Abstract
Biomineralization is regulated by inorganic pyrophosphate (PPi), a potent physiological inhibitor of hydroxyapatite crystal growth. Progressive ankylosis protein (ANK) and ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1) act to increase local extracellular levels of PPi, inhibiting mineralization. The periodontal complex includes 2 mineralized tissues, cementum and alveolar bone (AB), both essential for tooth attachment. Previous studies demonstrated that loss of function of ANK or ENPP1 (reducing PPi) resulted in increased cementum formation, suggesting PPi metabolism may be a target for periodontal regenerative therapies. To compare the effects of genetic ablation of Ank, Enpp1, and both factors concurrently on cementum and AB regeneration, mandibular fenestration defects were created in Ank knockout (Ank KO), Enpp1 mutant (Enpp1asj/asj), and double KO (dKO) mice. Genetic ablation of Ank, Enpp1, or both factors increased cementum regeneration compared to controls at postoperative days (PODs) 15 and 30 (Ank KO: 8-fold, 3-fold; Enpp1asj/asj: 7-fold, 3-fold; dKO: 11-fold, 4-fold, respectively) associated with increased fluorochrome labeling and expression of mineralized tissue markers, dentin matrix protein 1 (Dmp1/DMP1), osteopontin (Spp1/OPN), and bone sialoprotein (Ibsp/BSP). Furthermore, dKO mice featured increased cementum thickness compared to single KOs at POD15 and Ank KO at POD30. No differences were noted in AB volume between genotypes, but osteoblast/osteocyte markers were increased in all KOs, partially mineralized osteoid volume was increased in dKO versus controls at POD15 (3-fold), and mineral density was decreased in Enpp1asj/asj and dKOs at POD30 (6% and 9%, respectively). Increased numbers of osteoclasts were present in regenerated AB of all KOs versus controls. These preclinical studies suggest PPi modulation as a potential and novel approach for cementum regeneration, particularly targeting ENPP1 and/or ANK. Differences in cementum and AB regeneration in response to reduced PPi conditions highlight the need to consider tissue-specific responses in strategies targeting regeneration of the entire periodontal complex.
Keywords: biomineralization, cementogenesis, bone regeneration, periodontium, extracellular matrix, osteoclast
Introduction
Periodontal disease affects approximately 45% of US adults and 20% to 50% of the global population, featuring destruction of tooth root cementum, periodontal ligament (PDL), and alveolar bone, leading to early tooth loss in advanced cases (Kassebaum et al. 2014; Eke et al. 2016). Cementum, located along the tooth root, includes acellular cementum at the cervical aspect and cellular cementum at the apical aspect. Acellular cementum is critical for PDL attachment to the tooth root, while cellular cementum is believed to adjust posteruption tooth position (Bosshardt 2005; Foster et al. 2007; Yamamoto et al. 2010). Although several studies provide data on factors important for periodontal regeneration, existing regenerative therapies are unpredictable, and cementum is especially challenging to regenerate due to gaps in knowledge about cementum formation (Bosshardt 2005; Foster et al. 2007).
Biomineralization is regulated by the ratio of inorganic phosphate (Pi), a component of hydroxyapatite (HA) mineral, to inorganic pyrophosphate (PPi), a physiological inhibitor of HA formation (Orriss et al. 2016; Chande and Bergwitz 2018). PPi regulators include progressive ankylosis protein (ANK: Ank in mice and ANKH in humans), a transmembrane protein that regulates PPi transport; ectonucleotide pyrophosphatase phosphodiesterase 1 (ENPP1: Enpp1), an ecto-enzyme that generates extracellular PPi from nucleotides; and tissue-nonspecific alkaline phosphatase (TNAP: Alpl), an ecto-enzyme that hydrolyzes PPi to Pi (Ho et al. 2000; Rutsch et al. 2003; Millan 2006; Szeri et al. 2020). Our group and others have shown that factors controlling Pi/PPi levels play significant roles in the formation of acellular cementum and alveolar bone. Previous studies indicate that loss of either ANK or ENPP1 (increasing extracellular Pi/PPi ratio) results in a striking hypercementosis phenotype, while loss of TNAP (decreasing extracellular Pi/PPi ratio) results in minimal cementum formation (Nociti et al. 2002; Foster et al. 2012; Thumbigere-Math et al. 2018; Chu et al. 2020).
Insights into the important role of Pi/PPi ratio in directing cementum formation prompted us to investigate the potential for Pi/PPi modulation to promote periodontal regeneration. In a proof-of-principle pilot study using a fenestration periodontal defect model, increased Pi/PPi ratio increased cementum formation in Ank knockout (KO) versus control mice (Rodrigues et al. 2011). This study did not address bone regeneration. Recognizing differences between bone and cementum in response to loss of Ank versus Enpp1 during development, parallel assessment of alveolar bone and cementum regenerative capabilities is imperative. The expanded studies here address important gaps in knowledge, including a direct comparison of cementum regeneration under the influence of genetic ablation of Ank versus Enpp1 or concurrent deletion of both factors, using a mouse fenestration defect model. For the first time, we contrast effects on cementum versus alveolar bone regeneration in response to reduced PPi concentrations. These data provide insights for strategies to promote regeneration of the periodontal complex.
Materials and Methods
Animals
Animal procedures were performed in accordance with guidelines of the Animal Care and Use Committee (ACUC), National Institutes of Health (NIH). Mice were maintained on a C57BL/6 background and fed a normal rodent diet (NIH-31). Preparation and genotyping of mice were described (Chu et al. 2020). Mice double heterozygote (dHet) for Ank and Enpp1 were bred to produce homozygote Ank KO and Enpp1asj/asj mice, double-deficient (dKO) mice, and littermate wild-type (WT) or dHet mice were used as controls (Control).
Fenestration Defects
A modified fenestration root surface defect model was used to remove alveolar bone and acellular cementum, as detailed in Appendix Figure 1A (King et al. 1997; Rodrigues et al. 2011). Surgeries were performed on 5-wk-old mice. Mandibles were harvested for analysis at postoperative days (PODs) 15 and 30 (n = 4 mice/genotype at POD15 and POD30 for micro–computed tomography [CT] and histology; n = 3 to 4 mice/genotype at POD30 for double fluorochrome labeling). Male and female mice were combined because no significant sex-related differences in cementum or alveolar bone were noted in a previous study (Chu et al. 2020).
Micro-CT
Mandibles were fixed in 10% formalin for 48 h at room temperature and scanned in 70% ethanol in a µCT 50 (ScancoMedical) at 70 kVp, 85 µA, 0.5-mm Al filter, 900-ms integration time, and 6-µm voxel size. Samples were analyzed using a combination of semiautomatic and manual methods with AnalyzePro 1.0 (AnalyzeDirect), as detailed in the Appendix.
Histology
After micro-CT analyses, mandibles were demineralized in an acetic acid, formalin, and sodium chloride (AFS) solution for 4 wk, as described (Ao et al. 2017). Mandibles were embedded in paraffin for 5-µm serial section and analyzed by hematoxylin and eosin (H&E), in situ hybridization (ISH), immunohistochemistry (IHC), and tartrate-resistant acid phosphatase (TRAP) as described (Ao et al. 2017; Chu et al. 2020). Double fluorochrome labeling using calcein and alizarin red was performed, as described in the Appendix. Regenerated cementum thickness and proportion of root coverage (PRC; %) were measured by H&E staining (Appendix Fig. 1B). For measurement of positive ISH staining, regenerated cementum and alveolar bone were selected in each section under 200× magnification and measured using ImageJ (NIH), as shown in Appendix Figure 2. To count TRAP+ and cathepsin K+ cells, the wound-healing area was selected, as detailed in the Appendix and Appendix Figure 3.
Statistical Analysis
Results (mean ± SD) were analyzed with Student’s t test for independent samples or 1-way analysis of variance followed by Tukey’s post hoc test for pairwise comparisons (GraphPad Prism 7; GraphPad Software). Values of P < 0.05 were considered statistically significant.
Results
Increased and Additive Cementum Regeneration with Loss of ANK and/or ENPP1
First, cementum regeneration was evaluated. Cementum thickness was measured at 3 points within the fenestration defect: mesial (M), central (C), and distal (D), and PRC was determined. Representative histological images of specimens obtained at POD15 and POD30 are shown in Figure 1A. The 3 KO models showed significantly increased regenerated cementum versus control mice at both POD15 and POD30 (Ank KO: 8-fold and 3-fold; Enpp1asj/asj: 7-fold and 3-fold; dKO: 11-fold and 4-fold, respectively; POD15: P < 0.01, POD30: P < 0.001). Regenerated cementum in all 3 KO models had cellular inclusions as reported in developmental studies of these KO models (Chu et al. 2020). Importantly, ankylosis was never observed in any of these KOs, consistent with previous studies (Rodrigues et al. 2011; Wolf et al. 2018; Chu et al. 2020). At POD15, dKO mice showed increased cementum thickness at all points versus single KO mice (dKO vs. Ank KO at 1.4-fold; dKO vs. Enpp1asj/asj at 1.7-fold, P < 0.05), although there were no significant differences between single KOs. Some differences were observed between KO models at selected locations or time points. At POD30, cementum thickness was greater in Enpp1asj/asj versus Ank KO mice at the distal point (1.2-fold, P < 0.01). In addition, dKO cementum was significantly increased versus Ank KO at all 3 locations at both POD15 and POD30 (1.4-fold, P < 0.05). No differences were observed between dKO and Enpp1asj/asj mice at POD30. PRC was increased in Ank KO, Enpp1asj/asj, and dKO mice versus controls (P < 0.01), with no differences between KOs. These findings indicate loss of function of ANK and ENPP1 has additive effects on cementum regeneration at an early stage, with loss of ENPP1 activity exhibiting a greater effect on cementum regeneration than loss of ANK.
Figure 1.
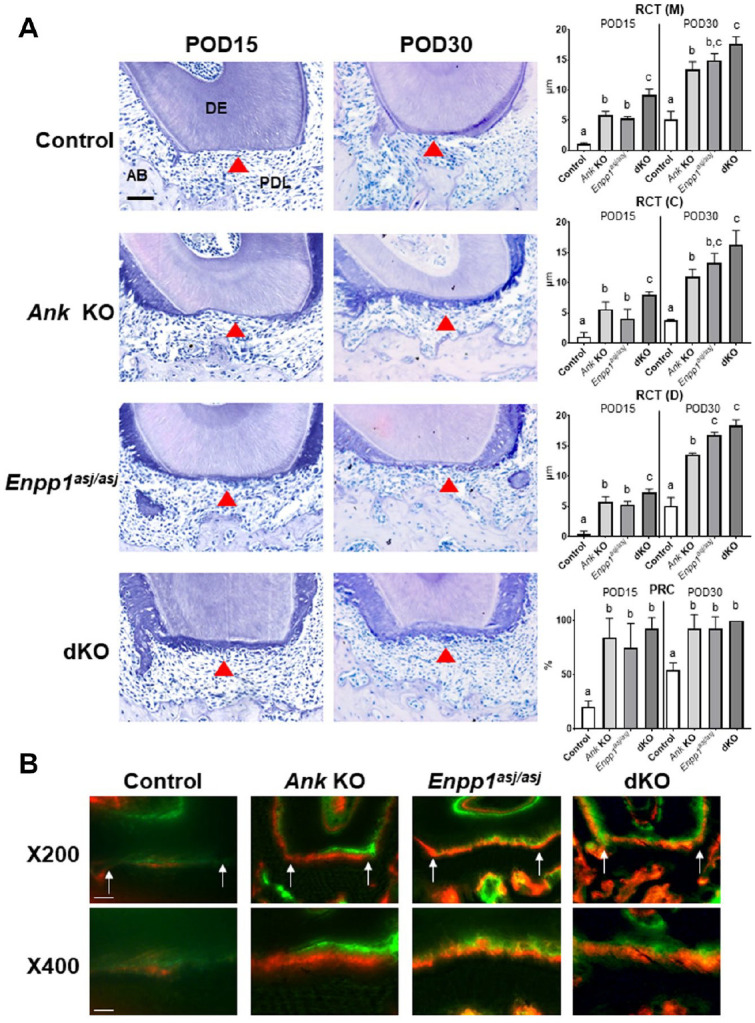
Increased cementum regeneration in Ank knockout (KO), Enpp1asj/asj, and double knockout (dKO) mice. (A) Representative images of distal root of first molars at postoperative days (PODs) 15 and 30 (n = 4 per genotype). Arrowhead, regenerated cementum; AB, alveolar bone; DE, dentin; PDL, periodontal ligament. Scale bar: 50 µm. Regenerated cementum thickness (RCT) was measured at 3 locations on the distal root of first molars: mesial (M), center (C), and distal (D). The proportion of root coverage (PRC) was measured in wild-type (WT)/dHet, Ank KO, Enpp1asj/asj, and dKO. Experimental groups marked by different letters (a, b, or c) within POD15 or POD30 are significantly different (P < 0.01, by one-way analysis of variance), while groups sharing the same letter are not different (P > 0.05). (B) Double fluorochrome labeling by injection of calcein (green) at POD7 and alizarin red (red) at POD14 (tissues harvested at POD30) reveals new cementum apposition. Arrows indicate wound boundaries. Scale bar: 50 µm in 200× and 25 µm in 400×.
Cementum regeneration was assessed further by double fluorochrome labeling using calcein and alizarin red injections (injected at POD7 and POD14, respectively). Calcein labeling had similar intensity in all genotypes, while alizarin red exhibited greater intensity in all 3 KO models versus controls, suggestive of rapid mineral deposition (Fig. 1B).
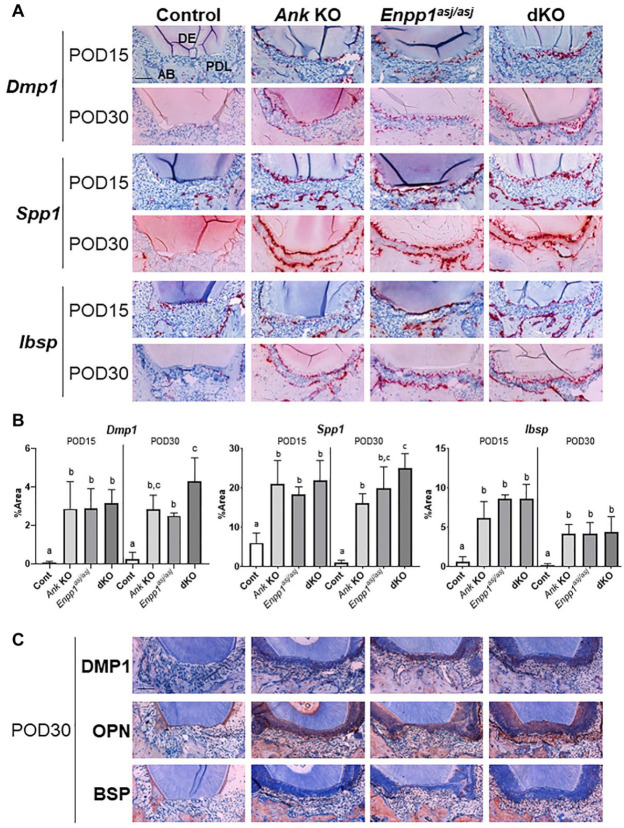
To confirm the identity of regenerated tissue and provide insights into the regenerative cellular response, ISH and IHC were used to assay expression of 3 cementoblast markers, bone sialoprotein (Ibsp/BSP), osteopontin (Spp1/OPN), and dentin matrix protein (Dmp1/DMP1). Stronger expression of transcripts for Dmp1, Spp1, and Ibsp were observed in root-lining cells of Ank KO, Enpp1asj/asj, and dKO mice at POD15, with continued strong expression at POD30, versus minimal signals in control mice (Fig. 2A). Quantitative measurement of ISH staining area along healing root surfaces indicated these 3 markers were significantly increased in all KO versus controls at both time points (P < 0.01) (Fig. 2B). Some differences were observed between single KO and dKO at POD30. Dmp1 and Spp1 expression was increased in dKO versus Enpp1asj/asj and Ank KO mice, respectively (1.7-fold and 1.5-fold, respectively; P < 0.05). Comparable results were noted by IHC. OPN robustly immunolocalized to the new cementum in Ank KO, Enpp1asj/asj, and dKO mice, with more modest immunostaining for DMP1 and BSP. Control mice exhibited minimal staining for OPN on root surfaces and no detectable DMP1 or BSP.
Figure 2.
Increased expression of cementum markers coincides with increased cementogenesis in knockout (KO) and double knockout (dKO) mice. Representative images of (A) in situ hybridization (ISH) and (C) immunohistochemistry (IHC) staining for dentin matrix protein (Dmp1/DMP1), osteopontin (Spp1/OPN), and bone sialoprotein (Ibsp/BSP) at postoperative day (POD) 15 and POD30 (n = 4 per genotype). Single KOs and dKO mice exhibited greater expression and localization of all markers in association with cementum regeneration. AB, alveolar bone; DE, dentin; PDL, periodontal ligament. Scale bar: 50 µm. (B) Statistical analyses of ISH staining. All 3 markers are increased in regenerated cementum of all KO models versus controls at both POD15 and POD30 (Dmp1: Ank KO: 40-fold, 11-fold; Enpp1asj/asj: 40-fold, 10-fold; dKO: 44-fold, 16-fold; Spp1: Ank KO: 3-fold, 17-fold; Enpp1asj/asj: 3-fold, 21-fold; dKO: 4-fold, 27-fold; Ibsp: Ank KO: 10-fold, 22-fold; Enpp1asj/asj: 13-fold, 21-fold; dKO: 13-fold, 22-fold, respectively, P < 0.01). At POD30, Dmp1 and Spp1 expression levels were significantly increased in dKO versus Enpp1asj/asj and Ank KO mice, respectively (1.7-fold and 1.5-fold, respectively, P < 0.05). Experimental groups marked by different letters (a, b, or c) within POD15 or POD30 are significantly different (P < 0.05, by one-way analysis of variance), while groups sharing the same letter are not different (P > 0.05).
Increased Osteoid Volume and Bone Marker Expression in Alveolar Bone of KO and dKO Mice
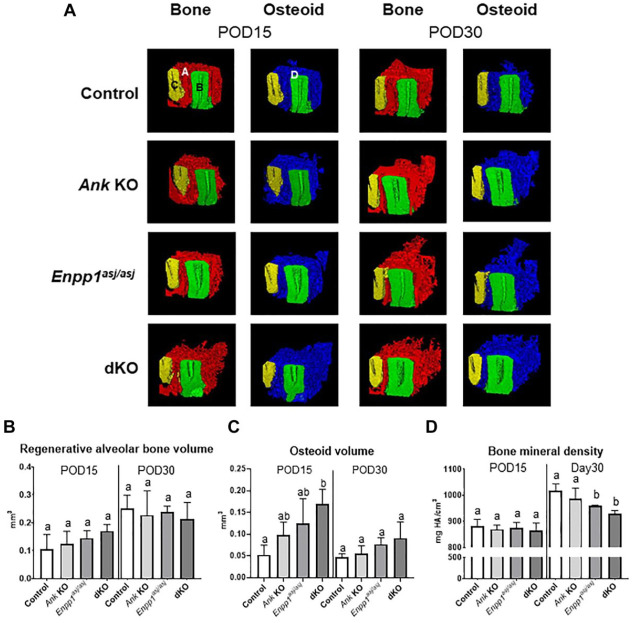
Quantity and quality of regenerated alveolar bone were evaluated by micro-CT analysis (Fig. 3A). No differences were noted in regenerated bone volume between any genotypes at POD15 or POD30 (Fig. 3B). Partially mineralized osteoid (i.e., incompletely mineralized bone matrix) was identified based on anatomical location and quantified using a lower density threshold. The osteoid volume was significantly increased in dKO versus control mice at POD15 (3-fold, P < 0.01), with similar trends in single KO mice, but differences between genotypes were not observed at POD30 (Fig. 3C). Regenerated alveolar bone density showed an inverse trend compared to osteoid volume at POD30, with Enpp1asj/asj and dKO mice exhibiting decreased bone mineral density versus controls at POD30, while a similar trend was observed in Ank KO mice (6%, P < 0.05; 9%, P < 0.01, respectively) (Fig. 3D).
Figure 3.
Ank and/or Enpp1 ablation does not increase alveolar bone volume. (A) Representative micro-computed tomography (CT) images of regenerated mineralized alveolar bone (red) and osteoid (blue) (n = 4 per genotype). A (red), regenerated alveolar bone; B (green), distal root of first molar; C (yellow), mesial root of second molar; D (blue), partially mineralized osteoid. No differences between genotypes were found for (B) regenerated alveolar bone volume, but (C) partially mineralized osteoid volume was increased in double knockout (dKO) mice at postoperative day (POD) 15. (D) Bone mineral density was reduced in Enpp1asj/asj and dKO mice at POD30 (6%, P < 0.05 and 9%, P < 0.01, respectively). Experimental groups marked by different letters (a, b, or c) within POD15 or POD30 are significantly different (P < 0.05, by one-way analysis of variance), while groups sharing the same letter are not different (P > 0.05).
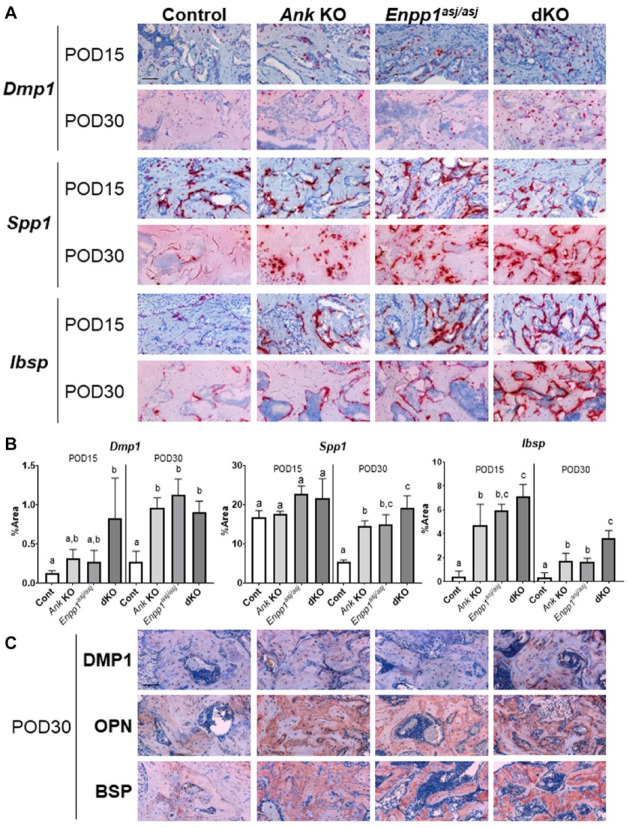
Regenerated bone was analyzed by ISH and IHC using established markers. In contrast to minimal expression of Dmp1/DMP1, Spp1/OPN, and Ibsp/BSP in cementum of control mice, expression levels for all markers were robust in regenerating alveolar bone of control mice at both POD15 and POD30, suggesting the process of bone regeneration was more active than cementum regeneration (Fig. 4). In all mice, Dmp1 localized primarily to osteocytes within the bone matrix and DMP1 to osteocyte perilacunar matrix, while Spp1 and Ibsp localized to osteoblasts on the surface and OPN and BSP immunolabeled throughout the regenerated bone (Fig. 4A, C). Quantitative measurement of ISH staining confirmed these 3 markers were significantly increased in regenerated bone of all 3 KO models versus controls at POD30 (P < 0.05). At POD15, Dmp1 was significantly increased in dKO versus control (6-fold, P < 0.05), while a trend was observed in single KOs. Ibsp was significantly increased in all 3 KO models (P < 0.001), with no significant difference observed for Spp1 (Fig. 4B). Some differences were observed between single KO and dKO at POD15 and POD30. Spp1 at POD30 and Ibsp at POD15 were significantly increased in dKO versus Ank KO (1.4-fold and 1.3-fold, respectively, P < 0.05), and Ibsp POD30 was increased in dKO versus single KOs (2-fold, P < 0.01). IHC staining showed increased immunostaining for BSP, OPN, and DMP1 in alveolar bone matrix at POD30. In control mice, in contrast to other markers that were expressed in bone at both POD15 and POD30, Spp1 was strongly expressed at POD15 with lower expression at POD30. These differences in the expression pattern of OPN between KOs and control mice coincide with findings of increased osteoid volume in KOs versus controls (Fig. 3), suggesting a role for OPN in modulating bone remodeling.
Figure 4.
Increased expression of alveolar bone markers in knockout (KO) and double knockout (dKO) mice. Representative images of (A) in situ hybridization (ISH) and (C) immunohistochemistry (IHC) staining for dentin matrix protein (Dmp1/DMP1), osteopontin (Spp1/OPN), and bone sialoprotein (Ibsp/BSP) at postoperative day (POD) 15 and POD30 (n = 4 per genotype). Single KOs and dKO mice exhibited greater expression and localization of all markers in association with alveolar bone regeneration. Scale bar: 50 µm. (B) Statistical analyses of ISH staining. At POD15, Ibsp was significantly increased in all 3 KOs versus controls (Ank KO: 12-fold; Enpp1asj/asj: 15-fold; dKO: 15-fold, P < 0.001). Although Dmp1 was increased in dKO (6-fold, P < 0.05) with a similar trend in single KOs and no difference was observed in Spp1 at POD15, all 3 markers were significantly increased in regenerated alveolar bone of all KO models versus controls at POD30 (Dmp1: Ank KO: 4-fold; Enpp1asj/asj: 4-fold; dKO: 3-fold; Spp1: Ank KO: 6-fold; Enpp1asj/asj: 5-fold; dKO: 9-fold; Ibsp: Ank KO: 5-fold; Enpp1asj/asj: 5-fold; dKO: 11-fold, P < 0.05). Spp1 at POD30 and Ibsp at POD15 were significantly increased in dKO versus Ank KO (1.4-fold and 1.3-fold, respectively, P < 0.05). Ibsp in dKO at POD30 was significantly increased versus single KOs (2-fold, P < 0.01). Experimental groups marked by different letters (a, b, or c) within POD15 or POD30 are significantly different (P < 0.05, by one-way analysis of variance), while groups sharing the same letter are not different (P > 0.05).
Increased Osteoclast Numbers in Single KOs and dKO Mice
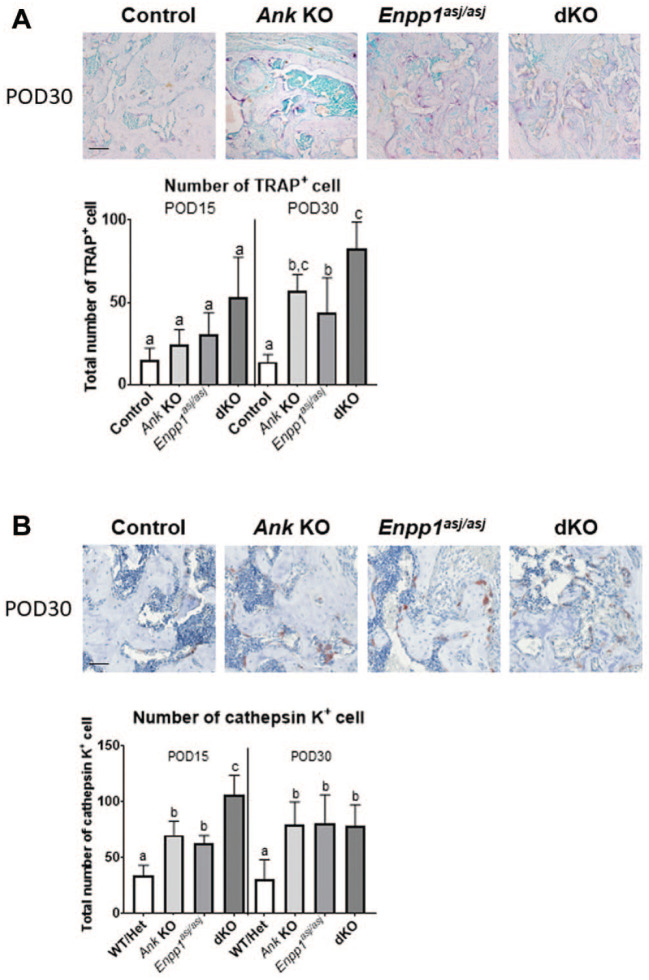
Decreased bone mineral density and altered IHC staining in Enpp1asj/asj and dKO mice at POD30 prompted us to examine osteoclasts at healing sites. TRAP staining revealed no differences in TRAP+ osteoclast numbers at POD15, but at POD30, all KO models featured increased osteoclasts versus controls (P < 0.001). Furthermore, dKO mice showed significantly increased osteoclast numbers compared to Enpp1asj/asj mice (2-fold, P < 0.05) (Fig. 5A). IHC indicated the number of cathepsin K+ cells was increased in all 3 KO models versus controls at both time points (P < 0.05). Moreover, dKO showed increased cathepsin K+ cells versus single KOs at POD15 (vs. Ank KO: 1.5-fold; vs. Enpp1asj/asj: 1.7-fold; P < 0.01).
Figure 5.
Increased osteoclast numbers in alveolar bone of knockout (KO) and double knockout (dKO) mice. (A) Representative images of tartrate-resistant acid phosphatase (TRAP) staining in all genotypes at postoperative day (POD) 30 (n = 4 per genotype). Scale bar: 50 µm. Increased numbers of TRAP+ osteoclasts were localized to regenerated alveolar bone in Ank KO, Enpp1asj/asj, and dKO versus control mice at POD30 (Ank KO: 4-fold, P < 0.01; Enpp1asj/asj: 3-fold, P < 0.05; dKO: 6-fold, P < 0.001). Increased TRAP+ osteoclasts were observed in dKO versus Enpp1asj/asj (2-fold, P < 0.05). (B) Representative immunohistochemistry (IHC) images for cathepsin K in all genotypes at POD30 (n = 4 per genotype). Scale bar: 50 µm. The number of cathepsin K+ osteoclasts was significantly increased in all KO models versus controls at both POD15 (Ank KO: 2-fold, P < 0.01; Enpp1asj/asj: 1.8-fold, P < 0.05; dKO: 3-fold, P < 0.0001) and POD30 (Ank KO, Enpp1asj/asj, dKO: 3-fold, P < 0.05); furthermore, dKO showed more increased cathepsin K+ osteoclasts versus single KOs at POD15 (vs. Ank KO: 1.5-fold; vs. Enpp1asj/asj: 1.7-fold, P < 0.01). Experimental groups marked by different letters (a, b, or c) within POD15 or POD30 are significantly different (P < 0.05, by one-way analysis of variance), while groups sharing the same letter are not different (P > 0.05).
Discussion
Previous studies, focused on defining the role of factors modulating Pi/PPi during periodontal development, demonstrated that Ank KO, Enpp1asj/asj, and dKO mice featured similarly increased cementum. Here, we asked whether altered PPi regulation during wound healing would parallel developmental findings. Using a fenestration wound-healing model, we show that ablation of Ank or Enpp1 increased reparative cementogenesis versus controls, including increased expression of mineralized tissue extracellular matrix markers. In contrast, loss of ANK or ENPP1 did not increase alveolar bone regeneration, leading to a transient increase in osteoid and decreased bone mineral density. Effects on cementum and osteoid formation from concurrent ANK and ENPP1 loss were additive. Notably, we observed increased numbers of TRAP+ and cathepsin K+ osteoclasts within the regenerated alveolar bone in all KO models versus control mice.
Reduction of PPi Promotes Regeneration of Cementum
PPi regulators, ANK and ENPP1, modulate cementogenesis and osteogenesis (Rodrigues et al. 2011; Foster et al. 2012; Thumbigere-Math et al. 2018; Chu et al. 2020). PPi dysregulation has clear implications in humans and mice models. For example, individuals with generalized arterial calcification of infancy (GACI) exhibit hypercementosis due to loss of ENPP1 (Thumbigere-Math et al. 2018). Previous studies reported that Enpp1asj/asj mice have reduced serum PPi levels (Albright et al. 2015), and loss of ANK resulted in intracellular PPi accumulation and extracellular PPi reduction (Ho et al. 2000). Furthermore, in vitro studies revealed 50% to 60% decreased extracellular PPi in Ank KO versus WT primary osteoblasts (Harmey et al. 2004). In a proof-of-principle study, ANK loss of function promoted cementum regeneration (Rodrigues et al. 2011), and here we expand the scope to determine effects from ENPP1 loss and concurrent loss of ANK and ENPP1. All KO models in this study demonstrated accelerated and increased cementum regeneration versus controls. Differences were noted in response between Ank KO and Enpp1asj/asj mice (i.e., Enpp1asj/asj mice showed increased cementum thickness compared to Ank KO mice at POD30). Notably, developmentally there were no differences in cementum thicknesses between all KO models, suggesting that during development, there is a limit to the effects of PPi on cementogenesis (Chu et al. 2020). In regeneration, ENPP1 loss of function has a more potent effect than ANK ablation. Moreover, dKO mice showed significantly increased regenerated cementum versus single KOs, suggesting additive effects of ANK and ENPP1. Factors beyond the Pi/PPi ratio may be contributing to this effect, warranting further investigation.
Regenerated cementum in all KOs exhibited robust expression of mineralized tissue extracellular matrix markers, Dmp1, Spp1, and Ibsp, coinciding with increased cementum thickness. There appeared to be a trend of increased BSP in all KOs versus controls, but differences were not as clear as with ISH, confirming previous studies (Foster et al. 2011; Rodrigues et al. 2011). In addition to serving as cementum markers, increased expression of these ECM proteins confirms previous studies demonstrating that modulators of Pi/PPi levels affect expression of DMP1, OPN, and BSP (Addison et al. 2007; Foster et al. 2012; Nishino et al. 2017).
We and others demonstrated that PPi regulation of cementogenesis is evolutionarily conserved across species, including mouse models of ANK, ENPP1, and TNAP loss-of-function and individuals with ENPP1 or ALPL loss-of-function mutations (Foster et al. 2012; Zweifler et al. 2015; Thumbigere-Math et al. 2018; Chavez et al. 2020), supporting PPi modulation as a novel approach to promote cementum regeneration. Backing this concept, we reported that pharmacologic manipulation of PPi through an ENPP1-Fc fusion protein regulates cementum growth (Chu et al. 2020). Previous findings bolstered by new data here support further studies of therapeutic interventions targeting PPi metabolism.
Altered Alveolar Bone Regeneration Associated with Reduced PPi
Although reduced levels of PPi promoted cementum regeneration, alveolar bone did not exhibit a similar regenerative response. These findings are not surprising because during wound healing, alveolar bone is in an active phase of formation and resorption, while cementum exhibits limited remodeling (Kusumbe et al. 2014). Critical processes in bone formation include osteoblast-mediated deposition of collagen-rich osteoid ECM, which mineralizes to form an ossification center (Kenkre and Bassett 2018). We noted increased osteoid volume at POD15 in dKO versus controls with a trend in single KOs, but by POD30, no differences were noted in osteoid volume between any of the phenotypes. No differences in bone volume were observed between phenotypes at POD15 or POD30. The initial increase in osteoid volume suggests delayed mineralization at early stages of regeneration. While speculative, this may be related to limited levels of PPi at the regenerative site. In this regard, hyperosteoidosis with hypomineralization of other tissues has been linked with inadequate Pi/PPi metabolism (Cundy et al. 2015; Kamiya et al. 2017). Further evidence of perturbed mineralization includes increased ectopic calcifications and reduced femur length in Ank, Enpp1 dKO versus single KO mice (Harmey et al. 2004; Chu et al. 2020). In the appendicular skeleton, osteopenia in adult Enpp1 KO mice is exacerbated over time, suggesting that ENPP1 loss alters bone remodeling (Mackenzie et al. 2012). Increased osteoid may also be related to increased Spp1/OPN expression in regenerating alveolar bone of all KOs. OPN is considered an inhibitor of mineralization (Hunter et al. 1994; Boskey et al. 2002; Harmey et al. 2006). Decreased expression of Spp1/OPN at POD30 in control mice may reflect normal healing. In contrast, all KOs continued to exhibit strong expression of Spp1/OPN at POD30, suggesting OPN may affect bone remodeling, perhaps related to decreased levels of PPi at sites of healing. Past studies suggest comparable effects of increased OPN expression in impairment of the mineralization process (Boskey et al. 2002; Harmey et al. 2006; Barros et al. 2013; Narisawa et al. 2013). Such a local response, if transient as would be expected with a local delivery of a regeneration factor, may prove to be beneficial (e.g., may slow down remodeling and thereby promote increased mineral formation fostered by the increased osteoid).
The altered regenerated bone profile in these animal models prompted us to assess osteoclast activity. Existing data from in vivo and in vitro models indicate that differentiation processes of osteoblasts and osteoclasts are influenced by Pi/PPi ratio (Beck et al. 2000; Hayashibara et al. 2007; Nishino et al. 2017). Osteoclast differentiation markers (i.e., TRAP and cathepsin K) (Zhao et al. 2007) were significantly increased in all 3 KOs versus control. Furthermore, dKO mice showed increased number of TRAP+ cells versus Enpp1asj/asj and cathepsin K+ cells versus both single KOs. During development, Ank KO, Enpp1asj/asj, and dKO mice also exhibited increased TRAP+ osteoclasts on alveolar bone surfaces, but differences in bone mineral density were not detected between KO models and controls (Chu et al. 2020). These differences between bone formed during development versus wound healing indicate that in “challenge” models, loss of ANK and/or ENPP1 perturbs the balance between osteogenesis and osteoclastogenesis, which can lead to reduced mineral density. Previous studies focused on ANK support a role for this factor in modulating osteoblastic-osteoclastic homeostasis (Kim et al. 2010). It is intriguing to speculate that additional events related to wound healing may contribute to osteoclast function and further signify nonredundant roles for ANK and ENPP1.
Our results emphasize mechanistic differences between cementum and bone regeneration, possibly attributable to high sensitivity of cementogenesis to alterations in PPi, in conjunction with limited remodeling. This serves as a reminder to consider tissue-specific responses during periodontal tissue regeneration. Taken together, our preclinical studies suggest PPi modulation as a potential approach for cementum regeneration, but large animal studies must be completed to build on these findings.
Author Contributions
A. Nagasaki, K. Nagasaki, E.Y. Chu, B.L. Foster, M.J. Somerman, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; B.D. Kear, contributed to design, data acquisition, and analysis, drafted and critically revised the manuscript; W.D. Tadesse, S.E. Ferebee, contributed to design, data acquisition, and analysis, drafted the manuscript; L. Li, contributed to data acquisition, drafted the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, sj-pdf-1-jdr-10.1177_0022034520981854 for Ablation of Pyrophosphate Regulators Promotes Periodontal Regeneration by A. Nagasaki, K. Nagasaki, E.Y. Chu, B.D. Kear, W.D. Tadesse, S.E. Ferebee, L. Li, B.L. Foster and M.J. Somerman in Journal of Dental Research
Acknowledgments
We thank Dr. Francisco Nociti (State University of Campinas, Brazil) for assistance with fenestration defect surgical techniques, Dr. Renny Franceschi (University of Michigan) for providing polyclonal rabbit anti-mouse bone sialoprotein antibody, Dr. Vardit Kram for assistance with micro-CT scanning (National Institute of Dental and Craniofacial Research/NIH), and Dr. Davide Randazzo, Dr. Evelyn Ralston, and Ms. Aster Kinea (Light Imaging Section, National Institute of Arthritis and Musculoskeletal and Skin Diseases/NIH) for assistance in slide scanning.
Footnotes
A supplemental appendix to this article is available online.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by NIAMS Intramural Research Program, Japan Society for the Promotion of Science (JSPS) to A. Nagasaki (JSPS-NIH Research Fellow, JSPS Research Fellowship for Japanese Biomedical and Behavioral Researchers at NIH), K99/R00 grant AR073926 to E.Y. Chu, and R03DE028411 and R01DE027639 from National Institute of Dental and Craniofacial Research/NIH to B.L. Foster.
ORCID iDs: A. Nagasaki  https://orcid.org/0000-0002-7655-9658
https://orcid.org/0000-0002-7655-9658
B.L. Foster  https://orcid.org/0000-0003-3444-0576
https://orcid.org/0000-0003-3444-0576
References
- Addison WN, Azari F, Sorensen ES, Kaartinen MT, McKee MD. 2007. Pyrophosphate inhibits mineralization of osteoblast cultures by binding to mineral, up-regulating osteopontin, and inhibiting alkaline phosphatase activity. J Biol Chem. 282(21):15872–15883. [DOI] [PubMed] [Google Scholar]
- Albright RA, Stabach P, Cao W, Kavanagh D, Mullen I, Braddock AA, Covo MS, Tehan M, Yang G, Cheng Z, et al. 2015. ENPP1-Fc prevents mortality and vascular calcifications in rodent model of generalized arterial calcification of infancy. Nat Commun. 6:10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ao M, Chavez MB, Chu EY, Hemstreet KC, Yin Y, Yadav MC, Millan JL, Fisher LW, Goldberg HA, Somerman MJ, et al. 2017. Overlapping functions of bone sialoprotein and pyrophosphate regulators in directing cementogenesis. Bone. 105:134–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros NM, Hoac B, Neves RL, Addison WN, Assis DM, Murshed M, Carmona AK, McKee MD. 2013. Proteolytic processing of osteopontin by PHEX and accumulation of osteopontin fragments in Hyp mouse bone, the murine model of X-linked hypophosphatemia. J Bone Miner Res. 28(3):688–699. [DOI] [PubMed] [Google Scholar]
- Beck GR, Jr, Zerler B, Moran E. 2000. Phosphate is a specific signal for induction of osteopontin gene expression. Proc Natl Acad Sci USA. 97(15):8352–8357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskey AL, Spevak L, Paschalis E, Doty SB, McKee MD. 2002. Osteopontin deficiency increases mineral content and mineral crystallinity in mouse bone. Calcif Tissue Int. 71(2):145–154. [DOI] [PubMed] [Google Scholar]
- Bosshardt DD. 2005. Are cementoblasts a subpopulation of osteoblasts or a unique phenotype? J Dent Res. 84(5):390–406. [DOI] [PubMed] [Google Scholar]
- Chande S, Bergwitz C. 2018. Role of phosphate sensing in bone and mineral metabolism. Nat Rev Endocrinol. 14(11):637–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez MB, Kramer K, Chu EY, Thumbigere-Math V, Foster BL. 2020. Insights into dental mineralization from three heritable mineralization disorders. J Struct Biol. 212(1):107597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu EY, Vo TD, Chavez MB, Nagasaki A, Mertz EL, Nociti FH, Aitken SF, Kavanagh D, Zimmerman K, Li X, et al. 2020. Genetic and pharmacologic modulation of cementogenesis via pyrophosphate regulators. Bone. 136:115329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundy T, Michigami T, Tachikawa K, Dray M, Collins JF, Paschalis EP, Gamsjaeger S, Roschger A, Fratzl-Zelman N, Roschger P, et al. 2015. Reversible deterioration in hypophosphatasia caused by renal failure with bisphosphonate treatment. J Bone Miner Res. 30(9):1726–1737. [DOI] [PubMed] [Google Scholar]
- Eke PI, Zhang X, Lu H, Wei L, Thornton-Evans G, Greenlund KJ, Holt JB, Croft JB. 2016. Predicting periodontitis at state and local levels in the United States. J Dent Res. 95(5):515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster BL, Nagatomo KJ, Bamashmous SO, Tompkins KA, Fong H, Dunn D, Chu EY, Guenther C, Kingsley DM, Rutherford RB, et al. 2011. The progressive ankylosis protein regulates cementum apposition and extracellular matrix composition. Cells Tissues Organs. 194(5):382–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster BL, Nagatomo KJ, Nociti FH, Jr, Fong H, Dunn D, Tran AB, Wang W, Narisawa S, Millan JL, Somerman MJ. 2012. Central role of pyrophosphate in acellular cementum formation. PLoS One. 7(6):e38393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster BL, Popowics TE, Fong HK, Somerman MJ. 2007. Advances in defining regulators of cementum development and periodontal regeneration. Curr Top Dev Biol. 78:47–126. [DOI] [PubMed] [Google Scholar]
- Harmey D, Hessle L, Narisawa S, Johnson KA, Terkeltaub R, Millan JL. 2004. Concerted regulation of inorganic pyrophosphate and osteopontin by akp2, enpp1, and ank: an integrated model of the pathogenesis of mineralization disorders. Am J Pathol. 164(4):1199–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmey D, Johnson KA, Zelken J, Camacho NP, Hoylaerts MF, Noda M, Terkeltaub R, Millan JL. 2006. Elevated skeletal osteopontin levels contribute to the hypophosphatasia phenotype in Akp2(−/−) mice. J Bone Miner Res. 21(9):1377–1386. [DOI] [PubMed] [Google Scholar]
- Hayashibara T, Hiraga T, Sugita A, Wang L, Hata K, Ooshima T, Yoneda T. 2007. Regulation of osteoclast differentiation and function by phosphate: potential role of osteoclasts in the skeletal abnormalities in hypophosphatemic conditions. J Bone Miner Res. 22(11):1743–1751. [DOI] [PubMed] [Google Scholar]
- Ho AM, Johnson MD, Kingsley DM. 2000. Role of the mouse ank gene in control of tissue calcification and arthritis. Science. 289(5477):265–270. [DOI] [PubMed] [Google Scholar]
- Hunter GK, Kyle CL, Goldberg HA. 1994. Modulation of crystal formation by bone phosphoproteins: structural specificity of the osteopontin-mediated inhibition of hydroxyapatite formation. Biochem J. 300(Pt 3):723–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya N, Yamaguchi R, Aruwajoye O, Kim AJ, Kuroyanagi G, Phipps M, Adapala NS, Feng JQ, Kim HK. 2017. Targeted disruption of NF1 in osteocytes increases FGF23 and osteoid with osteomalacia-like bone phenotype. J Bone Miner Res. 32(8):1716–1726. [DOI] [PubMed] [Google Scholar]
- Kassebaum NJ, Bernabe E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. 2014. Global burden of severe periodontitis in 1990–2010: a systematic review and meta-regression. J Dent Res. 93(11):1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenkre JS, Bassett J. 2018. The bone remodelling cycle. Ann Clin Biochem. 55(3):308–327. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Minashima T, McCarthy EF, Winkles JA, Kirsch T. 2010. Progressive ankylosis protein (ANK) in osteoblasts and osteoclasts controls bone formation and bone remodeling. J Bone Miner Res. 25(8):1771–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King GN, King N, Cruchley AT, Wozney JM, Hughes FJ. 1997. Recombinant human bone morphogenetic protein-2 promotes wound healing in rat periodontal fenestration defects. J Dent Res. 76(8):1460–1470. [DOI] [PubMed] [Google Scholar]
- Kusumbe AP, Ramasamy SK, Adams RH. 2014. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 507(7492):323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie NC, Zhu D, Milne EM, van’t Hof R, Martin A, Quarles DL, Millan JL, Farquharson C, MacRae VE. 2012. Altered bone development and an increase in FGF-23 expression in Enpp1(−/−) mice. PLoS One. 7(2):e32177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan JL. 2006. Alkaline phosphatases: structure, substrate specificity and functional relatedness to other members of a large superfamily of enzymes. Purinergic Signal. 2(2):335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narisawa S, Yadav MC, Millan JL. 2013. In vivo overexpression of tissue-nonspecific alkaline phosphatase increases skeletal mineralization and affects the phosphorylation status of osteopontin. J Bone Miner Res. 28(7):1587–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino J, Yamazaki M, Kawai M, Tachikawa K, Yamamoto K, Miyagawa K, Kogo M, Ozono K, Michigami T. 2017. Extracellular phosphate induces the expression of dentin matrix protein 1 through the FGF receptor in osteoblasts. J Cell Biochem. 118(5):1151–1163. [DOI] [PubMed] [Google Scholar]
- Nociti FH, Jr, Berry JE, Foster BL, Gurley KA, Kingsley DM, Takata T, Miyauchi M, Somerman MJ. 2002. Cementum: a phosphate-sensitive tissue. J Dent Res. 81(12):817–821. [DOI] [PubMed] [Google Scholar]
- Orriss IR, Arnett TR, Russell RG. 2016. Pyrophosphate: a key inhibitor of mineralisation. Curr Opin Pharmacol. 28:57–68. [DOI] [PubMed] [Google Scholar]
- Rodrigues TL, Nagatomo KJ, Foster BL, Nociti FH, Somerman MJ. 2011. Modulation of phosphate/pyrophosphate metabolism to regenerate the periodontium: a novel in vivo approach. J Periodontol. 82(12):1757–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutsch F, Ruf N, Vaingankar S, Toliat MR, Suk A, Hohne W, Schauer G, Lehmann M, Roscioli T, Schnabel D, et al. 2003. Mutations in ENPP1 are associated with ‘idiopathic’ infantile arterial calcification. Nat Genet. 34(4):379–381. [DOI] [PubMed] [Google Scholar]
- Szeri F, Lundkvist S, Donnelly S, Engelke UFH, Rhee K, Williams CJ, Sundberg JP, Wevers RA, Tomlinson RE, Jansen RS, et al. 2020. The membrane protein ANKH is crucial for bone mechanical performance by mediating cellular export of citrate and ATP. PLoS Genet. 16(7):e1008884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thumbigere-Math V, Alqadi A, Chalmers NI, Chavez MB, Chu EY, Collins MT, Ferreira CR, FitzGerald K, Gafni RI, Gahl WA, et al. 2018. Hypercementosis associated with ENPP1 mutations and GACI. J Dent Res. 97(4):432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M, Ao M, Chavez MB, Kolli TN, Thumbigere-Math V, Becker K, Chu EY, Jager A, Somerman MJ, Foster BL. 2018. Reduced orthodontic tooth movement in Enpp1 mutant mice with hypercementosis. J Dent Res. 97(8):937–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Li M, Liu Z, Guo Y, Hasegawa T, Masuki H, Suzuki R, Amizuka N. 2010. Histological review of the human cellular cementum with special reference to an alternating lamellar pattern. Odontology. 98(2):102–109. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Shao J, Chen W, Li YP. 2007. Osteoclast differentiation and gene regulation. Front Biosci. 12:2519–2529. [DOI] [PubMed] [Google Scholar]
- Zweifler LE, Patel MK, Nociti FH, Jr, Wimer HF, Millan JL, Somerman MJ, Foster BL. 2015. Counter-regulatory phosphatases TNAP and NPP1 temporally regulate tooth root cementogenesis. Int J Oral Sci. 7(1):27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jdr-10.1177_0022034520981854 for Ablation of Pyrophosphate Regulators Promotes Periodontal Regeneration by A. Nagasaki, K. Nagasaki, E.Y. Chu, B.D. Kear, W.D. Tadesse, S.E. Ferebee, L. Li, B.L. Foster and M.J. Somerman in Journal of Dental Research