Abstract
The aim of this article was to synopsize platelet-rich plasma (PRP) use in musculoskeletal pathologies through evidence-based assessment of the preparation, classification, mechanism of action and applications of PRP, thereby answering which PRP type is best for each clinical indication.
The literature search was performed using Medline, EMBASE and Cochrane Reviews databases for papers containing the key terms “platelet-rich plasma” AND “orthopaedics” AND (“classification” OR “mechanism of action” OR “preparation” OR “clinical application”). Generated papers were evaluated for pertinence in following areas: preparation, classification, mechanism of action, clinical application within orthopaedics. Non-English papers were excluded. Included studies were evaluated for quality.
Sixty studies were included in our review. There are many commercial PRP preparation kits with differing component concentrations. There is no consensus on optimal component concentrations. Multiple PRP classifications exist but none have been validated. Platelet-rich plasma acts via growth factors (GFs) released from α-granules within platelets. Growth factors have been shown to be beneficial in healing. Grossly elevated concentrations of GFs may have inhibitory effects on healing. Multiple systematic reviews show efficacy of PRP in tendinopathies, early osteoarthritis, acute muscle injuries and in combination with rotator cuff repair and anterior cruciate ligament reconstruction.
The literature suggests leukocyte-rich PRP (L-PRP) is more beneficial in tendinopathies and pure PRP (P-PRP) is more beneficial in cartilage pathology. However, different PRP preparations have not been directly compared in any pathology. Classification of PRP type is frequently not stated in research. Standardization of PRP research parameters is needed to streamline findings and generate clear indications for PRP types to yield maximum clinical benefit.
Cite this article: EFORT Open Rev 2021;6:225-235. DOI: 10.1302/2058-5241.6.200017
Keywords: orthopaedics, osteoarthritis, platelet-rich plasma, soft tissue, sports and exercise medicine
Introduction
Arthritis and soft tissue pathology make up the majority of orthopaedic referrals. An increasing proportion of patients are developing these pathologies at an earlier age, thereby producing a growing societal cost on healthcare and reduced productivity.1–4 This has led the drive to find treatments that can delay, or preferably cure, diagnoses that would otherwise require surgical intervention at an age or time when it would not typically be undertaken.
Platelet-rich plasma (PRP) is an autologous blood product acquired from part of the plasma fraction created via centrifugation of whole blood. By definition it has a platelet concentration above that of normal physiological levels.5
The term PRP originated in the 1970s by haematologists describing plasma with a platelet count higher than peripheral blood,6 which at the time was being used as a transfusion product in thrombocytopaenic patients.5 Since then it has been applied in multiple fields including plastic surgery, paediatric surgery, cardiac surgery, gynaecology, urology and ophthalmology.7 However, it is within the musculoskeletal field where there has been a surge of PRP use for multiple pathologies, largely due to widespread commercial interest following PRP use in professional sport.8
This review aims to synopsise PRP use in musculoskeletal pathologies through evidence-based assessment of the preparation, classification systems, mechanism of action and clinical applications of PRP and thereby answer, ‘What type of PRP is best for different clinical indications?’ We attempt to interpret the current viability of PRP within orthopaedics to help direct the focus of future research.
Methods
A literature search was performed using the Medline, EMBASE and Cochrane Reviews databases for all papers published between 1978 and 2019 containing the following key terms: “platelet-rich plasma” AND “orthopaedics” AND (“classification” OR “mechanism of action” OR “preparation” OR “clinical application”).
Selection criteria
Yielded papers were evaluated independently by two authors and selected if containing pertinence to PRP regarding one or more of the following areas: preparation, classification, mechanism of action, or clinical application within trauma and orthopaedics. Papers not written in English and animal studies relating to applications were excluded. The authors acknowledge an element of language bias; however, using more modern publications limits the extent of exclusion.
Literature grading and analysis
Studies were independently rated by two authors using the Oxford Centre for Evidence-based Medicine ‘Levels of evidence’ document9 and the Coleman modified score (CMS) when applicable.
Results
Sixty studies5,10–68 were included in our analysis published between 1978 and 2019. Details of the literature search and data extraction can be seen in a flow diagram (Fig. 1).
Fig. 1.
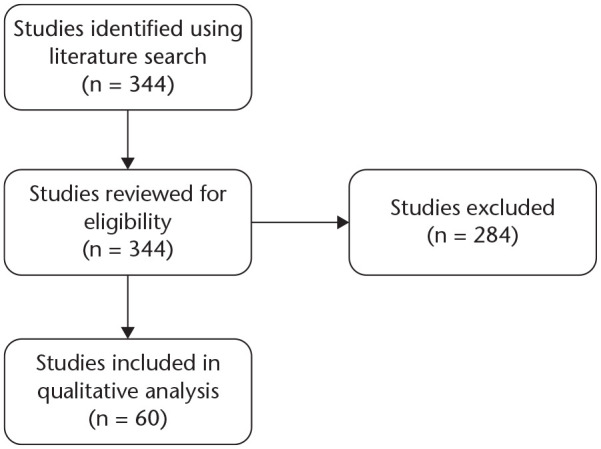
Study flow diagram.
Preparations
Platelet-rich plasma can be formulated in multiple ways with no consensus on a definitive protocol that could be used internationally to standardize the formulation. The following section is based on level 5 evidence surrounding the preparation of PRP. The basis of the preparations relies on the concept of differential centrifugation.10 Each component of whole blood has a different specific gravity and, when spun in a centrifuge, separates into distinct layers.
There are two principle methods of producing PRP, the PRP method and the buffy-coat method.10 The PRP method uses fresh blood from venepuncture, which is placed in a centrifuge for a ‘soft’ spin to separate the red blood cells (RBCs). The supernatant plasma is then centrifuged in a ‘hard’ spin at higher speeds to obtain the platelet concentrate. The buffy-coat technique utilizes whole blood, pre-stored at room temperature (i.e. 20–24oC). It undergoes a ‘hard’ spin to separate it into three layers: RBCs, platelets and white blood cells (WBCs), and platelet-poor plasma. The supernatant plasma is removed, and the buffy-coat is separated. This layer undergoes a second low-speed spin to separate the WBCs, or a leukocyte filter can be used.
Centrifugation rate has proved important in determining the optimal platelet yield. Most PRP production involves two spins – for separation and then concentration; the main factors of these spins are the speed in rotations per minute (RPM) and duration. Sabarish et al11 studied three differing protocols involving spin rates from 1000–3600 RPM lasting 4 to 15 minutes respectively. They found that lower spin rates had higher platelet yields, hypothesizing that high rates could cause platelet clumping or disintegration.
Lansdown et al12 highlighted that patient factors also play a role in platelet concentration. Fasting patients had lower concentrations than those who ate a high-fat meal. The timings of venepuncture can also affect concentration, with the optimal time being in the afternoon. They also surmise from other literature that there appears to be an optimal platelet concentration. If it is too low, i.e. 0.5–1.5x whole blood concentration, there is no enhancement in bone regeneration. If too high, i.e. 6–11x whole blood concentration, then there is a paradoxical inhibitory effect on bone regeneration.
Arora et al14 reviewed some of the technical aspects in relation to PRP preparation. They stressed the importance of anticoagulants in preventing the coagulation cascade of collected blood. Ethylenediaminetetraacetic acid (EDTA) suppresses platelet degranulation and therefore is not recommended for PRP. Conversely, heparin can cause spontaneous aggregation of platelets in some individuals. Anticoagulant citrate dextrose-A (ACD-A) is the most commonly used in commercial kits. It maintains the optimal pH for platelets at 7.2 while the citrate binds to calcium preventing the coagulation cascade. They also recommend PRP be kept in small diameter tubes with caps (to minimize surface area to atmosphere) as the pH can increase by diffusion of CO2 out of the plasma, potentially causing spontaneous aggregation.
Etulain et al15 proposed an optimized protocol for PRP preparation. Their three-pronged approach centred on dilution, 4oC incubation and plasma cryoprecipitate supplementation. The combination of these had an additive effect with greater angiogenesis, greater secretion of vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), interleukin-17 (IL-17) and interleukin-8 (IL-8) which translated into faster and more efficient mouse-skin wound repair vs. non-optimized PRP.
Preparation of PRP is vitally important, as it will have a direct impact on the final composition of PRP. Degen et al16 looked into the compositional differences of five commercially available PRP systems. They found that platelet concentration and capture efficiency was similar amongst all systems, WBC concentration was significantly elevated in all systems compared to whole blood, and variation was seen with pH, RBC and neutrophil levels. The exact implications of differing compositional elements are still unknown and will be considered later in the article. What is clear is the heterogeneity amongst differing commercial PRP systems (Table 1). It is therefore inferable that this heterogeneity limits conclusions drawn from pooled PRP studies containing multiple PRP preparation systems.
Table 1.
Component and preparation profile of commercial platelet-rich plasma (PRP) systems
| Arthrex ACP Double Syringe (Arthrex, USA) | Arthrex Angel System (Arthrex, USA) | RegenKit A-PRP (RegenLab, Switzerland) | MyCells (UK)/ Tropocells (UK)/ Cellenis PRP (Estar Medical, Israel) | PRGF / Endoret (BTI, Spain) | Glo PRP (Glofinn, Finland) | |
|---|---|---|---|---|---|---|
| PRP type | Plasma-based | Buffy-coat | Variant | Variant | Plasma-based | Variant |
| Starting volume | 15 ml | 40–180 ml | 8 ml | 10 ml | 9 ml | 9 ml |
| Platelets | 2–3x (~2.5x) | Up to 18x | 1.6x | 2–5x (4.5x in 2ml) | 2x | 4–9x |
| WBCs | Reduction | Adjustable | Reduction | Reduction | Reduction | Increase |
| RBCs | Adjustable | Adjustable | Reduction | Reduction | Adjustable | No reduction possibility |
| PRP yield | 4–6 ml | 2–20 ml depending on composition | 4 ml | 2–3 ml | 2 ml | Adjustable |
| Closed system | Yes | Yes | No | No | No | No |
| Needles involved | No | No | Yes | Yes | Yes | Yes |
| Principle | Centrifugation – closed transfer of PRP | Centrifugation with sensor/valve technology (light absorption) – PRP automatically collected in syringe | Separation gel – open needle transfer of PRP | Separation gel | Manual | Manual |
| Separation gel | No | No | Yes | Yes | No | No |
| Anticoagulant | No | Yes | Yes | Yes | Yes | Yes |
| Centrifugation steps | One | One | One | One | One | Two |
| Spinning parameters | 1500 rpm / 5 min | Depending on program, 3000 rpm or 3500 rpm, 15–30 min | 3400 rpm / 5min | 1500 g / 10 min | 580 / 8 min (+20 min clotting time) | 1200 g / 5 min 1200 g / 10 min |
| Preparation time | 10 min | 25–40 min | 10 min | 25 min | 30 min | 25 min |
| Handling steps | ⩽ 5 | 5–8 | ⩽ 5 | > 10 | 5–8 | 8–10 |
| Centrifuge | Specific | Specific | Specific | Specific | Specific | Specific |
| Ortho.pras (Proteal, Spain) | Genesis CS (EmCyte, USA) | PurePRP II (EmCyte, USA) | Y-PRP (Ycellbio Medical, South Korea) | Dr. PRP (SDD Medical Group, UK) | |
|---|---|---|---|---|---|
| PRP type | Variant | Buffy-coat | Buffy-coat | Buffy-coat | Buffy-coat |
| Starting volume | 20 / 40 ml | 30 / 60 ml | 60 / 120 ml | 15 ml | 20 ml |
| Platelets | 2.2x (in 4 ml PRP) | ? | 8x | 7–9x | ? |
| WBCs | Adjustable | Increase | Adjustable | Increase | Adjustable |
| RBCs | Adjustable | No reduction possibility | Adjustable | Reduction | Adjustable |
| PRP yield | 4–10 ml | 3–4 / 7 ml | 7 / 14 ml | 1–2 ml | 5 ml |
| Closed system | No | Yes | Yes | No | No |
| Needles involved | No | No | No | Yes | Yes |
| Principle | Manual | Manual | Manual, PRP transferred to a separate container after first centrifugation | Manual | Manual, after first centrifugation plasma and RBC container are separated |
| Separation gel | No | No | No | No | No |
| Anticoagulant | Yes | Yes | Yes | Yes | Yes |
| Centrifugation steps | One | One | Two | One | Two |
| Spinning parameters | 1800 rpm / 8 min | 4400 rpm / 5 min | 3800 rpm / 1.5 min; 3800 rpm / 5 min | 3200–3600 rpm / 4 min | 3400 rpm / 4 min; 3500 rpm / 2 min |
| Preparation time | 20 min | 15 min | 20 min | 20 min | 20 min |
| Handling steps | 8–10 | 5–8 | 5–8 | 5–8 | > 10 |
| Centrifuge | Specific | Specific | Specific | Specific | Specific |
| SW-PRP (Seawon meditech, South Korea) | Biomet GPS (Zimmer Biomet, USA) | Harvest SmartPReP (Terumo, USA) | CPunT (Eltek Group, Italy) | Magellan (Arteriocyte Medical Systems, USA) | |
|---|---|---|---|---|---|
| PRP type | Buffy-coat | Buffy-coat | Buffy-coat | Buffy-coat | Buffy-coat |
| Starting volume | 25 ml | 30 / 60 ml | 20 / 60 ml | 50 ml | 30–160 ml |
| Platelets | ? | 9.3x | 4.3–6.6x | 4–5x | ? |
| WBCs | Increase | Increase | Increase | Adjustable | Adjustable |
| RBCs | No reduction possibility | No reduction possibility | No reduction possibility | Adjustable | Adjustable |
| PRP yield | 2 ml | 3 / 6 ml | 3 / 7 / 10 ml | 10 ml | 3–10 ml |
| Closed system | No | No | No | Yes | Yes |
| Needles involved | No | No | Yes | Yes | No |
| Principle | Manual, after first centrifugation plasma and RBC container are separated | Dual buoy system – extraction of PRP through separate luer port | Two chamber bucket + floating shelf – open needle transfer of PPP and PRP | Centrifugation – first separation automated in electromechanical device, second separation manual | Centrifugation with sensor/valve technology (light absorption) – PRP automatically collected in syringe |
| Separation gel | No | No | No | No | No |
| Anticoagulant | Yes | Yes | Yes | Yes | Yes |
| Centrifugation steps | Two | One | One | Two | One |
| Spinning parameters | 3850 rpm / 7 min; 3850 rpm / 8 min | 3200 rpm / 15 min | 1000 g / 14 min | 1200 rpm / 10 min; 1900 rpm / 10 min | Depending on programme, 12–17 min |
| Preparation time | 40 min | 30 min | 20 min | 30 min | 25–30 min |
| Handling steps | > 10 | 5–8 | 8–10 | > 10 | 5–8 |
| Centrifuge | Specific | Specific | Specific | Specific | Specific |
Note. ACP, autologous conditioned plasma; PRGF, plasma rich in growth factors; BTI, biotechnology institute; WBC, white blood cell; RBC, red blood cell. GPS, gravitational platelet system.
At a commercial level, there is a vast array of kits available to prepare PRP with varying concentration of platelet yields and no overall consensus on optimal component concentrations.17 A systematic review by Chahla et al18 found that, of 105 studies using PRP in orthopaedics, only 11 provided comprehensive reporting of the preparation protocol that could be used by subsequent investigators. Only 17 studies provided qualitative metrics on the composition of their PRP products. Greater transparency in the reporting of PRP used in clinical and laboratory studies is needed. This must be in conjunction with a unified classification system with international consensus. This must be the direction of future research if the full potential of PRP is to be realized.
Classifications
Over the past decade, PRP use has grown significantly with numerous formulations currently available. Several authors have attempted to classify these preparations in order to give the orthopaedic community the means of comparing formulations, to find the optimal preparations for specific pathologies. The first such classification was proposed in 2009 by Ehrenfest et al19 (Table 2) who divided preparations by the presence of cell content and fibrin architecture. This qualitative classification gave a starting point but did not take into consideration other subpopulations of cells such as RBCs or neutrophils, which have an important role in the mechanism of action of PRP.
Table 2.
Ehrenfest classification
| Pure platelet-rich plasma (P-PRP) | Leukocyte-poor, low-density fibrin network |
| Leukocyte and platelet-rich plasma (L-PRP) | Contains leukocytes and low-density fibrin network |
| Pure platelet-rich fibrin (P-PRF) | Without leukocytes and high-density fibrin network |
| Leukocyte and platelet-rich fibrin (L-PRF) | Contains leukocytes and high-density fibrin network |
DeLong et al20 developed on this classification and introduced a quantitative element. The PAW classification (Platelets, Activation, White blood cells) (Table 3) provides a nomenclature based on platelet concentration, activation and WBC count, including the neutrophil subgroup.
Table 3.
PAW (Platelets, Activation, White blood cells) classification
| Platelets | Concentration (/µL) | ⩽ baseline > baseline – 750,000 > 750,000 – 1,250,000 > 1,250,000 |
P1 P2 P3 P4 |
| Activation | Exogenous | X | |
| White blood cells (WBCs) | Total WBCs Neutrophils |
Above baseline ⩽ baseline Above baseline ⩽ baseline |
A B α β |
Mautner et al,21 however, argued that the PAW system did not address the effects of the RBC content on PRP preparations and recommended the PLRA classification (Platelet count, Leukocyte content, RBC content, Activation) (Table 4). This system was the first to recommend documentation of the volume of PRP used and the absolute platelet concentration. The authors also suggested the frequency of PRP treatments be recorded if multiple treatments were delivered.
Table 4.
PLRA (Platelet count, Leukocyte content, RBC content, Activation) classification
| Criteria | Final Score | |
|---|---|---|
| P Platelet count | ____P Volume Injected |
_____M Cells/µL |
| L Leucocyte content* | > 1% < 1% |
+ – |
| R Red blood cell content | > 1% < 1% |
+ – |
| A Activation** | Yes No |
+ – |
If white blood cells are present (+), percentage of neutrophils should be reported.
The method of exogenous activation should be reported.
Magalon et al22 proposed a classification system (Table 5) focused on the quality of the preparation. The DEPA classification (Dose, Efficiency, Purity, Activation) analyses aspects of the production process that were not previously taken into consideration. However, it does not analyse the content of the PRP based on different cell types to the same quantitative level as the PLRA classification.
Table 5.
DEPA (Dose, Efficiency, Purity, Activation) classification
| Subgroup | Description | |
|---|---|---|
| Dose of injected platelets | Very high High Medium Low |
> 5 Billion injected platelets 3–5 Billion 1–3 Billion < 1 Billion |
| Efficiency of production | High Medium Low Poor |
Recovery rate in platelets > 90% 70–90% 30–70% < 30% |
| Purity of PRP | Very pure Pure Heterogeneous Whole-blood |
Platelets in PRP > 90% 70–90% 30–70% < 30% |
| Activation process | Autologous thrombin Calcium chloride |
The latest PRP classification system is MARSPILL (Method, Activation, RBCs, Spin, Platelet concentration, Image guided, Leukocyte concentration, Light activation), developed by Lana et al23 (Table 6). This is an amalgamation of previous systems, incorporating aspects of the manufacturing process as well as analysing the subgroups of cell types. They believe the focus on peripheral blood mononuclear cells is as important as platelet concentration, and they also incorporate newer concepts such as light activation and the use of image guidance to provide more elements to the classification system.
Table 6.
MARSPILL classification
| M | Method | Handmade Machine |
H M |
| A | Activation | Activated Non-activated |
A+ A– |
| R | Red blood cells | Rich Poor |
RBC-R RBC-P |
| S | Spin | One spin Two spins |
Sp1 Sp2 |
| P | Platelet concentration | PL 2–3 PL 4–6 PL 6–8 PL 8–10 |
|
| I | Image guided | Guided Not-guided |
G+ G– |
| L | Leukocyte concentration | Rich Poor |
Lc-R Lc-P |
| L | Light activation | Activated Not-activated |
A+ A– |
Numerous authors have developed classification systems for PRP, with each new generation taking into account additional factors that their predecessors had not considered. Due to the rapidly evolving nature of this field and the increased complexity of each preparation, there has not been widespread uptake of a single classification. In turn, none of the aforementioned classifications have been validated at an international consensus level. Barriers to widespread use of these classifications include the problem of oversimplification, such that researchers are wary of the earlier classification systems, which may classify their formulation as equal to other products that have not had favourable outcomes. Alternatively, if the trend of classifications becomes ever more complex, it may pose financial barriers to smaller research groups who may not have the resources to analyse their preparations to the same standard as large-scale pharmaceutical corporations.13
Mechanism of action
Platelets are anucleated cytoplasmic fragments of megakaryocytes that differentiate down the myeloid cell lineage.24 They contain α-granules, often thought of as the storage units of platelets,25 which studies suggest contain an abundance of growth factors (GFs). These are believed to influence inflammation, angiogenesis, stem cell migration and cell proliferation.5 Platelets are well known to be the initiators of the healing process; however, not all tissues have a rich blood supply, for example tendons, ligaments and cartilage. This results in relatively low levels of GFs being available to these tissues to enact effective healing. Application of PRP to these, and other, areas can therefore introduce supra-physiological levels of GFs to theoretically stimulate resolution of chronic pathological processes. Commercial ELISA (Vector Laboratories, Burlingame, CA; Quantikine Immunoassay, R&D Systems, Minneapolis, Minnesota) and Luminex kits (Luminex Corporation, Austin, Texas) were used to accurately quantify GFs in software based statistical analysis in the following section.
Once recruited to an area of injury, platelet adhesion is facilitated through adhesive glycoproteins secreted by α-granules,26 including vitronectin, fibronectin, thrombospondin and von Willebrand factor.27,28 Once the clot is formed the platelets are activated,29 allowing the release of the GFs from α-granules to stimulate healing.
There are myriad GFs contained within α-granules, of which the complex interchange amongst them is hypothesized to be of additional benefit to the healing process beyond simply introducing a higher concentration of platelets at hypovascular sites.24
Growth factors enact their functions primarily via ligand binding to associated extracellular cell surface receptors, which signal intracellular cytoplasmic proteins to attach to phosphorylated tyrosine. This is followed by multiple phosphorylation and activation steps of protein kinases within the cytoplasm, finally leading to translocation of a phosphorylated kinase to the cell nucleus. This phosphorylates transcription factors enabling gene transcription and ultimately the execution of the encoded function.30,31
Growth factors contained within α-granules thought to be crucial to the efficacy of PRP include platelet-derived growth factor (PDGF), VEGF, the transforming growth factor-β superfamily (TGF-β), fibroblast growth factor (FGF) and insulin-like growth factor (IGF). PDGF is able to initiate callus formation via chemotaxis and mitogenesis of fibroblasts and chondrocytes,32,33 along with chemotaxis of mesenchymal stem cells (MSCs).34 The promotion of endothelial cell proliferation by PDGF also has an important role in angiogenesis.35 VEGF is involved in neovascularization through its strong endothelial chemokine and mitogenic properties.36 TGF-β is well established as a promoter of chondrogenesis,37 but has also been shown to: stimulate osteogenic MSC differentiation38 and undifferentiated mesenchymal cell proliferation; regulate the mitogenic effects of other GFs; and inhibit macrophage and lymphocyte proliferation.39 The FGF family is involved in multiple biological processes including osteoblastogenesis,38,40 growth and differentiation of chondrocytes and MSCs.39 IGF regulates the proliferation and maturation of chondrocytes41,42 and IGF-1 may down-regulate expression of programmed cell death 5 (PDCD5), thereby inhibiting apoptosis of osteoarthritic chondrocytes.43
In addition to GF release following platelet activation, Xie et al44 demonstrated that PRP also forms a fibrin gel, which acts as a conductive bioscaffold to allow incorporation of migrating cells for tendon healing. Entrapment of GFs within a fibrin matrix45,46 may hold the key to controlled release of GFs at the intended site of action. However, it is important to note that cellular response to GFs is limited by number of target receptors available on cell surfaces, therefore high platelet concentrations and subsequent GF release may not be of benefit.26 This may explain why PRP preparations with GFs over six times the physiological concentration may have an inhibitory effect.47
This leads on to an important point, that while there are many GFs that have been shown to have beneficial effects on cartilage, tendons, bone and other tissues, there are other components that can have negative effects such as pro-inflammatory cytokines, matrix metalloproteinases (MMPs) and interleukin-1β (IL-1β).48 For example, Browning et al49 demonstrated an increase in MMP-1 and MMP-3 in osteoarthritis (OA) synoviocytes incubated with PRP. Thereby suggesting PRP application to joints may lead to accelerated cartilage breakdown due to a pro-inflammatory response. Most in vitro studies support PRP use in cartilage tissue because of the ability to increase chondrocyte proliferation and production of matrix molecules whilst not affecting chondrogenic phenotype.50 However, the importance of platelet-derived GF dosage has also been highlighted through the different results they can produce.51
Perhaps the biggest area of controversy surrounding PRP is the concentration of cellular components, particularly leucocytes. There has been debate around whether leucocytes are adverse because of cytokines causing inflammation and subsequent weaker fibrotic tissue and/or proteases and reactive oxygen species they release,50 or beneficial as a result of cytokines that can prevent infection and improve healing.16 This is something we will explore in the following section.
Applications
The ubiquitous nature of the mechanism of action of PRP suggests that, in theory, it can be applied to multiple pathologies to aid the body’s natural healing processes. We will look at these pathologies in detail and the type of PRP used (see Table 7). Unless stated, all the evidence included in this section is either level 1 (systematic review of randomized controlled trials [RCTs] or individual RCT) or level 2 (systematic review of cohort studies and RCTs). The Coleman Modified Scores given are the average of the papers analysed.
Table 7.
Summarized platelet-rich plasma (PRP) evidence by indication and PRP type
| Indication | Findings | PRP type studied |
|---|---|---|
| Osteoarthritis |
Laver et al
Improved symptoms with PRP at 12-month follow up. Significantly improved in 7 of the 9 RCTs included. Trend towards improved outcomes in younger patients/early OA changes with PRP. Chang et al Improved functional outcomes with PRP up to a year post treatment. Less severe OA showed more benefit from PRP. |
|
| Lateral epicondylitis |
Ben-Nafa and Munro
Improved outcomes for up to 2 years post treatment with PRP compared to corticosteroid injection. |
 |
| Achilles tendinopathy |
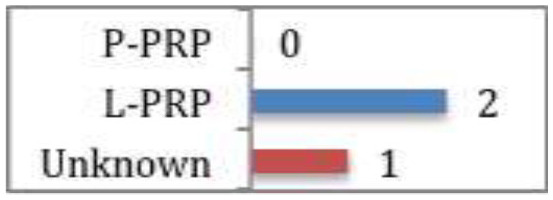
Gholami et al
No clinical benefit shown between PRP and saline placebo injection or eccentric loading programme. |
 |
| Patella tendinosis |
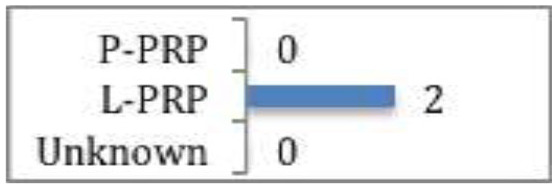
Dupley and Charalambous
Statistically significant improvement in functional scores at 6 months post treatment with PRP compared to dry needling or extracorporeal shockwave therapy (ESWT). |
 |
| Rotator cuff disease |
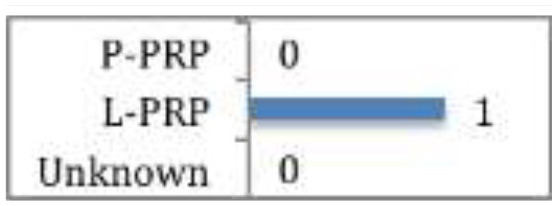
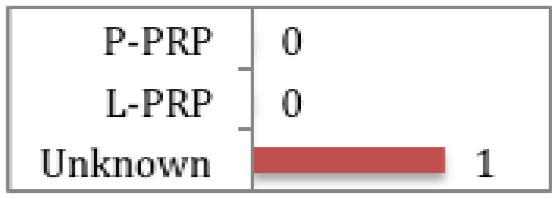
Kesikburun et al
No difference demonstrated between PRP and saline at any follow up point (followed up for 1 year) for rotator cuff tendinopathy or partial tears. Rha et al Significant functional improvement and greater symptomatic relief at 6 weeks to 6 months post treatment with PRP compared to dry needling for partial tears. |
|
| Acute muscle injury |
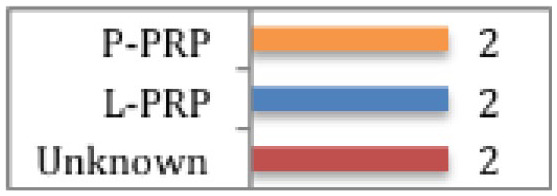
Grassi et al
Meta-analysis demonstrated statistically significant reduction in return to sport time (7.17 days) with PRP compared to controls (none/haematoma evacuation/saline injection/platelet-poor plasma (PPP) injection). Subgroup analysis of only double-blinded RCTs (both using P-PRP) showed no difference between PRP and controls (haematoma evacuation/saline injection). Subgroup analysis of hamstring injuries (2 using L-PRP, 1 using P-PRP) showed no difference between PRP and controls (none/saline injection/PPP injection). |
 |
| Surgical augmentation: rotator cuff repair |
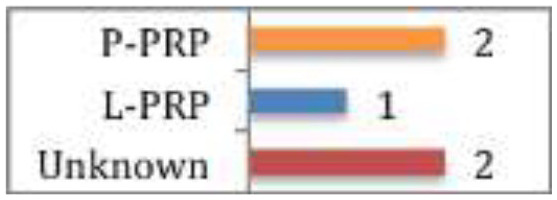
Cohn et al
Of the 5 studies included, 1 showed less pain in the early post-operative period and increased strength of external rotation at 3 months post op with L-PRP + surgery. Another study showed a 20% reduction in re-rupture rate and significant improvement in shoulder function post op in PRP + surgery (PRP type unknown). The other 3 studies showed no significant differences with the addition of PRP. |
 |
| Surgical augmentation: ACL reconstruction |
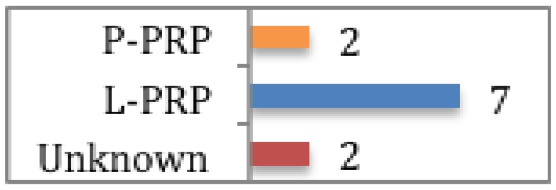
Figueroa et al
Of the 9 RCTs included, 2 studies showed PRP might reduce graft maturity time (one used L-PRP, the other type was unknown). |
 |
| Sacroiliac joint instability |
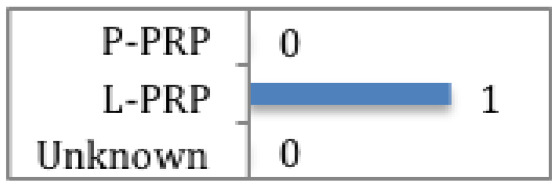
Ko et al
Clinically and statistically significant improvement in pain at 12 months post treatment with L-PRP. Clinically significant improvement still present at 4 years post treatment. |
 |
Note. P-PRP, pure platelet-rich plasma; L-PRP, leukocyte-rich platelet-rich plasma; RCT, randomized controlled trial; ACL, anterior cruciate ligament.
Tendinopathies
The majority of research into PRP treatment for tendinopathy centres on lateral epicondylitis, where PRP has been shown through systematic review52 to have a better, albeit delayed, therapeutic effect compared to corticosteroid injection for up to two years post injection (CMS 53). Three of the five RCTs analysed used leukocyte-rich PRP (L-PRP), the others did not document the type of PRP used. On further analysis, the RCTs that showed the most significant improvements compared to corticosteroid, were those documenting L-PRP was used.
Systematic review and meta-analyses of studies assessing PRP efficacy in Achilles tendinopathy53 showed that PRP conferred no clinical benefit when compared to saline placebo or an eccentric loading programme (CMS 65). Two of the studies used L-PRP, the other did not document the type of PRP used.
A systematic review and meta-analysis of two RCTs assessing L-PRP efficacy for patellar tendinosis54 suggested that PRP was statistically better than dry needling or extracorporeal shockwave therapy at six months post treatment (CMS 66).
There have been two RCTs assessing PRP versus saline injection55 and dry needling56 respectively in the treatment of rotator cuff disease (tendinopathy or partial tears). Rha et al56 found that PRP provided more symptomatic relief and functional improvement (based on greater reduction in shoulder pain and disability index) at six weeks to six months post injection than dry needling (CMS 66). The type of PRP was not documented. Whereas, Kesikburun et al55 found no difference between L-PRP and saline injections at any follow-up point up to a year post injection (CMS 71).
The combined evidence for PRP efficacy in tendinopathies shows that in the studies where PRP has shown statistical improvement to control measures, it is L-PRP that has been used.
Cartilage pathology
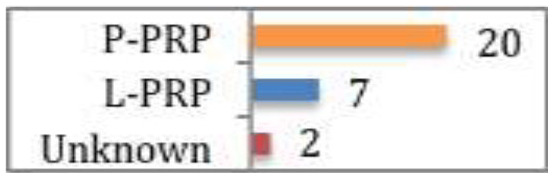
Laver et al57 reviewed all studies that assessed PRP for the treatment of degenerative cartilage pathology. A total of 29 studies were included, nine prospective RCTs, four prospective comparative studies, 14 case series, and two retrospective comparative studies. Of the nine RCTs, all reported improved symptoms with PRP groups at the final 12-month follow up, seven of which were significantly superior results. Generally, all studies appear to show overall positive results and clinical benefit from PRP, irrespective of methodological variation. Interestingly, there was a trend towards improved outcomes in either patients of younger age or early OA changes. Only one study followed up patients beyond 12 months (to two years). In this study, while there was symptomatic improvement at 12 months follow up; there was significant decrease in functional scores at two years, albeit still higher than the baseline level (CMS 61). Twenty studies used pure PRP (P-PRP), seven studies used L-PRP and two studies did not document PRP leukocyte content. Of the nine RCTs reporting improved outcomes, eight used P-PRP, while one used L-PRP. Whilst not directly investigated, these findings suggest P-PRP is more suitable to intra-articular pathology.
Further review and meta-analysis by Chang et al58 reinforced the findings of Laver et al.57 Specifically that less severe OA benefits more from PRP, and PRP is likely to be superior to hyaluronic acid for functional outcomes and have longer duration of action (up to a year).
A case series by Ko et al59 (level 4) has even shown L-PRP can significantly reduce chronic low back pain in patients with sacroiliac joint (SIJ) instability when injected under ultrasound guidance into the SIJ, lasting up to four years (CMS 59).
Acute muscle injuries
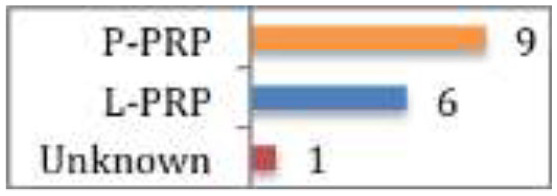
A systematic review and meta-analysis of six RCTs assessing the effectiveness of PRP in reducing return to sport times, demonstrated that when taking into account all six studies, the return to sport time was significantly shorter (by 7.17 days) in the PRP group (CMS 67).60 However, when only the double-blinded studies or studies including only hamstring injury were included in the analysis, no significant difference was noted. In addition, re-injury rates were similar between PRP and controls across studies. There were no significant differences regarding pain, muscle strength, flexibility, muscle function or healing (on ultrasound scan or magnetic resonance imaging).60 Two studies used P-PRP, two used L-PRP, and two did not document PRP type. These findings suggest that when return to play as early as possible is the primary motivation (such as for professional sport) it can be worth using PRP. However, the results are varied and the type of PRP best suited is unknown.
Surgical augmentation
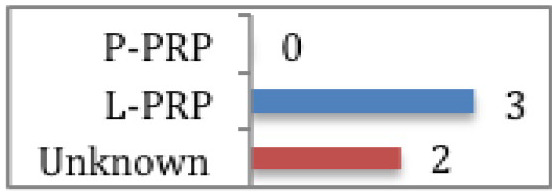
Multiple studies have looked at the use of PRP as an augmentation for surgery to expedite healing and recovery time. The majority of studies assessing this are focussed on rotator cuff repair and anterior cruciate ligament (ACL) surgery. Cohn et al61 reviewed five RCTs assessing the effect of PRP versus no treatment in conjunction with rotator cuff repair. Only two of the studies showed any benefit. Randelli et al62 demonstrated less pain in the early post-operative period and increased strength of external rotation at three months post-operatively in the L-PRP group (CMS 76). Interestingly, subgroup analysis of grade 1 and 2 tears showed greater strength of external rotation from 3 to 24 months post-operatively, suggesting milder tears may benefit more from L-PRP. Jo et al63 looked at PRP efficacy in large rotator cuff tears and found that re-rupture was 20% lower in the PRP + surgery group compared with surgery alone, as well as the overall shoulder function being significantly better (CMS 73). However, the type of PRP used was not described. The other RCTs showed no significant differences in peri-operative morbidity, clinical outcomes of structural integrity between PRP + surgery and surgery alone. Two of the studies used P-PRP while the other did not specify the PRP classification. Overall, these results show L-PRP may be of benefit in rotator cuff repair. A 20% reduction in large tear re-rupture is certainly worth the addition of PRP. However, the type was not documented. Interestingly, of the three RCTs showing no benefit with these tendon injuries, two used P-PRP and the other was unspecified.
A systematic review of nine RCTs and two cohort studies assessing PRP use in ACL surgery64 (level 3) showed there is evidence that adding PRP to the graft or tunnels could be beneficial in expediting graft maturity (CMS 60). Seven studies used L-PRP, two used P-PRP and two did not document PRP type. Similarly to muscle injuries, where early return to play is a crucial, these finding suggest the addition of PRP during ACL reconstruction may be of benefit. However, the type of PRP is again unclear.
Discussion
The breadth of applications for PRP in orthopaedics is vast. There have been encouraging results in a multitude of studies focussing on different potential indications. However, the sheer scale of heterogeneity across studies makes it difficult to draw clear conclusions from promising results. In addition, many studies will group soft tissue injuries together in their analysis, thereby further compounding the heterogeneity and potentially obscuring the true impact that PRP may have on specific soft tissue pathologies.
Too often the classification of PRP is not made clear, making it difficult to establish trends of PRP efficacy for differing pathologies. This is especially important when many clinicians are matching the type of PRP to specific pathologies, based on loose clinical indications from studies where the primary aim was not the comparison of PRP types for specific pathologies. Throughout the applications section we have highlighted the PRP type used in each study and, in conjunction with the results, thereby suggested which PRP type appears more effective for each indication based on the analysed evidence. However, it must be emphasized that no specific trends of impaired or improved outcomes of one PRP type over another have been observed for any indication. This is due to the methodologies of the analysed studies not being specifically designed to answer the question ‘Which PRP type is best for this indication?’ Therefore, as there is no direct comparison between these two PRP formulations for any indication, definitive conclusions cannot be made. This highlights the importance for future research to compare PRP formulation efficacy across applications, or at least state clearly what PRP formulation is being used so it can be accurately classified.
While there has been no direct comparison of PRP types for different applications within the literature, L-PRP appears to be more effective in chronic tendinopathies. This is due to the natural first stage of tendon healing including inflammation from leucocytes and catabolic cytokines.66 In contrast, P-PRP seems to be more beneficial in cartilage pathology.65,67 This may be because L-PRP has been shown to cause a significantly greater acute inflammatory response and increased synoviocyte cell death.67,68
Conclusion
Going forward, there needs to be standardization of certain parameters regarding PRP research. Murray et al69 have produced a comprehensive 23-statement checklist that all future clinical studies in PRP should adhere to, with the aim of streamlining PRP research towards yielding robust evidence.
Footnotes
ICMJE Conflict of interest statement: The authors declare no conflict of interest relevant to this work.
OA licence text: This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (CC BY-NC 4.0) licence (https://creativecommons.org/licenses/by-nc/4.0/) which permits non-commercial use, reproduction and distribution of the work without further permission provided the original work is attributed.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Pereira D, Peleteiro B, Araújo J, Branco J, Santos RA, Ramos E. The effect of osteoarthritis definition on prevalence and incidence estimates: a systematic review. Osteoarthritis Cartilage 2011;19:1270–1285. [DOI] [PubMed] [Google Scholar]
- 2. Weinstein AM, Rome BN, Reichmann WM, et al. Estimating the burden of total knee replacement in the United States. J Bone Joint Surg Am 2013;95:385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wielage RC, Myers JA, Klein RW, Happich M. Cost-effectiveness analyses of osteoarthritis oral therapies: a systematic review. Appl Health Econ Health Policy 2013;11:593–618. [DOI] [PubMed] [Google Scholar]
- 4. Hiligsmann M, Cooper C, Arden N, et al. Health economics in the field of osteoarthritis: an expert’s consensus paper from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Semin Arthritis Rheum 2013;43:303–313. [DOI] [PubMed] [Google Scholar]
- 5. Alves R, Grimalt R. A review of platelet-rich plasma: history, biology, mechanism of action, and classification. Skin Appendage Disord 2018;4:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andia I, Abate M. Platelet-rich plasma: underlying biology and clinical correlates. Regen Med 2013;8:645–658. [DOI] [PubMed] [Google Scholar]
- 7. Andia I, Rubio-Azpeitia E, Martin JI, Abate M. Current concepts and translational uses of platelet rich plasma biotechnology. In: Ekinci D, ed. Biotechnology. eBook: InTechOpen, 2015. [Google Scholar]
- 8. Sheth U, Dwyer T, Smith I, et al. Does platelet-rich plasma lead to earlier return to sport when compared with conservative treatment in acute muscle injuries? A systematic review and meta-analysis. Arthroscopy 2018;34:281–288.e1. [DOI] [PubMed] [Google Scholar]
- 9. Oxford Centre for Evidence-based Medicine. Levels of evidence, 2009. https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/ (date last accessed March 2009).
- 10. Dhurat R, Sukesh M. Principles and methods of preparation of platelet-rich plasma: a review and author’s perspective. J Cutan Aesthet Surg 2014;7:189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sabarish R, Lavu V, Rao SR. A comparison of platelet count and enrichment percentages in the platelet rich plasma (PRP) obtained following preparation by three different methods. J Clin Diagn Res 2015;9:ZC10–ZC12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lansdown DA, Fortier LA. Platelet-rich plasma: formulations, preparations, constituents, and their effects. Oper Tech Sports Med 2017;25:7–12. [Google Scholar]
- 13. Rossi LA, Murray IR, Chu CR, Muschler GF, Rodeo SA, Piuzzi NS. Classification systems for platelet-rich plasma. Bone Joint J 2019;101-B:891–896. [DOI] [PubMed] [Google Scholar]
- 14. Arora S, Agnihotri N. Platelet derived biomaterials for therapeutic use: review of technical aspects. Indian J Hematol Blood Transfus 2017;33:159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Etulain J, Mena HA, Meiss RP, Frechtel G, Gutt S, Negrotto S, et al. An optimised protocol for platelet-rich plasma preparation to improve its angiogenic and regenerative properties. Sci Rep 2018. 24;8:1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Degen RM, Bernard JA, Oliver KS, Dines JS. Commercial separation systems designed for preparation of platelet-rich plasma yield differences in cellular composition. HSS J 2017;13:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oudelaar BW, Peerbooms JC, Huis In ’t Veld R, Vochteloo AJH. Concentrations of blood components in commercial platelet-rich plasma separation systems: a review of the literature. Am J Sports Med 2019;47:479–487. [DOI] [PubMed] [Google Scholar]
- 18. Chahla J, Cinque ME, Piuzzi NS, et al. A call for standardization in platelet-rich plasma preparation protocols and composition reporting: a systematic review of the clinical orthopaedic literature. J Bone Joint Surg Am 2017;99:1769–1779. [DOI] [PubMed] [Google Scholar]
- 19. Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol 2009;27:158–167. [DOI] [PubMed] [Google Scholar]
- 20. DeLong JM, Russell RP, Mazzocca AD. Platelet-rich plasma: the PAW classification system. Arthroscopy 2012;28:998–1009. [DOI] [PubMed] [Google Scholar]
- 21. Mautner K, Malanga GA, Smith J, et al. A call for a standard classification system for future biologic research: the rationale for new PRP nomenclature. PM R 2015;7:S53–S59. [DOI] [PubMed] [Google Scholar]
- 22. Magalon J, Chateau AL, Bertrand B, et al. DEPA classification: a proposal for standardising PRP use and a retrospective application of available devices. BMJ Open Sport Exerc Med 2016;2:e000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lana JFSD, Purita J, Paulus C, et al. Contributions for classification of platelet rich plasma – proposal of a new classification: MARSPILL. Regen Med 2017;12:565–574. [DOI] [PubMed] [Google Scholar]
- 24. Lee KS. Ultrasound-guided platelet-rich plasma treatment: application and technique. In: Lee K, ed. Seminars in musculoskeletal radiology. New York, NY: Thieme Medical Publishers, 2016:422–431. [DOI] [PubMed] [Google Scholar]
- 25. Marx R. Platelet-function – assays and events: introduction. Suppl Thromb Haemost 1978;63:65–80. [PubMed] [Google Scholar]
- 26. Malhotra A, Pelletier MH, Yu Y, Walsh WR. Can platelet-rich plasma (PRP) improve bone healing? A comparison between the theory and experimental outcomes. Arch Orthop Trauma Surg 2013;133:153–165. [DOI] [PubMed] [Google Scholar]
- 27. Blair P, Flaumenhaft R. Platelet alpha-granules: basic biology and clinical correlates. Blood Rev 2009;23:177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rendu F, Brohard-Bohn B. The platelet release reaction: granules’ constituents, secretion and functions. Platelets 2001;12:261–273. [DOI] [PubMed] [Google Scholar]
- 29. Wroblewski AP, Mejia HA, Wright VJ. Application of platelet-rich plasma to enhance tissue repair. Oper Tech Orthop 2010;20:98–105. [Google Scholar]
- 30. Kawabata M, Imamura T, Miyazono K. Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev 1998;9:49–61. [DOI] [PubMed] [Google Scholar]
- 31. Bolsover SR, Hyams JS, Shephard EA, White HA, Wiedemann CG. Cell biology. Hoboken, NJ: John Wiley & Sons, 2003. http://doi.wiley.com/10.1002/047146158X (date last accessed 21 March 2019). [Google Scholar]
- 32. Ross R. Platelet-derived growth factor. Annu Rev Med 1987;38:71–79. [DOI] [PubMed] [Google Scholar]
- 33. Fujii H, Kitazawa R, Maeda S, Mizuno K, Kitazawa S. Expression of platelet-derived growth factor proteins and their receptor alpha and beta mRNAs during fracture healing in the normal mouse. Histochem Cell Biol 1999;112:131–138. [DOI] [PubMed] [Google Scholar]
- 34. Rasubala L, Yoshikawa H, Nagata K, Iijima T, Ohishi M. Platelet-derived growth factor and bone morphogenetic protein in the healing of mandibular fractures in rats. Br J Oral Maxillofac Surg 2003;41:173–178. [DOI] [PubMed] [Google Scholar]
- 35. Battegay EJ, Rupp J, Iruela-Arispe L, Sage EH, Pech M. PDGF-BB modulates endothelial proliferation and angiogenesis in vitro via PDGF beta-receptors. J Cell Biol 1994;125:917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bauer SM, Bauer RJ, Velazquez OC. Angiogenesis, vasculogenesis, and induction of healing in chronic wounds. Vasc Endovascular Surg 2005;39:293–306. [DOI] [PubMed] [Google Scholar]
- 37. Joyce ME, Roberts AB, Sporn MB, Bolander ME. Transforming growth factor-beta and the initiation of chondrogenesis and osteogenesis in the rat femur. J Cell Biol 1990;110:2195–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ng F, Boucher S, Koh S, et al. PDGF, TGF-β, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood 2008;112:295–307. [DOI] [PubMed] [Google Scholar]
- 39. Everts PAM, Knape JTA, Weibrich G, et al. Platelet-rich plasma and platelet gel: a review. J Extra Corpor Technol 2006;38:174–187. [PMC free article] [PubMed] [Google Scholar]
- 40. Fei Y, Xiao L, Doetschman T, Coffin DJ, Hurley MM. Fibroblast growth factor 2 stimulation of osteoblast differentiation and bone formation is mediated by modulation of the Wnt signaling pathway. J Biol Chem 2011;286:40575–40583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fisher MC, Meyer C, Garber G, Dealy CN. Role of IGFBP2, IGF-I and IGF-II in regulating long bone growth. Bone 2005;37:741–750. [DOI] [PubMed] [Google Scholar]
- 42. Lieberman JR, Daluiski A, Einhorn TA. The role of growth factors in the repair of bone: biology and clinical applications. J Bone Joint Surg Am 2002;84:1032–1044. [DOI] [PubMed] [Google Scholar]
- 43. Yin Z, Yang X, Jiang Y, et al. Platelet-rich plasma combined with agarose as a bioactive scaffold to enhance cartilage repair: an in vitro study. J Biomater Appl 2014;28:1039–1050. [DOI] [PubMed] [Google Scholar]
- 44. Xie X, Wang Y, Zhao C, et al. Comparative evaluation of MSCs from bone marrow and adipose tissue seeded in PRP-derived scaffold for cartilage regeneration. Biomaterials 2012;33:7008–7018. [DOI] [PubMed] [Google Scholar]
- 45. Laurens N, Koolwijk P, de Maat MPM. Fibrin structure and wound healing. J Thromb Haemost 2006;4:932–939. [DOI] [PubMed] [Google Scholar]
- 46. Clark RA. Fibrin and wound healing. Ann N Y Acad Sci 2001;936:355–367. [DOI] [PubMed] [Google Scholar]
- 47. El-Sharkawy H, Kantarci A, Deady J, et al. Platelet-rich plasma: growth factors and pro- and anti-inflammatory properties. J Periodontol 2007;78:661–669. [DOI] [PubMed] [Google Scholar]
- 48. Oh JH, Kim W, Park KU, Roh YH. Comparison of the cellular composition and cytokine-release kinetics of various platelet-rich plasma preparations. Am J Sports Med 2015;43:3062–3070. [DOI] [PubMed] [Google Scholar]
- 49. Browning SR, Weiser AM, Woolf N, et al. Platelet-rich plasma increases matrix metalloproteinases in cultures of human synovial fibroblasts. J Bone Joint Surg Am 2012;94:e1721–e1727. [DOI] [PubMed] [Google Scholar]
- 50. Filardo G, Kon E, Roffi A, Di Matteo B, Merli ML, Marcacci M. Platelet-rich plasma: why intra-articular? A systematic review of preclinical studies and clinical evidence on PRP for joint degeneration. Knee Surg Sports Traumatol Arthrosc 2015;23:2459–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Torricelli P, Fini M, Filardo G, et al. Regenerative medicine for the treatment of musculoskeletal overuse injuries in competition horses. Int Orthop 2011;35:1569–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ben-Nafa W, Munro W. The effect of corticosteroid versus platelet-rich plasma injection therapies for the management of lateral epicondylitis: a systematic review. SICOT J 2018;4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gholami M, Ravaghi H, Salehi M, Yekta AA, Doaee S, Jaafaripooyan E. A systematic review and meta-analysis of the application of platelet rich plasma in sports medicine. Electron Physician 2016;8:2325–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dupley L, Charalambous CP. Platelet-rich plasma injections as a treatment for refractory patellar tendinosis: a meta-analysis of randomised trials. Knee Surg Relat Res 2017;29:165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kesikburun S, Tan AK, Yilmaz B, Yaşar E, Yazicioğlu K. Platelet-rich plasma injections in the treatment of chronic rotator cuff tendinopathy: a randomized controlled trial with 1-year follow-up. Am J Sports Med 2013;41:2609–2616. [DOI] [PubMed] [Google Scholar]
- 56. Rha DW, Park G-Y, Kim Y-K, Kim MT, Lee SC. Comparison of the therapeutic effects of ultrasound-guided platelet-rich plasma injection and dry needling in rotator cuff disease: a randomized controlled trial. Clin Rehabil 2013;27:113–122. [DOI] [PubMed] [Google Scholar]
- 57. Laver L, Marom N, Dnyanesh L, Mei-Dan O, Espregueira-Mendes J, Gobbi A. PRP for degenerative cartilage disease: a systematic review of clinical studies. Cartilage 2017;8:341–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chang K-V, Hung C-Y, Aliwarga F, Wang T-G, Han D-S, Chen W-S. Comparative effectiveness of platelet-rich plasma injections for treating knee joint cartilage degenerative pathology: a systematic review and meta-analysis. Arch Phys Med Rehabil 2014;95:562–575. [DOI] [PubMed] [Google Scholar]
- 59. Ko GD, Mindra S, Lawson GE, Whitmore S, Arseneau L. Case series of ultrasound-guided platelet-rich plasma injections for sacroiliac joint dysfunction. J Back Musculoskelet Rehabil 2017;30:363–370. [DOI] [PubMed] [Google Scholar]
- 60. Grassi A, Napoli F, Romandini I, et al. Is platelet-rich plasma (PRP) effective in the treatment of acute muscle injuries? A systematic review and meta-analysis. Sports Med 2018;48:971–989. [DOI] [PubMed] [Google Scholar]
- 61. Cohn CS, Lockhart E, McCullough JJ. The use of autologous platelet-rich plasma in the orthopedic setting. Transfusion 2015;55:1812–1820. [DOI] [PubMed] [Google Scholar]
- 62. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg 2011;20:518–528. [DOI] [PubMed] [Google Scholar]
- 63. Jo CH, Shin JS, Lee YG, et al. Platelet-rich plasma for arthroscopic repair of large to massive rotator cuff tears: a randomized, single-blind, parallel-group trial. Am J Sports Med 2013;41:2240–2248. [DOI] [PubMed] [Google Scholar]
- 64. Figueroa D, Figueroa F, Calvo R, Vaisman A, Ahumada X, Arellano S. Platelet-rich plasma use in anterior cruciate ligament surgery: systematic review of the literature. Arthroscopy 2015;31:981–988. [DOI] [PubMed] [Google Scholar]
- 65. Meheux CJ, McCulloch PC, Lintner DM, Varner KE, Harris JD. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy 2016;32:495–505. [DOI] [PubMed] [Google Scholar]
- 66. Mishra A, Woodall J, Jr, Vieira A. Treatment of tendon and muscle using platelet-rich plasma. Clin Sports Med 2009;28:113–125. [DOI] [PubMed] [Google Scholar]
- 67. Braun HJ, Kim HJ, Chu CR, Dragoo JL. The effect of platelet-rich plasma formulations and blood products on human synoviocytes: implications for intra-articular injury and therapy. Am J Sports Med 2014;42:1204–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dragoo JL, Braun HJ, Durham JL, et al. Comparison of the acute inflammatory response of two commercial platelet-rich plasma systems in healthy rabbit tendons. Am J Sports Med 2012;40:1274–1281. [DOI] [PubMed] [Google Scholar]
- 69. Murray IR, Geeslin AG, Goudie EB, Petrigliano FA, LaPrade RF. Minimum information for studies evaluating biologics in orthopaedics (MIBO): platelet-rich plasma and mesenchymal stem cells. J Bone Joint Surg Am. 2017;99(10):809-819. [DOI] [PubMed] [Google Scholar]


