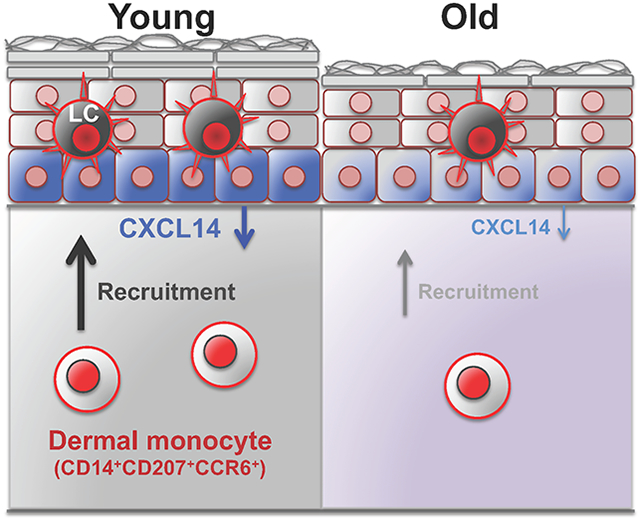
Abstract
Skin provides the first line of physical and immunological defense against the environmental insults. However, the age-related changes in the immune function of the human skin are unclear. Here, we investigated the age-related changes in epidermal Langerhans cells (LCs), which play a sentinel role in the initiation of the immune responses in the skin. We found a significant reduction in the number of epidermal LCs in the sun-protected skin with age. Among the possible explanations for this reduction, we found that the number of CD14+ CD207+ CCR6+ dermal-resident monocytes that can differentiate into epidermal LCs were markedly reduced with age (p = 0.0057). Among the chemokines that can recruit these cells into the skin, the expression of CXCL14 was significantly down-regulated in the epidermal keratinocytes with age. Importantly, we discovered that young skin recruited significantly more monocytic THP-1 cells compared to old skin ex vivo. This recruitment was blocked by CXCL14 neutralizing antibody and was conversely promoted by CXCL14 treatment. Collectively, our findings indicate that decreased CXCL14-mediated recruitment of CD14+ monocytes in the human skin results in the reduction of epidermal LCs with age, and CXCL14 may provide a novel therapeutic target for the prevention of age-related LC reduction.
Keywords: Langerhans cell, CD14+ monocyte, CXCL14, Aging, Human skin
Graphical Abstract
INTRODUCTION
Aging is accompanied by a decline in the function of the immune system, known as “immunosenescence”, leading to increased susceptibility to bacterial and viral infections, and increased risk of cancer (Goronzy and Weyand, 2013). The regulation of the immune system is most crucial in the homeostasis of the barrier organs like lung, gastrointestinal track and skin, which are constantly under environmental insults (Eyerich et al., 2018). The proper function of the immune system is paramount to the maintenance of the barrier organ’s homeostatic state and a defective immune response at barrier sites can cause severe local and systemic diseases (Dimitrov et al., 2013, Huber-Lang et al., 2018). Therefore, it is important to determine the age-related alterations in the immune responses that predispose to pathology at barrier sites.
Skin is the largest organ of the body and acts as the primary interface between the body and the environment, providing a first line of defense against microbial pathogens and physical and chemical insults (Eyerich et al., 2018). Skin immune system encompasses a complex network of immune cells and factors including antigen presenting cells (APCs), T cells and innate lymphoid cells (Heath and Carbone, 2013, Kim, 2015, Nestle et al., 2009). The immune barrier is compromised in the aging skin leading to high susceptibility to pathogens like Staphylococcus aureus skin infection in elderly (Suaya et al., 2014). However, the age-related changes in the immune components of the human skin that account for this immunocompromised state are largely unknown. It is important to determine the age-related immune changes in the skin in order to block the adverse effects of aging on our immune barrier.
Langerhans cell (LC) is a skin-resident APC, which plays a sentinel role in maintaining the skin immune barrier as the first immune cell confronting the environmental insults in the epidermis (Deckers et al., 2018). Activated epidermal LCs capture foreign antigens by extending their dendrites through epidermal tight junctions and initiate a preemptive humoral immunity against potentially pathogenic skin microbes, such as Staphylococcus aureus, before such pathogens can invade through the epidermal barrier (Kubo et al., 2009, Ouchi et al., 2011). Similar to tissue resident macrophages, LCs arise from embryonic precursors and are maintained within the epidermis by local self-renewal under steady-state conditions (Doebel et al., 2017). However, in contrast to other tissue resident macrophages, LCs display dendritic cell (DC)-like functions and constitutively migrate to peripheral lymph nodes to prime naïve T cells to initiate adaptive immune responses (Deckers et al., 2018). The sentinel role of LC in maintaining skin health highlight the importance of investigating the impact of aging on LC biology in human skin (Bhushan et al., 2002, Bhushan et al., 2004, Gilchrest et al., 1982, Thiers et al., 1984).
To determine the mechanism of age-related changes in LCs in human skin, we identified cohorts of sun-protected skin from young and old women. We found a significant reduction in epidermal LCs in the sun-protected skin with age. Among the mechanisms that could explain this reduction, we found a significant reduction in CD14+ CD207+ CCR6+ dermal-resident monocytes that can differentiate into epidermal LCs and their recruiting chemokine, CXCL14, with age. Importantly, blockade of CXCL14 with a neutralizing antibody inhibited CD14+ THP-1 cell recruitment into the young skin, and conversely, addition of CXCL14 promoted CD14+ THP-1 cell recruitment into the skin. We conclude that the age-related LC reduction is mediated by an age-dependent decline in CXCL14-mediated recruitment of CD14+ monocytes in human skin.
RESULTS
Overview of study cohorts.
In order to determine the intrinsic age-related changes in epidermal LCs in the human skin, we screened over 400 human skin samples from various ages and anatomical sites and identified cohorts of sun-protected breast skin from young (n = 20, average age: 22.8) and old (n = 21, average age: 63.3) women (p < 0.0001, Table 1). To confirm the biological evidence of aging in the skin samples, we measured the epidermal thickness, which is known to be reduced with age (Lavker et al., 1987). As expected, epidermal thickness was significantly reduced in our old compared with young skin samples (p = 0.0001, Table 1). Skin adnexal composition can have a significant impact on the immune environment of the skin as it is known that T cells tend to cluster around hair follicles and other adnexal structures in the skin (Paus et al., 1999). In order to exclude this confounding factor and establish an optimal age-specific comparison, we picked only skin from the same anatomical site (i.e., breast) and confirmed that there was no difference in the hair follicle density between the young and old skin samples (Table 1). Therefore, the selected cohorts of young and old skin samples were suitable for the determination of the intrinsic effects of aging on human epidermal LCs independent of the effects of ultraviolet (UV) radiation.
Table 1. Skin samples characteristics.
The epidermal thickness and the number of hair follicle unit are determined from hematoxylin and eosin (H&E) stained tissue sections.
| Young skin (n = 20) |
Old skin (n = 21) |
p value | |
|---|---|---|---|
| Age, years | |||
| Mean (SD) | 22.8 (3.8) | 63.3 (6.6) | < 0.0001 |
| Range | 16-28 | 53-74 | |
| Gender, number (%) | - | ||
| Male | 0 (0) | 0 (0) | |
| Female | 20 (100) | 21 (100) | |
| Epidermal thickness, mean (SD), μm | 41.8 (6.2) | 31.9 (2.5) | 0.0001 |
| No. of hair follicle units per mm skin, mean (SD) | 0.19 (0.17) | 0.16 (0.17) | 0.560 |
Epidermal LCs are significantly reduced with age.
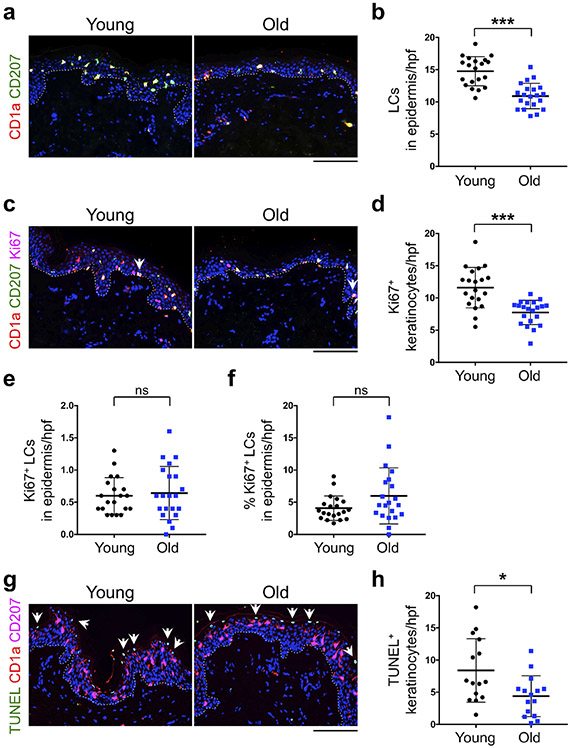
To determine whether the number of LCs is changed with age, we stained the human skin tissues with CD1a and CD207 (Langerin), which are human LC surface markers (Figure 1a). CD1a and CD207 staining marked the same population of cells in the epidermis (i.e., LCs). The number of CD1a+ CD207+ LCs in the epidermis was significantly reduced with age (p < 0.0001, Figure 1b). The majority of LCs in the human skin are distributed horizontally in the spinous layer of the epidermis in a plane parallel to the epidermal surface (Hashemi et al., 2012, Seite et al., 2003). Quantification of epidermal CD1a+ CD207+ LCs distributed horizontally in a plane parallel to the epidermal surface per 1 mm epidermal length also showed a significant reduction in epidermal LCs with age (Supplementary Figure S1). We utilized de-identified normal human skin tissues for our studies. Therefore, racial or ethnic information for our cohorts were not available. Nonetheless, there were no significant differences in the number of epidermal LCs in heavily pigmented skin compared with other skin samples in young or old cohorts (Supplementary Figure S2). In contrast to the reduction in epidermal LCs, the number of CD1a+ CD207+ dermal migrating LCs was not changed with age (Supplementary Figure S3). Reduced number of epidermal LCs in old skin under steady-state condition can be explained by either a decrease in LC’s self-renewal capacity, an increased LC apoptosis, and/or a reduced supply of LC precursors. To address this question, we investigated the three possible mechanisms of LC reduction by age.
Figure 1. Epidermal LCs are reduced with age.
(a) Representative CD1a (red) and CD207 (green) immunofluorescence (IF) stained young and old human skin samples. (b) The quantitation of epidermal CD1a+ CD207+ LCs in the epidermis per high power field (hpf) image. (c) Representative IF staining of CD1a (red), CD207 (green), and Ki67 (magenta, marker of cell proliferation) in young and old skin samples. The arrows point to Ki67+ LCs in the epidermis. (d) The quantitation of Ki67+ keratinocytes in the epidermis per hpf image. (e, f) The number (e) and percentage (f) of epidermal CD1a+ CD207+ Ki67+ cells indicative of proliferative LCs per hpf image. (g) TUNEL staining of human skin samples. Young and old skin tissues are stained with CD1a (red), CD207 (magenta) and TUNEL signal (green) to mark the apoptotic cells. The arrows point to TUNEL+ keratinocytes in the epidermis. Note the absence of TUNEL signal in CD1a+ CD207+ epidermal LCs. (h) The quantitation of TUNEL+ keratinocyte per hpf image (n = 15 per group). Nuclei are stained with DAPI (blue), dotted lines mark the basement membrane separating the epidermis from dermis, cells are counted blindly and averaged across 10 randomly selected hpf images per skin sample, all data are expressed as the mean ± SD, n = 20 in young group and n = 21 in old group, * p < 0.05, and *** p < 0.0001, ns: not significant, scale bars: 100 μm.
Epidermal LC’s self-renewal capacity is not altered with age.
Epidermal LCs in steady-state condition can self-renew to maintain their frequency (Doebel et al., 2017). To determine whether LC self-renewal capacity was reduced with age, we compared the proliferation capacity of LCs between young and old skin samples. Ki67 staining was used as a proliferation marker to measure the self-renewal ability of LCs in the skin (Figure 1c). Although the number of Ki67+ keratinocytes was significantly reduced with age (Figure 1d), we did not observe any significant change in the number or the percentage of CD1a+ CD207+ Ki67+ epidermal LCs between young and old skin samples (Figure 1e and f). Furthermore, we stained a randomly selected subset of human skin samples with proliferating cell nuclear antigen (PCNA) to further look for alterations in LC self-renewal capacity (Supplementary Figure S4a). Consistent with Ki67 staining results, PCNA+ keratinocytes were also significantly reduced with age (Supplementary Figure S4b), however, we did not observe a significant change in the number or the percentage of PCNA+ epidermal LCs between young and old skin samples (Supplementary Figure S4c and d). Therefore, our findings indicate that epidermal LC’s self-renewal capacity is not altered with age in the human skin.
LC apoptosis is not detected in the human skin.
To determine whether increased LC apoptosis was the reason for reduced epidermal LC counts with age, we evaluated the number of apoptotic LCs in the skin using TUNEL assay. We could not detect any TUNEL positive CD1a+ CD207+ LCs in the skin (Figure 1g). However, we found reduction in TUNEL positive keratinocyte with age (Figure 1h). This is in agreement with a previous report showing that cleaved caspase 3+ or TUNEL+ LCs are not found in normal human epidermis (Kolgen et al., 2002). Further, LCs resist apoptosis in response to genotoxic stress, such as ionizing irradiation (IR), through prompt DNA damage repair machinery via cyclin-dependent kinase inhibitor CDKN1A (p21) (Price et al., 2015). These data indicate that LC apoptosis cannot account for reduced epidermal LC numbers with age.
Dermal CD14+ CD207+ CCR6+ cells are significantly reduced with age
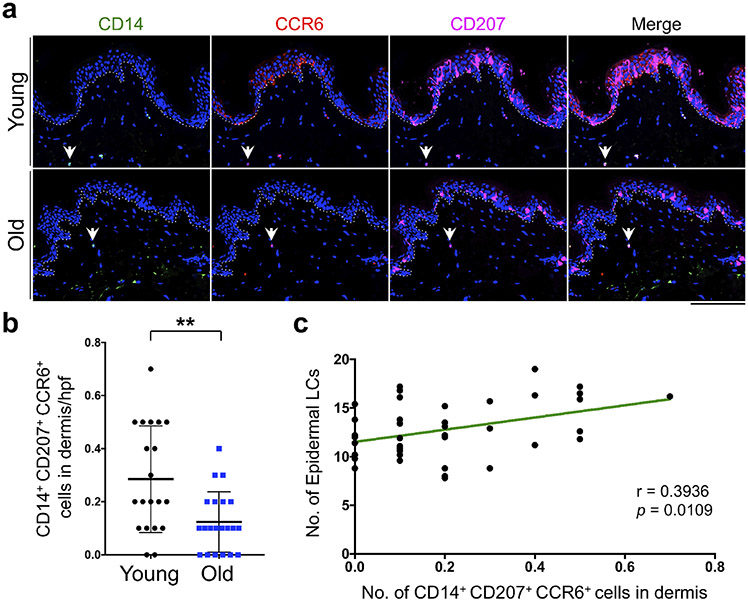
To investigate whether a reduction in LC precursor supplies in the skin could explain the loss of epidermal LCs with age, we quantified the number of LC precursors in young and old skin. Dermal-resident cells that can give rise to epidermal LCs express CD14, CD207, and CCR6 under steady-state condition in human skin (Larregina et al., 2001). Therefore, we stained the young and old skin samples with CD14, CD207, and CCR6 antibodies (Figure 2a). Importantly, the number of CD14+ CD207+ CCR6+ cells was significantly reduced in the dermis of old compared with young skin (p = 0.0057, Figure 2b). Furthermore, we found a significant positive correlation between the number of dermal CD14+ CD207+ CCR6+ cells and epidermal LC counts (r = 0.3936, p = 0.0109, Figure 2c). These findings indicate that the age-related reduction in epidermal LCs may be due to the reduced supply of CD14+ monocytes from the dermis.
Figure 2. Dermal CD14+ CD207+ CCR6+ cells are reduced with age.
(a) Representative IF staining of CD14 (green), CD207 (magenta), and CCR6 (red) in young and old human skin samples. The arrows point to a CD14+ CD207+ CCR6+ cell in dermis. (b) The quantitation of dermal CD14+ CD207+ CCR6+ cells per hpf image. (c) Correlation between the number of dermal CD14+ CD207+ CCR6+ cells and epidermal LC numbers across young and old skin samples. n = 20 in young group and n = 21 in old group, nuclei are stained with DAPI (blue), dotted lines mark the basement membrane separating the epidermis from dermis, cells are counted blindly and averaged across 10 randomly selected hpf images per skin sample, all data are expressed as the mean ± SD, ** p < 0.01, scale bar: 100 μm.
CXCL14 is down-regulated in epidermal keratinocytes with age.
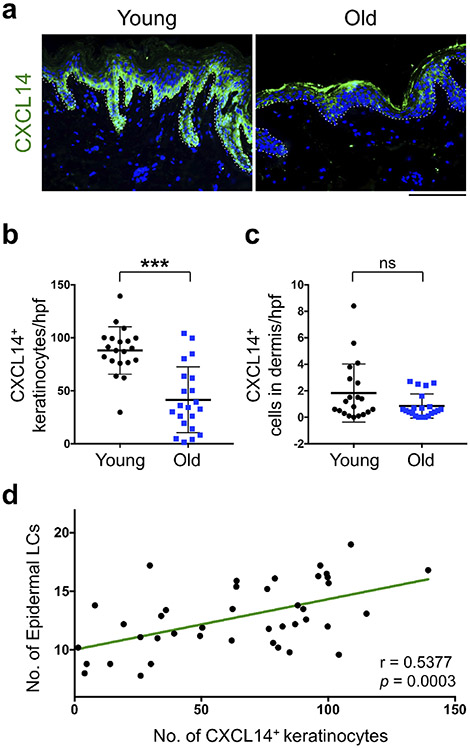
To understand why dermal LC precursors were reduced with age, we focused on chemokines, CCL2, CCL20 and CXCL14, and cytokine, interleukin (IL)-34, which have been implicated in LC biology (Greter et al., 2012, Nagao et al., 2012, Schaerli et al., 2005). Among them, CXCL14 was mainly expressed by basal layer keratinocytes in the epidermis (Figure 3a). In contrast, we did not detect a measurable CCL2 or CCL20 in the skin samples (Supplementary Figure S5). Although IL-34 was expressed in the human skin epidermis, there was no significant difference in IL-34 levels between young and old skin samples (Supplementary Figure S6). Interestingly, CXCL14 expression level was markedly down-regulated in basal keratinocytes of the old compared with young skin (Figure 3a). Quantitative analysis revealed that the number of CXCL14+ keratinocytes was significantly decreased in old versus young epidermis (Figure 3b). We did not detect any change in the rare CXCL14+ cells found in the dermis with age (Figure 3c). Furthermore, we found a significant positive correlation between the number of CXCL14+ keratinocytes and epidermal LCs (r = 0.5377, p = 0.0003, Figure 3d). Therefore, age-related reduction of dermal LC precursors may be due to a reduction of CXCL14 expression in keratinocytes of the old skin. Notably, epidermal thickness had no impact on CXCL14 expression, the number of epidermal LCs or dermal CD14+ monocytes independent of age (Supplementary Figure S7).
Figure 3. CXCL14 is down-regulated in the epidermal keratinocytes of the old skin.
(a) Representative IF staining of CXCL14 (green) in young and old human skin tissues. Nuclei are stained with DAPI (blue) and dotted lines highlight the basement membrane (scale bar: 100 μm). (b) The quantitation of CXCL14+ keratinocytes per hpf image. (c) The quantitation of dermal CXCL14+ cells per hpf. (d) Correlation between the number of CXCL14+ keratinocytes and the number epidermal LCs across young and old skin samples. n = 20 in young group and n = 21 in old group, cells are counted blindly and averaged across 10 randomly selected hpf images per skin sample, all data are expressed as the mean ± SD, *** p < 0.0001, ns: not significant.
Young skin recruits THP-1 cells in a CXCL14 dependent manner.
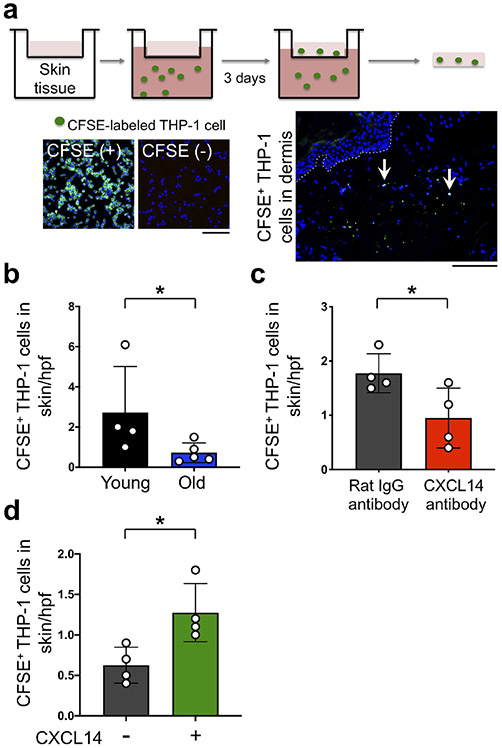
To test whether young skin has a higher capacity for recruitment of LC precursor-like monocytes compared with old skin, and CXCL14 contributes to this recruitment, we performed an ex vivo migration assay of CD14+ THP-1 monocytes into human skin tissue (Figure 4a and Supplementary Figure S8). The number of carboxyfluorescein succinimidyl ester (CFSE)-labeled THP-1 cells that migrated into young skin was significantly higher than old skin (p = 0.0317, Figure 4b). The addition of CXCL14 neutralization antibody (50 μg/mL) into the culture media, which was used to block secreted CXCL14 chemokine, inhibited this recruitment (Figure 4c). In contrast, subepidermal injection of recombinant human CXCL14 (5 μg/mL) into human old skin tissues promoted THP-1 cell recruitment into the skin (Figure 4d). These findings indicate that young skin recruits more monocytic THP-1 cells than old skin, and CXCL14 is both required and sufficient in mediating this recruitment.
Figure 4. Young skin recruits more THP-1 cells compared to old skin ex vivo, which is inhibited by CXCL14 blockade and induced by CXCL14 chemokine addition.
(a) The experimental scheme for ex vivo THP-1 migration into human skin. Nuclei are stained with DAPI (blue). The arrows point to CFSE+ THP-1 cells that have migrated into the dermis. Dotted line marks the basement membrane, scale bars: 100 μm. (b) The quantitation of CFSE+ THP-1 cells that have migrated into the skin per hpf image (n = 4 young and 5 old skin samples). (c) The effect of CXCL14 neutralizing antibody (50 μg/mL) treatment on the recruitment of CFSE+ THP-1 cells into young skin samples (n = 4 per group). Note that CXCL14 neutralizing antibody was added to the THP-1 media in the lower chamber of the transwell assay system. (d) The effect of CXCL14 recombinant protein (5 μg/mL) treatment on the recruitment of CFSE+ THP-1 cells into old skin samples (n = 4 per group). Note that recombinant human CXCL14 or PBS (carrier control) was injected subepidermally into the skin in the top well of the transwell assay system. Cells are counted blindly and averaged across 10 randomly selected hpf images per skin sample, all data are expressed as the mean ± SD, * p < 0.05.
DISCUSSION
In this report, we identify a mechanism for the age-related reduction of epidermal LCs in the human skin. CD14+ CD207+ CCR6+ dermal-resident monocytes that can be a source for epidermal LCs are markedly reduced with age. Concurrently, the CD14+ monocyte-recruiting chemokine, CXCL14, is down-regulated in the old skin keratinocytes. Furthermore, young skin recruits more CD14+ THP-1 monocytes compared to old skin ex vivo. This recruitment is promoted by CXCL14 addition and inhibited by CXCL14 blockade. These findings suggest boosting CXCL14 expression in the epidermal keratinocytes can have a beneficial effect on restoring the epidermal LC population in the aging skin.
Epidermal LCs have self-renewal capacity and can arise from CD14+ dermal cells to maintain their constant frequency under the steady-state condition (Doebel et al., 2017). We investigated whether aging has any effect on the epidermal LC population in a sun-protected skin. We chose the sun-protected breast skin from women for our studies to exclude the impact of UV radiation, anatomical site variation and gender differences on LC population. We observed that the number of epidermal LCs is reduced with age. Consistent with our observation, previous reports have shown the age-related reduction in human epidermal LCs (Bhushan et al., 2002, Bhushan et al., 2004, Gilchrest et al., 1982, Thiers et al., 1984). However, the mechanism behind LC reduction by age remained unknown. Among the possible explanation of this reduction, LC self-renewal capacity is not altered, and apoptotic LC is not detected with age. Adult murine epidermal LCs have a slow turnover, which is maintained by their self-renewal capacity under steady-state condition (Ghigo et al., 2013). However, murine LC self-renewal capacity is not changed with age (Xu et al., 2012). Based on our findings in the human skin, we conclude that alteration in LC self-renewal capacity is an unlikely explanation for the age-related reduction in epidermal LCs in the human skin. Therefore, we pursued the following possibilities: (a) a decrease in the influx of LC precursors into skin, or (b) an increase in efflux of LCs out of the epidermis.
Antigen-loaded LCs migrate to the draining lymph nodes in order to present antigen to T cells and initiate immune responses (Deckers et al., 2018). Although we could not assess the LCs in the subjects’ lymph nodes, we did not observe any significant difference in the number of dermal LCs, suggesting no change in migrating LCs between young and old skin under steady-state condition. Considering that the capacity of LCs to migrate in response to tumor necrosis factor (TNF)-α is decreased with age (Bhushan et al., 2002), we conclude that LC migration out of epidermis is an unlikely explanation for the age-related reduction in epidermal LCs.
We find that dermal-resident CD14+ CD207+ CCR6+ cells are reduced with age, suggesting that a decrease in supply of new LCs from the dermis results in the age-related reduction in epidermal LCs. Monocyte-like LC precursors in mice can be recruited into the epidermis in response to stress but this gives rise to short-lived LCs (Kaplan, 2017). A recent publication has revealed that monocyte-like LC precursors can also differentiate into mature long-lasting epidermal LCs for rebuilding the mature LC network after immune injury in mice (Ferrer et al., 2019). Therefore, not only self-renewal of LCs but also CD14+ CD207+ CCR6+ cells supply may contribute to the maintenance of long-term LC frequency in the human epidermis.
To substantiate this finding, we investigated the candidate factors that are implicated in the recruitment of CD14+ dermal-resident monocytes. Trafficking and tissue localization of the immune cells are controlled by chemokines, which are composed of over 50 members (Zlotnik and Yoshie, 2012). Chemokines are divided into two functional groups: inflammatory chemokines are induced locally in response to inflammatory stimuli, where they recruit immune cells to the site of infection or injury (Zlotnik and Yoshie, 2012). In contrast, homeostatic chemokines are constitutively expressed in normal tissues, where they control the homeostatic trafficking and maintenance of immune cells during peripheral tissue immune surveillance (McCully et al., 2018). Among of homeostatic chemokines, CXCL14 (also known as breast and kidney-expressed chemokine (BRAK)) is an ELR-negative CXC chemokine with unknown receptor selectivity (Maerki et al., 2009). CXCL14 is constitutively expressed in epithelial tissues such as epidermis (Maerki et al., 2009). Further, in contrast to inflammatory chemokines, CXCL14 expression is down-regulated in the skin inflammatory diseases, such as atopic dermatitis and psoriasis (Maerki et al., 2009). CXCL14 contributes to maintain epidermal LC through chemoattractant of dermal CD14+ dermal cells under steady-state condition (Schaerli et al., 2005). In addition, it has an unconventional function as a strong broad-spectrum antimicrobial agent against cutaneous Gram-positive and negative bacteria (Maerki et al., 2009). Thus, CXCL14 fulfills a critical role in the front-line immune barrier of the skin through homeostatic replenishment of epidermal LCs and fighting against pathogenic microbes.
In the present study, we find that CXCL14 expression is down-regulated in human skin epidermis with age. In contrast to CXCL14, other LC precursor-recruiting chemokine CCL2 and CCL20, which are expressed under inflammatory condition (Nagao et al., 2012), are undetectable in the normal human skin under steady-state condition. IL-34 is an essential cytokine for the replenishment of epidermal LCs after resolution of inflammation (Greter et al., 2012). Although it is constitutively expressed in the human skin epidermis, there is no significant difference in IL-34 expression level between young and old skin. Our ex vivo migration assays demonstrate that CXCL14 is required for the migration of monocytic THP-1 cells into the young skin and its addition is sufficient to promote this migration. Although immortalized THP-1 cells are not primary monocytes, these CD14+ cells have been extensively used as a model to investigate human monocyte structure and function in both health and disease (Chanput et al., 2014). Therefore, age-related down-regulation of CXCL14 in keratinocytes provides an explanation for the impaired CD14+ dermal monocytes recruitment into the skin and a possible mechanism for reduced epidermal LCs with age.
CXCL14 can be down-regulated in human keratinocytes in response to inflammatory cytokines such as IL-1β and TNF-α (Schaerli et al., 2005). These cytokines may represent insults-induced factors that accumulate in the skin with aging. We speculate that the cumulative insults throughout life result in the age-related impairment of CXCL14-mediated recruitment of CD14+ monocytes into the skin. Human blood monocytes are capable of acquiring an LC-like phenotype in culture assays (Geissmann et al., 1998, Hoshino et al., 2005, Otsuka M. et al., 2018, Otsuka Y. et al., 2018). However, the precise contribution of CD14+ monocytes to the maintenance of epidermal LCs in the human skin and its direct recruitment from the circulation require future investigations. In order to further elucidate the importance of epidermal CXCL14-dermal LC precursor axis in the skin and the precise route of CXCL14-mediated recruitment of CD14+ monocytes, the identification of CXCL14 receptor is required, which will also provide insights into the broader function of CXCL14 in maintaining the skin homoeostasis.
In conclusion, our findings contribute to a better understanding of the mechanism of age-related immune dysfunction in the human skin and provide a potential therapeutic target for the prevention of age-related reduction in epidermal LCs. Skin is a major route for vaccination, and our mechanistic findings on epithelial-immune crosstalk may facilitate vaccination and percutaneous modulation of the systemic immunity and thus enhance the competence of the immune system in elderly population.
MATERIALS AND METHODS
Human skin tissue study
Study of de-identified normal adult human skin samples was reviewed and approved by Massachusetts General Hospital IRB. Patient consent for experiments was not required because US laws consider human tissue left over from surgery as discarded material.
Immunofluorescence (IF) staining of human breast skin tissues
Human skin samples were fixed by 4% PFA, and embedded in paraffin. 5 μm sections were cut and deparaffinized. After permeabilized with 0.2% Triton-X in phosphate buffered saline (PBS), sections were boiled in Vector antigen unmasking solutions (Vector Labs) for 20 min. Sections were blocked with 5% normal goat serum and 5% bovine serum albumin in PBS. Then, sections were incubated overnight at 4°C with primary antibodies against CCR6 (1:100, R&D, MAB195), CD1a (1:50, Dako, M3571), CD14 (1:500, abcam, ab133335), CD207 (1:100, Novus Biologicals, DDX0362P-100), CXCL14 (1:50, abcam, ab36622), and Ki67 (1:500, abcam, ab15580), and corresponding second antibodies with labeled with Alexa Fluor 488, 568, and 638. Nuclear counterstaining was done with DAPI. The stained tissues were imaged with a ZEISS confocal microscope. Cell counts are reported as the average number of cells across 10 randomly selected high power fields (hpf, 200x magnification) images per skin sample in each group.
TUNEL assay
TUNEL signals in formalin-fixed paraffin-embedded human skin tissue sections were detected with Click-iT Plus TUNEL Assay for in Situ Apoptosis Detection, Alexa Fluor™ 488 dye (Thermo Fisher Scientific), according to the manufacturer’s protocol. The sections were co-stained with CD1a and CD207. Nuclear counterstaining was done with DAPI. The sections were imaged with a ZEISS confocal microscope.
THP-1 migration assay
THP-1 cells (ATCC) were seeded at a density of 3–5 × 104 cells/cm2 into 75 cm2 cell culture flasks and cultured in RPMI 1640 medium (Gibco) including 10% fetal bovine serum (FBS), 1% penicillin/streptomycin, and 1% L-glutamine at 37 °C Human breast skin tissues were punch-biopsied at 8 mm diameter. Subcutaneous fat was removed, and then the biopsied skin was placed in the top well of the 24 well transwell plate (Corning). To monitor THP-1 cell migration, THP-1 cells were labeled with Carboxyfluorescein succinimidyl ester (CFSE, abcam). Briefly, THP-1 cells were incubated in 10 μM CFSE solution for 15 min at 37 °C Then, CFSE-labeled THP-1 cells were seeded at a density of 1.5 x 105 cells/well into the bottom well, and cultured in RPMI 1640 medium without FBS. After 3 days, the skin tissue in the top well was collected and embedded in Tissue-Tek™ OCT compound (Sakura Finetek). Frozen OCT tissue sections were stained with DAPI, and CFSE+ THP-1 cells in dermis were counted. In CXCL14 antibody neutralization assay, 50 μg/mL CXCL14 neutralizing antibody (R&D systems, AF866 (Lu et al., 2015)) or Rat IgG antibody (R&D systems, 6-001-A) was added to the lower chamber of the transwell assay. In CXCL14 induction test, biopsied skin samples were injected subepidermally with 60 μL recombinant human CXCL14 (5 μg/mL, R&D systems, 866-CX) or PBS (carrier only) immediately before placing them in the top well of the transwell assay plates containing CFSE+ THP-1 cells in the bottom chamber.
Statistical analysis
Column scatter graphs show mean ± SD. Two-tailed Mann-Whitney U test was used as the test of significance for cell counts and protein expression levels. Student's t test for the Pearson correlation coefficient was used as the test of significance for the linear regression in the scatter plots. p value < 0.05 was considered significant.
Supplementary Material
ACKNOWLEDGMENTS
Shadmehr Demehri, M.D., Ph.D., holds a Career Award for Medical Scientists award from the Burroughs Wellcome Fund. TH and JH were supported by Shiseido Co. Ltd. ZF was supported by the Department of Obstetrics and Gynecology, Peking University First Hospital, Beijing, China. TH, ZF, ZY, KN and SD were supported by grants from the Burroughs Wellcome Fund, Sidney Kimmel Foundation, Cancer Research Institute CLIP Award and NIH (K08AR068619 and DP5OD021353).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.
REFERENCES
- Bhushan M, Cumberbatch M, Dearman RJ, Andrew SM, Kimber I, Griffiths CE. Tumour necrosis factor-alpha-induced migration of human Langerhans cells: the influence of ageing. Br J Dermatol 2002;146(1):32–40. [DOI] [PubMed] [Google Scholar]
- Bhushan M, Cumberbatch M, Dearman RJ, Kimber I, Griffiths CE. Exogenous interleukin-1beta restores impaired Langerhans cell migration in aged skin. Br J Dermatol 2004;150(6):1217–8. [DOI] [PubMed] [Google Scholar]
- Chanput W, Mes JJ, Wichers HJ. THP-1 cell line: an in vitro cell model for immune modulation approach. Int Immunopharmacol 2014;23(1):37–45. [DOI] [PubMed] [Google Scholar]
- Deckers J, Hammad H, Hoste E. Langerhans Cells: Sensing the Environment in Health and Disease. Front Immunol 2018;9:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov JD, Planchais C, Roumenina LT, Vassilev TL, Kaveri SV, Lacroix-Desmazes S. Antibody polyreactivity in health and disease: statu variabilis. J Immunol 2013;191(3):993–9. [DOI] [PubMed] [Google Scholar]
- Doebel T, Voisin B, Nagao K. Langerhans Cells - The Macrophage in Dendritic Cell Clothing. Trends Immunol 2017;38(11):817–28. [DOI] [PubMed] [Google Scholar]
- Eyerich S, Eyerich K, Traidl-Hoffmann C, Biedermann T. Cutaneous Barriers and Skin Immunity: Differentiating A Connected Network. Trends Immunol 2018;39(4):315–27. [DOI] [PubMed] [Google Scholar]
- Ferrer IR, West HC, Henderson S, Ushakov DS, Santos ESP, Strid J, et al. A wave of monocytes is recruited to replenish the long-term Langerhans cell network after immune injury. Sci Immunol 2019;4(38). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissmann F, Prost C, Monnet JP, Dy M, Brousse N, Hermine O. Transforming growth factor beta1, in the presence of granulocyte/macrophage colony-stimulating factor and interleukin 4, induces differentiation of human peripheral blood monocytes into dendritic Langerhans cells. J Exp Med 1998;187(6):961–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghigo C, Mondor I, Jorquera A, Nowak J, Wienert S, Zahner SP, et al. Multicolor fate mapping of Langerhans cell homeostasis. J Exp Med 2013;210(9):1657–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrest BA, Murphy GF, Soter NA. Effect of chronologic aging and ultraviolet irradiation on Langerhans cells in human epidermis. The Journal of investigative dermatology 1982;79(2):85–8. [DOI] [PubMed] [Google Scholar]
- Goronzy JJ, Weyand CM. Understanding immunosenescence to improve responses to vaccines. Nat Immunol 2013;14(5):428–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greter M, Lelios I, Pelczar P, Hoeffel G, Price J, Leboeuf M, et al. Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia. Immunity 2012;37(6):1050–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi P, Pulitzer MP, Scope A, Kovalyshyn I, Halpern AC, Marghoob AA. Langerhans cells and melanocytes share similar morphologic features under in vivo reflectance confocal microscopy: a challenge for melanoma diagnosis. Journal of the American Academy of Dermatology 2012;66(3):452–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath WR, Carbone FR. The skin-resident and migratory immune system in steady state and memory: innate lymphocytes, dendritic cells and T cells. Nat Immunol 2013; 14(10):978–85. [DOI] [PubMed] [Google Scholar]
- Hoshino N, Katayama N, Shibasaki T, Ohishi K, Nishioka J, Masuya M, et al. A novel role for Notch ligand Delta-1 as a regulator of human Langerhans cell development from blood monocytes. J Leukoc Biol 2005;78(4):921–9. [DOI] [PubMed] [Google Scholar]
- Huber-Lang M, Lambris JD, Ward PA. Innate immune responses to trauma. Nat Immunol 2018;19(4):327–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DH. Ontogeny and function of murine epidermal Langerhans cells. Nat Immunol 2017;18(10):1068–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BS. Innate lymphoid cells in the skin. J Invest Dermatol 2015;135(3):673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolgen W, Both H, van Weelden H, Guikers KL, Bruijnzeel-Koomen CA, Knol EF, et al. Epidermal langerhans cell depletion after artificial ultraviolet B irradiation of human skin in vivo: apoptosis versus migration. J Invest Dermatol 2002;118(5):812–7. [DOI] [PubMed] [Google Scholar]
- Kubo A, Nagao K, Yokouchi M, Sasaki H, Amagai M. External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J Exp Med 2009;206(13):2937–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larregina AT, Morelli AE, Spencer LA, Logar AJ, Watkins SC, Thomson AW, et al. Dermal-resident CD14+ cells differentiate into Langerhans cells. Nat Immunol 2001;2(12): 1151–8. [DOI] [PubMed] [Google Scholar]
- Lavker RM, Zheng PS, Dong G. Aged skin: a study by light, transmission electron, and scanning electron microscopy. J Invest Dermatol 1987;88(3 Suppl):44s–51s. [DOI] [PubMed] [Google Scholar]
- Lu J, Song G, Tang Q, Zou C, Han F, Zhao Z, et al. IRX1 hypomethylation promotes osteosarcoma metastasis via induction of CXCL14/NF-kappaB signaling. The Journal of clinical investigation 2015;125(5):1839–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maerki C, Meuter S, Liebi M, Muhlemann K, Frederick MJ, Yawalkar N, et al. Potent and broad-spectrum antimicrobial activity of CXCL14 suggests an immediate role in skin infections. J Immunol 2009;182(1):507–14. [DOI] [PubMed] [Google Scholar]
- McCully ML, Kouzeli A, Moser B. Peripheral Tissue Chemokines: Homeostatic Control of Immune Surveillance T Cells. Trends Immunol 2018;39(9):734–47. [DOI] [PubMed] [Google Scholar]
- Nagao K, Kobayashi T, Moro K, Ohyama M, Adachi T, Kitashima DY, et al. Stress-induced production of chemokines by hair follicles regulates the trafficking of dendritic cells in skin. Nat Immunol 2012;13(8):744–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol 2009;9(10):679–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka M, Egawa G, Kabashima K. Uncovering the Mysteries of Langerhans Cells, Inflammatory Dendritic Epidermal Cells, and Monocyte-Derived Langerhans Cell-Like Cells in the Epidermis. Front Immunol 2018;9:1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka Y, Watanabe E, Shinya E, Okura S, Saeki H, Geijtenbeek TBH, et al. Differentiation of Langerhans Cells from Monocytes and Their Specific Function in Inducing IL-22-Specific Th Cells. J Immunol 2018;201(10):3006–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi T, Kubo A, Yokouchi M, Adachi T, Kobayashi T, Kitashima DY, et al. Langerhans cell antigen capture through tight junctions confers preemptive immunity in experimental staphylococcal scalded skin syndrome. J Exp Med 2011;208(13):2607–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus R, Christoph T, Muller-Rover S. Immunology of the hair follicle: a short journey into terra incognita. J Investig Dermatol Symp Proc 1999;4(3):226–34. [DOI] [PubMed] [Google Scholar]
- Price JG, Idoyaga J, Salmon H, Hogstad B, Bigarella CL, Ghaffari S, et al. CDKN1A regulates Langerhans cell survival and promotes Treg cell generation upon exposure to ionizing irradiation. Nat Immunol 2015;16(10):1060–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaerli P, Willimann K, Ebert LM, Walz A, Moser B. Cutaneous CXCL14 targets blood precursors to epidermal niches for Langerhans cell differentiation. Immunity 2005;23(3):331–42. [DOI] [PubMed] [Google Scholar]
- Seite S, Zucchi H, Moyal D, Tison S, Compan D, Christiaens F, et al. Alterations in human epidermal Langerhans cells by ultraviolet radiation: quantitative and morphological study. The British journal of dermatology 2003;148(2):291–9. [DOI] [PubMed] [Google Scholar]
- Suaya JA, Mera RM, Cassidy A, O'Hara P, Amrine-Madsen H, Burstin S, et al. Incidence and cost of hospitalizations associated with Staphylococcus aureus skin and soft tissue infections in the United States from 2001 through 2009. BMC Infect Dis 2014;14:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiers BH, Maize JC, Spicer SS, Cantor AB. The effect of aging and chronic sun exposure on human Langerhans cell populations. J Invest Dermatol 1984;82(3):223–6. [DOI] [PubMed] [Google Scholar]
- Xu YP, Qi RQ, Chen W, Shi Y, Cui ZZ, Gao XH, et al. Aging affects epidermal Langerhans cell development and function and alters their miRNA gene expression profile. Aging (Albany NY) 2012;4(11):742–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity 2012;36(5):705–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.