Abstract
Vascular dysfunctions, including arterial stiffness and endothelial dysfunction, are prevalent in hypertensive subjects. We aimed to study their relations to subclinical intracranial vascular health in this study. A total of 200 older hypertensive males without overt cardiovascular or cerebrovascular diseases were recruited. Arterial elasticity was measured as carotid-femoral pulse wave velocity (cfPWV) and endothelial function was measured as digital reactive hyperemia index (RHI). Cerebrovascular health was evaluated using MRI in four aspects: intracranial atherosclerosis, brain perfusion as cerebral blood flow (CBF), vascular rarefaction analyzed as visible arterial branches on angiography using a custom-developed analysis technique and small vessel disease measured as white matter hyperintensity (WMH). There was a significant negative association between cfPWV and CBF, suggesting a link between arterial stiffness and CBF decline. Higher cfPWV was also associated with presence of intracranial stenotic plaque and greater WMH volume. RHI was positively related to CBF, indicating that endothelial dysfunction was associated with reduced CBF. All the associations remained significant after adjustment for confounding variables. Arterial stiffness and endothelial dysfunction are associated with reduced brain perfusion in older hypertensive males. Arterial stiffness is also associated with global cerebral vascular injury, affecting both small and medium-to-large arteries.
Keywords: Arterial stiffness, cerebrovascular health, endothelial function, MRI, vascular function
Introduction
Vascular dysfunctions, including arterial stiffness and endothelial dysfunction, are tightly associated with hypertension and may serve as indicators of typical target-organ damage in essential hypertension. In turn, they can promote further increases in blood pressure (BP) and contribute to adverse cardiovascular outcomes.1–4 In light of their considerable pathophysiological and clinical implications, a number of non-invasive methods have been developed to assess arterial stiffness and endothelial dysfunction. For arterial stiffness, pulse wave velocity (PWV) has been recommended as the “gold-standard” measurement with several commercially available devices in the market.5 For endothelial dysfunction, ultrasonography-based flow mediated dilation (FMD) has been widely used since its introduction in the early 1990s.6 More recently, the pulse amplitude tonometry (PAT) technique has received increasing interest for measurement of digital endothelial function given its operator-independence and higher reproducibility compared to FMD.7 These techniques, when used in combination, provide a comprehensive evaluation to study vascular function in vivo.
The associations between vascular dysfunctions and extracranial hypertensive target-organ damage have been well documented.8–12 However, the relationship of arterial stiffness and endothelial dysfunction to intracranial vascular health has rarely been explored, despite the fact that hypertension is a well-established risk factor for stroke and vascular cognitive impairment. A few prior attempts have linked arterial stiffness to small vessel diseases, such as white matter hyperintensity (WMH) and microbleeds.13–15 Nevertheless, medium-to-large arteries, as the other important component of the cerebrovascular system, have largely been neglected. Meanwhile, the lack of techniques for direct assessment of intracranial vascular structural and functional properties, rather than merely indications based on structural magnetic resonance imaging (MRI), leaves a large gap in information on cerebrovascular vascular health. These limitations can be partly attributed to the technical challenges in assessing intracranial vascular health via imaging, due to the presence of the skull and extremely tortuous vessels.
Here, we used a series of cutting-edge MRI techniques and custom-developed analysis tools to examine the relationship of vascular function (both arterial stiffness and endothelial dysfunction) with global intracranial vascular health in a pilot cohort study. Vascular health is defined as a combination of information from the intracranial vessel wall, blood flow in large and intermediate arteries, tissue perfusion and small vessel disease. We want to test the hypothesis that arterial stiffness and endothelial dysfunction are associated with subclinical cerebrovascular injuries in hypertensive subjects and this relationship is not limited to small vessel diseases. To the best of our knowledge, our work represents one of the most comprehensive evaluations to date of the association between vascular dysfunction and intracranial vascular health.
Material and methods
Study participants
This study analyzes the baseline data of the Brain and Vascular Health in the Elderly (BRAVE-1) study. The BRAVE-1 study aimed to use multimodal MRI to study the interrelationship between vascular health and brain aging. The study consecutively recruited 200 older males (≥55 years old) with essential hypertension and without clinically overt neurological, cardio- or cerebrovascular disease. Subjects with psychological disorders or contraindications to MRI were excluded. Full eligibility criteria for the study are included in the Supplements. All participants provided written informed consent. The study protocol was approved by the Institutional Ethics Committee of The Second Affiliated Hospital of Nanjing Medical University. All procedures were conducted according to the Declaration of Helsinki.
Vascular function
Vascular functions and BP were measured in the morning in a quiet test room with the room temperature set at 22°C–25°C. Participants were instructed to refrain from meals, alcohol, coffee or tea before the measurements. Antihypertensive medications and short-acting nitrates were not allowed within 2 h, and long-acting nitrates were for 12 h before measurement to minimize the influence of their vasoactive effect on the tests. Participants seated for at least 5 min before a research staff measured their BP using an automatic device (Omron HEM-7130; Omron Healthcare Co. Ltd, Japan) thrice. The results were averaged for current analysis.
Carotid-femoral PWV (cfPWV) was measured using the Complior Analyzer device (Artech Medical, France) by an operator with over five years’ experience. With subjects in the supine position, three probes were placed over the palpable pulse of the carotid, femoral and radial arteries. Transit time was averaged over 10 consecutive recordings using the intersecting tangent algorithm. Carotid–femoral distance was measured and multiplied by 0.8. PWV was calculated as distance divided by transit time. Any measurement with a tolerance value ≥3 was considered invalid. We only used cfPWV in our analysis, as it is the current “gold standard” for measuring arterial stiffness and has been validated as superior to brachial (carotid-radial) PWV for predicting cardiovascular outcomes.16
PAT was performed using the EndoPAT 2000 device (Itamar Medical, Israel). Two finger probes were placed on the index finger of each hand. After 6 min of baseline measurement, pulsatile arterial flow was occluded through inflation of a blood pressure cuff on the test arm for 5 min. The cuff was inflated starting at a pressure of 250 mmHg and increased until a complete occlusion was achieved as judged by the PAT signal or to a maximum of 300 mmHg. The signal was recorded for another 5 min after occlusion. The reactive hyperemia index (RHI), which is a ratio of the average amplitude of the PAT signal over a 1-min time interval starting 1 min after cuff deflation divided by the average amplitude of the PAT signal over a 3.5-min period before cuff inflation (baseline signal), was calculated automatically by the system algorithm.17 Reproducibility and accuracy of the PAT measurement for assessing endothelial function have been validated in prior work.17,18
MRI protocol
MRI was performed with a 3 Tesla GE scanner (Signa HDxt, GE Healthcare). We assessed cerebrovascular health in the following four dimensions: intracranial atherosclerosis, vascular rarefaction, cerebral blood flow (CBF) and small vessel disease (as measured by WMH). The following sequences were used and analyzed for intracranial vascular health evaluation: 3D T1-weighted fast spin echo (FSE) intracranial vessel wall imaging, 3D time-of-flight (TOF) angiography, 3D pseudo-continuous arterial spin labeling (ASL), 3D magnetization prepared rapid acquisition with gradient echo (MP-RAGE) imaging and T2-weighted fluid-attenuated inversion recovery (FLAIR) imaging. Sequence protocols are presented in Table S1 in the Supplements.
MRI image analysis
Image review processes are introduced briefly below. For a detailed description of the review protocol as well as the tools we used for the analysis, please refer to MRI review process section in the Supplements.
Intracranial atherosclerosis was assessed using vessel wall imaging (3D T1-weighted fast spin echo) in combination with angiography (3D time-of-flight). Atherosclerotic plaques, defined as visible focal thickening on vessel wall imaging, were identified on proximal intracranial artery segments up to and including the ICA, BA, A1, M1 and P1 segments. A stenotic plaque was defined as ≥30% stenosis on MRA.
Vascular rarefaction was analyzed using the custom-developed iCafe (intraCranial Artery Feature Extraction) technique.19 While rarefaction has been generally used to indicate reduced density of capillaries and precapillary arterioles, we used it here to describe less discernible vessels on MRA. This phenomenon we have identified in the process of aging, which indicates that vascular rarefaction may actually develop at more proximal arterial segments and is not exclusively restricted to microvessel in the brain.20 A trained reader used the software to process TOF images. Artery regions were first automatically traced using an open-curve active contour model and then the reader manually corrected the traces when needed. The arteries were then labelled with anatomical names. Vascular rarefaction was assessed using branch number, which was calculated as the total number of artery branches excluding the major large segments (i.e. internal carotid arteries, vertebral arteries, basilar artery, M1, P1 and A1).
CBF was calculated from 3D arterial spin labeling images using the recommended hemodynamic model,21 after co-registration to structural images (3D magnetization prepared rapid acquisition with gradient echo). WMH volume was quantified on T2-weighted fluid attenuated inversion recovery images using an automatic pipeline adapted from Roura et al.22 The results were reviewed to manually correct errors and exclude lesions detected in the cerebellum and brainstem.
General data collection
Each participant was interviewed by a research staff with a structured data collection form. Blood tests were performed in an independent central laboratory (KingMed Diagnostics, Nanjing, China) using fasting blood samples. Physical activity was evaluated using the Global Physical Activity Questionnaire (GPAQ) (Chinese version 2) and subjects were categorized into low, moderate and high physical activity according to the analysis guide. Those with low physical activity were considered as physical inactive for the analysis. Uncontrolled hypertension was defined as systolic BP ≥ 140 mm Hg or diastolic BP ≥ 90 mm Hg.
Statistical analyses
Numerical variables and categorical variables were presented as mean ± sd or median (IQR) and number (%), respectively. Normal distribution of variables was determined using the Shapiro–Wilk’s test. WMH volume was expressed as percentage of intracranial volume, and was then log-transformed due to the skewed distribution. RHI was also natural log transformed (LnRHI) in the association analyses. We used logistic regression analysis to analyze the associations between vascular dysfunctions and intracranial atherosclerosis (plaque and stenotic plaque). The associations of vascular function measures with brain perfusion (CBF), vascular rarefaction (branch number) and small vessel disease (WMH) were analyzed using multivariable linear regression. Confounding variables including age, hypertension control, diabetes, renal function, smoking, body mass index (BMI), high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, use of antihypertensive medication, statins and physical inactivity were adjusted to justify the independent associations between vascular dysfunction and cerebrovascular health metrics. We also adjusted endothelial function for arterial elasticity and vice versa. All statistical analyses were performed using R software, version 3.4.3 (R Foundation for Statistical Computing, www.R-project.org). A two-tailed p value of 0.05 was considered statistically significant.
Results
General characteristics of the study population
Among all the 200 participants enrolled in the BRAVE-1 study, MRI scans were not acquired in 11 subjects. The remaining 189 subjects were included in the current analysis. There was one subject with missing cfPWV and another with missing RHI due to unsuccessful measurement. For each cerebrovascular health metric analysis, 6, 11 and 9 cases were excluded for CBF, intracranial atherosclerosis and WMH, respectively, due to unavailable MRI images or insufficient image quality. Representative illustrations of image review are presented in Figure 1 . The general characteristics for the study population are presented in Table 1 . Mean age was 64.9 ± 7.2 years. Average systolic and diastolic BP level was 143.0 ± 15.9 mm Hg and 88.6 ± 9.4 mm Hg, respectively. Mean CBF was 37.7 ± 6.1 ml/100 g/min. There were 44 (24.7%) subjects with intracranial plaque and 21 (11.8%) subjects with stenotic plaque. Average number of intracranial arterial branches was 92 ± 11 and median WMH volume was 0.55% (0.37%–0.83%). Average cfPWV and RHI level was 9.8 ± 2.3 m/s and 2.0 ± 0.6, respectively.
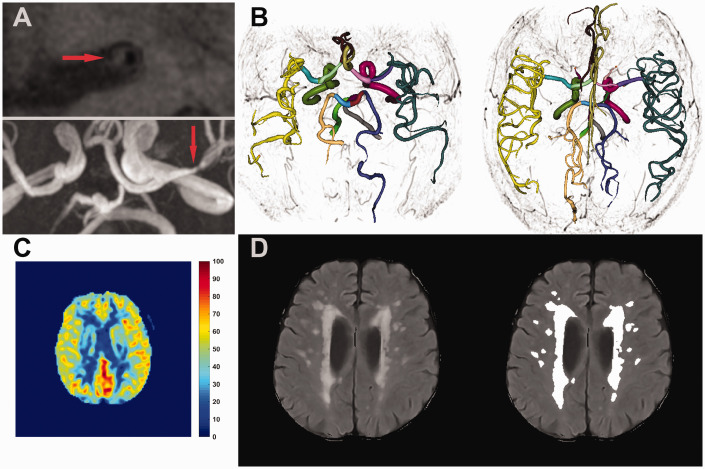
Figure 1.
Illustrations of intracranial vascular MR image review. (a) Cross-sectional view of the left middle cerebral artery (M1 segment) with an identified plaque (Upper panel). The location of the plaque was marked on time-of-flight image (lower panel). (b) Representative cases for iCafe analysis. The left case showed significant vascular rarefaction compared to the right one. Different colors indicate different artery segments as labelled in iCafe. (c) A representative cerebral blood flow map obtained from arterial spin labeling images. (d) White matter hyperintensity lesions detected using the automatic pipeline. The right panel presents the raw image with the left panel showing lesions detected and highlighted by the automatic pipeline.
Table 1.
Characteristics of the study population (N = 189).
| Mean ± SD / number (%) | |
|---|---|
| Age, years | 64.9 ± 7.2 |
| BMI, kg/m2 | 25.4 ± 2.6 |
| Current smoker | 56 (29.6%) |
| Diabetes | 38 (20.1%) |
| Antihypertensive medication | 185 (97.9%) |
| Statins | 28 (14.8%) |
| Systolic BP, mm Hg | 143.0 ± 15.9 |
| Diastolic BP, mm Hg | 88.6 ± 9.4 |
| Uncontrolled hypertension | 131 (69.3%) |
| Physical inactivity | 85 (45.0%) |
| Total cholesterol, mmol/L | 4.66 ± 0.81 |
| Triglycerides, mmol/L | 1.67 ± 1.19 |
| HDL cholesterol, mmol/L | 1.25 ± 0.30 |
| LDL cholesterol, mmol/L | 2.87 ± 0.76 |
| eGFR, ml/min/1.73 m2 | 82.3 ± 14.3 |
| Cerebrovascular health metrics | |
| CBF, ml/100 g/min | 37.7 ± 6.1 |
| Plaque / stenotic plaque | 44 (24.7%) / 21 (11.8%) |
| Number of arterial branch | 92 ± 11 |
| WMHa | 0.55 (0.37–0.83) |
| cfPWV | 9.8 ± 2.3 |
| RHI | 2.0 ± 0.6 |
WMH (%) was expressed as (total WMH volume/intracranial volume) × 100.
BMI: body mass index; BP: blood pressure; HDL: high-density lipoprotein; LDL: low-density lipoprotein; eGFR: estimated glomerular filtration rate; CBF: cerebral blood flow; WMH: white matter hyperintensity; cfPWV: carotid-femoral pulse wave velocity; RHI: reactive hyperemia index.
Arterial elasticity and cerebrovascular health
The associations between arterial elasticity and cerebrovascular health metrics are summarized in Table 2 . In the first model with adjustment for age, BP control, diabetes and renal function, there was a significant negative association between cfPWV and CBF (β = −0.53, p = 0.018), suggesting a link between worse arterial elasticity and CBF decline. When more potential covariates (smoking, body mass index, HDL cholesterol, LDL cholesterol, use of antihypertensive medication, statins and physical inactivity) were included, the association was still significant (β = −0.57, p = 0.009). Finally, adding endothelial function (LnRHI) as a covariate in the analysis did not alter the significance of the result (β = −0.60, p = 0.006).
Table 2.
Associations between arterial elasticity and cerebrovascular health metrics.
|
CBF |
Plaque |
Stenotic plaque |
Branch number |
WMH |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β (se) | p | OR (95% CI) | p | OR (95% CI) | p | β (se) | p | β (se) | p | |
| Model-1 | −0.53 (0.22) | 0.018 | 1.03 (0.86–1.22) | 0.772 | 1.24 (1.01–1.54) | 0.043 | 0.29 (0.40) | 0.468 | 0.03 (0.01) | 0.003 |
| Model-2 | −0.57 (0.22) | 0.009 | 1.05 (0.88–1.26) | 0.556 | 1.26 (1.02–1.59) | 0.032 | 0.28 (0.40) | 0.487 | 0.03 (0.01) | 0.001 |
| Model-3 | −0.60 (0.21) | 0.006 | 1.06 (0.89–1.27) | 0.498 | 1.28 (1.03–1.62) | 0.029 | 0.27 (0.41) | 0.509 | 0.03 (0.01) | 0.001 |
OR: odds ratio.
Model-1: Adjusted for age, control of hypertension, diabetes and renal function (eGFR).
Model-2: Model-1 + smoking + BMI + HDL cholesterol + LDL cholesterol + use of antihypertensives + statins + physical inactivity.
Model-3: Model-2 + LnRHI.
There was no association between cfPWV and the presence of total plaque. Instead, higher cfPWV was associated with increased risk of having stenotic plaque (all p ≤ 0.043). In addition, there was a significant association of increased cfPWV with more severe WMH (all p ≤ 0.003). We did not note any association between arterial elasticity and vascular rarefaction as measured by branch number.
Endothelial function and cerebrovascular health
The associations between endothelial function and cerebrovascular health metrics are presented in Table 3 . There was a positive association between LnRHI and CBF (Model-1: β = 4.33, p = 0.007; Model-2: β = 3.63, p = 0.025), indicating that worse endothelial function was associated with a decline in CBF. This association remained significant after including cfPWV as a covariate (β = 3.98, p = 0.012). Endothelial function was not associated with the presence of plaque/stenotic plaque, branch number or WMH.
Table 3.
Associations between endothelial function and cerebrovascular health metrics.
|
CBF |
Plaque |
Stenotic plaque |
Branch number |
WMH |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β (se) | p | OR (95% CI) | p | OR (95% CI) | p | β (se) | p | β (se) | p | |
| Model-1 | 4.33 (1.58) | 0.007 | 0.53 (0.14–1.90) | 0.331 | 0.40 (0.07–2.12) | 0.285 | 1.54 (2.92) | 0.600 | −0.04 (0.08) | 0.618 |
| Model-2 | 3.63 (1.60) | 0.025 | 0.48 (0.12–1.85) | 0.293 | 0.24 (0.03–1.54) | 0.143 | 0.67 (3.04) | 0.827 | −0.05 (0.08) | 0.523 |
| Model-3 | 3.98 (1.58) | 0.012 | 0.46 (0.11–1.78) | 0.266 | 0.20 (0.03–1.38) | 0.115 | 0.47 (3.06) | 0.879 | −0.06 (0.08) | 0.428 |
OR: odds ratio.
Model-1: Adjusted for age, control of hypertension, diabetes and renal function (eGFR);
Model-2: Model-1 + smoking + BMI + HDL cholesterol + LDL cholesterol + use of antihypertensives + statins + physical inactivity;
Model-3: Model-2 + cfPWV.
Discussion
Understanding systemic modifiable contributors to impaired cerebrovascular health is important for identifying early interventional targets to maintain a healthy brain. In this study, we explored the association of systemic vascular function, including arterial elasticity and endothelial function, with subclinical cerebrovascular health metrics in a group of hypertensive males without clinically overt cerebro- or cardiovascular disease. Our results demonstrated that (1) both decreased arterial elasticity and endothelial dysfunction were associated with diminished brain perfusion as measured by CBF, and (2) decreased arterial elasticity was also associated with the presence of stenotic intracranial arterial plaque and increased WMH volume. Our data suggest that vascular dysfunction may contribute to brain hypoperfusion in hypertension and that the presence of arterial stiffness may also lead to more severe intracranial atherosclerosis and small vessel disease.
The adequate functioning of the brain highly depends on cerebral blood supply, which accounts for 20% of total cardiac output. The brain has a set of orchestrated autoregulation mechanisms to maintain a constant blood flow, impairment of which will eventually result in tissue damage and brain dysfunction. Here, our data establish a link between systemic vascular dysfunction with intracranial blood flow decline which may shed light on understanding the pathophysiological mechanisms underlying CBF decline in hypertension.
Arterial stiffness, as characterized by loss of elastin and accumulation of abnormal collagen within the medial layer of the large vessels, compromises the buffering capacity of the aorta for the pulsatile flow ejected from the heart and thus increases the transmission of pulsatile energy into the intracranial circulation.23 This detrimental pressure pulsatility can induce adaptive remodeling of the brain vessels to increase intracranial vascular resistance and reduce CBF. In addition, there is evidence suggesting that diminished microvessel density may also contribute to the association between carotid stiffness and decreased CBF.24
Endothelial dysfunction is mainly characterized by reduced vasodilatory capacity as well as a proinflammatory and prothrombic state of the endothelium.25 The endothelium plays a critical role in the modulation of vascular tone through the release of a variety of vasoactive factors, such as nitric oxide. Injury to endothelial cells and loss of normal function can lead to impaired cerebral vasoreactivity and enhanced constriction of the arterioles, which promote the reduction of CBF as found in our study.26,27 To the best of our knowledge, the current study is among the first to identify a link between extracranial endothelial function measured in the peripheral vascular bed and intracranial blood flow.
Besides CBF, we also found significant associations between arterial stiffness with presence of stenotic plaque and increased WMH volume. It is interesting to note that elevated cfPWV is only related to increased risk of having stenotic plaque but not general plaque, possibly indicating that arterial stiffness has a more prominent role in the progression of atherosclerosis and arterial remodeling than in the initiation of plaque formation. The association between cfPWV and WMH volume supports the aforementioned hypothesis that arterial stiffness increases the transmission of harmful pulsatile energy into the cerebral circulation, resulting in arteriole remodeling and hypoperfusion. The pathogenesis role of increased pulsatility in intracranial small vessels disease has also been revealed by studies measuring cerebrovascular pulsatility directly.28,29
Of note, although endothelial dysfunction has been suggested as a key early step in the pathophysiology of atherosclerosis,30 we did not find an association with intracranial atherosclerosis in our study. The reason for this might be that the small numbers of subjects having plaque in a relatively healthy study population limit the power to detect a significant association. It may also be possible that the endothelial function measured at the digital vascular beds is too remote to reflect the local situation in the cerebral circulation. In fact, some previous studies have tried to measure cerebrovascular reactivity using MRI and found that its impairment is involved in a variety of intracranial vascular pathogenesis, including both small and large vessel diseases.31–34 These data indicate the importance of directly measuring local intracranial endothelial function. However, questions remain as to what extent these cerebrovascular reactivity measurements rely on intracranial endothelial cells since very few of these investigations had used direct endothelial-dependent stimuli (e.g. L-arginine). Meanwhile, how systematic endothelial dysfunction affects intracranial endothelial health may also be deserving of further exploration.
For a better interpretation, our results need to be considered together with prior findings. In the study by Jefferson et al.,35 higher aortic PWV was associated with lower frontal lobe CBF in 155 older adults with normal cognition and without stroke or heart failure. In another small study by Tarumi et al.,36 cfPWV was inversely associated with cerebral perfusion in deep subcortical frontal white matter among 35 adults without cardiovascular or cerebrovascular disease. There are few explorations into the association between endothelial function and brain vascular measures. In the Framingham Offspring Study, Tsao et al.15 found no association between brachial FMD and brain structural and vascular measures including brain volume, WMH volume and cerebral infarcts. However, cerebral blood flow was not measured in their study. The associations between arterial stiffness and intracranial stenotic plaque as well as WMH volume are also supported by previous data.37–39
The findings of our study are clinically relevant. The data indicate the potentially important role of vascular dysfunction in the pathophysiological pathway linking hypertension and impaired cerebrovascular health. Arterial stiffness and endothelial dysfunction may serve as screening indicator for subclinical cerebrovascular injury in hypertensive patients. They are also potential targets for early intervention to prevent irreversible brain outcomes if the causative relationships are confirmed.
Our study has several strengths. First, we combined PWV and RHI measurement to provide non-invasive evaluation of extracranial vascular function in a relatively large aging cohort. This enables us to study not only arterial stiffness but also endothelial dysfunction for their association with cerebrovascular health. Second, we used a series of cutting-edge imaging and analysis techniques to generate a comprehensive map of cerebrovascular health metrics. With these metrics, we acquired a detailed phenotyping of intracranial vascular condition at the subclinical stage. In contrast to previous studies which usually used surrogate structural measures or were limited to small vessel disease, our approach covers both the small vessels and the medium-to-large vessels, with direct visualization of the vessels. This comprehensive evaluation provides us an advantage of acquiring a global picture on the effect of systemic vascular dysfunction on cerebrovascular health. Lastly, the extensive data collection in the BRAVE-1 study enable us to perform adjustment for a variety of potential confounding factors, which strengthens the independent associations found in our study.
There are also some limitations that need to be pointed out. First, the cross-sectional nature of this analysis cannot help to establish causality or temporal relationship. The results warrant further validation in prospective studies or clinical trials. Second, the inclusion of only older hypertensive males suggests that the findings may not be generalizable to females or other populations. Lastly, we used quantitative features from MRA images to describe “rarefaction” of medium-to-large vessels in our analysis. While this measurement provides unique and complementary information on global cerebrovascular health over small vessel disease markers, the nature of MRA indicates that this measure represents functional rarefaction, which means a lack of effective flow in those arteries, rather than structural abnormalities.
In conclusion, arterial stiffness and endothelial dysfunction are both related to declined CBF in older hypertensive males without overt stroke or cardiovascular diseases. Arterial stiffness also relates to the presence of intracranial stenotic plaque and greater volume of WMH. Our results suggest that vascular dysfunctions may provide further implications over BP control for targeted intervention to maintain cerebrovascular health and brain function at subclinical stage.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study is supported by a grant from Jiangsu Science and Technology Department (BE2017762) to Dr. Junwei Yang.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X20956950 for Arterial elasticity, endothelial function and intracranial vascular health: A multimodal MRI study by Wenjin Liu, Zhensen Chen, Dakota Ortega, Xuebing Liu, Xiaoqin Huang, Lulu Wang, Li Chen, Jie Sun, Thomas S Hatsukami, Chun Yuan, Haige Li and Junwei Yang in Journal of Cerebral Blood Flow & Metabolism
Acknowledgements
We would like to thank all the participants of the BRAVE-1 study.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Wenjin Liu is supported by NVIDIA Corporation with the donation of the Titan V GPU used for this research. For others, none to declare.
Authors’ contributions: JY, HL and CY designed the study. WL, XL, XH and LW conducted the study. WL, ZC, DO, LC and JS analyzed and/or interpreted the data. WL wrote the paper. TH, CY, HL and JY revised the paper.
ORCID iD: Li Chen https://orcid.org/0000-0003-0233-4576
Supplemental material: Supplemental material for this article is available online.
References
- 1.Mitchell GF. Arterial stiffness and hypertension. Hypertension 2014; 64: 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franklin SS. Arterial stiffness and hypertension. Hypertension 2005; 45: 349–351. [DOI] [PubMed] [Google Scholar]
- 3.Dharmashankar K, Widlansky ME. Vascular endothelial function and hypertension: insights and directions. Curr Hypertens Rep 2010; 12: 448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mordi I, Mordi N, Delles C, et al. Endothelial dysfunction in human essential hypertension. J Hypertens 2016; 34: 1464–1472. [DOI] [PubMed] [Google Scholar]
- 5.Laurent S Cockcroft J Van Bortel L European Network for Non-invasive Investigation of Large Arteries, , et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006; 27: 2588–2605. [DOI] [PubMed] [Google Scholar]
- 6.Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 1992; 340: 1111–1115. [DOI] [PubMed] [Google Scholar]
- 7.Hamburg NM, Benjamin EJ. Assessment of endothelial function using digital pulse amplitude tonometry. Trends Cardiovasc Med 2009; 19: 6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsui Y, Ishikawa J, Shibasaki S, et al. Association between home arterial stiffness index and target organ damage in hypertension: comparison with pulse wave velocity and augmentation index. Atherosclerosis 2011; 219: 637–642. [DOI] [PubMed] [Google Scholar]
- 9.Bruno RM, Cartoni G, Stea F, et al. Carotid and aortic stiffness in essential hypertension and their relation with target organ damage: the CATOD study. J Hypertens 2017; 35: 310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coutinho T, Turner ST, Kullo IJ. Aortic pulse wave velocity is associated with measures of subclinical target organ damage. JACC Cardiovasc Imaging 2011; 4: 754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghiadoni L, Taddei S, Virdis A, et al. Endothelial function and common carotid artery wall thickening in patients with essential hypertension. Hypertension 1998; 32: 25–32. [DOI] [PubMed] [Google Scholar]
- 12.Treasure CB, Klein JL, Vita JA, et al. Hypertension and left ventricular hypertrophy are associated with impaired endothelium-mediated relaxation in human coronary resistance vessels. Circulation 1993; 87: 86–93. [DOI] [PubMed] [Google Scholar]
- 13.Poels MMF, Zaccai K, Verwoert GC, et al. Arterial stiffness and cerebral small vessel disease. Stroke 2012; 43: 2637–2642. [DOI] [PubMed] [Google Scholar]
- 14.Ding J, Mitchell GF, Bots ML, et al. Carotid arterial stiffness and risk of incident cerebral microbleeds in older people. Arterioscler Thromb Vasc Biol 2015; 35: 1889–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsao CW, Seshadri S, Beiser AS, et al. Relations of arterial stiffness and endothelial function to brain aging in the community. Neurology 2013; 81: 984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Bortel LM Laurent S Boutouyrie P European Network for Noninvasive Investigation of Large Arteries, , et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens 2012; 30: 445–448. [DOI] [PubMed] [Google Scholar]
- 17.Bonetti PO, Pumper GM, Higano ST, et al. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol 2004; 44: 2137–2141. [DOI] [PubMed] [Google Scholar]
- 18.Brant LC, Barreto SM, Passos VM, et al. Reproducibility of peripheral arterial tonometry for the assessment of endothelial function in adults. J Hypertens 2013; 31: 1984–1990. [DOI] [PubMed] [Google Scholar]
- 19.Chen L, Mossa-Basha M, Balu N, et al. Development of a quantitative intracranial vascular features extraction tool on 3D MRA using semiautomated open-curve active contour vessel tracing. Magn Reson Med 2018; 79: 3229–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen L, Sun J, Hippe DS, et al. Quantitative assessment of the intracranial vasculature in an older adult population using iCafe. Neurobiol Aging 2019; 79: 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the european consortium for ASL in dementia. Magn Reson Med 2015; 73: 102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roura E, Oliver A, Cabezas M, et al. A toolbox for multiple sclerosis lesion segmentation. Neuroradiology 2015; 57: 1031–1043. [DOI] [PubMed] [Google Scholar]
- 23.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol 2005; 25: 932–943. [DOI] [PubMed] [Google Scholar]
- 24.Muhire G, Iulita MF, Vallerand D, et al. Arterial stiffness due to carotid calcification disrupts cerebral blood flow regulation and leads to cognitive deficits. J Am Heart Assoc 2019; 8: e011630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Endemann DH, Schiffrin EL. Endothelial dysfunction. J Am Soc Nephrol 2004; 15: 1983–1992. [DOI] [PubMed] [Google Scholar]
- 26.Lavi S, Gaitini D, Milloul V, et al. Impaired cerebral CO2 vasoreactivity: association with endothelial dysfunction. Am J Physiol Heart Circ Physiol 2006; 291: H1856–H1861. [DOI] [PubMed] [Google Scholar]
- 27.Hannah RM, Dunn KM, Bonev AD, et al. Endothelial SKCa and IKCa channels regulate brain parenchymal arteriolar diameter and cortical cerebral blood flow. J Cereb Blood Flow Metab 2011; 31: 1175–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Birnefeld J, Wahlin A, Eklund A, et al. Cerebral arterial pulsatility is associated with features of small vessel disease in patients with acute stroke and TIA: a 4D flow MRI study. J Neurol 2020; 267: 721–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geurts LJ, Zwanenburg JJM, Klijn CJM, et al. Higher pulsatility in cerebral perforating arteries in patients with small vessel disease related stroke, a 7T MRI study. Stroke 2019; 50: 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004; 109: III-27–III-32. [DOI] [PubMed] [Google Scholar]
- 31.van Opstal AM, van Rooden S, van Harten T, et al. Cerebrovascular function in presymptomatic and symptomatic individuals with hereditary cerebral amyloid angiopathy: a case-control study. Lancet Neurol 2017; 16: 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cogswell PM, Davis TL, Strother MK, et al. Impact of vessel wall lesions and vascular stenoses on cerebrovascular reactivity in patients with intracranial stenotic disease. J Magn Reson Imaging 2017; 46: 1167–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhogal AA, De Vis JB, Siero JCW, et al. The BOLD cerebrovascular reactivity response to progressive hypercapnia in young and elderly. Neuroimage 2016; 139: 94–102. [DOI] [PubMed] [Google Scholar]
- 34.Blair GW, Doubal FN, Thrippleton MJ, et al. Magnetic resonance imaging for assessment of cerebrovascular reactivity in cerebral small vessel disease: a systematic review. J Cereb Blood Flow Metab 2016; 36: 833–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jefferson AL, Cambronero FE, Liu D, et al. Higher aortic stiffness is related to lower cerebral blood flow and preserved cerebrovascular reactivity in older adults. Circulation 2018; 138: 1951–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tarumi T, Shah F, Tanaka H, et al. Association between central elastic artery stiffness and cerebral perfusion in deep subcortical gray and white matter. Am J Hypertens 2011; 24: 1108–1113. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J, Li Y, Wang Y, et al. Arterial stiffness and asymptomatic intracranial large arterial stenosis and calcification in hypertensive Chinese. Am J Hypertens 2011; 24: 304–309. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell GF, van Buchem MA, Sigurdsson S, et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the age, gene/environment susceptibility – Reykjavik study. Brain 2011; 134: 3398–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohmine T, Miwa Y, Yao H, et al. Association between arterial stiffness and cerebral white matter lesions in community-dwelling elderly subjects. Hypertens Res 2008; 31: 75–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X20956950 for Arterial elasticity, endothelial function and intracranial vascular health: A multimodal MRI study by Wenjin Liu, Zhensen Chen, Dakota Ortega, Xuebing Liu, Xiaoqin Huang, Lulu Wang, Li Chen, Jie Sun, Thomas S Hatsukami, Chun Yuan, Haige Li and Junwei Yang in Journal of Cerebral Blood Flow & Metabolism