Abstract
Oxygen supply for ischemic brain tissue during stroke is critical to neuroprotection. Remote ischemic conditioning (RIC) treatment is effective for stroke. However, it is not known whether RIC can improve brain tissue oxygen supply. In current study, we employed a mouse model of stroke created by middle cerebral artery occlusion (MCAO) to investigate the effect of RIC on oxygen supply to the ischemic brain tissue using a hypoxyprobe system. Erythrocyte oxygen-carrying capacity and tissue oxygen exchange were assessed by measuring oxygenated hemoglobin and oxygen dissociation curve. We found that RIC significantly mitigated hypoxic signals and decreased neural cell death, thereby preserving neurological functions. The tissue oxygen exchange was markedly enhanced, along with the elevated hemoglobin P50 and right-shifted oxygen dissociation curve. Intriguingly, RIC markedly elevated 2,3-biphosphoglycerate (2,3-BPG) levels in erythrocyte, and the erythrocyte 2,3-BPG levels were highly negatively correlated with the hypoxia in the ischemic brain tissue. Further, adoptive transfusion of 2,3-BPG-rich erythrocytes prepared from RIC-treated mice significantly enhanced the oxygen supply to the ischemic tissue in MCAO mouse model. Collectively, RIC protects against ischemic stroke through improving oxygen supply to the ischemic brain tissue where the enhanced tissue oxygen delivery and exchange by RIC-induced 2,3-BPG-rich erythrocytes may play a role.
Keywords: Remote ischemic conditioning; stroke; 2,3-biphosphoglycerate/2,3-diphosphoglycerate; oxygen dissociation curve; hypoxia
Introduction
Remote ischemic conditioning (RIC) is one of the few effective therapeutic options for ischemic stroke.1,2 RIC has been used to treat patients with high-risk, new-onset, or post-onset stroke3 and it has been reported that patients with acute stroke who receive RIC in the ambulance en route to hospital have better clinical outcomes than their counterparts who do not.4 The mechanism underlying the effectiveness of RIC has been investigated extensively, and three protective pathways associated with humoral factors, neurological regulation, and anti-inflammatory activity have been suggested.5–7 However, the mechanism has not been fully clarified.
Adequate oxygen supply is critical for vital organs, including the brain and heart,8,9 and even very transient disruption can have devastating consequences. Given the evidence that emergent RIC significantly improves the clinical outcome of new-onset stroke,2,4 it would be of interest to know whether or not RIC improves the oxygen supply to ischemic brain tissue.
Oxygen is delivered to tissues in the body by hemoglobin in mature red blood cells (RBCs), also known as erythrocytes. The hemoglobin molecules in RBCs are composed of two α subunits and two β subunits.10 Each hemoglobin molecule carries four oxygen molecules, and the rate of association and dissociation between oxygen and hemoglobin molecules depends on the partial pressure of oxygen (PO2) in the microenvironment.11 For example, the lung is normally with a high PO2, which allows hemoglobin within RBCs in the venous blood delivered via the pulmonary artery to be loaded with oxygen. When oxygen-loaded, these RBCs circulate back to the heart and are subsequently delivered to all organs where the PO2 is low after metabolic consumption and carbon dioxide pressure (PCO2) is high, which leads to association of carbon dioxide and dissociation of oxygen with hemoglobin, ensuring adequate oxygen supply to the tissues. The oxygen-binding capacity of hemoglobin is also regulated by other factors, including temperature, pH, and 2,3-biphosphoglycerate (2,3-BPG).11–15 It is conceivable that application of RIC to the limbs would lead to transient distal ischemia in the distal part of the limbs whereby enhanced glycolysis would occur because of lack of oxygen. As a result, one of the intermediate glycolic products, 2,3-BPG, would be elevated. It is well known that 2,3-BPG in erythrocytes plays an important part in regulating the interaction between hemoglobin and oxygen.13 2,3-BPG interacts with hemoglobin causing the latter to undergo a conformational change allowing for bound oxygen prone to be dissociated with hemoglobin and released in the tissues.13,16–20 However, it has been unknown whether RIC improves oxygen supply to the ischemic brain tissue in stroke. In the present study, we hypothesize that RIC protects against stroke through enhancing the oxygen supply to ischemic brain tissue, and the enhanced oxygen-delivering capacity of RBCs containing elevated levels of 2,3-BPG may play an important role. Our findings in the current research strongly support our hypothesis. To our knowledge, this is the first research to demonstrate that RIC protects against stroke by improving the oxygen supply to ischemic brain tissue.
Materials and methods
Experimental animals and their use
Adult male wild-type C57BL/6 mice (aged 8–10 weeks, Charles River, Beijing China) were maintained at the Animal Center of Xuanwu Hospital, Capital Medical University, Beijing, China. The mice were housed in ventilated plastic cages with soft bedding and free access to food and water in an environmentally controlled and specific pathogen-free room (22–26°C, 56–70% humidity). All experimental procedures were approved by the Capital Medical University Institutional Animal Care and Use Committee (SCXK 2006-0009) and performed according to the Guide for the Care and Use of Laboratory Animals. The animal related experimental data in this study are in accordance with the Animal Research: Reporting in Vivo Experiments (ARRIVE) guidelines. The detailed information of animal groups and use was provided in each individual experiment as indicated elsewhere.
Middle cerebral artery occlusion
The mice were chosen with body weight no less than 21 g for better tolerance to surgery. The mice were anesthetized with 3–5% isoflurane in 70% nitrous oxide and 30% oxygen and maintained with 1–2% isoflurane. For the middle cerebral artery occlusion (MCAO) model, ischemia and reperfusion were established as previously described.21,22 During surgery, the right common carotid artery, right internal carotid artery (ICA), and right external carotid artery were exposed. A silica gel-coated nylon suture (Doccol Corporation, Sharon, MA, USA) with a diameter of 0.22 mm was inserted into the ICA. An 8–12-mm length of suture was inserted from the bifurcation of the external carotid artery to block the middle cerebral artery for ischemic 70 min and was withdrawn after occlusion for reperfusion 24 h to allow reopening of the middle cerebral artery. A laser speckle blood flow imaging system (LSF, Perimed, Jarfalla, Sweden) was used to monitor changes in cerebral blood flow before and after MCAO. Mice with less than a 50% reduction in cerebral blood flow after MCAO were excluded.23 Rectal temperature was maintained at 37 ± 0.5°C during the entire procedure.
Remote ischemic conditioning
RIC was performed by simultaneously tying both hind limbs with strip gauze bandages to occlude the blood circulation for 10 min and then released for 10 min to allow reperfusion. The occlusion–reperfusion cycle was repeated three times as described previously.24,25 RIC was performed immediately after inserting the suture into the ICA in all experiments.
Measurement of 2,3-biphosphoglycerate content in erythrocytes
The 2,3-BPG content in erythrocytes was measured using kits purchased from Roche following the instructions of the manufacturer (Roche, Penzberg, Germany).26–28 Briefly, 1 mL of fresh blood with heparin as an anticoagulant was well mixed with 5 mL of cold perchloric acid (0.6 M) on ice. The homogenate was centrifuged at 5000 r/min for 10 min. A 4 mL volume of supernatant was neutralized with 0.5 mL of K2CO3 (2.5 M) and placed in an ice bath for 60 min. The mixture was then centrifuged at 5000 r/min for 5 min at 4°C using a refrigerated centrifuge (Beckman Coulter, Krefeld, Germany). A 100 µL volume of supernatant was used to quantify the 2,3-BPG content. The working solutions were prepared according to the manufacturer’s instructions and added sequentially. The absorbance value was measured at 340 nm after adding the third working solution to obtain the first value. After adding the fifth working solution, the absorbance value was re-measured at 340 nm to obtain the second value. The concentration of 2,3-BPG in RBCs was calculated based on the difference between the two absorbance values following the manufacturer’s instructions. For those with collected blood less than 1 mL, we proportionally reduced the reagent usage based on the blood volume for 2,3-BPG measurement.
Concentrated RBC preparation and transfusion
Heparin-anticoagulated whole blood was collected from donor mice. The pooled blood was centrifuged using a centrifuge (Ika, Königswinter, Germany) at 4000 r/min for 5 min. The supernatant as well as the buffy coat was then removed. The remaining RBCs were mixed thoroughly by pipetting the cells up and down. Immediately after insertion of the suture into the ICA, the mice with MCAO received an intravenous injection of concentrated RBCs (250 µL/mouse) or normal saline (NS; 250 µL/mouse) via the tail vein.
Measurement of erythrocyte oxygen release capacity and blood gas analysis
Blood gas analysis was performed to measure pH, blood oxygen saturation (SO2), and blood oxyhemoglobin saturation (HbO2) using Radiometer ABL90 (Radiometer America Inc, Brea, CA, USA) before and after RIC. For measurement of the P50, an approximately 30 µL aliquot of whole blood with heparin as an anticoagulant was mixed with 4 mL of BLOODOX solution, 20 µL of antifoaming reagent (Sigma-Aldrich, St Louis, MO, USA), and 20 µL of 22% bovine serum albumin (Sigma-Aldrich). The mixture was then drawn into a BLOODOX-2018 oxygenation-dissociation analyzer (Softron Biotechnology, Beijing, China), under the guidance of the team led by Professor Hong Zhou at the Chinese Academy of Military Medical Sciences, for measurement of the oxygen dissociation curve at a temperature of 37°C and an atmospheric pressure of 150 mmHg. BLOODOX solution includes normal saline (NS, Chinese Medicine Group Chemical Agent Company, Nanjing, China), 30 mmol/L TES (Sigma-Aldrich), and 5 mmol/L KCl (Chinese Medicine Group Chemical Agent Company, Beijing, China) and has a pH of 7.4 ± 0.01.
Behavioral tests of stroke mice
The mice were assessed for behavioral changes by the adhesive removal test and the Longa score system before MCAO operation and after animal ischemia for 70 min and reperfusion for 24 h. The adhesive removal test, which has been described previously,25,29 was performed to evaluate the effect of RIC on neurological function after stroke. Briefly, the left claw palm of each mouse was fixed to a small rectangular tape that was of a constant size to ensure that all adhesive tapes had the same strength. The mice were then returned to the observation cage, and the time taken for each mouse to touch the tape with mouth and the time taken to tear it off was recorded. Mice in each study group were trained using the adhesive removal test for three days before surgery, and those that could remove the tape within 120 s were entered into the study. The Longa scoring system grades behavioral defects using the following 5-point system: 0, no defect; 1, failure to extend left forepaw; 2, circling to the left; 3, falling to the left; 4, failure to walk spontaneously and loss of consciousness.30
Assessment of infarct volume of stroke mice
Infarct volume was assessed using the method described previously.22,25 In brief, the brain tissue infarct volume was measured by staining with 2,3,5-triphenyl-2H-tetrazolium chloride (TTC, Sigma-Aldrich) after animal ischemia for 70 min and reperfusion for 24 h. For TTC staining, the brains were removed after mice were euthanized and sectioned coronally at the layer of the optic chiasm at 1 mm intervals by cutting two pieces in front of the optic chiasm and four pieces in the back of the optic chiasm and generating a total of six sections. The sections were stained with 2% TTC. Both nonischemic and ischemic hemispheres were examined, and the images were analyzed by ImageJ software (National Institutes of Health, Bethesda, MD, USA). The ischemic area was calculated based on the difference between the area of the nonischemic hemisphere and the nonischemic region in the ischemic hemisphere.
Assessment of brain tissue infarct by MAP2 immunofluorescence staining for live cells and TUNEL staining for apoptotic cells of stroke mice
Microtubule-associated protein 2 staining (MAP2, GeneTex Inc, San Antonio, TX, USA)31,32 was used to assess live cells and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining33,34 was used to assess apoptotic cells in brain tissue. After ischemia for 70 min and reperfusion for 24 h, the brain tissues were removed and 10 µm coronal cryo-sections at 1 mm intervals between two adjacent sections were prepared. Taking the optic chiasm as a reference two slices before the optic chiasm, and four slices after the optic chiasm were taken. Rabbit anti-MAP2 (1:200) was used as the primary antibody and the fluorescently conjugated anti-rabbit IgG antibody Alexa594 (1:300, Abcam Inc., Cambridge, MA, USA) as the secondary antibody. Immunofluorescent staining was performed following the manufacturers’ protocols. TUNEL staining was performed using an in situ cell death detection kit (Roche, Indianapolis, IN, USA) following the manufacturer’s instructions. After MAP2 and TUNEL staining, the section slides were mounted with a medium containing 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI; Molecular Probes, Eugene, OR, USA) as a counterstain. A fully automated slice scanning microscope (Pannoramic, 3DHISTECH Ltd, Budapest, Hungary) was used for panoramic scanning. All images were analyzed with ImageJ software for living (MAP2+) and apoptotic (TUNEL+) cells.
Hypoxyprobe detection of hypoxic brain tissue
As previously described,26 tissue hypoxia levels were assessed by hypoxyprobe immunofluorescent staining (Hypoxyprobe, Burlington, MA, USA). The hypoxyprobe system can detect hypoxic tissues with a PO2 below 10 mmHg.26,35 Briefly, after ischemic for 60 min in MCAO, hypoxyprobe (60 mg/kg body weight) was injected intraperitoneally. The mice were sacrificed 30 min later after hypoxyprobe injection and the brains were removed. Then, the brains were placed into a cryo-embedding medium (Solarbio, Beijing, China), and stored at −80°C. The cryo-sections were prepared with 10 µm thickness and 1 mm apart between adjacent two sections. For the assay, the tissue sections were incubated with anti-hypoxyprobe (1:100, FITC-conjugated mouse IgG monoclonal antibody) overnight at 4°C. After thorough washing, all section slides were mounted with a medium containing DAPI as a counterstain. The fluorescent signals and areas with fluorescent staining were quantified using the ImageJ software. The fluorescent intensity varied substantially in staining experiments performed at different times. Therefore, we attempted to perform the comparison analysis for fluorescent intensity between groups with the staining experiment done at the same time.
Statistical analysis
All values were shown as the mean ± standard deviation (SD). The statistical analysis was performed using GraphPad Prism 6 (GraphPad Software, La Jolla, CA, USA). Kolmogoroc–Smirnov D test (K–S test) was used to test the normality of data in this study. The Student’s t-test or non-parametric test (Mann–Whitney test) was used to analyze the difference between two groups. Multiple groups were compared using non-parametric test (Kruskal–Wallis test) or one-way analysis of variance (ANOVA) followed by Turkey test as post hoc test. Pearson correlation analysis was employed to analyze the relationship between the levels 2,3-BPG in erythrocytes and the ischemic brain tissue oxygen supply, including hypoxia area and hypoxia intensity, in stroke mice. During the Pearson correlation analysis, first, we combined the data from RIC-RBCs and non-RIC-RBCs groups to analyze the relationship between erythrocyte 2,3-BPG levels and hypoxia status in ischemic brain tissues. Secondly, since non-RIC-RBCs had uniformly lower 2,3-BPG levels in contrast to RIC-RBCs, with the amounts of 2,3-BPG being at the similar levels, whereas, RIC-RBCs possessed elevated levels of 2,3-BPG at a wider range relative to non-RIC-RBCs, we exclusively analyzed RIC-RBCs group with regard to the relationship between the 2,3-BPG levels of RIC-RBCs and the ischemic brain tissue hypoxia signals to further determine the potential role of 2,3-BPG for RIC-RBC transfusion in enhancing oxygen supply to ischemic brain tissue. A p value <0.05 (two-sided) was considered statistically significant.
Results
RIC significantly improves the oxygen supply to ischemic brain tissue in a mouse model of stroke
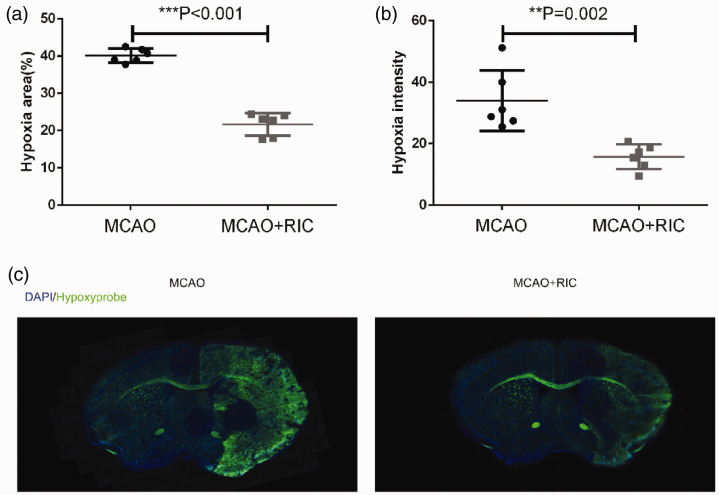
To determine whether or not RIC improves oxygen supply to ischemic brain tissue, we employed a hypoxyprobe system to assess the oxygen supply in the brain tissue as described in the Materials and methods section. We found that the signal detected by hypoxyprobe was significantly weaker in the MCAO + RIC group than MCAO control group. Moreover, the hypoxic areas were smaller in MCAO + RIC group mice than in control mice (Figure 1(a) to (c)). To rule out the impact of the variation of blood supply after MCAO in different animals on the data analysis, we performed the blood flow analysis using laser speckle blood flow imaging system, and found no difference between the two groups after MCAO and reperfusion (Supplemental Figure 1).
Figure 1.
Remote ischemic conditioning increases the oxygen supply to ischemic brain tissue. (a) The average hypoxic areas (%) of all mice between the two groups (6 mice per group) were compared by the Student’s t-test. The hypoxic area was calculated using the following formula: [area of nonischemic hemisphere (hypoxyprobe negative) − area of the hypoxyprobe negative region in the ischemic hemisphere]/2 × area of nonischemic hemisphere × 100%. (b) The average hypoxic intensities of all mice between the two groups were compared by the Student’s t-test. (c) Representative images at the same level (brain optic chiasm level) of brain from each group were depicted. **p < 0.01, ***p < 0.001. The findings were similar when the same experiment was repeated twice independently.
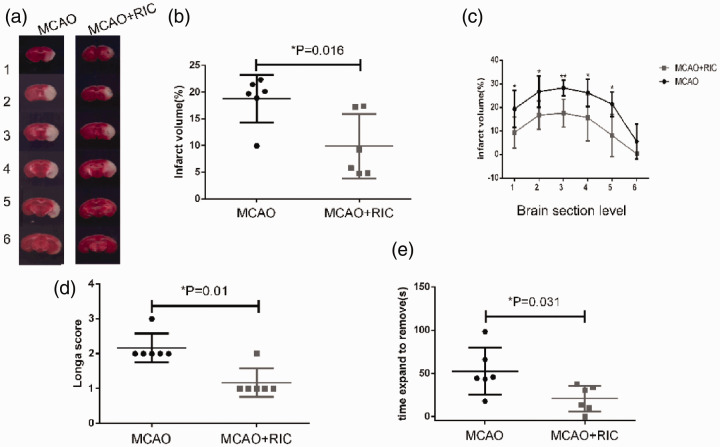
RIC significantly decreases infarct volume and has a beneficial effect on neurological function
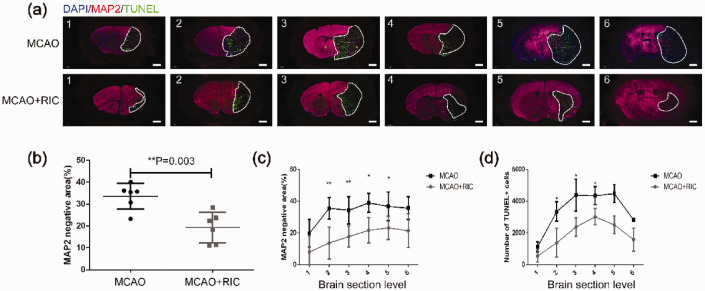
To determine whether or not the improved oxygen supply protected neural cells from cell death, we first compared the infarct volume between the MCAO + RIC group and MCAO group mice 24 h after MCAO and reperfusion as described in the Materials and methods section. We found that the infarct volume was markedly smaller in the MCAO + RIC group than in MCAO group (Figure 2(a) to (c)). As expected, both the Longa scores (Figure 2(d)) and adhesive removal test results (Figure 2(e)) indicated greater preservation of neurological function in the MCAO + RIC group than in MCAO group. These results were further confirmed by the TUNEL staining results for apoptosis and MAP2 staining for live neural cells in brain tissue (Figure 3(a) to (d)).
Figure 2.
Remote ischemic conditioning decreases the infarct volume and improves behavioral function. (a) Representative TTC staining images of different sections of brain tissues from two groups (6 mice per group) were shown. The numbers 1 to 6 represented coronal sections at different planes of each mouse brain. The brain tissue with pale TTC staining was the infarct region. (b) Total infarct volume (%) for six sections of each brain was evaluated by TTC staining in the different groups, and compared between the two groups by the Student’s t-test. (c) Infarct volume (%) by TTC staining in each of the six sections was compared by the Student’s t-test. Infarct volume was calculated using the following formula: [(volume of nonischemic hemisphere) − (volume of nonischemic region of ischemic hemisphere)/2 × volume of nonischemic hemisphere × 100%]. (d) Neurological function was assessed by the Longa scoring system in the different groups, and compared by the non-parametric test (Mann–Whitney test). (e) Delay time in stick strip removal during the adhesive removal test was compared between the groups by the Student’s t-test. *p < 0.05.**p < 0.01. The findings were similar when the same experiment was repeated independently.
Figure 3.
Remote ischemic conditioning decreases infarction verified by MAP2 staining for live cells and neural cell apoptosis by TUNEL staining. (a) Representative images of brain tissue in the two groups as indicated (6 mice per group) with MAP2 and TUNEL immunofluorescence staining (scale bar, 1 mm) were depicted. The numbers of 1 to 6 represented coronal sections at different planes of each mouse brain. The brain tissue negative for MAP2 staining and positive for TUNEL (dead tissue) as marked by the white line was shown. (b) Average total infarct area (%) of six sections from each brain evaluated by MAP2 staining in the different groups was compared by the Student’s t-test. The infarct areas were calculated using the following formula: [(MAP2 positive area (live cells) of the nonischemic hemisphere) − (area of the MAP2 positive region in the ischemic hemisphere)/2 × area of the nonischemic hemisphere ×100%]. (c) Infarct area (%) evaluated by MAP2 staining in each of six sections was compared between the two groups by the Student’s t-test. (d) Apoptotic cells were evaluated by TUNEL immunofluorescent staining of different sections and counted for positive cells. The data were processed as described in the Materials and methods section and compared by the Student’s t-test. *p < 0.05, **p < 0.01. The similar findings were obtained when the same experiment was repeated independently.
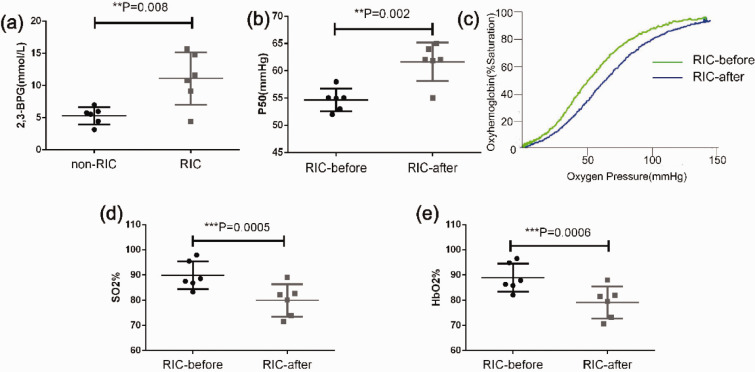
RIC increases erythrocyte 2,3-BPG levels and the P50 value for hemoglobin oxygenation, shifts the oxygen dissociation curve to the right, and decreases the oxygenated hemoglobin level in venous blood
The association and dissociation of oxygen with hemoglobin in RBCs plays a key role in delivering oxygen inhaled from lung to various organs with different oxygen demands, and 2,3-BPG is an important modulator of the binding of oxygen with hemoglobin.14 An increase in erythrocyte 2,3-BPG content facilitates the dissociation of oxygen from hemoglobin, thereby increasing the oxygen supply to tissues.16 To determine whether or not erythrocyte 2,3-BPG was potentially involved in the improved oxygen supply to ischemic brain tissue in the RIC group, we measured 2,3-BPG levels in RBCs obtained from the RIC and non-RIC control groups and found that 2,3-BPG levels were significantly elevated in the RIC group (Figure 4(a)). Consistent with this finding, we found that the P50 value was considerably elevated after RIC (Figure 4(b)), leading to a shift in the oxygen dissociation curve to the right (Figure 4(c)). To assess whether or not RIC increased the oxygen supply to the tissues, we measured the levels of venous blood SO2 and HbO2 before and after RIC. If there was more oxygen release in the tissues, a drop in both parameters would be observed after RIC. Predictably, both SO2 and HbO2 in the venous blood were significantly decreased after RIC (Figure 4(d) and (e)), suggesting that RIC promoted oxygen release in the tissues. Notably, RIC increased the erythrocyte 2,3-BPG levels in MCAO mice (Supplemental Figure 2) as well, suggesting its role in enhancing oxygen supply in ischemic brain tissue in RIC treated mice shown in Figure 1.
Figure 4.
Remote ischemic conditioning increases the 2,3-BPG level in red blood cells, leading to an increased P50 value, a shift of the oxygen dissociation curve (ODC) to the right, and more oxygen exchange in tissues. (a) The 2,3-BPG levels in red blood cells of RIC-treated group and non-RIC-treated control group mice were measured using the procedure described in the Materials and methods section and compared by the Student’s t-test (6 mice per group). (b) P50 was measured before and after RIC and compared by the paired t-test (n = 6). (c) A representative ODC before and after RIC showed that RIC led to a shift in the ODC to the right (blue line). (d) Venous blood SO2 levels before and after RIC. The data were compared using the paired t-test (n = 6). (e) Venous blood HbO2 levels before and after RIC. The data were compared using the paired t-test (n = 6). **p < 0.01, ***p < 0.001. The findings were similar when the same experiment was repeated on two occasions independently. 2,3-BPG: 2,3-biphosphoglycerate. HbO2: blood oxyhemoglobin; ODC: oxygen dissociation curve; P50: partial pressure of oxygen at 50% oxyhemoglobin saturation; SO2: oxygen saturation.
Transfusion of RBCs from RIC-treated mice confers a neuroprotective effect by increasing oxygen supply to ischemic brain tissue in a mouse model of stroke
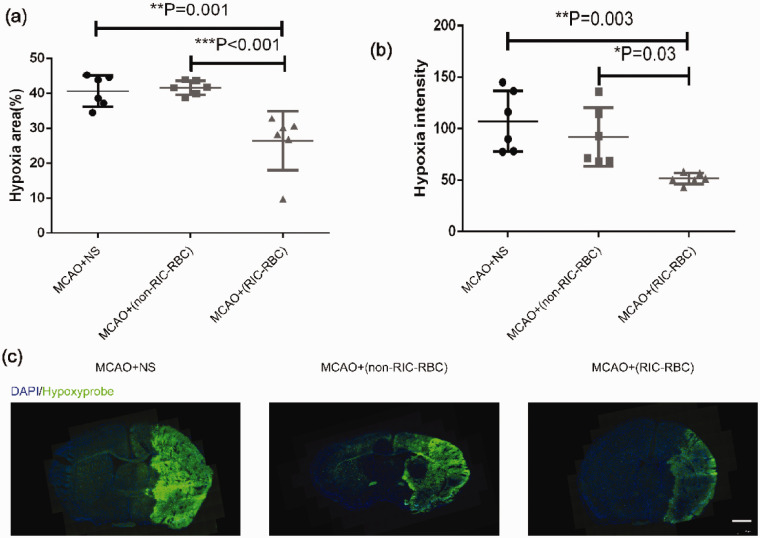
To confirm that the neuroprotection afforded by RIC was the result of the increased oxygen supply delivered by 2,3-BPG-rich RBCs, we prepared RBCs from RIC-treated mice and non-RIC-treated mice and then adoptively transferred these RBCs to the mice that had undergone MCAO. Before RBC transfusion, we tested all donor RBCs in terms of 2,3-BPG levels, and reproduced the results as shown in Figure 4(a). The oxygen supply status was evaluated by hypoxyprobe staining. Hypoxic signal intensities and hypoxic areas were found to be markedly reduced after adoptive transfer of RBCs prepared from RIC-treated mice than after adoptive transfer of RBCs from non-RIC-treated mice or NS (Figure 5(a) to (c)), indicating that 2,3-BPG-rich RBCs prepared from RIC-treated mice improved the oxygen supply to ischemic brain tissue. Of note, the difference was not significant between non-RIC-RBCs groups and NS group (Figure 5(a) to (c)). Again, the blood flow was comparable among the groups after MACO and after reperfusion, similar to the results shown in Supplemental Figure 1 (data not depicted).
Figure 5.
Transfusion of 2,3-BPG-rich red blood cells prepared from RIC-treated mice increases the oxygen supply to ischemic brain tissue. (a) The average hypoxic areas (%) of all mice between the groups (6 mice per group) were compared by the one-way ANOVA followed by the post hoc test. The hypoxic area was calculated using the following formula: [area of nonischemic hemisphere (hypoxyprobe negative) − area of the hypoxyprobe negative region in the ischemic hemisphere]/2 × area of nonischemic hemisphere × 100%. (b) The average hypoxic intensities of all mice between the groups were compared by one-way ANOVA followed by the post hoc test. (c) Representative images of brain section at the same level (brain optic chiasm level) from each group were depicted (scale bar 1 mm). *p < 0.05, **p < 0.01, ***p < 0.001. The findings were similar when the same experiment was repeated independently. Twelve C57BL/6 mice were used as RBC donors for each experiment.
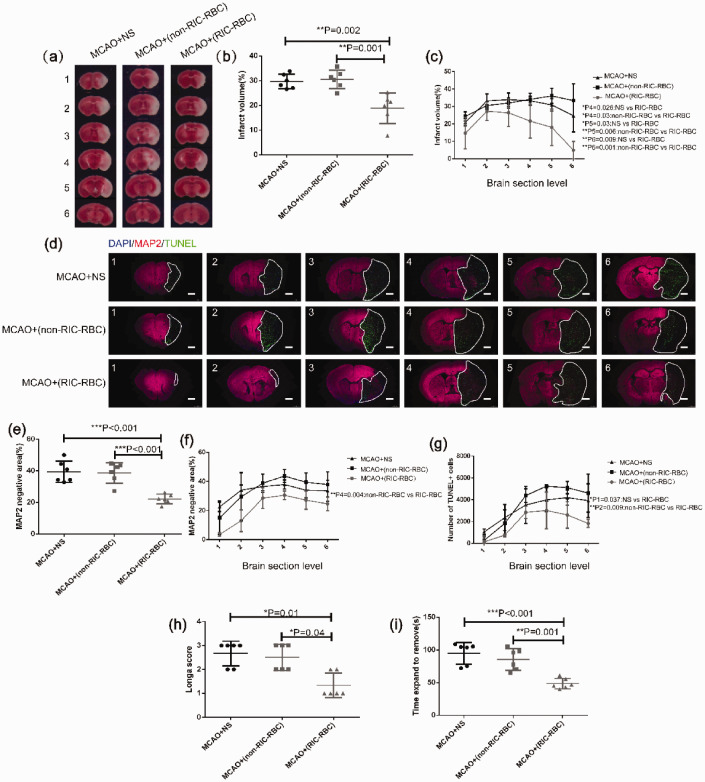
Transfusion of RBCs prepared from RIC-treated mice protects ischemic brain cells against cell death and has a beneficial effect on neurological function
We also tested whether or not brain tissue infarction was attenuated by adoptive transfer of 2,3-BPG-rich RBCs derived from RIC-treated mice in the MCAO mouse model as a result of enhanced oxygen supply by comparing with the group receiving transfusion of RBCs prepared from non-RIC treated mice, or the NS group. The TTC staining results demonstrated that transfusion of RBCs prepared from RIC-treated mice markedly reduced the brain infarct volume when compared with the other two groups (Figure 6(a) to (c)). The infarct area evaluated by MAP2 staining also demonstrated that transfusion of RBCs derived from RIC-treated mice markedly protected brain tissue from ischemic damage. In line with these findings, cell apoptosis in the ischemic brain tissue was significantly reduced in mice that had undergone MCAO and received a transfusion of RBCs from RIC-treated mice when compared to the other two groups (Figure 6(d) to (g)). As expected, in the behavioral tests, we observed greater preservation of neurological function in MCAO mice that received a transfusion of RBCs prepared from RIC-treated mice when compared with the control groups with regard to both Longa scores (Figure 6(i)) and the adhesive removal test results (Figure 6(h)).
Figure 6.
Transfusion of 2,3-BPG-rich RBCs prepared from RIC-treated donor mice alleviates infarct size and apoptosis of brain cells, and improves behavioral function in MCAO mice. (a) Representative TTC staining images of different sections of brain tissue from one mouse in each group as indicated (6 mice per group). The numbers 1 to 6 represented coronal sections at different planes of mouse brain. The brain tissue with pale TTC staining was the infarct region. (b) The average total infarct volume (%) evaluated by TTC staining of six sections in the different groups was compared by one-way ANOVA followed by the post hoc test. (c) Infarct volume (%) by TTC staining of individual sections compared by one-way ANOVA followed by the post hoc test. P1–P6 represent the p values when the six individual sections were compared as indicated in the figure (only showing the sections with a significant difference). (d) Representative images of different sections of brain tissue MAP2 and TUNEL immunofluorescent staining were shown (scale bar 1 mm) in each group as indicated (6 mice per group). The numbers of 1 to 6 represented six coronal sections at different planes of mouse brain with 1 mm apart between two adjacent sections. The white line marked area was infarct with MAP2 staining negative and TUNEL staining positive. (e) The average total infarct area (%) evaluated by MAP2 immunofluorescent staining of six sections of all mice in different groups was compared by one-way ANOVA followed by post hoc test. (f) Infarct area (%) by MAP2 immunofluorescent staining of different individual sections was compared by one-way ANOVA followed by post hoc test. P1–P6 represent the p values when the six individual sections were compared as indicated in the figure (only showing the sections with a significant difference). (g) Apoptotic cells examined by TUNEL immunofluorescent staining and analyzed as described in the Materials and methods. The total TUNEL+ cells at different sections were compared by one-way ANOVA followed by the post hoc test. P1–P6 represented the p values when the six individual sections were compared as indicated in the figure (only showing the sections with significant difference). (h) Neurological function assessed by the Longa scoring system compared between the different groups by non-parametric test (Kruskal–Wallis test). (i) Delay time in stick strip removal during the adhesive removal test compared between the different groups by one-way ANOVA followed by the post hoc test. *p < 0.05, **p < 0.01. The findings were similar when the same experiment was repeated independently.
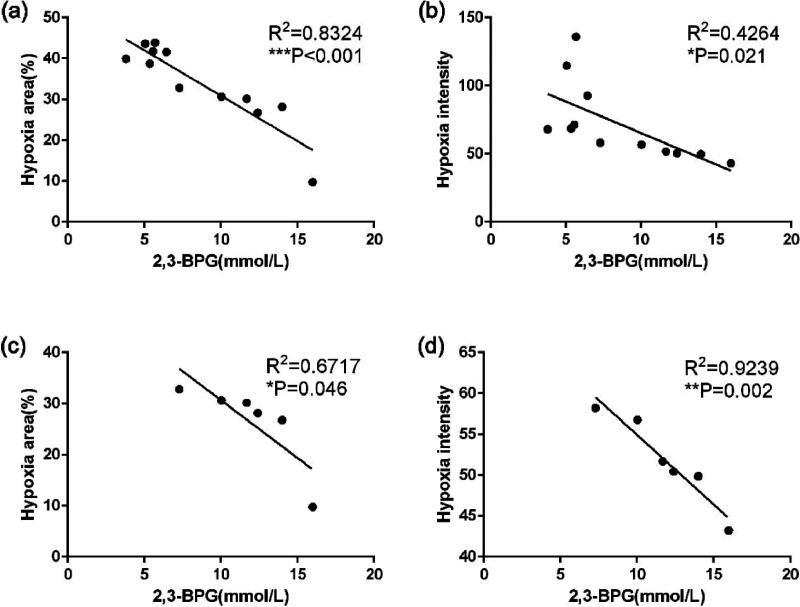
The levels of 2,3-BPG in erythrocytes are closely correlated with ischemic brain tissue oxygen supply by transfusion of RBCs
To further address whether the levels of erythrocyte 2,3-BPG were associated with the increase of oxygen supply to ischemic brain tissue, we performed a Pearson correlation analysis between the 2,3-BPG levels of the transfused erythrocytes and hypoxia signals in the ischemic brain tissues for the data shown in Figure 5. Intriguingly, we found that the levels of 2,3-BPG in erythrocytes had highly negative correlation with hypoxic areas and hypoxic signal intensities when combining the data from both RIC-RBCs and non-RIC-RBCs groups (Figure 7(a)). Notably, the negative correlation between 2,3-BPG levels and hypoxia remained in RIC-RBCs group (Figure 7(b)), albeit less significant than that with the data from two groups combined together. The above findings suggest that the neuroprotection induced by erythrocyte transfusion might be attributed to 2,3-BPG contained in erythrocytes, and 2,3-BPG levels in erythrocytes might determine the degree of neuroprotection of 2,3-BPG-rich erythrocytes induced by RIC treatment.
Figure 7.
Relationship between erythrocyte 2,3-BPG levels and ischemic brain tissue oxygen supply impacted by erythrocyte transfusion. Pearson correlation analysis was performed to analyze the relationship between erythrocyte 2,3-BPG levels and hypoxic signals for the data shown in Figure 5. (a) The correlation was shown between 2,3-BPG levels of transfused RBCs and hypoxic areas in the ischemic brain tissues, combining data from RIC-RBCs and non-RIC-RBCs groups. (b) The correlation was shown between 2,3-BPG levels of transfused RBCs and hypoxic signal intensities in the ischemic brain tissues, combining data from RIC-RBCs and non-RIC-RBCs groups. (c) The correlation was shown between 2,3-BPG levels of RIC-RBCs and hypoxic areas in the ischemic brain tissues from the data exclusively from RIC-RBCs group. (d) The correlation was shown between 2,3-BPG levels of RIC-RBCs and hypoxic intensities in the ischemic brain tissues from the data exclusively from RIC-RBCs group. *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
An adequate oxygen supply to the organs is essential for an organism to be able to function normally.8,36–38 It is known that interruption of the oxygen supply for just 5 min can cause irreversible brain damage in humans.9 Blockage of blood flow by thrombosis in ischemic stroke dramatically decreases the oxygen supply to the area distal to the thrombus. If the thrombosis cannot be resolved rapidly enough, the neurons in the ischemic area will inevitably die, causing various clinical manifestations depending on the site of thrombosis.39 Thus far, thrombolysis and thrombectomy are the only effective rescue interventions for stroke that can restore blood flow and the oxygen supply to ischemic brain tissue.40 Unfortunately, only a small percentage of patients can receive these rescue treatments because of limited facilities as well as the restricted time window for implementation.9 Our present findings strongly suggest that RIC could serve as a rescue regimen and potentially widen the time window for thrombolysis or thrombectomy in stroke patients who would otherwise have permanent disability. Even patients with acute stroke who are unable to receive thrombolysis or thrombectomy would be likely to be benefited from RIC, given our data showing that RIC significantly protects brain cells from apoptosis in the MCAO model (Figure 3). There is mounting evidence indicating that RIC considerably improves the clinical outcomes of stroke and myocardial infarction.2,4,41,42 In the present study, we specifically investigated whether RIC affected the oxygen supply to the ischemic brain tissue in a mouse model of stroke. We demonstrated for the first time that RIC augmented the oxygen supply not only in the penumbra but also in the core area after stroke and preserved neurological function. These findings appeared to be contradictory to the previous study reporting that ischemic core could not be rescued sufficiently by oxygen supply.43 The discrepancy between this study and ours would be attributable to the different levels of oxygen release in the ischemic brain tissues. As stated elsewhere, RIC promotes more oxygen release to the ischemic brain tissue. This is an important new mechanistic insight into the therapeutic efficacy of RIC treatment in stroke.
The mouse model of stroke created by temporary insertion of a suture in the middle cerebral artery is widely used to study stroke.44,45 This model was used in the present study to determine whether or not RIC increases the oxygen supply in the acute phase of stroke, and whether or not it can preserve neurological function. We chose to use an MCAO ischemia mouse model, which had been shown to cause severe brain damage.21,22 Surprisingly, even if the occlusion was not resolved before reperfusion, the oxygen supply in the ischemic area during MCAO was significantly improved. This finding suggests that RIC promotes RBCs circulating to the area adjacent to ischemic tissue to release more oxygen into the tissues, and that the increased oxygen in the ischemic area is likely dissipated from the surrounding area with normal blood circulation. For the experiments assessing death of brain cells and neurological functioning, we withdrew the suture from the ICA to allow for restoration of blood flow upon completion of the RIC regimen after MCAO for 70 min (Supplemental Figure 1). This was done to determine (1) whether or not RIC performed in the acute stage of stroke was associated with a better outcome after blood reperfusion (like thrombolysis or thrombectomy in the clinical setting) and (2) whether or not restoration of blood flow containing oxygen-loaded RBCs would provide sustained neuroprotection by augmenting release of oxygen into the brain tissue. The results of these experiments showed that brain cell death was markedly alleviated and that neurological function was significantly preserved. These findings suggest that RIC-induced improvement of oxygen supply to ischemic brain tissue in the acute stage of stroke likely plays an important role in protecting neuronal cells, thereby preserving neurological function, and that a continuous improvement in the oxygen supply after reperfusion might also contribute to the overall protective effect induced by RIC treatment.
The observation that RIC of the limbs alters the ability of RBCs to deliver oxygen to ischemic brain tissue is intriguing. When performing a distal RIC maneuver, the most marked effect would be temporary large reduction of blood flow, which would lead to dramatic metabolic changes in the temporarily ischemic limbs. One of the major metabolic changes during RIC would be an increased rate of glycolysis in RBCs under the hypoxic condition, which would lead to an increased level of 2,3-BPG, one of the intermediate metabolic products in glycolysis.19,20,46 2,3-BPG in RBCs has a leading role in the regulation of association and dissociation between oxygen and hemoglobin.47,48 It is known that binding of 2,3-BPG to hemoglobin results in a change in the conformation of the hemoglobin molecule, thereby allowing for easier dissociation of oxygen from hemoglobin. This change is particularly important in an ischemic organ with a high oxygen demand, such as ischemic brain tissue in stroke. It is worth noting that the rate of glycolysis in brain tissue subjected to stroke is increased because of lack of oxygen, which likely leads to an acidic microenvironment.49,50 The lowered pH in ischemic brain tissue may also facilitate the release of oxygen to increase the tissue oxygen supply.11,14 Sun et al. recently reported that high-altitude hypoxia upregulated sphingosine-1-phosphate, which plays an important role in upregulation of 2,3-BPG.26 Therefore, it is possible that the temporary ischemic and hypoxic conditions during the RIC maneuver in the limbs trigger more production of sphingosine-1-phosphate, which in turn upregulates 2,3-BPG. Further studies are needed to investigate this possibility.
Another interesting finding in this study was the dramatic decrease in venous blood SO2 and HbO2 levels after RIC (Figure 4), which provides further evidence of RIC leading to more oxygen release in tissues during exchange of oxygen between RBCs and tissue cells. Although this feature would improve the oxygen supply to all tissues, it would be particularly important in ischemic and hypoxic tissues, in which an oxygen supply is critical for tissue viability and function, as in acute stroke. Furthermore, this feature may be exploited to attenuate the adverse effects of altitude hypoxia, where the main reason for impairment of the oxygen supply to tissues is a low PO2. The elevated 2,3-BPG induced by RIC would increase the oxygen P50 and force more oxygen release under a low oxygen pressure in the hypoxic environment at high altitude.26,47,51 The same principle would apply to pulmonary diseases and heart defects that cause tissue hypoxia as well.
Finally, to confirm that RBCs are indeed the key players in the improved oxygen supply to ischemic brain tissue during RIC, adoptive transfer of RBCs was included in our MCAO model. As expected, adoptive transfer of 2,3-BPG-rich RBCs prepared from RIC-treated mice, but not from non-RIC-treated mice, led to a significant increase in oxygen supply to the ischemic brain tissue. Consequently, brain cell death was profoundly mitigated, and neurological functioning was markedly preserved. These findings suggest that RIC has a considerable impact on the ability of RBCs to deliver oxygen to the tissues, and that enhanced oxygen delivery by RBCs may play an important role in RIC-induced neuroprotection. In addition, no significant difference between non-RIC-RBCs and NS groups suggests that the RBC transfusion itself does not have significant impact on oxygen delivery if RBCs are not collected from RIC-treated animals. Given the evidence showing that white blood cells or platelets have neuroprotective effect,52–55 to eliminate the impact of white blood cells or platelets, we carefully prepared the concentrated RBCs by centrifuging the blood and then removing plasma (partial platelets) and buffy coat (white blood cells and most platelets).
Of note, there is a study in humans underway at our center that is testing the same hypothesis proposed in the current mouse study. The preliminary findings of this study indicate that RIC also increases the RBC 2,3-BPG content in humans and promotes oxygen release in the tissues, as evidenced by a decrease in venous blood SO2 and HbO2 levels after RIC (Supplemental Figure 3). These findings support our hypothesis that RIC augments the tissue oxygen supply not only in mice but also in humans. The above data suggest that the elevated 2,3-BPG in erythrocytes may play an important role in neuroprotection induced by RIC treatment, which is further supported by the correlation analysis between 2,3-BPG levels in erythrocytes and the oxygen supply induced by the transfusion of 2,3-BPG-rich erythrocytes from RIC-treated mice (Figure 7(c) and (d)).
The present study investigated how RIC in the limbs affects the oxygen supply to ischemic brain tissue, thereby providing a previously unrecognized important mechanistic insight into how RIC affords neuroprotection in ischemic stroke. Our findings indicate that RIC may be an alternative rescue strategy for patients with acute stroke by increasing the oxygen supply through enhancing erythrocyte 2,3-BPG levels. One study has linked 2,3-BPG-rich RBCs to more energy supply to ischemic brain tissues in stroke;56 however, there is no further mechanistic investigation in that study to show how 2,3-BPG-rich RBCs enhances tissue energy supply. This phenomenon could be well explained by our findings in the current study that 2,3-BPG-rich RBCs deliver more oxygen to ischemic brain tissue and subsequently promote energy production in the environment with more oxygen provided. Importantly, induction of 2,3-BPG-rich RBCs by RIC completely avoids the ex vivo process to make 2,3-BPG-rich RBCs as described in the study mentioned above,56 and therefore, avoid the potential risks of using 2,3-BPG-rich RBCs prepared ex vivo. Several issues need to be further addressed, such as, how RIC raises erythrocyte 2,3-BPG levels, how long the increased 2,3-BPG level is maintained, what is the optimal frequency of implementing RIC, and how well these findings are relevant to clinical settings. Nevertheless, the current research paves a new avenue for development of RIC-based therapeutic strategies for ischemic stroke, and other ischemic or hypoxic conditions.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X20952264 for Remote ischemic conditioning enhances oxygen supply to ischemic brain tissue in a mouse model of stroke: Role of elevated 2,3-biphosphoglycerate in erythrocytes by Lin Wang, Changhong Ren, Yang Li, Chen Gao, Ning Li, Haiyan Li, Di Wu, Xiaoduo He, Changqing Xia and Xunming Ji in Journal of Cerebral Blood Flow & Metabolism
Acknowledgements
We sincerely thank the team led by Professor Hong Zhou at the Chinese Academy of Military Medical Sciences for generously providing the oxygenation-dissociation analyzer used in this study and for technical support in testing the P50 for oxygen.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of China (81971114, 81801313) and the Science and Technology Development Project of Beijing Municipal Health Commission and National Key R&D Program of China (2017YFC1308402).
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: Lin Wang involved in the investigation, methodology; Changhong Ren: formal analysis, investigation, methodology, and funding acquisition; Yang Li: formal analysis, methodology; Chen Gao, Ning Li, Haiyan Li, Di Wu, Xiaoduo He: methodology; Changqing Xia: conceptualization, data curation, formal analysis, supervision, methodology; Xunming Ji: conceptualization, data curation, formal analysis, funding acquisition, investigation, project administration.
ORCID iD: Changqing Xia https://orcid.org/0000-0002-3832-1091
Supplemental material: Supplemental material for this article is available online.
References
- 1.Hess DC, Blauenfeldt RA, Andersen G, et al. Remote ischaemic conditioning-a new paradigm of self-protection in the brain. Nat Rev Neurol 2015; 11: 698–710. [DOI] [PubMed] [Google Scholar]
- 2.Landman TRJ, Schoon Y, Warle MC, et al. Remote ischemic conditioning as an additional treatment for acute ischemic stroke. Stroke 2019; 50: 1934–1939. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Reis C, Applegate R, et al. Ischemic conditioning-induced endogenous brain protection: applications pre-, per- or post-stroke. Exp Neurol 2015; 272: 26–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hougaard KD, Hjort N, Zeidler D, et al. Remote ischemic perconditioning as an adjunct therapy to thrombolysis in patients with acute ischemic stroke: a randomized trial. Stroke 2014; 45: 159–167. [DOI] [PubMed] [Google Scholar]
- 5.Heusch G.Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res 2015; 116: 674–699. [DOI] [PubMed] [Google Scholar]
- 6.Xia C, Ji X.Perspectives on mechanisms underlying remote ischemic conditioning against ischemic stroke. J Transl Neurosci 2019; 4: 1–14. [Google Scholar]
- 7.Al Kasab S, Hess DC, Chimowitz MI.Rationale for ischemic conditioning to prevent stroke in patients with intracranial arterial stenosis. Brain Circ 2016; 2: 67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giordano FJ.Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest 2005; 115: 500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 update: a report from the American Heart Association. Circulation 2019; 139: e56–e528. [DOI] [PubMed] [Google Scholar]
- 10.Shibayama N.Functional analysis of hemoglobin molecules locked in doubly liganded conformations. J Mol Biol 1999; 285: 1383–1388. [DOI] [PubMed] [Google Scholar]
- 11.Collins JA, Rudenski A, Gibson J, et al. Relating oxygen partial pressure, saturation and content: the haemoglobin -oxygen dissociation curve. Breathe 2015; 11: 194--201. [DOI] [PMC free article] [PubMed]
- 12.Di Caprio G, Stokes C, Higgins JM, et al. Single-cell measurement of red blood cell oxygen affinity. Proc Natl Acad Sci U S A 2015; 112: 9984–9989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brewer GJ.2,3-DPG and erythrocyte oxygen affinity. Annu Rev Med 1974; 25: 29–38. [DOI] [PubMed] [Google Scholar]
- 14.Barvitenko NN, Adragna NC, Weber RE.Erythrocyte signal transduction pathways, their oxygenation dependence and functional significance. Cell Physiol Biochem 2005; 15: 1–18. [DOI] [PubMed] [Google Scholar]
- 15.Benesch R, Benesch RE.The effect of organic phosphates from the human erythrocyte on the allosteric properties of hemoglobin. Biochem Biophys Res Commun 1967; 26: 162–167. [DOI] [PubMed] [Google Scholar]
- 16.diBella G, Scandariato G, Suriano O, et al. Oxygen affinity and Bohr effect responses to 2,3-diphosphoglycerate in equine and human blood. Res Vet Sci 1996; 60: 272–275. [DOI] [PubMed] [Google Scholar]
- 17.Juel R. 2,3-Diphosphoglycerate: its role in health and disease. CRC Crit Rev Clin Lab Sci 1979; 10: 113–146. [DOI] [PubMed] [Google Scholar]
- 18.Kauffman KJ, Pajerowski JD, Jamshidi N, et al. Description and analysis of metabolic connectivity and dynamics in the human red blood cell. Biophys J 2002; 83: 646–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulquiney PJ, Bubb WA, Kuchel PW.Model of 2,3-bisphosphoglycerate metabolism in the human erythrocyte based on detailed enzyme kinetic equations: in vivo kinetic characterization of 2,3-bisphosphoglycerate synthase/phosphatase using 13C and 31P NMR. Biochem J 1999; 342: 567–580. [PMC free article] [PubMed] [Google Scholar]
- 20.Cho J, King JS, Qian X, et al. Dephosphorylation of 2,3-bisphosphoglycerate by MIPP expands the regulatory capacity of the Rapoport-Luebering glycolytic shunt. Proc Natl Acad Sci U S A 2008; 105: 5998–6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koronowski KB, Dave KR, Saul I, et al. Resveratrol preconditioning induces a novel extended window of ischemic tolerance in the mouse brain. Stroke 2015; 46: 2293–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng X, Zhao H, Yan F, et al. Limb remote ischemic post-conditioning mitigates brain recovery in a mouse model of ischemic stroke by regulating reactive astrocytic plasticity. Brain Res 2018; 1686: 94–100. [DOI] [PubMed] [Google Scholar]
- 23.Dai X, Chen J, Xu F, et al. TGFalpha preserves oligodendrocyte lineage cells and improves white matter integrity after cerebral ischemia. J Cereb Blood Flow Metab 2020; 40: 639--655. [DOI] [PMC free article] [PubMed]
- 24.Li X, Ren C, Li S, et al. Limb remote ischemic conditioning promotes myelination by upregulating PTEN/Akt/mTOR signaling activities after chronic cerebral hypoperfusion. Aging Dis 2017; 8: 392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Ren C, Li H, et al. Role of exosomes induced by remote ischemic preconditioning in neuroprotection against cerebral ischemia. Neuroreport 2019; 30: 834–841. [DOI] [PubMed] [Google Scholar]
- 26.Sun K, Zhang Y, D'Alessandro A, et al. Sphingosine-1-phosphate promotes erythrocyte glycolysis and oxygen release for adaptation to high-altitude hypoxia. Nat Commun 2016; 7: 12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Dai Y, Wen J, et al. Detrimental effects of adenosine signaling in sickle cell disease. Nat Med 2011; 17: 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ericson A, de Verdier CH.A modified method for the determination of 2,3-diphosphoglycerate in erythrocytes. Scand J Clin Lab Invest 1972; 29: 84–90. [PubMed] [Google Scholar]
- 29.Bouet V, Boulouard M, Toutain J, et al. The adhesive removal test: a sensitive method to assess sensorimotor deficits in mice. Nat Protoc 2009; 4: 1560–1564. [DOI] [PubMed] [Google Scholar]
- 30.Longa EZ, Weinstein PR, Carlson S, et al. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989; 20: 84–91. [DOI] [PubMed] [Google Scholar]
- 31.Shalavadi MH, Chandrashekhar VM, Muchchandi IS.Neuroprotective effect of convolvulus pluricaulis choisy in oxidative stress model of cerebral ischemia reperfusion injury and assessment of MAP2 in rats. J Ethnopharmacol 2019; 249: 112393. [DOI] [PubMed] [Google Scholar]
- 32.Liang D, He XB, Wang Z, et al. Remote limb ischemic postconditioning promotes motor function recovery in a rat model of ischemic stroke via the up-regulation of endogenous tissue kallikrein. CNS Neurosci Ther 2018; 24: 519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Y, Zhou Y, Ma X, et al. DDAH-1 via HIF-1 target genes improves cerebral ischemic tolerance after hypoxic preconditioning and middle cerebral artery occlusion–reperfusion. Nitric Oxide 2020; 95: 17–28. [DOI] [PubMed] [Google Scholar]
- 34.Wu LR, Liu L, Xiong XY, et al. Vinpocetine alleviate cerebral ischemia/reperfusion injury by down-regulating TLR4/MyD88/NF-kappaB signaling. Oncotarget 2017; 8: 80315–80324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raleigh JA, Calkins-Adams DP, Rinker LH, et al. Hypoxia and vascular endothelial growth factor expression in human squamous cell carcinomas using pimonidazole as a hypoxia marker. Cancer Res 1998; 58: 3765–3768. [PubMed] [Google Scholar]
- 36.Carlsson R, Ozen I, Barbariga M, et al. STAT3 precedes HIF1alpha transcriptional responses to oxygen and oxygen and glucose deprivation in human brain pericytes. PLoS One 2018; 13: e0194146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pitoni S, D'Arrigo S, Grieco DL, et al. Tidal volume lowering by instrumental dead space reduction in brain-injured ARDS patients: effects on respiratory mechanics, gas exchange, and cerebral hemodynamics. Neurocrit Care 2020; 1--10. [DOI] [PMC free article] [PubMed]
- 38.Ratcliffe PJ.Oxygen sensing and hypoxia signalling pathways in animals: the implications of physiology for cancer. J Physiol 2013; 591: 2027–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pan J, Li X, Peng Y.Remote ischemic conditioning for acute ischemic stroke: dawn in the darkness. Rev Neurosci 2016; 27: 501–510. [DOI] [PubMed] [Google Scholar]
- 40.Ferrari J, Krebs S, Sykora M.Intravenous thrombolysis and mechanical thrombectomy in patients with minor or rapidly improving neurological deficits. Curr Opin Neurol 2019; 32: 13–18. [DOI] [PubMed] [Google Scholar]
- 41.Botker HE, Kharbanda R, Schmidt MR, et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet 2010; 375: 727–734. [DOI] [PubMed] [Google Scholar]
- 42.Chong J, Bulluck H, Yap EP, et al. Remote ischemic conditioning in ST-segment elevation myocardial infarction – an update. Cond Med 2018; 1: 13–22. [PMC free article] [PubMed] [Google Scholar]
- 43.Liu S, Shi H, Liu W, et al. Interstitial pO2 in ischemic penumbra and core are differentially affected following transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab 2004; 24: 343–349. [DOI] [PubMed] [Google Scholar]
- 44.Kronenberg G, Uhlemann R, Richter N, et al. Distinguishing features of microglia- and monocyte-derived macrophages after stroke. Acta Neuropathol 2018; 135: 551–568. [DOI] [PubMed] [Google Scholar]
- 45.Spescha RD, Shi Y, Wegener S, et al. Deletion of the ageing gene p66(Shc) reduces early stroke size following ischaemia/reperfusion brain injury. Eur Heart J 2013; 34: 96–103. [DOI] [PubMed] [Google Scholar]
- 46.Kono N, Kuwajima M, Tarui S.Alteration of glycolytic intermediary metabolism in erythrocytes during diabetic ketoacidosis and its recovery phase. Diabetes 1981; 30: 346–353. [DOI] [PubMed] [Google Scholar]
- 47.Lenfant C, Torrance J, English E, et al. Effect of altitude on oxygen binding by hemoglobin and on organic phosphate levels. J Clin Invest 1968; 47: 2652–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benesch R, Benesch RE, Enoki Y.The interaction of hemoglobin and its subunits with 2,3-diphosphoglycerate. Proc Natl Acad Sci U S A 1968; 61: 1102–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McKenna MC, Scafidi S, Robertson CL.Metabolic alterations in developing brain after injury: knowns and unknowns. Neurochem Res 2015; 40: 2527–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geng J, Zhang Y, Li S, et al. Metabolomic profiling reveals that reprogramming of cerebral glucose metabolism is involved in ischemic preconditioning-induced neuroprotection in a rodent model of ischemic stroke. J Proteome Res 2019; 18: 57–68. [DOI] [PubMed] [Google Scholar]
- 51.Liu H, Zhang Y, Wu H, et al. Beneficial role of erythrocyte adenosine A2B receptor-mediated AMP-activated protein kinase activation in high-altitude hypoxia. Circulation 2016; 134: 405–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu ZJ, Chen C, Li XR, et al. Remote ischemic preconditioning-mediated neuroprotection against stroke is associated with significant alterations in peripheral immune responses. CNS Neurosci Ther 2016; 22: 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang J, Balkaya M, Beltran C, et al. Remote postischemic conditioning promotes stroke recovery by shifting circulating monocytes to CCR2(+) proinflammatory subset. J Neurosci 2019; 39: 7778–7789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shan LY, Li JZ, Zu LY, et al. Platelet-derived microparticles are implicated in remote ischemia conditioning in a rat model of cerebral infarction. CNS Neurosci Ther 2013; 19: 917–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rise N, Kristiansen J, Hvas AM, et al. Effect of remote ischaemic conditioning on platelet aggregation and platelet turnover. J Thromb Thrombolysis 2018; 46: 528–533. [DOI] [PubMed] [Google Scholar]
- 56.Kimura H, Hamasaki N, Yamamoto M, et al. Circulation of red blood cells having high levels of 2,3-bisphosphoglycerate protects rat brain from ischemic metabolic changes during hemodilution. Stroke 1995; 26: 1431–1436; discussion 6–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X20952264 for Remote ischemic conditioning enhances oxygen supply to ischemic brain tissue in a mouse model of stroke: Role of elevated 2,3-biphosphoglycerate in erythrocytes by Lin Wang, Changhong Ren, Yang Li, Chen Gao, Ning Li, Haiyan Li, Di Wu, Xiaoduo He, Changqing Xia and Xunming Ji in Journal of Cerebral Blood Flow & Metabolism