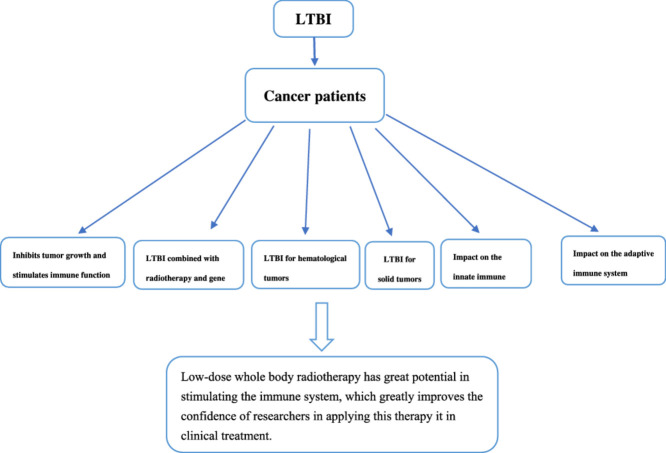
Highlights
-
l
LTBI (low-dose total-body irradiation) can change the immune microenvironment of tumor.
-
l
LTBI (low-dose total-body irradiation) can regulate a variety of signal pathways (such as nuclear factor-κ B, p38 / MAPK, c-jun), thereby enhancing the expression and function of immune cells in the body, and it may even change the immune microenvironment of human body through an unknown signal pathway, such as enhancing the connection between PD-1 and PD-L1 and promoting the low expression of CTLA4.
-
l
LTBI (low-dose total-body irradiation) can stably stimulate the immune function of cancer patients.
-
l
LTBI (low-dose total-body irradiation) can be widely used as a new comprehensive anti-tumor therapy.
Keywords: Low dose total-body irradiation, Tumor, Immune microenvironment, New tumor comprehensive treatment, Radiotherapy
Abstract
The history of low-dose total-body irradiation (LTBI) as a means of radiotherapy for treating malignant tumors can be traced back to the 1920s. Despite this very low total dose, LTBI can induce long-term remissions. Tumor cells are known to change and maintain their own survival and development conditions through autocrine and paracrine signaling. LTBI can change the tumor microenvironment, enhance the infiltration of activated T cells, and trigger inflammatory processes. LTBI-mediated immune response can exert systemic long-term anti-tumor effects, and can induce tumor regression at the primary site and metastatic sites. With a continuous improvement in the anti-tumor immune microenvironment in the field of tumor therapy, LTBI provides more choices to comprehensively treat of tumors. The present study aimed to explore the experimental research mechanism of LTBI and immune microenvironment, and discuss the difficulties and development prospects of applying LTBI to tumor treatment.
Graphical abstract
Introduction
According to the United Nations Scientific Committee on Atomic Radiation Effects, low-dose radiation (LDR) refers to low-linear-energy-transfer (LET) radiation within 0.2 GY or high LET radiation within 5 cGY; the dose rate is within 0.005 cGY/min. Since Luckey and Olivieri et al. proposed that LDR could induce organisms to produce hormesis and adaptive effects, some scholars carried out extensive systematic research on LDR [1,2]. Recent studies revealed that innate immunity initially sensed the presence of transformed cells and exercised the first line of anticancer defense. Soon after the activation, the elements of the innate immune system promoted the induction of adaptive (specific) anti-tumor responses. Tumors might become “ignorant” to immune effectors through the loss or aberrant expression of the major histocompatibility complex (MHC) class I antigens or of other molecules on cancer cells involved in triggering of the innate and/or adaptive immune responses [3,4]. This is one of the most widely used mechanisms of tumor cell immune escape. LDR induces anti-tumor immune mechanisms including scavenging of reactive chemical intermediates, stimulating of the repair of the DNA damage, mitigating of inflammation, triggering of selective apoptosis or senescence of aberrant cells, and upregulating both the innate and adaptive arms of the anticancer immune system [5], [6], [7], [8]. Until recently, the possibility of using low-dose total-body irradiation (LTBI) alone as an immunoadjuvant to stimulate a systemic anti-tumor immune response was not a realistic clinical opportunity. In tumors, the body of preclinical evidence combining LTBI and other anti-tumor therapies stimulated clinical efforts, from which promising results have begun to emerge [7]. An increasing number of studies found that LTBI could inhibit the occurrence, development, and metastasis of tumors [9], [10], [11]; improve the efficacy of radiotherapy and chemotherapy [12,13]; and increase the cure rate of tumors. The potential of such a comprehensive anti-tumor treatment method with broad therapeutic prospects needs immediate exploration in clinical treatment [9].
Basic research and clinical research on LTBI
Animal experimental research
LTBI inhibits tumor growth and stimulates immune function
Studies on LTBI date back to as early as the 1990s. Wang et al. administered a uniform irradiation of 0.05 GY (dose rate 0.05 GY/ min) to the whole body of tumor-bearing mice before local irradiation and tumor cell implantation, which could reduce the tumorigenic rate of tumor cells and inhibit the growth of tumors [14]. They further proved that pre-administering of LBTI before local radiotherapy could improve the proliferative response of T lymphocytes to mitogens, facilitating the signal transmission pathway of T lymphocytes and eliminating part of the inhibitory T lymphocyte subsets that were more sensitive to radiation. T-cell subsets, as an important part of the body's cellular immune function, are very important in anti-tumor immunity and tumor immune escape. Subsequently, in 1997, the natural killer (NK) activity of spleen cells in tumor-bearing mice was significantly enhanced within 24 h of 10cGY γ-ray whole-body irradiation, and the serum tumor necrosis factor (TNF) levels in mice increased after 24 h of whole-body irradiation [15]. The NK cells swiftly killed multiple adjacent cells if they showed surface markers associated with oncogenic transformation. The increase in the number of NK cells amplified the immune response of organisms. In 2000, some scholars found that the expression of the mouse B-cell lymphoma 2 (Bcl-2) protein gradually increased after 75 mGY x-ray whole-body irradiation, peaked after 12–24 h, and returned to basic levels after 72 h [16]. Apoptosis is crucial in immune responses is a better approach to battle tumors because of the following realistic views as shown in table 1. The Bcl-2 family is a key regulator of the mitochondrial response to apoptotic signals in the intrinsic pathway. The high expression of Bcl-2 inhibits the death of immune cells without affecting their proliferation, and increases the stability of mitochondria, while reducing the apoptosis of normal lymphocytes. LTBI inhibits tumor growth in two fields: First,it controls tumor growth in two ways: first, it increases T lymphocytes and B lymphocytes,while increasing the stability of mitochondria, while reducing the apoptosis of normal lymphocytes.
Table 1.
Reported Animal experimental research.
| Reports | Xianli Wang.et al. 1996 | Hailin Tian.et al. 1997 | Hong Wan.et al. 2000 |
|---|---|---|---|
| Histological subtype (n) | Lewis lung cancer, S180 Sarcoma | Ehrlich ascites carcinoma | Healthy mice |
| No. of (evaluable) animals | 105 | 75 | 40 |
| LTBI dose (GY) | 0.05GY | 0–0.05GY | 0.075GY |
| Tumor weight remission rate | 61.36% | 72% | – |
| Immune cell | Increased T lymphocytes | Increased NK cell activity and Tlymphocyte | Increased expression of Bcl-2 protein on immune organs |
| Tumor markers | – | Increased TNF levels | – |
| Ref. | [14] | [15] | [16] |
LTBI combined with radiotherapy and gene therapy
Tumor-bearing mice were treated with conventional radiotherapy (2GY × 6) combined with LTBI (0.075 GY) and gene therapy (intratumor injection of pEgr-IL-18-B7.1 plasmid) [17]. The results showed that cancer control most significantly improved in the group receiving local radiotherapy combined with LTBI and gene therapy as shown by the prolongation of mean survival time by 60.4%, reduction in average tumor weight by 70.8%, decrease in pulmonary metastasis by 66.9%, and decrease in intratumor angiogenesis by 64.8% compared with local radiotherapy alone (P < 0.05).After LTBI,t he upregulation of host immunity manifested as stimulated NK and cytotoxic T lymphocyte (CTL) activity, interferon-gamma (IFN-γ) and TNF-α secretion, protein kinase C-θ activation, and lysosomal-associated membrane protein-1 expression. So that the tumor can be effectively controlled.Another research also revealed that NK,CTL, IFN-γ, TNF-α, protein kinase could be regulated by LTBI.Liu et al. found that the combination of hypo-fractionated radiotherapy (H-RT) and LTBI could enhance immunity by infiltrating CD8+T cells and changing the immunosuppressive microenvironment of unirradiated subcutaneous tumor lesion–related reactions. With the production of tumor-specific CD8+T cells, the secretion of IFN-γ significantly inhibited the growth of secondary tumors. H-RT could only significantly delay the growth of primary tumors, while LTBI was vital in the combination therapy for inducing immune effects and causing regression of distant tumors. The LTBI and H-RT combined treatment reduced the apoptosis and DNAdamage, and led to the infiltration of CD8+Tcells, IFN-γ+CD8+Tcells, and dendritic cells (DCs) of unirradiated tumors, which enhancing the immunity and inhibiting the growth of primary tumors [18]. These studies suggests that LTBI can stimulate some molecules (NK,CTL,IFN-γ, TNF-α)in tumor immune microenvironment.
A large number of animal experiments provided strong evidence for the therapeutic potential of LTBI in cancer. The anti-tumor effects of immune cells, antioxidant defense system enzymes, and red blood cell system were measured using different doses of gamma rays at different irradiation intervals [19], [20], [21]. The results revealed that (1)LTBI decreased apoptosis in the splenocyte subpopulations studied most prominently in NK cells and DCs; (2) the organisms pre-exposed to LTBI had a lower tumor formation rate compared with those without LTBI; (3) the induction of endogenous glutathione immediately after exposure to low-dose gamma irradiation might be beneficial in protecting the cells from the reactive oxygen species (ROS)-induced oxidative stress in various ROS-related diseases; (4) LTBI, within a certain period of time, decreased the expression of hypoxia-inducible factor EPO and VEGFR, which might improve the situation of tumor hypoxia and radiosensitivity of the tumor itself. However,conventional radiotherapy combined LTBI could reduce the total radiation dose and simultaneously improve the treatment efficacy of cancer accompanied by upregulated host anticancer immunity. Based on the aforementioned basic research, the impact of LTBI on the immune system has been investigated in detail.
Clinical research
LTBI for hematological tumors
LTBI was first used to treat lymphoma and myeloma clinically. Simple LTBI or combination chemotherapy achieved good clinical efficacy. Eight consecutive patients with a histological diagnosis of lymphosarcoma (excluding reticulum cell sarcoma) received total-body irradiation in 1967. All patients had generalized lymph node involvement, and extranodal (visceral or bone marrow) disease was present in four of the eight patients Three patients (B, D, and G) received prior chemotherapy or radiotherapy, and the remaining five were previously untreated. The results showed that six of the eight patients had at least a 90% decrease in the size of the measurable tumor lesions following their initial course of irradiation [22].Owing to the effective control of blood system tumor by LTBI,later, in a group of 35 patients with relapsed or chemo-resistant non-Hodgkin's lymphoma (NHL), LTBI (involved-field radiotherapy to bulky sites) achieved a complete remission rate of 29%, 2-year progression-free survival of 32%, and median progression-free survival of 12 months. The 2-year average survival was 42%, and the median survival was 17 months. Immunostaining and flow cytometry of peripheral blood in 14 patients showed that LTBI led to a significant increase in the percentage of CD4+ cells with a consequent significant increase in the CD4+/CD8+ ratio [23]. However, more clinical trials tend to focus on molecular signaling pathways and neglect to explore the radiation dose of LTBI that can be used in clinic [24]. Therefore, it is of great significance to explore the most suitable radiation dose for LTBI
In addition, Chaffey et al. [25], Choi et al. [26], and Safwat et al. [27] reported that LTBI significantly improved the treatment effect on patients with malignant lymphoma. The results indicated the following: (1) miR-30a and miR-30b, which effectively inhibited plasminogen activator inhibitor-1 (PAI-1), were overexpressed after treatment with LTBI for non-small-cell lung cancer cells. Phosphorylation of protein kinase B (Akt) and ERK, the downstream survival signals of PAI-1, was decreased by PAI-1 inhibition [25].The tumor growth and aggressiveness were efficiently decreased by LTBI treatment followed by radiotherapy due to Phosphorylation of protein kinase B (Akt) and ERK was decreased. (2) A total of 39 patients with advanced NHL were treated with LTBI with a minimum follow-up of 8 months. In conclusion, LTBI was an excellent induction agent for stages III and IV nodular and diffuse lymphocytic lymphomas. Given the good response (CR 50%, PR 40%) of the relapse after LTBI to the subsequent chemotherapy and local radiotherapy, maintenance chemotherapy after LTBI might be of value in prolonging the duration of remission and possibly improving survival [26,27]. see table 2.The inhibition of LTBI on hematolymph system tumors starts from molecular mechanism, and eventually leads to the decrease of tumor cells and the increase of immune cells. It show that LTBI has a greater effect on the immune microenvironment of patients with malignant lymphoma.
Table 2.
Reported clinical cases of LTBI.
| Reports | Johnson R E et al.1967 | Safwat A et al.2003 | Travis L B et al.1996 | Choi N C et al.1979 |
|---|---|---|---|---|
| Patients (n) | 8 | 35 | 61 | 39 |
| Pathological type | Lymphosarcoma (excluding reticulum cell sarcoma) | Non-Hodgkin's lymphoma | Non-Hodgkin's lymphoma (NHL) | Non-Hodgkin's lymphoma |
| Distant metastasis (n) | Generalized lymph node involvement 4 | Bulky disease (nodalor extranodal)16 | – | 38 patients with lymphocytic lymphoma and 1 patient with mixed lymphocytic and his- tiocytic lymphoma |
| TLBI regimen | 0. 1–0.2 Gy (Twice a month) | 0.1 – 0.25GY per fraction three to five fractions per week | 0.1GY per week | 0.15GY with two treatments per week. |
| Hematology index | White blood cells (WBC) , hemoglobin, blood cells | Lymphocytic subtype:CD45, CD16, CD56, CD4, CD8, CD3, CD95 | Acute nonlymphocytic leukemia (6%) | WBC,Nadir platelet |
| Pathologic response rate | Measurable lesion volume reduction 90% | A 2-years progression-free survival of 32%; a median progression- free survival time of 12 months | solid tumors relapse (13%) | – |
| Patients outcomes | Complete response 1 | Complete response 10 | – | Complete response 33 |
| Partial response 5 | Partial response 15 | Partial response 6 | ||
| Progressive disease 2 | Stable disease 5 | |||
| Progressive disease 5 | ||||
| Ref. | [22] | [23] | [25] | [26] |
LTBI for solid tumors
Among the reported preliminary cases, the exploratory operation in the case of a 49-year-old woman with an ovarian tumor revealed that tumor cells had infiltrated into the sigmoidal, rectal, and peritoneal regions with ascites. A combined treatment of LTBI (10 cGY) and local irradiation was planned for this patient, which comprised of a single dose of 1.5 GY,given to the whole abdominal region, given 5 or 6 h after 10 cGY of LTBI. This combined treatment was given three times a week and repeated for 5 weeks. The total dose of LTBI did not exceed 150 cGY, and the total dose of local irradiation was 37.5 GY. The results were remarkable [11]. What's clear that there is a variation in patients and several forms of neoplasm exist.In another case with CA125 as the evaluation index,the value of CA125 which was used as the tumor marker for ovarian tumors, was 140 u / ml before treatment. During the course of the treatment, its value decreased to 35 u / ml (normal value ≤50 u/mL) and was maintained at that value for 3 months after the completion of the therapy [28].In patients with advanced cancer,Lakimova et al. used 0.1 GY whole-body irradiation before local radiotherapy for treating of cervical cancer. They found that the annual survival rate of patients with stage III tumors increased by 14%; radiotherapy caused hypothyroidism, bone marrow suppression, leukopenia, or cell function suppression, and obviously reduced the incidence of other complications [29]. It is understand that most of the effects of LTBI on human body are closely related to the changes of immune microenvironment. And Sonveaux found that LTBI could stimulate local vascular endothelial cells in the tumor tissues to secrete NO, causing vasodilation, thereby significantly increasing the blood flow in the tumor, increasing the oxygen content of the tumor tissue, and sensitizing the subsequent high-dose radiotherapy [30]. So how can we better understand the immune stimulation of LTBI considering a single form of neoplasm. These can be achieved based on LTBI changes the CA125,tumor internal hypoxia, and combine H-RT anti-tumor therapy effective [31].
In the study of male tumors,Prostate specific antigen (PSA) increased in one patient with prostate cancer after prostatectomy. His-PSA value began to decline immediately after low-dose x-ray irradiation with a dose of 150 mGY, and has remained at a low level since then. A patient with prostate cancer with bone metastasis received 150 mGY repeated low-dose x-ray irradiation, His-PSA level dropped to near normal level within 3 months after treatment, and remained at a low level after the end of stimulation therapy. His-bone metastases almost disappeared [32]. In the study of female tumors,Sakamoto et al. performed LTBI (0.1GY) 6 h before local 1.5GY radiotherapy in patients with advanced ovarian cancer, the ovarian tumor marker CA125 decreased from 140 u / ml to 35 u / ml (normal value ≤ 50 u / ml), and was maintained for 3 months after treatment [10].Their research deserve that LTBI has brought benefits to human beings. Nevertheless, scientists continue to investigate different rules of LTBI could enhance the susceptibility of resistant ovarian cancer cells to cisplatin (DDP). Compared with the control and conventional-dose groups, LTBI resulted in significant apoptosis of tumor cells, as detected by flow cytometry (P < 0.05). The relative mRNA expression levels of excision repair cross-complementing group 1 (ERCC1) and Bcl-2 were significantly lower in the low-dose group than in the control and conventional-dose groups (P < 0.05) [33].So, LTBI differed from the routine high-dose radiation in that its target might be the cytoplasm, cell membrane, and some of conductive systems rather than DNA. The application value of the LTBI in clinic was prospected as follows: (1)Combined with radiotherapy [34], DNA chemotherapy [27], and DNA vaccination [35], and so on, LTBI could inhibit tumor growth and metastasis [36]. LTBI before and after conventional surgery, radiotherapy, and chemotherapy might reduce the chance of tumor recurrence and metastasis [37]. (2)LTBI might improve the therapeutic effect of immunosuppressive checkpoint inhibitors (such as PD1 / PD-L1, CTLA-4) [38,39], Although immunosuppressive therapy can improve the survival rate of patients with advanced cancer, the overall effectiveness of immunosuppressive therapy is low. Since LTBI can regulate a variety of signal pathways (such as nuclear factor-κ B, p38 / MAPK, c-jun), thereby enhancing the expression and function of immune cells in the body, and it may even change the immune microenvironment of human body through an unknown signal pathway, such as enhancing the connection between PD-1 and PD-L1 and promoting the low expression of CTLA4, so that LTBI may be used as a new method of radiotherapy combined with immunosuppressive therapy in the future to provide cancer patients with a greater chance of survival [40], [41], [42].The low immunity of the patients with advanced cancer may be an important reason for immune tolerance. LTBI can excite the immune system [43,44], so that the combination of LTBI and immunosuppressant can be considered. (3)Immunotherapy based on DC vaccination is considered as a way to treat metastatic and hormone-resistant cancer so as to improve the curative effect of tumor vaccine [45,46]. Patients’ immune systems can be used to eliminate tumor cells. However, the therapeutic effect of immunotherapy on tumors is limited in clinical practice due to reduced interleukin-12 (IL-12) production and migration during the DC vaccine generation process. The tumor immunity reaction to immunotherapy is solely based on the availability of the drug at the cancer site and it is effective with the use of LTBI to improve the vaccine for DCs uptake [47,48] .(4) LTBI can promote the production of IL-12 and the migration of DC, which provides hope for the preparation of the DC vaccine in vitro. (5) LTBI exhibits a long-lasting and stable effect of modulating the body's immune response, and has the advantages of simple operation and low price. See Fig. 1. Therefore, LTBI as a new method of anti-tumor treatment, has extraordinary potential in the application of clinical tumor comprehensive treatment of tumors in the clinic.
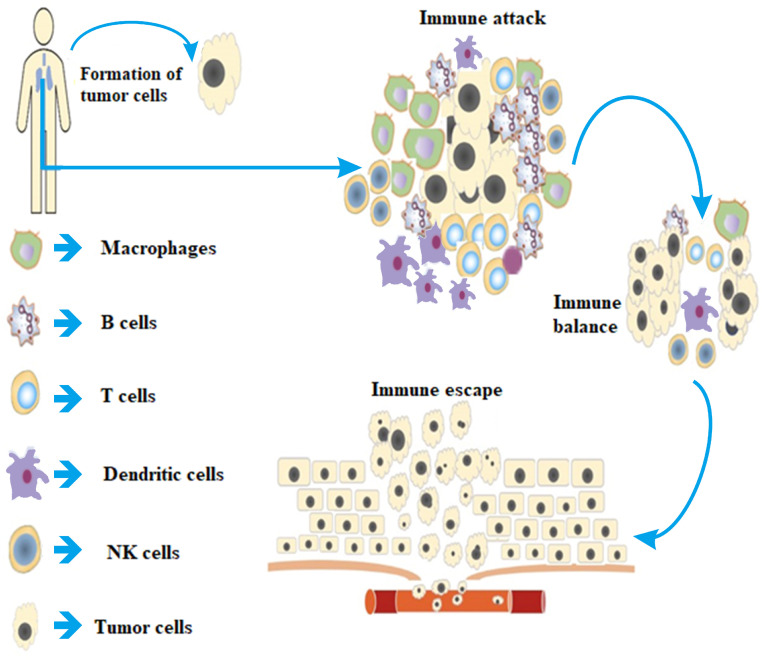
Fig. 1.
When tumor cells attack body, T cells, B cells, NK cells, Macrophages, and Dendritic cells in the immune system are activated by tumor antigens through a variety of ways to recognize and attack tumor cells, so that the tumor immune microenvironment reaches an immune balance status. In order to reproduce and grow, tumor cells wisely adopt a variety of strategies to avoid the immune system, survive all stages of the anti-tumor immune response and produce immune escape.
LTBI in the tumor microenvironment
Impact on the innate immune system
The immune system is very important in the body's control of tumor development [49].Enhancing anti-neoplastic immunity may be an important mechanism of the cancer-inhibitory effects of LTBI [26,36]. First, the immune enhancement induced by LTBI promotes the proliferation of NK cells, macrophages, and DCs, thereby promoting the intercellular reaction within immune synapses and stimulating the expression of many surface molecules and cytokines to regulate secretion [50,51]. As the main effector cells of the nonspecific immune system, they are vital in monitoring tumors, killing tumor cells, and starting adaptive immunity [52,53]. Many animal experiments showed that the impact of low-dose ionizing radiation exposure on the tumor microenvironment involved NK lymphocytes (partially mediated by the perforin and/or Fas ligand receptor ligand pathway) and/or activated macrophages. The anti-tumor cytotoxic response of cells (through the production of nitric oxide) was significantly upregulated [54]. Also, Hee suggested that the increase in NK cytotoxicity caused by LTBI in the body was not due to changes in NK activating receptors NK1.1, NKG2D, CD69, and 2B4 or changes in early or late apoptosis rates [55]. Subsequently, it was reported that LTBI could enhance the cytotoxicity of NK cells in vitro by reducing NK cell apoptosis and increasing the production of IFN-γ, TNF-α, perforin, and granzyme [19]. In addition, a study found that LTBI could increase the proliferation index and killing activity of NK cells through augmentation the p38/mitogen-activated protein kinase (MAPK) signaling pathway both in vitro and in vivo [56]. All of these basic studies showed that LTBI could promote the immune system. It need to further study the main molecular mechanisms, signaling pathways and even the most appropriate radiation dose that LTBI can improve the tumor microenvironment.
The function of macrophages is mainly to process antigens and present them to T and B cells, stimulate the proliferation of B cells, and differentiate into mature plasma cells that secrete specific antibodies. At the same time, macrophages promote the proliferation and differentiation of T lymphocytes into helper/inducible T cells (TH) and inhibitory cytotoxic T cells (CTL or Tc) [57]. Macrophages have two activation phenotypes, M1 and M2. M1 macrophages mainly activate T helper type 1 (Th1) cells to enhance the immune response. M2 macrophages are mainly mediated by the anti-inflammatory response of T helper type 2 (Th2) cells, and promote tumor cell growth, angiogenesis, invasion, and metastasis, and hence are termed as tumor-associated macrophages (TAMs) [58]. TAMs are important inflammatory cells in the tumor microenvironment. They have a high degree of plasticity and are important in regulating tumor tissue immune function [59]. Interestingly, LTBI not only induces endothelial cell activation and Th1 chemokine expression but also inhibits angiogenesis, immunosuppression, and tumor growth factor production, and uses iNOS to program the differentiation of iNOS (+) M1 macrophages, thereby causing CTL recruitment and killing in solid tumors (such as melanoma) [60,61]. It also affects oxidative burst activity and superoxide production in macrophages and inhibits Akt and p38/MAPK phosphorylation [62]. Interestingly, LTBI can induce the transformation of the M2 phenotype into the M1 phenotype [63]. Although the molecular mechanism of LTBI inducing the transformation of macrophages from M2 to M1 is not very clear, recent studies showed that macrophages were activated by LTBI. During the process, Rac2 GTPase was activated downstream of α4β1 integrin and Macrophage colony stimulating factor (MCSF) receptors to control the differentiation of macrophages into M2 type. In other words, LTBI induced an increase in the ratio of IFN-γ/ IL-4. These experimental results strongly suggested that LTBI had a positive effect on macrophage polarization and function to move the immune balance toward Th1 [64,65]. They also showed that the innate immune system and the adaptive immune system complemented each other and promoted each other in anti-tumor immunity.Human tumor immune microenvironment can be seen in Fig. 1. After exploring the effects of LTBI on NK, CTL, M1 and M2, the researchers were curious about DC, so they did the following research.Akio showed that the expression of MHC or costimulatory molecules (CD1b, CD40, CD80, CD86, ICAM-a, LFA-1, and MHC class II molecules on DC) did not increase, but the levels of DC-related cytokines (IL-2, IL-12, and IFN-γ) increased after 0.05 GY irradiation, thereby allowing the transfer of naive helper T cells to Th1 cells [66]. Similarly, in vitro x-ray irradiation of DC (0.2 GY) significantly increased DC migration and IL-12 production, and upregulated CCR7 expression. However, CCR7-neutralizing antibodies could attenuate DC migration, indicating that LTBI might mediate DC migration through CCR7 [33]. See Fig. 2. Reports on the effect of LTBI on DC in vitro were conflicting due to the special and complex role of DC in the immune system. Therefore, the mechanism of LTBI on DC deserves further exploration.
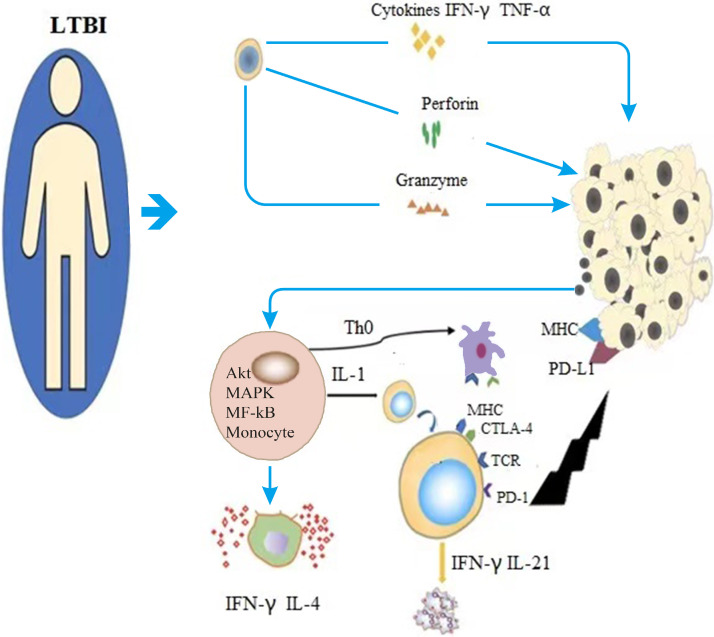
Fig. 2.
Monocytes in the immune system rapidly differentiate into various types of immune cells after low-dose total body radiotherapy in tumor patients. For instance, NK cells produce IFN-γ, TNF-α, perforin and Granzyme through three pathways to enhance the body's non-specific anti-tumor activity. Macrophages release cytokines (such as IL-4 and IFN-γ) to stimulate the proliferation and differentiation of T cells, enhance the activity of NK cells, and kill tumor cells. Dendritic cells present tumor antigens to T cells and activate T cells. T lymphocytes promote the proliferation of B lymphocytes and dendritic cells by secreting cytokines and signaling targets, and kill tumor cells directly.
Impact on the adaptive immune system
Adaptive immunity mainly includes cellular immunity and humoral immunity, which involve T cells and B cells, respectively. Adaptive immunity occurs after innate immunity, mainly to produce immunological memory after reacting to pathogens, and encountering the same pathogen again can completely eliminate the pathogen and prevent reinfection.
T-cell subsets are the main forms of an immune response in cellular immunity. They are mainly divided into three categories: helper T cells (Th), CTL or Tc, and regulatory cells (Treg). Under normal circumstances, these T cells are in a state of dynamic equilibrium. LTBI can enhance the response of helper CD4 + T cells and CD8 + CTL [67], [68], [69]. The potential molecular mechanism of T cell−mediated immune regulation of LTBI might involve activating survival/signaling proteins (nuclear factor-κB, p38/MAPK, and c-Jun N-terminal kinase) and increasing the ability of T cells to produce immune-enhancing cytokines (IL-2, IL-4, and IFN-γ) while reducing the production of major immunosuppressive cytokines (transforming growth factor β1, Foxp3 (+), and IL-10), so as to mobilize anti-tumor immunity [37,70,71] .Recently, the evaluation of Treg (CD4, CD25) lymphocyte counts and functional evaluation showed the main parameters that could represent the anti-tumor immune function status of patients with tumors. After LTBI irradiation, the expression of CTLA-4 on the surface of CD4(+) CD25(+) Treg cells in mice decreased, and its inhibitory effect on CD4 + CD25-T cell proliferation reduced. Therefore, the body's immune function was enhanced [72,73].
The B cells are mainly involved in humoral immunity and circulate in the plasma and lymph to produce antibodies. As early as 1988, B cells were found to be radiation sensitive, and LTBI could stimulate the proliferation of B cells [74]. Another study found that LTBI might increase the phosphorylation levels of the Ikaros gene protein by activating casein kinase 2 and Akt, thereby enhancing the proliferation of B lymphoblasts, secreting special cytokines, and activating related cell pathways. LTBI induced immune cells to aggregate like tumors, thereby killing tumor cells [75,76].
The occurrence of malignant tumors was closely related to the increase in ROS in the body, changes in redox balance, and imbalance in redox signals [77]. In 2011, low-dose (0.25–0.5 GY) gamma rays increased the levels of ATP-mediated thioredoxin (Trx-1) in various organs of mice. Radiation-mediated ATP release might promote the production of ROS through purine energy signaling, further leading to cells. The increase in the levels of internal antioxidants (Trx-1) was an adaptive response to oxidative stress [76]. LTBI could improve the immune function of red blood cells and increase the activity of superoxide dismutase (SOD) in red blood cells through the activation of several members (c-Raf, MEK, and ERK) in the MAPK/ERK signaling pathway [78], [79], [80]. Liu et al. also found that after exposure to 0.05 GY combined with high-dose irradiation, the level of lipid peroxide in liver tissues reduced, while the activity of glutathione peroxidase and SOD increased by [81,82]. Some studies found that a single LTBI could stimulate the activity of SOD in the kidney [83]. This also suggested that LTBI could be applied to clinical patients with tumors for mobilizing the immune organs of the body to exert a lasting and stable immune effect,maintain of hypoxia in tumor and hence attack tumor cells. Moreover, LTBI did not induce an excitatory effect on prostate cancer (PC-3) cells [84], human lung adenocarcinoma cells (A549) [85], cervical cancer cells (HeLaS3), breast cancer cells (EMT6) [86], colon carcinoma cells (HRT18, HT29, and HCT116) [87], villous tumor and lymphoma cells [86], and other tumor cells. It only induced the excitatory and adaptive responses of normal cells, strongly indicating the protective effect of LTBI on normal tissues, which also showed that LTBI was safe and effective.
Current doubts in LTBI
Although many studies reported on the broad application prospects of LTBI, the following problems still need to be addressed. (1) At present, many anti-tumor immune mechanisms are stimulated by LTBI, but the main mechanism of action is not clear. (2) The optimal dose at which LTBI can stimulate the immune response of patients also needs to be explored. (3) No unified immunological detection index exists regarding how to judge the stimulation of the immune response of patients by LTBI. (4) Whether the response of LTBI to tumors in different parts of the body (lung, liver, and bone) is different and whether the response of lymph node system tumors and solid tumors are different is not known. (5) How to change the anti-tumor response from rare, inconsistent, and accidentally discovered response to intentionally induced response is unclear. (6) Finally, the ethical problems associated with LTBI need immediate attention.
LTBI application prospects
The global update of anti-tumor treatment technology and drugs indicated that the single anti-tumor treatment could not meet the expectations. LTBI can also be used as an anti-tumor therapy, which can not only kill tumor cells directly, but also improve the efficiency of immunotherapy, improve the hypoxia in tumor, and reduce the tumor volume significantly. Especially low-dose whole body radiotherapy has great potential in stimulating the immune system, which greatly improves the confidence of researchers in applying this therapy it in clinical treatment. Low-dose total-body radiotherapy also has the advantages of low treatment cost, low toxicity, and good patient tolerance, which does not affect the implementation of operation, radiotherapy, chemotherapy, immunotherapy, and other treatment methods. LTBI can improve the survival of tumor patients by changing the tumor immune microenvironment, increasing the sensitivity of tumor patients to immunotherapy, chemotherapy and radiotherapy.Therefore, we speculate that LTBI may become the first choice of comprehensive anti-tumor therapy in the future.
Author contributions
All authors made a significant contribution to the work reported, Zhuo Chen: formulation or evolution of overarching research goals and aims and performed writing – review and editing; Zhouxue Wu and Tobias Achu Muluh: Ideas; conceptualization; Shaozhi Fu and Jingbo Wu supervision; Acquisition of the financial support for the project leading to this publication.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The Union Project of Luzhou City and the Southwest Medical University (Nos. 14JC0144, 2018LZXNYD-ZK06) is appreciated for the grants covering this work, the authors wish to thank the members of Department of Oncology and Laboratory of Cancer research Institute of Southwest Medical University, Luzhou 64600, PRC. for stimulating discussions and inspiring research environment for this review.
References
- 1.Luckey and D.J.H.P. T., Physiological benefits from low levels of ionizing radiation. 1982. 43(6): p. 771–89. [DOI] [PubMed]
- 2.Olivieri G., Bodycote J., Wolff S.J.E. Adaptive response of human lymphocytes to low concentrations of radioactive thymidine. 1984;223(4636):594–597. doi: 10.1126/science.6695170. [DOI] [PubMed] [Google Scholar]
- 3.Algarra I. The selection of tumor variants with altered expression of classical and nonclassical MHC class I molecules: implications for tumor immune escape. 2004;53(10):904–910. doi: 10.1007/s00262-004-0517-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aptsiauri N. MHC class I antigens and immune surveillance in transformed cells. 2007;256:139–189. doi: 10.1016/S0074-7696(07)56005-5. [DOI] [PubMed] [Google Scholar]
- 5.Feinendegen, L.E., M. Pollycove, and R.D. Neumann, Hormesis by Low Dose Radiation Effects: low-Dose Cancer Risk Modeling Must Recognize Up-Regulation of Protection. 2012. [DOI] [PMC free article] [PubMed]
- 6.Scott, R.J.J.o.C.C. Bobby, and Signaling, Radiation-hormesis phenotypes, the related mechanisms and implications for disease prevention and therapy. 2014. 8(4): p. 341–52. [DOI] [PMC free article] [PubMed]
- 7.Farooque A. Low-dose radiation therapy of cancer: role of immune enhancement. 2011;11(5):791–802. doi: 10.1586/era.10.217. [DOI] [PubMed] [Google Scholar]
- 8.Minglong S. Multiple Low-Dose Radiation Prevents Type 2 Diabetes-Induced Renal Damage through Attenuation of Dyslipidemia and Insulin Resistance and Subsequent Renal Inflammation and Oxidative Stress. 2014;9(3):e92574. doi: 10.1371/journal.pone.0092574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lemon, J.A., N. Phan, and D.R.J.R.R. Boreham, Single CT Scan Prolongs Survival by Extending Cancer Latency in Trp53 Heterozygous Mice. 2017: p. RR14576.1. [DOI] [PubMed]
- 10.Sakamoto and K.J.N.B.T. Med, Radiobiological Basis for Cancer Therapy by Total or Half-Body Irradiation. 2004. 2(4): p. 293–316. [DOI] [PMC free article] [PubMed]
- 11.Kaushik, N., et al., Low-dose radiation decreases tumor progression via the inhibition of the JAK1/STAT3 signaling axis in breast cancer cell lines. 2017. 7: p. 43361. [DOI] [PMC free article] [PubMed]
- 12.Yu, H.S., et al., Low Dose Radiation Increased the Therapeutic Efficacy of Cyclophosphamide on S180 Sarcoma Bearing Mice. 2007. [DOI] [PubMed]
- 13.W P.C., A S.J. The Linear No-Threshold Model of Low-Dose Radiogenic Cancer: a Failed Fiction. %J Dose-response: a publication of International Hormesis Society. 2019;17(1) doi: 10.1177/1559325818824200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xianli W. Effects of low-dose ionizing radiation on tumor-bearing mice and on radiotherapy. 1996;16 [Google Scholar]
- 15.Hailin, T.J.A.A.M.S., Studies of Immunal Effects Induced by Low Dose Radiation on Tumor Bearing Mice. 1997.
- 16.Hong, W. and L.J.J.o.N.B.U.o.M.e. Shuzheng, Effect of low dose radiation on Bcl-2 protein expressions in mouse immune system. 2000. 26.
- 17.Wu N. Increase in efficacy of cancer radiotherapy by combination with whole-body low dose irradiation. 2008;84(3):201. doi: 10.1080/09553000801902133. [DOI] [PubMed] [Google Scholar]
- 18.Jing L. Low-Dose Total Body Irradiation Can Enhance Systemic Immune Related Response Induced by Hypo-Fractionated Radiation. %J Frontiers in immunology. 2019:10. doi: 10.3389/fimmu.2019.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.EN B. Effects of low-dose radiation on the immune system of mice after total-body irradiation. 2010;174(4):480–489. doi: 10.1667/RR2160.1. [DOI] [PubMed] [Google Scholar]
- 20.Pathak C., Avti P., Khanduja K.J.M.P. Differential Modulation of Antioxidant Defense System in Various Organs of Balb/c Mice by Whole Body Exposure to Low-Dose Gamma Radiation. 2011;38(6):3489. [Google Scholar]
- 21.Yu H.S. Low-dose Radiation Induces Antitumor Effects and Erythrocyte System Hormesis. 2013;14(7):4121–4126. doi: 10.7314/apjcp.2013.14.7.4121. [DOI] [PubMed] [Google Scholar]
- 22.Johnson R.E. Treatment of lymphosarcoma with fractionated total body irradiation. 1967;20(4):482–485. doi: 10.1002/1097-0142(1967)20:4<482::aid-cncr2820200404>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Safwat A. The potential palliative role and possible immune modulatory effects of low-dose total body irradiation in relapsed or chemo-resistant non-Hodgkin's lymphoma. 2003;69(1):33–36. doi: 10.1016/s0167-8140(03)00247-0. [DOI] [PubMed] [Google Scholar]
- 24.Liu S.Z. Signal transduction in lymphocytes after low dose radiation. 1994;107(6):431–436. [PubMed] [Google Scholar]
- 25.Travis L.B. Leukemia following low-dose total body irradiation and chemotherapy for non-Hodgkin’s lymphoma. J. Clin. Oncol. 1996;14(2):565–571. doi: 10.1200/JCO.1996.14.2.565. [DOI] [PubMed] [Google Scholar]
- 26.Choi N.C. Low dose fractionated whole body irradiation in the treatment of advanced non-Hodgkin's lymphoma. 1979;43(5):1636–1642. doi: 10.1002/1097-0142(197905)43:5<1636::aid-cncr2820430512>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 27.Safwat, A., The role of low-dose total body irradiation in treatment of non-Hodgkin\"s lymphoma: a new look at an old method. 2000. 56(1): p. 1–8. [DOI] [PubMed]
- 28.Duffy M.J. CA125 in ovarian cancer: european Group on Tumor Markers guidelines for clinical use. Int. J. Gynecol. Cancer. 2005;15(5):679–691. doi: 10.1111/j.1525-1438.2005.00130.x. [DOI] [PubMed] [Google Scholar]
- 29.Iakimova T.P. [The results of the radiation treatment of cervical cancer using low, sensitizing doses of radiation] Vopr. Onkol. 1990;36(6):678–682. [PubMed] [Google Scholar]
- 30.Sonveaux P. Modulation of the tumor vasculature functionality by ionizing radiation accounts for tumor radiosensitization and promotes gene delivery. FASEB J. 2002;16(14):1979–1981. doi: 10.1096/fj.02-0487fje. [DOI] [PubMed] [Google Scholar]
- 31.Frank, P., et al., NF-kappa B, cytokines, proteasomes, and low-dose radiation exposure. 2002 (suppl_1): p. 66–7. [PubMed]
- 32.Kojima S. Treatment of Cancer and Inflammation With Low-Dose Ionizing Radiation: Three Case Reports. 2017;15(1) doi: 10.1177/1559325817697531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu X. Low-dose radiation enhances susceptibility to cisplatin in resistant ovarian cancer cells via downregulation of ERCC1 and Bcl-2. %J Oncology and Translational Medicine. 2016;2(02):84–89. [Google Scholar]
- 34.Iakimova, T.P., et al., [The results of the radiation treatment of cervical cancer using low, sensitizing doses of radiation]. 1990. 36(6): p. 678. [PubMed]
- 35.Lerret, N.M.J.D. and T.-. Gradworks, DNA Vaccination Combined with Low-Dose Total Body Irradiation Induces Long-Term Breast Tumor Regression. 2011.
- 36.Safwat and A.J.R. Research, The immunobiology of low-dose total-body irradiation: more questions than answers. 2000. 153(5): p. 599–604. [DOI] [PubMed]
- 37.H., et al., The suppression of metastases and the change in host immune response after low dose total body irradiation to tumor-bearing rats. 1998. [PubMed]
- 38.Pfannenstiel L.W. Combination PD-1 blockade and irradiation of brain metastasis induces an effective abscopal effect in melanoma. 2018;8:1–12. doi: 10.1080/2162402X.2018.1507669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trommer M. Abscopal Effects in Radio-Immunotherapy—Response Analysis of Metastatic Cancer Patients With Progressive Disease Under Anti-PD-1 Immune Checkpoint Inhibition. 2019;10 doi: 10.3389/fphar.2019.00511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.M T.J. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. %J Clinical cancer research: an official journal of the American Association for Cancer Research. 2014;20(19) doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elsas M.J.V., Hall T.v., Burg S.H.v.d. Future Challenges in Cancer Resistance to. Immunotherapy%J Cancers. 2020;12(4) doi: 10.3390/cancers12040935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitchel R.E. Low-Dose Radiation Exposure and Atherosclerosis in ApoE /Mice. 2011;175(5):665–676. doi: 10.1667/RR2176.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu, X., et al., Low-dose radiation enhances susceptibility to cisplatin in resistant ovarian cancer cells via downregulation of ERCC1 and Bcl-2. 2016.
- 44.Hee, S.C., et al., Augmentation of natural cytotoxicity by chronic low-dose ionizing radiation in murine natural killer cells primed by IL-2. (6): p. 823–829. [DOI] [PMC free article] [PubMed]
- 45.Nakamura A. CTCs as tumor antigens: A pilot study using ex-vivo expanded tumor cells to be used as lysate for DC vaccines. 2019;8:27–31. [Google Scholar]
- 46.Ho, H.H. and H.F. Wong, TNP-470 skews DC differentiation to Th1-stimulatory phenotypes and can serve as a novel adjuvant in a cancer vaccine. 2018. [DOI] [PMC free article] [PubMed]
- 47.Sinian, et al., Exposure to Low-Dose Radiation Enhanced the Antitumor Effect of a Dendritic Cell Vaccine. 2019. [DOI] [PMC free article] [PubMed]
- 48.Tseng C.W. Low-dose radiation enhances therapeutic HPV DNA vaccination in tumor-bearing hosts. 2009;58(5):737. doi: 10.1007/s00262-008-0596-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Corthay and A.J.F.i. Immunology, Does the Immune System Naturally Protect Against Cancer? 2014. 5: p. 197. [DOI] [PMC free article] [PubMed]
- 50.Liu and S.-Z.J.N.B.T. Med, Nonlinear Dose-Response Relationship in the Immune System Following Exposure to Ionizing Radiation: mechanisms and Implications. 2003. 1(1): p. 71–92. [DOI] [PMC free article] [PubMed]
- 51.Xia M. Dexamethasone enhances CTLA-4 expression during T cell activation. 1999;55(12):1649–1656. doi: 10.1007/s000180050403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lodoen M.B., Lanier L.L. Natural killer cells as an initial defense against pathogens. 2006;18(4):391–398. doi: 10.1016/j.coi.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joshi S. Rac2 controls tumor growth, metastasis and M1-M2 macrophage differentiation in vivo. %J PLoS ONE. 2017;9(4) doi: 10.1371/journal.pone.0095893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nowosielska E.M. Effect of low doses of low-let radiation on the innate anti-tumor reactions in radioresistant and radiosensitive mice. 2012;10(4):500. doi: 10.2203/dose-response.12-018.Nowosielska. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sonn, C.H., et al., Augmentation of natural cytotoxicity by chronic low-dose ionizing radiation in murine natural killer cells primed by IL-2. 2012. [DOI] [PMC free article] [PubMed]
- 56.Liyun, Y.J.J.o.R., Effect of Low Dose Radiation(LDR) on Biological Activity of NK Cell. 2006.
- 57.Mantovani A. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017;14(7):399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paola I., Diana B. From Monocytes to M1/M2 Macrophages: phenotypical vs. Functional Differentiation. %J Frontiers in immunology. 2014;5 doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pathria P., Louis T.L., Varner J.A. Targeting Tumor-Associated Macrophages in Cancer. Trends Immunol. 2019;40(4):310–327. doi: 10.1016/j.it.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 60.Klug F. Low-Dose Irradiation Programs Macrophage Differentiation to an iNOS + /M1 Phenotype that Orchestrates Effective T. Cell Immunotherapy%J Cancer Cell. 2013;24(5) doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 61.Nadella V. Low dose radiation primed iNOS + M1macrophages modulate angiogenic programming of tumor derived endothelium. Mol. Carcinog. 2018;57(11):1664–1671. doi: 10.1002/mc.22879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schaue D. The effects of low-dose X-irradiation on the oxidative burst in stimulated macrophages. 2002;78(7):567–576. doi: 10.1080/09553000210126457. [DOI] [PubMed] [Google Scholar]
- 63.Kojima S.J.C. Induction of Glutathione and Activation of Immune Functions by Low-Dose. Whole-Body Irradiation with γ-Rays. 2007;38 doi: 10.1248/yakushi.126.849. [DOI] [PubMed] [Google Scholar]
- 64.Son C.H. Enhanced dendritic cell-based immunotherapy using low-dose cyclophosphamide and CD25-targeted antibody for transplanted Lewis lung carcinoma cells. J. Immunother. 2015;38(3):107–115. doi: 10.1097/CJI.0000000000000068. [DOI] [PubMed] [Google Scholar]
- 65.Joshi S. Rac2 controls tumor growth, metastasis and M1-M2 macrophage differentiation in vivo. PLoS ONE. 2014;9(4):e95893. doi: 10.1371/journal.pone.0095893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shigematsu, A., et al., Effects of Low-dose Irradiation on Enhancement of Immunity by Dendritic Cells. 2007. [DOI] [PubMed]
- 67.Ishii, K., et al., Enhanced mitogen-induced proliferation of rat splenocytes by low-dose whole-body X-irradiation. 1995. 27(1): p. 17–23. [PubMed]
- 68.Analysis of immune cell populations and cytokine profiles in murine splenocytes exposed to whole-body low-dose irradiation. %J International Journal of Radiation Biology. 2015;91(10):795. doi: 10.3109/09553002.2015.1068461. [DOI] [PubMed] [Google Scholar]
- 69.Sainis K.B. Low dose radiation induced immunomodulation: effect on macrophages and CD8+ T cells. 2005;81(11):801–812. doi: 10.1080/09553000500531886. [DOI] [PubMed] [Google Scholar]
- 70.N S.R. Cure with low-dose total-body irradiation of the hematological disorder induced in mice with the Friend virus: possible mechanism involving interferon-gamma and interleukin-2. %J Lymphokine and cytokine research. 1991;10(1–2) [PubMed] [Google Scholar]
- 71.Rizvi, A., et al., Low-dose γ-rays modify CD4(+) T cell signalling response to simulated solar particle event protons in a mouse model. 2011. 87(1): p. 24–35. [DOI] [PubMed]
- 72.Lissoni, P., et al., Effects of the conventional antitumor therapies surgery, chemotherapy, radiotherapy and immunotherapy on regulatory T lymphocytes in cancer patients. 2009. 29(5): p. 1847–1852. [PubMed]
- 73.Li, B., et al., Low-dose splenic radiation Inhibits liver tumor development of rats through functional changes In CD4+CD25+Treg cells. 2014. [DOI] [PubMed]
- 74.Stewart C.C., Stevenson A.P., Habbersett R.C. The effect of low-dose irradiation on unstimulated and PHA-stimulated human lymphocyte subsets. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1988;53(1):77–87. doi: 10.1080/09553008814550441. [DOI] [PubMed] [Google Scholar]
- 75.Cho, S.J., et al., Site-Specific Phosphorylation of Ikaros Induced by Low-Dose Ionizing Radiation Regulates Cell Cycle Progression of B Lymphoblast Through CK2 and AKT Activation. 2016. 94(5): p. 1207–1218. [DOI] [PubMed]
- 76.Ohshima, Y., et al., Induction of extracellular ATP mediates increase in intracellular thioredoxin in RAW264.7 cells exposed to low-dose γ-rays. 2011. 51(6): p. 1240–1248. [DOI] [PubMed]
- 77.Seema, et al., Reactive Oxygen Species: a Key Constituent in Cancer Survival. 2018. [DOI] [PMC free article] [PubMed]
- 78.Xinyue, L., et al., The low-dose ionizing radiation stimulates cell proliferation via activation of the MAPK/ERK pathway in rat cultured mesenchymal stem cells. (3): p. 380–6. [DOI] [PubMed]
- 79.Yu H.S. Effects of low-dose radiation on tumor growth, erythrocyte immune function and SOD activity in tumor-bearing mice. 2004;117(7):1036. [PubMed] [Google Scholar]
- 80.Li W. Low-dose radiation (LDR) induces hematopoietic hormesis: LDR-induced mobilization of hematopoietic progenitor cells into peripheral blood circulation. 2004;32(11):1088–1096. doi: 10.1016/j.exphem.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 81.Low-Dose Radiation Induces Cell Proliferation in Human Embryonic Lung Fibroblasts but not in Lung Cancer Cells: importance of ERK1/2 and AKT Signaling Pathways. %J Dose Response A Publication of International Hormesis Society. 2016 doi: 10.1177/1559325815622174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang S.Y., Cui Y.F.J.C.J.o.C.R. Effect of electromagnetic radiation of low dosage on immune system of organism: Research and application. 2006;10(13):154–157. [Google Scholar]
- 83.Zhang C. Low-dose radiation induces renal SOD1 expression and activity in type 1 diabetic mice. 2014;90(3):224–230. doi: 10.3109/09553002.2014.877174. [DOI] [PubMed] [Google Scholar]
- 84.Li . 2018. Low-dose Irradiation Inhibits Proliferation of the p53null Type Human Prostate Cancer Cells Through the ATM/p21 Pathway. [DOI] [PubMed] [Google Scholar]
- 85.Yang G. Distinct biological effects of low-dose radiation on normal and cancerous human lung cells are mediated by ATM signaling. 2016;7(44):71856–71872. doi: 10.18632/oncotarget.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miyamoto A. Absence of radioadaptive responses in four cell-lines in vitro as determined by colony formation assay. 2006;53(1–2):1. doi: 10.2739/kurumemedj.53.1. [DOI] [PubMed] [Google Scholar]
- 87.Zhao X. Effects of low-dose radiation on adaptive response in colon cancer stem cells. 2017;19(7):907–914. doi: 10.1007/s12094-017-1624-3. [DOI] [PubMed] [Google Scholar]