOI] [PubMed] [Google Scholar]
- 14.Aboonabi A., Meyer R.R., Singh I., Aboonabi A. Temporary removal: anthocyanins reduce inflammation and improve glucose and lipid metabolism associated with inhibiting nuclear factor-kappaB activation and increasing PPAR-gamma gene expression in metabolic syndrome subjects. Free Radic Biol Med. 2020;150:30–39. doi: 10.1016/j.freeradbiomed.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Chang C.C., Hsieh M.S., Liao S.T., Chen Y.H., Cheng C.W., Huang P.T. Hyaluronan regulates PPARgamma and inflammatory responses in IL-1beta-stimulated human chondrosarcoma cells, a model for osteoarthritis. Carbohydr Polym. 2012;90:1168–1175. doi: 10.1016/j.carbpol.2012.06.071. [DOI] [PubMed] [Google Scholar]
- 16.Liu L., Pan Y., Zhai C., Zhu Y., Ke R., Shi W. Activation of peroxisome proliferation-activated receptor-gamma inhibits transforming growth factor-beta1-induced airway smooth muscle cell proliferation by suppressing Smad-miR-21 signaling. J Cell Physiol. 2018;234:669–681. doi: 10.1002/jcp.26839. [DOI] [PubMed] [Google Scholar]
- 17.Yang Y., Zhang N., Lan F., Van Crombruggen K., Fang L., Hu G. Transforming growth factor-beta 1 pathways in inflammatory airway diseases. Allergy. 2014;69:699–707. doi: 10.1111/all.12403. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y.J., Shen M., Wang S., Wen X., Han X.R., Zhang Z.F. Inhibition of the TGF-beta1/Smad signaling pathway protects against cartilage injury and osteoarthritis in a rat model. Life Sci. 2017;189:106–113. doi: 10.1016/j.lfs.2022.120531. [DOI] [PubMed] [Google Scholar]
- 19.Chen R., Mian M., Fu M., Zhao J.Y., Yang L., Li Y. Attenuation of the progression of articular cartilage degeneration by inhibition of TGF-beta1 signaling in a mouse model of osteoarthritis. Am J Pathol. 2015;185:2875–2885. doi: 10.1016/j.ajpath.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng Z., Gao X., Sun X., Amra S., Lu A., Cui Y. Characterization of articular cartilage homeostasis and the mechanism of superior cartilage regeneration of MRL/MpJ mice. Faseb J. 2019;33:8809–8821. doi: 10.1096/fj.201802132RR. [DOI] [PubMed] [Google Scholar]
- 21.Goldring M.B., Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011;23:471–478. doi: 10.1097/BOR.0b013e328349c2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeon H., Im G.I. Autophagy in osteoarthritis. Connect Tissue Res. 2017;58:497–508. doi: 10.1080/03008207.2016.1240790. [DOI] [PubMed] [Google Scholar]
- 23.Glyn-Jones S., Palmer A.J., Agricola R., Price A.J., Vincent T.L., Weinans H. Osteoarthritis. Lancet. 2015;386:376–387. doi: 10.1016/S0140-6736(14)60802-3. [DOI] [PubMed] [Google Scholar]
- 24.Li C., Bo L., Li P., Lu X., Li W., Pan L. Losartan, a selective antagonist of AT1 receptor, attenuates seawater inhalation induced lung injury via modulating JAK2/STATs and apoptosis in rat. Pulm Pharmacol Therapeut. 2017;45:69–79. doi: 10.1016/j.pupt.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Chen X., Zhang C., Wang X., Huo S. Juglanin inhibits IL-1beta-induced inflammation in human chondrocytes. Artif Cells Nanomed Biotechnol. 2019;47:3614–3620. doi: 10.1080/21691401.2019.1657877. [DOI] [PubMed] [Google Scholar]
- 26.Neri M., Cantatore S., Pomara C., Riezzo I., Bello S., Turillazzi E. Immunohistochemical expression of proinflammatory cytokines IL-1beta, IL-6, TNF-alpha and involvement of COX-2, quantitatively confirmed by Western blot analysis, in Wernicke's encephalopathy. Pathol Res Pract. 2011;207:652–658. doi: 10.1016/j.prp.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Zheng W., Tao Z., Cai L., Chen C., Zhang C., Wang Q. Chrysin attenuates IL-1beta-induced expression of inflammatory mediators by suppressing NF-kappaB in human osteoarthritis chondrocytes. Inflammation. 2017;40:1143–1154. doi: 10.1007/s10753-017-0558-9. [DOI] [PubMed] [Google Scholar]
- 28.Li Y.S., Xiao W.F., Luo W. Cellular aging towards osteoarthritis. Mech Ageing Dev. 2017;162:80–84. doi: 10.1016/j.mad.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 29.Nystrom A., Thriene K., Mittapalli V., Kern J.S., Kiritsi D., Dengjel J. Losartan ameliorates dystrophic epidermolysis bullosa and uncovers new disease mechanisms. EMBO Mol Med. 2015;7:1211–1228. doi: 10.15252/emmm.201505061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koh E.J., Yoon S.J., Lee S.M. Losartan protects liver against ischaemia/reperfusion injury through PPAR-gamma activation and receptor for advanced glycation end-products down-regulation. Br J Pharmacol. 2013;169:1404–1416. doi: 10.1111/bph.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monemdjou R., Vasheghani F., Fahmi H., Perez G., Blati M., Taniguchi N. Association of cartilage-specific deletion of peroxisome proliferator-activated receptor gamma with abnormal endochondral ossification and impaired cartilage growth and development in a murine model. Arthritis Rheum. 2012;64:1551–1561. doi: 10.1002/art.33490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qu Y., Zhou L., Wang C. Mangiferin inhibits IL-1beta-induced inflammatory response by activating PPAR-gamma in human osteoarthritis chondrocytes. Inflammation. 2017;40:52–57. doi: 10.1007/s10753-016-0451-y. [DOI] [PubMed] [Google Scholar]
- 33.Jingbo W., Aimin C., Qi W., Xin L., Huaining L. Betulinic acid inhibits IL-1beta-induced inflammation by activating PPAR-gamma in human osteoarthritis chondrocytes. Int Immunopharm. 2015;29:687–692. doi: 10.1016/j.intimp.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 34.Vaamonde-Garcia C., Malaise O., Charlier E., Deroyer C., Neuville S., Gillet P. 15-Deoxy-Delta-12, 14-prostaglandin J2 acts cooperatively with prednisolone to reduce TGF-beta-induced pro-fibrotic pathways in human osteoarthritis fibroblasts. Biochem Pharmacol. 2019;165:66–78. doi: 10.1016/j.bcp.2019.03.039. [DOI] [PubMed] [Google Scholar]
- 35.Zhu D., Zhao J., Lou A., Huang Q., OuYang Q., Zhu J. Transforming growth factor beta1 promotes fibroblast-like synoviocytes migration and invasion via TGF-beta1/Smad signaling in rheumatoid arthritis. Mol Cell Biochem. 2019;459:141–150. doi: 10.1007/s11010-019-03557-0. [DOI] [PubMed] [Google Scholar]