Abstract
Background
Tendons are the force transferring tissue that enable joint movement. Excessive mechanical loading is commonly considered as a primary factor causing tendinopathy, however, an increasing body of evidence supports the hypothesis that overloading creates microdamage of collagen fibers resulting in a localized decreased loading on the cell population within the damaged site. Heterotopic ossification is a complication of late stage tendinopathy, which can significantly affect the mechanical properties and homeostasis of the tendon. Here, we the examine the effect of mechanical underloading on tendon ossification and investigate its underlying molecular mechanism.
Method
Rabbit Achilles tendons were dissected and cultured in an underloading environment (3% cyclic tensile stain,0.25 Hz, 8 h/day) for either 10, 15 or 20 days. Using isolated tendon-derived stem cells (TDSCs) 3D constructs were generated, cultured and subjected to an underloading environment for 6 days. Histological assessments were performed to evaluate the structure of the 3D constructs; qPCR and immunohistochemistry were employed to study TDSC differentiation and the β-catenin signal pathway was investigated by Western blotting. Mechanical testing was used to determine ability of the tendon to withstand force generation.
Result
Tendons cultured for extended times in an environment of underloading showed progressive heterotopic ossification and a reduction in biomechanical strength. qPCR revealed that 3D TDSCs constructs cultured in an underloading environment exhibited increased expression of several osteogenic genes: these include RUNX2, ALP and osteocalcin in comparison to tenogenic differentiation markers (scleraxis and tenomodulin). Immunohistochemical analysis further confirmed high osteocalcin production in 3D TDSCs constructs subject to underloading. Western blotting of TDSC constructs revealed that β-catenin accumulation and translocation were associated with an increase in phosphorylation at Ser552 and decrease phosphorylation at Ser33.
Conclusion
These findings unveil a potential mechanism for heterotopic ossification in tendinopathy due to the underloading of TDSCs at the damage sites, and also that β-catenin could be a potential target for treating heterotopic ossification in tendons.
The Translational potential
Tendon heterotopic ossification detrimentally affect quality of life especially for those who has atheletic career. This study reveals the possible mechanism of heterotpic ossification in tendon related to mechanical loading. This study provided the possible to develop a mechanical stimulation protocol for preventive and therapeutic purpose for tendon heterotopic ossification.
Keywords: Bioreactor, Mechanical loading, Heterotopic ossification, Tendon-derived stem cell, Mechanobiology
1. Background
Tendinopathy is a common and significant health problem, whose prevalence is increasing in due to the rising numbers of sporting injuries and the burgeoning ageing population [1]. Tendon pathologies including Achilles tendinopathy and rotator cuff tears cause considerable pain, loss of function, joint failure and an increased risk for the development of secondary osteoarthritis, which poses a substantial social and economic burden. Heterotopic ossification (HO) often manifests in the later stages of tendinopathy, where it can be either asymptomatic or present as discomfort due to localized ‘inflammation’ [2]. Most importantly, HO in tendons significantly reduces their strength, which may lead to tendon rupture [3]. Treatments for calcific tendinopathy are very limited, although long-term physiotherapy is commonly used [3,4]. While increased loading can be beneficial for regeneration, it may lead to further damage of the degenerated tendon [5]. Treatment outcomes for tendinopathy are often unsatisfactory as the physiological processes associated with tendon calcification are not well understood.
Tendons are constantly transferring mechanical load from muscle to bone and overuse is considered the main cause of tendinopathy [6]. However, several studies now report that tendinopathy results from a combination of overuse and underuse mechanisms; with overloading in particular creating collagen fiber microdamage resulting in a decreased loading of the resident cell population [[7], [8], [9]].
Tendon healing includes three phases: inflammation, proliferation, and remodeling [10]. During the pathophysiological progression of tendinopathy, resident cells including tenocytes and tendon-derived stem cell (TDSCs) proliferate but fail to regenerate tendon tissue [11]. Mature tenocytes can fully differentiate from either their progenitor/stem cells (TDSCs) or alternatively from undifferentiated precursors including mesenchymal stem cells (MSCs). Apart from their capacity to form tendon-like tissue ex vivo [12], TDSCs have multi-lineage differentiation potential to develop into cartilage, bone and fat [13,14]. Thus, during the process of tendinopathy the conspicuous adipose tissue infiltration, glycosaminoglycan accumulation and HO accumulation within tendon tissue [15,16] is consistent with the hypothesis that TDSC are the cell type responsible for this aberrant tissue formation.
Although the molecular mechanism of HO during chronic tendinopathy remains unclear, recent evidence indicates that overactivation of the Wnt–β-catenin signaling pathways contributes to HO in multiple organ systems including tendon [17]. The Wnt–β-catenin signaling pathway regulates differentiation of MSCs in embryonic development as well as in adult tissue homeostasis [18]. In the absence of Wnt ligand binding, β-catenin is steadily phosphorylated at Ser33 by glycogen synthase kinase 3 (GSK3) in a degradation complex, that is subsequently removed via the proteasomal pathway [19]. In addition, Akt preferentially phosphorylates β-catenin at Ser552, inducing β-catenin translocation to the nucleus [20]. In the nucleus, β-catenin is known to interact with T-cell factor/lymphoid enhancing factor (TCF/LEF) and activate transcription of Wnt/β-catenin target genes. It is well understood that mechanical stimulation [22] activates Wnt/β-catenin signaling which is essential for bone formation [21]. However, in loading-induced tendon ossification little is known about the role of the Wnt/β-catenin signaling pathway.
In this study, rabbit Achilles tendon was cultured in a bioreactor and subjected to an underloading environment; we found multiple HO within this tendon tissue. We also generated 3D TDSC constructs and subjected these to an underloading environment, finding that mechanical underloading led to osteogenic differentiation and activation of the GSK3/β-catenin pathway.
2. Materials and methods
2.1. Tissue preparation and culture
Animal tissues were obtained immediately post-euthanasia as by-products of control group (non-experimental) animals conducted in the School of Anatomy, Physiology and Human Biology at the University of Western Australia.
Full-length Achilles tendons, including the enthesis and myo-tendinous junction were dissected from the hindlimbs of 15 weeks old (3–3.5 kg weight) specific pathogen free female New Zealand white rabbits (Oryctolagus cuniculus).
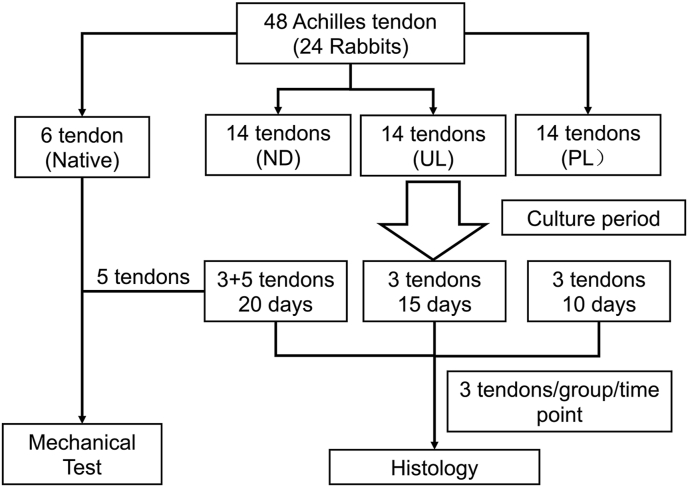
In total, 48 Achilles tendons from 24 rabbits were randomly allocated to 4 groups (see Fig. 1). In the native group, 6 Achilles tendons were dissected from the rabbits and immediately frozen at −80 °C prior to mechanical testing. In static/loading groups, 42 tendons were subjected to static or dynamic loading culture regimes as previously reported [8]. In brief, tendons were secured to the tissue hook in the culture chamber using surgical suture and grown in growth medium (DMEM/F-12, 10% fetal bovine serum and 50 μg/ml Gentamicin) in the bioreactor while being subjected to either static (0% strain) or dynamic loading (3% or 6% strain at 0.25 Hz for 8 h followed by 16 h rest) for 10 (n = 3/group), 15 (3/group) or 20 days (8/group).
Fig. 1.
Flowchart of experimental plan. The rabbit Achilles tendons were allocated in four groups which are native tendon group for mechanical test, and NL (no loading), UL (underloading) and PL (physiological loading) groups in bioreactor.
Following the static or loading regimes, 3 samples from each time (10, 15 & 20 days) & loading regime (0, 3 or 6% load; Fig. 1) resulted in a total of 27 samples being fixed in 4% paraformaldehyde (PFA) overnight for histological or immunohistochemical analysis. In addition, a total of 5 samples from each of the 0, 3 & 6% loading regimes (n = 15) that were subjected to 20 days of culture (Fig. 1) were stored at −80 °C for further mechanical testing.
2.1. Isolation and characterization of TDSCs
The use of mice for these experiments was approved by the University of Western Australia Animal Ethics Committee. A total of 48 6-8-week-old mice were used in this study. After euthanasia, the patellar tendons and Achilles tendons were excised. TDSCs isolation was performed as previously described [23]. Briefly, the tissues were digested with type I collagenase (3 mg/ml; Sigma) for 3 h and the suspension was passed through a 70 μm cell strainer to yield single-cell suspensions. The cells were cultured in complete medium (α-Modified Eagle Medium supplemented with 10% fetal bovine serum (FBS), 100U/ml penicillin, 100 μg/ml streptomycin). After 7–10 days in culture, the cells were detached by incubation at 37 °C in a solution of 0.1% trypsin, the digestion was terminated with complete medium, cell suspension subsequently washed with media and TDSC's reseeded into T75 flasks at low density (400 cells/cm2). The medium was changed every 3 days and TDSCs were sub-cultured when they reached 80% confluence. Passage 4 TDSCs were used for all our experiments.
2.1.1. Flow cytometry assay
Single-cell suspensions of TDSCs were prepared in PBS/1% FBS and incubated with the relevant fluorochrome-conjugated monoclonal antibody for 1hr at 4 °C, washed twice with PBS/1% FBS then resuspended in PBS/1% FBS/5 mM EDTA for analysis by flow cytometry. The antibodies used were against mouse CD90.2-FITC, CD34-FITC, CD44-FITC, CD45-PE (all from BD Biosciences). Data was collected using the FACS Canto II flow cytometer and FACSDiva software (BD Biosciences) and further analyzed using FlowJo software (Tree Star, Inc., Ashland, OR).
2.2. TDSC colony formation and differentiation assays
2.2.1. Colony formation
Triplicate samples of TDSCs were seeded at either 100, 1000 or 10000 cells per 11 cm2 dish and cultured in complete medium (α-Modified Eagle Medium supplemented with 10% fetal bovine serum (FBS), 100U/ml penicillin, 100 μg/ml streptomycin)for 21 days. The cells were then stained with 0.5% crystal violet (Sigma) to visualize cell colonies.
2.2.2. Osteogenic differentiation assay
TDSCs were seeded at 105 cells per well in a six-well plate and cultured in complete culture medium until the cells reached confluence. They were then incubated for 28 days in either complete medium or osteogenic medium which consisted of Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 100U/ml penicillin, 100 mg/ml streptomycin, 1 nM dexamethasone, 50 mM ascorbic acid, and 10 mM β-glycerolphosphate (all from Sigma, Australia). The cells were then fixed with 4% PFA and stained with Alizarin-red (Sigma) to detect calcium nodules [14].
2.2.3. Adipogenic differentiation assay
TDSCs were plated at 105 cells/well and cultured until they reached confluence. Then the medium was replaced with complete medium or adipogenic medium, consisting of high glucose Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 100U/ml penicillin, 100 μg/ml streptomycin, 500 nM dexamethasone, 0.5 mM isobutylmethylxanthine, 50 μM indomethacin, and 10 μg/ml insulin (all from Sigma–Aldrich), and cultured for a further 21 days. At the end of culture, the cells were fixed with 4% PFA and stained with a X% solution of 0.5% Oil red-O (Sigma) for oil droplets [14].
2.2.4. Chondrogenic differentiation assay
A total of 106 TDSCs were placed into a 15 ml conical polypropylene tube, centrifuged at 1200 g for 5 min and the resulting pellet cultured in complete medium (see above) or chondrogenic medium which consisted of low-glucose Dulbecco's modified Eagle's medium, supplemented with 10 ng/ml transforming growth factor-β3 (R&D Systems), 500 ng/ml bone morphogenetic protein-2 (R&D Systems), 100 nM dexamethasone, 50 μg/ml ascorbate-2-phosphate, 40 μg/ml proline, 100 mg/ml pyruvate (all from Sigma–Aldrich), and 1:100 diluted ITS + Premix (6.25 mg/ml insulin, 6.25 mg/ml transferrin, 6.25 mg/ml Selenous acid, 1.25 mg/ml bovine serum albumin, and 5.35 mg/ml linoleic acid; all obtained from Becton Dickinson, Franklin Lakes, NJ). After 21 days culture, the pellets were fixed with 4% PFA and stained with 1% Alcian Blue (Sigma) [14].
2.3. 3D TDSC constructs in a bioreactor and subjected to mechanical loading
The 3D TDSC constructs were fabricated according to our previous description [12]. In brief, TDSCs were grown to confluence as a monolayer, and then stimulated to promote extracellular matrix deposition by culturing in the presence of 25 ng/ml connective tissue growth factor (CTGF) (Sigma) and 4.4 μg/ml ascorbic acid for 6 days. The cell sheets formed at the end of the incubation period were detached using 0.25% trypsin, and attached to the tissue hooks to form 3D constructs. The 3D constructs placed into the bioreactor were then cultured for 6 days either with or without (static control) cyclic tensile loading, similar to the loading regime used for ex vivo rabbit Achilles tendon (described above) to ensure consistency between ex vivo and 3D-construct experimental conditions (3% strain, 0.25 Hz for 8 h followed by 16 h rest).
2.4. Western blotting and analyses
Total cellular proteins were extracted from tendon-like tissues using RIPA lysis buffer (50 mM Tris–HCl pH7.5, 150 mM NaCl, 1% Nonidet P-40, 0.1% SDS, 1% sodium deoxycholate) supplemented with 50 μg/ml phenylmethylsulfonyl fluoride (PMSF), 1 mM sodium orthovanadate (Na3VO4), and a protease inhibitor cocktail (Roche). Lysates were cleared by centrifugation at 12,000g for 10 min at 4 °C and supernatants containing proteins collected. Aliquots of extracted proteins (15 μg each) were resolved on 10% SDS-PAGE gels and then electroblotted onto nitrocellulose membranes (Protran, Perkin–Elmer). Membranes were blocked with 5% skim milk in TBS-0.1% Tween (TBST) for 1 h and then probed with primary antibodies: namely anti-Phospho-GSK3β (Ser9), anti-Phospho-β-Catenin (Ser33), anti-Phospho-β-Catenin (Ser552), anti-β-Catenin and anti-pGSK3β (1:1000, Cell Signaling Technology) diluted in 1% (w/v) skim milk powder in TBST for either 2 h at room temperature or overnight at 4 °C. Membranes were subsequently washed TBST and then incubated with the appropriate horseradish peroxidase-conjugated anti-mouse secondary antibodies (Sigma), and immunoreactivity was detected using the Western Lightning Ultra Detection Kit (Perkin–Elmer) using the FujiFilm LAS-4000 Gel Documentation System (Japan) and its associated software. Each Western Blot was performed in triplicate using 3 individual samples and a representative blot used for quantitation.
2.5. Quantitative real-time polymerase chain reaction
Total cellular RNAs from 3D TDSCs constructs were extracted using TRIzol® reagent (Invitrogen, USA) as previously described [24]. The RNA concentration was quantified using a NanoDropTM 2000 spectrophotometer (ThermoFisher, Australia). mRNA was reverse-transcribed into cDNA using the SuperScript® III First-Strand Synthesis System (Invitrogen, USA) according to the manufacturer's instructions. The cDNA was amplified and quantified by RT-PCR using the iQ™ SYBR® Green Supermix (Bio-Rad, USA) as previously described [24]. Primer sequences for the genes of interest are listed in previous publication [12]. The housekeeping gene 36B4 was used as an internal control. A cycle threshold (Ct) value was obtained from each reaction and the comparative 2-△△Ct method was used to calculate the relative expression of each target gene [25].The RT-PCR was repeated using 5 individual samples from each group and performed in triplicate in every RT-PCR setting. The average ± SD expression levels were calculated and used for comparison.
2.6. Histology and immunohistochemistry
The rabbit Achilles tendon and 3D TDSCs constructs were fixed in 4% PFA, dehydrated in a series of increasing grade ethanols, critically embedded in paraffin and 5 μm thick longitudinal sections were prepared. The sections were stained with hematoxylin and eosin (H&E) for general histological assessment. Staining of sections with the von Kossa method was used to examine heterotopic ossification. Histological scoring was performed by 3 individuals (blinded to sample identification) as previously described [26]. A total of five elements (cell density, cell roundness, fiber structure, fiber arrangement and calcification) were assessed using scoring scale of 0–3: where 0 is normal and 3 severely abnormal as described in a previous study [27]. The combined score average (from the 3 independent individuals) for each parameter was used for comparison.
Immunohistochemistry was performed as described previously [24] using antibodies specific against osteocalcin and tenomodulin (Santa Cruz, USA). Biotinylated goat anti-mouse IgG, rabbit anti-goat IgG and goat anti-rabbit IgG (Sigma, USA) were used at a dilution of 1:200 as secondary antibodies. The sections were developed to visualize positive staining using the Diaminobenzidine (DAB) kit (DAKO, Glostrup, Denmark), and counterstained with hematoxylin solution. Positive staining was quantified as staining intensity using ImageJ.
2.7. Mechanical testing
The rabbit Achilles tendons (5 from each group) were thawed at room temperature on the day of testing. The enthesis and myotendinous junction ends were individually clamped into either side of custom-designed cryogrips. To prevent tissue sliding, both ends were snap-frozen by liquid nitrogen. A thermometer was used to monitor the temperature of the tissue between two cryogrips, to avoid the “region of interest” from freezing. The grips were then fixed into the Instron mechanical testing system (model 5566, Instron, China), and the portion of the Achilles tendon between the clamps was subjected to tensile loading to failure [9]. The samples were preloaded at 1 N as the starting point, then gradually pulled at a speed of 2 mm/s until the ultimate force was reached. During the experiment, the samples were closely monitored to ensure that each tested tendon was broken at the middle portion but not the point near the grip. A force versus displacement curve was recorded, and the ultimate load was recorded. Mean stiffness (N/mm) was calculated based on the linear region of the loading curve.
2.8. Statistical analysis
All the data are presented as mean ± SEM. Each experiment was performed at least three times. Either a two-tailed Student's t-test or one-way analysis of variance (ANOVA) followed by Tukey post hoc test (GraphPad Software 5.0, USA) were used for determining the statistical significance between the two-groups or multi-groups comparison, respectively. Statistical significance was accepted as a p < 0.05.
3. Results
3.1. Mechanical underloading induces heterotopic ossification within tendons
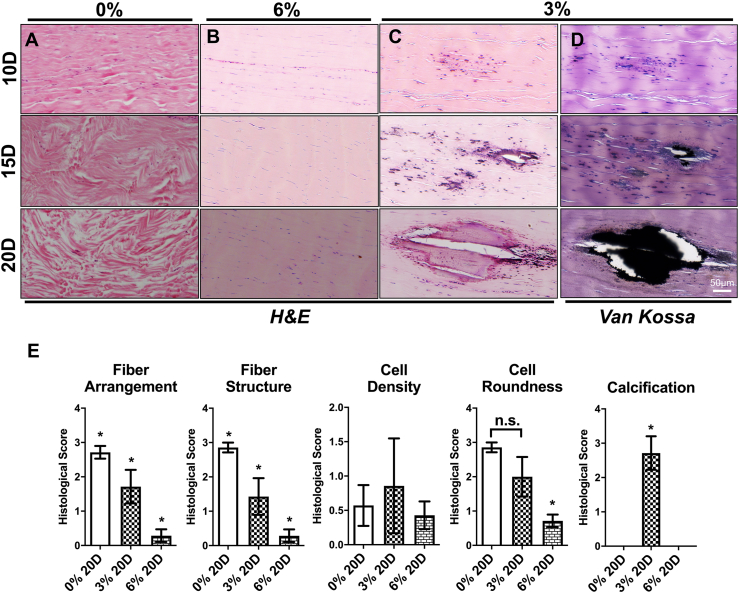
Mechanical stimulation is one of the key factors for tendon homeostasis. We hypothesised that underloading of tendons contributes to the progression of tendinopathy. Therefore, we examined histological changes that occur in ex vivo cultured tendons under different loading regimes (0, 3 and 6% tensile strain). In static cultured tendons, an increasing level of collagen disorientation and degradation were observed from days 10–20 during the culture period (Fig. 2A). Severe collagen fiber rupture was shown after 20 days of static culture (Fig. 2A) which is consistent with a previous study in which tendons showed degenerative changes after immobilization [28]. In contrast, well organized collagen fibers were shown in tendons subjected to 6% strain (p < 0.05) (Fig. 2B, E). Furthermore, minor collagen fiber disorientation was exhibited without rupture (p < 0.05) (Fig. 2C, E). Interestingly, minor heterotopic ossification was started to show in tendon subject to low magnitude loading for 15 days, and ossification area increased after 20 days underloading culture (p < 0.05) (Fig. 2D).
Fig. 2.
Histological assessment of rabbit Achilles tendons stained with either H&E or the von Kossa method. The tendons cultured without loading over a period of 10-20 days showed a pattern of progressive fiber disorientation and rupture (A); Normal morphology was shown in tendon subjected to 6% loading (B); Tendons subjected to 3% loading exhibited wavy collagen fibers (B) and progressive ossification confirmed by von Kossa staining (C, D). Average histological scores of tendon pathological changes (E), A score of 0 for each parameter represents normal and healthy structure, whereas 3 represents severe abnormality. Results are expressed as the mean±SEM. One-way ANOVA significance values were ∗p < 0.05 n = 5.
3.2. Heterotopic ossification reduces the mechanical properties of tendons
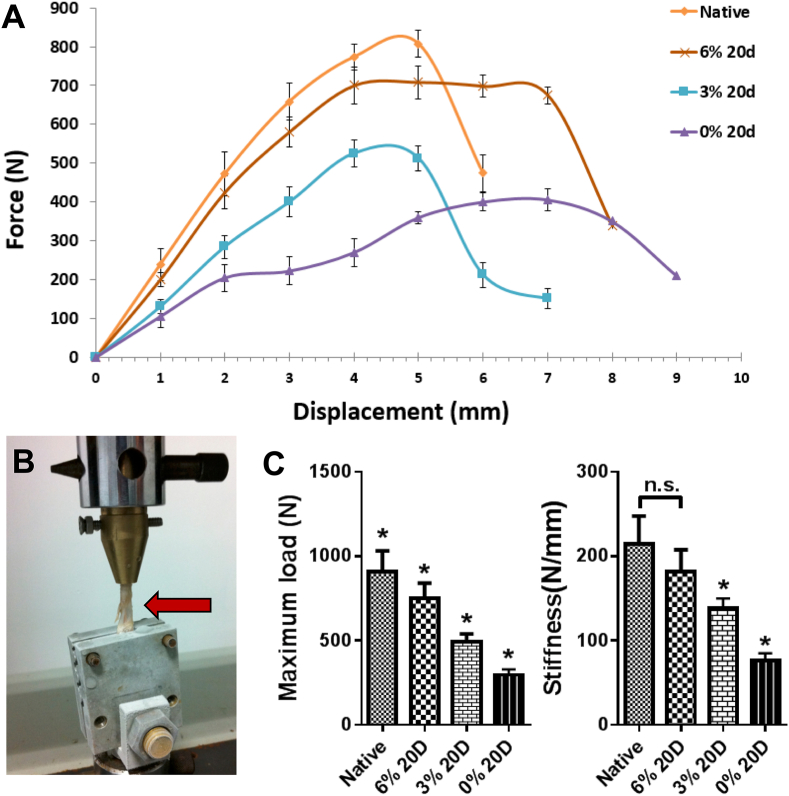
In order to investigate the overall impact of culture environment on tendon mechanical properties, we performed mechanical tests on tendon samples by assembling the tissue into the loading system (Fig. 3A). A loading curve was created as shown in Fig. 3B. The loading curves increased linearly along the length of displacement in native tendon as well as in the 0, 3 & 6% loading groups. Peak force was reached at around 4.5 mm extension, with the average ultimate tensile force in native tendons 910.5 ± 109.1 N, being significantly higher than the 0 (830.4 ± 41.2 N), 3 (491.9 ± 43.1 N) and 6% loading group (750.5 ± 81.5 N). No significant difference was shown in the stiffness of the native tendon (214.5 ± 29.6 N/mm) and 6% loaded tendons (181.6 ± 23.4 N/mm), but the tendons cultured within loading deprivation (76.5 ± 7.4 N/mm) and underloading mechanical environment (138 ± 10.8 N/mm).
Fig. 3.
Mechanical testing of rabbit Achilles tendons. (A) The Force versus displacement curves. (B) Mechanicl testing machine and loaded samples. (C) Maximum load and mean stiffness showed reduce mechanical properties of Achilles tendon tissue cultured ex vivo compared to native tendon tissue. Mechanical underloading and deprivation culture of tendon further reduced the mechanical properties compared to the one under physiological loading culture. Results are expressed as the mean ± SEM (n = 5). One-way ANOVA significance values were ∗p < 0.05.
3.3. TDSC identification and characterization
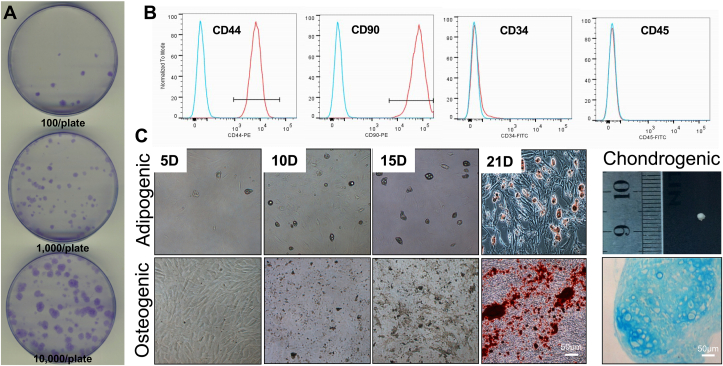
All our experiments utilized either tendon-derived stem cells (TDSCs) or 3D TDSCs constructs as described in Materials and Methods. To confirm the identity of isolated TDSCs, colony formation, surface stem cell marker expression and multilinear differentiation assays were performed (Fig. 4). TDSCs were able to form colonies 21 days after initial seeding densities between 102 – 104 cells/plate (Fig. 4A). Consistent with Bi et al. (2007) [29], more than 90% of purified TDSCs expressed MSC surface markers such as CD44 and CD90, whereas very few cells expressed either the endothelial cell marker CD34 and/or the hematopoietic marker CD45 (Fig. 4B). Calcium nodules indicative of osteoblast formation were detected by Alizarin-red staining after 21 days of culturing TDSCs in osteogenic media (Fig. 4C; lower panel), whereas lipid droplets were formed in adipogenic culture, as detected by Oil Red O staining (Fig. 4C; upper panel). Likewise, the pellet formed in chondrogenic medium stained positively for Alcian blue, indicating that mouse TDSCs can also differentiate into chondrocytes (Fig. 4C). No positive staining was observed in any of the control groups. Thus, we were able to isolate TDSCs with predicted cell surface markers and differentiation potential characteristics.
Fig. 4.
Tendon derived stem cells identification. (A) Formation of cell colonies TDSCs after culturing for 21 days at different seeding density of 100, 1000, 10000 cell/90mm petri dish. (B) Flow cytometry showing expression of mesenchymal stem cell marker (CD44, CD90), endothelial cell marker (CD34) and hematopoietic cell marker (CD45). (C) Differentiation potential of TDSCs after 21 days conditional medium stimulation. Calcium deposition was formed in osteogenic medium and stained by Alizarin Red. Lipid vacuoles were form in adipogenic medium and visualized by Oil red-O staining. Cell pellets were formed in chondrogenic medium and proteoglycan was stained by Alcian Blue.
3.4. Mechanical underloading induces osteogenic differentiation in 3D TDSC constructs
To further evaluate the effect of mechanical underloading on TDSCs differentiation in 3D environment, we generated 3D TDSC constructs, cultured them in a bioreactor and subjected them to static or loading conditions as shown as Fig. 5A. TDSC constructs without loading exhibited randomly oriented extracellular matrix and rounded cell nuclei. In an underloading environment, the 3D construct showed wavy but aligned ECM morphology and the cell nuclei were slightly elongated. Low levels of tenomodulin and osteocalcin were detected in the static cultured samples, although strong positive staining for osteocalcin was seen in under stimulated samples (p < 0.01; n = 3???).
Fig. 5.
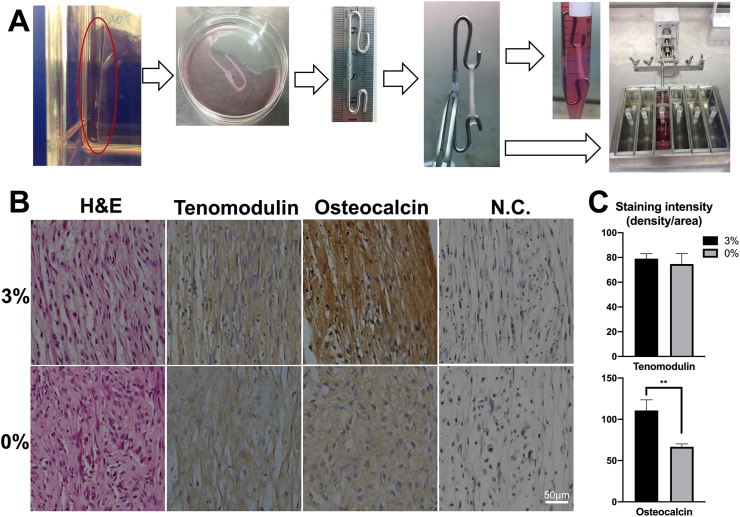
(A) Photographs of 3D TDSCs construct culture. The cell sheet was formed and detached from T75 culture flask, which was then rolled over the hooks forming tendon-like 3D construct. The tissues were either cultured without loading in 15ml tube for 6 days or subjected to 3% cyclic tensile strain at 0.25Hz, 8h/day for 6 days in the bioreactor system. (B) Histological and immunohistochemical assessment comparison of 3D constructs. The sample cultured with 3% loading exhibited aligned collagen fibers with higher positive staining of osteocalcin (∗∗p<0.01) than the tissue without loading. Staining of Tenomodulin showed no difference between two groups. The tissue formed without loading exhibited random extracellular matrix formation without specific differentiation pattern. Samples stained without primary antibodies acted as negative control group (N.C.). (C) Quantitation of immunohistochemical staining were performed as staining intensity using ImageJ software.
Next, we compared markers of osteogenic and tenogenic differentiation using qPCR. The results showed that mechanical underloading did not increase tenogenic marker gene expression for scleraxis and tenomodulin (Fig. 6A), however, collagen I gene expression was upregulated (p < 0.05) (Fig. 6A). In contrast, markers of osteogenic gene expression including ALP, RUNX2 and Osteocalcin were increased consistent with the hypothesis that 3D constructs are prone to osteogenic rather than tenogenic differentiation (p < 0.05) (Fig. 6A).
Fig. 6.
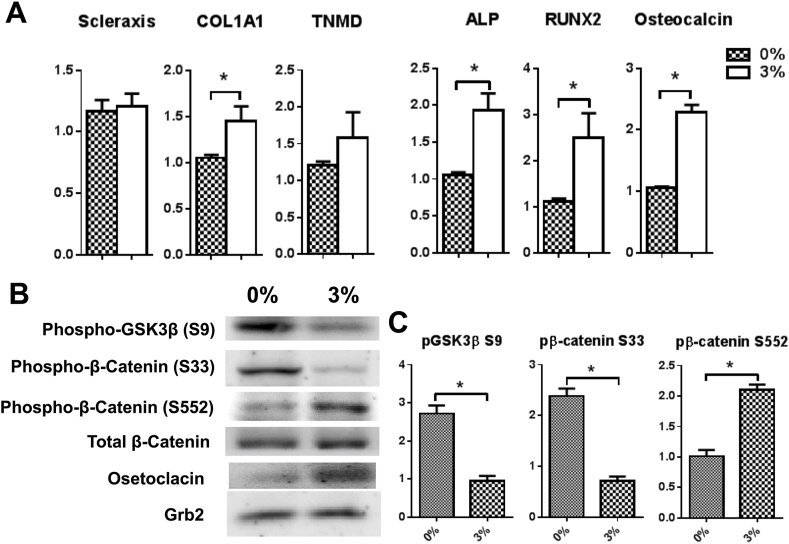
(A) Comparison of differentiation-related markers gene expression on 3D TDSCs construct. 3% mechanical loading induced upregulation of COL1A1, ALP, RUNX2 and osteocalcin. The gene expression level is normalized against 36B4. Result are expressed as the mean±SEM. One-way ANOVA significance values were ∗p < 0.05 n=5; (B) Western blot analysis of protein phosphorylation and expression levels in 3D TDSC constructs cultured for 6 d, with or without 3% uniaxial loading. Total cell lysates were immunoblotted with the indicated antibodies. (C) Densitometric quantitation of bands (∗p<0.05, n = 3).
3.5. Mechanical underloading induces β-catenin accumulation
As β-catenin accumulation is central to osteoblast differentiation and mineralization, abnormal β-catenin accumulation may be the key to heterotopic ossification of tendons. Therefore, we further investigated β-catenin phosphorylation in TDSC constructs. We determined that mechanical underloading significantly inhibits phosphorylation of GSK3β at Ser9, which further reduces β-catenin degradation by the inhibition of phosphorylation at Ser33 (p < 0.05) (Fig. 6B and C). The phosphorylation of β-catenin at Ser552 was significantly upregulated (p < 0.05) (Fig. 6B and C) in response to 3% loading, suggesting increased accumulation and translocation of β-catenin into cell nuclei.
4. Discussion
Heterotopic ossifications of tendons is a severe pathological change at the end stage of tendinopathy, which often leads to tendon rupture. In our previous study, we have demonstrated that reduction of mechanical loading for short period (3% tensile strain, 0.25 Hz, 8 h/d, 6 days) caused minor pathological changes in Achilles tendon similar to those observbed in the progression of tendinopathy [8]. Using the 3% loading protocol in culture for up to 20 days we found multiple ossification sites within tendon tissues. To further investigate the underlying mechanism, we generated a 3D TDSCs construct and evaluated the differentiation induction ability of mechanical underloading to TDSCs. Consistently, we found mechanical underloading induced osteogenic differentiation over tenogenic differentiation, which is mediated by stabilizing the β-catenin accumulation.
HO is bone formation within soft tissues such as tendon, with an unknown patho-mechanism that often leads to deleterious outcomes [30,31]. The pathogenesis of HO is believed to be correlated with progenitor cells with osteogenic differentiation ability in affected soft tissue, however, the underlying mechanism remained unclear due to lack of in vitro model. The majority of HO studies are based on in vivo models developed by McClure et al. [32]. They did an Achilles tenotomy with a blunt dissection, and most of the animals developed HO between 5 and 10 weeks post operation [32]. However, tenotomy might not represent pathogenesis in human tendon, thus, the mechanism might be different.
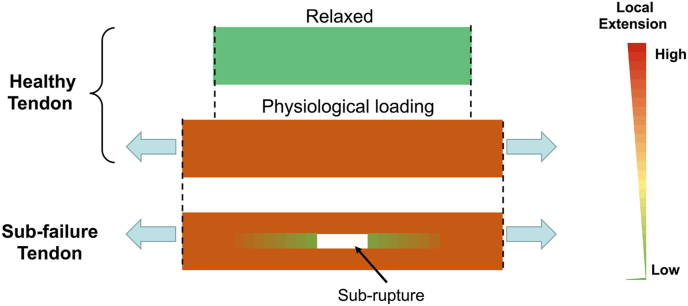
Tendons are force-transferring tissue that enable joint movement. Due to its physiological function, overuse of tendon is often considered as the primary factor of tendinopathy. Numerous studies were conducted to investigate the effect of overloading environment on tendon by applying high strain loading on tenocytes or TDSCs [[33], [34], [35], [36], [37]]. However, tendons are often subject to uni-axial tensile loading of up to 6% strain [38], and Arnoczky et al. showed that local strain and cellular deformation are only around 65–85% of overall strain on tendon tissue [39]. Therefore, physiological loading on resident cells in tendon should be around 6%. Sudden and high extension of tendon often leads to the local rupture of collagen fiber leaving the sub-failure site. The tendon cells at the sub-failure site proliferate and are designated to repair the damage, if patient continue to apply the loading on the sub-failure tendon [7], the proliferated cells then experience underloading environment as shown as Fig. 7. Interestingly, recent study of Wang et al. demonstrated that percutaneous Achilles tendon puncture would induce HO [40], which is consistent with our hypothetical model. Our previous study showed that 6 days of underloading environment led to cell proliferation and minor disruption of collagen structure that similar to early tendinopathy [8]. Here, we further demonstrated that prolonged mechanical underloading was the key of heterotopic ossification in tendon.
Fig. 7.
Heatmap of extension distribution in tendon. In healthy tendon, extension is evenly distributed through the tendon. However, reduced local tension is formed in sub-ruptured site in the sub-failure tendon.
Multiple signaling pathways were revealed to be associated with HO. HO was observed in rare genetic diseases such as fibrodysplasia ossificans progressiva (FOP) and progressive osseous heteroplasia (POH), which are due to mutation of BMP receptors and the disruption of BMP signaling [41,42]. Recent study demonstrated that mTORC1 signaling was proposed to induce HO in rat tendon tissues as well [43,44]. In addition, overactivation of TGF-β induced and promoted ectopic bone formation in Achilles tendon [40]. The signaling pathways above are all closely associated with osteoblast differentiation. Wnt/β-catenin signaling pathway has been demonstrated as a key regulator for bone formation, which has been shown to crosstalk to BMP, TGF-β and mTORC signaling [[45], [46], [47], [48]]. Activation of Wnt leads to its binding with a dual receptor composed of LRP5/6 and FZD receptors, resulting in the inactivation of GSK-3β and accumulation of cytosolic β-catenin, mutation of LRP5 has been reported to associated with high bone density in human [49]. Our results showed that mechanical underloading inactivated GSK-3β leading to disruption of β-catenin degradation. In addition, it further induced β-catenin phosphorylation at Ser552, which induced β-catenin translocation into nuclei. The dual effect of mechanical underloading on TDSCs led to increased accumulation and translocation of β-catenin, eventually caused HO in tendon tissue.
5. Conclusion
To our knowledge, this study is the first to show that in a culture environment underloading, tendons would develop HO. In addition, our 3D TDSCs loading model further demonstrated that mechanical underloading induced osteogenic differentiation instead of tenogenic differentiation, which is mediate by accumulation and translocation of β-catenin. These findings shed light on a potential mechanism for heterotopic ossification in tendinopathy due to the underloading of TDSCs at the damage sites, which could be mediated by dis-regualtion of β-catenin signalling.
Availability of data and materials
Not applicable.
Consent for publication
Not applicable.
Authors’contributions
T. Wang and P. Chen had major contribution in study design, performance, and writing. L. Chen and Y. Zhou carried out parts of the experiment, A. Wang, T. Leys, C.A. Mitchell and R. Tuan assisted with data analysis and manuscript revision. M.H. Zheng and Q.J. Zheng coordinated and designed this study and contributed in paper writing. All authors approved the final manuscript and submission.
Funding
This work is supported by the National Natural Science Foundation of China (81802214), Australia Research Council Linkage Grant (LP150100905), and Guangdong Science and Technology Department (2015B020225007).
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
We would like to thank for the technical assistance from the Centre for Microscopy, Characterisation & Analysis. The authors acknowledge the support from Australian Research Council Industrial Transformation Training Centre for Personalised Therapeutics Technologies (IC170100016).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jot.2021.03.004.
Contributor Information
Tao Wang, Email: tao.wang@uwa.edu.au.
Peilin Chen, Email: peilin.chen@research.uwa.edu.au.
Lianzhi Chen, Email: Lianzhi.chen@research.uwa.edu.au.
Yinghong Zhou, Email: y26.zhou@qut.edu.au.
Allan Wang, Email: allanwang@aapt.net.au.
Qiujian Zheng, Email: zqj650@126.com.
Toby Leys, Email: tobyleys@gmail.com.
Rocky S. Tuan, Email: tuanr@cuhk.edu.hk.
Ming H. Zheng, Email: minghao.zheng@uwa.edu.au.
Abbreviations list
- HO
Heterotopic ossification
- TDSC
Tendon derived stem cell
- CTGF
connective tissue growth factor
Appendix A. Supplementary data
The following is the Supplementary data to this article:
figs1.
References
- 1.Sharma P., Maffulli N. Current concepts review tendon injury and tendinopathy: healing and repair. J Bone Jt Surg Am Vol. 2005;87a(1):187–202. doi: 10.2106/JBJS.D.01850. [DOI] [PubMed] [Google Scholar]
- 2.Raynor K.J. Ossification of the achilles-tendon. J Am Podiatr Med Assoc. 1986;76(12):688–690. doi: 10.7547/87507315-76-12-688. [DOI] [PubMed] [Google Scholar]
- 3.Richards P.J. Achilles tendon ossification: pathology, imaging and aetiology. Disabil Rehabil. 2008;30(20–22):1651–1665. doi: 10.1080/09638280701785866. [DOI] [PubMed] [Google Scholar]
- 4.Xu R.S. Heterotopic ossification: mechanistic insights and clinical challenges. Bone. 2018;109:134–142. doi: 10.1016/j.bone.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 5.Frohm A. Eccentric treatment for patellar tendinopathy: a prospective randomised short-term pilot study of two rehabilitation protocols. Br J Sports Med. 2007;41(7) doi: 10.1136/bjsm.2006.032599. e7-e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang T. Bioreactor design for tendon/ligament engineering. Tissue Eng B Rev. 2013;19(2):133–146. doi: 10.1089/ten.teb.2012.0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galloway M.T., Lalley A.L., Shearn J.T. The role of mechanical loading in tendon development, maintenance, injury, and repair. J Bone Jt Surg Am Vol. 2013;95a(17):1620–1628. doi: 10.2106/JBJS.L.01004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang T. Programmable mechanical stimulation influences tendon homeostasis in a bioreactor system. Biotechnol Bioeng. 2013;110(5):1495–1507. doi: 10.1002/bit.24809. [DOI] [PubMed] [Google Scholar]
- 9.Wang T. Cyclic mechanical stimulation rescues achilles tendon from degeneration in a bioreactor system. J Orthop Res. 2015;33(12):1888–1896. doi: 10.1002/jor.22960. [DOI] [PubMed] [Google Scholar]
- 10.Thomopoulos S. Mechanisms of tendon injury and repair. J Orthop Res. 2015;33(6):832–839. doi: 10.1002/jor.22806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma P., Maffulli N. Biology of tendon injury: healing, modeling and remodeling. J Musculoskelet Neuronal Interact. 2006;6(2):181–190. [PubMed] [Google Scholar]
- 12.Wang T. 3D uniaxial mechanical stimulation induces tenogenic differentiation of tendon-derived stem cells through a PI3K/AKT signaling pathway. Faseb J. 2018;32(9):4804–4814. doi: 10.1096/fj.201701384R. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J., Wang J.H. Characterization of differential properties of rabbit tendon stem cells and tenocytes. BMC Muscoskel Disord. 2010;11:10. doi: 10.1186/1471-2474-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rui Y.F. Isolation and characterization of multipotent rat tendon-derived stem cells. Tissue Eng. 2010;16(5):1549–1558. doi: 10.1089/ten.TEA.2009.0529. [DOI] [PubMed] [Google Scholar]
- 15.Abate M. Achilles tendinopathy in amateur runners: role of adiposity (Tendinopathies and obesity) Muscles Ligaments Tendons J. 2012;2(1):44–48. [PMC free article] [PubMed] [Google Scholar]
- 16.Lui P.P. Chondrocyte phenotype and ectopic ossification in collagenase-induced tendon degeneration. J Histochem Cytochem. 2009;57(2):91–100. doi: 10.1369/jhc.2008.952143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Regard J.B. Activation of Hedgehog signaling by loss of GNAS causes heterotopic ossification. Nat Med. 2013;19(11):1505–+. doi: 10.1038/nm.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nusse R. Wnt signaling in disease and in development. Cell Res. 2005;15(1):28–32. doi: 10.1038/sj.cr.7290260. [DOI] [PubMed] [Google Scholar]
- 19.Birchmeier W. The Wnt/beta-catenin signaling pathway in development and disease. Pathol Res Pract. 2007;203(5) 341-341. [Google Scholar]
- 20.Fang D.X. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem. 2007;282(15):11221–11229. doi: 10.1074/jbc.M611871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baron R., Rawadi G. Minireview: targeting the Wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology. 2007;148(6):2635–2643. doi: 10.1210/en.2007-0270. [DOI] [PubMed] [Google Scholar]
- 22.Case N. Mechanical activation of beta-catenin regulates phenotype in adult murine marrow-derived mesenchymal stem cells. J Orthop Res. 2010;28(11):1531–1538. doi: 10.1002/jor.21156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang T. 3D uniaxial mechanical stimulation induces tenogenic differentiation of tendon-derived stem cells through a PI3K/AKT signaling pathway. Faseb J. 2018;32(9):4804–4814. doi: 10.1096/fj.201701384R. [DOI] [PubMed] [Google Scholar]
- 24.Wang T. Programmable mechanical stimulation influences tendon homeostasis in a bioreactor system. Biotechnol Bioeng. 2013;110(5):1495–1507. doi: 10.1002/bit.24809. [DOI] [PubMed] [Google Scholar]
- 25.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Chen J. Autologous tenocyte therapy for experimental Achilles tendinopathy in a rabbit model. Tissue Eng. 2011;17(15–16):2037–2048. doi: 10.1089/ten.TEA.2010.0492. [DOI] [PubMed] [Google Scholar]
- 27.Chen J.M. Autologous tenocyte therapy using porcine-derived bioscaffolds for massive rotator cuff defect in rabbits. Tissue Eng. 2007;13(7):1479–1491. doi: 10.1089/ten.2006.0266. [DOI] [PubMed] [Google Scholar]
- 28.Kannus P. Effects of training, immobilization and remobilization on tendons. Scand J Med Sci Sports. 1997;7(2):67–71. doi: 10.1111/j.1600-0838.1997.tb00121.x. [DOI] [PubMed] [Google Scholar]
- 29.Bi Y. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13(10):1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 30.Vanden Bossche L., Vanderstraeten G. Heterotopic ossification: a review. J Rehabil Med. 2005;37(3):129–136. doi: 10.1080/16501970510027628. [DOI] [PubMed] [Google Scholar]
- 31.Mavrogenis A.F., Soucacos P.N., Papagelopoulos P.J. Heterotopic ossification revisited. Orthopedics. 2011;34(3) doi: 10.3928/01477447-20110124-08. [DOI] [PubMed] [Google Scholar]
- 32.Mcclure J. The effect of diphosphonates on heterotopic ossification in regenerating achilles-tendon of the mouse. J Pathol. 1983;139(4):419–430. doi: 10.1002/path.1711390403. [DOI] [PubMed] [Google Scholar]
- 33.Mousavizadeh R. Mechanical loading modulates angiogenic factors in tendon cells. Br J Sports Med. 2013;47(9) e2-e2. [Google Scholar]
- 34.Mousavizadeh R. Cyclic strain alters the expression and release of angiogenic factors by human tendon cells. PloS One. 2014;9(5) doi: 10.1371/journal.pone.0097356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andersson G., Backman L. BMJ Publishing Group Ltd and British Association of Sport and Exercise Medicine; 2014. 13 fibrotic regulators Ccn1 and Ccn2 respond to mechanical loading of tendon cells. [Google Scholar]
- 36.Zhu L.X., Li C.Y., Ji C.F. Dual action of substance-P in nociception and pain modulation at spinal level. Sci Sin B Chem Biol Agric Med Earth Sci. 1987;30(7):727–738. [PubMed] [Google Scholar]
- 37.Spang C. Glutamate signaling through the NMDA receptor reduces the expression of scleraxis in plantaris tendon derived cells. BMC Muscoskel Disord. 2017;18(1):218. doi: 10.1186/s12891-017-1575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Screen H.R.C. An investigation into the effects of the hierarchical structure of tendon fascicles on micromechanical properties. Proc IME H J Eng Med. 2004;218(H2):109–119. doi: 10.1243/095441104322984004. [DOI] [PubMed] [Google Scholar]
- 39.Arnoczky S.P. In situ cell nucleus deformation in tendons under tensile load; a morphological analysis using confocal laser microscopy. J Orthop Res. 2002;20(1):29–35. doi: 10.1016/S0736-0266(01)00080-8. [DOI] [PubMed] [Google Scholar]
- 40.Wang X. Inhibition of overactive TGF-beta attenuates progression of heterotopic ossification in mice. Nat Commun. 2018;9(1):551. doi: 10.1038/s41467-018-02988-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shafritz A.B. Overexpression of an osteogenic morphogen in fibrodysplasia ossificans progressiva. N Engl J Med. 1996;335(8):555–561. doi: 10.1056/NEJM199608223350804. [DOI] [PubMed] [Google Scholar]
- 42.Kaplan F.S., Hahn G.V., Zasloff M.A. Heterotopic ossification: two rare forms and what they can teach us. J Am Acad Orthop Surg. 1994;2(5):288–296. doi: 10.5435/00124635-199409000-00007. [DOI] [PubMed] [Google Scholar]
- 43.Jiang H.J. Leptin accelerates the pathogenesis of heterotopic ossification in rat tendon tissues via mTORC1 signaling. J Cell Physiol. 2018;233(2):1017–1028. doi: 10.1002/jcp.25955. [DOI] [PubMed] [Google Scholar]
- 44.Chen G.R. Mechanical loading modulates heterotopic ossification in calcific tendinopathy through the mTORC1 signaling pathway. Mol Med Rep. 2017;16(5):5901–5907. doi: 10.3892/mmr.2017.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raisz L.G. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest. 2005;115(12):3318–3325. doi: 10.1172/JCI27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCubrey J.A. Roles of GSK-3 and microRNAs on epithelial mesenchymal transition and cancer stem cells. Oncotarget. 2017;8(8):14221–14250. doi: 10.18632/oncotarget.13991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thouverey C., Caverzasio J. Sclerostin inhibits osteoblast differentiation without affecting BMP2/SMAD1/5 or Wnt3a/beta-catenin signaling but through activation of platelet-derived growth factor receptor signaling in vitro. BoneKEy Rep. 2015;4:757. doi: 10.1038/bonekey.2015.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou S. TGF-beta regulates beta-catenin signaling and osteoblast differentiation in human mesenchymal stem cells. J Cell Biochem. 2011;112(6):1651–1660. doi: 10.1002/jcb.23079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boyden L.M. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346(20):1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.