Summary
The study objective was to the assess level of detectable severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies in the urban population of Qatar. Antibody testing was performed on residual blood specimens for 112,941 individuals (∼10% of Qatar's urban population) attending for routine/other clinical care between May 12 and September 9, 2020. Seropositivity was 13.3% (95% confidence interval [CI] = 13.1–13.6%) and was independently associated with sex, age, nationality, clinical care encounter type, and testing date. Median optical density (antibody titer) among antibody-positive persons was 27.0 (range = 1.0–150.0), with higher values associated with age, nationality, clinical care encounter type, and testing date. Seropositivity by nationality was positively correlated with the likelihood of having higher antibody titers (Pearson correlation coefficient = 0.85; 95% CI = 0.47–0.96). Less than two in every 10 individuals in Qatar's urban population had detectable antibodies against SARS-CoV-2, suggesting this population is still far from herd immunity and at risk of subsequent infection waves. Higher antibody titer appears to be a biomarker of repeated exposures to the infection.
Subject areas: Health informatics, Public health, Infection control in health technology, Immunology
Graphical abstract
Highlights
-
•
Less than 20% of Qatar's urban population had detectable antibodies for SARS-CoV-2
-
•
Qatar's urban population is still at risk of future epidemic waves
-
•
Antibody titers varied by age, nationality, and over time
-
•
Higher antibody titers appear to be a biomarker of re-exposures to the infection
Health informatics; Public health; Infection control in health technology; Immunology
Introduction
With the breakthrough development of highly efficacious vaccines against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Polack et al., 2020; Jackson et al., 2020; Voysey et al., 2020), determining the population's cumulative infection exposure and current immunity level is critical to inform national vaccine roll-out strategies.
Qatar, located in the Arabian Peninsula, with a multinational population of 2.8 million people, nearly all living in the capital city, Doha, had a significant first wave of coronavirus disease 2019 (COVID-19) that peaked in late May 2020 (Planning and Statistics Authority- State of Qatar, 2020; Planning and Statistics Authority-State of Qatar, 2019). As of December 23, 2020, >60,000 infections per million population (number of infections divided by total population) had been laboratory confirmed (Hamad Medical Corporation, 2020b; Ministry of Public Health-State of Qatar, 2021). Qatar has a unique socio-demographic structure, in which single-unit and family households, including children, adults, and/or older adults, account for only 40% of the total population, with adults in this “urban population” often being part of the professional or service workforce (Planning and Statistics Authority- State of Qatar, 2020; Ministry of Interior-State of Qatar, 2020; Planning and Statistics Authority- State of Qatar, 2017). The remaining 60% of the population consists of craft and manual workers (CMWs) (Planning and Statistics Authority- State of Qatar, 2020; Ministry of Interior-State of Qatar, 2020; Planning and Statistics Authority- State of Qatar, 2017)—mostly single, young men working in development projects (Planning and Statistics Authority- State of Qatar, 2017) and typically living in large, shared accommodations (De Bel-Air, 2018).
Infection transmission in Qatar was first documented among CMWs on March 6, 2020 (Al Kuwari et al., 2020), who were subsequently most affected by this epidemic (Abu-Raddad et al., 2021a). A recently completed nationwide, population-based survey assessing “every” infection among the CMW population found that six out of every ten persons had detectable antibodies against SARS-CoV-2 (Al-Thani et al., 2021; Jeremijenko et al., 2021), suggesting that this population is at or near herd immunity for the variants circulating at this time (Jeremijenko et al., 2021; Anderson et al., 2020; Britton et al., 2020). In the present study, the first objective was to assess the level of infection exposure among the rest of the population of Qatar, that of the “urban population” of this country. The urban population was defined as the complement of the CMW population, that is, the population that lives in single-unit or family households (not shared accommodations) and is part of the professional or service workforce. This part of the population is highly diverse and includes over 150 nationalities in addition to Qataris. The second objective was to identify predictors for infection and for having higher antibody titers.
Results
In all, 112,941 individuals were tested for SARS-CoV-2 antibodies, representing ∼10% of the urban population of Qatar (Planning and Statistics Authority- State of Qatar, 2020) (Table 1). Of these, 51.6% were men. Two-thirds (66%) of tested persons were 20–49 years of age. Qatari (25.8%) and Indian nationals (16.5%) were most heavily represented in the sample, reflecting their representation in the urban population (Ministry of Interior-State of Qatar, 2020; Priya Dsouza Communications, 2019; Planning and Statistics Authority-State of Qatar, 2019). Blood specimens were collected in the course of routine clinical care during home care visits (34.2%), outpatient visits (28.5%), inpatient hospital stays (21.0%), and emergency department visits (16.4%). Overall, the sample mirrored the urban population demographics (Table S1 of supplemental information (SI)) (Primary Health Care Corporation, 2020, Ministry of Interior-State of Qatar, 2020; Planning and Statistics Authority- State of Qatar, 2017; Planning and Statistics Authority-State of Qatar, 2019).
Table 1.
Characteristics of tested individuals (112,941) and antibody positivity
| Characteristics | Tested |
Antibody positive |
Univariable regression analysis |
F Test |
Multivariable regression analysis |
|||
|---|---|---|---|---|---|---|---|---|
| N (%) | N (%a) | p value | ORa (95% CI) | p value | p valueb | AORa (95% CI) | p valuec | |
| Sex | ||||||||
| Women | 54,707 (48.4) | 4,387 (8.0) | <0.001 | 1.00 | 1.00 | |||
| Men | 58,234 (51.6) | 14,457 (18.3) | 2.59 (2.44–2.73) | <0.001 | <0.001 | 2.07 (1.95–2.21) | <0.001 | |
| Age (years) | ||||||||
| <10 | 3,384 (3.0) | 243 (7.1) | <0.001 | 1.00 | 1.00 | |||
| 10–19 | 5,557 (4.9) | 407 (7.3) | 1.04 (0.87–1.25) | 0.633 | <0.001 | 1.21 (1.00–1.46) | 0.049 | |
| 20–29 | 19,271 (17.1) | 2,867 (15.0) | 2.33 (2.03–2.69) | <0.001 | 2.04 (1.76–2.36) | <0.001 | ||
| 30–39 | 31,622 (28.0) | 5,533 (16.8) | 2.67 (2.33–3.06) | <0.001 | 2.21 (1.91–2.55) | <0.001 | ||
| 40–49 | 23,582 (20.9) | 4,876 (18.1) | 2.91 (2.53–3.34) | <0.001 | 2.47 (2.14–2.85) | <0.001 | ||
| 50–59 | 16,363 (14.5) | 3,220 (17.9) | 2.87 (2.50–3.31) | <0.001 | 2.46 (2.13–2.85) | <0.001 | ||
| 60–69 | 8,639 (7.6) | 1,281 (15.0) | 2.32 (2.00–2.69) | <0.001 | 2.12 (1.82–2.46) | <0.001 | ||
| 70–79 | 3,192 (2.8) | 315 (10.5) | 1.54 (1.29–1.84) | <0.001 | 1.73 (1.44–2.07) | <0.001 | ||
| 80+ | 1,331 (1.2) | 102 (7.5) | 1.06 (0.83–1.36) | 0.621 | 1.42 (1.10–1.83) | 0.007 | ||
| Nationality | ||||||||
| All other nationalitiesd | 24,799 (22.0) | 2,203 (8.0) | <0.001 | 1.00 | <0.001 | 1.00 | ||
| Bangladeshi | 7,773 (6.9) | 3,471 (41.9) | 8.32 (7.66–9.04) | <0.001 | 5.05 (4.63–5.50) | <0.001 | ||
| Nepalese | 4,962 (4.4) | 2,236 (38.2) | 7.10 (6.43–7.85) | <0.001 | 4.26 (3.87–4.69) | <0.001 | ||
| Pakistani | 5,114 (4.5) | 1,419 (23.9) | 3.61 (3.23–4.04) | <0.001 | 3.45 (3.07–3.87) | <0.001 | ||
| Indian | 18,590 (16.5) | 4,114 (17.5) | 2.44 (2.24–2.65) | <0.001 | 1.95 (1.80–2.13) | <0.001 | ||
| Sri Lankan | 2,252 (2.0) | 556 (17.3) | 2.40 (2.06–2.81) | <0.001 | 1.85 (1.60–2.13) | <0.001 | ||
| Filipino | 7,085 (6.3) | 1,100 (12.1) | 1.59 (1.42–1.77) | <0.001 | 1.59 (1.41–1.78) | <0.001 | ||
| Sudanese | 3,954 (3.5) | 466 (11.3) | 1.47 (1.25–1.73) | <0.001 | 1.36 (1.15–1.61) | <0.001 | ||
| Egyptian | 9,329 (8.3) | 1,150 (10.7) | 1.38 (1.23–1.53) | <0.001 | 1.33 (1.19–1.48) | <0.001 | ||
| Qatari | 29,083 (25.8) | 2,129 (7.1) | 0.88 (0.80–0.97) | 0.008 | 0.95 (0.86–1.04) | 0.266 | ||
| Clinical care encounter type | ||||||||
| Emergency | 18,473 (16.4) | 3,333 (14.2) | <0.001 | 1.00 | <0.001 | 1.00 | ||
| Inpatient | 23,720 (21.0) | 6,308 (19.4) | 1.45 (1.35–1.57) | <0.001 | 1.19 (1.10–1.28) | <0.001 | ||
| Outpatient | 32,146 (28.5) | 5,264 (13.1) | 0.91 (0.85–0.98) | 0.011 | 0.87 (0.81–0.94) | 0.001 | ||
| Home care/follow-up consultations | 38,602 (34.2) | 3,939 (9.2) | 0.61 (0.56–0.66) | <0.001 | 0.72 (0.66–0.78) | <0.001 | ||
| Antibody test date | ||||||||
| Calendar date (a linear term) | 112,941 (100.0) | 18,844 (13.3) | <0.001 | 0.999 (0.998–1.000) | 0.123 | 0.123 | 1.002 (1.001–1.003) | <0.001 |
AOR, adjusted odds ratio; CI, confidence interval; OR, odds ratio.
Estimates are proportions of antibody-positive persons among those tested, weighted by sex, age, and nationality.
Covariates with p value ≤0.2 in the univariable analysis were included in the multivariable analysis.
Covariates with p value ≤0.05 in the multivariable analysis were considered strong predictors of anti-SARS-CoV-2 positivity after adjustment for sex, age, nationality, clinical care encounter type, and antibody test date.
These include other nationalities residing in Qatar.
A total of 18,844 individuals had detectable SARS-CoV-2 antibodies—a weighted antibody positivity of 13.3% (95% confidence interval [CI]: 13.1–13.6%). Seropositivity was independently associated with sex, age, nationality, clinical care type, and calendar date of the antibody test in the multivariable regression analysis (Table 1). Men had two-fold higher odds of being seropositive (adjusted odds ratio [AOR] of 2.07; 95% CI: 1.95–2.21) than women. Similarly, the AOR was two-fold higher for adults aged 20–79 years than for children <10 years. Seropositivity varied by nationality. Compared to other nationalities, AOR was 5.05 (95% CI: 4.63–5.50) for Bangladeshis, 4.26 (95% CI: 3.87–4.69) for Nepalese, 3.45 (95% CI: 3.07–3.87) for Pakistanis, 1.95 (95% CI: 1.80–2.13) for Indians, 1.85 (95% CI: 1.60–2.13) for Sri Lankans, 1.59 (95% CI: 1.41–1.78) for Filipinos, 1.36 (95% CI: 1.15–1.61) for Sudanese, 1.33 (95% CI: 1.19–1.48) for Egyptians, and 0.95 (95% CI: 0.86–1.04) for Qataris. Compared to emergency department attendees, AOR was 0.87 (95% CI: 0.81–0.94) for outpatients and 0.72 (95% CI: 0.66–0.78) for patients with home care visits or follow-up consultations and 1.19 (95% CI: 1.10–1.28) for inpatients. There was evidence of increasing seropositivity over time (Table 1 and Table S2 of supplemental information) but at a slow rate. The AOR (per day) was only 1.002 (95% CI: 1.001–1.003; Table 1). Figure S1 of supplemental information shows seropositivity month by month, which was largely stable over the study duration.
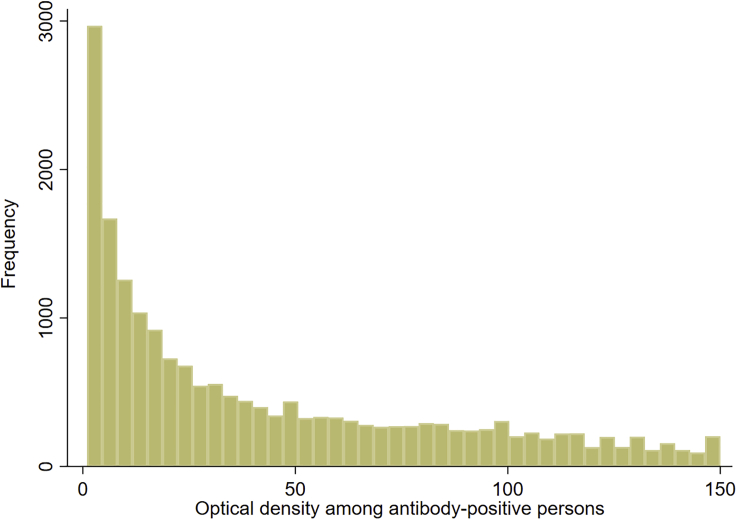
Figure 1 illustrates the distribution of antibody titers (optical density values) among the 18,844 antibody-positive persons. Optical density values ranged from 1.0 to 150.0 with a median of 27.0. Having higher antibody titers than the median was not associated with sex, but in the multivariable regression analysis, they were independently associated with age, nationality, clinical care type, and the calendar date of the antibody test (Table 2). Compared to those aged 20–29 years, the AOR was higher in children <10 years and adults aged 40–79 years. There were significant differences by nationality. AOR was 1.68 (95% CI: 1.45–1.94) for Bangladeshis, 1.54 (95% CI: 1.32–1.80) for Nepalese, 1.30 (95% CI: 1.05–1.61) for Filipinos, 1.22 (95% CI: 1.05–1.43) for Indians, 1.19 (95% CI: 0.98–1.44) for Pakistanis, 1.12 (95% CI: 0.87–1.44) for Sri Lankans, 1.04 (95% CI: 0.77–1.41) for Sudanese, 0.82 (95% CI: 0.67–1.01) for Egyptians, and 0.78 (95% CI: 0.65–0.94) for Qataris. Compared to emergency department attendees, inpatients had an AOR for higher antibody positivity of 0.38 (95% CI: 0.34–0.43), while no difference was found for outpatients or for patients with home care visits or follow-up consultations. Having higher antibody titers increased with time (Tables 2 and S3 of supplemental information), with an AOR (per day) of 1.011 (95% CI: 1.010–1.013; Table 2).
Figure 1.
Distribution of antibody titers (optical density values) among the 18,844 antibody-positive individuals identified in this study
Table 2.
Associations of antibody titers (optical densities) higher than the median value (≥27.0) among the 18,844 antibody-positive individuals
| Characteristics | Tested |
Higher antibody titers than the median |
Univariable regression analysis |
F Test |
Multivariable regression analysis |
|||
|---|---|---|---|---|---|---|---|---|
| N (%) | N (%a) | p value | ORa (95% CI) | p value | p valueb | AORa (95% CI) | p valuec | |
| Sex | ||||||||
| Women | 4,387 (23.3) | 2,175 (52.1) | 0.702 | 1.00 | 0.702 | – | – | |
| Men | 14,457 (76.7) | 7,244 (51.6) | 0.98 (0.88–1.09) | 0.702 | – | – | ||
| Age (years) | ||||||||
| 20–29d | 2,867 (15.2) | 1,360 (47.6) | <0.001 | 1.00 | <0.001 | 1.00 | ||
| <10 | 243 (1.3) | 170 (70.0) | 2.57 (1.92–3.45) | <0.001 | 2.85 (2.12–3.83) | <0.001 | ||
| 10–19 | 407 (2.2) | 210 (54.0) | 1.30 (1.02–1.65) | 0.036 | 1.19 (0.90–1.56) | 0.220 | ||
| 30–39 | 5,533 (29.4) | 2,701 (48.1) | 1.02 (0.92–1.13) | 0.692 | 0.98 (0.88–1.09) | 0.678 | ||
| 40–49 | 4,876 (25.9) | 2,442 (50.1) | 1.11 (1.00–1.22) | 0.051 | 1.13 (1.02–1.26) | 0.025 | ||
| 50–59 | 3,220 (17.1) | 1,634 (50.8) | 1.14 (1.02–1.27) | 0.020 | 1.20 (1.07–1.35) | 0.002 | ||
| 60–69 | 1,281 (6.8) | 700 (54.2) | 1.31 (1.14–1.50) | <0.001 | 1.46 (1.26–1.70) | <0.001 | ||
| 70–79 | 315 (1.7) | 158 (49.7) | 1.09 (0.86–1.38) | 0.480 | 1.38 (1.07–1.77) | 0.012 | ||
| 80+ | 102 (0.5) | 44 (41.7) | 0.79 (0.52–1.19) | 0.254 | 1.27 (0.82–1.97) | 0.281 | ||
| Nationality | ||||||||
| All other nationalitiese | 2,203 (11.7) | 1,064 (51.7) | <0.001 | 1.00 | <0.001 | 1.00 | ||
| Bangladeshi | 3,471 (18.4) | 1,946 (56.2) | 1.20 (1.04–1.38) | 0.012 | 1.68 (1.45–1.94) | <0.001 | ||
| Nepalese | 2,236 (11.9) | 1,099 (49.3) | 0.91 (0.78–1.06) | 0.210 | 1.54 (1.32–1.80) | <0.001 | ||
| Filipino | 1,100 (5.8) | 507 (46.2) | 0.80 (0.65–0.99) | 0.038 | 1.30 (1.05–1.61) | 0.017 | ||
| Indian | 4,114 (21.8) | 2,027 (51.0) | 0.97 (0.84–1.13) | 0.699 | 1.22 (1.05–1.43) | 0.011 | ||
| Pakistani | 1,419 (7.5) | 731 (55.5) | 1.16 (0.96–1.41) | 0.124 | 1.19 (0.98–1.44) | 0.076 | ||
| Sri Lankan | 556 (3.0) | 242 (45.2) | 0.77 (0.59–0.99) | 0.044 | 1.12 (0.87–1.44) | 0.392 | ||
| Sudanese | 466 (2.5) | 264 (53.2) | 1.06 (0.78–1.44) | 0.712 | 1.04 (0.77–1.41) | 0.789 | ||
| Egyptian | 1,150 (6.1) | 535 (49.0) | 0.90 (0.73–1.10) | 0.283 | 0.82 (0.67–1.01) | 0.062 | ||
| Qatari | 2,129 (11.3) | 1,004 (51.8) | 1.00 (0.84–1.21) | 0.959 | 0.78 (0.65–0.94) | 0.010 | ||
| Clinical care encounter type | ||||||||
| Emergency | 3,333 (17.7) | 1,866 (58.5) | <0.001 | 1.00 | <0.001 | 1.00 | ||
| Outpatient | 5,264 (27.9) | 3,189 (60.7) | 1.10 (0.97–1.25) | 0.136 | 1.06 (0.94–1.21) | 0.334 | ||
| Home care/follow-up consultations | 3,939 (20.9) | 2,195 (58.3) | 0.99 (0.86–1.14) | 0.915 | 1.00 (0.87–1.15) | 0.987 | ||
| Inpatient | 6,308 (33.5) | 2,169 (35.2) | 0.39 (0.34–0.44) | <0.001 | 0.38 (0.34–0.43) | <0.001 | ||
| Antibody test date | ||||||||
| Calendar date (a linear term) | 18,844 (100.0) | 9,419 (51.8) | <0.001 | 1.014 (1.012–1.016) | <0.001 | <0.001 | 1.011 (1.010–1.013) | <0.001 |
AOR, adjusted odds ratio; CI, confidence interval; OR, odds ratio.
Estimates are proportions of persons with antibody titers higher than the median among those antibody-positive, weighted by sex, age, and nationality.
Covariates with p value ≤0.2 in the univariable analysis were included in the multivariable analysis.
Covariates with p value ≤0.05 in the multivariable analysis were considered strong predictors of anti-SARS-CoV-2 positivity after adjustment for age, nationality, clinical care encounter type, and antibody test date.
The 20–29 age group was chosen as the reference group (instead of the <10 age group) because of the larger sample size and for epidemiological relevance.
These include other nationalities residing in Qatar.
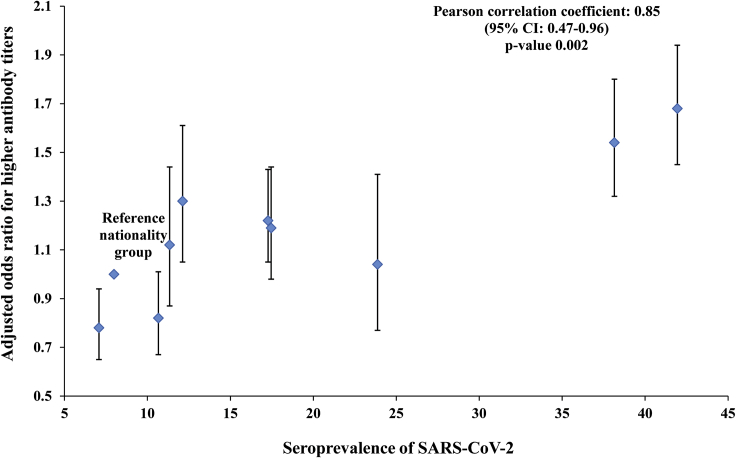
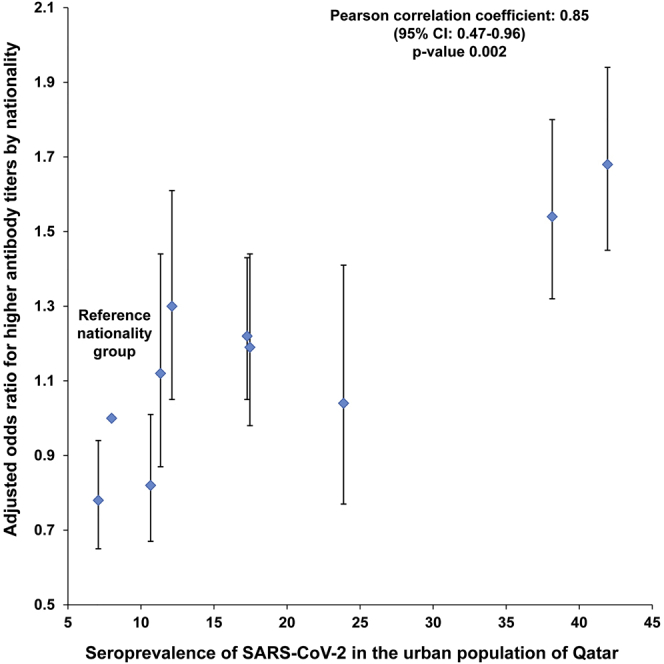
There was a strong correlation between the AOR for higher antibody titers in each nationality and the corresponding SARS-CoV-2 seroprevalence of that nationality (Figure 2). The Pearson correlation coefficient was 0.85 (95% CI: 0.47–0.96), possibly indicating that higher antibody titers correlate with repeated exposures to this coronavirus.
Figure 2.
Adjusted odds ratios for higher antibody titers (optical densities higher than the median value of 27.0) for each nationality (Table 2) versus the corresponding SARS-CoV-2 seroprevalence for that nationality (Table 1). Error bars indicate 95% confidence intervals.
Of the 18,844 antibody-positive persons, 9,375 had a polymerase chain reaction (PCR)-confirmed diagnosis prior to the antibody-positive test—47.1% (95% CI: 46.1–48.2%) (Table 3). Meanwhile, 1,085 of the 18,844 antibody-positive persons had or progressed to a severe infection and 393 had or progressed to critical infection. Thus, the infection severity rate was 3.9% (95% CI: 3.7–4.2%), the infection criticality rate was 1.3% (95% CI: 1.1–1.4%), and the combined infection severity or criticality rate was 5.2% (95% CI: 4.9–5.5%). With exactly 100 COVID-19 deaths recorded among the antibody-positive persons, the infection fatality rate was 0.3% (95% CI: 0.2–0.3%).
Table 3.
Key SARS-CoV-2 epidemiological measures assessed in the study.
| Epidemiological measure | Sample (denominator) | Positive for outcome (numerator) | Estimate in percentage (95% CI) |
|---|---|---|---|
| Sample antibody positivity (seropositivity) | 112,941 | 18,844 | 16.7 (16.5–16.9) |
| Weighted antibody positivity (seropositivity) | 112,941 | 18,844 | 13.3 (13.1–13.6)a |
| Proportion with prior PCR-confirmed diagnosisb | 18,844 | 9,375 | 47.1 (46.1–48.2)a |
| Infection severity ratec | 18,844 | 1,085 | 3.9 (3.7–4.2)a |
| Infection criticality rated | 18,844 | 393 | 1.3 (1.1–1.4)a |
| Infection severity or criticality ratee | 18,844 | 1,478 | 5.2 (4.9–5.5)a |
| Infection fatality ratef | 18,844 | 100 | 0.3 (0.2–0.3)a |
PCR, polymerase chain reaction; CI, confidence interval.
Estimates weighted by sex, age, and nationality.
Proportion of antibody-positive persons who had a prior SARS-CoV-2 PCR-confirmed diagnosis.
Number of infections that were severe per World Health Organization criteria (World Health Organization, 2020) over the total number of antibody-positive persons.
Number of infections that were critical per World Health Organization criteria (World Health Organization, 2020) over the total number of antibody-positive persons.
Number of infections that were severe or critical per World Health Organization criteria (World Health Organization, 2020) over the total number of antibody-positive persons.
Number of COVID-19 deaths per World Health Organization criteria (World Health Organization, 2020) over the total number of antibody-positive persons.
Discussion
The above results indicate that <20% of the urban population of Qatar, which constitutes ∼40% of the total population and includes nearly all older adults, manifests evidence of prior infection. This seroprevalence is substantially less than that in the CMW part of the population, which was estimated recently in a nationwide survey at 55.3% (Al-Thani et al., 2021), with the leading risk factor being living in a large shared accommodation (Al-Thani et al., 2021; Abu-Raddad et al., 2021a; Jeremijenko et al., 2021; Al Kuwari et al., 2020).
This finding suggests that the lockdown and imposed social and physical distancing restrictions have been more successful in slowing transmission in the urban population compared to the CMW population. Building on the totality of evidence on the Qatar epidemic (Abu-Raddad et al., 2021a; Al Kuwari et al., 2020; Al-Thani et al., 2021; Ayoub et al., 2021; Jeremijenko et al., 2021), this appears to be due to the dwelling structure, in that the urban population lives mostly in single-unit or family households that each includes a small number of individuals. Meanwhile, the CMW population lives mostly in large shared accommodations that each includes a large number of individuals. While the lockdown forced individuals to stay more at place of residence, it is typical to have more social contacts every day in a large shared accommodation than in a single-unit or family household. This outcome highlights the role of the “boarding school” effect in respiratory infection transmission, seen often in the intense influenza outbreaks in regular and boarding schools (Jackson et al., 2013; Glatman-Freedman et al., 2012). This effect has been also seen in the large SARS-CoV-2 outbreaks in nursing homes in Europe and the United States (Arons et al., 2020; Burton et al., 2020; Ladhani et al., 2020).
With a seroprevalence of <20%, the urban population of Qatar remains far below the herd immunity threshold, estimated at 60–70% infection exposure (Anderson et al., 2020; Britton et al., 2020; Jeremijenko et al., 2021). Accordingly, there should exist potential for subsequent waves of infection in this part of the population, but no second wave materialized from May 2020 up to end of this year (Abu-Raddad et al., 2021a; Ayoub et al., 2021), that is, before the introduction and expansion of the B.1.1.7 and B.1.351 variants of concern (Abu-Raddad et al., 2021b). On the contrary, only a slow increase in seroprevalence has occurred since the peak of the first wave (Table S2 of supplemental information), reflecting the actual low incidence of infection in Qatar up to end of 2020 (Ayoub et al., 2021). The absence of a new wave, despite the lack of a lockdown and easing of many social distancing restrictions, may be explained by an “immunity shield” effect (Weitz et al., 2020) arising from the social mixing between the urban and CMW populations. With most CMWs being immune, the infection transmission chains had difficulty in sustaining themselves, as they were interrupted by the presence of immune persons who were not getting reinfected (Abu-Raddad et al., 2021b, 2021c; Abu-Raddad et al., 2020. ciaa1846. https://doi.org/10.1093/cid/ciaa1846). The effective implementation of “ tuning,” an adjustment of restrictions by national policymakers based on the value, may have also contributed to preventing a new wave (Ayoub et al., 2021).
There were significant differences in seropositivity by sex, age, and nationality. These are probably not due to biological differences but to differences in the likelihood of exposure to the infection. Indeed, a small proportion of the specimens tested in this study belonged to CMWs who had a higher risk of exposure to the infection than the urban population (Al-Thani et al., 2021; Jeremijenko et al., 2021). While Hamad Medical Corporation (HMC) provides healthcare primarily to the urban population and other providers cater to the CMW population, HMC is a main tertiary care center in Qatar and was also the nationally designated provider for COVID-19 healthcare needs. Thus, it is likely that a small proportion of specimens, which cannot be estimated precisely, were drawn from CMWs who were hospitalized for COVID-19 or other reasons. This may explain the higher antibody positivity of young Bangladeshi, Indian, and Nepalese men (Table 1), who form the bulk of the CMW population (Al-Thani et al., 2021; Jeremijenko et al., 2021). This may also explain the higher seroprevalence in the blood specimens drawn during inpatient or emergency clinical care, which are more likely to be COVID-19 related, than those drawn during outpatient or home care/follow-up consultation clinical care (Table 1). The higher exposure among men aged 20–69 years probably reflects their more frequent work and other activities outside the home, whereas men aged ≥70 years, urged through public health messaging to remain at home, were more likely to do so, out of concern about infection severity.
The proportion of those antibody positive who had a PCR-confirmed diagnosis prior to the antibody-positive test was 47.1% (Table 3), much higher than the 9.3% in the CMW population (Al-Thani et al., 2021), and that estimated for the total population of Qatar (11.6%) (Ayoub et al., 2021). This is probably because study specimens were drawn from individuals receiving healthcare, including those hospitalized for COVID-19, people more likely to have been tested for the infection. This fact, along with the difference in age structure between the urban and CMW populations (Planning and Statistics Authority- State of Qatar, 2020; Ministry of Interior-State of Qatar, 2020; Planning and Statistics Authority- State of Qatar, 2017; Al-Thani et al., 2021), may have resulted in higher estimates of infection severity, criticality, and fatality rates in this study (Table 3), compared to the study of the CMW population (Al-Thani et al., 2021), or model predictions for the entire population of Qatar (Seedat et al., 2020).
Strikingly, having a higher antibody titer varied by nationality, clinical care type, and time (Table 2). Variation by nationality is probably an indirect biomarker of re-exposure to infection, resulting in repeated immune system reactivation. This is suggested by the very strong positive correlation between the odds of having a higher antibody titer and seroprevalence across the nationalities (Figure 2). Lower antibody titers were found in inpatients, but this may reflect COVID-19 hospitalizations for recent infections so that there was not sufficient time for higher antibody titers to develop. There was a trend of increasing “higher antibody titers” over time, which may reflect the growing pool of infected persons who have had more time to develop higher levels of detectable antibodies after infection or alternatively to being re-exposed to the infection.
Limitations of the study
This study has some limitations. The sample included individuals attending HMC for routine or other clinical care, but this population may not necessarily be representative of the wider urban population of Qatar. Though HMC is the main public healthcare provider and is widely accessible at minimal cost to nationals and residents, the sample may still have missed persons inclined to seek healthcare in the private sector or who avoided contact with healthcare during the COVID-19 pandemic. Some specimens may have been drawn from CMWs, who were not part of our intended study sample. However, the large sample size, equivalent to ∼10% of the urban population of Qatar, as well as the probabilistic weighting used in the analysis may have reduced inherent biases in our sample.
Laboratory methods were based on high quality, validated commercial platforms, such as the Roche platform used for serological testing (The Roche Group, 2020; Jahrsdörfer et al., 2020). The Roche platform is one of the most extensively used and investigated commercial platforms, with a specificity ≥99.8% (The Roche Group, 2020; Public Health England, 2020; Oved et al., 2020) and a sensitivity ≥89% (Jahrsdörfer et al., 2020; Abu-Raddad et al., 2021a; Oved et al., 2020). However, it is possible that the less than perfect sensitivity, especially for those with recent infections, may have underestimated the actual seroprevalence as it may take up to few weeks before recently infected individuals develop antibodies at detectable levels (Nasrallah et al., 2020; Wajnberg et al., 2020). Indeed, a recent investigation of the performance of three automated, commercial, serological platforms in Qatar, including the Roche platform, found that each of them missed ≥20% of individuals with past or current infections (Nasrallah et al., 2020). Factoring the less than perfect sensitivity and specificity (Sempos and Tian, 2021) would have increased the measured seroprevalence to 14.8% instead of 13.3%. The fatality rate may have been underestimated in this study as some individuals may have died before being tested or before developing detectable antibody levels.
In conclusion, fewer than two in every 10 individuals in the urban population of Qatar had detectable antibodies against SARS-CoV-2, suggesting that this population is still well below the herd immunity threshold and is potentially at risk from a subsequent epidemic wave. This emphasizes the need to maintain current social and physical distancing restrictions while SARS-CoV-2 vaccinations are being scaled up throughout the country. The findings also suggest that higher antibody titers appear to be a biomarker of repeated exposures to the infection.
STAR★methods
Key resources table
| REAGENT OR RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Residual blood specimens | Hamad Medical Corporation | Not applicable |
| Critical commercial assays | ||
| Roche Elecsys® anti-SARS-CoV-2 | Roche, Switzerland | https://diagnostics.roche.com/global/en/products/params/elecsys-anti-sars-cov-2.html |
| TaqPath™ COVID-19 Combo Kit | Thermo Fisher Scientific, USA | https://www.thermofisher.com/order/catalog/product/A47814 |
| AccuPower SARS-CoV-2 real-time RT-PCR Kit | Bioneer, Korea | https://us.bioneer.com/pagecat1/diagnostic/AccuPower-SARS-CoV2-RealTime-RT-PCR-Kit |
| cobas® SARS-CoV-2 test | Roche, Switzerland | https://diagnostics.roche.com/global/en/products/params/cobas-sars-cov-2-test.html |
| Deposited data | ||
| Antibody testing data | This paper | Not applicable |
| PCR testing data | This paper | Not applicable |
| Software and algorithms | ||
| STATA/SE 16.1 | StataCorp, USA | https://www.stata.com/ |
Resource availability
Lead contact
Further information and requests should be directed to lead contacts, Peter V. Coyle, Email: PCoyle@hamad.qa and Laith J. Abu-Raddad, Email: lja2002@qatar-med.cornell.edu.
Material availability
The study did not generate new reagents.
Data and code availability
All data are included in an aggregate form within the manuscript and its supplemental information.
Data sources
A retrospective analysis of residual blood specimens collected from May 12 to September 9, 2020 was conducted to assess the level of and associations with antibody positivity in the urban population of Qatar. Residual blood specimens were collected from individuals receiving routine and other clinical care at Hamad Medical Corporation (HMC), a main provider of healthcare to the urban population of this country and the nationally designated provider for Coronavirus Disease 2019 (COVID-19) healthcare needs. Qatar has a universal, quality, and modern healthcare system that is heavily subsidized and equally accessible to nationals and residents. The public healthcare system is organized into several internationally accredited entities, with HMC and the Primary Health Care Corporation (PHCC) centers typically serving the urban population, and the Qatar Red Crescent Society centers typically serving the CMW population (Al-Thani et al., 2021). It follows that the tested population in this study is broadly representative of the urban population, but not of the CMW population of Qatar.
Each person in this study contributed only one antibody test, the last test performed during the study period. Antibody data generated during the study were subsequently linked to the national centralized SARS-CoV-2 polymerase chain reaction (PCR) testing and hospitalization database, which includes records for all PCR testing and COVID-19 hospitalizations in Qatar since the start of the epidemic (Hamad Medical Corporation, 2020a). The database further includes the severity classification of hospitalized cases, based on individual chart reviews completed by trained medical personnel using the World Health Organization (WHO) criteria (World Health Organization, 2020). The study was approved by the HMC and Weill Cornell Medicine-Qatar Institutional Review Boards. The study was conducted following the ethics review boards guidelines and regulations.
Laboratory methods
Roche Elecsys Anti-SARS-CoV-2 (99.5% sensitivity (Muench et al., 2020), 99.8% specificity (The Roche Group, 2020; Muench et al., 2020); Roche, Switzerland), an electrochemiluminescence immunoassay, was used for antibody detection in the serological samples. Result interpretation followed manufacturer instructions: reactive for optical density (a proxy for antibody titer) cutoff index ≥1.0 and non-reactive for cutoff index <1.0 (The Roche Group, 2020).
Current infection was assessed using PCR testing of aliquots of Universal Transport Medium (UTM) used for nasopharyngeal and oropharyngeal swab collection (Huachenyang Technology, China). Aliquots were extracted on the QIAsymphony platform (QIAGEN, USA) and tested with real-time reverse-transcription PCR (RT-qPCR) using the TaqPath COVID-19 Combo Kit (100% sensitivity and specificity (Thermo Fisher Scientific, 2020); Thermo Fisher Scientific, USA) on an ABI 7500 FAST (Thermo Fisher, USA). Samples were also extracted using a custom protocol (Kalikiri et al., 2020) on a Hamilton Microlab STAR (Hamilton, USA) and tested using the AccuPower SARS-CoV-2 Real-Time RT-PCR Kit (100% sensitivity and specificity (Kubina and Dziedzic, 2020); Bioneer, Korea) on an ABI 7500 FAST, or loaded directly into a Roche cobas 6800 system and assayed with the cobas SARS-CoV-2 Test (95% sensitivity, 100% specificity (US Food and Drug Administration, 2020); Roche, Switzerland).
All laboratory testing was conducted at HMC Central Laboratory following standardized protocols.
Statistical analysis
Frequency distributions were used to describe sample characteristics and optical density among antibody-positive persons. Probability weights were applied to generate estimates representing the wider urban population. Weights were developed using population distributions by sex, age group, and nationality in the PHCC database (Primary Health Care Corporation, 2020). Since the PHCC caters mainly to the urban population of Qatar through 27 geographically-distributed centers, this database, which includes 1,468,837 registered users, describes the demographics of the urban population (Primary Health Care Corporation, 2020).
Associations with anti-SARS-CoV-2 positivity, as well as with higher antibody titers (defined as optical density higher than the median value) were investigated using chi-square tests and univariable logistic regression. Covariates with p values ≤0.2 in univariable regression analysis were included in the multivariable model. Covariates with p values ≤0.05 in the multivariable analysis were regarded as strong evidence for an association with the outcome. Odds ratios, adjusted ORs, 95% confidence intervals, and p values were reported.
The antibody database was linked to the SARS-CoV-2 PCR testing and hospitalization database to enable estimation of other epidemiologic metrics. The latter included the proportion of antibody-positive persons who had a diagnosis of SARS-CoV-2 confirmed by PCR prior to the antibody test. Numbers of infections that were classified as severe, critical, or fatal, according to WHO criteria (World Health Organization, 2020), among all antibody-positive persons, were used to estimate severity, criticality, and fatality rates.
All analyses were performed in STATA/SE 16.1 (StataCorp, 2019).
Acknowledgments
We thank Her Excellency Dr. Hanan Al Kuwari, Minister of Public Health, for her vision, guidance, leadership, and support. We also thank Dr. Saad Al Kaabi, Chair of the System Wide Incident Command and Control (SWICC) Committee for the COVID-19 national healthcare response, for his leadership, for analytical insights, and for his instrumental role in enacting data information systems that made these studies possible. We further extend our appreciation to SWICC Committee and Scientific Reference and Research Taskforce (SRRT) members for their informative input, scientific technical advice, and enriching discussions. We also thank Dr. Mariam Abdulmalik, CEO of the Primary Health Care Corporation and the Chairperson of the Tactical Community Command Group on COVID-19, as well as members of this committee, for providing support to the teams that worked on the field surveillance. We further thank Dr. Nahla Afifi, Director of Qatar Biobank (QBB), Ms. Tasneem Al-Hamad, Ms. Eiman Al-Khayat, and the rest of the QBB team for their unwavering support in retrieving and analyzing samples and in compiling and generating databases for COVID-19 infection, as well as Dr. Asmaa Al-Thani, Chairperson of the Qatar Genome Programme Committee and Board Vice Chairperson of QBB, for her leadership of this effort. We also acknowledge the dedicated efforts of the Clinical Coding Team and the COVID-19 Mortality Review Team, both at Hamad Medical Corporation, and the Surveillance Team at the Ministry of Public Health. The authors are grateful for support from Hamad Medical Corporation, the Ministry of Public Health, and the Biomedical Research Program and the Biostatistics, Epidemiology, and Biomathematics Research Core, both at Weill Cornell Medicine-Qatar. The statements made herein are solely the responsibility of the authors.
Author contributions
P.V.C. conceived and designed this study and led its implementation and antibody testing. H.C. managed the databases, performed the statistical data analyses, and co-wrote the first draft of the manuscript. L.J.A. led the statistical analyses and co-wrote the first draft of the article. All authors contributed to development of the study protocol, data collection, and acquisition, database development, discussions and interpretation of the results, and to the writing of the manuscript. All authors have read and approved the final manuscript.
Published: June 25, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102646.
Contributor Information
Peter V. Coyle, Email: pcoyle@hamad.qa.
Laith J. Abu-Raddad, Email: lja2002@qatar-med.cornell.edu.
Supplemental information
References
- Abu-Raddad L.J., Chemaitelly H., Ayoub H.H., Al Kanaani Z., Al Khal A., Al Kuwari E., Butt A.A., Coyle P., Jeremijenko A., Kaleeckal A.H. Characterizing the Qatar advanced-phase SARS-CoV-2 epidemic. Sci. Rep. 2021;11:6233. doi: 10.1038/s41598-021-85428-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Raddad, L.J., Chemaitelly, H., Ayoub, H.H., Coyle, P., Malek, J.A., Ahmed, A.A., Mohamoud, Y.A., Younuskunju, S., Tang, P., Kanaani, Z.A., et al 2021b. Epidemiologic Characterization of Introduction and Expansion of the SARS-CoV-2 B.1.1.7 Variant and its Reinfections in a National Population. Under review. [DOI] [PMC free article] [PubMed]
- Abu-Raddad L.J., Chemaitelly H., Coyle P., Malek J.A., Ahmed A.A., Mohamoud Y.A., Younuskunju S., Ayoub H.H., Al Kanaani Z., Al Kuwari E. SARS-CoV-2 reinfection in a cohort of 43,000 antibody-positive individuals followed for up to 35 weeks. E Clin. Med. 2021;35:100861. doi: 10.1016/j.eclinm.2021.100861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Raddad L.J., Chemaitelly H., Malek J.A., Ahmed A.A., Mohamoud Y.A., Younuskunju S., Ayoub H.H., Al Kanaani Z., Al Khal A., Al Kuwari E. Assessment of the risk of SARS-CoV-2 reinfection in an intense re-exposure setting. Clin. Infect. Dis. 2020:ciaa1846. doi: 10.1093/cid/ciaa1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Kuwari H.M., Abdul Rahim H.F., Abu-Raddad L.J., Abou-Samra A.-B., Al Kanaani Z., Al Khal A., Al Kuwari E., Al Marri S., Al Masalmani M., Al Romaihi H.E. Epidemiological investigation of the first 5685 cases of SARS-CoV-2 infection in Qatar, 28 February–18 April 2020. BMJ Open. 2020;10:e040428. doi: 10.1136/bmjopen-2020-040428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Thani M.H., Farag E., Bertollini R., Al Romaihi H.E., Abdeen S., Abdelkarim A., Daraan F., Ismail A., Mostafa N., Sahl M. SARS-CoV-2 infection is at herd immunity in the majority segment of the population of Qatar. Open Forum Infectious Diseases. 2021 doi: 10.1093/ofid/ofab221. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R.M., Heesterbeek H., Klinkenberg D., Hollingsworth T.D. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet. 2020;395:931–934. doi: 10.1016/S0140-6736(20)30567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arons M.M., Hatfield K.M., Reddy S.C., Kimball A., James A., Jacobs J.R., Taylor J., Spicer K., Bardossy A.C., Oakley L.P. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N. Engl. J. Med. 2020;382:2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoub H.H., Chemaitelly H., Seedat S., Makhoul M., Al Kanaani Z., Al Khal A., Al Kuwari E., Butt A.A., Coyle P., Jeremijenko A. Mathematical modeling of the SARS-CoV-2 epidemic in Qatar and its impact on the national response to COVID-19. J. Glob. Health. 2021;11:05005. doi: 10.7189/jogh.11.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton T., Ball F., Trapman P. A mathematical model reveals the influence of population heterogeneity on herd immunity to SARS-CoV-2. Science. 2020;369:846–849. doi: 10.1126/science.abc6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton J.K., Bayne G., Evans C., Garbe F., Gorman D., Honhold N., Mccormick D., Othieno R., Stevenson J.E., Swietlik S. Evolution and effects of COVID-19 outbreaks in care homes: a population analysis in 189 care homes in one geographical region of the UK. Lancet Healthy Longev. 2020;1:e21–e31. doi: 10.1016/S2666-7568(20)30012-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bel-Air F. Gulf Labour Markets and Migration; 2018. Demography, Migration, and Labour Market in Qatar.https://www.researchgate.net/publication/323129801_Demography_Migration_and_Labour_Market_in_Qatar-_UPDATED_June_2017 [Google Scholar]
- Glatman-Freedman A., Portelli I., Jacobs S.K., Mathew J.I., Slutzman J.E., Goldfrank L.R., Smith S.W. Attack rates assessment of the 2009 pandemic H1N1 influenza A in children and their contacts: a systematic review and meta-analysis. PLoS One. 2012;7:e50228. doi: 10.1371/journal.pone.0050228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamad Medical Corporation 2020a. National SARS-CoV-2 PCR Testing, Infection Severity, and Hospitalization Database.
- Hamad Medical Corporation 2020b. SARS-CoV-2 Hospitalizations and Care.
- Jackson C., Vynnycky E., Hawker J., Olowokure B., Mangtani P. School closures and influenza: systematic review of epidemiological studies. BMJ Open. 2013;3:e002149. doi: 10.1136/bmjopen-2012-002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., Mccullough M.P., Chappell J.D., Denison M.R., Stevens L.J. An mRNA vaccine against SARS-CoV-2 - preliminary report. N. Engl. J. Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahrsdörfer B., Kroschel J., Ludwig C., Corman V.M., Schwarz T., Körper S., Rojewski M., Lotfi R., Weinstock C., Drosten C. Independent side-by-side validation and comparison of four serological platforms for SARS-CoV-2 antibody testing. J. Infect. Dis. 2020;223:796–801. doi: 10.1093/infdis/jiaa656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeremijenko A., Chemaitelly H., Ayoub H.H., Abdulla M.A.H., Abou-Samra A.B., Al Ajmi J.A.A.A., Al-Ansari N.A.A., Al Kanaani Z., Al Khal A., Al Kuwari E. Evidence for and level of herd immunity against SARS-CoV-2 infection: the ten-community study. Emerging Infect. Dis. 2021;27:1343–1352. doi: 10.3201/eid2705.204365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalikiri M.K.R., Hasan M.R., Mirza F., Xaba T., Tang P., Lorenz S. High-throughput extraction of SARS-CoV-2 RNA from nasopharyngeal swabs using solid-phase reverse immobilization beads. medRxiv. 2020 2020.04.08.20055731. [Google Scholar]
- Kubina R., Dziedzic A. Molecular and serological tests for COVID-19 a comparative review of SARS-CoV-2 coronavirus laboratory and point-of-care diagnostics. Diagnostics (Basel) 2020;10:434. doi: 10.3390/diagnostics10060434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladhani S.N., Chow J.Y., Janarthanan R., Fok J., Crawley-Boevey E., Vusirikala A., Fernandez E., Perez M.S., Tang S., Dun-Campbell K. Investigation of SARS-CoV-2 outbreaks in six care homes in London, April 2020. EClinicalMedicine. 2020;26:100533. doi: 10.1016/j.eclinm.2020.100533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Interior-State of Qatar 2020. Population Distribution by Sex, Age, and Nationality: Results of Kashef Database.
- Ministry of Public Health-State of Qatar Coronavirus Disease 2019 (COVID-19) 2021. https://covid19.moph.gov.qa/EN/Pages/default.aspx
- Muench P., Jochum S., Wenderoth V., Ofenloch-Haehnle B., Hombach M., Strobl M., Sadlowski H., Sachse C., Torriani G., Eckerle I., Riedel A. Development and validation of the Elecsys anti-SARS-CoV-2 immunoassay as a highly specific tool for determining past exposure to SARS-CoV-2. J. Clin. Microbiol. 2020;58:e01694-20. doi: 10.1128/JCM.01694-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah G.K., Dargham S.R., Shurrab F., Al-Sadeq D.W., Al-Jighefee H., Chemaitelly H., Al Kanaani Z., Al Khal A., Al Kuwari E., Coyle P. Are commercial antibody assays substantially underestimating SARS-CoV-2 ever infection? An analysis on a population-based sample in a high exposure setting. medRxiv. 2020 2020.12.14.20248163. [Google Scholar]
- Oved K., Olmer L., Shemer-Avni Y., Wolf T., Supino-Rosin L., Prajgrod G., Shenhar Y., Payorsky I., Cohen Y., Kohn Y. Multi-center nationwide comparison of seven serology assays reveals a SARS-CoV-2 non-responding seronegative subpopulation. EClinicalMedicine. 2020;29:100651. doi: 10.1016/j.eclinm.2020.100651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planning and Statistics Authority- State of Qatar Labor force sample survey. 2017. https://www.psa.gov.qa/en/statistics/Statistical%20Releases/Social/LaborForce/2017/statistical_analysis_labor_force_2017_En.pdf
- Planning and Statistics Authority- State of Qatar Qatar monthly Statistics. 2020. https://www.psa.gov.qa/en/pages/default.aspx
- Planning and Statistics Authority-State of Qatar The simplified census of population, housing & establishments. 2019. https://www.psa.gov.qa/en/statistics/Statistical%20Releases/Population/Population/2018/Population_social_1_2018_AE.pdf
- Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Perez Marc G., Moreira E.D., Zerbini C. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primary Health Care Corporation 2020. Primary Health Care Corporation Services Registered Users Database.
- Priya Dsouza Communications Population of Qatar by nationality - 2019 report. 2019. https://priyadsouza.com/population-of-qatar-by-nationality-in-2017/
- Public Health England Evaluation of Roche Elecsys AntiSARS-CoV-2 serology assay for the detection of anti-SARS-CoV-2 antibodies. 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/891598/Evaluation_of_Roche_Elecsys_anti_SARS_CoV_2_PHE_200610_v8.1_FINAL.pdf
- Seedat S., Chemaitelly H., Ayoub H., Makhoul M., Mumtaz G.R., Al Kanaani Z., Al Khal A., Al Kuwari E., Butt A.A., Coyle P. SARS-CoV-2 infection hospitalization, severity, criticality, and fatality rates. medRxiv. 2020 doi: 10.1038/s41598-021-97606-8. 2020.11.29.20240416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sempos C.T., Tian L. Adjusting coronavirus prevalence estimates for laboratory test Kit error. Am. J. Epidemiol. 2021;190:109–115. doi: 10.1093/aje/kwaa174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp . Stata Corporation; 2019. Statistical Software: Release 16.1. [Google Scholar]
- The Roche Group Roche’s COVID-19 antibody test receives FDA Emergency Use Authorization and is available in markets accepting the CE mark. 2020. https://www.roche.com/media/releases/med-cor-2020-05-03.htm
- Thermo Fisher Scientific TaqPath™ COVID-19 CE-IVD RT-PCR Kit instructions for use. 2020. https://assets.thermofisher.com/TFS-Assets/LSG/manuals/MAN0019215_TaqPathCOVID-19_CE-IVD_RT-PCR%20Kit_IFU.pdf
- US Food and Drug Administration Cobas® SARS-CoV-2: qualitative assay for use on the cobas® 6800/8800 systems. 2020. https://www.fda.gov/media/136049/download
- Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2020;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajnberg A., Mansour M., Leven E., Bouvier N.M., Patel G., Firpo-Betancourt A., Mendu R., Jhang J., Arinsburg S., Gitman M. Humoral response and PCR positivity in patients with COVID-19 in the New York City region, USA: an observational study. Lancet Microbe. 2020;1:e283–e289. doi: 10.1016/S2666-5247(20)30120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitz J.S., Beckett S.J., Coenen A.R., Demory D., Dominguez-Mirazo M., Dushoff J., Leung C.Y., Li G., Magalie A., Park S.W. Modeling shield immunity to reduce COVID-19 epidemic spread. Nat. Med. 2020;26:849–854. doi: 10.1038/s41591-020-0895-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization Clinical management of COVID-19. 2020. https://www.who.int/publications-detail/clinical-management-of-covid-19 [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are included in an aggregate form within the manuscript and its supplemental information.